Abstract
Case reports and preclinical data suggest radiotherapy and immunotherapy may synergize to generate “abscopal” responses outside the radiation field. This phenomenon remains relatively unexplored, prompting our systematic evaluation of metastatic melanoma patients treated with the CTLA-4 inhibitor ipilimumab and palliative radiation therapy. We evaluated 47 consecutive metastatic melanoma patients treated with ipilimumab and 65 courses of radiation. Responses of index lesions outside the radiation field were compared before and after radiotherapy, and parameters associated with favorable response were assessed. Median survival was 28 months, with an estimated 20% 5-y survival. Index lesions shrank in 7 instances prior to radiation therapy (11%), compared with 16 instances (25%) after radiation therapy; in 11 of the latter instances (69%), the index lesion had been increasing in size prior to radiotherapy (P = 0.03). In 68% of cases, radiotherapy was associated with an improved rate of index lesion response (P = 0.006). Radiation fraction size ≤ 3 Gy was the only parameter identified associated with favorable index lesion response (P = 0.014). Our systematic review of melanoma patients treated with radiotherapy and ipilimumab suggests that a subset of patients may have more favorable out-of-field responses following treatment with radiation. Interestingly, we found that multiple fraction radiation regimens were associated with a more favorable response. These results are encouraging regarding potential synergies between radiation and immunotherapy, but suggest that attention and even prospective testing of radiation parameters critical to producing abscopal effects in human patients would be of value.
Keywords: abscopal effect, immunotherapy, ipilimumab, melanoma, radiation therapy
Abbreviations
- (Gy)
gray
- (IMRT)
intensity-modulated radiation therapy
- (IQR)
interquartile range
Introduction
Malignant melanoma remains a devastating disease despite recent advances in treatment. This year, an anticipated 76,000 new cases will be diagnosed, with 9,710 associated deaths.1 Prior to the development of the CTLA-4 inhibitor ipilimumab, no therapy had consistently improved overall survival in patients with metastatic melanoma, 2-4 and prognosis was dismal with median overall survival less than 1 y and 5 y overall survival under 10%.5 However, the success of ipilimumab and other forms of immunotherapy heralds a new era in more effective melanoma treatment.2
Palliative radiotherapy is often indicated in metastatic melanoma patients, and, at least in the case of intracranial metastases, may confer a survival benefit in patients treated with ipilimumab.6 Small case series and preclinical data suggest that, in select patients, combined use of ipilimumab and radiotherapy may synergize via immune-mediated mechanisms.7-12
Of particular interest is the possibility that targeted radiation and immunotherapy may synergize to result in abscopal effects, defined as tumor regression in response to radiation at a site distant from the radiation treatment field.13 In light of the aforementioned observations of abscopal responses in practice,7,8,12 there is growing interest in quantifying the frequency of this effect, and the radiotherapy parameters associated with its occurrence. No large cohort studies addressing these questions have been undertaken; therefore, the optimal design of prospective trials designed to capitalize on this effect is uncertain. Consequently, we sought to systematically evaluate abscopal responses to radiotherapy in a large cohort of patients at our institution who had metastatic melanoma treated with ipilimumab.
Results
Patient characteristics and survival
Table 1 illustrates clinical characteristics for the 47 individuals who were treated with the combination of radiation and ipilimumab. The majority of the cohort was male (72%) and patients were diagnosed with metastatic melanoma at a median age of 57 (interquartile range, IQR: 50–65). BRAF mutations were present in 14 patients' tumors (30%). The median number of cycles of ipilimumab received was 4 (range: 1–18). There were eight patients that received additional cycles of ipilimumab: one on a protocol and seven others at their physicians' discretion (generally because of eventual progression after initial response). Four patients received ipilimumab at a dose of 10 mg/kg because they were treated with ipilimumab on an investigational protocol; the remainder received a dose of 3 mg/kg. The median number of radiotherapy courses per patient was 2 (range: 1–8). With a median follow-up of 24 months (range: 24–112 months), the median survival was 28 months (IQR: 15–48 months, Fig. 1). There was an estimated 5-y survival of 20%. Ten patients (21%) were alive at most recent follow-up.
Table 1.
Description of the 47 individuals with metastatic melanoma treated with ipilimumab and 65 courses of radiation therapy
| Clinical factor | n = 47 |
|---|---|
| Median age at metastatic diagnosis, year (IQR) | 57 (50, 65) |
| Gender (male, female) | (34, 13) |
| Median number of ipilimumab cycles administered (range) | 4 (1, 18) |
| Median number of RT courses administered (range) | 2 (1,8) |
Abbreviations: IQR, interquartile range; RT, radiation therapy.
Figure 1.
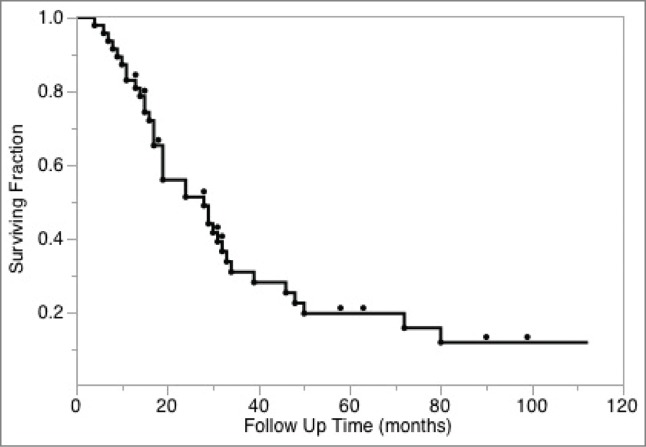
Kaplan-Meier analysis of overall survival of study cohort.
Radiotherapy treatment courses
The 47 identified patients received 65 courses of palliative radiotherapy. The majority of patients received either one (38%) or two (23%) courses of radiation, and only 13% of patients received more than three courses. In 53% of cases radiation treatments were administered within three months of a dose of ipilimumab. In 25 instances (22 patients) ipilimumab was also given following radiation treatment.
The median radiotherapy dose delivered across all sites irradiated was 26 Gy (range: 8–68 Gy) and the median fraction size was 4 Gy (range: 1.8–25 Gy). In a minority of instances (11%), two palliative sites were treated concurrently (Table 2).
Table 2.
Characteristics of the radiation courses included on index lesion analysis (n = 65)
| (n = 65 ) | |
|---|---|
| Total RT dose, median (range), Gy | 30 (8, 66) |
| RT fraction size, median (range), Gy | 3 (2, 25) |
| Use of multiple RT fields, % | 11 |
| Median time between RT and ipilimumab, months | <1 |
Abbreviations: Gy, Gray; RT, radiation therapy.
The irradiated sites are summarized in Table 3. The most common type and site of radiation administered was stereotactic radiosurgery performed on intracranial lesions (34% of radiotherapy courses), followed by whole brain radiation (WBRT) (19%), and radiation to metastatic soft tissue deposits (17%). There were no significant associations between the time elapsed from the diagnosis of metastatic disease and either site irradiated, radiation fraction size and total radiation dose administered.
Table 3.
Radiation therapy courses delivered by treatment field
| Radiation treatment field | # of RT courses (%) | Total RT dose, median (range), Gy | RT fraction size, median (range), Gy |
|---|---|---|---|
| Whole brain | 15 (23) | 30 (30, 37.5) | 3 (2.5, 3) |
| Brain directed stereotactic radiosurgery / therapy | 18 (28) | 20 (18, 25) | 19.5 (5, 25) |
| Spine | 7 (11) | 30 (20, 37.5) | 3 (2, 4) |
| Intrathoracic | 3 (5) | 24 (24, 30) | 4 (3, 4) |
| Bone | 8 (12) | 30 (8, 36) | 3.5 (3, 8) |
| Soft tissue | 13(20) | 35 (24, 66) | 3 (2, 6) |
| Abdominovisceral | 1 (2) | 36( 36, 36) | 3 (3, 3) |
Abbreviations: Gy, Gray; RT, radiation therapy.
Analysis of treatment response
Characteristics of the 65 radiation courses included in the analysis are shown in Tables 2 and 3. The median timing of restaging imaging was 33 d prior to radiation therapy and 81 d following radiation. The median time between the first dose of ipilimumab and restaging performed prior to radiation was 93 d. Index lesions were typically intrathoracic (51%) or abdominovisceral (34%) (Table S1). There were no appreciable differences in the location and size of index lesions between the stereotactic, whole brain, and other patient groups (P = 0.24 and 0.8, respectively, Table S2). Mean size of index lesions was 4 cm in both the SRS and non-SRS groups. Index lesions shrank in 7 instances prior to radiation therapy (11%) as compared with 16 instances (25%) after radiation therapy; in 11 of these 16 instances (69%) the index lesion had been increasing in size prior to radiotherapy (P = 0.03). Radiotherapy was also associated with an improved rate of index lesion response (“delta–delta”) in 68% of cases (P = 0.006). In contrast, there was no association between improved rate of index response and time elapsed from diagnosis of metastatic disease or time elapsed from first cycle of ipilimumab administered. There was also no association between the temporal proximity of the first dose of ipilimumab to radiation and the response of the index lesions.
Radiation and clinical parameters including BRAF mutational status, total radiation dose, site irradiated, timing of ipilimumab in relation to radiation therapy, receipt of ipilimumab following radiation, and treatment of multiple radiation fields were tested for their association with a favorable change in rate of index lesion response. The only parameter associated with favorable response was radiation fraction size (Fig. 2). A radiation fraction size ≤ 3 Gy was associated with an improved rate of index lesion response as compared with hypofractionated treatment including stereotactic radiosurgery/therapy (81% favorable as compared with 52%, P = 0.014). The association between fraction size and response remained significant even if stereotactic radiosurgery/therapy patients were excluded from the analysis (Table S2). In the 47 cases of non-stereotactic radiosurgery/therapy treatments, there were 29/36 instances with favorable index lesion velocity following ≤ 3 Gy fractions compared with 5/11 instances of favorable index lesion velocity following >3 Gy fractions, P = 0.02 In contrast, in the stereotactic group, the rate of favorable velocity change was 10/18 (56%, P = 0.8), although power to detect a favorable impact of radiation on response rate was more limited in this subgroup. The association between fraction size and favorable change in response rate remained significant on multivariate analysis (P = 0.027) adjusting for total radiation dose, site irradiated, timing of ipilimumab in relation to radiation therapy, and time from diagnosis to radiation treatment.
Figure 2.
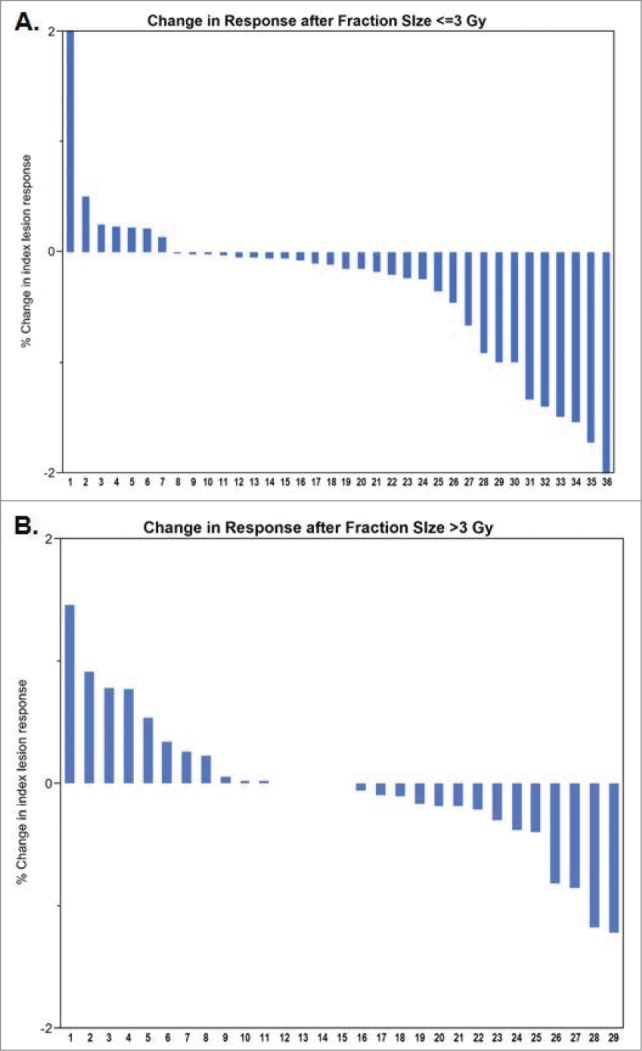
Change in index lesion response by fraction size.
Discussion
Our evaluation of patients treated with the combination of radiation and ipilimumab is notable in several respects. First, we present the largest systematic analysis of abscopal responses to radiotherapy in a cohort of metastatic melanoma patients treated with ipilimumab. Prior evidence of these responses is limited to case reports and smaller retrospective cohorts.7,8,12 In our cohort, more than two-thirds of instances in which index lesions showed a favorable response occurred after radiotherapy. The majority of these index lesions had been progressing prior to radiotherapy. Additionally, radiotherapy was associated with an improved rate of index lesion response in the majority of cases. Second, we report a median overall survival of 28 months, which compares favorably with an 11.4 month median overall survival reported in the most recent extended follow-up and pooled analysis of ipilimumab trials.14 Our data confirm that long-term survival with ipilimumab can be achieved in clinical practice, as evidenced by our estimated 5 y overall survival of 20%. Although our favorable results may relate to patient selection, 23% of the population received WBRT, and 16 were treated on a compassionate use protocol. We do think it is notable that in this relatively poor prognosis group median survival following WBRT was 17 months in the setting of ipilimumab therapy.
In our exploration of predictors of favorable response following radiation therapy among the 65 cases, we found no association between abscopal responses and either timing of ipilimumab in relation to radiotherapy, or duration from first dose of ipilimumab to initiation of radiotherapy. Interestingly, the parameter that we observed to be significantly associated with abscopal responses was multiple fraction regimens, specifically with radiation fraction size ≤3 Gy.
While the biological mechanism underlying abscopal effects remains unproven, it likely hinges on radiation-induced immunomodulation.15 Preclinical studies have shown that ionizing radiation contributes to systemic antitumor immunity by inducing a variety of changes in tumor cells, including increased chemokine production, antigen release, and MHC class I expression.16,17 Accordingly, a mouse model of breast carcinoma demonstrated abscopal responses only after in vivo dendritic cell expansion, a result that furthermore proved to be T cell-dependent.9
Radiation-induced immunomodulation also likely provides the conduit for synergy between radiotherapy and immunotherapy, which to date has been most clearly observed with CTLA-4 inhibitors, such as ipilimumab.18,19 For example, in mice with breast carcinoma, combined treatment with radiotherapy and anti-CTLA-4 antibodies resulted in improved systemic antitumor responses and prolonged overall survival.10 Case reports have shown similar radiation-induced immune phenomena in patients with metastatic melanoma treated with combined radiation and ipilimumab, with decreased numbers of inhibitory myeloid-derived suppressor cells and increased levels of circulating antitumor antibodies and activated T cells.7,8,20
Preclinical studies have demonstrated mixed results with regard to the optimal fractionation for use in conjunction with immunotherapy. In one mouse model of breast carcinoma, an ablative dose of radiation (15–20 Gy delivered in a single fraction), but not a more prolonged fractionation scheme (20 Gy delivered in four fractions over 2 weeks), resulted in enhanced abscopal, CD8+ T cell-dependent responses, with synergy noted upon the addition of an immunotherapeutic.21 Contrastingly, in the mice with breast carcinoma treated with combined radiotherapy and anti-CTLA-4 antibodies, abscopal responses were observed only with fractionated radiation (24 Gy delivered in three fractions or 30 Gy delivered in five fractions, each given in consecutive days) as opposed to a single ablative dose of 20 Gy.11
In light of these conflicting results, it is important to note that there are inherent limitations to extrapolating conclusions drawn from animal models regarding equivalent radiation dosing in human patients. It is difficult to equate radiation doses across species,22 especially as dose parameters are dependent on the absolute size and number of cells that are being targeted. Moreover, ideal radiation fractionation will likely vary based on tumor type and individual patient characteristics. That said, our findings may relate to previous observations pertaining to vaccines aimed at boosting CD8+ T cell immunity. In mice, sequential immunizations have been shown to generate more antigen-specific CD8+ T cells than a single administration.23 A similar immunologic phenomenon may underlie our observed association between sequentially delivered, fractionated radiation and abscopal responses. It is worth noting that the abscopal responses in human patients that have been described in the literature have all used multifraction radiation regimens.7,8,20,24 Additionally, in a recent phase III trial, patients with metastatic castration-resistant prostate cancer received a single 8 Gy fraction of palliative radiation and were then randomized to either ipilimumab or placebo. Significance was not reached for the primary endpoint of overall survival despite signs of drug activity that were observed.25
The optimum timing and sequencing of immunomodulation and radiation therapy in humans is undefined. Because the effects of ipilimumab and other types of immunotherapy can be both delayed in onset and prolonged over many years,14,26 a waning immune response could potentially be boosted by radiation therapy even some time after the drug is discontinued. Despite our observation that the sequencing of the two treatments did not change the likelihood of a more favorable response, which treatment is given first may have implications for what immunologic mechanism predominates. For example, radiation given prior to ipilimumab could ostensibly liberate antigen and recruit T-cells to the tumor microenvironment as a priming event, which would later be amplified by checkpoint blockade. In the case of ipilimumab delivered first, radiotherapy could boost immunogenic cell death, as the host would have tumor-reactive T cells activated by initial treatment with checkpoint blockade. Further studies will be needed to understand and exploit such mechanisms.
There are potential limitations to our study. It is unclear if our results are generalizable to the treatment of other malignancies besides melanoma, or the use of other types of immunotherapeutic agents, such as those that inhibit the programmed death (PD)-1 axis. Given that our study was retrospective, we cannot account for all potential sources of bias. Patients could have received heterogeneous treatments including surgery, chemotherapy, targeted therapy, immunotherapy, and other experimental agents at other points in their care. We were also unable to comprehensively determine overall and immune-related response following radiation given this would potentially require an additional sequential scan following the completion of treatment. Additionally, immune response in the setting of radiation therapy targeting a variable number of lesions has not been rigorously defined, precluding standardized assessment. However, supporting our hypothesis that changes in index lesions may be indicative of an overall favorable response, there was a trend toward improved survival following radiation in instances with favorable index lesion velocity (hazard ratio for death 0.6, P = 0.08).
Although our data favor the use of fractionated radiation in promoting abscopal responses, we are unable to recommend a specific regimen for this purpose due to a lack of patients receiving stereotactic body radiotherapy and alternative and specifically intermediate radiation fractionation regimens for comparison, such as 24 Gy in three fractions or 30 Gy in five fractions, the regimens associated with robust abscopal responses in mice.11 Conversely, heterogeneity of radiation dose and fractionation also makes it difficult to draw conclusions about radiation dose fractionation; however, among patients with favorable index lesion responses following radiation, only the minority had received prior course of radiation (38%), and even fewer had received treatments with a different radiation fractionation (25%).
We used pre-radiotherapy imaging as a control. The fact that out-of-field responses were less prominent in patients that received larger fraction sizes also served as an internal control that argues against systematic bias. Although delayed responses can be seen after immunotherapy,26 in our study the response of index lesions was not associated with time from initial ipilimumab administration on univariate analysis, and the association we observed between radiation fractionation and favorable response rate remained significant on multivariate analysis adjusting for time; therefore, this too is unlikely to be a source of bias.
In summary, our study demonstrates significantly better systemic responses in patients with metastatic melanoma treated with ipilimumab and radiotherapy. Nonetheless, we demonstrate the abscopal effect remains a rare clinical entity even in the setting of CTLA-4 blockade, and, therefore, further studies aimed at increasing the frequency of out-of-field responses driven by the synergistic effects of radiation and immunotherapy are warranted. Our study comprehensively evaluated a cohort of melanoma patients treated with ipilimumab and radiotherapy and, enabled the identification of multiple fraction regimens, specifically with radiation fraction size ≤3 Gy, as a potential therapeutic parameter of interest. Prospective studies should evaluate different radiation fractionation regimens to optimize the synergistic effects of radiation and immunotherapy. Additionally, as ongoing phase II trials examining combined treatment with radiotherapy and ipilimumab accrue, it is worth noting that radiation fraction size may influence findings.
Materials and Methods
Patient population
We retrospectively identified 47 consecutive patients with metastatic melanoma who were treated with both ipilimumab and radiation therapy at our institution from October 2007 to June 2014. Patients were identified from a comprehensive database of all patients treated with palliative radiotherapy within our institution dating back to 2007. Patients treated after June 2011 received radiotherapy from our radiation oncology department's dedicated palliative care service. To be included in this analysis, patients needed to have received ipilimumab prior to restaging scans performed after palliative radiotherapy so that we could assess the systemic effects of the two treatments administered. Patients who received adjuvant radiation with definitive intent following resection of localized or locoregionally advanced melanoma were not included in this study.
The 47 identified patients received a total of 65 courses of palliative radiation treatment. A radiation course was defined as a sequential series of radiation treatments to one or more lesions delivered at the same time. Radiation fields were, in general, designed with the aid of a CT simulator, administered with a linear accelerator and delivered using conventional treatment with individualized blocking, conformal radiotherapy, or intensity-modulated radiation therapy (IMRT), as clinically appropriate. Stereotactic radiosurgery or fractionated stereotactic radiotherapy was generally given for patients with four or less brain metastases for which urgent radiation was not indicated. The use of WBRT was recommended for patients for whom the competing risk of the development of elsewhere brain metastases was high relative to the extracranial disease progression risk. The decision to use WBRT or not in these contexts was done after thorough discussion with individual patients regarding their preferences, however. When used, stereotactic radiosurgery was performed on individual brain lesions using a Novalis linear accelerator-based radiosurgery platform (Varian Medical Systems, Palo Alto, CA). Before 2009, all patients were immobilized with the use of a fixed head frame; starting in April 2009, a frameless approach using the thermoplastic BrainLAB immobilization system (BrainLAB, Inc.., Westchester, IL) was used.
Restaging was generally performed before initiating ipilimumab therapy and then approximately every three months thereafter or as clinically indicated. Ipilimumab was typically administered intravenously every 3 weeks for four cycles, and then, in select patients (n = 10), as maintenance every 12 weeks. Patients were followed regularly by medical oncology and radiation oncology as appropriate. This study was approved by the Dana Farber/Harvard Cancer Center Institutional Review Board.
Response assessment
Overall survival was calculated from the date patients were diagnosed with metastatic melanoma using the Kaplan-Meier method (Fig. 1). To assess “out-of-field” responses potentially impacted by the combination of ipilimumab and radiation therapy, we sought to evaluate serial responses of the largest “index” lesion outside the radiation treatment field for each of the 65 courses of palliative radiation reviewed. Individual courses were analyzed as opposed to patient level data since patients received multiple radiation courses over time.
To assess out-of-field responses, we abstracted the largest diameter of index lesions from radiology reports or, rarely, by direct measurement of images reviewed on our institutional PACS system. The difference in the largest diameters of the index lesions were calculated over two time intervals (Fig. S1). The first interval compared the size of the index lesion on the two consecutive imaging studies performed prior to radiation therapy. The second interval compared the imaging study performed immediately prior to radiation with the first imaging performed after radiation therapy. A favorable response was defined as any absolute decrease in the size of the index lesion.
Statistical methods
For each index lesion, we tabulated the number of favorable responses before and after radiation therapy and compared these responses using McNemar's test, which compares concordance between two states based on an exposure of interest. In this case, favorable versus non-favorable responses were compared in relation to the timing of radiotherapy. We also calculated the difference in response on the two imaging studies prior to radiation as compared to imaging performed before and after radiation therapy by calculating the difference between the percent changes in index lesion diameter (“delta–delta”). We analyzed favorable changes in these percent differences using the binomial test. Finally, we used Pearson's chi-squared test, Pearson's correlations, and univariate and multivariate logistic regression to assess associations between the time elapsed from initial diagnosis of metastatic disease, a favorable change in response rate of index lesions, and radiation treatment parameters (timing in relation to ipilimumab administration, fraction size, site irradiated, and total radiation dose administered). Clinical parameters of interest were included in our multivariate model (radiation dose, radiation fraction size, site irradiated, timing of ipilimumab in relation to radiation therapy, and time from diagnosis to radiation treatment) regardless of their univariate significance. A two-sided P value of less than 0.05 was considered significant in all cases. JMP version 11.0 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the pub-lisher's website.
References
- 1.Siegel R, Jiemin M, Zhaohui Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2013; 64:9-29; PMID:24399786; http://dx.doi.org/ 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Eggermont AMM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer 2011; 47:2150-7; PMID:21802280; http://dx.doi.org/ 10.1016/j.ejca.2011.06.052 [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/ 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 5.Balch CM, Buzaid AC, Soong SJ et al.. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19:3635-48; PMID:1150475 [DOI] [PubMed] [Google Scholar]
- 6.Knisely JPS, Yu JB, Flanigan J Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012; 117:227-33; PMID:22702482; http://dx.doi.org/ 10.3171/2012.5.JNS111929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E et al.. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366:925-31; PMID:22397654; http://dx.doi.org/ 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamell EF, Wolchok JD, Gnjatic S Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85:293-5; PMID:22560555; http://dx.doi.org/ 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demaria S, Ng B, Devitt ML Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune-mediated. Int J Radiat Oncol Biol Phys 2004; 58:862-70; PMID:14967443; http://dx.doi.org/ 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 10.Demaria S, Kawashima N, Yang AM Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005; 11:728-34; PMID:15701862 [PubMed] [Google Scholar]
- 11.Dewan MZ, Galloway AE, Kawashima N Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15:5379-88; PMID:19706802; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, Giugliano FM, Sandomenico F, Petrillo A, Curvietto M et al.. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014; 3:e28780; PMID:25083318; http://dx.doi.org/ 10.4161/onci.28780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mole RH. Whole body irradiation: radiobiology or medicine? Br J Radiol 1953; 26:234-41; PMID:13042090; http://dx.doi.org/ 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 14.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015. Jun 10; 33(17):1889-94. doi: 10.1200/JCO.2014.56.2736. Epub 2015 Feb 9. PMID: 2566729518713980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake C: Radiation-induced immune modulation, in DeWeese TL, Laiho M (eds.): Molecular determinants of radiation response. New York, NY, Springer, 2011, 251-63; http://dx.doi.ogr/1007/978-1-4419-8044-1. [Google Scholar]
- 16.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML et al.. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181:3099-107; PMID:18713980; http://dx.doi.org/ 10.4049/jimmunol.181.5.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reits E, Hodge J, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J et al.. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunity. J Exp Med 2006; 203:1259-71; PMID:16636135; http://dx.doi.org/ 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst 2013; 105:256-65; PMID:23291374; http://dx.doi.org/ 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy A, Chargari C, Cheminant M, Simon N, Bourgier C, Deutsch E. Radiation therapy and immunotherapy: Implications for a combined cancer treatment. Crit Rev Oncol Hematol 2012; 85:278-87; PMID:23036459; http://dx.doi.org/ 10.1016/j.critrevonc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 20.Teulings HE, Tjin EPM, Willemsen KJ. Radiation induced melanoma-associated-leukoderma, systemic anti-melanoma immunity and disease-free survival in an advanced stage melanoma patient: A case report and immunological analysis. Br J Dermatol 2013; 168:733-8; PMID:23421690; http://dx.doi.org/ 10.1111/bjd.12136 [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T et al.. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009; 114:589-95; PMID:19349616; http://dx.doi.org/ 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM et al.. Animal models for medical countermeasures to radiation exposure. Radiat Res 2010; 173:557-78; PMID:20334528; http://dx.doi.org/ 10.1667/RR1880.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatsis N, Lin S, Harris-McCoy K Garber DA, Feinberg MB, Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology 2007; 367:156-67; PMID:17590405; http://dx.doi.org/ 10.1016/j.virol.2007.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden EB, Demaria S, Schiff PB Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013; 1:365-72; PMID:24563870; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon E, Drake C, Scher H, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H et al.. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15:700-12; PMID:24831977; http://dx.doi.org/ 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G et al.. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 2009; 15:7412-20; PMID:19934295; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


