Abstract
Background
Cytomegalovirus (CMV) is the leading infectious agent causing congenital sensorineural hearing loss and psychomotor retardation. CMV vaccine is currently unavailable and treatment options in pregnancy are limited. Susceptible pregnant women caring for children are at high risk for primary infection. CMV educational and hygienic measures have the potential to prevent primary maternal infection.
Methods
A mixed interventional and observational controlled study was conducted to investigate the effectiveness of hygiene information among pregnant women at risk for primary CMV infection for personal/occupational reasons. In the intervention arm, CMV-seronegative women, identified at the time of maternal serum screening for fetal aneuploidy at 11–12 weeks of gestation, were given hygiene information and prospectively tested for CMV until delivery. The comparison arm consisted of women enrolled at delivery who were neither tested for nor informed about CMV during pregnancy, and who had a serum sample stored at the screening for fetal aneuploidy. By design, groups were homogeneous for age, parity, education, and exposure to at least one risk factor. The primary outcome was CMV seroconversion. Acceptance of hygiene recommendations was a secondary objective and was measured by a self-report.
Findings
Four out of 331 (1.2%) women seroconverted in the intervention group compared to 24/315 (7.6%) in the comparison group (delta = 6.4%; 95% CI 3.2–9.6; P < 0.001). There were 3 newborns with congenital infection in the intervention group and 8 in the comparison group (1 with cerebral ultrasound abnormalities at birth). Ninety-three percent of women felt hygiene recommendations were worth suggesting to all pregnant women at risk for infection.
Interpretation
This controlled study provides evidence that an intervention based on the identification and hygiene counseling of CMV-seronegative pregnant women significantly prevents maternal infection. While waiting for CMV vaccine to become available, the intervention described may represent a responsible and acceptable primary prevention strategy to reduce congenital CMV.
Keywords: Cytomegalovirus, Primary infection, Pregnancy, Prevention, Hygiene measures
Highlights
-
•
The rate of CMV seroconversion was 4/331 (1.2%) in the intervention group vs 24/315 (7.6%) in the comparison group
-
•
This study shows that hygiene information of CMV-seronegative pregnant women significantly prevents maternal infection.
-
•
93% participants in the study found hygiene recommendations worth suggesting to all pregnant women.
Cytomegalovirus is the leading infectious agent causing congenital sensorineural hearing loss and psychomotor retardation. Contacts with young children have been identified as the main source of virus transmission to mothers. While waiting for a CMV vaccine to become available, this study documents that an intervention based on the identification and hygiene counseling of pregnant women susceptible to be infected by CMV for the first time during pregnancy, significantly prevents maternal infection.
1. Introduction
Cytomegalovirus (CMV) is the most common intrauterine infection. The burden caused by congenital CMV disease reportedly exceeds that caused by other childhood diseases such as Down syndrome, fetal alcohol syndrome and spina bifida (Cannon and Davis, 2005). Mental retardation, motor disabilities and hearing loss are among the most severe sequelae of congenital CMV infection (McCracken et al., 1969; Reynolds et al., 1974; Hanshaw et al., 1976). CMV has been assigned the highest priority for vaccine development (Arvin et al., 2004), but presently there is no licensed CMV vaccine.
Studies have identified two sources of maternal CMV infection: sexual activity and contact with young children (Fowler and Pass, 2006). However, the latter is considered the most important one as the high circulation rate of CMV in infants and children of pre-school age puts seronegative pregnant women caring for young children at high risk for CMV infection (Stagno et al., 1984; Taber et al., 1985; Walmus et al., 1988; Pass et al., 1990). Transmission occurs through direct contact with infectious bodily fluids such as saliva and urine (Cannon et al., 2011). Molecular epidemiology studies have demonstrated horizontal transmission among children and from child to mother (Adler, 1986; Revello et al., 2008).
In the past, 2 small studies performed by the same group in the USA and one French study suggested that behavioral interventions and avoiding intimate contacts with young children could reduce CMV seroconversion in pregnant women (Adler et al., 1996, 2004; Vauloup-Fellous et al., 2009). However, each of these studies had important limitations in their design and/or implementation and the true effectiveness and/or actual feasibility of hygiene recommendations in terms of routine use in general practice could not be assessed.
Here a controlled trial was conducted to assess the effectiveness of an intervention aimed at identifying and educating CMV-seronegative pregnant women with hygiene measures for primary CMV prevention. The target population consisted of pregnant women with frequent contacts with young children and, thus, at high risk for primary CMV infection.
2. Methods
2.1. Design of the Study
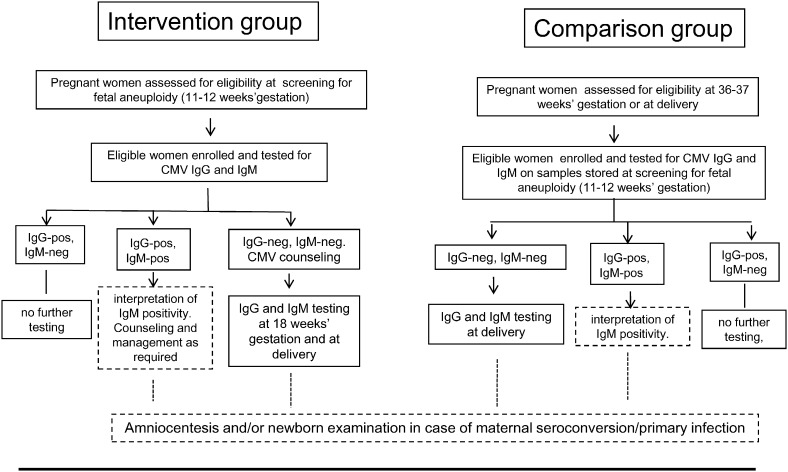
The study included a prospective intervention group and an observational comparison group (Fig. 1). Details regarding the identification of women at risk for CMV infection together with eligibility criteria are reported in the Suppl. data. Briefly, in the intervention group, all consecutive pregnant women undergoing serum screening for fetal aneuploidy at 11–12 weeks of gestation during the study period were assessed for risk factors for CMV infection. Women were considered at risk if they had their own child or had frequent contacts with children other than their own of less than 36 months of age. Pregnant women working with children of the same age group either full time or part time (health care or child care providers) together with pregnant women of less than 20 years of age were also considered at risk. Women, who were either CMV-seronegative or not previously tested for CMV immune status, were invited to participate in the study. Susceptible women received information about CMV and preventive measures, and were prospectively monitored for CMV antibody at 18 weeks of gestation and at delivery. In case of primary CMV infection diagnosed either at enrollment or at 18 weeks of gestation, the option of prenatal diagnosis was discussed and pregnancy managed accordingly. In the absence of maternal infection, women received standard prenatal care according to Italian guidelines (www.snlg-iss.it/lgn_gravidanza_fisiologica_agg_2011).
Fig. 1.
Overview of the study design. Participation in the study was restricted to adult (> 18 years of age) Italian pregnant women undergoing serum screening for fetal aneuploidy at 11–12 weeks of gestation and at risk for CMV infection. Interpretation of IgM-positive results and prenatal or neonatal diagnosis of congenital CMV infection in case of maternal seroconversion or primary infection in the first trimester of gestation were performed outside of the present study as part of routine care in case of suspected or confirmed primary CMV infection (dotted boxes).
Pregnant women in the comparison group were consecutively assessed for risk factors at pre-delivery visit at 36–37 weeks of gestation or at admission for delivery. Women at risk for CMV infection who had not been tested before and were unaware of CMV, were invited to participate in the study provided they had undergone fetal aneuploidy screening. Samples stored at 11–12 weeks of gestation were retrospectively tested for CMV antibody and CMV IgG-negative women were re-tested at delivery.
Enrollment of the intervention and comparison group took place during the same period of time. Pregnant women of the intervention group were enrolled at both sites (Pavia and Turin), whereas pregnant women of the comparison group were enrolled at the Turin center only. By design, study groups were homogeneous for age, parity, education and exposure to at least one risk factor for CMV infection. All newborns born to women with primary infection or seroconversion were examined for congenital infection.
Additional information about the study background and design, and enrollment are available (Suppl. data). The protocol of the study (in Italian) is available at the following website http://www.cittadellasalute.to.it/index.php?option=com_content&view=article&id=1809%3Aprogetti-regina-margherita-santanna&catid=138&Itemid=534, and also uploaded as an online component to this article.
The study was approved by the Ethics Committee of the two participating Italian centers, i.e., Department of Obstetrics and Gynecology, Fondazione IRCCS Policlinico San Matteo, Pavia (no. 20120040139) and Department of Obstetrics and Gynecology, Ospedale S. Anna, Torino (no. 474 61399/AZ.10). Pregnant women gave written informed consent to be included in the study before participation.
2.2. Objectives of the Study and Outcome Measures
The primary objective of the study was to investigate the effectiveness of CMV information and hygiene recommendations for prevention of CMV infection in seronegative pregnant women at risk for primary infection. The outcome measure was seroconversion, i.e., de novo appearance of CMV-specific IgG in a previously seronegative woman. The secondary end point was to assess acceptance of and adherence to hygiene recommendations, and was measured with a self-report.
2.3. Pre-Study Activities
To develop a common communication algorithm and optimize CMV information and hygiene counseling, midwives and physicians of the two participating centers involved in the study underwent a short, dedicated course on basic techniques of conscious communication, active listening and motivational communication (Istituto Change, Torino).
2.4. Intervention
Women were offered basic information about this study and CMV antibody testing. Consenting CMV-seronegative women received an information session (15–20 min) illustrating the nature of the virus, the potential consequences to the fetus, routes of transmission and ways of preventing the infection. Specifically, participants were invited to frequently wash their hands after exposure to young children's bodily fluids as well as surfaces touched by children (toys, high chair, stroller, etc.). Women were also invited to avoid kissing children on the mouth/cheeks and not to share utensils, food, drinks, washcloths etc. Use of gloves and avoidance of sleeping in the same bed were not suggested.
The above information was also provided in writing. In addition, a pictorial card showing protective and risky behaviors (Fig. 1 Suppl. data) was given to each woman with the recommendation to keep it in sight at home. Participants were free to ask questions during the session and afterwards. A short (about 5 min) additional session to reinforce hygiene recommendations was scheduled at follow-up visit at 18 weeks of gestation. Women were also invited to fill in a short questionnaire (Suppl. data) every 6 weeks starting at 18 weeks of gestation.
Women enrolled in the comparison group who were found to be CMV-seronegative at delivery were informed of the potential risks associated with CMV susceptibility in future pregnancies. They were advised to have their serostatus promptly checked in case of a new pregnancy and, if still seronegative, to follow the hygiene recommendations. Susceptible women also received written information and the pictorial card.
2.5. Laboratory Tests
Levels of virus-specific IgG and IgM were determined using Liaison CMV IgG II and CMV IgM II (DiaSorin), and Vidas CMV IgG and IgM (BioMérieux). IgG avidity was determined with BEIA CMV IgG Avidity (Techno Genetics). Viral DNA in amniotic fluid or urine was determined by real-time polymerase chain reaction (PCR) assay (CMV ELITe MGB Kit, ELITechGroup). Seroconversion was based on appearance of CMV-specific IgG in a previously seronegative woman. Diagnosis of primary CMV infection was based on detection of CMV-specific IgM and a low/intermediate IgG avidity index. An avidity index < 25% indicated a primary infection acquired since less than 3 months. Congenital CMV infection was diagnosed antenatally by detection of CMV DNA in amniotic fluid and postnatally by detection of viral DNA in urine samples collected within the first two weeks of life.
2.6. Statistical Analysis
We estimated that 308 seronegative women were needed in each group for the study to show a reduction in seroconversion rate from 5% to 0.5% with 80% power (alpha, two-sided = 1%). Data were described as median and range if continuous and as counts and percentage if categorical. Risk factors at baseline were compared between groups using the Chi-square or the Wilcoxon test where appropriated. The incidence of seroconversion and exact binomial 95% confidence interval was estimated for each group, and compared with the Fisher exact test. The risk difference, the number needed to treat/counsel, and the odds ratio (OR) with a 95% CI were computed by Stata 13.1 (StataCorp, College Station, TX, USA). Logistic regression model was used to estimate the effect of not receiving counseling on seroconversion, while adjusting for confounders. Age (continuous), parity (pluriparous), education (secondary and tertiary vs primary) and risk factors (to have their own child or frequent contacts with children < 36 months, to have an occupational risk) were used as covariates. A two-sided P value of less than 0.05 was considered statistically significant.
2.7. Role of the Funding Source
The funding source had no role in the study design, data collection, data analyses, or data interpretation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Enrollment and Outcomes
Enrollment took place between January 2013 and April 2014. The last woman enrolled in the intervention group completed the study in November 2014. Baseline characteristics of the two study groups are reported in Table 1. Median age was close in the 2 groups (33 vs 34 years, in intervention and comparison group, respectively), despite a statistically significant difference, and thus was not further considered as a confounder. Conversely, the distribution of risk factors was different in the 2 groups and their confounding effect was adjusted for in the multivariable logistic model.
Table 1.
Baseline characteristics.
| Variable | Intervention (n = 331) | Comparison (n = 315) | P |
|---|---|---|---|
| Age (years) | 33 (30–36) | 34 (32–37) | < 0.01 |
| Parity | |||
| Pluriparous | 266 (80) | 270 (86) | 0.07 |
| Risk factors | |||
| Contact with children < 36 months | |||
| Own child | 251 (76) | 261 (83) | 0.02 |
| Familiar or other childrena | 88 (27) | 53 (17) | < 0.01 |
| Occupationb | 33 (10) | 12 (4) | < 0.01 |
| Age < 20 yrs | 2 (1) | 0 | 0.49 |
| Education | 0.59 | ||
| Primary | 25 (8) | 21 (7) | |
| Secondary | 130 (39) | 134 (42) | |
| Tertiary | 176 (53) | 160 (51) | |
| Duration of the study (days)c | 194 (186–200) | 194 (188–201) | 0.30 |
Data are median (interquartile range) or n (%).
Family members other than own child or other children but outside of a child-related professional activity.
Health professionals or child care providers.
Duration of the study was calculated by subtracting the time of gestation (in days) at fetal aneuploidy test from the time of gestation at delivery or last control.
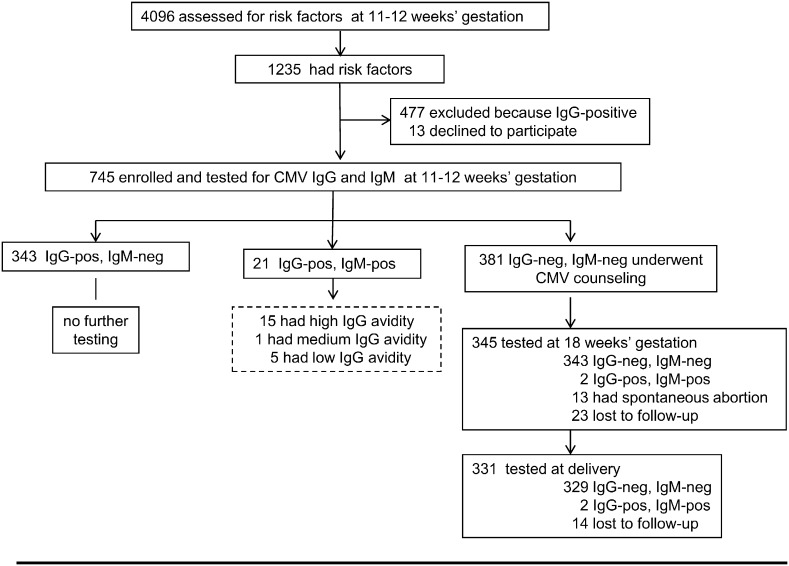
In the intervention arm, 4096 consecutive Italian pregnant women undergoing genetic screening were assessed for eligibility (Fig. 2). Overall, 745 were enrolled and tested for CMV antibody. Of these, 381 (51%) women were found to be CMV-seronegative and received CMV information and hygiene counseling. During the study, 13 women had a spontaneous abortion between 12 and 18 weeks of gestation. Four of these women were not further tested, whereas 9 were still CMV-seronegative about one year after completion of the study. In addition, 37 (23 + 14) women were lost to follow-up. Two women seroconverted between 12 and 18 weeks of gestation and 2 between 18 weeks and delivery. Overall, 4 of the 331 women who completed the study seroconverted (1.2%; 95% CI 0.3–3.1). Detailed information about these 4 women is reported in Table 1 (Suppl. data). In 5 women, CMV-specific IgM and IgG of low avidity indicating a primary CMV infection acquired in the first trimester of gestation were detected when they were first tested at 11–12 weeks of gestation (Fig. 2).
Fig. 2.
Enrollment and outcome. Interventio group. Twenty-one women found to be CMV IgM-positive at enrollment were further tested for IgG avidity (dotted box). Avidity index was high (> 45%), thus excluding a primary CMV infection, in the previous 3 months in 15 women. One woman had avidity in the intermediate range (25%–45%), indicating a possible primary infection in the past 6 months, and 5 women had low (< 25%) avidity indexes compatible with a recent (< 3 months) primary infection. Two women diagnosed with primary infection in the first trimester of gestation opted for prenatal diagnosis of congenital CMV infection and amniocentesis was performed at 20 weeks of gestation.
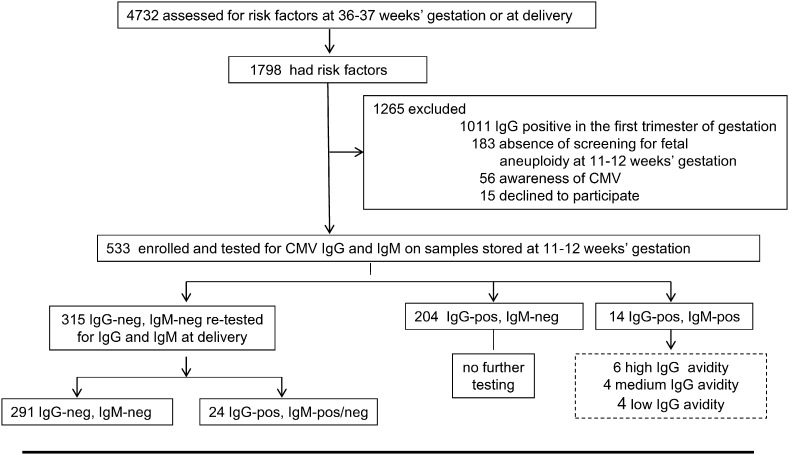
In the comparison arm, of the 4732 women assessed for eligibility at 36–37 weeks of gestation or at delivery, 533 were enrolled (Fig. 3). Of these women, 315 (59%) were found to be CMV-seronegative on serum samples stored at 11–12 weeks of gestation. When retested around or at delivery, it was found that 24 women had seroconverted during pregnancy (7.6%, 95% CI 4.9–11.1). Characteristics of these women are reported in Table 2 (Suppl. data). Four additional women were retrospectively diagnosed with a primary CMV infection acquired in the first trimester of gestation on the basis of CMV-specific IgM and IgG of low avidity (Fig. 3).
Fig. 3.
Enrollment and outcome. Comparison group. In the comparison group, 56 women were excluded because they were CMV-seronegative at the beginning of pregnancy, they received hygiene information and were monitored for CMV during pregnancy. Fourteen women who tested IgM-positive on stored serum samples at 11–12 weeks of gestation were also tested for avidity of CMV-specific IgG (dotted box). A low avidity index indicating a primary infection acquired in the first trimester of gestation was found in 4 of them. Of the 24 women who seroconverted during pregnancy, 4 were IgM-negative when tested at delivery. All the 291 CMV-seronegative women at delivery received CMV counseling.
Overall, 9 women suffered from primary CMV infection during the first trimester of gestation and did not receive any information. When they were included, the incidence of primary CMV infection in pregnancy among women not receiving information rose to 9%, i.e., 7.6% (24/315) plus 1.4% [9/(9 + 315 + 331)]. The cumulative incidence of seroconversion in susceptible pregnant women who did not receive CMV information was calculated as the incidence of seroconversion in the intervention and comparison group (during the first 12 weeks of gestation) plus the incidence of seroconversion after 12 weeks of gestation in the comparison group.
3.2. Primary Objective
The seroconversion rate in the intervention (1.2%) was significantly lower than in the comparison (7.6%) group (Δ = 6.4%, 95% CI 3.2–9.6; P < 0.001). The corresponding number needed to treat/counsel to prevent one seroconversion was 16 (1/0.064) women (95% CI 10–30). Susceptible pregnant women who received CMV information had a lower risk (1.2/7.6) of acquiring primary CMV infection than women who were not informed (crude OR 0.15; 95% CI 0.05–0.43). The risk of infection was significantly reduced also after adjustment for potential confounders (Adj OR 0.14; 95% CI 0.05–0.41).
3.3. Congenital Infection
Eleven of the 28 (39.3%) neonates (3 in the intervention and 8 in the comparison group) born to seroconverted mothers were found to be congenitally infected at birth (Fig. 2 Suppl. data). Ten infected infants were asymptomatic at birth and have remained so during the follow-up. One infected neonate born to a woman enrolled in the comparison group had cerebral vasculitis diagnosed by an ultrasound scan at birth and at 3 and 6 months of age. Presumed onset of maternal infection was around 14–15 weeks of gestation. This infant did not receive CMV therapy and appeared to be asymptomatic when last seen at 6 months of age.
Of the 5 women in the intervention group who were diagnosed with a primary CMV infection acquired in the first trimester of gestation, 2 women delivered uninfected babies and 3 opted for termination of the pregnancy. Virologic outcome of pregnancies was unknown for 2 of these women (including one carrying twins) who terminated pregnancy at 12 weeks of gestation (products of conception were not examined), while fetal infection was diagnosed at amniocentesis in the remaining woman (Fig. 2 Suppl. data). None of the 4 women in the comparison group who were retrospectively diagnosed with a first trimester primary infection delivered a CMV-infected newborn (Fig. 2 Suppl. data).
3.4. Secondary Objective
Overall, 932 questionnaires were returned and preliminary data indicate that, when following the recommendations, lack of time was the major problem, while knowledge of risks helped the most. In addition, 80% of respondents reported following the recommendations often (66%) or always (14%) during pregnancy. Finally, 93% of responders estimated the recommendations worth suggesting to all pregnant women at risk for infection.
4. Discussion
Our controlled study provides evidence that a primary prevention strategy based on the identification and provision of adequate information to susceptible pregnant women at risk for primary infection are highly effective in reducing the rate of maternal primary CMV infection and, ultimately, congenital CMV infection. In the past 20 years only a handful of primary studies have addressed the issue of prevention of primary CMV infection by educational interventions (Harvey and Dennis, 2008; Hamilton et al., 2014). Two small studies performed in Virginia, USA, in the 1990s by SP Adler and colleagues, reported that following hygiene education, pregnant women were significantly less likely to acquire CMV infection than were nonpregnant women (Adler et al., 1996, 2004). In the only subsequent study investigating the role of hygiene counseling in a large population of French pregnant women tested at 12 weeks of gestation, a reduction in CMV infection from 0.035% to 0.008% per pregnant-woman week was reported by comparing the number of seroconversions between 12 and 36 weeks gestation (i.e., following hygiene counseling) with the number of primary infections acquired between 0 and 12 weeks gestation (i.e., before counseling) (Vauloup-Fellous et al., 2009). However, in the above studies, the education interventions as well as study populations were quite different, and minimal or no demographics were provided.
One crucial and challenging aspect of our study was the identification of a suitable comparison group. We exploited the fact that the majority of Italian pregnant women undergo serum screening for fetal aneuploidy at 11–12 weeks of gestation (European Ligand Assay Society, 2009), and that on that occasion an aliquot of serum is routinely stored. On this basis, women homogenous for age, parity, education and exposure to risk factors could be enrolled in parallel in the prospective intervention and in the observational comparison arm. Importantly, the duration of the study in the two study groups was also the same (from 12 weeks of gestation to delivery), thus eliminating a potentially important variable such as difference in sexual activity during pregnancy (as in the French study, Vauloup-Fellous et al., 2009) as well as between pregnant and non-pregnant women (as in the US studies, Adler et al., 1996, 2004).
We devised a type of intervention that, if successful, could be further tested in larger trials and, ultimately, translated into general practice. We focused on pregnant women known to be at increased risk of infection, i.e., women caring for young children. These women were first offered CMV testing and, if seronegative, they were informed about CMV and prevention measures to be adopted. Information was kept as simple as possible. However, as hygiene recommendations implied substantial and sustained behavioral changes, active involvement was encouraged and a positive attitude in the woman was considered crucial for a successful intervention (see Suppl. data). Unlike the two previous studies performed in the USA (Adler et al., 1996, 2004), our intervention did not include an educational video. In addition, use of gloves and recommendation not to sleep in the same bed with the child was not suggested. Finally, liquid soap and disposable gloves were not provided, and home visits were not performed. As a result, an 84% reduction in seroconversion rate was observed: a finding strickingly similar to what previously reported by Adler et al. (2004) Reiteration of recommendations during antenatal classes may further improve the intervention effectiveness, as reported for toxoplasmosis (Breugelmans et al., 2004). We do not know which of the suggested recommendations was most effective in reducing the seroconversion rate in the intervention group. No difference was previously reported, for a number of protective or risky behaviors, between infected and uninfected mothers of children excreting CMV (Adler et al., 2004).
Adherence to suggested recommendations is still under examination. However, preliminary data indicate that 80% women reported substantial or complete compliance with suggested recommendations. According to one study, mothers were able to make and sustain behavioral changes for more than 6 months and were not necessarily concerned about reducing intimate contact with their children (Adler et al., 2004). Importantly, in our study, 93% of the women felt that the recommendations are worth suggesting to all women at risk for infection. This finding shows that an intervention like the one herein reported would be highly acceptable to the target population of women and confirms previous results (Cordier et al., 2012).
An additional finding of our study was that 9 primary maternal infections occurred in the first trimester of gestation, the highest risk period in case of virus transmission to the fetus (Enders et al., 2011). Based on results of our study, it is reasonable to hypothesize that some infections would have been avoided had these women been informed at an earlier stage of pregnancy. Ideally, all women should be tested for CMV antibody and informed before pregnancy. A further additional by-product of our study is that hundreds of women enrolled in the comparison group and found to be susceptible to CMV received post-partum CMV information. Should these women become pregnant again, it is less likely they will be inadvertently exposed to CMV. Indeed, in a population of women receiving fertility treatment, preconception screening and counseling seemed to have the potential to reduce CMV exposure in pregnancy (Reichman et al., 2014). Preconception testing would also reduce problems arising from the detection and interpretation of CMV-specific IgM antibody in an already pregnant woman (Revello and Gerna, 2002; Guerra et al., 2007).
Finally, in view of the 15–20% rate of spontaneous abortion reported in the general population in Italy (ISTAT, http://www.istat.it,2014), it is difficult to hypothesize whether CMV had an etiological role in the spontaneous abortion observed in the 4 women in the intervention group (representing about 1% of CMV-seronegative women initially enrolled) who could not be further tested.
Hopefully, an effective CMV vaccine will eventually limit congenital CMV (Griffiths et al., 2013) but this is not foreseen in the near future. In the meantime, based on the uncertain efficacy of the costly treatment with hyperimmune globulin (Nigro et al., 2005; Revello et al., 2014), reducing rates of primary infection in pregnancy by alternative prevention strategies should be carefully considered.
Contributors
MGR conceived the idea for the study and managed the project. MGR, CT, and TT designed the study. GM, VF, AS, AA, MF, acquired and analyzed the data. CK and MC did the statistical analyses and wrote the statistical analysis plan. MGR and GG wrote the paper. TT, AS, MF, and CT made substantial contribution to analysis and interpretation of data.
Declaration of Interests
No author declares conflict of interests.
Acknowledgments
This study was funded by the Fondazione Carlo Denegri, Torino, Italy. We thank all the women who participated in the study. We also thank Daniela Sartori for the assistance in the editing of the manuscript, and Laurene Kelly for the English revision.
Footnotes
Funding: Fondazione Carlo Denegri, Torino, Italy.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.08.003.
Appendix A. Supplementary Data
Supplementary material 1.
Supplementary material 2 Protocol of the study.
References
- Adler S.P. Molecular epidemiology of cytomegalovirus: evidence for viral transmission to parents from children infected at a day care center. Pediatr. Infect. Dis. 1986;5:315–318. [PubMed] [Google Scholar]
- Adler S.P., Finney J.W., Manganello A.M., Best A.M. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviours: a randomized controlled trial. Pediatr. Infect. Dis. J. 1996;15:240–246. doi: 10.1097/00006454-199603000-00013. [DOI] [PubMed] [Google Scholar]
- Adler S.P., Finney J.W., Manganello A.M., Best A.M. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J. Pediatr. 2004;145:485–491. doi: 10.1016/j.jpeds.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Arvin A.M., Fast P., Myers M., Plotkin S., Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the national vaccine advisory committee. Clin. Infect. Dis. 2004;39:233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- Breugelmans M., Naessens A., Foulon W. Prevention of toxoplasmosis during pregnancy — an epidemiological survey over 22 consecutive years. J. Perinat. Med. 2004;32:211–214. doi: 10.1515/JPM.2004.039. [DOI] [PubMed] [Google Scholar]
- Cannon M.J., Davis K.F. Washing our hands of the congenital cytomegalovirus epidemics. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M.J., Hyde T.B., Schmid D.S. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol. 2011;21:240–255. doi: 10.1002/rmv.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier A.G., Guitton S., Vauloup-Fellous C., Grangeot-Keros L., Ayoubi J.M., Benachi A., Picone O. Awareness of cytomegalovirus infection among pregnant women in France. J. Clin. Virol. 2012;53:332–337. doi: 10.1016/j.jcv.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Enders G., Daiminger A., Bäder U., Exler S., Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestastional age. J. Clin. Virol. 2011;52:244–246. doi: 10.1016/j.jcv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- European Ligand Assay Society Sezione Italiana Screening e diagnosi prenatale. Ligandassay. 2009;14(2) (june 2009 — Editor Biomedia) [Google Scholar]
- Fowler K.B., Pass R.F. Risk factor for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics. 2006;118:e286–e292. doi: 10.1542/peds.2005-1142. [DOI] [PubMed] [Google Scholar]
- Griffiths P., Plotkin S., Mocarski E. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine. 2013;31S:B197–B203. doi: 10.1016/j.vaccine.2012.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra B., Simonazzi G., Lazzarotto T. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am. J. Obstet. Gynecol. 2007;196(3) doi: 10.1016/j.ajog.2006.08.039. (221.e1-221.e6) [DOI] [PubMed] [Google Scholar]
- Hamilton S.T., van Zuylen W., Shand A.B. Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: a systematic review. Rev. Med. Virol. 2014;24:420–433. doi: 10.1002/rmv.1814. [DOI] [PubMed] [Google Scholar]
- Hanshaw J.B., Scheiner A.P., Moxley A.W., Gaev L., Abel V., Scheiner B. School failure and deafness after “silent” congenital cytomegalovirus infection. N. Engl. J. Med. 1976;295:468–470. doi: 10.1056/NEJM197608262950902. [DOI] [PubMed] [Google Scholar]
- Harvey J., Dennis C.-L. Hygiene interventions for prevention of cytomegalovirus infection among childbearing women: systematic review. J. Adv. Nurs. 2008;63:440–450. doi: 10.1111/j.1365-2648.2008.04726.x. [DOI] [PubMed] [Google Scholar]
- McCracken G.J., Shinefield H.R., Cobb K., Rausen A.R., Dische M.R., Eichenwald H.F. Congenital cytomegalic inclusion disease. A longitudinal study of 20 patients. Am. J. Dis. Child. 1969;117:522–539. doi: 10.1001/archpedi.1969.02100030524005. [DOI] [PubMed] [Google Scholar]
- Nigro G., Adler S.P., La Torre R., Best A.M. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 2005;353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- Pass R.F., Hutto C., Lyon M.D., Cloud G. Increased rate of cytomegalovirus infection among day care center workers. Pediatr. Infect. Dis. J. 1990;9:465–470. doi: 10.1097/00006454-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Reichman O., Miskin I., Sharoni L. Preconception screening for cytomegalovirus: an effective preventive approach. BioMed Res. Int. 2014:135416. doi: 10.1155/2014/135416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revello M.G., Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 2002;15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revello M.G., Campanini G., Piralla A. Molecular epidemiology of primary human cytomegalovirus infection in pregnant women and their families. J. Med. Virol. 2008;80:1415–1425. doi: 10.1002/jmv.21243. [DOI] [PubMed] [Google Scholar]
- Revello M.G., Lazzarotto T., Guerra B. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N. Engl. J. Med. 2014;370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- Reynolds D.W., Stagno S., Stubbs K.G. Inapparent congenital cytomegalovirus infection with elevated cord IgM levels: causal relationship with auditory and mental deficiency. N. Engl. J. Med. 1974;209:291–296. doi: 10.1056/NEJM197402072900601. [DOI] [PubMed] [Google Scholar]
- Stagno S., Cloud G., Pass R.F., Britt W.J., Alford C.A. Factors associated with primary cytomegalovirus infection during pregnancy. J. Med. Virol. 1984;13:347–353. doi: 10.1002/jmv.1890130405. [DOI] [PubMed] [Google Scholar]
- Taber L.H., Frank A.L., Yow M.D., Bagley A. Acquisition of cytomegaloviral infections in families with young children: a serological study. J. Infect. Dis. 1985;151:948–952. doi: 10.1093/infdis/151.5.948. [DOI] [PubMed] [Google Scholar]
- Vauloup-Fellous C., Picone O., Cordier A.-G. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J. Clin. Virol. 2009;46S:S49–S53. doi: 10.1016/j.jcv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Walmus B.F., Yow M.D., Lester J.W., Leeds L., Thompson P.K., Woodward R.M. Factors predictive of cytomegalovirus immune status in pregnant women. J. Infect. Dis. 1988;157:172–177. doi: 10.1093/infdis/157.1.172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1.
Supplementary material 2 Protocol of the study.