Abstract
Phosphatidylinositol-specific phospholipase C (PI-PLC) hydrolyses phosphatidylinositol-4,5-bisphosphate to produce diacylglycerol and inositol 1,4,5-trisphosphate. It plays an important role in plant development and abiotic stress responses. However, systematic analysis and expression profiling of the phospholipase C (PLC) gene family in soybean have not been reported. In this study, 12 putative PLC genes were identified in the soybean genome. Soybean PLCs were found on chromosomes 2, 11, 14 and 18 and encoded 58.8–70.06 kD proteins. Expression pattern analysis by RT-PCR demonstrated that expression of the GmPLCs was induced by PEG, NaCl and saline-alkali treatments in roots and leaves. GmPLC transcripts accumulated specifically in roots after ABA treatment. Furthermore, GmPLC transcripts were analyzed in various tissues. The results showed that GmPLC7 was highly expressed in most tissues, whereas GmPLC12 was expressed in early pods specifically. In addition, subcellular localization analysis was carried out and confirmed that GmPLC10 was localized in the plasma membrane in Nicotiana benthamiana. Our genomic analysis of the soybean PLC family provides an insight into the regulation of abiotic stress responses and development. It also provides a solid foundation for the functional characterization of the soybean PLC gene family.
Introduction
The PLC (phospholipase C) gene family is a subfamily of the phospholipase superfamily, which includes phospholipase D, phospholipase C, phospholipase A1 and phospholipase A2. PLCs are divided into phosphatidylinositol-specific phospholipase C (PI-PLC) and non-specific phospholipase (NPC) by different hydrolysis sites [1]. NPC hydrolyzes the membrane lipids phosphatidylcholine and phosphatidylethanolamine in a calcium-independent manner [1]. It plays an important role in the supply of inorganic phosphate in the root plasma membrane during phosphate deprivation [2]. PI-PLC, also named PLC, exists in both animals and plants. PLC-catalyzed production of the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) is a key event in the transduction of agonist-dependent signals over the plasma membrane [3, 4]. IP3 was shown to release Ca2+ from internal stores to the cytoplasm in animals. However, an IP3 receptor has not been found in plants. Furthermore, protein kinase C (PKC), which is the target of DAG in animals, is lacking from plant genomes [5]. In plants, DAGs can be phosphorylated by DAG kinase (DGK) and converted into phosphatidic acids (PAs), which have emerged as a new class of lipid mediators involved in diverse cellular functions in plants [6]. IP3 is rapidly converted into IP6 and causes the release of Ca2+ in plants [7]. The function of PLC is carried out mainly by the PA and IP6 pathways in plants.
Two different types of PI-PLC enzymes, soluble and membrane-bound, were identified in the cells of several plants in early studies [8–13]. Only membrane-bound PI-PLCs were characterized to hydrolyze phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) and phosphatidylinositol 4-phosphate (PI4P) [14], although the activities of these PI-PLCs were measured from crude protein extracts. Nonetheless, in vitro PLC activity has been confirmed for recombinant Arabidopsis PLCs. All five AtPLC fusion proteins tested required micromolar levels of Ca2+ for PI(4,5)P2 hydrolyzing activity with the exception of AtPLC4 [15]. However, our research showed that the activity of OsPLC1 was inhibited under high Ca2+ concentrations (more than 1 mM) (data not shown). This should be caused mainly by feedback in the PLC signal pathway. Two messengers produced by PLC are thought to play signaling roles in guard cells [7], pathogen responses, Nod factor signaling and carbon fixation in C4 plants [16–18]. In Arabidopsis, there are nine known PI-PLC sequences in the genome [19]. Pokotylo et al. [20] characterized six NPCs in Arabidopsis and NPC involvement has been identified in the regulation of diverse cellular processes including root development and hormone signaling [21], Al stress signaling [22], abscisic acid (ABA) sensing, and tolerance to hyperosmotic and salt stresses [1, 23]. Both PI-PLCs and NPCs have been found and characterized in the rice and tomato genomes [24, 25].
Soybean PLC was first cloned and characterized in 1995 [26]. Soluble enzyme fractions that hydrolyzed phosphoinositides were separated from soybean sprouts using Matrex green gel column chromatography [27]. Functional analysis of soybean PLC was carried out using the PI-PLC specific inhibitor U-73122 and suggested that PI-PLC may negatively regulate the expression of defense genes [28]. In this study, we identified and characterized 12 PLC genes in the soybean genome. The expression profiles of soybean PLC genes were examined in different tissues and under various abiotic stresses. Moreover, subcellular localization analysis was carried out and showed that GmPLC10 was located in the plasma membrane.
Materials and Methods
Genomic search and sequence analysis
We searched for soybean PLC genes in the Phytozome v9.1 database (http://www.phytozome.org/). The key words “phospholipase C” were also searched using the browse tool on the NCBI website (www.ncbi.nlm.nih.gov/). The conserved domains of GmPLC proteins were identified by CDSEARCH/cddv3.13 (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Arabidopsis PLC sequences were obtained from TAIR 10.0 (The Arabidopsis Information Resource). Rice PLC sequences were obtained from RGAP (Rice Genome Annotation Project). The loci of PLCs from Arabidopsis and rice are shown in S1 Table. A phylogenetic tree was constructed by MEGA5.1 with the default parameters [29].
Plant growth and abiotic stress treatment
Soybean (Glycine max, Williams 82) seeds were grown in the natural environment on 5 May of 2014 at Jilin Agricultural University, Changchun, Jilin, China. The soil type is black soil and the row spacing is 30 cm. For expression analysis of GmPLCs in various tissues, leaves, roots and stems were harvested 30 d after sowing. Flowers were harvested about 80 d after sowing. Early and late pods were harvested 7 and 30 d after flowering. Immature and mature seeds were collected at 20 and 40 d after flowering. In addition, soybean seeds were grown in hydroponic pots that contained Hoagland’s solution under a 12 h light/12 h dark cycle, a constant temperature of 25°C, and a humidity of 70%. The concentrations of Hoagland’s solution for each element are 210 ppm N, 235 ppm K, 200 ppm Ca, 31 ppm P, 64 ppm S, 48 ppm Mg, 0.5 ppm B, 0.5 ppm Mn, 0.05 ppm Zn, 0.02 ppm Cu, 0.01 ppm Mo and 1 to 5 ppm Fe. The nutrient solution was replaced every 2 d. When the G. max seedlings were at the four-leaf stage (about 10 d), they were transferred into various stress solutions, salt (110 mM NaCl), alkali (100 mM NaHCO3), saline-alkali (70 mM NaCl + 50 mM NaHCO3), PEG (8% PEG8000) or ABA (100 μM ABA), for 0, 1, 3, 6, 9 and 12 h. Control plants were grown in Hoagland’s solution. The roots and leaves were collected at about 10 AM and stored at −80°C until further use.
Extraction of total RNA and cDNA synthesis
Total RNA was isolated from the collected tissue samples separately using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocols. The quality and quantity of the total RNA were determined using a NanoDrop 2000 (ThermoFisher Scientific, Beijing, China). cDNA was synthesized from 2 μg of total RNA using the PrimeScript RT reagent kit (Takara, Japan).
Quantitative real-time PCR
Gene-specific primers for the GmPLCs and reference genes were designed using Primer Express and are shown in S2 Table [30]. For expression analysis under abiotic stresses, Actin11 was selected as the reference gene. EF1A was chosen for the expression analysis in different tissues. RT-PCR was performed on a Stratagene Mx3000P thermocycler (Agilent) with the following program: 95°C for 15 s, followed by 40 cycles of 95°C for 15s and annealing at 60°C for 30 s. The expression level was calculated using the 2−ΔΔCt method for abiotic stress treatments and the 2ΔCt method for different tissues. Triplicates of each reaction were performed.
Promoter analysis
The promoters of the GmPLCs, which were 1 kb upstream from the translation start site, were mapped in the soybean genome. The cis-acting regulatory elements were identified in each promoter. Among these, we chose abiotic stress response and development related elements for expression analysis.
Nicotiana benthamiana transient transformation and subcellular localization
For subcellular localization analysis of soybean PLC, GmPLC10 was introduced into the binary expression vector pCAMBIA1302. The expression vector was transformed into Agrobacterium tumefaciens cells of the EHA105 strain. The transient transformation of N. benthamiana leaves was carried out following Marion et al. [31], with minor revision. For confocal microscopy, tobacco leaves were imaged using an inverted TCS-SPE spectral confocal laser scanning microscope (Leica, Germany). GFP fluorescence was excited with 488 nm argon laser lines with an emission band of 495–540 nm. Triplicate tobacco infiltrations were performed for the subcellular localization experiment.
Results and Discussion
Identification of PLC genes in soybean
The genome of soybean was sequenced recently [32]. We searched for soybean phospholipase C in both the genome and GenBank. Twelve PLCs were identified after removing redundant sequences. We named these 12 PLC genes GmPLC1 to GmPLC12 according to their physical locations on chromosomes 1–20 (Table 1). There are four, six and nine PLCs in the rice, tomato and Arabidopsis genomes, respectively [24, 25, 33]. The greater number of PLCs in soybean implied the importance and complexity of GmPLCs. The soybean PLCs were spread over chromosomes 2, 11, 14 and 18 equally (S1 Fig). Interestingly, all three PLC genes in each chromosome were located adjacent to one another, with the exception of GmPLC9 in chromosome 14. A similar phenomenon was found in Arabidopsis; AtPLC1, AtPLC4 and AtPLC5 are located in a 12 kb fragment on chromosome 5 [33]. The open reading frames (ORFs) of the soybean PLC genes ranged from 1623 to 1833 bp and encoded proteins of 540 to 610 amino acids. The theoretical isoelectric points of the soybean PLC proteins ranged from 5.57 to 9.11 with molecular masses ranging from 58.80 to 70.06 kD. The average length of PLC proteins is about 600 amino acids in plants. The smallest PLC is OsPLC2 from rice, which is composed of 491 residues [24]. In soybean, GmPLC5 and GmPLC11 were smaller than the others (Table 1). Furthermore, the coding sequences were analyzed and we found that all of the soybean PLC genes contained nine exons, except GmPLC4 and GmPLC12. The number of exons in the soybean PLCs was greater than that in rice and Arabidopsis (S2 Table).
Table 1. List of 12 PLC genes identified in soybean.
| Name | Gene Identifier | Chr. | Location coordinates (5'-3') | ORF length (bp) | Protein | Exons | ||
|---|---|---|---|---|---|---|---|---|
| Length (aa.) | pI | Mol. Wt. (kD) | ||||||
| GmPLC1 | Glyma02g42410 | 2 | 47442411–47446810 | 1833 | 610 | 6.59 | 70.06 | 9 |
| GmPLC2 | Glyma02g42420 | 2 | 47450458–47455299 | 1713 | 570 | 8.44 | 65.00 | 9 |
| GmPLC3 | Glyma02g42430 | 2 | 47463027–47467886 | 1803 | 600 | 5.70 | 58.80 | 9 |
| GmPLC4 | Glyma11g35290 | 11 | 36978149–36982608 | 1791 | 596 | 9.11 | 68.56 | 10 |
| GmPLC5 | Glyma11g35300 | 11 | 36984672–36990916 | 1623 | 540 | 6.01 | 61.17 | 9 |
| GmPLC6 | Glyma11g35310 | 11 | 36993479–36998177 | 1779 | 592 | 5.57 | 67.29 | 9 |
| GmPLC7 | Glyma14g06450 | 14 | 4706397–4711170 | 1803 | 600 | 5.73 | 68.70 | 9 |
| GmPLC8 | Glyma14g06460 | 14 | 4715979–4720795 | 1767 | 588 | 6.57 | 66.83 | 9 |
| GmPLC9 | Glyma14g37290 | 14 | 46572314–46578539 | 1731 | 576 | 6.42 | 66.38 | 9 |
| GmPLC10 | Glyma18g03090 | 18 | 2033312–2038120 | 1779 | 592 | 5.90 | 67.56 | 9 |
| GmPLC11 | Glyma18g03100 | 18 | 2042085–2047648 | 1671 | 556 | 5.70 | 63.22 | 9 |
| GmPLC12 | Glyma18g03110 | 18 | 2048710–2053428 | 1815 | 604 | 8.83 | 68.99 | 10 |
GmPLC domain analysis and phylogenetic analysis
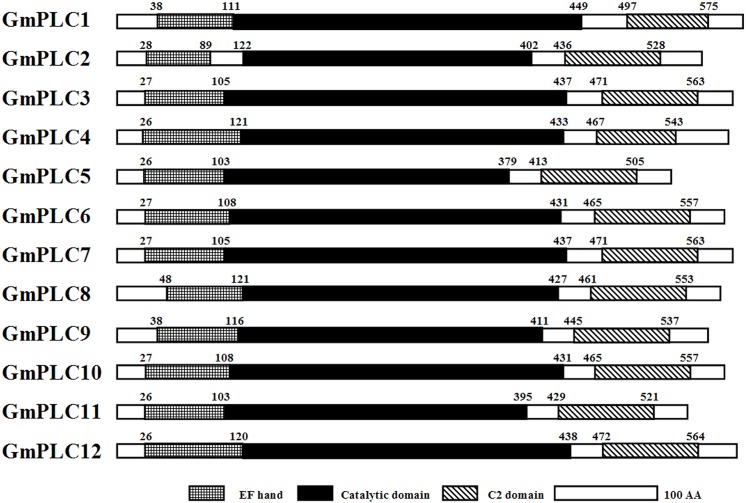
To characterize the soybean PLC genes, their domain structure was analyzed and a phylogenetic tree was constructed. PLC proteins contain a Pleckstrin Homology (PH) domain, EF hand, catalytic domain and C2 domain in animals. Some also contain a PDZ binding motif, Ras-binding domain, guanine-nucleotide-exchange factor for the Ras domain and Src homology domain [5]. However, in plants, such as soybean, PLCs only contain an EF hand, catalytic domain and C2 domain (Fig 1). The EF hand, which also found in DGK, has a key role in Ca2+ binding [34]. The catalytic domain, which contains X and Y domains, is conserved among eukaryotic PLCs [35], especially soybean PLC (S2 Fig). The C2 domain can bind Ca2+ and other effectors, including phospholipids, inositol phosphates, and proteins [36–38]. The C2 domain appears at the beginning of phospholipase D [39], but at the end of soybean PLC (Fig 1).
Fig 1. Domain analysis of soybean PLCs.
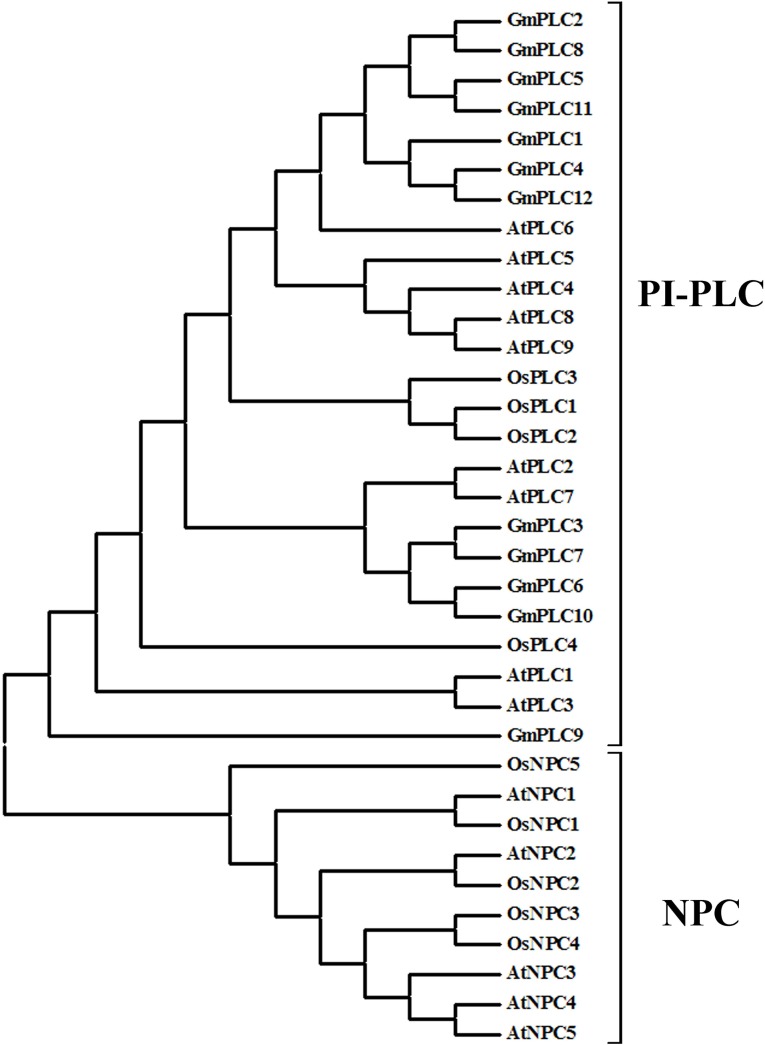
There are two types of phospholipase C (PI-PLC and NPC) in plants. However, no NPCs were found in the soybean genome. Phylogenetic analysis was carried out on the plant PLC and NPC gene families in Arabidopsis, rice and soybean. Soybean PLCs showed high homology to PI-PLCs from Arabidopsis and rice. On the other hand, OsNPCs and AtNPCs shared similarity with each other (Fig 2). The differences between the PLC types derived from diversity in structure and amino acid composition. These results suggest that soybean PLC genes belong to the PLC gene family and should be classified as PI-PLCs.
Fig 2. Phylogenetic analysis of the PLC family in soybean, Arabidopsis and rice.
Expression analysis of GmPLCs under abiotic stresses
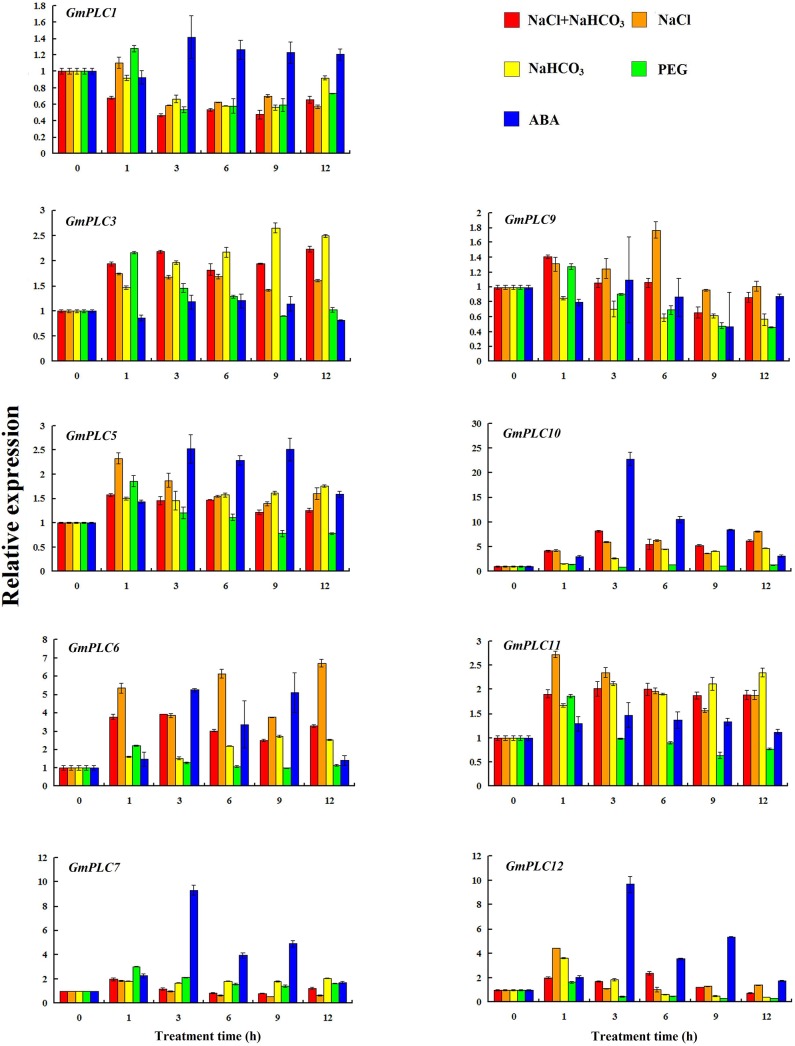
To investigate the expression patterns of the soybean PLC genes, qRT-PCR was used to detect their transcript levels in leaves and roots under PEG, ABA, salt, alkali and saline-alkaline treatments. Our result indicated that the expression of GmPLC1, GmPLC2, GmPLC4 in leaves and GmPLC2, GmPLC4, GmPLC8 in roots cannot be detected. In leaves, GmPLC6 transcripts accumulated dramatically under saline-alkali and ABA treatments (Fig 3). In pea leaves, PsPLC transcription was also stimulated after 30 min induction by ABA [40]. It has been reported that PLC plays a role in the events associated with the inhibition of stomatal opening by ABA [41]. This implies that PLC takes part in the regulation of drought stress in plants. On the other hand, the expression levels of GmPLC9 were induced by PEG stress, specifically. Transcript levels of GmPLC10 were increased after alkali treatment. After induction by salt, GmPLC12 transcript levels were more than 3-fold greater than in the control (Fig 3). Recently, TaPLC1 was characterized to have biological functions in regulating seedling growth and responses to drought and salinity stress [42]. The expression of TaPLC1 and AtPLC5 could be induced by salt and PEG treatments [15, 42]. The overexpression of AtPLC9 improved thermotolerance in Arabidopsis [43]. These results show that plant PLCs play a positive role in abiotic stress responses. However, the expression levels of GmPLC3 and GmPLC9 decreased under ABA treatment, and GmPLC6 transcripts were decreased under PEG treatment (Fig 3). This phenomenon was also observed in rice, in which OsPLC4 transcripts decreased during salt, cold and drought stresses [24]. It was also shown that one family member had dominant negative effects on the function of the others in Arabidopsis [44]. It is essential to investigate the mechanism by which PLCs function in the regulation of plant abiotic stress responses.
Fig 3. Expression of soybean PLCs in leaves under abiotic stress.
The expression levels of soybean PLCs, which were shown in Y-axis, were compared with the control (0 h). Please note that the expression levels of different PLCs are scaled differently.
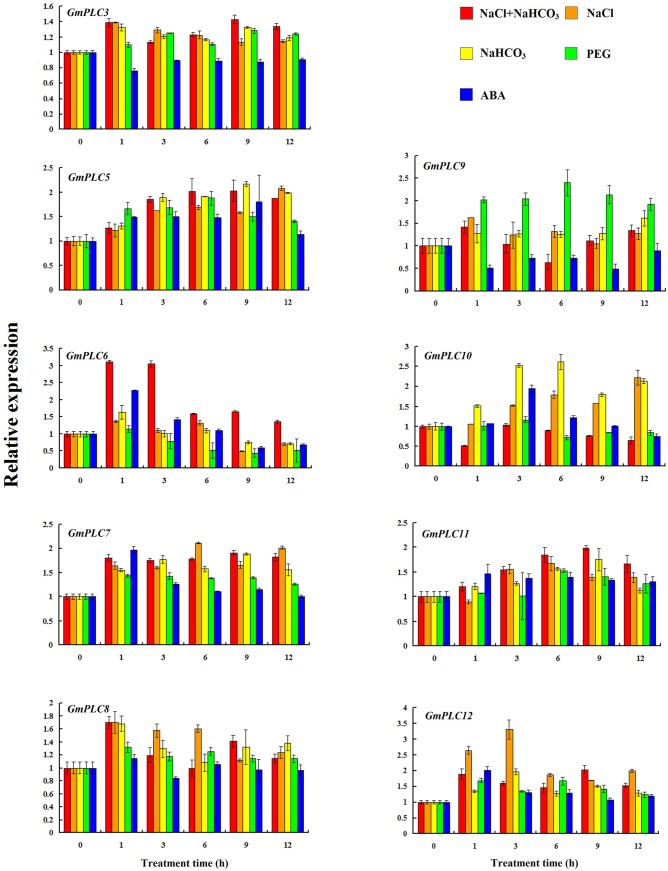
PA reportedly binds to a number of proteins that play a role during water limiting conditions, such as drought and salinity stress, and has been shown to play an important role in maintaining root system architecture [45]. In addition, DAG, which is a product of PLC, can be subsequently phosphorylated to PA by DGK. Therefore, the expression profiles of the soybean PLC genes were also analyzed in roots. The expression of GmPLC1 decreased under salt, saline-alkali, alkali and PEG treatments in roots. GmPLC3, GmPLC5, GmPLC6, GmPLC10, GmPLC11 and GmPLC12 transcripts were induced by salt stress (Fig 4). The expression levels of GmPLC3, GmPLC5, GmPLC6, GmPLC10, GmPLC11 and GmPLC12 were increased under alkali treatment. GmPLC3, GmPLC5, GmPLC6, GmPLC10 and GmPLC11 were expressed under saline-alkaline treatment. ABA treatment stimulated the transcription of GmPLC5, GmPLC6, GmPLC7, GmPLC10 and GmPLC12, especially GmPLC7, GmPLC10 and GmPLC12 (Fig 4). Although the expression profiles of PLCs in roots have not been analyzed before, PLC signaling has been characterized in the activation of plasmolysis in root cells [46]. Moreover, most of the soybean PLC genes were induced by abiotic stress, especially ABA treatment. This suggests that soybean PLC genes are involved in various stress pathways.
Fig 4. Expression of soybean PLCs in roots under abiotic stress.
The expression levels of soybean PLCs, which were shown in Y-axis, were compared with the control (0 h). Please note that the expression levels of different PLCs are scaled differently.
Expression analysis of GmPLCs in various organs
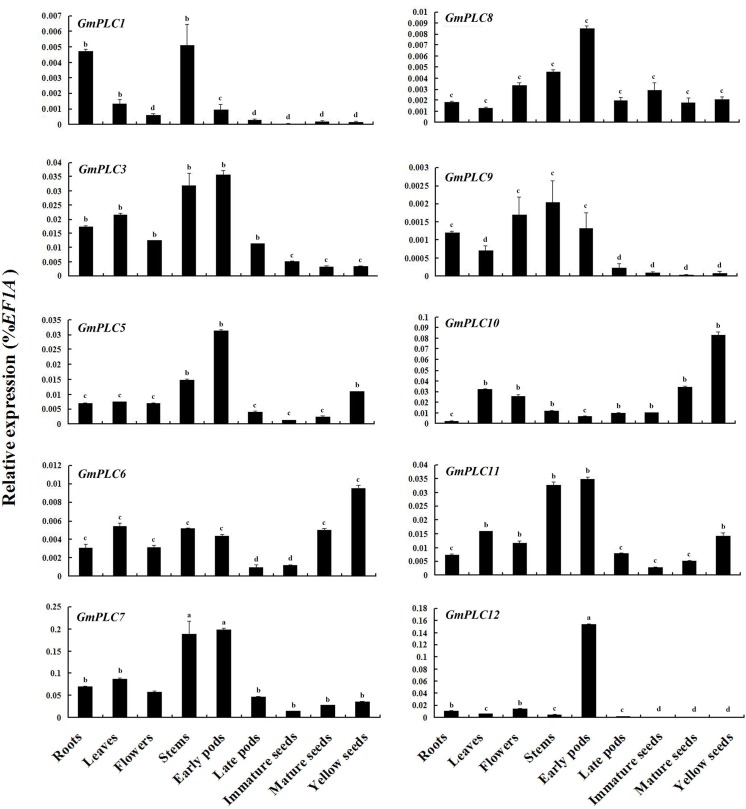
Plant PLC members have been characterized in various cellular processes and signaling networks, and are triggered in response to developmental events in different plant species [47]. To investigate the steady-state expression patterns of the PLC genes in soybean, qRT-PCR was used to detect transcript levels in various organs. High transcript levels of GmPLC7 were detected in all organs, especially stems and early pods. GmPLC12 expression was found in early pods specifically, although low transcript levels were found in other organs (Fig 5). In Arabidopsis, AtPLC2 was highly expressed in leaves, stems, roots and flowers [33]. Recently, microarray analysis of the Arabidopsis PI-PLC gene family was carried out and confirmed that AtPLC2 transcripts were highly expressed in all organs [48]. GmPLC3, GmPLC5, GmPLC10 and GmPLC11 had medium transcript levels in all organs. The expression levels of GmPLC1, GmPLC6, GmPLC8 and GmPLC9 were lower than the others (Fig 5). In rice, OsPLC1 and OsPLC3 were highly expressed in all organs, but lower expression of OsPLC2 was detected in various organs [24]. The varied expression patterns of the soybean PLC gene family imply that GmPLCs are involved in different stages during soybean development.
Fig 5. Expression of GmPLCs in various organs.
The expression levels of soybean PLCs was compared with EF1A which the expression level of EF1A was defined as “1”. a: expression level > 0.1; b: expression level from 0.01 to 0.1; c: expression level from 0.001 to 0.01; d: expression level < 0.001. Please note that the expression levels of different PLCs are scaled differently.
Cis-regulatory elements in the promoters of soybean PLC genes
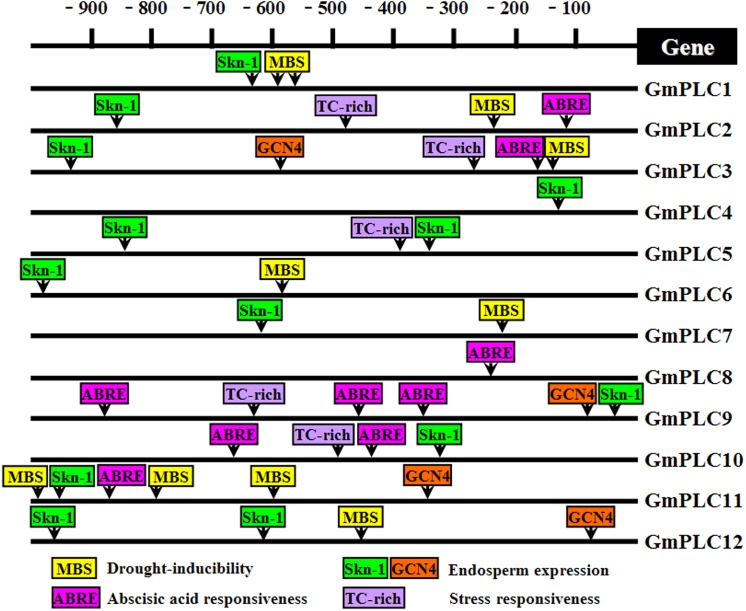
Gene expression can be regulated at several levels, such as transcription and post-translational modification. The promoter is the region of DNA that initiates the transcription of a particular gene. Cis-regulatory elements are functional DNA sequences that precisely control the temporal and spatial expression patterns of the genes expressed in higher plants [49]. In this study, soybean PLC genes were mapped on the genome and their promoter sequences were obtained. The cis-regulatory elements, such as MBS (MYB binding site), ABRE (abscisic acid responsive element), TC-rich, Skn-1 and GCN4, were analyzed. These cis-regulatory elements are known to regulate various stress responses and plant development [50–53]. Each soybean PLC gene had 1–6 cis-regulatory elements in its promoter region (Fig 6). The promoter of GmPLC10, which was highly expressed in all organs and induced strongly by abiotic stresses, contained one TC-rich and Skn-1, and two ABRE motifs. GmPLC3, GmPLC11 and GmPLC12 had more cis-regulatory elements, which might account for their responsiveness to abiotic stresses and development (Fig 6, S3 Table). Surprisingly, six cis-regulatory elements were found in the promoter of GmPLC9, but GmPLC9 transcripts were low in all organs and insensitive to nearly all abiotic stresses. This is in agreement with previous data on Glu-A1-1, -B1-1 and -B1-2 [54]. This result implies that other mechanisms modulate the expression of GmPLC9.
Fig 6. Cis-regulatory elements in the promoters of the soybean PLC gene family.
The values of -900 to -100 represent the upstream region (from translation start site) of the promoter of all the PLC genes. Various elements such as ABRE, MBS, TC-rich, Skn-1 and GCN4 are present in the promoter.
Subcellular localization of GmPLC10
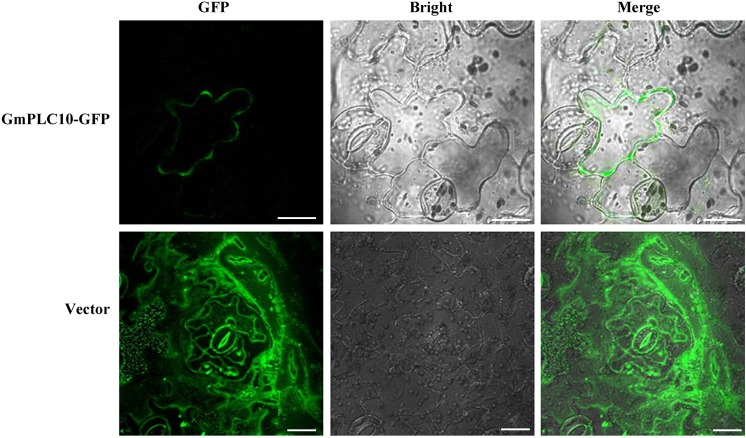
Previously, it was reported that the substrates of PLC, PI4P and PI(4,5)P2, are located in the plasma membrane [55]. To analyze the location of soybean PLC proteins, GmPLC10 was selected for subcellular localization analysis. GmPLC10 was fused to the N-terminus of GFP and transformed into N. benthamiana transiently. In confocal observation, GmPLC10-GFP fluorescence was located in the plasma membrane, whereas fluorescence of the vector control was observed everywhere (Fig 7). This result is consistent with research on AtPLC9 [43]. However, Singh et al. [24] reported that OsPLC1 and OsPLC4 were distributed throughout the cytoplasm and nucleus. The diverse localization of plant PLCs implies that they might take part in different cellular processes during development and abiotic stress.
Fig 7. Subcellular localization of GmPLC10 in Nicotiana benthamiana cells.
Bar = 20 μm.
Conclusions
In conclusion, we identified 12 GmPLC genes from the soybean genome in this study. Conversed domain and phylogenetic analyses were carried out and verified that all soybean PLC genes belong to the PI-PLC subfamily. The expression profiles of the soybean PLC genes were analyzed during development and various stresses. Together with cis-regulatory elements analysis, our results suggest that soybean PLCs are involved in various cellular processes. Moreover, subcellular localization analysis confirmed that GmPLC10 was located in the plasma membrane. Taken together, these results suggest that soybean PLCs is important for plant development and adaptation to environmental stress conditions.
Supporting Information
(XLS)
(XLS)
(XLS)
(TIF)
(TIF)
Acknowledgments
We thank Dr. Haiyue Sun (College of Horticulture, Jilin Agricultural University) for help with the subcellular localization experiment.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the National Natural Science Foundation of China (31201144, 31271746, 31401403, 31101091), the Special Program for Research of Transgenic Plants (2014ZX08010-002), The Ministry of Education doctoral program funding: Young Teacher Funding Project of China (20122223120003) and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (201410193036). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kocourkova D, Krckova Z, Pejchar P, Veselkova S, Valentova O, Wimalasekera R, et al. (2011) The phosphatidylcholine-hydrolysing phospholipase C NPC4 plays a role in response of Arabidopsis roots to salt stress. J Exp Bot 62(11): 3753–3763. 10.1093/jxb/err039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakamura Y, Awai K, Masuda T Yoshioka Y, Takamiya K, Ohta H (2005) A novel phosphatidylcholine-hydrolyzing phospholipase C induced by phosphate starvation in Arabidopsis . J Biol Chem 280: 7469–7476. [DOI] [PubMed] [Google Scholar]
- 3. Berridge MJ (1987) Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem 56: 159–193. [DOI] [PubMed] [Google Scholar]
- 4. Meldrum E, Parker PJ, Carozzi A (1991) The PtdIns-PLC superfamily and signal transduction. Biochim Biophys Acta 1092: 49–71. [DOI] [PubMed] [Google Scholar]
- 5. Munnik T, Testerink C (2009) Plant phospholipid signaling–‘in a nutshell’. J Lipid Res 50: S260–265. 10.1194/jlr.R800098-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang XM, Devaiah SP, Zhang WH, Welti R (2006) Signaling functions of phosphatidic acid. Prog Lipid Res 45(3): 250–278. [DOI] [PubMed] [Google Scholar]
- 7. Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, Manison NF, Brownlee C, Skepper JN, et al. (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100: 10091–10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMurry WC, Irvine RF (1987) Phosphatidylinositol 4,5-bisphosphate phosphodiesterase in higher plants. Biochem J 249: 877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melin PM, Sommarin M, Sandelius AS, Jergil B (1987) Identification of Ca2+-stimulated polyphosphoinositide phospholipase C in isolated plant plasma membranes. FEBS Lett 223: 87–91. [DOI] [PubMed] [Google Scholar]
- 10. Pfaffmann H, Hartmann E, Brightman AO, Morre DJ (1987) Phosphatidylinositol specific phospholipase C of plant stems. Membrane associated activity concentrated in plasma membranes. Plant Physiol 85: 1151–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tate BF, Schaller GE, Sussman MR, Crain RC (1989) Characterization of a polyphosphoinositide phospholipase C from the plasma membrane of Avena sativa . Plant Physiol 91: 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamada Y, Muto S (1991) Ca2+ regulation of phosphatidylinositol turnover in the plasma membrane of tobacco suspension culture cells. Biochim Biophys Acta 1093: 72–79. [DOI] [PubMed] [Google Scholar]
- 13. Yotsushima K, Nakamura K, Mitsui T, Igaue I (1992) Purification and characterization of phosphatidylinositol-specific phospholipase C in suspension-cultured cells of rice (Oryza sativa L.). Biosci Biotech Biochem 56: 1247–1251. [Google Scholar]
- 14. Yotsushima K, Mitsui T, Takaoka T, Hayakawa T, Igaue I (1993) Purification and characterization of membrane-bound inositol phospholipid-specific phospholipase C from suspension-cultured rice (Oryza sativa L.) cells (Identification of a regulatory factor). Plant Physiol 102(1): 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunt L, Otterhag L, Lee JC, Lasheen T, Hunt J, Seki M, et al. (2004) Gene-specific expression and calcium activation of Arabidopsis thaliana phospholipase C isoforms. New Phytol 162: 643–654. [DOI] [PubMed] [Google Scholar]
- 16. Charron D, Pingret JL, Chabaud M, Journet EP, Barker DG (2004) Pharmacological evidence that multiple phospholipid signaling pathways link Rhizobium nodulation factor perception in Medicago truncatula root hairs to intracellular responses, including Ca2+ spiking and specific ENOD gene expression. Plant Physiol 136: 3582–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engstrom EM, Ehrhardt DW, Mitra RM, Long SR (2002) Pharmacological analysis of nod factor-induced calcium spiking in Medicago truncatula. Evidence for the requirement of type IIA calcium pumps and phosphoinositide signaling. Plant Physiol 128: 1390–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coursol S, Pierre JN, Vidal J (2000) Role of the phosphoinositide pathway in the light-dependent C4 phosphoenolpyruvate carboxylase phosphorylation cascade in Digitaria sanguinalis protoplasts. Biochem Soc Trans 6: 821–823. [PubMed] [Google Scholar]
- 19. Mueller-Roeber B, Pical C (2002) Inositol phospholipid metabolism in Arabidopsis, characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol 130: 22–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pokotylo I, Pejchar P, Potocky M, Kocourkova D, Krckova Z, Ruelland E, et al. (2013) The plant non-specific phospholipase C gene family. Novel competitors in lipid signalling. Prog Lipid Res 52(2013): 62–79. [DOI] [PubMed] [Google Scholar]
- 21. Wimalasekera R, Pejchar P, Holk A, Martinec J, Scherer GF (2010) Plant phosphatidylcholine-hydrolyzing phospholipases C NPC3 and NPC4 with roles in root development and brassinolide signaling in Arabidopsis thaliana . Mol Plant 3: 610–625. 10.1093/mp/ssq005 [DOI] [PubMed] [Google Scholar]
- 22. Pejchar P, Potocky M, Novotna Z, Veselkova S, Kocourkova D, Valentova O, et al. (2010) Aluminium ions inhibit the formation of diacylglycerol generated by phosphatidylcholine-hydrolysing phospholipase C in tobacco cells. New Phytol 188: 150–160. 10.1111/j.1469-8137.2010.03349.x [DOI] [PubMed] [Google Scholar]
- 23. Peters C, Li M, Narasimhan R, Roth M, Welti R, Wang X (2010) Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis . Plant Cell 22: 2642–2659. 10.1105/tpc.109.071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh A, Kanwar P, Pandey A, Tyagi AK, Sopory SK, Kapoor S, et al. (2013) Comprehensive genomic analysis and expression profiling of phospholipase C gene family during abiotic stresses and development in rice. PLoS One 8(4): e62494 10.1371/journal.pone.0062494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vossen JH, Abd-El-Haliem A, Fradin EF, van den Berg GC, Ekengren SK, Meijer HJ, et al. (2010) Identification of tomato phosphatidylinositol-specific phospholipase-C (PI-PLC) family members and the role of PLC4 and PLC6 in HR and disease resistance. Plant J 62(2): 224–239. 10.1111/j.1365-313X.2010.04136.x [DOI] [PubMed] [Google Scholar]
- 26. Shi J, Gonzales RA, Bhattacharyya MK (1995) Characterization of a plasma membrane-associated phosphoinositide-specific phospholipase C from soybean. Plant J 8(3): 381–390. [DOI] [PubMed] [Google Scholar]
- 27. Park SK, Lee JR, Lee SS, Son HJ, Yoo JY, Moon JC, et al. (2002) Partial purification and properties of a phosphatidylinositol 4,5-bisphosphate hydrolyzing phospholipase C from the soluble fraction of soybean sprouts. Mol Cells 13(3): 377–384. [PubMed] [Google Scholar]
- 28. Chou WM, Shigaki T, Dammann C, Liu YQ, Bhattacharyya MK (2004) Inhibition of phosphoinositide-sepficif phospholipase C results in the induction of pathogenesis-related genes in soybean. Plant Biol 6: 664–672. [DOI] [PubMed] [Google Scholar]
- 29. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2012) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 10: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh A, Pandey GK (2015) Primer design using Primer Express for SYBR Green-based quantitative PCR. Methods Mol Biol 1275: 153–164. 10.1007/978-1-4939-2365-6_11 [DOI] [PubMed] [Google Scholar]
- 31. Marion J, Bach L, Bellec Y, Meyer C, Gissot L, Faure JD (2008) Systematic analysis of protein subcellular localization and interaction using high-throughtput transient transformation of Arabidopsis seedlings. Plant J 56: 469–179. [DOI] [PubMed] [Google Scholar]
- 32. Schmutz J, Cannon SB, Schlueter J, Ma JX, Mitros T, Nelson W, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. 10.1038/nature08670 [DOI] [PubMed] [Google Scholar]
- 33. Tasma IM, Brendel V, Whitham SA, Bhattacharyya MK (2008) Expression and evolution of the phosphoinositide-specific phospholipase C gene family in Arabidopsis thaliana . Plant Physiol Biochem 46: 627–637. 10.1016/j.plaphy.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 34. Katagiri T, Mizoguchi T, Shinozaki K (1996) Molecular cloning of a cDNA encoding diacylglycerol kinase (DGK) in Arabidopsis thaliana . Plant Mol Biol 30: 647–653. [DOI] [PubMed] [Google Scholar]
- 35. Katan M (1998) Families of phosphoinositide-specific phospholipase C: structure and function. Biochim Biophy Acta 1436: 5–173 [DOI] [PubMed] [Google Scholar]
- 36. Nalefski EA, Falke JJ (1996) The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci 5: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rizo J, Sudhof TC (1998) C2-domains, structure and function of a universal Ca2+-binding domain. J Bio Chem 273: 15879–15882. [DOI] [PubMed] [Google Scholar]
- 38. Zheng L, Krishnamoorthi R, Zolkiewski M, Wang X (2000) Distinct Ca2+ binding properties of novel C2 domains of plants phospholipase D α and β. J Biol Chem 275: 19700–19706. [DOI] [PubMed] [Google Scholar]
- 39. Zhao JZ, Zhou D, Zhang Q, Zhang WH (2012) Genomic analysis of phospholipase D family and characterization of GmPLDαs in soybean (Glycine max). J Plant Res 125: 569–578. 10.1007/s10265-011-0468-0 [DOI] [PubMed] [Google Scholar]
- 40. Liu HT, Liu YY, Pan QH, Yang HR, Zhan JC, Huang WD (2006) Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J Exp Bot 57(12): 3337–3347. [DOI] [PubMed] [Google Scholar]
- 41. Mills LN, Hunt L, Leckie CP, Aitken FL, Wentworth M, McAinsh MR, et al. (2004) The effects of manipulating phospholipase C on guard cell ABA-signalling. J Exp Bot 55(395): 199–204. [DOI] [PubMed] [Google Scholar]
- 42. Zhang K, Jin CC, Wu LZ, Hou MY, Dou SJ, Pan YY, et al. (2014) Expression analysis of a stress-related phosphoinositide-specific phospholipase C gene in wheat (Triticum aestivum L.). PLoS One 9(8): e105061 10.1371/journal.pone.0105061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng SZ, Liu YL, Li B, Shang ZL, Zhou RG, Sun DY (2012) Phosphoinositide-specific phospholipase C9 is involved in the thermotolerance of Arabidopsis. Plant J 69: 689–700. 10.1111/j.1365-313X.2011.04823.x [DOI] [PubMed] [Google Scholar]
- 44. Park S, Lee CM, Doherty CJ, Gilmour SJ, Kim Y, Thomashow MF (2015) Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J 82(2): 193–207. 10.1111/tpj.12796 [DOI] [PubMed] [Google Scholar]
- 45. McLoughlin F, Testerink C (2013) Phosphatidic acid, a versatile water-stress signal in roots. Front Plant Sci 4: 525 10.3389/fpls.2013.00525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Komis G, Galatis B, Quader H, Galanopoulou D, Apostolakos P (2008) Phospholipase C signaling involvement in macrotubule assembly and activation of the mechanism regulating protoplast volume in plasmolyzed root cells of Triticum turgidum . New Phytol 178(2): 267–282. 10.1111/j.1469-8137.2007.02363.x [DOI] [PubMed] [Google Scholar]
- 47. Singh A, Bhatnagar N, Pandey A, Pandey GK (2015) Plant phospholipase C family: Regulation and functional role in lipid signaling. Cell Calcium 58(2): 139–146. 10.1016/j.ceca.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 48. Pokotylo I, Kolesnikov Y, Kravets V, Zachowski A, Ruelland E (2014) Plant phosphoinositide-dependent phospholipases C: Variations around a canonical theme. Biochimie 96: 144–157. 10.1016/j.biochi.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 49. Vedel V, Scotti I. (2011) Promoting the promoter. Plant Sci 180: 182–189. 10.1016/j.plantsci.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 50. Narusaka Y, Nakashima K, Shinwari ZK, Sakuma Y, Furihata T, Abe H, et al. (2003) Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J 34: 137–148. [DOI] [PubMed] [Google Scholar]
- 51. Takaiwa F, Oono K, Kato A (1991) Analysis of the 5’ flanking region responsible for the endosperm-specific expression of a rice glutelin chimeric gene in transgenic tobacco. Plant Mol Biol 16: 49–58. [DOI] [PubMed] [Google Scholar]
- 52. Kim SY, Wu R (1990) Multiple protein factors bind to a rice glutelin promoter region. Nucleic Acids Res 18: 6845–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abe H, Urao T, Ito T, Seki M, Shinozaki K, et al. (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ravel C, Fiquet S, Boudet J, Dardevet M, Vincent J, Merlino M, et al. (2014) Conserved cis-regulatory modules in promoters of genes encoding wheat high-molecular-weight glutenin subunits. Front Plant Sci 5: 621 10.3389/fpls.2014.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Munnik T, Irvine RF, Musgrave A (1998) Phospholipid signaling in plants. Biochim Biophys Acta 1389: 222–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
(XLS)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.