Inhibitory neurotransmission in the spinal dorsal horn is at the foundation of the Gate Control Theory of Pain and has been the subject of intense investigation [16; 21]. The dorsal horn is richly endowed with GABAergic and glycinergic neurons and their ionotropic receptors. GABAA receptors are located presynaptically on afferent terminals and both glycine and GABAA receptors are found postsynaptically on dorsal horn neurons [15]. Pharmacologically blocking those receptors can reproduce many features of persistent pain, including mechanical allodynia and spontaneous pain. There is now unequivocal evidence that inhibition in the spinal dorsal horn is pathologically reduced after peripheral nerve injury and persistent inflammation [6; 12; 14]. Importantly, disinhibition can develop via distinct mechanisms. Here we concisely review how disinhibition occurs, focusing on mechanisms affecting postsynaptic inhibition in dorsal horn neurons. We also discuss emerging therapeutic opportunities to restore normal inhibition.
Ion flux and effective cellular inhibition
Effective inhibition requires (1) that GABAA or glycine receptors are activated and (2) that receptor activation hyperpolarizes the postsynaptic neuron or at least prevents (i.e. shunts) depolarization caused by concurrent excitatory input. Disinhibition can result from disruption of either step. Therapeutic efforts to restore inhibition have focused largely on replacing lost transmitter and/or enhancing the receptor activation caused by residual transmitter (via positive allosteric modulators). Indeed, there is evidence that inhibitory transmitters or their receptors are downregulated after nerve injury. But if the problem lies with the second step, modulating the first step may fail to reverse disinhibition and may even be counterproductive in certain circumstances. So how exactly do GABAA and glycine receptors mediate hyperpolarization and how can that process be pathologically undermined?
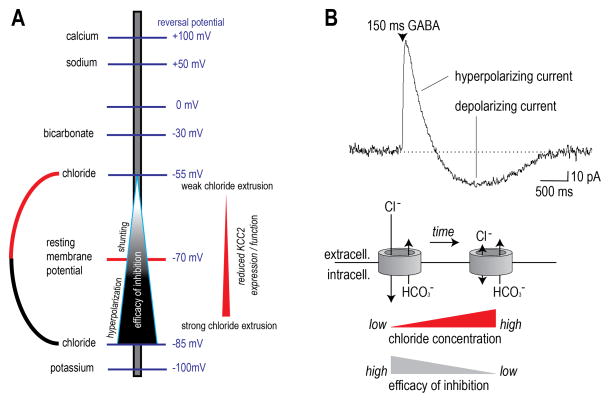
GABAA and glycine receptors are permeable to chloride and, to a lesser extent, bicarbonate. When these receptors open, chloride and bicarbonate ions flow across the membrane down their electrochemical gradients. The gradient for each ion depends on the relative concentration of the ion inside and outside the neuron, as calculated using the Nernst equation and expressed as the “reversal potential”. Chloride moves into the cell because its intracellular concentration is low, whereas bicarbonate leaves the cell because its intracellular concentration is high (Fig 1A). Notably, bicarbonate is maintained at a high intracellular level because it is replenished by the enzyme carbonic anhydrase. Chloride influx produces a hyperpolarizing current that is usually only partially offset by the small depolarizing current produced by bicarbonate efflux; the net current is therefore hyperpolarizing. But if chloride influx were to decrease because of abnormally high intracellular chloride levels, the hyperpolarizing current it produces could become equal to or even smaller than the depolarizing current produced by bicarbonate efflux; in the latter case, GABAA and glycine receptor activation produces paradoxical depolarization on account of bicarbonate efflux [4; 7; 11]. Notably, this is different from primary afferent depolarization (PAD) in which GABAA receptor activation causes depolarization via chloride efflux because chloride is actively loaded into primary afferents. In the case of spinal neurons, pathological changes reduce the capacity to remove intracellular chloride but chloride is not actively loaded into those cells [21]. This constitutes an important difference between pre- and postsynaptic inhibition.
Figure 1. KCC2 expression and chloride extrusion capacity determine the efficacy of postsynaptic inhibition.
A) The chloride reversal potential is set by the concentration of intracellular chloride. When intracellular chloride concentration is low, which is usually paralleled by high KCC2 expression, inhibition is very efficacious. As intracellular chloride concentrations increase, usually due to decreased KCC2 expression or function, inhibitory efficacy decreases. B) A GABAA current is shown with an initial hyperpolarizing current followed by a longer lasting depolarization. The depolarization arises due to chloride flooding wherein the chloride gradient is decreased or absent and bicarbonate currents become prominent leading to paradoxical depolarization.
In most central neurons, chloride is normally maintained at a low intracellular level by a K+/Cl− cotransporter known as KCC2 [17]. KCC2 moves chloride out of the cell, against its electrochemical gradient, by allowing those anions to piggyback potassium cations moving down their electrochemical gradient. If chloride is not extruded via KCC2, it accumulates intracellularly because of its passive influx via numerous chloride channels including activated GABAA and glycine receptors [11]. Importantly, peripheral nerve injury, chronic inflammation and long-term exposure to opioids all reduce KCC2 in the spinal dorsal horn [6; 9; 14]. KCC2 is not expressed on primary afferent terminals and so presynaptic inhibition is impervious to changes in KCC2.
Based on the above discussion, one should appreciate that effective postsynaptic inhibition relies on chloride influx. This is even true of so-called shunting inhibition where depolarizing current caused by excitatory input is counterbalanced (i.e. shunted) by hyperpolarizing chloride current (Fig 1A). Chloride influx would necessarily deplete the gradient were the gradient not constantly replenished by KCC2 [7]. Even when KCC2 is functioning properly, its chloride extrusion capacity can be transiently overwhelmed during intense inhibitory input, which is to say that the cell gets flooded with chloride because KCC2 simply cannot clear it fast enough. Any reduction in KCC2 function makes these floods more likely [7; 11] and exacerbates their consequences (Fig 1B). If chloride extrusion by KCC2 fails, postsynaptic inhibition will be reduced because activation of GABAA or glycine receptors will not produce sufficient hyperpolarization for effective inhibition. Even though some shunting effects may remain simply due to conductance of anions through open GABAA or glycine channels, this loss of hyperpolarizing current reduces the impact of inhibitory circuitry on spiking, which is the ultimate output feature of neuronal circuits.
Inhibitory control of spinal circuits
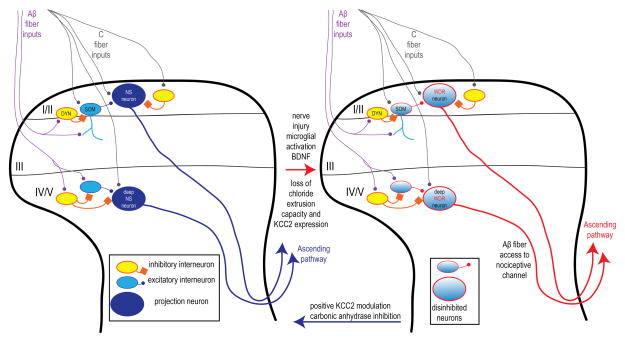
The spinal dorsal horn comprises projection neurons and both excitatory and inhibitory interneurons that form circuits that are still not fully understood [22]. KCC2 is expressed by most central neurons so most dorsal horn neurons are presumably susceptible to chloride dysregulation caused by KCC2 downregulation [11]. Chloride dysregulation in projection neurons has the most obvious consequences because this directly affects signals to the brain [12; 14]. In the case of lamina I projection neurons, chloride dysregulation leads to the unmasking of low threshold inputs, consistent with the development of mechanical allodynia (Fig 2, [12]). In deep dorsal horn, a similar effect has been observed where formerly nociceptor-specific (NS) neurons now receive low-threshold inputs[14]. Detailed quantitative analysis suggests that disinhibiton is not associated with increased input to neurons that are normally wide dynamic range. For both superficial and deep NS neurons, de novo low-threshold input is likely due to reduced feedforward inhibition that previously masked the ability of Aβ-fibers to activate this subset of neurons. But at least some of that unmasking likely occurs on account of chloride dysregulation amongst excitatory interneurons that lie upstream of the projection neurons and which themselves receive Aβ input (Fig 2, [23] and reviewed in [19]).
Figure 2. Disinhibition opens the dorsal horn gate for Aβ-fiber access to a normally nociceptive-dedicated channel.
Lamina I/II and deep dorsal horn nociceptive specific (NS) projection neurons receive C-fiber inputs as well as Aβ-fiber inputs that are normally suppressed by feed-forward inhibition. In lamina I/II the genetic identity of these neurons has recently been identified with excitatory interneurons expressing somatostatin (SOM) and feed-forward inhibitory interneurons expressing dynorphin (DYN). Postsynaptic disinhibition mediated by decreased KCC2 expression (red arrow) can open this “gate” allowing for Aβ-access to the previously nociceptive-specific channel. In superficial and deep dorsal horn neurons this converts them from NS to wide-dynamic range (WDR) like properties. Drugs that increase KCC2 activity or block carbonic anhydrase activity may normalize the system (blue arrow).
A recently published landmark study has shed light on subsets of interneurons forming a local circuit responsible for mechanical allodynia [8]. Some excitatory interneurons in lamina II identified by their expression of somatostatin (SOM neurons) send dendrites ventrally into lamina III where they receive inputs from Aβ-fibers. These neurons are directly excited by C-fiber input that causes them to fire action potentials. They are likewise excited by Aβ inputs but do not fire action potentials in response due to feed-forward inhibition from GABAergic and glycinergic interneurons. Crucially, the genetically targeted ablation of SOM neurons completely prevented the development of mechanical allodynia after nerve injury. The same study described a subset of inhibitory interneurons that can be identified by their expression of dynorphin (DYN neurons) and which also receive Aβ inputs. These neurons provide the feed-forward inhibition for SOM neurons as their genetically targeted ablation causes SOM neurons to fire action potentials in response to Aβ input. Remarkably, loss of DYN neurons promotes mechanical allodynia even in the absence of any injury [8]. It is tempting to speculate that loss of KCC2 expression in SOM neurons would profoundly influence the impact of feed-forward inhibition from DYN neurons (Fig 2). Future elucidation of such a mechanism would provide a molecular and network level explanation for allodynia that has thus far been elusive.
Implications for therapeutic intervention
While chloride dysregulation linked to reduced KCC2 expression and/or function is now thought to play a key role in pathological pain, it is also clear that increasing GABAergic tone with positive allosteric modulators (PAMs, [13]) or by implanting GABA-producing stem cells [3] can reduce pathological pain. Do these two observations contradict one another? The answer is no. Part of the explanation lies in the preserved efficacy of presynaptic inhibition on primary afferent terminals; there is strong evidence that the analgesic efficacy of GABAA receptor PAMs is mediated primarily through these presynaptic receptors [24]. Beyond the retained efficacy of presynaptic inhibition, postsynaptic inhibition becomes weaker but does not abruptly stop as KCC2 function is diminished. The reduced hyperpolarization due to mild chloride dysregulation may be mitigated by increased glycine or GABAA receptor activation [7; 20]. Notably, however, this risks increasing the chloride flooding alluded to above and will eventually fail if chloride dysregulation worsens [2; 7]. This has prompted efforts to target KCC2 or carbonic anhydrase (Fig 2, blue arrow).
The most obvious way to address chloride dysregulation is to target the source directly at KCC2. Recently PAMs for KCC2 were described that increase KCC2 activity in vitro [10], alleviate some aspects of neuropathic pain in preclinical models [10] and transiently reverse the acquisition of low-threshold input by deep dorsal horn neurons after peripheral nerve injury [14]. This route of pharmacological modulation of KCC2 has great potential for furthering our understanding of the function of these co-transporters but may ultimately be limited by the current view that proteolytic metabolism of KCC2 is a fundamental mechanism for downregulation of the co-transporter. On the other hand, it may be possible to target proteolytic metabolism of KCC2 via calpain, the key intracellular regulator of KCC2 [11]. The upstream signals responsible for triggering KCC2 downregulation make BDNF and microglial activation good therapeutic targets as well [5; 12].
One should, of course, bear in mind that reduced transmitter release and/or receptor function can contribute to postsynaptic disinhibition. Therefore pharmacological approaches to restore inhibitory tone are attractive. Moreover, various disinhibitory mechanisms can co-exist, as there is no fundamental reason for them to be mutually exclusive. This has important implications for choosing the optimal therapeutic intervention. Combination therapy may achieve the most efficacious results because the underlying pathological mechanisms are multifaceted. But even if chloride dysregulation were the sole contributing disinhibitory mechanism, combination therapy makes sense. Computer simulations and behavioral experiments have shown that GABAA receptor PAMs become less effective when chloride extrusion is compromised [1; 2; 7]. As explained above, enhancing GABAA receptor activation may enhance inhibition if chloride dysregulation is not too far-gone; by extension, restoring chloride extrusion capacity (e.g. with KCC2 PAMs) even just a little may help restore the efficacy of GABAA receptor PAMs.
An attractive alternative target that emerges from this notion is blockade of bicarbonate efflux through glycine and GABAA receptors. As explained above, bicarbonate efflux mediates a depolarizing current that becomes prominent when hyperpolarizing chloride current is reduced because of intracellular chloride accumulation. This depolarizing current can be blocked by depleting intracellular bicarbonate through inhibition of the enzyme responsible for its generation, namely carbonic anhydrase. There is already experimental evidence for a synergistic effect of combining carbonic anhydrase inhibitors with GABAA receptor PAMs for the alleviation of pain in a variety of preclinical models [1; 2]. Similar synergism would be expected with modulators of glycinergic transmission or even with a neural stem cell-based approach. Likewise synergism would be expected between GABAA receptor PAMs and drugs directly targeting KCC2, whereas modulating KCC2 while blocking carbonic anhydrase would likely be redundant [18].
Conclusions and outlook
Spinal disinhibition has emerged as a central focus for research into pathological pain mechanisms and therapeutics. As we have explained, synaptic inhibition in the spinal dorsal horn involves both pre and postsynaptic mechanisms, each involving various mechanisms that are differentially susceptible to pathological insults. Precise understanding of these multifaceted mechanisms is needed in order to intervene most effectively. A more detailed understanding of these molecular, cellular, and circuit level events holds great promise for a new therapeutic approach to pathological pain. It is our view that the realization of these opportunities is rapidly approaching and in some cases, such as carbonic anhydrase inhibitors with GABAA PAMs, are testable in clinical trials with drugs that are already approved for human use.
Acknowledgments
This work was supported by NIH grants: R01NS065926 (TJP), R01GM102575 (TJP), The University of Texas STARS program research support grant (TJP), Canadian Institutes of Health Research New Investigator Award (SAP) and the SickKids Chase an Idea in Pediatric Neuroscience grant (SAP).
Footnotes
The authors declare no conflicts of interest.
REFERENCES CITED
- 1.Asiedu M, Ossipov MH, Kaila K, Price TJ. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148(2):302–308. doi: 10.1016/j.pain.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asiedu MN, Mejia GL, Hubner CA, Kaila K, Price TJ. Inhibition of carbonic anhydrase augments GABAA receptor-mediated analgesia via a spinal mechanism of action. J Pain. 2014;15(4):395–406. doi: 10.1016/j.jpain.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74(4):663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: impact of chloride extrusion capacity. J Neurosci. 2005;25(42):9613–9623. doi: 10.1523/JNEUROSCI.1488-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 6.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 7.Doyon N, Prescott SA, Castonguay A, Godin AG, Kroger H, De Koninck Y. Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLoS Comput Biol. 2011;7(9):e1002149. doi: 10.1371/journal.pcbi.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159(6):1417–1432. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrini F, Trang T, Mattioli TA, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(−) homeostasis. Nat Neurosci. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19(11):1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15(10):637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller, Beggs, Salter, Koninck D. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Molecular Pain. 2007;3(1):27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy J-M, Rudolph U, Mohler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451(7176):330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 14.Lavertu G, Cote SL, De Koninck Y. Enhancing K-Cl co-transport restores normal spinothalamic sensory coding in a neuropathic pain model. Brain. 2014;137(Pt 3):724–738. doi: 10.1093/brain/awt334. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo LE, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva A, De Koninck Y. Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABA(A) receptors. J Neurosci. 2014;34(24):8300–8317. doi: 10.1523/JNEUROSCI.0159-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 17.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26(4):199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 18.Prescott SA. Synaptic inhibition and disinhibition in the spinal dorsal horn. In: Price TJ, Dussor G, editors. Molecular Biology of Pain, Progress in Molecular and Translational Science. Elsevier; in press. [DOI] [PubMed] [Google Scholar]
- 19.Prescott SA, Ma Q, De Koninck Y. Normal and abnormal coding of somatosensory stimuli causing pain. Nat Neurosci. 2014;17(2):183–191. doi: 10.1038/nn.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prescott SA, Sejnowski TJ, De Koninck Y. Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: towards a biophysical basis for neuropathic pain. MolPain. 2006:2. doi: 10.1186/1744-8069-2-32. [DOI] [PMC free article] [PubMed]
- 21.Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60(1):149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26(6):1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, Kuner R, Keist R, Rudolph U, Zeilhofer HU. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011;31(22):8134–8142. doi: 10.1523/JNEUROSCI.6328-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]