Abstract
Transcutaneous electrical nerve stimulation (TENS) is a non-invasive, inexpensive, self-administered technique to relieve pain.
There are few side effects and no potential for overdose so patients can titrate the treatment as required.
TENS techniques include conventional TENS, acupuncture-like TENS and intense TENS. In general, conventional TENS is used in the first instance.
The purpose of conventional TENS is to selectively activate large diameter non-noxious afferents (A-beta) to reduce nociceptor cell activity and sensitization at a segmental level in the central nervous system.
Pain relief with conventional TENS is rapid in onset and offset and is maximal when the patient experiences a strong but non-painful paraesthesia beneath the electrodes. Therefore, patients may need to administer TENS throughout the day.
Clinical experience suggests that TENS may be beneficial as an adjunct to pharmacotherapy for acute pain although systematic reviews are conflicting. Clinical experience and systematic reviews suggest that TENS is beneficial for chronic pain.
Introduction
Transcutaneous electrical nerve stimulation (TENS) is a non-invasive peripheral stimulation technique used to relieve pain. During TENS pulsed electrical currents are delivered across the intact surface of the skin to activate underlying nerves. Patients can self-administer TENS and titrate dosage as required because there is no potential for overdose and there are few side effects or drug interactions. Maximal analgesia occurs when TENS generates a strong but non-painful electrical paraesthesia beneath the electrodes. Effects are generally rapid in onset and offset so patients are encouraged to administer TENS as needed and throughout the day. TENS is cheap when compared to long term drug therapy and TENS devices can be purchased over the counter and without medical prescription in the UK. However, a practitioner experienced in TENS principles should supervise patients using TENS for the first time. A point of contact to troubleshoot any problems should also be provided (1, 2).
Historical context
Using electricity for pain relief is an age old technique dating back to the ancient Egyptians (2,500BC) who applied electrogenic fish to painful body parts. In the 18th century electrostatic generators helped to popularise electrotherapy but increasing use of pharmacological treatments meant that use of electrotherapy had declined by the late 19th century. In 1965 interest in the use of electricity to relieve pain was re-kindled following the publication of Melzack and Wall's “Pain Mechanisms: A New Theory”. They suggested that electrical stimuli could be used to activate large diameter peripheral afferents in order to inhibit central transmission of noxious information. Clinical observations confirmed that electrical stimulation of peripheral afferents, dorsal columns and descending pain inhibitory pathways were able to reduce pain in patients. Initially TENS was used to predict the success of dorsal column stimulation implants until it was realised that TENS could be used as a successful modality on its own.
TENS as a Peripheral Stimulation Technique
Definition
By strict definition TENS is any technique that passes electrical currents across the intact surface of the skin to activate underlying nerves. Health care professionals use the term to describe currents generated by a ‘standard TENS device’ (Fig 1). This consists of a battery-powered hand-held stimulating device which generates pulsed electrical currents which are delivered through the skin using electrode pads attached to the skin surface. Users can adjust the pulse amplitude (mA), frequency (pulses per second - pps), width or duration (μs) and pattern of the currents. A variety of TENS-like devices are also available on the market but evidence to support their effectiveness is very limited(3).
Figure 1.
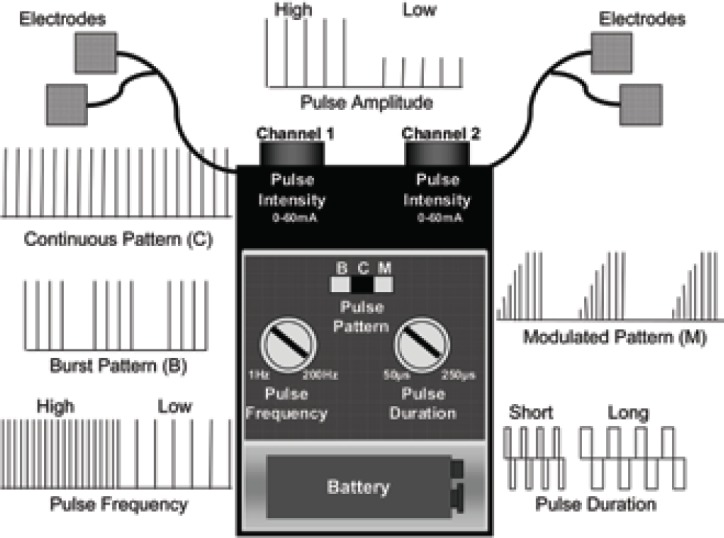
A standard TENS device
TENS techniques
TENS nomenclature focuses on the output of the device rather than the physiological intention of currents. This has led to inappropriate clinical practice. Different TENS techniques are used to selectively activate populations of nerve fibres to elicit mechanisms leading to pain relief (Table 1). The main techniques are:
Table 1.
The characteristics of different TENS techniques
| Physiological Intention | Clinical Technique | |
|---|---|---|
| Conventional TENS | To selectively activate large diameter non-noxious afferents to elicit segmental analgesia | Low-intensity / high-frequency TENS at site of pain to produce “strong but comfortable TENS paraesthesia”. Administer whenever in pain |
| Acupuncture-like TENS | To produce muscle twitches to activate small diameter motor afferents to elicit extrasegmental analgesia | High-intensity / low-frequency TENS over muscles, acupuncture points or trigger points to produce “strong but comfortable muscle contractions”. Administer for 15–30 minutes at a time |
| Intense TENS | To activate small diameter noxious afferents to elicit peripheral nerve blockade and extrasegmental analgesia | High-intensity/high-frequency TENS over nerves arising from painful site to produce “maximum tolerable (painful) TENS paraesthesia”. Administer for a few minutes at a time |
Conventional TENS (low-intensity, high-frequency)
Acupuncture-like TENS (high-intensity, low-frequency)
Intense TENS (high-intensity, high-frequency)
Conventional TENS is most the commonly used technique and for most patients is selected in the first instance.
Conventional TENS
The International Association for the Study of Pain (IASP) describes conventional TENS as “High-frequency (50–100 Hz), low-intensity (paraesthesia, not painful), small pulse width (50–200μs)” (4). The intention of conventional TENS is to stimulate selectively large diameter, low threshold non-noxious afferents (A-beta) in dermatomes related to the pain (Fig 2a). This inhibits activity in second order nociceptive transmission neurones in the central nervous system and is achieved by increasing TENS pulse amplitude to generate a strong, comfortable, non-painful paraesthesia beneath the electrodes. Further increases in pulse amplitude leads to high threshold A-delta afferent activity and a painful paraesthesia beneath the electrodes. This is not the desired outcome for conventional TENS.
Figure 2a.
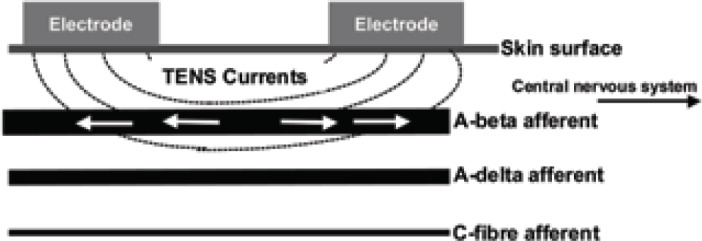
- Conventional TENS.
TENS-induced impulses (arrows) travel towards central and peripheral structures.
Acupuncture-like TENS (AL-TENS)
AL-TENS is a form of hyperstimulation described by Sjölund and colleagues in the 1970s. It can be used if patients do not respond to conventional TENS (5). IASP defines the characteristics of AL-TENS as “Low-frequency (2–4Hz), higher intensity (to tolerance threshold), longer pulse width (100–400μs)” (4). Low-frequency trains or bursts (2–4Hz) of high-frequency pulses (100–200pps) are often used in clinical practice. The intention of AL-TENS is to stimulate small diameter, high threshold peripheral afferents (A-delta) in order to activate extrasegmental descending pain inhibitory pathways. Non-painful muscle twitches occur during stimulation causing activity in small diameter muscle afferents (Fig 2b). Electrodes are positioned over myotomes, trigger points and acupuncture points. The term AL-TENS is used loosely in literature making synthesis of research findings difficult. Patients are advised to administer AL-TENS less frequently than conventional TENS e.g. 20 minutes, 3 times a day (5).
Figure 2b.
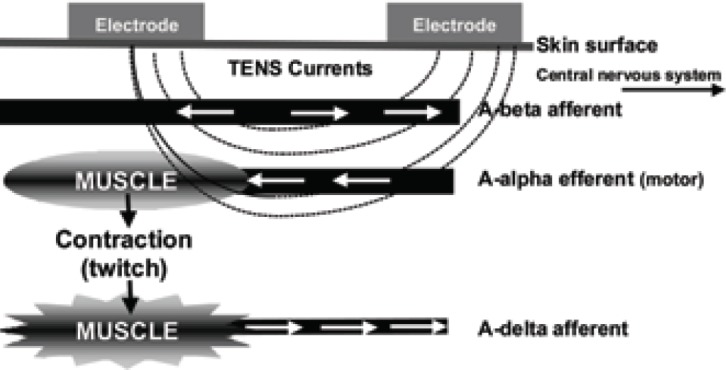
- Acupuncture-like TENS.
Arrows indicate direction of impulses.
Intense TENS
The intention of intense TENS is to stimulate small diameter, high threshold cutaneous afferents (A-delta) to block transmission of nociceptive information in peripheral nerves and to activate extrasegmental analgesic mechanisms. High frequencies (up to 200 pps) and high intensities that are just tolerable to the patient are used but only delivered for short periods of time (Fig 2c). Intense TENS acts as a counter irritant and is useful for minor procedures such as wound dressing and suture removal.
Figure 2c.
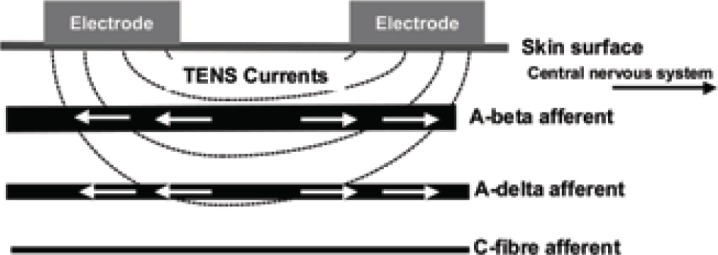
- Intense TENS
TENS-induced impulses (arrows) travel towards central and peripheral structures.
Postulated Mechanisms
Low-intensity, non-noxious TENS paraesthesiae (conventional TENS) relieves pain by a segmental mechanism. Higher intensity TENS increases the likelihood of activating extrasegmental descending pain inhibitory pathways and diffuse noxious inhibitory controls via counter-irritant effects. TENS will also cause peripheral blockade of afferent impulses that have arisen from a peripheral structure (i.e. ‘busy line-effect’) (2, 6).
Segmental mechanisms
Evidence from animal studies shows that TENS reduces ongoing nociceptor cell activity and sensitization in the central nervous system when applied to somatic receptive fields and after spinal cord transection. TENS-induced A-delta activity causes long-term depression of central nociceptor cell activity for up to 2 hours.
Extrasegmental mechanisms
TENS-induced activity in small diameter afferents (A-delta) leads to activation of the midbrain periaqueductal grey and rostral ventromedial medulla (i.e. descending pain inhibitory pathways) and inhibition of descending pain facilitatory pathways. Larger effects have been observed when muscle rather than skin afferents were activated.
Peripheral mechanisms
Antidromic activation of peripheral nerves by TENS generates nerve impulses that have been shown to collide and extinguish afferent impulses arising from peripheral structures. Peripheral blockade of nociceptive impulses is more likely when TENS activates A-delta fibres (i.e. intense TENS). TENS-induced activity in large diameter afferents (i.e. conventional TENS) will block afferent activity in large diameter fibres that may be contributing to pain.
Neurochemicals
TENS effects are mediated by many neurochemicals including opioids, serotonin, acetylcholine, noradrenaline and gamma-aminobutyric acid (GABA) (6). Low but not high-frequency TENS has been shown to involve mu opioid and 5-HT2 and 5-HT3 receptors. High but not low-frequency TENS has been shown to involve delta opioid receptors and reduce aspartate and glutamate levels in the spinal cord (6).
Whether the physiological effects observed in animal experiments translate into meaningful effects in humans is a matter of debate. Evidence from experimental pain studies using pain-free volunteers suggests that TENS hypoalgesia is greater than sham TENS but the relationship between pulse frequency and outcome is equivocal (Fig 3).
Figure 3.
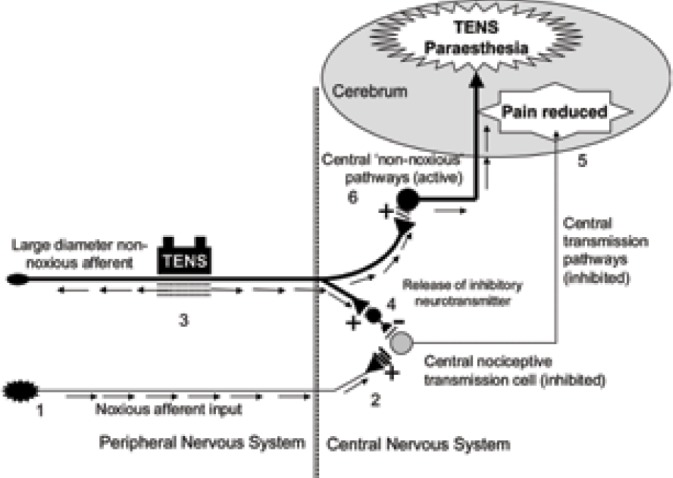
Activity in peripheral nociceptive afferents [1] releases excitatory neurotransmitters causing activity in second order nociceptive transmission cells in the central nervous system [2] which leads to pain. Activation of large diameter non-noxious afferents by TENS [3] causes the release of inhibitory neurotransmitters which reduce activity in second order nociceptive transmission cells [4]. The reduction in nociceptive input to the brain results in pain relief [5]. TENS induced activity in non-noxious transmitting pathways in the central nervous system results in a sensation of electrical paraesthesiae [6].
Clinical Application
New TENS patients should undergo a pain assessment and should be given clear, simple instructions on the safe use of TENS. The patient should be observed using TENS to ensure competence. Conventional TENS is used in the first instance starting with a continuous pulse pattern, and ‘mid-range’ pulse frequencies (e.g. 80pps-100pps) and durations (100–200μs). However, patients should be encouraged to take a trial and error approach on each treatment session to establish the most comfortable pattern, frequency and duration for their pain at that moment in time. Maximal pain relief occurs in the presence of a strong but comfortable electrical paraesthesia so TENS should be delivered as often as needed throughout the day.
Location of electrodes
Electrodes must be positioned on healthy sensate skin which should be checked prior to application. Accurate placement of pads can be time-consuming. Electrodes are positioned on relevant dermatomes so that paraesthesia can be directed into the painful area. Exceptions include:
Hyperaesthesia and mechanical allodynia because TENS may aggravate pain
Hypoaesthesia because TENS is not effective in non sensate skin and may cause skin irritation due to accidental use of excessively high intensities
Absence of a body part (i.e. phantom pain), damaged or frail skin (e.g. wounds, eczema)
In these situations electrodes are positioned along the main nerves proximal to the site of pain in the first instance. Electrodes can also be positioned paravertebrally at spinal segments related to the pain or at contralateral ‘mirror’ sites. Dual channel TENS devices with 4 electrodes are used for large or multiple pains.
Inappropriate electrode positions include:
Anterior neck because carotid sinus stimulation may cause a hypotensive response and laryngeal nerve stimulation may cause a laryngeal spasm
Over the eyes because it may increase intraocular pressure
Through the chest (anterior and posterior positions) because stimulation may affect electrical conductivity of the heart and interfere with intercostal muscle activity
Internally except using devices designed for dental, vaginal and anal stimulation.
The position of the red and black lead usually has little effect in clinical practice as biphasic waveforms with zero net current flow are often used in devices.
Electrical Characteristics
Pulse amplitude is the key determinant of response because of its relationship to fibre recruitment. Patients should titrate pulse amplitude and use TENS sensation to establish the type of fibre activated. Non-painful paraesthesia indicates large diameter cutaneous afferent activity, painful paraesthesia indicates small diameter cutaneous afferent activity and muscle twitching small diameter muscle afferent activity.
Claims about differential effects according to pulse frequencies, durations and patterns of TENS are based on poor quality research which fails to isolate the influence of individual parameters per se. Theoretically, selective activation of A-beta fibres is best achieved using frequencies of 10–200pps and durations of 50–200μs. Also, large diameter fibres have short refractory periods so they are able to generate higher rates of nerve impulses in response to higher frequencies of TENS and this may result in more profound inhibition of central nociceptive transmission. Longer pulse durations are sometimes used during AL-TENS and intense TENS because they activate small diameter fibres at lower pulse amplitudes. In practice a trial and error approach is used to establish pulse pattern, frequency and duration based on the patient's report of comfort.
Analgesic time course and dosing
Clinical experience suggests that pain relief with conventional TENS is rapid in onset and offset, although post-TENS effects vary considerably in reports and may be due to natural fluctuations in symptoms and patient expectation rather than TENS hypoalgesia.
Long term depression of central nociceptive cells and activation of descending pain inhibitory pathways is more likely to contribute to post-TENS analgesia at higher intensities of TENS. TENS effects may decline over time due to habituation to TENS or a worsening pain problem. Experimenting with electrode placements and TENS settings or temporarily withdrawing TENS treatment may resolve the problem.
Contraindications, precautions and adverse events
Manufacturers list cardiac pacemakers, pregnancy and epilepsy as contraindications because it may be difficult to exclude TENS as a potential cause of a problem from a legal perspective. It is possible to use TENS for these patients providing the advice of the appropriate medical specialist has been sought and electrodes are not applied over the chest, abdomen and head or neck respectively. Electrodes should not be positioned over an active tumour for a patient whose tumour is treatable. Decisions are left to the discretion of the medical practitioner and progress needs to be very carefully monitored. TENS should not be delivered close to transdermal drug delivery systems.
Serious adverse events from TENS are rare. Skin irritation and contact dermatitis beneath the electrodes may occur so attention to skin care is important. Nausea and feeling faint may occur in some patients. Children as young as 4 years can tolerate TENS but patients with cognitive impairment may not be able to comprehend instructions. TENS should not be used while operating motor vehicles. TENS can be used whilst going to sleep providing the device has a timer so that it automatically switches off.
Efficacy
Clinical experience suggests that TENS is useful for acute and chronic pain of nociceptive, neuropathic and musculoskeletal origin. Many of the 400 clinical trials cited in PubMed [28 March 2007] have methodological shortcomings.
TENS and acute pain
Opinion is divided about the effectiveness of TENS for acute pain. TENS is of limited benefit as a stand alone treatment for severe to moderate pain but maybe useful in combination with pharmacotherapy. Systematic reviews on TENS and postoperative pain and labour pain have reported negative outcomes (7, 8). A meta-analysis demonstrated that TENS reduced post-operative analgesic consumption and that adequate TENS technique was critical to outcome (9). One Cochrane review concluded that high but not low-frequency TENS was superior to sham at reducing pain associated with primary dysmenorrhoea (10). RCTs suggest that TENS may be beneficial for acute orofacial pain, painful dental procedures, fractured ribs, acute lower back pain. TENS can be used for angina by placing electrodes over the painful area of the chest.
TENS and chronic pain
Clinical experience suggests that TENS may be useful for any type of chronic pain. RCTs suggest that TENS is beneficial for localised muscle pain, post-herpetic neuralgia, trigeminal neuralgia, phantom limb and stump pain, diabetic neuropathies and entrapment neuropathies, radiculopathies (cervical, thoracic and lumbar), complex regional pain syndromes type I (reflex sympathetic dystrophy) and type II (causalgia). TENS may also be beneficial for cancer and cancer related pain. Cochrane reviews on chronic low back pain, rheumatoid arthritis of the hand, whiplash and mechanical neck disorders, post-stroke shoulder pain and chronic recurrent headache are inconclusive. One Cochrane review reported that TENS relieved pain and stiffness associated with knee osteoarthritis (294 patients, (11)). A recent meta-analysis of transcutaneous and percutaneous electrical nerve stimulation included 38 studies and concluded that electrical nerve stimulation was an effective treatment for chronic musculoskeletal pain (12).
References
- 1.Walsh D. (1997). TENS. Clinical applications and related theory. 1st ed. New York: Churchill Livingstone. [Google Scholar]
- 2.Johnson MI. (2002). Transcutaneous Electrical Nerve Stimulation. In: Kitchen S, editor. Electrotherapy: Evidence-Based Practice. Edinburgh: Churchill Livingstone, 259–286. [Google Scholar]
- 3.Johnson MI. (2003) Pain Reviews, 8, 121–128. [Google Scholar]
- 4.Charlton J. (2005) Core Curriculum for Professional Education in Pain. 3rd ed. Seattle: IASP press, 93–96. [Google Scholar]
- 5.Johnson MI. (1998) Physical Therapy Reviews, 3, 73–93. [Google Scholar]
- 6.Sluka KA, Walsh D. (2003) J Pain, 4, 3, 109–21. [DOI] [PubMed] [Google Scholar]
- 7.Carroll D, Tramer M, McQuay H, et al. (1996) Br J Anaesth, 77, 6, 798–803. [DOI] [PubMed] [Google Scholar]
- 8.Carroll D, Tramer M, McQuay H, et al. (1997) Br J Obstet Gynaecol, 104, 2, 169–75. [DOI] [PubMed] [Google Scholar]
- 9.Bjordal JM, Johnson MI, Ljunggreen AE. (2003) Eur J Pain, 7, 2, 181–8. [DOI] [PubMed] [Google Scholar]
- 10.Proctor ML, Smith CA, Farquhar CM, et al. (2003) Cochrane Database of Systematic Reviews, 1, CD002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osiri M, Welch V, Brosseau L, et al. (2000), Cochrane Database of Systematic Reviews, 4, CD002823. [DOI] [PubMed] [Google Scholar]
- 12.Johnson M, Martinson M. (2007) Pain, 130, 1–2, 157–65. [DOI] [PubMed] [Google Scholar]


