Abstract
The crystal structure of Escherichia coli asparaginase II (EC 3.5.1.1), a drug (Elspar) used for the treatment of acute lymphoblastic leukemia, has been determined at 2.3 A resolution by using data from a single heavy atom derivative in combination with molecular replacement. The atomic model was refined to an R factor of 0.143. This enzyme, active as a homotetramer with 222 symmetry, belongs to the class of alpha/beta proteins. Each subunit has two domains with unique topological features. On the basis of present structural evidence consistent with previous biochemical studies, we propose locations for the active sites between the N- and C-terminal domains belonging to different subunits and postulate a catalytic role for Thr-89.
Full text
PDF

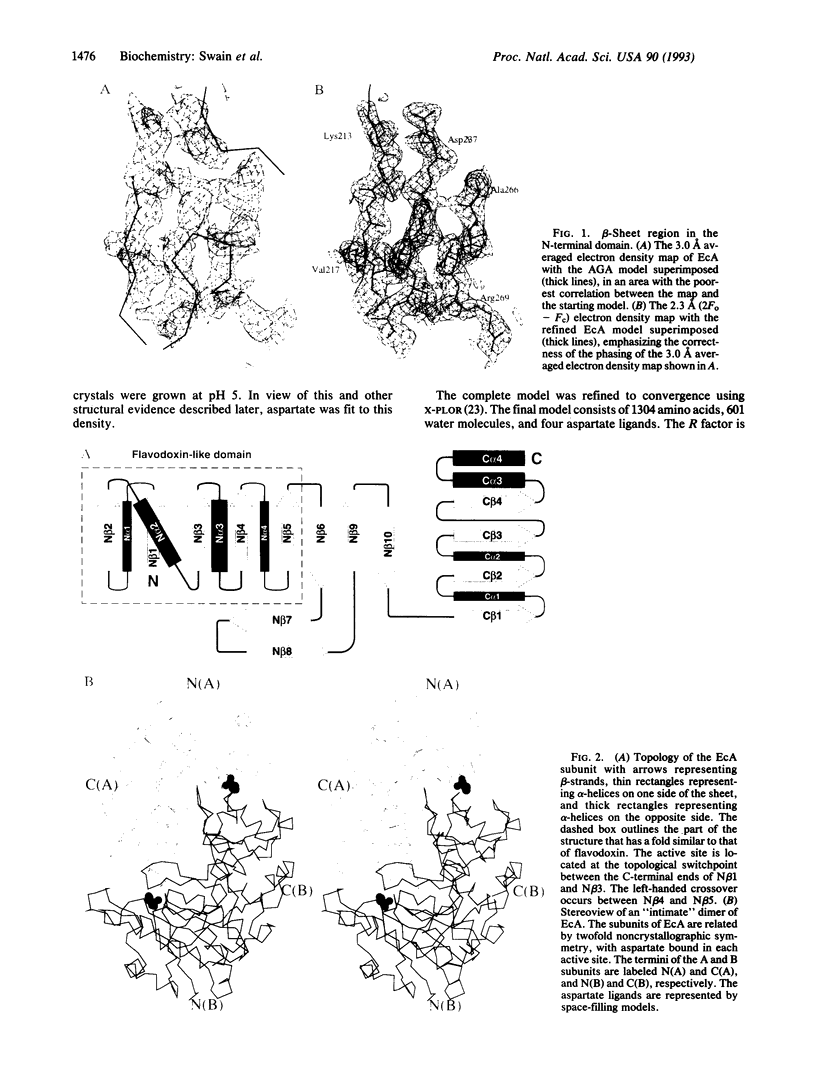
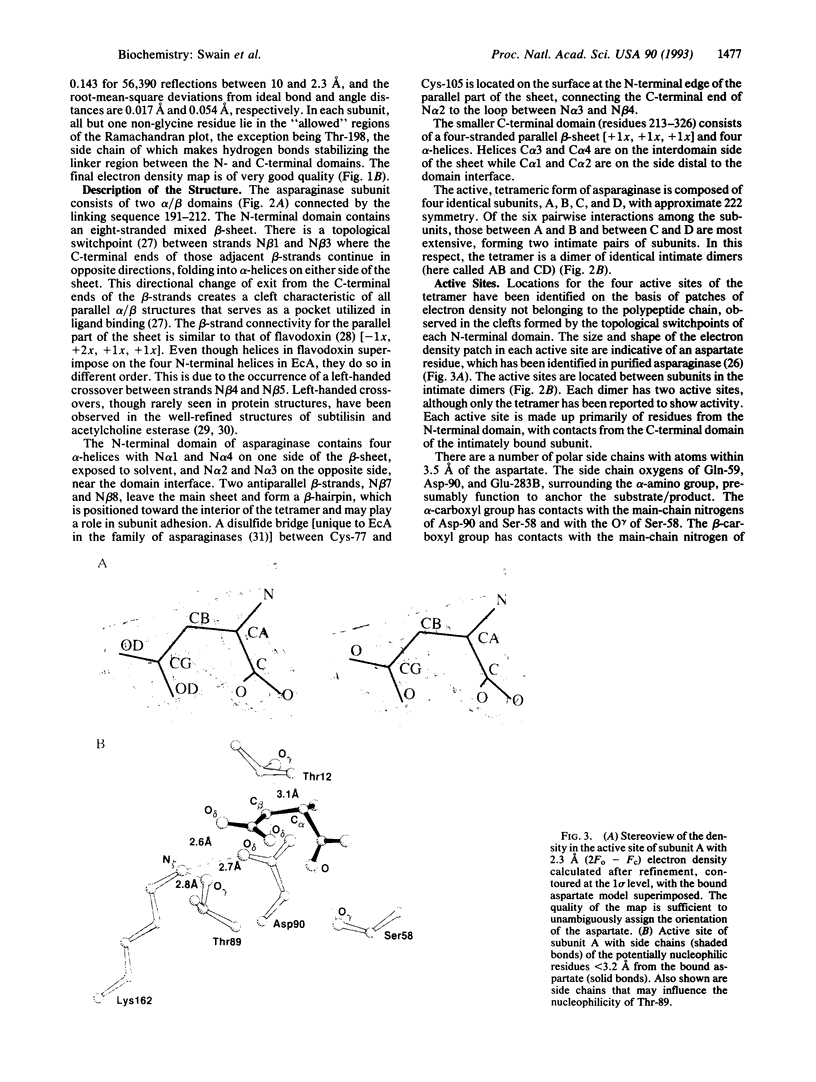

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. L., Murphy K. C., Chandrasekhar K., Wlodawer A. Preliminary crystallographic study of an L-asparaginase from Vibrio succinogenes. J Mol Biol. 1985 Jul 5;184(1):179–181. doi: 10.1016/0022-2836(85)90051-8. [DOI] [PubMed] [Google Scholar]
- Ammon H. L., Weber I. T., Wlodawer A., Harrison R. W., Gilliland G. L., Murphy K. C., Sjölin L., Roberts J. Preliminary crystal structure of Acinetobacter glutaminasificans glutaminase-asparaginase. J Biol Chem. 1988 Jan 5;263(1):150–156. [PubMed] [Google Scholar]
- Bagert U., Röhm K. H. On the role of histidine and tyrosine residues in E. coli asparaginase. Chemical modification and 1H-nuclear magnetic resonance studies. Biochim Biophys Acta. 1989 Nov 9;999(1):36–41. doi: 10.1016/0167-4838(89)90026-5. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Localization of the two-L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3753–3755. [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Production of L-asparaginase II by Escherichia coli. J Bacteriol. 1968 Dec;96(6):2043–2048. doi: 10.1128/jb.96.6.2043-2048.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derst C., Henseling J., Röhm K. H. Probing the role of threonine and serine residues of E. coli asparaginase II by site-specific mutagenesis. Protein Eng. 1992 Dec;5(8):785–789. doi: 10.1093/protein/5.8.785. [DOI] [PubMed] [Google Scholar]
- Dunphy J. E. Surgery's relevance to an understanding of basic biology. Tissue repair and cellular regeneration. JAMA. 1967 Oct 9;202(2):116–117. [PubMed] [Google Scholar]
- Epp O., Steigemann W., Formanek H., Huber R. Crystallographic evidence for the tetrameric subunit structure of L-asparaginase from Escherichia coli. Eur J Biochem. 1971 Jun 11;20(3):432–437. doi: 10.1111/j.1432-1033.1971.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Greenquist A. C., Wriston J. C., Jr Chemical evidence for identical subunits in L-asparaginase from Escherichia coli B. Arch Biochem Biophys. 1972 Sep;152(1):280–286. doi: 10.1016/0003-9861(72)90216-0. [DOI] [PubMed] [Google Scholar]
- Haskell C. M., Canellos G. P. l-asparaginase resistance in human leukemia--asparagine synthetase. Biochem Pharmacol. 1969 Oct;18(10):2578–2580. doi: 10.1016/0006-2952(69)90375-x. [DOI] [PubMed] [Google Scholar]
- Holcenberg J. S., Ericsson L., Roberts J. Amino acid sequence of the diazooxonorleucine binding site of Acinetobacter and Pseudomonas 7A glutaminase--asparaginase enzymes. Biochemistry. 1978 Feb 7;17(3):411–417. doi: 10.1021/bi00596a005. [DOI] [PubMed] [Google Scholar]
- Jayaram H. N., Cooney D. A., Huang C. Y. Interaction between L-aspartic acid and L-asparaginase from Escherichia coli: binding and inhibition studies. J Enzyme Inhib. 1986;1(2):151–161. doi: 10.3109/14756368609020113. [DOI] [PubMed] [Google Scholar]
- Jennings M. P., Beacham I. R. Analysis of the Escherichia coli gene encoding L-asparaginase II, ansB, and its regulation by cyclic AMP receptor and FNR proteins. J Bacteriol. 1990 Mar;172(3):1491–1498. doi: 10.1128/jb.172.3.1491-1498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer J. F., Moraga D. A., Schuster S. M. Comparison of glycine metabolism in mouse lymphoma cells either sensitive or resistant to L-asparaginase. Biochem Pharmacol. 1985 Feb 15;34(4):559–565. doi: 10.1016/0006-2952(85)90190-x. [DOI] [PubMed] [Google Scholar]
- Lee B., Yang H. J. Crystallographic studies on L-asparaginase from Proteus vulgaris. I. Preliminary crystal data. J Biol Chem. 1973 Nov 10;248(21):7620–7621. [PubMed] [Google Scholar]
- North A. C., Wade H. E., Cammack K. A. Physicochemical studies of L-asparaginase from Erwinia carotovora. Nature. 1969 Nov 8;224(5219):594–595. doi: 10.1038/224594a0. [DOI] [PubMed] [Google Scholar]
- Peterson R. G., Richards F. F., Handschumacher R. E. Structure of peptide from active site region of Escherichia coli L-asparaginase. J Biol Chem. 1977 Mar 25;252(6):2072–2076. [PubMed] [Google Scholar]
- Rogers K. S. L-asparaginase for treatment of lymphoid neoplasia in dogs. J Am Vet Med Assoc. 1989 Jun 1;194(11):1626–1630. [PubMed] [Google Scholar]
- Röhm K. H., Van Etten R. L. The 18O isotope effect in 13C nuclear magnetic resonance spectroscopy: mechanistic studies on asparaginase from Escherichia coli. Arch Biochem Biophys. 1986 Jan;244(1):128–136. doi: 10.1016/0003-9861(86)90101-3. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Reeves J. Y., Broome J. D. Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1516–1519. doi: 10.1073/pnas.56.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Watenpaugh K. D., Sieker L. C., Jensen L. H. The binding of riboflavin-5'-phosphate in a flavoprotein: flavodoxin at 2.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3857–3860. doi: 10.1073/pnas.70.12.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner A., Harms E., Jennings M. P., Beacham I. R., Derst C., Bast P., Röhm K. H. Site-specific mutagenesis of Escherichia coli asparaginase II. None of the three histidine residues is required for catalysis. Eur J Biochem. 1992 Sep 1;208(2):475–480. doi: 10.1111/j.1432-1033.1992.tb17210.x. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Hodgson K. O., Bensch K. Studies of two crystal forms of L-glutaminase-asparaginase from Acinetobacter glutaminasificans. J Mol Biol. 1975 Dec 5;99(2):295–299. doi: 10.1016/s0022-2836(75)80147-1. [DOI] [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]