Abstract
Carbon dioxide (CO2) is a key molecule in many biological processes. Studies in humans, mice, D. melanogaster, C. elegans, unicellular organisms and plants have shed light on the molecular pathways activated by elevated levels of CO2. However, the mechanisms that organisms use to sense and respond to high CO2 levels remain largely unknown. Previous work has shown that C. elegans quickly avoid elevated CO2 levels using mechanisms that involve the BAG, ASE and AFD neurons via cGMP- and calcium- signaling pathways. Here, we discuss our recent finding that exposure of C. elegans to high CO2 levels leads to a very rapid cessation in the contraction of the pharynx muscles. Surprisingly, none of the tested CO2 avoidance mutants affected the rapid pumping inhibition response to elevated CO2 levels. A forward genetic screen identified that the hid-1-mediated pathway of dense core vesicle maturation regulates the pumping inhibition, probably through affecting neuropeptide secretion. Genetic studies and laser ablation experiments showed that the CO2 response of the pharyngeal muscle pumping is regulated by the BAG neurons, the same neurons that mediate CO2 avoidance.
Keywords: BAG neurons, CO2 avoidance, Caenorhabditis elegans, dense core vesicles, pharynx
Introduction
The respiratory gases, carbon dioxide (CO2) and oxygen (O2), are key molecules in oxidative metabolism. In order to maintain cellular homeostasis, all organisms must adapt to changes in the levels of these gases. The CO2 and O2 homeostasis in our body is mainly achieved by CO2 chemoreceptors in the brain that promote respiratory responses to maintain normal CO2 and O2 levels in the blood. At the cellular level, under normal oxygen conditions, the transcription factor HIF-1α is hydroxylated in a conserved proline residue and subsequently targeted for proteasomal degradation. Under hypoxic conditions (low oxygen levels), HIF-1α is stabilized and orchestrates an adaptive response that maintains normal metabolism.1
Whether a master regulator that responds to changes in CO2 levels at the cellular level exists is not known. Several recent studies have shed new light on molecular pathways activated by elevated levels of CO2. In lungs, elevated levels of CO2 are associated with impaired fluid reabsorption as a consequence of Na,K- ATPase endocytosis.2 The CO2-mediated Na,K-ATPase endocytosis is partially regulated by activation of AMPK and subsequent activation of PKC-ζ.3 The soluble adenylyl cyclase (sAC) was also found to be activated by CO2/HCO3.4 In a feedback loop found in alveolar epithelial cells, sAC activation results in elevated cAMP levels which leads to PKA-1α-dependent phosphorylation of the actin cytoskeleton component α-adducin and endocytosis of the Na,K-ATPase.5 CO2 also inhibits cell proliferation by inducing mitochondrial dysfunction mediated by down regulation of the TCA cycle enzyme isocitrate dehydrogenase-2 (IDH2).6 In addition, elevated levels of CO2 impair innate immunity responses in mammalian cells. CO2 leads to nuclear translocation of RelB and IKKα, 2 central regulatory components of the NF-κB signaling pathway, which leads to significant attenuation of NF-kB signaling and altered inflammatory responses.7,8 Most importantly, all the effects mentioned above are pH-independent, suggesting specific cellular responses to CO2.
The nematode C. elegans, is a good model in which to study the physiological and molecular responses to high levels of CO2.9 In this model organism, high levels of CO2 induce an avoidance response, which is mediated by cGMP signaling pathway activation in the BAG neurons (Fig. 1).10,11 This response is modulated by the nutritional state of the worm. Starved worms do not avoid CO2 and worms mutated in insulin/IGF signaling, which mimics the starvation response, also do not avoid CO2.10,11 The homeostatic response to CO2 is also modulated by both temperature and O2 sensing neurons in that CO2 is less aversive to animals acclimated to 15°C compared to animals acclimated to 22°C.12 This difference requires the activation of temperature sensitive AFD neurons, which are also activated by CO2. In addition, signaling from the oxygen-sensing neuron URX inhibits CO2-mediated avoidance.12,13 Little is known about how CO2 is actually sensed in the BAG neurons, it was previously suggested that the guanylate cyclase receptor gcy-9, whose expression is directly controlled by the transcription factor ETS-5, serves as the CO2 sensor in these neurons.14 ETS-5 is also required for proper differentiation and proper CO2 responses of the BAG neurons.15,16 More recently, EGL-13 and EGL-46 were also found to be important for the differentiation of the CO2-sensing BAG neurons.17,18
Figure 1.
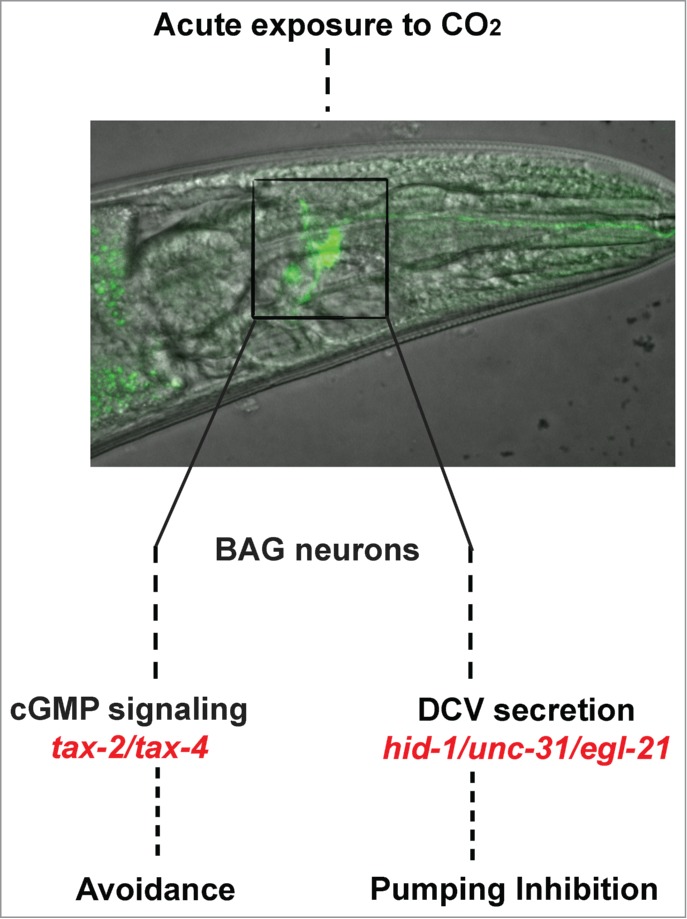
Acute exposure of Caenorhabditis elegans to elevated CO2 level causes animal avoidance and stops pharyngeal muscle contractions. Both responses are regulated by the BAG neurons but through separate signaling pathways. While avoidance requires cGMP signaling, the inhibition of muscle contraction in the pharynx is mediated by neuropeptide secretion. Both CO2 responses are decreased following starvation.
Further studies are required to fully understand the molecular mechanisms induced by high levels of CO2 and how these mechanisms regulate physiologic responses to elevated CO2. We discuss here our recent study of a previously uncharacterized behavioral response of C. elegans to elevated CO2, and a new component that is involved in C. elegans CO2 signaling.19
High Levels of CO2 Halt Pharynx Contractions
We previously described the effects of wild type C. elegans' exposure to chronic, high CO2 levels.20 These studies were performed to establish the potential use of C. elegans as a model organism for investigating the molecular mechanisms, at the whole organism level, that are activated in response to elevated levels of CO2. We found that when wild type C. elegans are maintained at high CO2 conditions they have a smaller brood size, delayed development, reduced motility that is coupled with striated muscle deterioration and a significant increase in life span. To gain a better insight into how worms respond to high levels of CO2 we set to study the immediate responses elicited when worms are exposed to high CO2 levels. We designed a small chamber, connected to a CO2 tank, and used it to expose wild type C. elegans to elevated levels of CO2 ranging from 5–20% CO2.21 We noticed that in CO2 concentrations higher than 10%, the pharynx, which normally contracts ∼200 times/min, almost completely stopped contracting after a few seconds of CO2 exposure. The contraction cessation is probably counteracted by other response mechanisms since after 2 min of continuous exposure we started to observe a recovery in the pharynx response. However, even after prolonged exposure to high levels of CO2 (30 min) a complete recovery of the pharynx contractions was not seen, suggesting a sustained response. Since CO2 might potentially change the pH of the growth medium we also tested the response of the pharynx under different media conditions. The pharynx response was not affected by the growth medium pH, suggesting the effect of elevated CO2 is probably not mediated by pH changes in the medium. It is well established that the nutritional state of the worm can significantly alter its responses to external environmental cues. Similarly, the nutritional state of the worm partially modulated the response of the pharynx to high levels of CO2. In 10% CO2, starved worms were able to partially contract the pharynx in contrast to well-fed animals where the pharynx contraction completely halted. However, in 20% CO2 both starved and well-fed animals had a complete cessation of the pharynx contractions. This dose dependency suggests the existence of several response mechanisms that are activated at different CO2 concentrations.
CO2-Mediated Pumping Inhibition Regulation is not Shared with CO2-Mediated Avoidance Components
We hypothesized that since the CO2-mediated pumping inhibition is quick and robust, similar to the CO2-mediated avoidance, the 2 behavioral responses probably share common components. However, all the CO2 avoidance mutants that we tested showed a similar inhibition of the pumping in response to an acute high CO2 exposure.19 The mutants that we tested included mutants in chemo sensation (tax-4), nutritional state of the worm (daf-2), ciliated neuron development (osm-3, che-10) and CO2 neuron specification (gcy-9,ets-5). To identify new components that mediate the response of the pharynx to high CO2, we performed a forward genetics screen after EMS mutagenesis. Specifically, we searched for mutants in which pumping inhibition is impaired when exposed to 10% CO2. One of the genes identified in this screen was hid-1. HID-1 was previously identified in a screen for mutants that induce dauer formation under high temperature conditions.22 HID-1 was also found to be an important component of dense core vesicle secretion that controls neuropeptide release.23-25 Interestingly, the response of this mutant to high CO2 is dose dependent. After exposure to 5% CO2, the pumping inhibition is completely rescued and pumping continues at the same rate as under normal atmospheric conditions. After exposure to 20% CO2, this mutation can no longer rescue the CO2-mediated pumping inhibition and pumping completely halts as in wild type worms.
Importantly, hid-1 is probably a component specifically involved in mediating the response of the pharynx to CO2 but not of other responses induced by high CO2. The development, fertility and brood size of hid-1 mutants are still impaired when chronically exposed to high levels of CO2.
HID-1 functions in C. elegans to regulate neuropeptide secretion by dense core vesicles. We therefore investigated whether neuropeptide secretion constitutes a fundamental component of C. elegans’ response to acute high CO2 exposure. We tested 2 mutants, unc-31, an essential player in the dense core vesicle secretion machinery and egl-21, which encodes a carboxypeptidase required for neuropeptide precursor processing. Like in hid-1, the response of these mutants to high CO2 was significantly impaired compared to that of wild type worms. In contrast, unc-13 and rab-3 mutants (Fig. 1), specifically involved in synaptic vesicle secretion, responded to high CO2 like the response of the wild type. Unfortunately, all of our attempts to identify single neuropeptide-encoding mutants in which the response to high CO2 is impaired failed, suggesting that there is probably more than one neuropeptide/receptor involved in mediating this response.
HID-1 Function is Required in the BAG Neurons
hid-1 was previously shown to be expressed in the gut and also in the nerve system of C. elegans.23,25 By tissue specifically expressing HID-1 in a hid-1 null background, either in the gut (under a ges-1 promoter) or in the nervous system (under a rab-3 promoter), we demonstrated that hid-1 is only needed in the nervous system to mediate pumping inhibition.19 We next searched for the neuronal subtype in which hid-1 mediates the effect of CO2 on the pharynx. We used the nlp-3 promoter, which is expressed in neurons that secrete DCVs, to drive the expression of hid-1. Indeed, overexpression of hid-1 in this subset of neurons in a hid-1 null background restored the CO2-mediated pumping inhibition to almost wild type levels. In addition, overexpression of hid-1 in the BAG neurons either under an flp-17 promoter or under a gcy-33 promoter restored the CO2-medited pumping inhibition to wild type levels. Laser ablation of BAG neurons in hid-1-null strains overexpressing HID-1 under a gcy-33 or flp-17 promoter eliminated the CO2-mediated pumping inhibition. This further supports that the expression of hid-1 is indeed required in BAG neurons. Our data support a model in which CO2 elicits 2 different responses in the BAG neurons, the first induces avoidance and the second, mediated by hid-1, induces pumping inhibition.
Concluding Remarks
Studying C. elegans’ response to both chronic and acute high CO2 levels has already yielded many insights into how cells react to this stressor and implications for patients suffering from pulmonary diseases. It is becoming clear that the nematode, Caenorhabditis elegans, presents a valuable model system to study the molecular sensing and response to changing levels of CO2. This response involves specific neurons that sense the changes in CO2 levels, neuropeptide secretion and activation of specific signaling pathways. Both the response and the recovery depend on the cell type and its metabolic state.
Many properties of organisms’ and cells’ response to elevated CO2 levels have yet to be determined. In C. elegans, these include the identification of the sensing molecules, understanding why the BAG neurons require 2 different responding pathways that react to high CO2 levels, discovering the responsible neuropeptides and their targets that respond to elevated CO2 levels and how the adaption to longer and chronic exposures to elevated CO2 levels occurs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant HL085534.
References
- 1. Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. . Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394:485-90; PMID:969777; http://dx.doi.org/ 10.1038/28867 [DOI] [PubMed] [Google Scholar]
- 2. Briva A, Vadász I, Lecuona E, Welch LC, Chen J, Dada LA, Trejo HE, Dumasius V, Azzam ZS, Myrianthefs PM, et al. . High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE 2007; 2:e1238; PMID:18043745; http://dx.doi.org/ 10.1371/journal.pone.0001238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vadász I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Tóth PT, Lecuona E, Witters LA, Schumacker PT, et al. . AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest 2008; 118:752-62; PMID:18188452; http://dx.doi.org/ 10.1172/JCI29723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000; 289:625-8; PMID:10915626; http://dx.doi.org/ 10.1126/science.289.5479.625 [DOI] [PubMed] [Google Scholar]
- 5. Lecuona E, Sun H, Chen J, Trejo HE, Baker MA, Sznajder JI. Protein kinase A-Ialpha regulates Na,K-ATPase endocytosis in alveolar epithelial cells exposed to high CO(2) concentrations. Am J Respirat Cell Mol Biol 2013; 48:626-34; PMID:23349050; http://dx.doi.org/ 10.1165/rcmb.2012-0373OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadász I, Chandel NS, Sznajder JI. Elevated CO2 Levels Cause Mitochondrial Dysfunction and Impair Cell Proliferation. J Biol Chem 2011; 286:37067-76; PMID:21903582; http://dx.doi.org/ 10.1074/jbc.M111.290056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, Scholz CC, Slattery C, Leonard MO, McLoughlin P, Taylor CT. NF-kappaB links CO2 sensing to innate immunity and inflammation in mammalian cells. J Immunol 2010; 185:4439-45; PMID:20817876; http://dx.doi.org/ 10.4049/jimmunol.1000701 [DOI] [PubMed] [Google Scholar]
- 8. Oliver KM, Lenihan CR, Bruning U, Cheong A, Laffey JG, McLoughlin P, Taylor CT, Cummins EP. Hypercapnia induces cleavage and nuclear localization of RelB protein, giving insight into CO2 sensing and signaling. J Biol Chem 2012; 287:14004-11; PMID:22396550; http://dx.doi.org/ 10.1074/jbc.M112.347971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharabi K, Lecuona E, Helenius IT, Beitel GJ, Sznajder JI, Gruenbaum Y. Sensing, physiological effects and molecular response to elevated CO2 levels in eukaryotes. J Cell Mol Med 2009; 13:4304-18; PMID:19863692; http://dx.doi.org/ 10.1111/j.1582-4934.2009.00952.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci 2008; 105:8044-9; PMID:18524954; http://dx.doi.org/ 10.1073/pnas.0707607105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci 2008; 105:8038-43; PMID:18524955; http://dx.doi.org/ 10.1073/pnas.0707469105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kodama-Namba E, Fenk LA, Bretscher AJ, Gross E, Busch KE, de Bono M. Cross-modulation of homeostatic responses to temperature, oxygen and carbon dioxide in C. elegans. PLoS Gen 2013; 9:e1004011; PMID:24385919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carrillo M, Guillermin M, Rengarajan S, Okubo R, Hallem E. O2-Sensing Neurons Control CO2 Response in C. elegans. J Neurosci 2013; 33:9675-83; PMID:23739964; http://dx.doi.org/ 10.1523/JNEUROSCI.4541-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, Rätsch G, Miller DM, Horvitz HR, Sternberg PW, Ringstad N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci 2011; 108:254-9; PMID:21173231; http://dx.doi.org/ 10.1073/pnas.1017354108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandt JP, Aziz-Zaman S, Juozaityte V, Martinez-Velazquez LA, Petersen JG, Pocock R, Ringstad N. A single gene target of an ETS-family transcription factor determines neuronal CO2 chemosensitivity. PLoS One 2012; 7:e34014; PMID:22479504; http://dx.doi.org/ 10.1371/journal.pone.0034014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guillermin ML, Castelletto ML, Hallem EA. Differentiation of carbon dioxide-sensing neurons in caenorhabditis elegans requires the ETS-5 transcription factor. Genetics 2011; 189:1327-39; PMID:21954162; http://dx.doi.org/ 10.1534/genetics.111.133835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gramstrup Petersen J, Rojo Romanos T, Juozaityte V, Redo Riveiro A, Hums I, Traunmuller L, Zimmer M, Pocock R. EGL-13/SoxD specifies distinct O2 and CO2 sensory neuron fates in Caenorhabditis elegans. PLoS Gen 2013; 9:e1003511; PMID:23671427; http://dx.doi.org/ 10.1371/journal.pgen [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rojo Romanos T, Gramstrup Petersen J, Redo Riveiro A, Pocock R. A novel role for the zinc-finger transcription factor EGL-46 in the differentiation of gas-sensing neurons in caenorhabditis elegans. Genetics 2014; PMID:25395666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharabi K, Charar C, Friedman N, Mizrahi I, Zaslaver A, Sznajder JI, Gruenbaum Y. The response to high CO2 levels requires the neuropeptide secretion component HID-1 to promote pumping inhibition. PLoS Gen 2014; 10:e1004529; PMID:25101962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharabi K, Hurwitz A, Simon AJ, Beitel GJ, Morimoto RI, Rechavi G, Sznajder JI, Gruenbaum Y. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc Natl Acad Sci 2009; 106:4024-9; PMID:19237558; http://dx.doi.org/ 10.1073/pnas.0900309106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zuela N, Friedman N, Zaslaver A, Gruenbaum Y. Measuring the effects of high CO(2) levels in Caenorhabditis elegans. Methods 2014; 68:487-91; PMID:24650565; http://dx.doi.org/ 10.1016/j.ymeth.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 22. Ailion M, Thomas JH. Isolation and characterization of high-temperature-induced dauer fFormation mutants in caenorhabditis elegans. Genetics 2003; 165:127-44; PMID:14504222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mesa R, Luo S, Hoover CM, Miller K, Minniti A, Inestrosa N, Nonet ML. HID-1, a new component of the peptidergic signaling pathway. Genetics 2011; 187:467-83; PMID:21115972; http://dx.doi.org/ 10.1534/genetics.110.121996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang L, Zhan Y, Song E, Yu Y, Jiu Y, Du W, Lu J, Liu P, Xu P, Xu T. HID-1 is a peripheral membrane protein primarily associated with the medial- and trans- Golgi apparatus. Protein Cell 2011; 2:74-85; PMID:21337012; http://dx.doi.org/ 10.1007/s13238-011-1008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu Y, Wang L, Jiu Y, Zhan Y, Liu L, Xia Z, Song E, Xu P, Xu T. HID-1 is a novel player in the regulation of neuropeptide sorting. Biochem J 2011; 434:383-90; PMID:21250940; http://dx.doi.org/ 10.1042/BJ20110027 [DOI] [PubMed] [Google Scholar]


