Abstract
Colorectal cancer (CRC) is a leading cause of cancer deaths worldwide. One of the fundamental processes driving the initiation and progression of CRC is the accumulation of a variety of genetic and epigenetic changes in colon epithelial cells. Over the past decade, major advances have been made in our understanding of cancer epigenetics, particularly regarding aberrant DNA methylation, microRNA (miRNA) and noncoding RNA deregulation, and alterations in histone modification states. Assessment of the colon cancer “epigenome” has revealed that virtually all CRCs have aberrantly methylated genes and altered miRNA expression. The average CRC methylome has hundreds to thousands of abnormally methylated genes and dozens of altered miRNAs. As with gene mutations in the cancer genome, a subset of these epigenetic alterations, called driver events, is presumed to have a functional role in CRC. In addition, the advances in our understanding of epigenetic alterations in CRC have led to these alterations being developed as clinical biomarkers for diagnostic, prognostic and therapeutic applications. Progress in this field suggests that these epigenetic alterations will be commonly used in the near future to direct the prevention and treatment of CRC.
Keywords: DNA methylation, MicroRNA, Long non-coding RNA, Histone modification
INTRODUCTION
Colorectal cancer (CRC) is common worldwide and remains the second leading cause of cancer-related deaths in Western countries1. Despite recent improvements in screening strategies and the development of more effective treatments for CRC, the prognosis of advanced CRC is still poor. Furthermore, the newest line of molecularly-targeted therapeutic agents, appear to only have activity in metastatic CRC and do not cure the patient, but they have exponentially increased treatment costs and the economic burden of CRC care. Therefore, robust diagnostic, prognostic and predictive biomarkers are clearly and urgently needed to detect advanced colon polyps and early stage CRC, which are most effectively treated with current therapies, and to identify the most effective treatments for specific CRC patients.
Gene mutations have long been known to be important in cancer formation. However, epigenetic alterations have only recently been recognized as significant contributors to cancer development. “Epigenetics” was first described by the developmental biologist Conrad H. Waddington, in 1942, as “the study of heritable changes in gene expression mediated by mechanisms other than alterations in primary nucleotide sequence of a gene” and is now considered as broadly referring to heritable alterations in gene expression that are not mediated by changes in the DNA sequence2, 3. Epigenetic alterations frequently found in cancer include aberrant DNA methylation, abnormal histone modifications, and altered expression levels of various non-coding RNAs, including microRNAs (miRNAs). With regards to the role of epigenetic alterations in the normal-polyp-cancer sequence, as with gene mutations, it appears that a subset of the hundreds-thousands of alterations found in the typical cancer cell drives the initiation and progression of CRC formation through the sequential accumulation of genetic and epigenetic changes in key tumor-suppressor and oncogenes4.
The prevailing consensus suggests that epigenetic alterations in CRC occur early and manifest more frequently than genetic alterations. In addition, advances in genomic technologies have led to the identification of a variety of specific epigenetic alterations as potential clinical biomarkers for CRC patients. This review briefly outlines the fundamental basis of epigenetic alterations in cancer, and details the current state of the field regarding the promise and clinical usefulness of various epigenetic alterations as biomarkers for early detection, diagnosis, prognosis and management of CRC patients.
DNA METHYLATION
Overview of DNA Methylation in Cancer
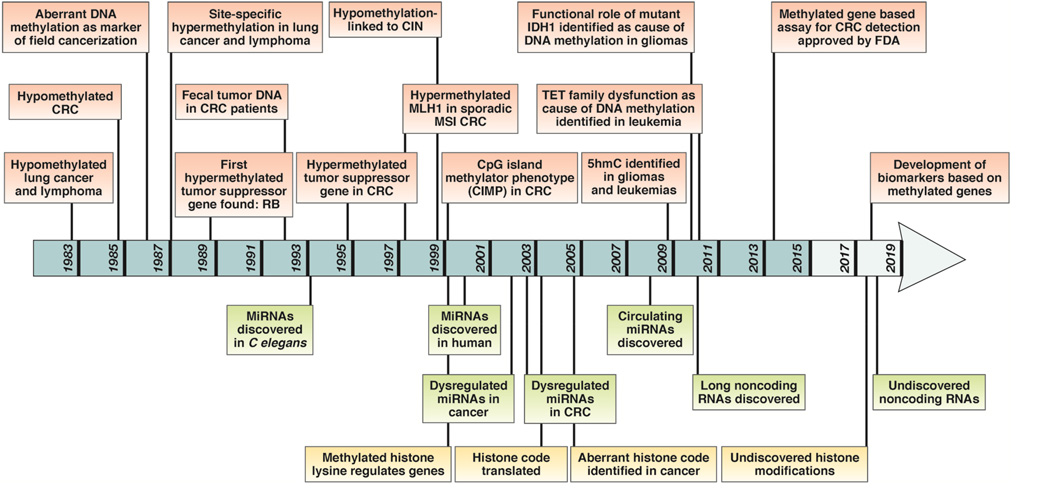
The most widely studied epigenetic alteration in cancer is aberrant DNA methylation. Although DNA hypermethylation has received the most attention recently, DNA hypomethylation was the first reported DNA methylation abnormality in human cancer (1983)5. As illustrated in Figure-1, at this time global DNA hypomethylation was identified in both colorectal adenomas and CRC6. It was not until several decades later, that Baylin and colleagues identified site-specific hypermethylation- of the Calcitonin (CALCA) gene in lung cancer and lymphoma7. Later, RB (Retinoblastoma), a known tumor suppressor gene, was found to silenced by aberrant DNA methylation, providing support for a functional role for epigenetic alterations in oncogenesis8–10. This landmark discovery was followed by the discovery of other hypermethylated tumor suppressor genes in cancer, including CDKN2A, MLH1, and CDH111–13.
Figure 1.
A historical perspective illustrating key milestones associated with the discovery of various epigenetic alterations in colorectal cancer from 1983 to the present. Individual epigenetic alterations are listed in color-coded boxes; aberrant DNA methylation (red), non-coding RNAs including microRNAs (green), and histone modifications (yellow).
DNA methylation is one of a host of epigenetic modifications that can regulate gene expression. In humans, DNA methylation occurs at cytosine residues that precede guanines, called CpG dinucleotides (C-phosphodiester-G bond)14, 15. The majority of CpG dinucleotides in the human genome are methylated, however, there are CpG rich sequences called CpG islands, which are typically unmethylated in normal healthy cells. CpG islands are found in the promoter regions of ~40–60% of tumor suppressor genes, are generally 200–2000 bps long, have a CG content >50%, and are involved in the regulation of gene expression14–16. Methylated CpG dinucleotides are usually found in gene bodies and large repetitive sequences (LINE-1, SINE/Alu sequences etc.) that are found in centromeres and other retrotransposon elements17–19. In one sense, this normal methylation pattern is essentially reversed as cancer cells.
DNA methylation is mediated by DNA methyltransferases (DNMTs) that facilitate the catalytic addition of methyl groups to the 5th position of the cytosine rings of CpG dinucleotides. DNA methyltransferases are classically considered to be either maintenance methyltransferases (e.g. DNMT1), which preserve existing methylation patterns during DNA replication, or de novo DNMTs (e.g. DNMT3A and DNMT3B), which preferentially catalyze the methylation of previously unmethylated CpGs3. Although the way in which methylation represses gene transcription is still under investigation, proposed mechanisms include alterations in chromatin complexes and the recruitment of methyl-CpG domain-binding proteins (MBD) around the CpG islands of the corresponding gene(s)20, 21. MBD proteins at gene promoters impede access of the regulatory proteins required for active gene transcription. DNA methylation can directly inhibit cis-binding elements, including the transcriptional factors Activating Protein 2 (AP-2), CCAAT enhancer-binding protein C/EBF, cAMP response element-binding protein (CREB), E2 promoter binding factor (E2F), and nuclear factor kappa-light-chain-enhancer of B cells (NF-κB). Furthermore, emerging evidence indicates that extensive DNA methylation changes at CpG island “shores” ― regions with relatively lower CpG density and located within 2 kb of CpG islands, strongly correlates with loss of gene expression22–24. CpG island shores are methylated in a tissue-specific manner, and contain 70% of the differentially methylated regions involved in cellular reprogramming. The mechanism behind differential methylation of CpG shores regulating gene expression remains poorly understood and is an area of active investigation.
Aberrant DNA Hypermethylation in Colorectal Cancer
Advances in our understanding of the molecular pathogenesis of CRC led to the initial observation that these neoplasms primarily arise through two major molecular pathways of genomic instability ― chromosomal instability (CIN) and microsatellite instability (MSI). However, more recently a third class of CRCs characterized by a high frequency of DNA hypermethylation has been identified. These cancers have been defined as having a “CpG island methylator phenotype (or CIMP)”, and their identification provided a significant advance in our understanding of the molecular mechanisms that orchestrate colorectal tumor formation.
The CIMP was first described in colorectal tumors in 1999 and was characterized as having an exceptionally high frequency of hypermethylated CpG dinucleotides25. Weisenberger and colleagues later introduced the prevailing method used to identify CIMP in CRC, which is based on the methylation status of five genes, CACNA1G, IGF2, NEUROG1, RUNX3, and SOCS126. CIMP-positive tumors exhibit unique clinicopathological and molecular features, including a predilection for proximal location in the colon, female gender, poor and mucinous histology, and the presence of frequent KRAS and BRAF mutations25, 27–30.
MSI generally results from inactivation of the DNA mismatch repair (MMR) system through hypermethylation (80% of MSI CRCs) or mutations in the genes MLH1, MSH2, MSH6, and PMS2 (20% of MSI CRCs). Inactivation of these genes results in the accumulation of DNA replication errors in repetitive microsatellite sequences, some of which are located in the exons of potential tumor suppressor genes. MSI CRCs represent ~12–15% of all tumors and >90% of familial Lynch syndrome CRCs, which account for 2–3% of all MSI CRC31, 32. The remaining MSI CRCs (10–12%) are sporadic33, 34. Eighty percent of sporadic CRCs with MSI harbor biallelic hypermethylated MLH1 alleles12, 26,12, 25.
While there exists a significant overlap in the clinical features between sporadic MSI and Lynch syndrome cancers, patients with sporadic MSI have an older age of onset, and higher frequency of the BRAF V600E mutations and hypermethylated MLH1, while Lynch patients are generally younger and harbor rare BRAF mutations 35, 36.
Aberrant DNA Methylation Occurs Early in “Traditional” and “Serrated” Polyp Pathways
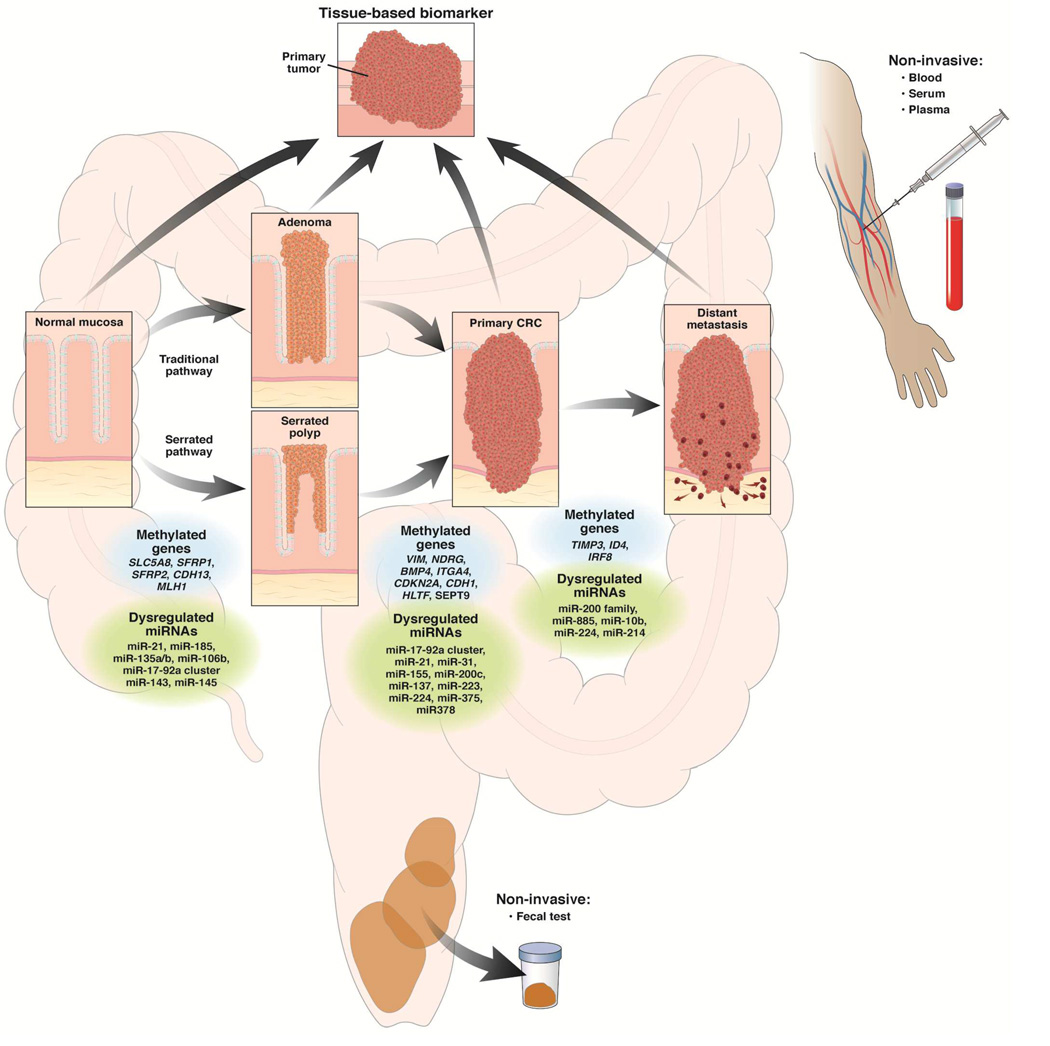
The conventional model of CRC formation as initially proposed by Fearon and Vogelstein describes a stepwise normal-adenoma-cancer progression and considers adenomatous polyps as the principal pre-neoplastic lesions leading to CRC4, 37. As described previously38, the transition from normal mucosa to adenomatous polyp is marked by both genetic and epigenetic alterations, some of which deregulate central molecular pathways39. These epigenetic alterations include hypermethylation of a variety of genes, such as SLC5A8, ITGA4, SFRP2, PTCH1, CDKN2A, HLTF, and MGMT, and some of these play a role in the initiation and progression of adenomas to CRC11, 40–45. The identification of methylated genes in colon polyps has led to their use as biomarkers in early detection assays for CRC46.
More recently recognized, the “serrated pathway” is another route for the formation CRC. It was originally described as “an alternative pathway” to the conventional adenoma-to-CRC pathway because of the unique morphological and histological characteristics of the sessile serrated polyps that give rise to CRCs via this route47. Mutations in BRAF and KRAS, along with CIMP, are common features of polyps in the serrated pathway. In contrast to classic adenomatous polyps, sessile serrated polyps and traditional serrated adenomas do not typically have genetic alterations in APC or CTNNB1 (gene for β-catenin)48–50 indicating that these two pathways are distinct and employ different molecular processes. In addition to BRAF/KRAS mutation-induced activation of the MAPK-ERK pathway51, serrated polyps evolve through methylation-mediated transcriptional inactivation of various genes belonging to the β-catenin/WNT pathway (SFRP family, CDX2, MCC)52–54, p53 signaling pathway (IGFBP7)55, cell cycle control proteins (CDKN2A)56, and DNA Mismatch repair (MLH1)55 family.
Aberrant DNA Hypomethylation in Colorectal Cancer
While DNA hypermethylation can silence tumor-suppressor genes, global DNA hypomethylation is believed to influence CRC development by inducing chromosomal instability and global loss of imprinting (Figure-2A)57–60. Genome-wide hypomethylation generally occurs within repetitive transposable DNA elements such as the LINE-1 or short interspersed nucleotide elements (SINE, or Alu) sequences in many cancers, including CRC61–65. LINE-1 hypomethylation inversely associates with MSI and/or CIMP65, 66. Furthermore, a number of studies have demonstrated that a high degree of LINE-1 hypomethylation correlates with worse patient survival67–70. One hypothesis is that hypomethylation of LINE/SINE sequences may induce inadvertent activation of potential proto-oncogenes71, which implies that LINE-1 hypomethylation has a functional role in CRC formation72.
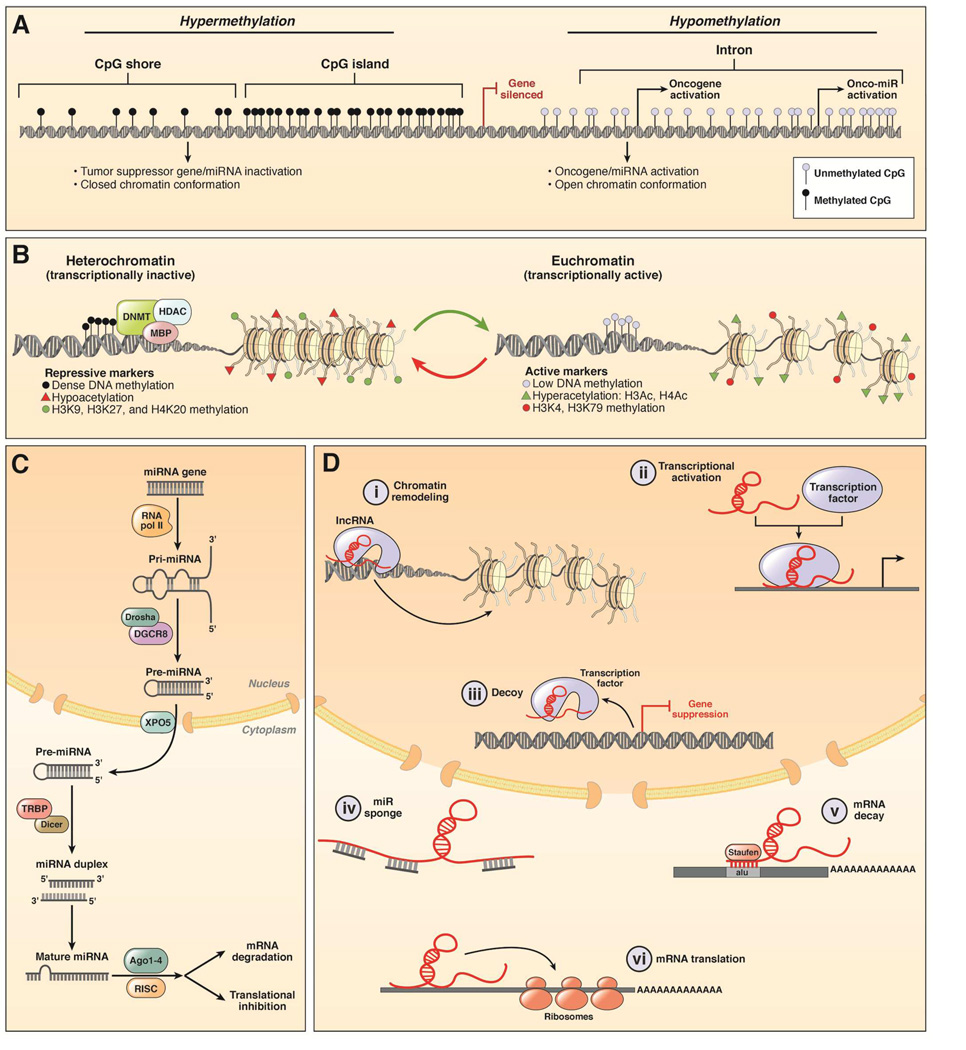
Figure 2.
An illustration of various epigenetic alterations in colorectal cancer. A) This figure illustrates the concept of aberrant DNA hypermethylation in the context of a “cancer cell”. Double helix DNA represents a tumor suppressor gene, with CpG islands and CpG shores in its promoter region. Hypermethylation of CpG sites (shown as black lollipops) leading to gene silencing and closed chromatin in the cancer cells is shown. In contrast, CpG dinucleotides within introns as well as in intergenic regions are frequently hypomethylated, which may lead to the increased expression of oncogenes and oncomiRNAs, and resulting open chromatin conformation. B) This figure illustrates histone modifications in a cancer cell. The left panel depicts heterochromatin, which is a closed chromatin conformation that is often associated with DNA methylation and inactive gene transcription. In contrast, the euchromatin state is in an open conformation and associates with active gene transcription, presumably secondary to increased transcription factor binding. C) A schematic demonstrating miRNA biogenesis in cancer cells and how miRNAs inhibit and/or cause degradation of their mRNA targets. D) This figure illustrates various activities of long-noncoding RNAs (lncRNAs) in cancer cells, including i) their ability to regulate chromatin conformation; ii) induce transcription by binding to appropriate transcription factors; iii) function as decoy and inhibit gene transcription; iv) act as miRNA sponges; v) cause mRNA decay and vi) induce mRNA translation.
With regards to the mechanism responsible for cancer associated DNA hypomethylation, the prevailing belief is that it results from a passive process secondary to inadequate maintenance of methylation during DNA replication in most cancers. Recent studies have demonstrated that DNA hypomethylation in cancer can be secondary to an active process in some tumors. The ten-eleven translocation enzymes (TET) can catalyze the formation of 5-hydroxymethylcytosine (5-hmC) from 5-methylcytosine, which is recognized by the Base Excision Repair (BER) proteins for removal and replacement with unmethylated cytosine. The ten-eleven translocation (TET) family members TET1 and TET2 are mutated in leukemia and a biochemically associated enzyme, isocitrate dehydrogenase (IDH1) is mutated in some gliomas. It has been proposed that mutant TET1 and TET2 and mutant IDH1 are the mechanisms for DNA hypermethylation in leukemias and gliomas, respectively.73–75. Of interest, global loss of 5-hmC is reported in several types of solid tumors, including CRC76. Recently, downregulation of TET1 expression was found in early stages of colon cancers, and loss of its expression was shown to inhibit WNT signaling pathway and suppression of tumor proliferation77, 78. However, the biological significance of 5-hmC and the TET proteins with regards to LINE-1 hypomethylation in CRC still remains unclear, and requires further investigation. IDH1 and TET1 or TET2 mutations do not appear to be a common cause of aberrant DNA methylation in CRC79.
Methylated DNA as a Biomarker for Colorectal Cancer
The term “biomarker” or “biological marker”80 was defined in 1998 by The National Institutes of Health Biomarkers Definition Working Group as “a characteristic that is objectively measured and evaluated as an indicator of normal biological and pathological processes, or pharmacologic responses to a therapeutic intervention”81. However, the definition of biomarker remains dynamic, and is constantly under revision in response to our evolving understanding of cancer. Based on current clinical criteria, CRC biomarkers can be described as substances: a) that are measured easily and inexpensively to identify a patient’s cancer; b) that identify a patient’s prognosis independent of conventional classifications (i.e. TNM classification and tumor markers) to improve their outcome; and c) that predict a patient’s response to a specific treatment, thereby improving their prognosis and quality of life.
Methylated DNA as a Diagnostic Biomarker
Despite recent advances in treatment options, early detection and removal of precancerous lesions (advanced colorectal adenomas, A-CRA) remains the most effective strategy for reducing mortality associated with CRC. However, current screening modalities are limited by invasiveness, expense and poor patient compliance, leading to the late detection of cancers and subsequently unfavorable prognoses. The most widely used fecal screening tests, FOBT and FIT, have suboptimal diagnostic accuracy, highlighting the need for more robust and reliable non-invasive biomarker assays for the detection of early CRC.
During early stages of colorectal carcinogenesis, epigenetic alterations appear to exceed the frequency of genetic mutations, suggesting their greater potential for the next generation of diagnostic biomarkers for the detection of colonic polyps and cancers.
Stool-based biomarkers
Specific biofluids, such as blood (plasma or serum) and feces are the most common analytes used in CRC screening tests. Since the initial discovery by Sidransky and colleagues of mutant KRAS in fecal specimens from patients with CRC82, numerous studies have supported using fecal DNA for potential screening assays for the early detection of CRC. Studies of methylated SFRP2, SFRP5, PGR, CALCA, and IGFBP2, in fecal DNA from two independent cohorts identified methylated SFRP2 as a diagnostic biomarker for CRC detection with high sensitivity (77–90%) and specificity (77%)83. A later study using 111 fecal specimens, including 21 colorectal adenomas, demonstrated that methylated SFRP2 can also identify patients with precancerous colonic polyps84.
Another well-studied fecal DNA biomarker for CRC is methylated VIM, the gene for Vimentin. Methylated VIM specifically occurs in CRC tissues and is detected in fecal DNA with reasonably high sensitivity (46%) and specificity (90%)85. A number of studies have demonstrated the potential for using methylated VIM as a biomarker for the early detection of CRC86, 87, and these studies led to the development of an assay that detects methylated VIM as one of the first commercial fecal-DNA screening test for CRC (ColoSure™, Lab Corp, Burlington, NC). To date, a large number of hypermethylated genes including APC, ATM, BMP3, CDKN2A, SFRP2, GATA4, GSTP1, HLTF, MLH1, MGMT, NDRG4, RASSF2A, SFRP2, TFPI2, VIM, and WIF1 have been analyzed in fecal DNA for the early detection of CRC85, 88–95.
As illustrated in Table 1, a large number of stool-based methylation biomarkers, for use in CRC early detection assays have been identified in Phase I and II biomarker studies96. Perhaps of most clinical importance, this line of investigation has culminated in the development of an FDA approved, clinically available stool-based CRC screening test – Cologuard® (Exact Sciences Corporation). This stool DNA based assay, which detects methylated BMP3, methylated NDRG4, and mutant KRAS, was recently compared to FIT and to colonoscopy in nearly 10,000 patients enrolled at 90 sites97. This fecal DNA based assay showed an overall sensitivity of 92% (95% CI, 83–97.5%) for CRC and 93% (95% CI 83.8–98.2%) for stage I-III CRC, compared to sensitivity of FIT at 74% (95% CI, 61.5–84%) and 73% (95% CI, 60.3–83.9%), respectively (p=0.002). For advanced adenomas and sessile serrated polyps, the sensitivity of the test increased proportionately with lesion size and grade. The molecular assay was significantly more sensitive than FIT for advanced adenomas: 42% (95% CI, 38.9–46%) vs. 24% (95% CI, 20.8–27%), respectively, for those ≥1 cm and 66% vs 43% for those ≥2 cm (p< 0.001). Sessile serrated polyps ≥1 cm were detected at a rate of 42% for the molecular assay compared 5% for FIT (p< 0.001); In this study the test specificity was based on the primary and secondary study endpoints, specifically the detection of CRC and advanced pre-cancers. For these endpoints combined, the specificity was 87%97.
Table 1.
List of aberrantly methylated genes/loci as potential non-invasive markers for diagnosing colorectal cancer
| Diagnostic markers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Markers | Source | Study Design |
Sample size |
Target: Adenoma or CRC |
Sensitivity (%) | Specificity (%) | Reference | |
| Cases | Control | |||||||
| Blood-based biomarker | ||||||||
| ALX4 | Serum | R | 30 | 52 | CRC | 83.3 | 70 | Ebert et al. 109 |
| ALK4 | Plasma | R V | 28/5 (CRC), 45/49 (polyp) | 55/22 | CRC, AD | 40 (CRC)/45(polyp) | 82 | Tänzer et al. 295 |
| HLTF | Serum | R | 49 | 41 | CRC | 32.7 | 92.7 | Leung et al. 110 |
| HLTF | Serum | R | 38 | 20 | CRC | 55 | 100 | Wallner et al. 111 |
| hMLH1 | Serum | R | 19 | - | CRC | 33 | 100 | Grady et al. 296 |
| hMLH1 | Serum | R | 49 | 41 | CRC | 42.9 | 97.6 | Leung et al. 110 |
| hMLH1 | Serum | R | 38 | 20 | CRC | 18.4 | 100 | Wallner et al. 111 |
| HPP1 | Serum | R | 38 | 20 | CRC | 21.1 | 100 | Wallner et al. 111 |
| NEUROG1 | Serum | R V | 252 (15/95/45/97) | 93 (32/-/16/45) | CRC | 61 | 91 | Herbst et al. 112 |
| SEPT9 | Plasma | R | 133 | 179 | CRC | 69 | 86 | Lofton-Day et al. 106 |
| SEPT9 | Plasma | R V | 252/126 (CRC),168 (polyp) | 102/183 | CRC, AD | 55.5(CRC), 14.9(AD) | 91 | Grützmann et al. 297 |
| SEPT9 | Plasma | R V | 97/90 | 172/155 | CRC | 72/68 | 93/89 | deVos et al. 298 |
| SEPT9 | Plasma | R V | 28/5 (CRC), 45/49 (polyp) | 55/22 | CRC, AD | 73(CRC), 29 (AD) | 91 | Tänzer et al. 295 |
| SEPT9 | Plasma | PT V | 50 (CRC) / 104 (AD) | 94/53 | CRC/AD | 90(CRC)/12 (AD) | 88/97 | Warren et al. 299 |
| SEPT9 | Plasma | R | 30 (CRC), 22 (AD) | 49 | CRC, AD | 60(CRC), 14 (AD) | 73 | Ahlquist et al. 300 |
| SEPT9 | Plasma | PT | 53 (CRC), 315 (AD) | 938 | CRC, AD | 48.2(CRC), 11.2(AD) | 91.5 | Church et al. 107 |
| SEPT9 | Plasma | R | 34 (CRC), 26 (AD) | 24 | CRC, AD | 88.2(CRC), 30.8(AD) | 91.7 | Tóth et al. 301 |
| SEPT9 | Plasma | PT | 1544 | CRC | 68 | 80 | Potter et al. 302 | |
| SEPT9 | Plasma | PT V | 102/2(CRC), 26(AD) | -/94 | CRC | 73.3 | 81.5 | Johnson et al. 303 |
| Vimentin | Plasma | R | 81 | 110 | CRC | 59 | 93 | Li et al. 113 |
| APC, MGMT, RASSF2A, WIF1 (panel) | Plasma | R | 243 (CRC), 64 (AD) | 276 | CRC, AD | 86.5(CRC), 74.6(AD) | 92.1(CRC), 91.3(AD) |
Lee et al. 95 |
| TMEFF2 | Plasma | R | 133 | 179 | CRC | 69 | 65 | Lofton-Day et al. 106 |
| NGFR | Plasma | R | 133 | 179 | CRC | 51 | 84 | Lofton-Day et al. 106 |
| Stool-based biomarker | ||||||||
| ALX4 | Stool | R | 90 (CRC+AD) | 157 | CRN (CRC+AD) | 10.6 | 98.7 | Amiot et al. 304 |
| CDKN2A | Stool | R | 29 | 25 | AD | 31 | 84 | Petko et al. 93 |
| CDKN2A | Stool | R | 25 | 20 | CRC | 20 | 100 | Abbaszadegan et al. 305 |
| CDKN2A | Stool | - | 30(CRC), 25(AD) | 31 | CRC, AD | 40 (CRC), 24 (AD) | 96.8 (panel) | Chang et al. 306 |
| GATA4 (Train set/Valid set) | Stool | PR V | 28/47 | 45/30 | CRC | 71/ 51 | 84/93 | Hellebrekers et al. 91 |
| ITGA4 | Stool | R | 13 | 28 | AD | 69 | 79 | Ausch et al. 307 |
| ITGA4 | Stool | - | 30(CRC), 25(AD) | 31 | CRC, AD | 36.7(CRC), 16 (AD) | 96.8 (panel) | Chang et al. 306 |
| MGMT | Stool | R | 29 | 25 | AD | 48 | 73 | Petko et al. 93 |
| MGMT | Stool | R | 52(CRC), 21(AD) | 24 | CRC, AD | 48.1 (CRC), 28.6 (AD) | 100 | Huang et al. 308 |
| MGMT | Stool | R | 60(CRC) 52(AD) | 37 | CRC, AD | 51.7 (CRC), 36.5 (AD) | - | Baek et al. 309 |
| NDRG4 (Train set/ Valid set) | Stool | R V | 28/46 | 45/30 | CRC | 61/53 | 93/100 | Melotte et al. 92 |
| SFRP2 (Train set/ Valid set) | Stool | R | 10/13 | 13/13 | CRC | 90/77 | 77/77 | Muller et al. 83 |
| SFRP2 | Stool | R | 52(CRC), 21(AD) | 24 | CRC, AD | 94.2 (CRC), 52.4 (AD) | 93 | Huang et al. 308 |
| SFRP2 | Stool | R | 69(CRC), 34(AD) | 30 | CRC, AD | 87.0 (CRC), 61.8 (AD) | 93.3 | Wang et al. 89 |
| SFRP2 | Stool | R | 29 (AD) | 6 | AD | 46 | 100 | Oberwalder et al. 310 |
| SFRP2 | Stool | R | 84(CRC), 56(AD) | 113 | CRC, AD | 63.1 (CRC), 32.1 (AD) | 92 | Nagasaka et al. 311 |
| SFRP2 | Stool | - | 30(CRC), 25(AD) | 31 | CRC, AD | 60 (CRC), 44 (AD) | 96.8 (panel) | Chang et al. 306 |
| SFRP2 | Stool | R | 48 (CRC), 35 (AD) | 30 | CRC, AD | 56.3(CRC), 51.4(AD) | 100 | Zhang et al. 312 |
| TFPI2 (Pilot/Training/Validation) | Stool | R V | 11/26/47(CRC), 7/19(AD) | 12/45/30 | CRC, AD | 73/89/76(CRC), 43/- /21(AD) |
100/79/93(CRC), 100/-/93(AD) |
Glockner et al. 90 |
| TFPI2 | Stool | R | 60(CRC), 20(AD) | 30 | CRN (CRC+AD) | 68.3 | 100 | Zhang et al. 313 |
| RASSF2 | Stool | R | 84(CRC), 56(AD) | 113 | CRC, AD | 45.3(CRC), 12.6(AD) | 94.7 | Nagasaka et al. 311 |
| Vimentin | Stool | R | 94 | 198 | CRC | 46 | 90 | Chen et al. 85 |
| Vimentin | Stool | R | 40 | 122 | CRC | 72.5 | 86.9 | Itzkowitz et al. 86 |
| Vimentin (Train set/Valid set) | Stool | PR V | 40/42 | 122/241 | CRC | 73/81 | 87/82 | Itzkowitz et al. 87 |
| Vimentin | Stool | R | 22(CRC), 20(AD) | 38 | CRC, AD | 41 (CRC), 45(AD) | 95 | Li et al. 113 |
| Vimentin | Stool | R | 60(CRC), 52(AD) | 37 | CRC, AD | 38.3 (CRC), 15.4 (AD) | - | Baek et al. 309 |
| Vimentin | Stool | R | 9 (UC-CRC)10 (UC-dysplasia) | 35 (25, UC; 10 ,CD) | UC-CRC/dysplasia | 89 | 94 | Kisiel et al. 314 |
| Vimentin | Stool | R | 90 (CRC+AD) | 157 | CRN (CRC+AD) | 32.6 | 100 | Amiot et al. 304 |
| WIF1 | Stool | R | 90 (CRC+AD) | 157 | CRN (CRC+AD) | 19.3 | 99.4 | Amiot et al. 314 |
| WIF1 | Stool | R | 48(CRC), 35(AD) | 30 | CRC, AD | 60.4(CRC), 45.7(AD) | 96.7 | Zhang et al. 312 |
| BMP3 NDRG4 TFPI2 Vimentin KRAS(mutation) (panel) |
Stool | R V | 170/82 (CRC), 89/44 (AD) | 197/96 | CRC, AD | 85(CRC), 56(AD)/78(CRC), 48(AD) |
90/85 | Ahlquist et al. 315 |
| BMP3 NDRG2 KRAS(mutation) (panel) | Stool | PT | 65(CRC), 757(precancerous) | 6274 | CRC, AD | 92.3(CRC), 42.4(AD) | 86.6 | Imperiale et al. 97 |
R, Retrospective study; PR, Retrospective analysis of prospectively collected samples in clinical trial; PT, Prospective trial; CRC, Colorectal cancer; CRN, colorectal neoplasia; AD, adenoma
Assays in bold have been approved by regulatory agencies in U.S., E.U. or China for clinical use.
Blood-based biomarkers
Due to accessibility and high patient acceptance, blood is invariably felt to be the most ideal analyte for cancer biomarkers. Table 1 summarizes aberrantly methylated genes discovered in the plasma or serum of CRC patients, which are candidate biomarkers consequently.
“Circulating DNA biomarkers” were first described in the 1970s when abnormally high concentrations of DNA were discovered in the sera of patients with various cancers98. Initial studies focused on discovering somatic mutations99, 100; however, since somatic mutations are relatively rare compared to DNA methylation alterations in the early stages of CRC tumorigenesis, a great deal of effort has gone into the development of blood-based diagnostic assays based on circulating methylated DNA.
Following the initial reports of methylated CDKN2A in circulating DNA in a variety of human cancers in 1999101–103, a growing number of studies have examined the potential of methylated genes to be a blood-based biomarkers for CRC patients. Currently, the most established methylated DNA blood biomarker is methylated Septin 9 (SEPT9), which belongs to the gene family that encodes a group of GTP-binding104 and filament-forming proteins105 involved in cytoskeletal formation. Lofton-Day and colleagues first identified methylated SEPT9 as a non-invasive diagnostic biomarker for CRC106. These authors reported that methylated SEPT9 had 69% sensitivity and 86% specificity for discriminating CRC patients from healthy individuals106. Subsequent studies validated the clinical significance of methylated SEPT9 as a potential biomarker for CRC screening, which is now commercially-offered as a blood-based screening test in various assays including EpiproColon® 1.0 (Epigenomics, Seattle, WA), ColoVantage® (Quest Diagnostics, Madison, NJ) and RealTime mS9 (Abbott Laboratories, Des Plaines, IL). Furthermore, a recent prospective clinical trial (PRESEPT) demonstrated a comparable sensitivity and specificity of this assay for CRC vis-à-vis the conventional fecal occult blood test (FOBT), confirming its potential usefulness as a blood-based biomarker for CRC107. This test was also recently approved the Chinese FDA for use as a CRC screening assay. However, methylated SEPT9 has a limited sensitivity for the detection of advanced adenomas (11%), underscoring the need for further improvement of this test for implementation for population-based screening of colorectal neoplasia. A recent study demonstrated that the methylated SEPT9 assay was superior to fecal immunochemical (FIT) at detecting CRC neoplasms, but both approaches were suboptimal for diagnosing patients with advanced adenomas108. To date, several other blood-based diagnostic methylation biomarkers have been identified for CRC detection, including ALX4109, APC95, CDKN2A93, HLTF110, HPP1111, hMLH1110, MGMT95, NEUROG1112, NGFR, RASSF2A95, SFRP2, VIM113, and WIF195. It is conceivable that a robust biomarker panel of methylated genes will be developed into a clinically viable CRC screening method in the near future.
Methylated DNA as a Prognostic Biomarker
Currently, the most accurate means for assessing CRC patient prognosis requires pathological staging of the tumor and the assessment of specific histological features of the tumor. However, the heterogeneity of survival times in patients with the same stage of CRC is well known and highlights the need for a more accurate system for determining CRC patient prognosis. As shown in Table 2, multiple, large and sufficiently powered clinical studies with independent external validation cohorts have demonstrated the feasibility of using specific methylated DNA signatures for developing prognostic biomarkers in CRC.
Table 2.
List of aberrantly methylated genes/loci as potential prognostic and predictive markers in colorectal cancer
| Prognostic markers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Study Design | Source | Sample size | Stage | Status associated with poor prognosis |
Assay | Survival Analysis |
Reference |
|
Tissue-based biomarker | ||||||||
| CDKN2A | PR | Tissues | 86 | Duke’s A-C | Hypermethylation | MSP | Univariate | Esteller et al. 316 |
| CDKN2A | PR | Tissues | 902 | I-IV | Hypermethylation | Methylight | Univariate | Shima et al. 317 |
| CDKN2A | R | Tissues | 90 | II-IV | Hypermethylation | Methylight | Univariate | Maeda et al. 318 |
| CDKN2A | R | Tissues | 84 | T3N0M0 (Dukes B2) | Hypermethylation | MSP | Univariate | Liang et al. 319 |
| CDKN2A | - | Tissues | 555 | I-IV | - | MSP | Univariate | Ward et al. 320 |
| CDKN2A | R | Tissues | 582 | I-IV | Hypermethylation | MSP | Univariate | Barault et al. 321 |
| CDKN2A | PR | Tissues | 188 | IV | Hypermethylation | MSP | Univariate | Shen et al. 114 |
| CDKN2A | R | Tissues | 422 | I-IV | Hypermethylation | Pyroseq and MSP | Multivariate | Bihl et al. 322 |
| CDKN2A | R | Tissues | 381 (Rectal cancer) | I-IV | Hypermethylation | Methylight | Multivariate | Kohonen-Corish et al. 323 |
| CDKN2A | R | Tissues | 285 (Rectal cancer:131) | I-IV | Hypermethylation | Pyrosequencing | Multivariate | Kim et al. 115 |
| CDKN2A | R | Tissues | 151 | I-IV | Hypermethylation | qMSP | Multivariate | Mitomi et al. 324 |
| CHFR | PR, V | Tissues | Trainig:173 (stageII:66); Validation:569 (Stage II:136) | I-IV | Hypermethylation | qMSP | Multivariate | Cleven et al. 325 |
| EVL | R | Tissues | 219 (Training 147; Validation:72) | I-IV | Hypermethylation | qMSP | Multivariate | Yi et al. 326 |
| IGFBP3 | R | Tissues | 219 (Training 147; Validation:72) | I-IV | Hypermethylation | qMSP | Multivariate | Yi et al. 326 |
| KiSS1 | R | Tissues | 342 (Training:62;Testing:100;Validation:190) | I-IV | Hypermethylation | MSP | - | Moya et al. 327 |
| LINE-1 | PR | Tissues | 643 | I-IV | Hypomethylation | Pyrosequencing | Multivariate | Ogino et al. 67 |
| LINE-1 | R | Tissues | 343 | I-IV | Hypomethylation | Pyrosequencing | Univariate | Antelo et al. 68 |
| LINE-1 | R | Tissues | 207(MSI) | I-IV | Hypomethylation | Pyrosequencing | Multivariate | Rhee et al. 70 |
| LINE-1 | R | Tissues | 161 | III | Hypomethylation | Pyrosequencing | Multivariate | Ahn et al. 69 |
| MGMT | R | Tissues | 111 | I-IV | Hypomethylation | Pyrosequencing | Univariate | Nilsson et al. 125 |
| MGMT | R | Tissues | 90 | I-IV | Hypomethylation | MSP | Multivariate | Nagasaka et al. 126 |
| IGF2 | PR | Tissues | 1033 | I-IV | Hypomethylation | Pyrosequencing | Multivariate | Baba et al. 124 |
| RET | PR, V | Tissues | Series 1:716, Series 2:393, Series3:294 | I-IV/II+III/II | Hypermethylation | qMSP and pyroseq | Multivariate | Draht et al. 127 |
| TFAP2E | R | Tissue | 311 | I-IV | Hypomethylation | MS-HRM | Multivariate | Zhang et al. 128 |
| TFAP2E | R | Tissue | 193 | I-III | Hypomethylation | MS-HRM | Multivariate | Park et al. 129 |
|
CIMP marker |
Study design | Specimens | Sample size |
Stage | Marker genes | CIMP category |
Significance | Survival analysis |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| CIMP | R + two arm study | Tissues | 206 | III | CDKN2A, MINT2, MDR1 | CIMP −/+ | CIMP(+) correlated with poor prognosis in surgery alone group |
Univariate | van Rijnsoever et al.118 |
| CIMP | - | Tissues | 605 | I-IV | MINT1,MINT2,MINT12, MINT31,CDKN2A | CIMP −/+ | CIMP(+) correlated with poor prognosis in MSS CRC |
- | Ward et al. 320 |
| CIMP | PR | Tissues | 816 | I-IV | MINT 1, MINT 2, MINT 31, CDKN2A, and hMLH1 | CIMP low/high | CIMP(+) correlated with poor prognosis in MSS CRC |
Univariate | Samowitz et al. 328 |
| CIMP | PR | Tissues | 31 | IV | CACNA1G, CDKN2A, CRABP1, IGF2,hMLH1, NEUROG1, RUNX3, SOCS1, MINT1, MINT31, IGFBP3, MGMT, WRN. |
CIMP 0/low/high |
CIMP High correlated with poor prognosis in metastatic CRC with MSS |
Univariate | Ogino et al. 116 |
| CIMP | PR | Tissues | 188 | IV | MINT1, MINT2, MINT31, hMLH1, p14, CDKN2A | CIMP −/+ | CIMP(+) correlated with poor prognosis in CRC |
Multivariate | Shen et al. 114 |
| CIMP | R | Tissues | 134 | I-IV | hMLH1, CDKN2A, MINT1, MINT2, and MINT31 | CIMP −/+ | CIMP-High correlated with poor survival (OS) in MSS CRC |
Univariate | Lee et al. 119 |
| CIMP | R | Tissues | 582 | I-IV | hMLH1, CDKN2A, MINT1, MINT2, and MINT31 | CIMP 0/low/high |
CIMP-High correlated with poor survival (OS) in MSS CRC |
Multivariate | Barault et al. 321 |
| CIMP | PR | Tissues | 649 | I-IV | CACNA1G, CDKN2A (p16), CRABP1, IGF2, hMLH1, NEUROG1, RUNX3, SOCS1 |
CIMP 0/low/high |
CIMP-High is independent factor of low colon-cancer mortality |
Multivariate | Ogino et al. 36 |
| CIMP | R | Tissues | 320 | I-IV | CACNA1G, CDKN2A (p16), CRABP1, IGF2, hMLH1, NEUROG1, RUNX3, and SOCS1. |
CIMP −/+ | CIMP(+) correlated with poor prognosis in CRC |
Univariate | Kim et al. 329 |
| CIMP | PR | Tissues | 604 | I-IV | hMLH1, CDKN2A, CACNA1G, NEUROG1, RUNX3, SOCS1, IGF2, CRRABP1 |
CIMP 0/low/high |
CIMP-High correlated with poor survival (CSS) in MSS CRC |
Multivariate | Dahlin et al. 330 |
| CIMP | R | Tissues | 161 | III | MINT1,MINT2,MINT31,hMLH1,CDKN2A,p14,SFRP1,SFRP2,WNT5A | CIMP −/+ | CIMP1 correlated with poor DFS in proximal CRC |
Multivariate | Ahn et al. 69 |
| CIMP | R | Tissues | 337 | - | CRABP1, hMLH1, CDKN2A, CACNA1G, NEUROG1 | CIMP 0/low/high |
CIMP-High correlated with poor prognosis in CRC |
Univariate | Zlobec et al. 117 |
| CIMP | PR | Tissues | 150 (Rectal) |
II/III | CACNA1G, IGF2, NEUROG1,RUNX3, SOCS1 | CIMP −/+ | CIMP(+) correlated with poor DFS in CRC |
Univariate | Jo et al. 331 |
| CIMP | R | Tissues | 207 (MSI) |
I-IV | CACNA1G, CDKN2A, CRABP1, IGF2,hMLH1, NEUROG1, RUNX3, and SOCS1 |
CIMP 0/low/high |
CIMP-High correlated with poor prognosis in MSI CRC |
Multivariate | Rhee et al. 70 |
| CIMP | PR | Tissues | 509 | I-IV | CACNA1G, IGF2, NEUROG1, RUNX3, SOCS1 | CIMP −/+ | CIMP(+) correlated with poor prognosis in early follow up timinig |
Multivariate | Simons et al. 332 |
| CIMP | R | Tissues | 734 | I-IV | CACNA1G, CDKN2A, CRABP1, IGF2, hMLH1, NEUROG1, RUNX3 and SOCS1 |
CIMP 0/low/high |
CIMP-High correlated with poor prognosis in rectal cancer |
Multivariate | Bae et al. 333 |
| CIMP | R | Tissues | 50 | II/III | p14, hMLH1, CDKN2A, MGMT, MINT1 | CIMP −/+ | CIMP(+) correlated with poor prognosis (DFS) in CRC |
Univariate | Wang et al. 334 |
| CIMP | PR+ two arm study | Tissues | 615 | III | CACNA1G, IGF2, NEUROG1, RUNX3, SOCS1 | CIMP −/+ | CIMP(+) correlated with poor prognosis in CRC |
Univariate | Shiovitz et al. 132 |
|
Blood-based biomarker | ||||||||
|---|---|---|---|---|---|---|---|---|
| CDKN2A | R | Serum | 99 | I-IV | Hypermethylation | qMSP | Univariate | Nakayama et al. 335 |
| HTLF | R | Serum | 77 | I-IV | Hypermethylation | qMSP | Multivariate | Wallner et al. 111 |
| HTLF | R | Serum | 106 | I-III | Hypermethylation | - | Multivariate | Herbst et al. 336 |
| HTLF | R | Serum | 311 | I-IV | Hypermethylation | qMSP | Multivariate | Philipp et al. 337 |
| HPP1 | R V | Serum | 142 (38,104) | I-IV | Hypermethylation | qMSP | Multivariate | Wallner et al. 111 |
| HPP1 | R | Serum | 311 | I-IV | Hypermethylation | qMSP | Multivariate | Philipp et al. 337 |
|
Predictive markers for response to treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Study design |
Specimen | Sample size | Purpose | Treatment | Significance | Endpoint | Reference |
|
Tissue-based biomarker | ||||||||
| TFAP2E | PR V | Tissues | 220 (CohortI:74, CohortII:36,CohortIII:42,CohortIV:68) |
Adjuvant/Palliative | 5-FU based chemotherapy |
Resistance to treatment with TFAP2E hypermethylation |
Response | Ebert et al. 134 |
| LINE-1 | R | Tissues | 131 (MSS/CIMP(-)) | Adjuvant | No benefit of 5-FU chemotherapy in patients with High LINE1 methylation in CRC |
Survival benefit | Kawakami et al. 338 | |
| EGFR | R | Tissues | 52 (EGFR-positive, K-RAS wild-type stage IV) |
Palliative | EGFR | No benefit if hypermethylation of EGFR | Survival benefit | Scartozzi et al. 339 |
| MGMT | PT | Tissues | 65 | Palliative | Dacarbazine | Hypermethylation correlates with good response |
Response | Amatu et al. 340 |
| MGMT | R | Tissues | 90 (I-IV) | Adjuvant/Palliative | oral fluoropyrimidines |
Unmethylated MGMT correlates with poor survival |
Survival benefit | Nagasaka et al. 126 |
| miR-148a | PR | Tissues | 273(II-IV) | Adjuvant/Palliative | 5-FU based chemotherapy |
Hypermethylation indicates poor response and prognosis |
Response and Survival benefit |
Takahashi et al. 273 |
| SRBC | R V | Tissues | 189 (IV; Discovery:131, Validation:58) | Palliative | Oxali-based chemotherapy |
Resistance to treatment with SRBC hypermethylation |
Survival benefit | Mountiho et al. 135 |
| PCDH10,SPARC,UCHL1 (panel) |
PR + two arm study |
Tissues | 143 (stage II) | Adjuvant | 5FU/LV or Surveillance |
Hypomethylation of panel could identify the stage II patients with benefit of treatment |
Survival benefit | Heitzer et al. 341 |
| CIMP marker | CIMP Marker | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CIMP | PR + two arm study |
Tissues | 206 (Stage III) | Adjuvant | 5-FU/LV | CDKN2A, MINT2, MDR1 | Treatment benefit in CIMP positive |
Survival benefit | Van Rijnsoever et al. 118 |
| CIMP | R | Tissues | 245 (Stage II/III: 196) | Adjuvant | 5-FU or Capecitabie |
CACNA1G, IGF2, NEUROG1,RUNX3, SOCS1 |
Treatment benefit in CIMP positive |
Survival benefit | Min et al. 130 |
| CIMP | PR + two arm study |
Tissues | 302 (Stage II/III: 196) | Adjuvant | 5-FU/LV or Surveillance |
CACNA1G, hMLH1, NEUROG1, RUNX3, SOCS1 |
No benefit in CIMP positive |
Survival benefit | Jover et al. 131 |
| CIMP | PR + two arm study |
Tissues | 615 (Stage III) | Adjuvant | 5FU/LV or IFL | CACNA1G, IGF2, NEUROG1, RUNX3, SOCS1 |
IrinotecanTreatment benefit in CIMP positive MSS CRC |
Survival benefit | Shiovitz et al. 132 |
Among all biomarker candidates, CIMP status has undoubtedly been the most promising indicator for prognosticating CRC patients. CIMP-positive cancers correlate with an overall unfavorable prognosis70, 114–117. Rijnsoever and colleagues showed in a cohort of 206 stage III CRC patients that CIMP-positive status associated with poor survival118. Another independent study analyzed more than 600 CRC patients and also found that CIMP associated with poor prognosis in MSS CRC patients119. Some studies suggested that poor prognosis in CIMP-positive CRCs is from coexisting V600E BRAF mutations36, 119–121, however, in addition to CIMP, MSI status remains an important confounding factor that likely underlies the difference in prognosis of CIMP-positive MSS vs. MSI cancers122, 123. These data highlight that the prognosis of patients with CIMP CRCs also depends on the MSI status of the tumor.
In addition to hypermethylation of various genes/loci, growing evidence suggests that DNA hypomethylation status associates with the prognosis of CRC patients. Ogino and colleagues have reported a correlation between LINE-1 hypomethylation and poor survival in prospective cohort studies of CRC patients67. Subsequent studies not only validated this association for LINE-1 hypomethylation and CRC prognosis68–70, but also identified other potential genes that correlate with adverse outcomes124–129. In aggregate, these studies provide evidence that aberrantly methylated DNA has potential for use as prognostic biomarkers for CRC; however, further investigation is required to develop clinically robust, “locked-down” assays based upon standardized and reproducible prognostic biomarkers in order for these assays to be used in clinical care.
Methylated DNA as a Predictive Biomarker for Response to Treatment
Despite recent advances in the development of cancer therapeutics, the current generation of chemotherapeutic drugs are suboptimal because of modest efficacy and intrinsic or acquired resistance. This limitation highlights the need for predictive biomarkers that can be used to identify patients with low or high likelihoods of a response to specific treatments in order to maximize the overall treatment success with these chemotherapeutic drugs.
Over the last decade, a number of aberrantly methylated genes have been proposed to serve as predictive biomarkers for CRC patients undergoing various chemotherapeutic regimens (Table 2). The majority of these studies have not progressed beyond Phase I/II discovery phase and thus will not be discussed further in this article. CIMP as a predictive marker has been intensively studied for more than a decade. In 2003, van Rijnsoever and colleagues first reported that CIMP-positive CRC patients benefited from 5-flurouracil (FU) based adjuvant chemotherapy and that this association with survival was independent of MSI or TP53 mutation status118. These findings were validated in a subsequent study when stage II and III CRC patients with CIMP-positive tumors were also shown to derive survival benefit following 5-FU treatment130. However, these results were challenged in a later study that examined a large patient cohort and reported that those patients with CIMP-positive tumors that did not receive 5-FU treatment survived longer when compared to patients with CIMP-negative CRCs131. Another study showed CIMP-positive CRC patients had a shorter DFS after 5-FU treatment compared with patients with CIMP-negative tumors131. Recently, in another prospective study, the addition of irinotecan to adjuvant 5FU/LV in stage III, MSS, and CIMP-positive colon cancers improved overall survival132. Although the differences between these studies may reflect differences in CIMP criteria or inherent differences between patient cohorts; these studies suggest promise for the use of CIMP as a predictive marker and also highlight the need for additional studies of the interaction between CIMP status and therapeutic response to various treatments.
Although still in the early phase of development, some promising pharmaco-epigenetic biomarkers, such methylated MGMT for temozolamide treatment in gliomas, have been identified in various cancers133. A recent study showed the feasibility of using hypermethylated Transcription Factor AP-2 Epsilon (TFAP2E) as a predictive biomarker for response to 5-FU based chemotherapy in CRC patients134. Furthermore, DNA methylation microarray profiling of oxaliplatin sensitive vs. resistant CRC cell lines revealed that oxaliplatin-resistant cells exhibited hypermethylation of the BRCA1 interactor SRBC gene; which was subsequently shown to associate with poor progression free survival (PFS) in CRC cohorts treated with oxaliplatin135. The ability to develop reliable assays for methylated genes and the results of early phase studies using methylated genes as predictive markers will continue to drive the investigation of methylated genes as response predictors for CRC therapy.
Methylated DNA as a Colorectal Cancer Risk Biomarker: “Field Cancerization” and ‘Epigenetic Drift’
The concept of “field cancerization” (or field effect) was first proposed in 1953 by Slaughter et al136. Field cancerization is characterized by the occurrence of genetic and epigenetic alterations in histologically normal-appearing tissues, and is believed to lead to an increased risk for synchronous or metachronous primary tumors. While genetic alterations are common in CRC cells, these are believed to be rare in normal cells. In contrast, some studies suggest somatic epigenetic dysregulation occurs not only in cancer tissues, but also in non-cancerous and pre-neoplastic tissues. Considering that epigenetic alterations could contribute to the early events predisposing to malignant transformation, these studies suggest epigenetic events are potentially more promising somatic CRC risk markers than are gene mutations. Methylation changes in tumor-suppressor genes and the estrogen receptor (ER) occurs in an age- and region-specific manner in normal colonic mucosa. The proposed age-related epigenetic alterations in tumor-suppressor genes may be one of the earliest events that predispose normal mucosa to tumorigenic transformation in CRC137. Loss of the insulin-like growth factor-II (IGF2) gene imprinting occurs at a higher frequency in the normal mucosa adjacent to cancer tissue, compared to normal mucosa in patients without CRC138, emphasizing the potential of IGF2 imprinting as a molecular signature to identify patients at greater risk for CRC development. In line with this concept, other studies have revealed that both hypermethylation of tumor-suppressive genes such as SFRP, ESR1, MYOD, and MGMT, as well as LINE-1 hypomethylation in normal colonic mucosa correlates with an increased risk of CRC, in contrast to patients without these traits139–142.
Accumulating evidence supports that the landscape of DNA methylation can be modified as a “function of age”. DNA methylation has been proposed to result from a gradual stochastic age-dependent dysregulation caused by a combination of external environmental factors and internal spontaneous random errors in the maintenance of methylation. This process of age-dependent alterations in methylation is defined as “epigenetic drift”143. Interestingly, such age-associated DNA methylation often targets the promoters of tumor-suppressive genes144, 145. In monozygotic twins, epigenetic divergence with age suggests the underlying epigenetic drift may in part help explain the disease discordance146–148. Since this new concept closely relates to the “field effect”, identification of biomarkers that overlap both the “epigenetic drift” and “field effect” in colorectal mucosa may allow development of next-generation biomarkers for determining risk for CRC development in the future.
HISTONE MODIFICATIONS: A POTENTIAL CLASS OF CRC BIOMARKERS
DNA in eukaryotic cells is found in chromatin, which is a complex of macromolecules consisting of DNA, RNA and protein. The primary functions of chromatin are to facilitate DNA compaction, to reinforce the DNA macromolecule during mitosis, to protect against DNA damage, and to control gene expression and DNA replication. The primary protein components of chromatin are histones, which regulate DNA compaction and gene expression. Histones are protein octamers that consist of two copies of four core proteins - H2A, H2B, H3, and H4. Each histone octamer contains approximately 147 bp of DNA to form the nucleosome149. As illustrated in Figure-2B, each core histone protein has specific histone tails, which are subject to modifications including acetylation, methylation, ubiquitination, phosphorylation, and sumoylation150. Various modifications alter the three-dimensional structure of the nucleosome and affect the transcriptional control of associated genes by creating an “inactive”, compacted, heterochromatin state, or an “active”, open chromatin, euchromatin conformation. For example, the active transcriptional state is recognized by di- and tri-methylation of histone H3 lysine 4 (H3K4me2, and H3K4me3), histone H3 lysine 36 (H3K36me2, and H3K36me3), and acetylation of H3 and H4, while trimethylation of H3 lysine 9 and 27 (H3K9me3 and H3K27me3) are enriched in heterochromatin and associate with inactive transcription151. After the initial discovery of the dysregulation of histone modifications in CRC152, studies have revealed that this dysregulation likely alters gene expression patterns in CRC. However, due to the technical limitations of assays that assess the post-translational histone modification state, it has been difficult to determine the histone modification state in primary cancer tissues. Therefore, efforts to determine if histone modifications can be used as disease biomarkers have been limited (Supplementary Table 1). Global alterations of specific-histones in primary tissues have been the focus for biomarker development in CRC. Studies of H3K4me2, H3K9ac, and H3K9me2 alterations detected by immunohistochemical staining in liver metastases suggest that high H3K4me2 expression levels inversely correlate with poor prognosis153. Additionally, other studies in CRC suggest that histone modifications, such as acetylation of H3 lysine 56 and di- or tri-methylation of H3 lysine 9 and 27, have potential to be prognostic markers in CRC.151–156. Similarly, studies of histone modifications in circulating nucleosomes have identified reduced levels of H3K9me3- and H4K20me3 as potential diagnostic biomarkers for CRC157, 158. However, thus far, these studies are all Phase I biomarker studies and should be considered “proof of principle”. Further research is needed to determine whether any of these modifications will be clinically useful diagnostic or prognostic biomarkers in CRC.
NONCODING RNAS
Noncoding RNA overview
The central dogma of molecular biology, which describes the sequential transfer of genetic information and the concept that “DNA makes RNA and RNA makes protein”, was developed in 1956 and provided a fundamental framework for modern molecular biology until recently159. Advances in our understanding of the regulation of gene expression has been provided by the Encyclopedia of DNA Elements Consortium (ENCODE) transcriptome project, which recently revealed that protein-coding genes represent less than 2% of total genome and approximately 80% of the genome is actively transcribed into non-coding RNA (ncRNA)160. Although ncRNAs were previously believed to be “background transcriptional noise” related to “junk DNA”, mounting evidence indicates that ncRNAs play a significant role in many biological processes, including the regulation of oncogenes and tumor suppressor genes in cancer161. NcRNAs are broadly categorized into two groups based upon their size: small ncRNAs, that are shorter than <200 nucleotides, comprising of miRNAs (miRNAs), piwi-interacting RNAs (piRNAs), and small nucleolar RNAs (snoRNAs); and long ncRNAs (lncRNA) that are longer than 200 nucleotides162. Recent studies have elucidated the functional role of ncRNAs, particularly miRNAs, in human cancers and have not only revolutionized our understanding of their biological contribution to cancer pathogenesis, but have also provided important insights into the feasibility of their use as clinically relevant biomarkers in cancer.
Overview of miRNAs
Over the last decade, in the family of ncRNAs, the role of miRNAs has been best established in the context of carcinogenesis. MiRNAs are endogenous single-stranded small RNAs that are 18–25 nucleotides in length that were first discovered in 1993 as negative post-transcriptional regulators in Caenorhabditis elegans163, 164. During their biogenesis, premature-miRNAs are exported from the nucleus to the cytoplasm. Subsequent processing of the pre-miRNA generates mature-miRNA, which binds to 3’UTR “seed sequence” of target mRNAs, a process that is catalyzed by the RNA-induced silencing complex (RISC). The binding of miRNA to the target mRNA can result in degradation of the target mRNA or inhibition of its translation into protein, with the degree of sequence complementarity between the miRNA and mRNA determining which mechanism is employed165–167 (Figure 2C). The role of miRNAs in cancer was first shown in 2002 by Croce and colleagues, who demonstrated decreased expression of miR-15 and miR-16 in patients with chronic lymphocytic leukemia168. Since then, hundreds of miRNAs have been shown to be deregulated in other human malignancies, some of these deregulated miRNAs appear to have a functional role in tumorigenesis by regulating the expression of important oncogenes and tumor-suppressor genes169.
Dysregulation of miRNA Expression in CRC: Role in “Traditional” and “Serrated” Pathways
Commonly deregulated miRNAs have been identified that appear to participate during each step of the “traditional” (normal-adenomatous polyp-cancer) and “serrated” (normal-serrated polyp-cancer) pathways of CRC development. For instance, the miR-17-92a cluster, miR-135b, miR-143, and miR-145 regulate WNT/β-Catenin signaling pathway, which is involved in colorectal neoplasia initiation 170–173. Genes associated with the RAS-MAPK and PI3K/AKT cascades, that appear to drive the transition from early to advanced adenoma, are found to be regulated by specific miRNAs (e.g. RAS-MAPK by miR-143, let-7, miR-21, and miR-31; and PI3K/AKT by miR-1, miR-21 and miR-143) 174–180. Likewise, p53, a tumor suppressor protein frequently inactivated/silenced during the evolution from advanced adenoma to adenocarcinoma, is controlled by miR-34a/b/c, miR-133a, miR-143, and miR-145174, 181–183. In contrast, genes such as LIN28 drive CRC progression through inhibition of let-7 miRNA biogenesis, highlighting the complexity of miRNA-gene interaction in cancer184, 185. In addition, miR-21, miR-155 and miR-200 family members regulate the TGF-ß pathway 186–188.
miRNAs also appear to play a role in the serrated pathway of carcinogenesis. The identification of increased expression of miR-21 and miR-181 in hyperplastic polyps (HP) and sessile serrated adenomas (SSAs), yielded the first clues for a potential role of miRNAs in the serrated pathway189. A recent, comprehensive miRNA expression profiling study of CRCs with or without BRAF mutations identified elevated miR-31-5p expression in BRAF mutant cancers190. A significant correlation between miR-31 overexpression and specific types of serrated polyps, sessile serrated adenomas (SSAs) vs. traditional serrated adenomas (TSAs) 191 highlights the possible functional role for this miRNA in the serrated pathway190. These findings were corroborated in a follow-up study that analyzed 381 serrated and 222 non-serrated adenomas and identified a CIMP-independent association between miR-31 overexpression and BRAF mutations192. Interestingly, expression of miR-31 was progressively increased in the histologically more advanced lesions arising from SSAs but not from TSAs. Furthermore, miR-31 expression, BRAF mutation, CIMP-positive status, and MLH1 methylation showed a gradual increase from rectum to cecum in SSA lesions 193, 194.
MiRNAs as Clinically Useful Biomarkers for Colorectal Cancer
Over the last five years, miRNA biomarker research in human cancer has increased exponentially. The underlying reasons for this burgeoning interest are based upon some of their unique characteristics. First, miRNAs are remarkably stable under a variety of experimental and laboratory conditions. Second, due to their small size and the hairpin-loop structure, miRNAs are protected from RNase-mediated degradation195, and thus are easily extractable from a wide variety of clinical specimens, including formalin-fixed paraffin embedded (FFPE) tissues, and a variety of body fluids including blood, saliva, urine, feces etc. Third, cell-free miRNAs are often protected from degradation because of being in high density lipoprotein particles, apoptotic bodies, microvesicles, and exosomes, and through their binding to argonaute-2 (Ago-2), which results in increased stability196–199. Fourth, in addition to the stability in various human specimens, miRNAs are actively secreted by cancer cells into the circulatory system and digestive tract200, 201. Taken together, the stability of miRNAs coupled with their presence in a variety of compartments in the body (blood, feces, cancer cells, cells near the cancer, etc.), has led to an intense line of research on the use of miRNAs as cancer biomarkers.
MiRNAs as Diagnostic Biomarkers in Colorectal Cancer
Detection of pre-malignant polyps and early-stage neoplasms currently is one of the major goals of CRC screening strategies. The identification and treatment of polyps and cancers in their earliest stages leads to the most successful outcomes. In the following sections and Table 3, we summarize some of the key findings with regards to circulating cell free-miRNAs and fecal-miRNAs and their potential as diagnostic biomarkers in CRC.
Table 3.
List of miRNAs as potential noninvasive diagnostic markers in colorectal cancer
| miRNA | Source | Study Design |
Sample size |
Endpoint: Adenoma or CRC |
Sensitivity (%) | Specificity (%) | AUC (95% CI) | Normalizer | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Control | |||||||||
| Blood based biomarker | ||||||||||
| miR-17-3p | plasma | R V | 5/25/90 | 5/20/50 | CRC | 64 | 70 | 0.72 | RNU6B | Ng et al. 203 |
| miR-18 | plasma | R | 78 | 86 | CRC | 73.1 | 79.1 | 0.8 | RNU6B | Zhang et al. 211 |
| miR-21 | plasma | R V | 30/20 | 30/20 | CRC | 90 | 90 | 0.82/0.91 | U6 | Kanaan et al. 207 |
| miR-21 | plasma | R | 29 | 29 | CRC | - | - | 0.65 | miR-16 | Zanutto et al. 342 |
| miR-21 | plasma | R | 49 | 49 | CRC | 76.2 | 93.2 | 0.88 | cel-miR-39 | Du et al. 343 |
| miR-21 | Serum | R V | 12/186(CRC), 43(AD) | 12/53 | CRC, AD | 91.9(CRC) 81.1(AD) | 81.1(CRC) 76.7(AD) | 0.92(CRC) 0.81(AD) | cel-miR-39 | Toiyama et al. 200 |
| miR-21 | Serum | R | 200(CRC), 50(AD) | 80 | CRC, AD | - | - | 0.8(CRC) 0.71(AD) | miR-16 | Liu et al. 208 |
| miR-21 | Serum (Exosome) |
R | 88 | 11 | CRC | 0.8 | miR-451 | Ogata-Kawata et al. 201 |
||
| miR-21 | Serum | R | 40 | 40 | CRC | 77 | 78 | 0.87 | RNU6B | Basati et al. 344 |
| miR-29a | plasma | R V | 20/100(CRC),37(AD) | 20/59 | CRC, AD | 69(CRC) 62.2(AD) | 89.1(CRC) 84.7(AD) | 0.84(CRC) 0.77(AD) | miR-16 | Huang et al. 204 |
| miR-92a | plasma | R V | 5/25/90 | 5/20/50 | CRC | 89 | 70 | 0.89 | RNU6B | Ng et al. 203 |
| miR-92a | plasma | R V | 20/100(CRC),37(AD) | 20/59 | CRC, AD | 84(CRC) 64.9(AD) | 71.2(CRC) 81.4(AD) | 0.84(CRC) 0.75(AD) | miR-16 | Huang et al. 204 |
| miR-92a | Serum | R | 200(CRC), 50(AD) | 80 | CRC, AD | - | - | 0.77(CRC) 0.7(AD) | miR-16 | Liu et al. 208 |
| miR-155 | Serum | - | 146 | 60 | CRC | - | - | 0.78 | - | Lv et al. 209 |
| miR-200c | plasma | R | 78 | 86 | CRC | 64.1 | 73.3 | 0.75 | RNU6B | Zhang et al. 211 |
| miR-221 | plasma | R | 103 | 37 | CRC | 86 | 41 | 0.61 | - | Pu et al. 210 |
| miR-18a,-20a,-21,-29a,-92a,- 106b, -133a,-143,-145 |
plasma | R V | 50/80(CRC), 50(AD) | 50/194 | CRC | - | - | 0.75 | miR-16 | Luo et al. 212 |
| miR-15b,-17,-142-3p, -195, - 331, -532,-532-3p, -652 |
plasma | R V | 20/45(CRC), 9/16(AD) | 12/26 | AD | 88 | 64 | 0.87(AD) | U6 | Kanaan et al. 207 |
| miR-21, 31, 92a, 181b, 203, let-7g (panel) |
Serum | R V | 30/83 | 30/59 | CRC | 96.4 | 88.1 | 0.92 | miR-16 | Wang et al. 214 |
| miR-7, -93, -409-3p (panel) | plasma | R V | 47/55/22 | 33/57/27 | CRC | 91/82 | 88/89 | 0.87/0.9 | miR-1228 | Wang et al. 215 |
| Fecal-based biomarker | ||||||||||
| miR-18 | Stool | R | 198 | 198 | CRC | 61 | 69 | 0.67 | - | Yau et al. 345 |
| miR-21 | Stool | R | 19 | 10 | CRN (CRC+AD) | - | - | - | miR-16/ miR-26b |
Link et al. 216 |
| miR-21 | Stool | R | 88 | 101 | CRC | 55.7 | 73.3 | 0.64 | - | Wu et al. 217 |
| miR-17-92 cluster | Fecal colonocyte |
R | 197 | 119 | CRC | 69.5 | 81.5 | - | U6 | Koga et al. 219 |
| miR-92a | Stool | R | 88 | 101 | CRC | 71.6 | 73.3 | 0.78 | - | Wu et al. 217 |
| miR-106a | Stool | R | 19 | 10 | CRN (CRC+AD) | - | - | - | miR-16/ miR-26b |
Link et al. 216 |
| miR-106a | Stool | R | 107 | 117 | CRC | 34.2 | 97.2 | - | miR-24 | Koga et al. 218 |
| miR-135b | Fecal colonocyte |
R | 197 | 119 | CRC | 45.7 | 95 | - | U6 | Koga et al. 219 |
| miR-135b | Stool | R | 104(CRC), 169(AD) | 109 | CRC | 78(CRC) 65(AD) | 68 | 0.79(CRC) 0.71(AD) | - | Wu et al. 346 |
| miR221 | Stool | R | 198 | 198 | CRC | 62 | 74 | 0.73 | - | Yau et al. 345 |
R, Retrospective study; PR, Retrospective analysis of prospectively collected samples in clinical trial; PT, Prospective trial; CRC, Colorectal cancer; CRN, colorectal neoplasia; AD, adenoma
Blood-based biomarkers
The discovery of miRNAs in extracellular body fluids202 triggered a growing number of studies that evaluated dysregulated expression of circulating miRNAs in various cancers. The first systematic and comprehensive miRNA expression profiling study was conducted by Ng and colleagues203, who evaluated miRNA expression alterations in tissue and plasma samples from CRC patients and healthy subjects. This study revealed that high expression of two miRNAs, miR-92a and miR-17-3p, could discriminate patients with CRC from healthy study subjects (sensitivity: 64%, 89%, specificity: 70%, 70%, AUC: 0.72, 0.89, for each miRNA respectively). This landmark study further reported that the plasma levels of both miRNAs decreased significantly following surgical resection of the primary tumors, and that plasma miR-92a levels were also elevated in patients with gastric cancer and inflammatory bowel disease. Their findings have been replicated in other studies of CRC patients204.
MiR-21 is another well-characterized oncogenic miRNA, which is considered as one of the promising non-invasive biomarkers for the early detection of CRC owing to the following attributes: a) dysregulation of miR-21 occurs frequently in early stages of the adenoma-carcinoma sequence205 b) miR-21 is one of the most highly expressed miRNAs in CRC206, and c) miR-21 is highly secreted by cancer cells and can be measured in exosomes or as free miRNAs in plasma or serum200, 201. One of the first studies utilizing miRNA expression profiling on primary CRC tissues and the adjacent normal mucosa identified miR-21 as differentially expressed in CRC (n=30)207. Validation studies in an independent set of plasma samples (“test” set; 20 CRCs and 20 healthy controls) demonstrated that plasma miR-21 could be used to discriminate patients with CRC from normal control patients with high sensitivity (90%) and specificity (90%). Furthermore, the diagnostic and prognostic potential of serum miR-21 in CRC patients was recently addressed in another study200, which revealed that tumor-derived circulating miR-21 expression not only accurately discriminated patients with CRC from healthy subjects, but allowed identification of patients with advanced adenomas, which are “bonafide target lesions” for an ideal CRC screening test (sensitivity: 91.1%, 81.1%, specificity: 81.1%, 76.7%, AUC: 0.92, 0.81, for cancer and adenoma detection, respectively). This study also confirmed the significant association between lower miR-21 expression in serum and CRC tissues following curative resection of the primary tumor. Consequently, several studies confirmed the potential of miR-21 for use as a single miRNA biomarker for the early detection of CRC201, 208.
Although increasing number of miRNAs have been identified as potential biomarkers for the early diagnosis of CRC204, 209–211, it seems unrealistic that a single miRNA will adequately capture the underlying disease heterogeneity in colorectal polyps and cancers. Accordingly, several studies have proposed combining miRNAs into a biomarker panel to improve the detection accuracy of colorectal neoplasms212–215.
Although miRNAs appear to be promising CRC biomarkers, there are several challenges that must be borne in mind while considering their potential as diagnostic CRC biomarkers. The lack of consistency between biomarker panels in independent studies highlights a major obstacle for the development of robust miRNA biomarkers. This variability in miRNA biomarkers/biomarker panels among studies may in part be due to lack of standardized sample handling and processing steps, use of inconsistent normalization approaches as well as to the differences in ethnic and racial makeup of patient populations in the various studies. Once these issues are resolved, validation studies utilizing standardized assays in large population-based cross-sectional and prospective cohort studies are needed to identify optimal miRNAs and marker panels that can be used in clinical care.
Stool-based biomarkers
Similar to blood-based biomarkers, the use of stool-based miRNA biomarker assays for the early detection of colorectal neoplasia has been assessed recently. In 2010, one of the first studies of stool-based miRNAs demonstrated the feasibility of a one-step miRNA extraction and amplification method defined as “direct miRNA analysis” (DMA), and showed dysregulated expression of miR-21 and miR-106a as potential candidate biomarkers for CRC screening216. A subsequent study that assessed fecal miR-21 and miR-92a levels found that fecal miR-92a expression could differentiate patients with CRC or adenoma from those with lower-risk polyps or healthy subjects217. A more recent study evaluated fecal miRNA expression in residual material collected from FOBT kits and determined that miR-106a expression enhanced the sensitivity of FOBT in identifying patients with CRC218.
Another potential stool-based miRNA biomarker assay employed the extraction of RNA from fecal colonocytes using immuno-magnetic beads conjugated with the epithelial cell adhesion molecule (EpCAM) monoclonal antibody. This assay was used to study a cohort of 197 CRC patients and 119 healthy volunteers219 and the expression of the miR-17-92a cluster and miR-135b in feces was found to discriminate patients with CRC from healthy subjects with a high sensitivity (69.5%, 45.7%) and specificity (81.5%, 95%), further highlighting the potential use of stool as a source for miRNA biomarkers in CRC screening tests.
MiRNAs as Prognostic Biomarkers in Colorectal Cancer
Recognition of the probable functional role miRNAs play in human cancers and their remarkable stability in a variety of clinical specimens has made them attractive candidates as prognostic biomarkers in CRC220. The first study in this regard was conducted by Schetter and colleagues in 2008205, who used a microarray-based approach to evaluate the expression levels of 389 miRNAs in 84 CRC and matched normal colonic tissues. This study identified and validated 37 differentially expressed miRNAs, including miR-20a, miR-21, miR-106a, miR-181b and miR-203. A seminal finding of this study was that high expression of miR-21 in CRC patients associated with poor survival, which has since been independently confirmed in several other reports200, 221–225. As shown in Table 4, although several other overexpressed (miR-10b, miR-17–92a cluster, miR-29a, miR-31, and miR-182)190, 226–236 and under-expressed miRNAs (miR-143 and miR-124)237–239 have been proposed to be prognostic biomarkers, currently miR-21 has the best potential to be a clinically useful miRNA-based prognostic biomarker in CRC.
Table 4.
List of miRNAs as potential prognostic and predictive markers in colorectal cancer
| Prognostic markers | ||||
|---|---|---|---|---|
| miRNA | Source | Dysregulation in CRC | Notable confirmed target genes* | |
| Tissue-based b iomarker | ||||
| Mature miRNA ID | Previous miRNA ID | |||
| miR-10b-5p | miR-10b | Tissue | Up regulated | TIP30, SDC1, NF1, KLF4, HOXD10, PPARA, CDKN2A, CDKN1A, TFAP2C, BCL2L11 |
| miR-17-5p | miR-17 | Tissue | Up regulated | PTEN, DLC1, ZBP1, p130, CDKN1A, PTPRO, PKD2, BCL2L11, E2F1, MAPK9, TGFBR2, VIM, CCND1 |
| miR-21-5p | miR-21 | Tissue | Up regulated | PTEN, PDCD, RECK, JAG1, BCL2, TIMP3 DKK2, SOX5, MTAP, TGFBR2, E2F1, TPM1, APAF1 |
| miR-29a-3p | miR-29a | Tissue | Up regulated | KLF4, DNMT3a, DNMT3b, CDK6, BACE1, MCL1, BCL2, PPM1D, LPL1 |
| miR-31-5p | miR-31 | Tissue | Up regulated | FIH1, RhoBTB1, RASA1, FOXP3, ITGA5, MMP16, RDX, FZD3, DKK1, CREG1, RASA1 |
| miR-92a -3p | miR-92a | Tissue | Up regulated | PTEN, P63, RECK, DUSP10, ITGA5, BMPR2, KAT2B |
| miR-155-5p | miR-155 | Tissue | Up regulated | MLH1, MSH2, MSH6, APC, TP53INP1, TAB2, MEIS1, MECP2, SOCS1, INPP5D, SMAD5, HIVEP2, BACH1 |
| miR-181a-5p | miR-181a | Tissue | Up regulated | PTEN, E2F5, SMAD7, ATM, BCL2, PROX1, KAT2B, CDKN1B, RNF2, RALA, KLF6 |
| miR-182-5p | miR-182 | Tissue | Up regulated | NDRG1, RECK, MTSS1, SMAD4, CDNK1A, FOXO3, FOXO1, MITF, CYLD, BCL2, CCND2 |
| miR-191-5p | miR-191 | Tissue | Up regulated | TIMP3, BASP1, CDK6, SATB1, NDST1 |
| miR-221-3p | miR-221 | Tissue | Up regulated | SUN2, RECK, CDKN1B, BMF, FOXO3, KIT, CDKN1C, TMED7, DDIT4, BNIP3L, TBK1 |
| miR-224-5p | miR-224 | Tissue | Up regulated | SMAD4, CXR4, PHLPP1, PHLPP2, KLK10, CDC42, AP15, EYA4, EDNRA, DIO1 |
| let-7i-5p | let-7i | Tissue | Down regulated | TLR4, SOCS1, BMP4, IL13 |
| miR-16 -5p | miR-16 | Tissue | Down regulated | BMI1, WIP1, CDK6, HMGA1, ACVR2A |
| miR-34a-5p | miR-34a | Tissue | Down regulated | CD44, MYCN, PD-L1, MET, JAG1, MYC, MET, CDK4, CCNE2, MYCN, CCND1, E2F3, SIRT1, BCL2, NOTCH1 |
| miR-124-3p | miR-124 | Tissue | Down regulated | SLUG, STAT3, EZH2, IL6R, EFNB1, CEBPA |
| miR-133a/b | miR-133a/b | Tissue | Down regulated | LASP1, RFFL, FSCN1, CXCR4, MET, KCNQ1, PNP, KCNH2, |
| miR-137 | miR-137 | Tissue | Down regulated | LSD1, FMNL2, CDC42, CDK6, KDM1A, NCOA2, CTBP1, MITF |
| miR-143-3p | miR-143 | Tissue | Down regulated | KRAS, DNMT3A, TLR2, MACC1, MYO6, MAPK7, FNDC3B, FSCN1, SERPINE1, JAG1, AKT1 |
| miR-148a-3p | miR-148a | Tissue | Down regulated | BCL2, MMP7, ROCK1, DNMT1, TG1F2, DNMT3, RPS6KA5 |
| miR-149-5p | miR-149 | Tissue | Down regulated | FOXM1, ZBTB2 |
| miR-200a-3p | miR-200a | Tissue | Down regulated | CTNNB1, ZEB2, SIRT1, ZEB1, ZFPM2, GDAP1, MAPK14, KEAP1 |
| miR-340-5p | miR-340 | Tissue | Down regulated | MET, PLAT |
| miR-378a-3p | miR-378 | Tissue | Down regulated | IGF1R, MYC, VEGFA, NPNT, GALNT7 |
| Blood-based biomarker | ||||
| miR-21-5p | miR-21 | Serum | Up regulated | PTEN, PDCD, RECK, JAG1, BCL2, TIMP3 DKK2, SOX5, MTAP, TGFBR2, E2F1, TPM1, APAF1 |
| miR-29c-3p | miR-29c | Serum | Up regulated | KLF4, DNMT3a, DNMT3b, CDK6, BACE1, MCL1, BCL2, PPM1D, LPL1 |
| miR-92a-3p | miR-92a | Serum | Up regulated | PTEN, P63, RECK, DUSP10, ITGA5, BMPR2, KAT2B |
| miR-141-3p | miR-141 | Plasma | Up regulated | ZEB2, ZEB1, SFPQ, CLOCK BRD3, UBAP1, PTEN, ZFPM2, E1F4E |
| miR-155-5p | miR-155 | Serum | Up regulated | MLH1, MSH2, MSH6, APC, TP53INP1, TAB2, MEIS1, MECP2, SOCS1, INPP5D, SMAD5, HIVEP2, BACH1 |
| miR-200c-3p | miR-200c | Serum | Up regulated | TUBB2, BMI1, ZEB2, ZEB1, FN1, ZFPM2, PTPN13, RNF2, BRD7 |
| miR-221-3p | miR-221 | Plasma | Up regulated | SUN2. RECK, CDKN1B, BMF, FOXO3, KIT, CDKN1C, TMED7, DDIT4, BNIP3L, TBK1 |
| miR-345-5p | miR-345 | Blood | Up regulated | CDKN1A, ABCC1 |
| miR-885-5p | - | Serum | Up regulated | CDK2, MCM5 |
| Predictive markers for response to treatment | |||||
|---|---|---|---|---|---|
| miRNA | Specimen | Purpose of therapy | Endpoint | Treatment | |
| Tissue-based biomarker | |||||
| Mature miRNA ID | Previous miRNA ID | ||||
| miR-21-5p | miR-21 | Tissue | NeoAdjuvant/Adjuvant/Palliative | Prognosis | 5FU-based chemotherapy |
| miR-148a-3p | miR-148a | Tissue | Adjuvant/Palliative | Prognosis | 5FU/5FU+oxaliplatin |
| miR-150-5p | miR-150 | Tissue | Adjuvant | Prognosis | 5FU with/without LV/levamisole/CDDP |
| - | miR-200 family | Tissue | Adjuvant | Prognosis | Fluoropyrimidines |
| - | miR-215 | Tissue | Adjuvant | Prognosis | 5FU-based chemotherapy |
| let-7c, g | let-7c, g | Tissue | Palliative | Response, Prognosis | S-1 with or without CDDP, anti-EGFR |
| miR-31-3p | miR-31* | Tissue | Palliative | Prognosis | anti-EGFR |
| miR-99a-5p,-3p | miR-99a, -99a* | Tissue | Palliative | Prognosis | 5FU-based chemotherapy, anti-EGFR |
| miR-107 | miR-107 | Tissue | Palliative | Prognosis | 5FU-based chemotherapy |
| miR-125b-5p | miR-125b | Tissue | Palliative | Prognosis | anti-EGFR |
| miR-126-3p | miR-126 | Tissue | Palliative | Response, Prognosis | XELOX, bevacizumab (anti-VEGF-A) |
| miR-181a,b | miR-181a,b | Tissue | Palliative | Response, Prognosis | S-1 with or without CDDP, anti-EGFR |
| miR-625-3p | miR-625* | Tissue | Palliative | Response, Prognosis | XELOX/FOLFOX |
| Blood-based predictive biomarker | |||||
| miR-27b, -106a, -130b, -148, -326, -484 | Plasma | Adjuvant | Response/Prognosis | 5FU+Oxaliplatin | |
| miR-19a-3p | miR-19a | Serum | Palliative | Response | FOLFOX |
miRTarBase Ver. 4.5 (http://mirtarbase.mbc.nctu.edu.tw) was used to list confirmed miRNA target genes
Based upon current guidelines, the majority of stage II CRC patients are treated surgically without adjuvant chemotherapy. However, a significant proportion (approximately 15%) of these patients experience tumor recurrence and death due to disease progression240, 241, highlighting the need for biomarkers that can identify high-risk stage II CRC patients who could benefit from adjuvant chemotherapy. Schepeler and colleagues found that miR-320 and miR-498 miRNA- distinguish high-risk from low-risk stage II patients242 and correlate with recurrence free survival. Similarly, miR-21 expression appears to identify high-risk population in stage II CRC patients, which has been confirmed in several independent investigations222, 243–245.
In addition to individual markers, a miRNA panel has been developed to identify stage II CRC patients with a high risk of recurrence. The expression of 1849 miRNA probes in 40 paired stage II colon tumors and adjacent normal mucosa tissues was examined225. A miRNA-based classifier comprising of miR-20a-5p, miR-21-5p, miR-103a-3p, miR-106a-5p, miR-143-5p and miR-215 was developed, which discriminated high risk of stage II CRC patients in a “testing cohort” (138 patients) and an independent “validation” cohort of patients (460 patients)225.
Although several miRNAs have thus far been identified as potential prognostic biomarkers in CRC, this field is still rapidly evolving. In addition to high-risk stage II patients, identification of low-risk stage III CRC patients is another potential use of miRNA risk stratification/prognostic biomarkers..
Predictive Biomarkers for Response to Treatment in Colorectal Cancer
The treatment options for advanced CRC patients have improved considerably over the last decade as a result of the development of novel targeted therapies246. 5-FU based regimens remain the mainstay for adjuvant therapy, while advanced metastatic disease is often treated with newer antibody-based drugs targeting the vascular endothelial growth factor (VEGFA) and epidermal growth factor receptor (EGFR)247–249. In spite of therapeutic advances, the prognosis of patients with unresectable CRC still remains poor, with the median overall survival of only 18–21 months250. The development of biomarkers that can accurately predict a patient’s response to a specific chemotherapeutic regimen prior to initiation of the chemotherapy is of clear value and an area of active investigation.
Currently, the majority of the results related to the role of miRNAs in drug resistance are based on in vitro studies and remain to be assessed in clinical sample sets (Table 4). MiRNAs shown to mediate 5-fluorouracil resistance include miR-10b226, miR-19b251, miR-20a252, miR-21253–255, miR-23a256, miR-31257, miR-34258, 259, miR-129260, miR-140261, miR-145262, miR-192/-215263, miR-200 family264, and miR-497265. MiRNAs have also been identified that mediate irinotecan-resistance (miR-21254 and miR-451266) and oxaliplatin resistance, (miR-20a252, miR-21254, miR-133a183, miR-143267, miR-153268, miR-203269 and miR-1915270). However, as noted above, most of these findings are primarily based upon in vitro studies, and clinical data supporting these miRNA biomarkers as drug resistance markers are limited or nonexistent. Let-7g and miR-181b expression in primary tissues has been shown to associate with S-1-based response to chemotherapy271, while overexpression of miR-21 in CRC tissues has been associated with a poor response to 5-FU-based chemotherapy205, 243, 272. Furthermore, both expression and methylation levels of miR-148a are linked with lack of response to 5-FU and oxaliplatin chemotherapies in advanced CRC patients273. A recent comprehensive array-based interrogation of tumor tissues collected from KRAS wild-type metastatic CRC (mCRC) patients treated with anti-EGFR therapy identified miR-31-3p as a negative predictor of progression free survival (PFS)274.
Importantly, the studies of predictive miRNA biomarkers have been done using retrospective study designs and archived tissue samples. This is a limitation in our understanding of miRNA biomarkers as these studies have not accounted for intratumoral heterogeneity in the primary tissues and carry the biases inherent in retrospective study designs. Nonetheless, based on the in-vitro studies and on their presence in blood, circulating miRNA biomarkers in serum or plasma have potential to be used in blood based assays for predicting or monitoring response to chemotherapy. Although in its infancy, the development of promising miRNA biomarkers for predicting response to chemotherapeutic treatment in CRC patients could usher in a new era in the era of personalized management of cancer patients.
Long Non-coding RNA Biomarkers in Colorectal Cancer
Recent improvements in our understanding of the role of ncRNAs in carcinogenesis have led to their assessment as CRC biomarkers. Long non-coding RNAs (lncRNAs) are the second most commonly studied ncRNAs, following miRNAs. In 2012, the ENCODE Project Consortium identified 9640 lncRNA loci in the human genome and this number continues to grow160, 275. LncRNAs are classified generally into five broad categories based on their orientation and genomic location with relation to protein-coding genes: sense, antisense, bidirectional, intergenic, and intronic275–277. Although the biological role of most lncRNAs still is unknown, a growing body of literature suggests that they have a wide variety of roles in controlling the expression of genes and miRNAs in cancer. These studies have shown that lncRNAs are involved in a variety of regulatory activities including chromatin remodeling, transcriptional activation, decoy (transcriptional repressor), and RNA degradation. They can also act as miR sponges and affect translational efficacy (Figure 2D)278.
In addition, dysregulation of lncRNA expression occurs in a tissue-specific and organ-specific manner279–281, and appear to contribute to CRC tumorigenesis282, as summarized in Supplementary Table 2. In 2001, lncRNA dysregulation was identified in CRC in the setting of loss of imprinting of the long QT intronic transcript 1 (LIT1/KCNQ1OT1)283. Emerging evidence indicates that the aberrant expression of lncRNAs may have a functional role in CRC pathogenesis which would have obvious clinical implications 284. For instance, the oncogenic lncRNA, HOX Antisense Intergenic RNA (HOTAIR) is a 2158 bp gene residing in the mammalian HOXC locus on chromosome 12q13.13. HOTAIR binds PRC2 and LSD1 complexes in the 5’ and 3’ regions, and represses transcription of the HOXD cluster by acting as a scaffold for histone282. Koga and colleagues were the first to demonstrate that high expression of HOTAIR significantly correlated with distant metastasis and poor prognosis in CRC patients285, a finding later validated by another group286. Another lncRNA, Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was first identified as a predictive biomarker of metastasis in non-small cell lung cancer patients287, and subsequently was found to be prognostic in CRC patients288. LncRNAs were recently detected in extracellular vesicles and were successfully measured in the serum or plasma289–294, indicating their potential to serve as minimally-invasive biomarkers in CRC.
CONCLUSION AND PERSPECTIVES
Advances in our understanding of the natural history of CRC and the epigenetics of colon polyps and CRC has led to the development of epigenetic biomarker assays for CRC diagnosis, prognosis, and prediction of treatment response (Figure 3). The last two decades of research have demonstrated the potential of aberrant DNA methylation and alterations in noncoding RNAs to be used as biomarkers for colon polyps and CRC. Continued investigation of these promising class of biomarkers promises to lead to an high performance assays that can be used to prevent and manage patients with CRC.
Figure 3.
A schematic view of bench-to-bedside aspects of colorectal cancer epigenetics. This figure illustrates how a normal colonic epithelium undergoes a series of genetic and epigenetic alterations and transitions into an adenomatous polyp (via the “traditional pathway), or a serrated polyp (via the “serrated pathway’). Thereafter, these polyps acquire additional epigenetic alterations and genetic alterations and develop into primary CRCs. This normal-polyp-cancer multi-step cascade is governed by the acquisition of gene mutations and epigenetic alterations, including aberrant DNA methylation and dysregulated expression of several miRNAs. The molecular alterations during each step of colorectal cancer development can be measured in tissues (tissue-based biomarkers), as well as non-invasively in serum/plasma (blood-based biomarkers) and stool (stool-based biomarkers) and thus have potential to be diagnostic, prognostic and predictive biomarkers for colorectal cancer.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Drs. Shusuke Toden, Wenhao Weng and Timothy Zumwalt, from the Center for Gastrointestinal Research for their advice, suggestion and tireless efforts in editing and improving the overall quality of this article. We would also like to thank R.A.C.E. Charities for their inspiration and support in biomarker development studies (WMG).
Grant support: This work was supported by grants R01 CA72851, CA181572, CA184792 and U01 CA187956 from the National Cancer Institute, National Institutes of Health, a pilot grant from Charles A Sammons Cancer Center, and funds from the Baylor Research Institute to AG and NIH grants (P30CA15704, UO1CA152756, R01CA194663, U54CA143862, P01CA077852), R.A.C.E. Charities, and a Burroughs Wellcome Fund Translational Research Award for Clinician Scientist to WMG
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: YO and AG have no conflict of interests to disclose. WMG has limited ownership interest in a patent application for methylated MLH1.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.CH W. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- 3.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 4.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 6.Goelz SE, Vogelstein B, Hamilton SR, Feinberg AP. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985;228:187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- 7.Baylin SB, Hoppener JW, de Bustros A, Steenbergh PH, Lips CJ, Nelkin BD. DNA methylation patterns of the calcitonin gene in human lung cancers and lymphomas. Cancer Res. 1986;46:2917–2922. [PubMed] [Google Scholar]
- 8.Greger V, Passarge E, Hopping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989;83:155–158. doi: 10.1007/BF00286709. [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, Toguchida J, Ohtani N, Yandell DW, Rapaport JM, Dryja TP. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991;48:880–888. [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtani-Fujita N, Fujita T, Aoike A, Osifchin NE, Robbins PD, Sakai T. CpG methylation inactivates the promoter activity of the human retinoblastoma tumor-suppressor gene. Oncogene. 1993;8:1063–1067. [PubMed] [Google Scholar]
- 11.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 12.Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 13.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A. 1995;92:7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 15.Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, Schubeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 16.Antequera F, Bird A. Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci U S A. 1993;90:11995–11999. doi: 10.1073/pnas.90.24.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takai D, Jones PA. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad Sci U S A. 2002;99:3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vlodrop IJ, Niessen HE, Derks S, Baldewijns MM, van Criekinge W, Herman JG, van Engeland M. Analysis of promoter CpG island hypermethylation in cancer: location, location, location! Clin Cancer Res. 2011;17:4225–4231. doi: 10.1158/1078-0432.CCR-10-3394. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17:330–339. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- 20.Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16(Spec No 1):R50–R59. doi: 10.1093/hmg/ddm018. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Serra L, Esteller M. Proteins that bind methylated DNA and human cancer: reading the wrong words. Br J Cancer. 2008;98:1881–1885. doi: 10.1038/sj.bjc.6604374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, Ladd-Acosta C, Rho J, Loewer S, Miller J, Schlaeger T, Daley GQ, Feinberg AP. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, Ji H, Potash JB, Sabunciyan S, Feinberg AP. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, Koh H, Simms L, Barker M, Leggett B, Levine J, Kim M, French AJ, Thibodeau SN, Jass J, Haile R, Laird PW. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 27.Toyota M, Ohe-Toyota M, Ahuja N, Issa JP. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci U S A. 2000;97:710–715. doi: 10.1073/pnas.97.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rijnsoever M, Grieu F, Elsaleh H, Joseph D, Iacopetta B. Characterisation of colorectal cancers showing hypermethylation at multiple CpG islands. Gut. 2002;51:797–802. doi: 10.1136/gut.51.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins N, Norrie M, Cheong K, Mokany E, Ku SL, Meagher A, O’Connor T, Ward R. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- 30.Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, Murtaugh MA, Wolff RK, Slattery ML. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 32.Ward R, Meagher A, Tomlinson I, O’Connor T, Norrie M, Wu R, Hawkins N. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut. 2001;48:821–829. doi: 10.1136/gut.48.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 34.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA, Panescu J, Fix D, Lockman J, LaJeunesse J, Comeras I, de la Chapelle A. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Cunningham JM, Winters JL, Guenther JC, French AJ, Boardman LA, Burgart LJ, McDonnell SK, Schaid DJ, Thibodeau SN. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res. 2003;63:5209–5212. [PubMed] [Google Scholar]
- 36.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 38.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colussi D, Brandi G, Bazzoli F, Ricciardiello L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci. 2013;14:16365–16385. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JK, Plass C, Markowitz SD. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci U S A. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi J, Zhu YQ, Luo J, Tao WH. Hypermethylation and expression regulation of secreted frizzled-related protein genes in colorectal tumor. World J Gastroenterol. 2006;12:7113–7117. doi: 10.3748/wjg.v12.i44.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moinova HR, Chen WD, Shen L, Smiraglia D, Olechnowicz J, Ravi L, Kasturi L, Myeroff L, Plass C, Parsons R, Minna J, Willson JK, Green SB, Issa JP, Markowitz SD. HLTF gene silencing in human colon cancer. Proc Natl Acad Sci U S A. 2002;99:4562–4567. doi: 10.1073/pnas.062459899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng L, Hu J, Li S, Wang Z, Xia B, Jiang B, Li B, Zhang Y, Wang J, Wang X. Aberrant methylation of the PTCH1 gene promoter region in aberrant crypt foci. Int J Cancer. 2013;132:E18–E25. doi: 10.1002/ijc.27812. [DOI] [PubMed] [Google Scholar]
- 44.Esteller M, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Watkins DN, Issa JP, Sidransky D, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000;60:2368–2371. [PubMed] [Google Scholar]
- 45.Chan AO, Broaddus RR, Houlihan PS, Issa JP, Hamilton SR, Rashid A. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol. 2002;160:1823–1830. doi: 10.1016/S0002-9440(10)61128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imperiale TF, Ransohoff DF, Itzkowitz SH. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;371:187–188. doi: 10.1056/NEJMc1405215. [DOI] [PubMed] [Google Scholar]
- 47.Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer--a morphological, mucin and lectin histochemical study. Pathology. 1992;24:233–242. doi: 10.3109/00313029209068874. [DOI] [PubMed] [Google Scholar]
- 48.Sawyer EJ, Cerar A, Hanby AM, Gorman P, Arends M, Talbot IC, Tomlinson IP. Molecular characteristics of serrated adenomas of the colorectum. Gut. 2002;51:200–206. doi: 10.1136/gut.51.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchida H, Ando H, Maruyama K, Kobayashi H, Toda H, Ogawa H, Ozawa T, Matsuda Y, Sugimura H, Kanno T, Baba S. Genetic alterations of mixed hyperplastic adenomatous polyps in the colon and rectum. Jpn J Cancer Res. 1998;89:299–306. doi: 10.1111/j.1349-7006.1998.tb00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto T, Konishi K, Yamochi T, Makino R, Kaneko K, Shimamura T, Ota H, Mitamura K. No major tumorigenic role for beta-catenin in serrated as opposed to conventional colorectal adenomas. Br J Cancer. 2003;89:152–157. doi: 10.1038/sj.bjc.6601070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 52.Dhir M, Yachida S, Van Neste L, Glockner SC, Jeschke J, Pappou EP, Montgomery EA, Herman JG, Baylin SB, Iacobuzio-Donahue C, Ahuja N. Sessile serrated adenomas and classical adenomas: an epigenetic perspective on premalignant neoplastic lesions of the gastrointestinal tract. Int J Cancer. 2011;129:1889–1898. doi: 10.1002/ijc.25847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo RJ, Funakoshi S, Lee HH, Kong J, Lynch JP. The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex. Carcinogenesis. 2010;31:159–166. doi: 10.1093/carcin/bgp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohonen-Corish MR, Sigglekow ND, Susanto J, Chapuis PH, Bokey EL, Dent OF, Chan C, Lin BP, Seng TJ, Laird PW, Young J, Leggett BA, Jass JR, Sutherland RL. Promoter methylation of the mutated in colorectal cancer gene is a frequent early event in colorectal cancer. Oncogene. 2007;26:4435–4441. doi: 10.1038/sj.onc.1210210. [DOI] [PubMed] [Google Scholar]
- 55.Kaji E, Uraoka T, Kato J, Hiraoka S, Suzuki H, Akita M, Saito S, Tanaka T, Ohara N, Yamamoto K. Externalization of saw-tooth architecture in small serrated polyps implies the presence of methylation of IGFBP7. Dig Dis Sci. 2012;57:1261–1270. doi: 10.1007/s10620-011-2008-0. [DOI] [PubMed] [Google Scholar]
- 56.Kriegl L, Neumann J, Vieth M, Greten FR, Reu S, Jung A, Kirchner T. Up and downregulation of p16(Ink4a) expression in BRAF-mutated polyps/adenomas indicates a senescence barrier in the serrated route to colon cancer. Mod Pathol. 2011;24:1015–1022. doi: 10.1038/modpathol.2011.43. [DOI] [PubMed] [Google Scholar]
- 57.Chen RZ, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–93. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- 58.Holm TM, Jackson-Grusby L, Brambrink T, Yamada Y, Rideout WM, 3rd, Jaenisch R. Global loss of imprinting leads to widespread tumorigenesis in adult mice. Cancer Cell. 2005;8:275–285. doi: 10.1016/j.ccr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki K, Suzuki I, Leodolter A, Alonso S, Horiuchi S, Yamashita K, Perucho M. Global DNA demethylation in gastrointestinal cancer is age dependent and precedes genomic damage. Cancer Cell. 2006;9:199–207. doi: 10.1016/j.ccr.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 61.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto E, Toyota M, Suzuki H, Kondo Y, Sanomura T, Murayama Y, Ohe-Toyota M, Maruyama R, Nojima M, Ashida M, Fujii K, Sasaki Y, Hayashi N, Mori M, Imai K, Tokino T, Shinomura Y. LINE-1 hypomethylation is associated with increased CpG island methylation in Helicobacter pylori-related enlarged-fold gastritis. Cancer Epidemiol Biomarkers Prev. 2008;17:2555–2564. doi: 10.1158/1055-9965.EPI-08-0112. [DOI] [PubMed] [Google Scholar]
- 63.Pattamadilok J, Huapai N, Rattanatanyong P, Vasurattana A, Triratanachat S, Tresukosol D, Mutirangura A. LINE-1 hypomethylation level as a potential prognostic factor for epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18:711–717. doi: 10.1111/j.1525-1438.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 64.Goel A, Xicola RM, Nguyen TP, Doyle BJ, Sohn VR, Bandipalliam P, Rozek LS, Reyes J, Cordero C, Balaguer F, Castells A, Jover R, Andreu M, Syngal S, Boland CR, Llor X. Aberrant DNA methylation in hereditary nonpolyposis colorectal cancer without mismatch repair deficiency. Gastroenterology. 2010;138:1854–1862. doi: 10.1053/j.gastro.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogino S, Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, Kirkner GJ, Fuchs CS. LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Int J Cancer. 2008;122:2767–2773. doi: 10.1002/ijc.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Estecio MR, Gharibyan V, Shen L, Ibrahim AE, Doshi K, He R, Jelinek J, Yang AS, Yan PS, Huang TH, Tajara EH, Issa JP. LINE-1 hypomethylation in cancer is highly variable and inversely correlated with microsatellite instability. PLoS One. 2007;2:e399. doi: 10.1371/journal.pone.0000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Chan AT, Schernhammer ES, Giovannucci EL, Fuchs CS. A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer. J Natl Cancer Inst. 2008;100:1734–1738. doi: 10.1093/jnci/djn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antelo M, Balaguer F, Shia J, Shen Y, Hur K, Moreira L, Cuatrecasas M, Bujanda L, Giraldez MD, Takahashi M, Cabanne A, Barugel ME, Arnold M, Roca EL, Andreu M, Castellvi-Bel S, Llor X, Jover R, Castells A, Boland CR, Goel A. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS One. 2012;7:e45357. doi: 10.1371/journal.pone.0045357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahn JB, Chung WB, Maeda O, Shin SJ, Kim HS, Chung HC, Kim NK, Issa JP. DNA methylation predicts recurrence from resected stage III proximal colon cancer. Cancer. 2011;117:1847–1854. doi: 10.1002/cncr.25737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhee YY, Kim MJ, Bae JM, Koh JM, Cho NY, Juhnn YS, Kim D, Kang GH. Clinical outcomes of patients with microsatellite-unstable colorectal carcinomas depend on L1 methylation level. Ann Surg Oncol. 2012;19:3441–3448. doi: 10.1245/s10434-012-2410-7. [DOI] [PubMed] [Google Scholar]
- 71.Wolff EM, Byun HM, Han HF, Sharma S, Nichols PW, Siegmund KD, Yang AS, Jones PA, Liang G. Hypomethylation of a LINE-1 promoter activates an alternate transcript of the MET oncogene in bladders with cancer. PLoS Genet. 2010;6:e1000917. doi: 10.1371/journal.pgen.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hur K, Cejas P, Feliu J, Moreno-Rubio J, Burgos E, Boland CR, Goel A. Hypomethylation of long interspersed nuclear element-1 (LINE-1) leads to activation of proto-oncogenes in human colorectal cancer metastasis. Gut. 2014;63:635–646. doi: 10.1136/gutjnl-2012-304219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer discovery. 2013;3:730–741. doi: 10.1158/2159-8290.CD-13-0083. [DOI] [PubMed] [Google Scholar]
- 74.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kudo Y, Tateishi K, Yamamoto K, Yamamoto S, Asaoka Y, Ijichi H, Nagae G, Yoshida H, Aburatani H, Koike K. Loss of 5-hydroxymethylcytosine is accompanied with malignant cellular transformation. Cancer Sci. 2012;103:670–676. doi: 10.1111/j.1349-7006.2012.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W, Liu M. Distribution of 5-hydroxymethylcytosine in different human tissues. J Nucleic Acids. 2011;2011:870726. doi: 10.4061/2011/870726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neri F, Dettori D, Incarnato D, Krepelova A, Rapelli S, Maldotti M, Parlato C, Paliogiannis P, Oliviero S. TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway. Oncogene. 2014 doi: 10.1038/onc.2014.356. [DOI] [PubMed] [Google Scholar]
- 79.Stachler MD, Rinehart E, Lindeman N, Odze R, Srivastava A. Novel molecular insights from routine genotyping of colorectal carcinomas. Human pathology. 2015;46:507–513. doi: 10.1016/j.humpath.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Paone JF, Waalkes TP, Baker RR, Shaper JH. Serum UDP-galactosyl transferase as a potential biomarker for breast carcinoma. J Surg Oncol. 1980;15:59–66. doi: 10.1002/jso.2930150110. [DOI] [PubMed] [Google Scholar]
- 81.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 82.Sidransky D, Tokino T, Hamilton SR, Kinzler KW, Levin B, Frost P, Vogelstein B. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science. 1992;256:102–105. doi: 10.1126/science.1566048. [DOI] [PubMed] [Google Scholar]
- 83.Muller HM, Oberwalder M, Fiegl H, Morandell M, Goebel G, Zitt M, Muhlthaler M, Ofner D, Margreiter R, Widschwendter M. Methylation changes in faecal DNA: a marker for colorectal cancer screening? Lancet. 2004;363:1283–1285. doi: 10.1016/S0140-6736(04)16002-9. [DOI] [PubMed] [Google Scholar]
- 84.Huang Z, Li L, Wang J. Hypermethylation of SFRP2 as a potential marker for stool-based detection of colorectal cancer and precancerous lesions. Dig Dis Sci. 2007;52:2287–2291. doi: 10.1007/s10620-007-9755-y. [DOI] [PubMed] [Google Scholar]
- 85.Chen WD, Han ZJ, Skoletsky J, Olson J, Sah J, Myeroff L, Platzer P, Lu S, Dawson D, Willis J, Pretlow TP, Lutterbaugh J, Kasturi L, Willson JK, Rao JS, Shuber A, Markowitz SD. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–1132. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
- 86.Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC, 3rd, Sontag S, Johnson D, Skoletsky J, Durkee K, Markowitz S, Shuber A. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Itzkowitz S, Brand R, Jandorf L, Durkee K, Millholland J, Rabeneck L, Schroy PC, 3rd, Sontag S, Johnson D, Markowitz S, Paszat L, Berger BM. A simplified, noninvasive stool DNA test for colorectal cancer detection. Am J Gastroenterol. 2008;103:2862–2870. doi: 10.1111/j.1572-0241.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- 88.Leung WK, To KF, Man EP, Chan MW, Hui AJ, Ng SS, Lau JY, Sung JJ. Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in fecal samples of patients with colorectal cancer or polyps. Am J Gastroenterol. 2007;102:1070–1076. doi: 10.1111/j.1572-0241.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang DR, Tang D. Hypermethylated SFRP2 gene in fecal DNA is a high potential biomarker for colorectal cancer noninvasive screening. World J Gastroenterol. 2008;14:524–531. doi: 10.3748/wjg.14.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glockner SC, Dhir M, Yi JM, McGarvey KE, Van Neste L, Louwagie J, Chan TA, Kleeberger W, de Bruine AP, Smits KM, Khalid-de Bakker CA, Jonkers DM, Stockbrugger RW, Meijer GA, Oort FA, Iacobuzio-Donahue C, Bierau K, Herman JG, Baylin SB, Van Engeland M, Schuebel KE, Ahuja N. Methylation of TFPI2 in stool DNA: a potential novel biomarker for the detection of colorectal cancer. Cancer Res. 2009;69:4691–4699. doi: 10.1158/0008-5472.CAN-08-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hellebrekers DM, Lentjes MH, van den Bosch SM, Melotte V, Wouters KA, Daenen KL, Smits KM, Akiyama Y, Yuasa Y, Sanduleanu S, Khalid-de Bakker CA, Jonkers D, Weijenberg MP, Louwagie J, van Criekinge W, Carvalho B, Meijer GA, Baylin SB, Herman JG, de Bruine AP, van Engeland M. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res. 2009;15:3990–3997. doi: 10.1158/1078-0432.CCR-09-0055. [DOI] [PubMed] [Google Scholar]
- 92.Melotte V, Lentjes MH, van den Bosch SM, Hellebrekers DM, de Hoon JP, Wouters KA, Daenen KL, Partouns-Hendriks IE, Stessels F, Louwagie J, Smits KM, Weijenberg MP, Sanduleanu S, Khalid-de Bakker CA, Oort FA, Meijer GA, Jonkers DM, Herman JG, de Bruine AP, van Engeland M. N-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst. 2009;101:916–927. doi: 10.1093/jnci/djp131. [DOI] [PubMed] [Google Scholar]
- 93.Petko Z, Ghiassi M, Shuber A, Gorham J, Smalley W, Washington MK, Schultenover S, Gautam S, Markowitz SD, Grady WM. Aberrantly methylated CDKN2A, MGMT, and MLH1 in colon polyps and in fecal DNA from patients with colorectal polyps. Clin Cancer Res. 2005;11:1203–1209. [PubMed] [Google Scholar]
- 94.Lidgard GP, Domanico MJ, Bruinsma JJ, Light J, Gagrat ZD, Oldham-Haltom RL, Fourrier KD, Allawi H, Yab TC, Taylor WR, Simonson JA, Devens M, Heigh RI, Ahlquist DA, Berger BM. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2013;11:1313–1318. doi: 10.1016/j.cgh.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 95.Lee BB, Lee EJ, Jung EH, Chun HK, Chang DK, Song SY, Park J, Kim DH. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res. 2009;15:6185–6191. doi: 10.1158/1078-0432.CCR-09-0111. [DOI] [PubMed] [Google Scholar]
- 96.Pepe MS, Alonzo TA. Comparing disease screening tests when true disease status is ascertained only for screen positives. Biostatistics. 2001;2:249–260. doi: 10.1093/biostatistics/2.3.249. [DOI] [PubMed] [Google Scholar]
- 97.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 98.Shapiro B, Chakrabarty M, Cohn EM, Leon SA. Determination of circulating DNA levels in patients with benign or malignant gastrointestinal disease. Cancer. 1983;51:2116–2120. doi: 10.1002/1097-0142(19830601)51:11<2116::aid-cncr2820511127>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 99.Nawroz H, Koch W, Anker P, Stroun M, Sidransky D. Microsatellite alterations in serum DNA of head and neck cancer patients. Nat Med. 1996;2:1035–1037. doi: 10.1038/nm0996-1035. [DOI] [PubMed] [Google Scholar]
- 100.Hibi K, Robinson CR, Booker S, Wu L, Hamilton SR, Sidransky D, Jen J. Molecular detection of genetic alterations in the serum of colorectal cancer patients. Cancer Res. 1998;58:1405–1407. [PubMed] [Google Scholar]
- 101.Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 102.Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, Lai PB, Lau WY, Hjelm NM, Johnson PJ. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 103.Silva JM, Dominguez G, Villanueva MJ, Gonzalez R, Garcia JM, Corbacho C, Provencio M, Espana P, Bonilla F. Aberrant DNA methylation of the p16INK4a gene in plasma DNA of breast cancer patients. Br J Cancer. 1999;80:1262–1264. doi: 10.1038/sj.bjc.6690495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. [DOI] [PubMed] [Google Scholar]
- 105.Sheffield PJ, Oliver CJ, Kremer BE, Sheng S, Shao Z, Macara IG. Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J Biol Chem. 2003;278:3483–3488. doi: 10.1074/jbc.M209701200. [DOI] [PubMed] [Google Scholar]
- 106.Lofton-Day C, Model F, Devos T, Tetzner R, Distler J, Schuster M, Song X, Lesche R, Liebenberg V, Ebert M, Molnar B, Grutzmann R, Pilarsky C, Sledziewski A. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414–423. doi: 10.1373/clinchem.2007.095992. [DOI] [PubMed] [Google Scholar]
- 107.Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, Castanos-Velez E, Blumenstein BA, Rosch T, Osborn N, Snover D, Day RW, Ransohoff DF Presept Clinical Study Steering Committee I, Study T. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–325. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin P, Kang Q, Wang X, Yang L, Yu Y, Li N, He YQ, Han X, Hang J, Zhang J, Song L, Han Y, Sheng JQ. Performance of a second generation methylated SEPT9 test in detecting colorectal neoplasm. J Gastroenterol Hepatol. 2014 doi: 10.1111/jgh.12855. [DOI] [PubMed] [Google Scholar]
- 109.Ebert MP, Model F, Mooney S, Hale K, Lograsso J, Tonnes-Priddy L, Hoffmann J, Csepregi A, Rocken C, Molnar B, Schulz HU, Malfertheiner P, Lofton-Day C. Aristaless-like homeobox-4 gene methylation is a potential marker for colorectal adenocarcinomas. Gastroenterology. 2006;131:1418–1430. doi: 10.1053/j.gastro.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 110.Leung WK, To KF, Man EP, Chan MW, Bai AH, Hui AJ, Chan FK, Sung JJ. Quantitative detection of promoter hypermethylation in multiple genes in the serum of patients with colorectal cancer. Am J Gastroenterol. 2005;100:2274–2279. doi: 10.1111/j.1572-0241.2005.50412.x. [DOI] [PubMed] [Google Scholar]
- 111.Wallner M, Herbst A, Behrens A, Crispin A, Stieber P, Goke B, Lamerz R, Kolligs FT. Methylation of serum DNA is an independent prognostic marker in colorectal cancer. Clin Cancer Res. 2006;12:7347–7352. doi: 10.1158/1078-0432.CCR-06-1264. [DOI] [PubMed] [Google Scholar]
- 112.Herbst A, Rahmig K, Stieber P, Philipp A, Jung A, Ofner A, Crispin A, Neumann J, Lamerz R, Kolligs FT. Methylation of NEUROG1 in serum is a sensitive marker for the detection of early colorectal cancer. Am J Gastroenterol. 2011;106:1110–1118. doi: 10.1038/ajg.2011.6. [DOI] [PubMed] [Google Scholar]
- 113.Li M, Chen WD, Papadopoulos N, Goodman SN, Bjerregaard NC, Laurberg S, Levin B, Juhl H, Arber N, Moinova H, Durkee K, Schmidt K, He Y, Diehl F, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW, Markowitz SD, Vogelstein B. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol. 2009;27:858–863. doi: 10.1038/nbt.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen L, Catalano PJ, Benson AB, 3rd, O'Dwyer P, Hamilton SR, Issa JP. Association between DNA methylation and shortened survival in patients with advanced colorectal cancer treated with 5-fluorouracil based chemotherapy. Clin Cancer Res. 2007;13:6093–6098. doi: 10.1158/1078-0432.CCR-07-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim JC, Choi JS, Roh SA, Cho DH, Kim TW, Kim YS. Promoter methylation of specific genes is associated with the phenotype and progression of colorectal adenocarcinomas. Ann Surg Oncol. 2010;17:1767–1776. doi: 10.1245/s10434-009-0901-y. [DOI] [PubMed] [Google Scholar]
- 116.Ogino S, Meyerhardt JA, Kawasaki T, Clark JW, Ryan DP, Kulke MH, Enzinger PC, Wolpin BM, Loda M, Fuchs CS. CpG island methylation, response to combination chemotherapy, and patient survival in advanced microsatellite stable colorectal carcinoma. Virchows Arch. 2007;450:529–537. doi: 10.1007/s00428-007-0398-3. [DOI] [PubMed] [Google Scholar]
- 117.Zlobec I, Bihl MP, Foerster A, Rufle A, Terracciano L, Lugli A. Stratification and Prognostic Relevance of Jass's Molecular Classification of Colorectal Cancer. Front Oncol. 2012;2:7. doi: 10.3389/fonc.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Rijnsoever M, Elsaleh H, Joseph D, McCaul K, Iacopetta B. CpG island methylator phenotype is an independent predictor of survival benefit from 5-fluorouracil in stage III colorectal cancer. Clin Cancer Res. 2003;9:2898–2903. [PubMed] [Google Scholar]
- 119.Lee S, Cho NY, Choi M, Yoo EJ, Kim JH, Kang GH. Clinicopathological features of CpG island methylator phenotype-positive colorectal cancer and its adverse prognosis in relation to KRAS/BRAF mutation. Pathol Int. 2008;58:104–113. doi: 10.1111/j.1440-1827.2007.02197.x. [DOI] [PubMed] [Google Scholar]
- 120.Pai RK, Jayachandran P, Koong AC, Chang DT, Kwok S, Ma L, Arber DA, Balise RR, Tubbs RR, Shadrach B, Pai RK. BRAF-mutated, microsatellite-stable adenocarcinoma of the proximal colon: an aggressive adenocarcinoma with poor survival, mucinous differentiation, and adverse morphologic features. Am J Surg Pathol. 2012;36:744–752. doi: 10.1097/PAS.0b013e31824430d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, Qian ZR, Morikawa T, Shen J, Meyerhardt JA, Fuchs CS, Ogino S. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suzuki H, Yamamoto E, Maruyama R, Niinuma T, Kai M. Biological significance of the CpG island methylator phenotype. Biochem Biophys Res Commun. 2014;455:35–42. doi: 10.1016/j.bbrc.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 123.Juo YY, Johnston FM, Zhang DY, Juo HH, Wang H, Pappou EP, Yu T, Easwaran H, Baylin S, van Engeland M, Ahuja N. Prognostic value of CpG island methylator phenotype among colorectal cancer patients: a systematic review and meta-analysis. Ann Oncol. 2014;25:2314–2327. doi: 10.1093/annonc/mdu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baba Y, Nosho K, Shima K, Huttenhower C, Tanaka N, Hazra A, Giovannucci EL, Fuchs CS, Ogino S. Hypomethylation of the IGF2 DMR in colorectal tumors, detected by bisulfite pyrosequencing, is associated with poor prognosis. Gastroenterology. 2010;139:1855–1864. doi: 10.1053/j.gastro.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nilsson TK, Lof-Ohlin ZM, Sun XF. DNA methylation of the p14ARF, RASSF1A and APC1A genes as an independent prognostic factor in colorectal cancer patients. Int J Oncol. 2013;42:127–133. doi: 10.3892/ijo.2012.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagasaka T, Sharp GB, Notohara K, Kambara T, Sasamoto H, Isozaki H, MacPhee DG, Jass JR, Tanaka N, Matsubara N. Hypermethylation of O6-methylguanine-DNA methyltransferase promoter may predict nonrecurrence after chemotherapy in colorectal cancer cases. Clin Cancer Res. 2003;9:5306–5312. [PubMed] [Google Scholar]
- 127.Draht MX, Smits KM, Tournier B, Jooste V, Chapusot C, Carvalho B, Cleven AH, Derks S, Wouters KA, Belt EJ, Stockmann HB, Bril H, Weijenberg MP, van den Brandt PA, de Bruine AP, Herman JG, Meijer GA, Piard F, Melotte V, van Engeland M. Promoter CpG island methylation of RET predicts poor prognosis in stage II colorectal cancer patients. Mol Oncol. 2014;8:679–688. doi: 10.1016/j.molonc.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang ZM, Wang Y, Huang R, Liu YP, Li X, Hu FL, Zhu L, Wang F, Cui BB, Dong XS, Zhao YS. TFAP2E hypermethylation was associated with survival advantage in patients with colorectal cancer. J Cancer Res Clin Oncol. 2014;140:2119–2127. doi: 10.1007/s00432-014-1766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park SJ, Kim SM, Hong YS, Lee JL, Kim JE, Kim KP, Hong SM, Jin DH, Kim CW, Yoon YS, Park IJ, Lim SB, Yu CS, Kim JC, Kim TW. TFAP2E methylation status and prognosis of patients with radically resected colorectal cancer. Oncology. 2015;88:122–132. doi: 10.1159/000362820. [DOI] [PubMed] [Google Scholar]
- 130.Min BH, Bae JM, Lee EJ, Yu HS, Kim YH, Chang DK, Kim HC, Park CK, Lee SH, Kim KM, Kang GH. The CpG island methylator phenotype may confer a survival benefit in patients with stage II or III colorectal carcinomas receiving fluoropyrimidine-based adjuvant chemotherapy. BMC Cancer. 2011;11:344. doi: 10.1186/1471-2407-11-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jover R, Nguyen TP, Perez-Carbonell L, Zapater P, Paya A, Alenda C, Rojas E, Cubiella J, Balaguer F, Morillas JD, Clofent J, Bujanda L, Rene JM, Bessa X, Xicola RM, Nicolas-Perez D, Castells A, Andreu M, Llor X, Boland CR, Goel A. 5-Fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology. 2011;140:1174–1181. doi: 10.1053/j.gastro.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shiovitz S, Bertagnolli MM, Renfro LA, Nam E, Foster NR, Dzieciatkowski S, Luo Y, Lao VV, Monnat RJ, Jr, Emond MJ, Maizels N, Niedzwiecki D, Goldberg RM, Saltz LB, Venook A, Warren RS, Grady WM Alliance for Clinical Trials in O. CpG island methylator phenotype is associated with response to adjuvant irinotecan-based therapy for stage III colon cancer. Gastroenterology. 2014;147:637–645. doi: 10.1053/j.gastro.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weller M, Stupp R, Hegi ME, van den Bent M, Tonn JC, Sanson M, Wick W, Reifenberger G. Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol. 2012;14(Suppl 4):iv100–iv108. doi: 10.1093/neuonc/nos206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ebert MP, Tanzer M, Balluff B, Burgermeister E, Kretzschmar AK, Hughes DJ, Tetzner R, Lofton-Day C, Rosenberg R, Reinacher-Schick AC, Schulmann K, Tannapfel A, Hofheinz R, Rocken C, Keller G, Langer R, Specht K, Porschen R, Stohlmacher-Williams J, Schuster T, Strobel P, Schmid RM. TFAP2E–DKK4 and chemoresistance in colorectal cancer. N Engl J Med. 2012;366:44–53. doi: 10.1056/NEJMoa1009473. [DOI] [PubMed] [Google Scholar]
- 135.Moutinho C, Martinez-Cardus A, Santos C, Navarro-Perez V, Martinez-Balibrea E, Musulen E, Carmona FJ, Sartore-Bianchi A, Cassingena A, Siena S, Elez E, Tabernero J, Salazar R, Abad A, Esteller M. Epigenetic inactivation of the BRCA1 interactor SRBC and resistance to oxaliplatin in colorectal cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt322. djt322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 137.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 138.Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6446. [PubMed] [Google Scholar]
- 139.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 140.Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Grieu F, Watanabe G, Iacopetta B. DNA hypermethylation in the normal colonic mucosa of patients with colorectal cancer. Br J Cancer. 2006;94:593–598. doi: 10.1038/sj.bjc.6602940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shen L, Kondo Y, Rosner GL, Xiao L, Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR, Einspahr JG, Buckmeier J, Alberts DS, Hamilton SR, Issa JP. MGMT promoter methylation and field defect in sporadic colorectal cancer. J Natl Cancer Inst. 2005;97:1330–1338. doi: 10.1093/jnci/dji275. [DOI] [PubMed] [Google Scholar]
- 142.Kamiyama H, Suzuki K, Maeda T, Koizumi K, Miyaki Y, Okada S, Kawamura YJ, Samuelsson JK, Alonso S, Konishi F, Perucho M. DNA demethylation in normal colon tissue predicts predisposition to multiple cancers. Oncogene. 2012;31:5029–5037. doi: 10.1038/onc.2011.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Issa JP. Aging and epigenetic drift: a vicious cycle. J Clin Invest. 2014;124:24–29. doi: 10.1172/JCI69735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–5494. [PubMed] [Google Scholar]
- 145.Ahuja N, Issa JP. Aging, methylation and cancer. Histol Histopathol. 2000;15:835–842. doi: 10.14670/HH-15.835. [DOI] [PubMed] [Google Scholar]
- 146.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bell JT, Spector TD. DNA methylation studies using twins: what are they telling us? Genome Biol. 2012;13:172. doi: 10.1186/gb-2012-13-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bell JT, Tsai PC, Yang TP, Pidsley R, Nisbet J, Glass D, Mangino M, Zhai G, Zhang F, Valdes A, Shin SY, Dempster EL, Murray RM, Grundberg E, Hedman AK, Nica A, Small KS, Mu TC, Dermitzakis ET, McCarthy MI, Mill J, Spector TD, Deloukas P. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. 2012;8:e1002629. doi: 10.1371/journal.pgen.1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andrews AJ, Luger K. Nucleosome structure(s) and stability: variations on a theme. Annu Rev Biophys. 2011;40:99–117. doi: 10.1146/annurev-biophys-042910-155329. [DOI] [PubMed] [Google Scholar]
- 150.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 151.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Perez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MA, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 153.Tamagawa H, Oshima T, Shiozawa M, Morinaga S, Nakamura Y, Yoshihara M, Sakuma Y, Kameda Y, Akaike M, Masuda M, Imada T, Miyagi Y. The global histone modification pattern correlates with overall survival in metachronous liver metastasis of colorectal cancer. Oncol Rep. 2012;27:637–642. doi: 10.3892/or.2011.1547. [DOI] [PubMed] [Google Scholar]
- 154.Benard A, Goossens-Beumer IJ, van Hoesel AQ, de Graaf W, Horati H, Putter H, Zeestraten EC, van de Velde CJ, Kuppen PJ. Histone trimethylation at H3K4, H3K9 and H4K20 correlates with patient survival and tumor recurrence in early-stage colon cancer. BMC Cancer. 2014;14:531. doi: 10.1186/1471-2407-14-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benard A, Goossens-Beumer IJ, van Hoesel AQ, Horati H, de Graaf W, Putter H, Zeestraten EC, Liefers GJ, van de Velde CJ, Kuppen PJ. Nuclear expression of histone deacetylases and their histone modifications predicts clinical outcome in colorectal cancer. Histopathology. 2015;66:270–282. doi: 10.1111/his.12534. [DOI] [PubMed] [Google Scholar]
- 156.Tamagawa H, Oshima T, Numata M, Yamamoto N, Shiozawa M, Morinaga S, Nakamura Y, Yoshihara M, Sakuma Y, Kameda Y, Akaike M, Yukawa N, Rino Y, Masuda M, Miyagi Y. Global histone modification of H3K27 correlates with the outcomes in patients with metachronous liver metastasis of colorectal cancer. Eur J Surg Oncol. 2013;39:655–661. doi: 10.1016/j.ejso.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 157.Leszinski G, Gezer U, Siegele B, Stoetzer O, Holdenrieder S. Relevance of histone marks H3K9me3 and H4K20me3 in cancer. Anticancer Res. 2012;32:2199–2205. [PubMed] [Google Scholar]
- 158.Gezer U, Ustek D, Yoruker EE, Cakiris A, Abaci N, Leszinski G, Dalay N, Holdenrieder S. Characterization of H3K9me3- and H4K20me3-associated circulating nucleosomal DNA by high-throughput sequencing in colorectal cancer. Tumour Biol. 2013;34:329–336. doi: 10.1007/s13277-012-0554-5. [DOI] [PubMed] [Google Scholar]
- 159.Crick FH, Barnett L, Brenner S, Watts-Tobin RJ. General nature of the genetic code for proteins. Nature. 1961;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- 160.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Costa FF. Non-coding RNAs: new players in eukaryotic biology. Gene. 2005;357:83–94. doi: 10.1016/j.gene.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 162.Pauli A, Rinn JL, Schier AF. Non-coding RNAs as regulators of embryogenesis. Nat Rev Genet. 2011;12:136–149. doi: 10.1038/nrg2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 164.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 165.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 166.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 167.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 168.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slaby O, Svoboda M, Michalek J, Vyzula R. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer. 2009;8:102. doi: 10.1186/1476-4598-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Motoyama K, Inoue H, Takatsuno Y, Tanaka F, Mimori K, Uetake H, Sugihara K, Mori M. Over- and under-expressed microRNAs in human colorectal cancer. Int J Oncol. 2009;34:1069–1075. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- 171.Nagel R, le Sage C, Diosdado B, van der Waal M, Oude Vrielink JA, Bolijn A, Meijer GA, Agami R. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68:5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 172.Arndt GM, Dossey L, Cullen LM, Lai A, Druker R, Eisbacher M, Zhang C, Tran N, Fan H, Retzlaff K, Bittner A, Raponi M. Characterization of global microRNA expression reveals oncogenic potential of miR-145 in metastatic colorectal cancer. BMC Cancer. 2009;9:374. doi: 10.1186/1471-2407-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oberg AL, French AJ, Sarver AL, Subramanian S, Morlan BW, Riska SM, Borralho PM, Cunningham JM, Boardman LA, Wang L, Smyrk TC, Asmann Y, Steer CJ, Thibodeau SN. miRNA expression in colon polyps provides evidence for a multihit model of colon cancer. PLoS One. 2011;6:e20465. doi: 10.1371/journal.pone.0020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pagliuca A, Valvo C, Fabrizi E, di Martino S, Biffoni M, Runci D, Forte S, De Maria R, Ricci-Vitiani L. Analysis of the combined action of miR-143 and miR-145 on oncogenic pathways in colorectal cancer cells reveals a coordinate program of gene repression. Oncogene. 2013;32:4806–4813. doi: 10.1038/onc.2012.495. [DOI] [PubMed] [Google Scholar]
- 175.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 176.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu L, Zhang Y, Wang H, Zhang G, Ding Y, Zhao L. Tumor suppressor miR-1 restrains epithelial-mesenchymal transition and metastasis of colorectal carcinoma via the MAPK and PI3K/AKT pathway. J Transl Med. 2014;12:244. doi: 10.1186/s12967-014-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xiong B, Cheng Y, Ma L, Zhang C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int J Oncol. 2013;42:219–228. doi: 10.3892/ijo.2012.1707. [DOI] [PubMed] [Google Scholar]
- 179.Josse C, Bouznad N, Geurts P, Irrthum A, Huynh-Thu VA, Servais L, Hego A, Delvenne P, Bours V, Oury C. Identification of a microRNA landscape targeting the PI3K/Akt signaling pathway in inflammation-induced colorectal carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G229–G243. doi: 10.1152/ajpgi.00484.2012. [DOI] [PubMed] [Google Scholar]
- 180.Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J, Zhang CY, Chen J, Zhang J. MicroRNA-31 activates the RAS pathway and functions as an oncogenic MicroRNA in human colorectal cancer by repressing RAS p21 GTPase activating protein 1 (RASA1) J Biol Chem. 2013;288:9508–9518. doi: 10.1074/jbc.M112.367763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vogt M, Munding J, Gruner M, Liffers ST, Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A, Hermeking H. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 182.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescencelike growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dong Y, Zhao J, Wu CW, Zhang L, Liu X, Kang W, Leung WW, Zhang N, Chan FK, Sung JJ, Ng SS, Yu J. Tumor suppressor functions of miR-133a in colorectal cancer. Mol Cancer Res. 2013;11:1051–1060. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 184.King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee JS, Rustgi AK. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71:4260–4268. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tu HC, Schwitalla S, Qian Z, LaPier GS, Yermalovich A, Ku YC, Chen SC, Viswanathan SR, Zhu H, Nishihara R, Inamura K, Kim SA, Morikawa T, Mima K, Sukawa Y, Yang J, Meredith G, Fuchs CS, Ogino S, Daley GQ. LIN28 cooperates with WNT signaling to drive invasive intestinal and colorectal adenocarcinoma in mice and humans. Genes Dev. 2015;29:1074–1086. doi: 10.1101/gad.256693.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164–8172. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 187.Liu F, Kong X, Lv L, Gao J. TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015;359:288–298. doi: 10.1016/j.canlet.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 188.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schmitz KJ, Hey S, Schinwald A, Wohlschlaeger J, Baba HA, Worm K, Schmid KW. Differential expression of microRNA 181b and microRNA 21 in hyperplastic polyps and sessile serrated adenomas of the colon. Virchows Arch. 2009;455:49–54. doi: 10.1007/s00428-009-0804-0. [DOI] [PubMed] [Google Scholar]
- 190.Nosho K, Igarashi H, Nojima M, Ito M, Maruyama R, Yoshii S, Naito T, Sukawa Y, Mikami M, Sumioka W, Yamamoto E, Kurokawa S, Adachi Y, Takahashi H, Okuda H, Kusumi T, Hosokawa M, Fujita M, Hasegawa T, Okita K, Hirata K, Suzuki H, Yamamoto H, Shinomura Y. Association of microRNA-31 with BRAF mutation, colorectal cancer survival and serrated pathway. Carcinogenesis. 2014;35:776–783. doi: 10.1093/carcin/bgt374. [DOI] [PubMed] [Google Scholar]
- 191.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O’Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ito M, Mitsuhashi K, Igarashi H, Nosho K, Naito T, Yoshii S, Takahashi H, Fujita M, Sukawa Y, Yamamoto E, Takahashi T, Adachi Y, Nojima M, Sasaki Y, Tokino T, Baba Y, Maruyama R, Suzuki H, Imai K, Yamamoto H, Shinomura Y. MicroRNA-31 expression in relation to BRAF mutation, CpG island methylation and colorectal continuum in serrated lesions. Int J Cancer. 2014;135:2507–2515. doi: 10.1002/ijc.28920. [DOI] [PubMed] [Google Scholar]
- 193.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–797. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 196.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 200.Toiyama Y, Takahashi M, Hur K, Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR, Goel A. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013;105:849–859. doi: 10.1093/jnci/djt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H, Watanabe M, Nakagama H, Yokota J, Kohno T, Tsuchiya N. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9:e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chim SS, Shing TK, Hung EC, Leung TY, Lau TK, Chiu RW, Lo YM. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482–490. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 203.Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 204.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 205.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, Liu CG, Calin GA, Croce CM, Harris CC. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schee K, Lorenz S, Worren MM, Gunther CC, Holden M, Hovig E, Fodstad O, Meza-Zepeda LA, Flatmark K. Deep Sequencing the MicroRNA Transcriptome in Colorectal Cancer. PLoS One. 2013;8:e66165. doi: 10.1371/journal.pone.0066165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kanaan Z, Rai SN, Eichenberger MR, Roberts H, Keskey B, Pan J, Galandiuk S. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012;256:544–551. doi: 10.1097/SLA.0b013e318265bd6f. [DOI] [PubMed] [Google Scholar]
- 208.Liu GH, Zhou ZG, Chen R, Wang MJ, Zhou B, Li Y, Sun XF. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol. 2013;34:2175–2181. doi: 10.1007/s13277-013-0753-8. [DOI] [PubMed] [Google Scholar]
- 209.Lv ZC, Fan YS, Chen HB, Zhao DW. Investigation of microRNA-155 as a serum diagnostic and prognostic biomarker for colorectal cancer. Tumour Biol. 2014 doi: 10.1007/s13277-014-2760-9. [DOI] [PubMed] [Google Scholar]
- 210.Pu XX, Huang GL, Guo HQ, Guo CC, Li H, Ye S, Ling S, Jiang L, Tian Y, Lin TY. Circulating miR-221 directly amplified from plasma is a potential diagnostic and prognostic marker of colorectal cancer and is correlated with p53 expression. J Gastroenterol Hepatol. 2010;25:1674–1680. doi: 10.1111/j.1440-1746.2010.06417.x. [DOI] [PubMed] [Google Scholar]
- 211.Zhang GJ, Zhou T, Liu ZL, Tian HP, Xia SS. Plasma miR-200c and miR-18a as potential biomarkers for the detection of colorectal carcinoma. Mol Clin Oncol. 2013;1:379–384. doi: 10.3892/mco.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Luo X, Stock C, Burwinkel B, Brenner H. Identification and evaluation of plasma microRNAs for early detection of colorectal cancer. PLoS One. 2013;8:e62880. doi: 10.1371/journal.pone.0062880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kanaan Z, Roberts H, Eichenberger MR, Billeter A, Ocheretner G, Pan J, Rai SN, Jorden J, Williford A, Galandiuk S. A plasma microRNA panel for detection of colorectal adenomas: a step toward more precise screening for colorectal cancer. Ann Surg. 2013;258:400–408. doi: 10.1097/SLA.0b013e3182a15bcc. [DOI] [PubMed] [Google Scholar]
- 214.Wang J, Huang SK, Zhao M, Yang M, Zhong JL, Gu YY, Peng H, Che YQ, Huang CZ. Identification of a circulating microRNA signature for colorectal cancer detection. PLoS One. 2014;9:e87451. doi: 10.1371/journal.pone.0087451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang S, Xiang J, Li Z, Lu S, Hu J, Gao X, Yu L, Wang L, Wang J, Wu Y, Chen Z, Zhu H. A plasma microRNA panel for early detection of colorectal cancer. Int J Cancer. 2015;136:152–161. doi: 10.1002/ijc.28136. [DOI] [PubMed] [Google Scholar]
- 216.Link A, Balaguer F, Shen Y, Nagasaka T, Lozano JJ, Boland CR, Goel A. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomarkers Prev. 2010;19:1766–1774. doi: 10.1158/1055-9965.EPI-10-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wu CW, Ng SS, Dong YJ, Ng SC, Leung WW, Lee CW, Wong YN, Chan FK, Yu J, Sung JJ. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739–745. doi: 10.1136/gut.2011.239236. [DOI] [PubMed] [Google Scholar]
- 218.Koga Y, Yamazaki N, Yamamoto Y, Yamamoto S, Saito N, Kakugawa Y, Otake Y, Matsumoto M, Matsumura Y. Fecal miR-106a is a useful marker for colorectal cancer patients with false-negative results in immunochemical fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2013;22:1844–1852. doi: 10.1158/1055-9965.EPI-13-0512. [DOI] [PubMed] [Google Scholar]
- 219.Koga Y, Yasunaga M, Takahashi A, Kuroda J, Moriya Y, Akasu T, Fujita S, Yamamoto S, Baba H, Matsumura Y. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res (Phila) 2010;3:1435–1442. doi: 10.1158/1940-6207.CAPR-10-0036. [DOI] [PubMed] [Google Scholar]
- 220.Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, Ju J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007;13:1668–1674. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shibuya H, Iinuma H, Shimada R, Horiuchi A, Watanabe T. Clinicopathological and prognostic value of microRNA-21 and microRNA-155 in colorectal cancer. Oncology. 2010;79:313–320. doi: 10.1159/000323283. [DOI] [PubMed] [Google Scholar]
- 222.Kjaer-Frifeldt S, Hansen TF, Nielsen BS, Joergensen S, Lindebjerg J, Soerensen FB, dePont Christensen R, Jakobsen A Danish Colorectal Cancer G. The prognostic importance of miR-21 in stage II colon cancer: a population-based study. Br J Cancer. 2012;107:1169–1174. doi: 10.1038/bjc.2012.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kulda V, Pesta M, Topolcan O, Liska V, Treska V, Sutnar A, Rupert K, Ludvikova M, Babuska V, Holubec L, Jr, Cerny R. Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet. 2010;200:154–160. doi: 10.1016/j.cancergencyto.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 224.Bovell LC, Shanmugam C, Putcha BD, Katkoori VR, Zhang B, Bae S, Singh KP, Grizzle WE, Manne U. The prognostic value of microRNAs varies with patient race/ethnicity and stage of colorectal cancer. Clin Cancer Res. 2013;19:3955–3965. doi: 10.1158/1078-0432.CCR-12-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J, Zhao HW, Chen W, He YL, Wang HY, Xie D, Luo JH. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14:1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 226.Nishida N, Yamashita S, Mimori K, Sudo T, Tanaka F, Shibata K, Yamamoto H, Ishii H, Doki Y, Mori M. MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Ann Surg Oncol. 2012;19:3065–3071. doi: 10.1245/s10434-012-2246-1. [DOI] [PubMed] [Google Scholar]
- 227.Hur K, Toiyama Y, Schetter AJ, Okugawa Y, Harris CC, Boland CR, Goel A. Identification of a Metastasis-Specific MicroRNA Signature in Human Colorectal Cancer. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/dju492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Valladares-Ayerbes M, Blanco M, Haz M, Medina V, Iglesias-Diaz P, Lorenzo-Patino MJ, Reboredo M, Santamarina I, Figueroa A, Anton-Aparicio LM, Calvo L. Prognostic impact of disseminated tumor cells and microRNA-17-92 cluster deregulation in gastrointestinal cancer. Int J Oncol. 2011;39:1253–1264. doi: 10.3892/ijo.2011.1112. [DOI] [PubMed] [Google Scholar]
- 229.Yu G, Tang JQ, Tian ML, Li H, Wang X, Wu T, Zhu J, Huang SJ, Wan YL. Prognostic values of the miR-17-92 cluster and its paralogs in colon cancer. J Surg Oncol. 2012;106:232–237. doi: 10.1002/jso.22138. [DOI] [PubMed] [Google Scholar]
- 230.Fang L, Li H, Wang L, Hu J, Jin T, Wang J, Yang BB. MicroRNA-17-5p promotes chemotherapeutic drug resistance and tumour metastasis of colorectal cancer by repressing PTEN expression. Oncotarget. 2014;5:2974–2987. doi: 10.18632/oncotarget.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Weissmann-Brenner A, Kushnir M, Lithwick Yanai G, Aharonov R, Gibori H, Purim O, Kundel Y, Morgenstern S, Halperin M, Niv Y, Brenner B. Tumor microRNA-29a expression and the risk of recurrence in stage II colon cancer. Int J Oncol. 2012;40:2097–2103. doi: 10.3892/ijo.2012.1403. [DOI] [PubMed] [Google Scholar]
- 232.Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y, Song C, Zhu S, Leng Y, Wang G, Chen W, Du P, Huang S, Zhou X, Kang J, Cui L. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer. 2014;110:450–458. doi: 10.1038/bjc.2013.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang MH, Yu J, Chen N, Wang XY, Liu XY, Wang S, Ding YQ. Elevated microRNA-31 expression regulates colorectal cancer progression by repressing its target gene SATB2. PLoS One. 2013;8:e85353. doi: 10.1371/journal.pone.0085353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Igarashi H, Kurihara H, Mitsuhashi K, Ito M, Okuda H, Kanno S, Naito T, Yoshii S, Takahashi H, Kusumi T, Hasegawa T, Sukawa Y, Adachi Y, Okita K, Hirata K, Imamura Y, Baba Y, Imai K, Suzuki H, Yamamoto H, Nosho K, Shinomura Y. Association of MicroRNA-31-5p with Clinical Efficacy of Anti-EGFR Therapy in Patients with Metastatic Colorectal Cancer. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4264-7. [DOI] [PubMed] [Google Scholar]
- 235.Liu H, Du L, Wen Z, Yang Y, Li J, Wang L, Zhang X, Liu Y, Dong Z, Li W, Zheng G, Wang C. Up-regulation of miR-182 expression in colorectal cancer tissues and its prognostic value. Int J Colorectal Dis. 2013;28:697–703. doi: 10.1007/s00384-013-1674-0. [DOI] [PubMed] [Google Scholar]
- 236.Wang S, Yang MH, Wang XY, Lin J, Ding YQ. Increased expression of miRNA-182 in colorectal carcinoma: an independent and tissue-specific prognostic factor. Int J Clin Exp Pathol. 2014;7:3498–3503. [PMC free article] [PubMed] [Google Scholar]
- 237.Guo H, Chen Y, Hu X, Qian G, Ge S, Zhang J. The regulation of Toll-like receptor 2 by miR-143 suppresses the invasion and migration of a subset of human colorectal carcinoma cells. Mol Cancer. 2013;12:77. doi: 10.1186/1476-4598-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang MJ, Li Y, Wang R, Wang C, Yu YY, Yang L, Zhang Y, Zhou B, Zhou ZG, Sun XF. Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. Int J Colorectal Dis. 2013;28:183–189. doi: 10.1007/s00384-012-1550-3. [DOI] [PubMed] [Google Scholar]
- 239.Jinushi T, Shibayama Y, Kinoshita I, Oizumi S, Jinushi M, Aota T, Takahashi T, Horita S, Dosaka-Akita H, Iseki K. Low expression levels of microRNA-124-5p correlated with poor prognosis in colorectal cancer via targeting of SMC4. Cancer Med. 2014;3:1544–1552. doi: 10.1002/cam4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005390.pub2. CD005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 242.Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjot L, Wiuf C, Sorensen FJ, Kruhoffer M, Laurberg S, Kauppinen S, Orntoft TF, Andersen CL. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 243.Oue N, Anami K, Schetter AJ, Moehler M, Okayama H, Khan MA, Bowman ED, Mueller A, Schad A, Shimomura M, Hinoi T, Aoyagi K, Sasaki H, Okajima M, Ohdan H, Galle PR, Yasui W, Harris CC. High miR-21 expression from FFPE tissues is associated with poor survival and response to adjuvant chemotherapy in colon cancer. Int J Cancer. 2014;134:1926–1934. doi: 10.1002/ijc.28522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hansen TF, Kjaer-Frifeldt S, Christensen RD, Morgenthaler S, Blondal T, Lindebjerg J, Sorensen FB, Jakobsen A. Redefining high-risk patients with stage II colon cancer by risk index and microRNA-21: results from a population-based cohort. Br J Cancer. 2014;111:1285–1292. doi: 10.1038/bjc.2014.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nielsen BS, Jorgensen S, Fog JU, Sokilde R, Christensen IJ, Hansen U, Brunner N, Baker A, Moller S, Nielsen HJ. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis. 2011;28:27–38. doi: 10.1007/s10585-010-9355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 247.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 248.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 249.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 250.Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, Nordlinger B, Adam R, Gruenberger T, Choti MA, Bilchik AJ, Van Cutsem EJ, Chiang JM, D’Angelica MI. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26:4828–4833. doi: 10.1200/JCO.2008.17.6453. [DOI] [PubMed] [Google Scholar]
- 251.Kurokawa K, Tanahashi T, Iima T, Yamamoto Y, Akaike Y, Nishida K, Masuda K, Kuwano Y, Murakami Y, Fukushima M, Rokutan K. Role of miR-19b and its target mRNAs in 5-fluorouracil resistance in colon cancer cells. J Gastroenterol. 2012;47:883–895. doi: 10.1007/s00535-012-0547-6. [DOI] [PubMed] [Google Scholar]
- 252.Chai H, Liu M, Tian R, Li X, Tang H. miR-20a targets BNIP2 and contributes chemotherapeutic resistance in colorectal adenocarcinoma SW480 and SW620 cell lines. Acta Biochim Biophys Sin (Shanghai) 2011;43:217–225. doi: 10.1093/abbs/gmq125. [DOI] [PubMed] [Google Scholar]
- 253.Valeri N, Gasparini P, Braconi C, Paone A, Lovat F, Fabbri M, Sumani KM, Alder H, Amadori D, Patel T, Nuovo GJ, Fishel R, Croce CM. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) Proc Natl Acad Sci U S A. 2010;107:21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Faltejskova P, Besse A, Sevcikova S, Kubiczkova L, Svoboda M, Smarda J, Kiss I, Vyzula R, Slaby O. Clinical correlations of miR-21 expression in colorectal cancer patients and effects of its inhibition on DLD1 colon cancer cells. Int J Colorectal Dis. 2012;27:1401–1408. doi: 10.1007/s00384-012-1461-3. [DOI] [PubMed] [Google Scholar]
- 255.Deng J, Lei W, Fu JC, Zhang L, Li JH, Xiong JP. Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochem Biophys Res Commun. 2014;443:789–795. doi: 10.1016/j.bbrc.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 256.Shang J, Yang F, Wang Y, Wang Y, Xue G, Mei Q, Wang F, Sun S. MicroRNA-23a antisense enhances 5-fluorouracil chemosensitivity through APAF-1/caspase-9 apoptotic pathway in colorectal cancer cells. J Cell Biochem. 2014;115:772–784. doi: 10.1002/jcb.24721. [DOI] [PubMed] [Google Scholar]
- 257.Wang CJ, Stratmann J, Zhou ZG, Sun XF. Suppression of microRNA-31 increases sensitivity to 5-FU at an early stage, and affects cell migration and invasion in HCT-116 colon cancer cells. BMC Cancer. 2010;10:616. doi: 10.1186/1471-2407-10-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Akao Y, Noguchi S, Iio A, Kojima K, Takagi T, Naoe T. Dysregulation of microRNA-34a expression causes drug-resistance to 5-FU in human colon cancer DLD-1 cells. Cancer Lett. 2011;300:197–204. doi: 10.1016/j.canlet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 259.Siemens H, Jackstadt R, Kaller M, Hermeking H. Repression of c-Kit by p53 is mediated by miR-34 and is associated with reduced chemoresistance, migration and stemness. Oncotarget. 2013;4:1399–1415. doi: 10.18632/oncotarget.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Karaayvaz M, Zhai H, Ju J. miR-129 promotes apoptosis and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cell Death Dis. 2013;4:e659. doi: 10.1038/cddis.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Song B, Wang Y, Xi Y, Kudo K, Bruheim S, Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, Fodstad O, Ju J. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang J, Guo H, Zhang H, Wang H, Qian G, Fan X, Hoffman AR, Hu JF, Ge S. Putative tumor suppressor miR-145 inhibits colon cancer cell growth by targeting oncogene Friend leukemia virus integration 1 gene. Cancer. 2011;117:86–95. doi: 10.1002/cncr.25522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Boni V, Bitarte N, Cristobal I, Zarate R, Rodriguez J, Maiello E, Garcia-Foncillas J, Bandres E. miR-192/miR-215 influence 5-fluorouracil resistance through cell cycle-mediated mechanisms complementary to its post-transcriptional thymidilate synthase regulation. Mol Cancer Ther. 2010;9:2265–2275. doi: 10.1158/1535-7163.MCT-10-0061. [DOI] [PubMed] [Google Scholar]
- 264.Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C, Shakibaei M, Boland CR, Goel A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015 doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Guo ST, Jiang CC, Wang GP, Li YP, Wang CY, Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, Thorne RF, Jin L, Zhang XD. MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene. 2013;32:1910–1920. doi: 10.1038/onc.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM, Fortes P, Garcia-Foncillas J. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–1671. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- 267.Qian X, Yu J, Yin Y, He J, Wang L, Li Q, Zhang LQ, Li CY, Shi ZM, Xu Q, Li W, Lai LH, Liu LZ, Jiang BH. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12:1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhang L, Pickard K, Jenei V, Bullock MD, Bruce A, Mitter R, Kelly G, Paraskeva C, Strefford J, Primrose J, Thomas GJ, Packham G, Mirnezami AH. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Res. 2013;73:6435–6447. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhou Y, Wan G, Spizzo R, Ivan C, Mathur R, Hu X, Ye X, Lu J, Fan F, Xia L, Calin GA, Ellis LM, Lu X. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol Oncol. 2014;8:83–92. doi: 10.1016/j.molonc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xu K, Liang X, Cui D, Wu Y, Shi W, Liu J. miR-1915 inhibits Bcl-2 to modulate multidrug resistance by increasing drug-sensitivity in human colorectal carcinoma cells. Mol Carcinog. 2013;52:70–78. doi: 10.1002/mc.21832. [DOI] [PubMed] [Google Scholar]
- 271.Nakajima G, Hayashi K, Xi Y, Kudo K, Uchida K, Takasaki K, Yamamoto M, Ju J. Non-coding MicroRNAs hsa-let-7g and hsa-miR-181b are Associated with Chemoresponse to S-1 in Colon Cancer. Cancer Genomics Proteomics. 2006;3:317–324. [PMC free article] [PubMed] [Google Scholar]
- 272.Liu K, Li G, Fan C, Zhou X, Wu B, Li J. Increased expression of microRNA-21and its association with chemotherapeutic response in human colorectal cancer. J Int Med Res. 2011;39:2288–2295. doi: 10.1177/147323001103900626. [DOI] [PubMed] [Google Scholar]
- 273.Takahashi M, Cuatrecasas M, Balaguer F, Hur K, Toiyama Y, Castells A, Boland CR, Goel A. The clinical significance of MiR-148a as a predictive biomarker in patients with advanced colorectal cancer. PLoS One. 2012;7:e46684. doi: 10.1371/journal.pone.0046684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Manceau G, Imbeaud S, Thiebaut R, Liebaert F, Fontaine K, Rousseau F, Genin B, Le Corre D, Didelot A, Vincent M, Bachet JB, Chibaudel B, Bouche O, Landi B, Bibeau F, Leroy K, Penault-Llorca F, Van Laethem JL, Demetter P, Tejpar S, Rossi S, Mosakhani N, Osterlund P, Ristamaki R, Sarhadi V, Knuutila S, Boige V, Andre T, Laurent-Puig P. Hsa-miR-31-3p expression is linked to progression-free survival in patients with KRAS wild-type metastatic colorectal cancer treated with anti-EGFR therapy. Clin Cancer Res. 2014;20:3338–3347. doi: 10.1158/1078-0432.CCR-13-2750. [DOI] [PubMed] [Google Scholar]
- 275.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigo R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 277.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yu G, Yao W, Wang J, Ma X, Xiao W, Li H, Xia D, Yang Y, Deng K, Xiao H, Wang B, Guo X, Guan W, Hu Z, Bai Y, Xu H, Liu J, Zhang X, Ye Z. LncRNAs expression signatures of renal clear cell carcinoma revealed by microarray. PLoS One. 2012;7:e42377. doi: 10.1371/journal.pone.0042377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gibb EA, Becker-Santos DD, Enfield KS, Guillaud M, Niekerk D, Matisic JP, Macaulay CE, Lam WL. Aberrant expression of long noncoding RNAs in cervical intraepithelial neoplasia. Int J Gynecol Cancer. 2012;22:1557–1563. doi: 10.1097/IGC.0b013e318272f2c9. [DOI] [PubMed] [Google Scholar]
- 281.Brunner AL, Beck AH, Edris B, Sweeney RT, Zhu SX, Li R, Montgomery K, Varma S, Gilks T, Guo X, Foley JW, Witten DM, Giacomini CP, Flynn RA, Pollack JR, Tibshirani R, Chang HY, van de Rijn M, West RB. Transcriptional profiling of long non-coding RNAs and novel transcribed regions across a diverse panel of archived human cancers. Genome Biol. 2012;13:R75. doi: 10.1186/gb-2012-13-8-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tanaka K, Shiota G, Meguro M, Mitsuya K, Oshimura M, Kawasaki H. Loss of imprinting of long QT intronic transcript 1 in colorectal cancer. Oncology. 2001;60:268–273. doi: 10.1159/000055328. [DOI] [PubMed] [Google Scholar]
- 284.Xu MD, Qi P, Du X. Long non-coding RNAs in colorectal cancer: implications for pathogenesis and clinical application. Mod Pathol. 2014;27:1310–1320. doi: 10.1038/modpathol.2014.33. [DOI] [PubMed] [Google Scholar]
- 285.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 286.Svoboda M, Slyskova J, Schneiderova M, Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z, Protivankova M, Vycital O, Liska V, Schwarzova L, Vodickova L, Vodicka P. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;35:1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 287.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 288.Zheng HT, Shi DB, Wang YW, Li XX, Xu Y, Tripathi P, Gu WL, Cai GX, Cai SJ. High expression of lncRNA MALAT1 suggests a biomarker of poor prognosis in colorectal cancer. Int J Clin Exp Pathol. 2014;7:3174–3181. [PMC free article] [PubMed] [Google Scholar]
- 289.Kogure T, Yan IK, Lin WL, Patel T. Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer. 2013;4:261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–3193. [PubMed] [Google Scholar]
- 292.Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J, Wei M, Xu C, Wu C, Zhang Z, Gao X, Liu Z, Hou J, Huang J, Sun Y. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer. 2013;49:2949–2959. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 293.Tong YS, Wang XW, Zhou XL, Liu ZH, Yang TX, Shi WH, Xie HW, Lv J, Wu QQ, Cao XF. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer. 2015;14:3. doi: 10.1186/1476-4598-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dong L, Qi P, Xu MD, Ni SJ, Huang D, Xu QH, Weng WW, Tan C, Sheng WQ, Zhou XY, Du X. Circulating CUDR, LSINCT-5 and PTENP1 long non-coding rnas in sera distinguish patients with gastric cancer from healthy controls. Int J Cancer. 2015 doi: 10.1002/ijc.29484. [DOI] [PubMed] [Google Scholar]
- 295.Tanzer M, Balluff B, Distler J, Hale K, Leodolter A, Rocken C, Molnar B, Schmid R, Lofton-Day C, Schuster T, Ebert MP. Performance of epigenetic markers SEPT9 and ALX4 in plasma for detection of colorectal precancerous lesions. PLoS One. 2010;5:e9061. doi: 10.1371/journal.pone.0009061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Grady WM, Rajput A, Lutterbaugh JD, Markowitz SD. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61:900–902. [PubMed] [Google Scholar]
- 297.Grutzmann R, Molnar B, Pilarsky C, Habermann JK, Schlag PM, Saeger HD, Miehlke S, Stolz T, Model F, Roblick UJ, Bruch HP, Koch R, Liebenberg V, Devos T, Song X, Day RH, Sledziewski AZ, Lofton-Day C. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, Steiger KV, Grutzmann R, Pilarsky C, Habermann JK, Fleshner PR, Oubre BM, Day R, Sledziewski AZ, Lofton-Day C. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337–1346. doi: 10.1373/clinchem.2008.115808. [DOI] [PubMed] [Google Scholar]
- 299.Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, Fang JC, Samowitz WS, Heichman KA. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133. doi: 10.1186/1741-7015-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ahlquist DA, Taylor WR, Mahoney DW, Zou H, Domanico M, Thibodeau SN, Boardman LA, Berger BM, Lidgard GP. The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin Gastroenterol Hepatol. 2012;10:272–277. doi: 10.1016/j.cgh.2011.10.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Toth K, Wasserkort R, Sipos F, Kalmar A, Wichmann B, Leiszter K, Valcz G, Juhasz M, Miheller P, Patai AV, Tulassay Z, Molnar B. Detection of methylated septin 9 in tissue and plasma of colorectal patients with neoplasia and the relationship to the amount of circulating cell-free DNA. PLoS One. 2014;9:e115415. doi: 10.1371/journal.pone.0115415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Potter NT, Hurban P, White MN, Whitlock KD, Lofton-Day CE, Tetzner R, Koenig T, Quigley NB, Weiss G. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem. 2014;60:1183–1191. doi: 10.1373/clinchem.2013.221044. [DOI] [PubMed] [Google Scholar]
- 303.Johnson DA, Barclay RL, Mergener K, Weiss G, Konig T, Beck J, Potter NT. Plasma Septin9 versus fecal immunochemical testing for colorectal cancer screening: a prospective multicenter study. PLoS One. 2014;9:e98238. doi: 10.1371/journal.pone.0098238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Amiot A, Mansour H, Baumgaertner I, Delchier JC, Tournigand C, Furet JP, Carrau JP, Canoui-Poitrine F, Sobhani I, Marne CRCgoVD. The detection of the methylated Wif-1 gene is more accurate than a fecal occult blood test for colorectal cancer screening. PLoS One. 2014;9:e99233. doi: 10.1371/journal.pone.0099233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Abbaszadegan MR, Tavasoli A, Velayati A, Sima HR, Vosooghinia H, Farzadnia M, Asadzedeh H, Gholamin M, Dadkhah E, Aarabi A. Stool-based DNA testing, a new noninvasive method for colorectal cancer screening, the first report from Iran. World J Gastroenterol. 2007;13:1528–1533. doi: 10.3748/wjg.v13.i10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chang E, Park DI, Kim YJ, Kim BK, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Kim HD, Kim DH, Kim YH. Detection of colorectal neoplasm using promoter methylation of ITGA4, SFRP2, and p16 in stool samples: a preliminary report in Korean patients. Hepatogastroenterology. 2010;57:720–727. [PubMed] [Google Scholar]
- 307.Ausch C, Kim YH, Tsuchiya KD, Dzieciatkowski S, Washington MK, Paraskeva C, Radich J, Grady WM. Comparative analysis of PCR-based biomarker assay methods for colorectal polyp detection from fecal DNA. Clin Chem. 2009;55:1559–1563. doi: 10.1373/clinchem.2008.122937. [DOI] [PubMed] [Google Scholar]
- 308.Huang ZH, Li LH, Yang F, Wang JF. Detection of aberrant methylation in fecal DNA as a molecular screening tool for colorectal cancer and precancerous lesions. World J Gastroenterol. 2007;13:950–954. doi: 10.3748/wjg.v13.i6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Baek YH, Chang E, Kim YJ, Kim BK, Sohn JH, Park DI. Stool methylation-specific polymerase chain reaction assay for the detection of colorectal neoplasia in Korean patients. Dis Colon Rectum. 2009;52:1452–1459. doi: 10.1007/DCR.0b013e3181a79533. discussion 1459-63. [DOI] [PubMed] [Google Scholar]
- 310.Oberwalder M, Zitt M, Wontner C, Fiegl H, Goebel G, Zitt M, Kohle O, Muhlmann G, Ofner D, Margreiter R, Muller HM. SFRP2 methylation in fecal DNA--a marker for colorectal polyps. Int J Colorectal Dis. 2008;23:15–19. doi: 10.1007/s00384-007-0355-2. [DOI] [PubMed] [Google Scholar]
- 311.Nagasaka T, Tanaka N, Cullings HM, Sun DS, Sasamoto H, Uchida T, Koi M, Nishida N, Naomoto Y, Boland CR, Matsubara N, Goel A. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst. 2009;101:1244–1258. doi: 10.1093/jnci/djp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang H, Zhu YQ, Wu YQ, Zhang P, Qi J. Detection of promoter hypermethylation of Wnt antagonist genes in fecal samples for diagnosis of early colorectal cancer. World J Gastroenterol. 2014;20:6329–6335. doi: 10.3748/wjg.v20.i20.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhang J, Yang S, Xie Y, Chen X, Zhao Y, He D, Li J. Detection of methylated tissue factor pathway inhibitor 2 and human long DNA in fecal samples of patients with colorectal cancer in China. Cancer Epidemiol. 2012;36:73–77. doi: 10.1016/j.canep.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 314.Kisiel JB, Yab TC, Nazer Hussain FT, Taylor WR, Garrity-Park MM, Sandborn WJ, Loftus EV, Wolff BG, Smyrk TC, Itzkowitz SH, Rubin DT, Zou H, Mahoney DW, Ahlquist DA. Stool DNA testing for the detection of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:546–554. doi: 10.1111/apt.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, Butz ML, Thibodeau SN, Rabeneck L, Paszat LF, Kinzler KW, Vogelstein B, Bjerregaard NC, Laurberg S, Sorensen HT, Berger BM, Lidgard GP. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142:248–256. doi: 10.1053/j.gastro.2011.10.031. quiz e25-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Esteller M, Gonzalez S, Risques RA, Marcuello E, Mangues R, Germa JR, Herman JG, Capella G, Peinado MA. K-ras and p16 aberrations confer poor prognosis in human colorectal cancer. J Clin Oncol. 2001;19:299–304. doi: 10.1200/JCO.2001.19.2.299. [DOI] [PubMed] [Google Scholar]
- 317.Shima K, Nosho K, Baba Y, Cantor M, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S. Prognostic significance of CDKN2A (p16) promoter methylation and loss of expression in 902 colorectal cancers: Cohort study and literature review. Int J Cancer. 2011;128:1080–1094. doi: 10.1002/ijc.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Maeda K, Kawakami K, Ishida Y, Ishiguro K, Omura K, Watanabe G. Hypermethylation of the CDKN2A gene in colorectal cancer is associated with shorter survival. Oncol Rep. 2003;10:935–938. [PubMed] [Google Scholar]
- 319.Liang JT, Chang KJ, Chen JC, Lee CC, Cheng YM, Hsu HC, Wu MS, Wang SM, Lin JT, Cheng AL. Hypermethylation of the p16 gene in sporadic T3N0M0 stage colorectal cancers: association with DNA replication error and shorter survival. Oncology. 1999;57:149–156. doi: 10.1159/000012023. [DOI] [PubMed] [Google Scholar]
- 320.Ward RL, Cheong K, Ku SL, Meagher A, O’Connor T, Hawkins NJ. Adverse prognostic effect of methylation in colorectal cancer is reversed by microsatellite instability. J Clin Oncol. 2003;21:3729–3736. doi: 10.1200/JCO.2003.03.123. [DOI] [PubMed] [Google Scholar]
- 321.Barault L, Charon-Barra C, Jooste V, de la Vega MF, Martin L, Roignot P, Rat P, Bouvier AM, Laurent-Puig P, Faivre J, Chapusot C, Piard F. Hypermethylator phenotype in sporadic colon cancer: study on a population-based series of 582 cases. Cancer Res. 2008;68:8541–8546. doi: 10.1158/0008-5472.CAN-08-1171. [DOI] [PubMed] [Google Scholar]
- 322.Bihl MP, Foerster A, Lugli A, Zlobec I. Characterization of CDKN2A(p16) methylation and impact in colorectal cancer: systematic analysis using pyrosequencing. J Transl Med. 2012;10:173. doi: 10.1186/1479-5876-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kohonen-Corish MR, Tseung J, Chan C, Currey N, Dent OF, Clarke S, Bokey L, Chapuis PH. KRAS mutations and CDKN2A promoter methylation show an interactive adverse effect on survival and predict recurrence of rectal cancer. Int J Cancer. 2014;134:2820–2828. doi: 10.1002/ijc.28619. [DOI] [PubMed] [Google Scholar]
- 324.Mitomi H, Fukui N, Tanaka N, Kanazawa H, Saito T, Matsuoka T, Yao T. Aberrant p16((INK4a)) methylation is a frequent event in colorectal cancers: prognostic value and relation to mRNA expression and immunoreactivity. J Cancer Res Clin Oncol. 2010;136:323–331. doi: 10.1007/s00432-009-0688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cleven AH, Derks S, Draht MX, Smits KM, Melotte V, Van Neste L, Tournier B, Jooste V, Chapusot C, Weijenberg MP, Herman JG, de Bruine AP, van Engeland M. CHFR promoter methylation indicates poor prognosis in stage II microsatellite stable colorectal cancer. Clin Cancer Res. 2014;20:3261–3271. doi: 10.1158/1078-0432.CCR-12-3734. [DOI] [PubMed] [Google Scholar]
- 326.Yi JM, Dhir M, Van Neste L, Downing SR, Jeschke J, Glockner SC, de Freitas Calmon M, Hooker CM, Funes JM, Boshoff C, Smits KM, van Engeland M, Weijenberg MP, Iacobuzio-Donahue CA, Herman JG, Schuebel KE, Baylin SB, Ahuja N. Genomic and epigenomic integration identifies a prognostic signature in colon cancer. Clin Cancer Res. 2011;17:1535–1545. doi: 10.1158/1078-0432.CCR-10-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Moya P, Esteban S, Fernandez-Suarez A, Maestro M, Morente M, Sanchez-Carbayo M. KiSS-1 methylation and protein expression patterns contribute to diagnostic and prognostic assessments in tissue specimens for colorectal cancer. Tumour Biol. 2013;34:471–479. doi: 10.1007/s13277-012-0572-3. [DOI] [PubMed] [Google Scholar]
- 328.Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 329.Kim JH, Shin SH, Kwon HJ, Cho NY, Kang GH. Prognostic implications of CpG island hypermethylator phenotype in colorectal cancers. Virchows Arch. 2009;455:485–494. doi: 10.1007/s00428-009-0857-0. [DOI] [PubMed] [Google Scholar]
- 330.Dahlin AM, Palmqvist R, Henriksson ML, Jacobsson M, Eklof V, Rutegard J, Oberg A, Van Guelpen BR. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res. 2010;16:1845–1855. doi: 10.1158/1078-0432.CCR-09-2594. [DOI] [PubMed] [Google Scholar]
- 331.Jo P, Jung K, Grade M, Conradi LC, Wolff HA, Kitz J, Becker H, Ruschoff J, Hartmann A, Beissbarth T, Muller-Dornieden A, Ghadimi M, Schneider-Stock R, Gaedcke J. CpG island methylator phenotype infers a poor disease-free survival in locally advanced rectal cancer. Surgery. 2012;151:564–570. doi: 10.1016/j.surg.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 332.Simons CC, Hughes LA, Smits KM, Khalid-de Bakker CA, de Bruine AP, Carvalho B, Meijer GA, Schouten LJ, van den Brandt PA, Weijenberg MP, van Engeland M. A novel classification of colorectal tumors based on microsatellite instability, the CpG island methylator phenotype and chromosomal instability: implications for prognosis. Ann Oncol. 2013;24:2048–2056. doi: 10.1093/annonc/mdt076. [DOI] [PubMed] [Google Scholar]
- 333.Bae JM, Kim JH, Cho NY, Kim TY, Kang GH. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109:1004–1012. doi: 10.1038/bjc.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wang Y, Long Y, Xu Y, Guan Z, Lian P, Peng J, Cai S, Cai G. Prognostic and predictive value of CpG island methylator phenotype in patients with locally advanced nonmetastatic sporadic colorectal cancer. Gastroenterol Res Pract. 2014;2014:436985. doi: 10.1155/2014/436985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Nakayama G, Hibi K, Kodera Y, Koike M, Fujiwara M, Nakao A. P16 methylation in serum as a potential marker for the malignancy of colorectal carcinoma. Anticancer Res. 2007;27:3367–3370. [PubMed] [Google Scholar]
- 336.Herbst A, Wallner M, Rahmig K, Stieber P, Crispin A, Lamerz R, Kolligs FT. Methylation of helicase-like transcription factor in serum of patients with colorectal cancer is an independent predictor of disease recurrence. Eur J Gastroenterol Hepatol. 2009;21:565–569. doi: 10.1097/MEG.0b013e328318ecf2. [DOI] [PubMed] [Google Scholar]
- 337.Philipp AB, Stieber P, Nagel D, Neumann J, Spelsberg F, Jung A, Lamerz R, Herbst A, Kolligs FT. Prognostic role of methylated free circulating DNA in colorectal cancer. Int J Cancer. 2012;131:2308–2319. doi: 10.1002/ijc.27505. [DOI] [PubMed] [Google Scholar]
- 338.Kawakami K, Matsunoki A, Kaneko M, Saito K, Watanabe G, Minamoto T. Long interspersed nuclear element-1 hypomethylation is a potential biomarker for the prediction of response to oral fluoropyrimidines in microsatellite stable and CpG island methylator phenotype-negative colorectal cancer. Cancer Sci. 2011;102:166–174. doi: 10.1111/j.1349-7006.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- 339.Scartozzi M, Bearzi I, Mandolesi A, Giampieri R, Faloppi L, Galizia E, Loupakis F, Zaniboni A, Zorzi F, Biscotti T, Labianca R, Falcone A, Cascinu S. Epidermal growth factor receptor (EGFR) gene promoter methylation and cetuximab treatment in colorectal cancer patients. Br J Cancer. 2011;104:1786–1790. doi: 10.1038/bjc.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Amatu A, Sartore-Bianchi A, Moutinho C, Belotti A, Bencardino K, Chirico G, Cassingena A, Rusconi F, Esposito A, Nichelatti M, Esteller M, Siena S. Promoter CpG island hypermethylation of the DNA repair enzyme MGMT predicts clinical response to dacarbazine in a phase II study for metastatic colorectal cancer. Clin Cancer Res. 2013;19:2265–2272. doi: 10.1158/1078-0432.CCR-12-3518. [DOI] [PubMed] [Google Scholar]
- 341.Heitzer E, Artl M, Filipits M, Resel M, Graf R, Weissenbacher B, Lax S, Gnant M, Wrba F, Greil R, Dietze O, Hofbauer F, Bohm G, Hofler G, Samonigg H, Schaberl-Moser R, Balic M, Dandachi N. Differential survival trends of stage II colorectal cancer patients relate to promoter methylation status of PCDH10, SPARC, and UCHL1. Mod Pathol. 2014;27:906–915. doi: 10.1038/modpathol.2013.204. [DOI] [PubMed] [Google Scholar]
- 342.Zanutto S, Pizzamiglio S, Ghilotti M, Bertan C, Ravagnani F, Perrone F, Leo E, Pilotti S, Verderio P, Gariboldi M, Pierotti MA. Circulating miR-378 in plasma: a reliable, haemolysis-independent biomarker for colorectal cancer. Br J Cancer. 2014;110:1001–1007. doi: 10.1038/bjc.2013.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Du M, Liu S, Gu D, Wang Q, Zhu L, Kang M, Shi D, Chu H, Tong N, Chen J, Adams TS, Zhang Z, Wang M. Clinical potential role of circulating microRNAs in early diagnosis of colorectal cancer patients. Carcinogenesis. 2014;35:2723–2730. doi: 10.1093/carcin/bgu189. [DOI] [PubMed] [Google Scholar]
- 344.Basati G, Emami Razavi A, Abdi S, Mirzaei A. Elevated level of microRNA-21 in the serum of patients with colorectal cancer. Med Oncol. 2014;31:205. doi: 10.1007/s12032-014-0205-3. [DOI] [PubMed] [Google Scholar]
- 345.Yau TO, Wu CW, Dong Y, Tang CM, Ng SS, Chan FK, Sung JJ, Yu J. microRNA-221 and microRNA-18a identification in stool as potential biomarkers for the non-invasive diagnosis of colorectal carcinoma. Br J Cancer. 2014;111:1765–1771. doi: 10.1038/bjc.2014.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wu CW, Ng SC, Dong Y, Tian L, Ng SS, Leung WW, Law WT, Yau TO, Chan FK, Sung JJ, Yu J. Identification of microRNA-135b in stool as a potential noninvasive biomarker for colorectal cancer and adenoma. Clin Cancer Res. 2014;20:2994–3002. doi: 10.1158/1078-0432.CCR-13-1750. [DOI] [PubMed] [Google Scholar]
- 347.Ge X, Chen Y, Liao X, Liu D, Li F, Ruan H, Jia W. Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med Oncol. 2013;30:588. doi: 10.1007/s12032-013-0588-6. [DOI] [PubMed] [Google Scholar]
- 348.Takahashi Y, Sawada G, Kurashige J, Uchi R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, Yamamoto H, Doki Y, Mori M, Mimori K. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110:164–171. doi: 10.1038/bjc.2013.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Deng Q, He B, Gao T, Pan Y, Sun H, Xu Y, Li R, Ying H, Wang F, Liu X, Chen J, Wang S. Up-regulation of 91H promotes tumor metastasis and predicts poor prognosis for patients with colorectal cancer. PLoS One. 2014;9:e103022. doi: 10.1371/journal.pone.0103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Han Y, Yang YN, Yuan HH, Zhang TT, Sui H, Wei XL, Liu L, Huang P, Zhang WJ, Bai YX. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46:396–401. doi: 10.1097/PAT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 351.Xu MD, Qi P, Weng WW, Shen XH, Ni SJ, Dong L, Huang D, Tan C, Sheng WQ, Zhou XY, Du X. Long non-coding RNA LSINCT5 predicts negative prognosis and exhibits oncogenic activity in gastric cancer. Medicine (Baltimore) 2014;93:e303. doi: 10.1097/MD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, Sevignani C, Fabbri M, Cimmino A, Lee EJ, Wojcik SE, Shimizu M, Tili E, Rossi S, Taccioli C, Pichiorri F, Liu X, Zupo S, Herlea V, Gramantieri L, Lanza G, Alder H, Rassenti L, Volinia S, Schmittgen TD, Kipps TJ, Negrini M, Croce CM. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–229. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 353.Qi P, Xu MD, Ni SJ, Huang D, Wei P, Tan C, Zhou XY, Du X. Low expression of LOC285194 is associated with poor prognosis in colorectal cancer. J Transl Med. 2013;11:122. doi: 10.1186/1479-5876-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Qi P, Xu MD, Ni SJ, Shen XH, Wei P, Huang D, Tan C, Sheng WQ, Zhou XY, Du X. Down-regulation of ncRAN, a long non-coding RNA, contributes to colorectal cancer cell migration and invasion and predicts poor overall survival for colorectal cancer patients. Mol Carcinog. 2014 doi: 10.1002/mc.22137. [DOI] [PubMed] [Google Scholar]
- 355.Shi D, Zheng H, Zhuo C, Peng J, Li D, Xu Y, Li X, Cai G, Cai S. Low expression of novel lncRNA RP11-462C24.1 suggests a biomarker of poor prognosis in colorectal cancer. Med Oncol. 2014;31:31. doi: 10.1007/s12032-014-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yin D, He X, Zhang E, Kong R, De W, Zhang Z. Long noncoding RNA GAS5 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Med Oncol. 2014;31:253. doi: 10.1007/s12032-014-0253-8. [DOI] [PubMed] [Google Scholar]
- 357.Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol. 2015 doi: 10.1007/s13277-015-3139-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.