Abstract
Parkinson disease (PD), once considered as a prototype of a sporadic disease, is now known to be considerably affected by various genetic factors, which interact with environmental factors and the normal process of aging, leading to PD. Large studies determined that the hereditary component of PD is at least 27%, and in some populations, single genetic factors are responsible for more than 33% of PD patients. Interestingly, many of these genetic factors, such as LRRK2, GBA, SMPD1, SNCA, PARK2, PINK1, PARK7, SCARB2, and others, are involved in the autophagy-lysosome pathway (ALP). Some of these genes encode lysosomal enzymes, whereas others correspond to proteins that are involved in transport to the lysosome, mitophagy, or other autophagic-related functions. Is it possible that all these factors converge into a single pathway that causes PD? In this review, we will discuss these genetic findings and the role of the ALP in the pathogenesis of PD and will try to answer this question. We will suggest a novel hypothesis for the pathogenic mechanism of PD that involves the lysosome and the different autophagy pathways.
Keywords: autophagy, GBA, genetics, LRRK2, LAMP2A, lysosome, mitophagy, Parkinson disease, SNCA
Abbreviations
- ALP
autophagy-lysosome pathway
- ATP
adenosine triphosphate
- ATG5
autophagy-related 5
- ATG7
autophagy-related 7
- ATP6AP2
ATPase, H+ transporting, lysosomal accessory protein 2
- ATP13A2
ATPase type 13A2
- CMA
chaperone-medicated autophagy
- DNA
DNA, DNAJC13, DnaJ (Hsp40) homolog, subfamily C, member 13
- FBXO7
F-box protein 7
- FBXW7
F-box and WD repeat domain containing 7, E3 ubiquitin protein ligase
- GAK
cyclin G associated kinase
- GBA
glucosidase, β, acid
- GD
Gaucher disease
- GLA
galactosidase, α
- GPNMB
glycoprotein (transmembrane) nmb
- GSK3A
glycogen synthase kinase 3 α
- GWAS
genome-wide association study
- LAMP2
lysosomal-associated membrane protein 2
- LAMP3
lysosomal-associated membrane protein 3
- LRRK2
leucine-rich repeat kinase 2
- MAPT
microtubule-associated protein tau
- MCCC1
methylcrotonoyl-CoA carboxylase 1 (α)
- MEF2D
myocyte enhancer factor 2D
- MFN1
mitofusin 1
- MFN2
mitofusin 2
- M6PR
mannose 6-phosphate receptor (cation dependent)
- mRMA
messenger ribonucleic acid
- NPC1
Niemann-Pick disease, type C1
- NPC2
Niemann-Pick disease, type C2
- NUCKS1
nuclear casein kinase and cyclin-dependent kinase substrate 1
- PARK2
parkin RBR E3 ubiquitin protein ligase
- PARK7
parkinson protein 7
- PARK16
Parkinson disease 16 (susceptibility)
- PINK1
PTEN induced putative kinase 1
- PM20D1
peptidase M20 domain containing 1
- RAB29
RAB29, member RAS oncogene family
- RNAi
RNA interference
- SCARB2
scavenger receptor class B, member 2
- SLC41A1
solute carrier family 41 (magnesium transporter), member 1
- SLC45A3
solute carrier family 45, member 3
- SMPD1
sphingomyelin phosphodiesterase 1, acid lysosomal
- SN
substantia nigra
- SNCA
synuclein, α (non A4 component of amyloid precursor)
- SQSTM1
sequestosome 1
- SREBF1
sterol regulatory element binding transcription factor 1
- SREBF2
sterol regulatory element binding transcription factor 2
- SYNJ1
synaptojanin 1
- TFEB
transcription factor EB
- TMEM175
transmembrane protein 175
- UCHL1
ubiquitin carboxyl-terminal esterase L1 (ubiquitin thiolesterase)
- UPS
ubiquitin-proteasome system
- VCP
valosin containing protein
- VPS35
VPS35 retromer complex component)
- WASH1
WAS protein family homolog 1.
Introduction
Parkinson disease (PD) is a common, age-related, neurodegenerative movement disorder, characterized by degeneration of dopaminergic neurons at the substantia nigra pars compacta, and accumulation of the SNCA protein within aggregates termed Lewy-Bodies.1 The lifetime risk for PD is 1–2%,2 with various environmental and genetic factors that affect PD susceptibility.1
For the past 2 decades, major advancements have been made in the understanding of the genetic basis of PD, transforming our notion of PD as a sporadic disease in which “it appears unlikely that heredity is an important determinant”3 into a disorder largely affected by genetics. Studies that aimed to determine the role of heredity in PD, suggested that at least 27% and up to 60% is attributed to genetic factors.4-6 With the development of new genetic methods, we now know of numerous genes and genetic loci that cause or affect the risk for PD.
Recently, the largest genetic study of PD thus far—a large scale meta-analysis of genome-wide association studies (GWAS), analyzed data from more than 19,000 PD patients and over 100,000 controls—identified 24 genetic loci across the genome that are associated with PD.7 Most of these genetic markers were identified in previous large GWASs,4,8-13 and 6 of them were novel. Interestingly, out of these 24 loci, at least 11 genes are involved in or disrupt various functions of the autophagy-lysosome pathway (ALP), namely SNCA,14 GBA,15,16 LRRK2,17,18 SCARB2,19,20 LAMP3,21 RAB29/RAB7L1,17,22,23, MAPT,24-26 GAK,27,28, SREBF1,29 GPNMB,30,31 and potentially TMEM175.32
Prior to and during the GWAS era, the study of familial parkinsonism and candidate gene approaches in clinical settings, led to the discovery of additional genes that cause PD or parkinsonian syndromes. The first gene that was identified was SNCA (synuclein, α [non A4 component of amyloid precursor]), in an Italian family with PD,33 followed by the identification of the PARK2,34 PINK135 and PARK7/DJ-136 genes in young onset recessively inherited PD. Additional autosomal recessive mutations that lead to atypical parkinsonism syndromes were identified in the genes ATP13A2,37 ATP6AP2,38 and SYNJ1,39,40 and mutations in DNAJC13 were suggested to cause autosomal dominant familial PD.41 Disease-causing mutations with reduced penetrance were also identified in familial settings and cohort studies in the LRRK242-45 and GBA46 genes, which are now recognized as the 2 most common genetic causes of PD worldwide. More recently, mutations in the VPS3547,48 and SMPD149,50 genes were also suggested to cause PD. All of these genes are also involved in various functions of the ALP.
The importance of the ALP in neuroprotection and neurodegeneration in general is well established.51 For example, ATG5 and ATG7, 2 important proteins in the ALP,52,53 are crucial for neuronal maintenance. Deficiency of ATG5 in mice leads to motor function deficiency and accumulation of neuronal inclusion bodies.54 Similarly, mice with selective ATG7 deficiency in the central nervous system have motor impairment, severe neurodegeneration, and formation of inclusion bodies from polyubiquitinated proteins.55 Whole-brain ATG7 deficiency results in accumulation of the PD-related proteins SNCA and LRRK2.56 SQSTM1/p62 also plays a central role in autophagy and may be involved in neurodegeneration.57-59 However, it is still not clear if the ATG5, ATG7 and SQSTM1 genes have a direct role in PD in humans, as no mutations or genetic risk markers have been found in these genes in PD patients.
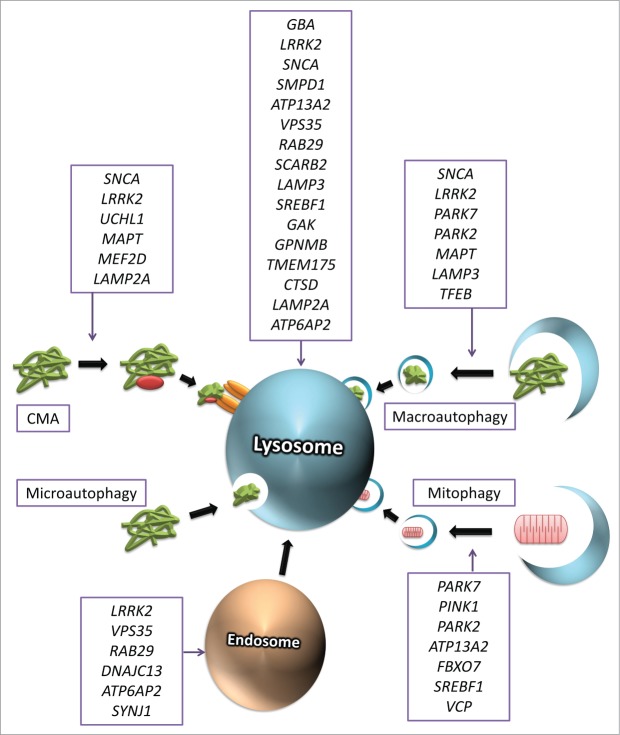
In this review we will discuss the findings in human genetic studies and their suggested roles in the ALP and in PD pathogenesis (Fig. 1). We will attempt to answer the question of whether these genes are involved in a specific ALP-related function that may lead to PD, or whether it is more probable that several ALP-related functions are involved. We will conclude by suggesting a novel hypothesis for one possible mechanism by which ALP dysfunction may lead to PD.
Figure 1.
Genes involved in Parkinson disease and in the autophagy-lysosome pathway. Figure 1 depicts genes that are associated with Parkinson disease and their area of effect in the autophagy lysosome pathway. Genes that are also involved in any lysosomal function that is not one of the forms of autophagy are depicted above the lysosome.
The Autophagy-Lysosome Pathway
Autophagy is a cellular degradation pathway involved in various processes in normal and diseased cells. The normal function of the ALP involves the transfer of various components into the lysosome for degradation, by 3 main pathways: chaperone-mediated autophagy (CMA), microautophagy, and macroautophagy (Fig. 1).51 CMA involves chaperone proteins that bind and direct specific targets (e.g., SNCA) to receptors on the lysosomal membrane (e.g., LAMP2A, to be discussed later), which in turn internalize these targets into the lysosome for degradation. Microautophagy is the engulfment and internalization of cytoplasmic components by the lysosomal membrane, a relatively less studied mechanism. Macroautophagy is a complex mechanism, characterized by the creation of a phagophore, a double-lipid bilayer that engulfs cellular cargo, such as proteins and organelles, to create an autophagosome, which in turn fuses with the lysosome for the degradation of its content.51 This process includes mitophagy, the selective degradation of mitochondria via a pathway involving specific signals.60
Genes Involved in Lysosomal Storage Disorders and Parkinson Disease
GBA—Gaucher disease
Mutations in the GBA gene may lead to the autosomal recessive Gaucher disease (GD) when inherited from both parents. GD is a lysosomal storage disorder that can be further classified into neuropathic (GD type II or III) or non-neuropathic (GD type I). In GD, the lysosomal enzyme encoded by the GBA gene, glucosidase β, acid/glucocerebrosidase, has a reduced or null activity, which leads to accumulation of glucocerebroside and lysosomal dysfunction. Thus far, approximately 300 GBA mutations have been described, including missense, frame-shift, splice-sites, and stop mutations, as well as recombinant alleles caused by recombination with the nearby and highly homologous pseudogene.61 The association between GBA mutations and PD was first suggested after several clinical reports described GD patients who developed PD.62 Although initially this association was controversial,63,64 it is now clear that GBA mutations are among the most common genetic factors for PD worldwide, found in about 3–20% of the patients in various populations, most common among Ashkenazi-Jews, but also found in Asians, Europeans, North and South Americans, and Africans.46,65 The classification of GBA mutations as “severe” or “mild” according to the type of GD that they lead to (severe mutations lead to the neuropathic GD types and mild mutations lead to the non-neuropathic type),66 is also valid for PD, as carriers of severe GBA mutations have higher risk and earlier onset compared to mild GBA mutation carriers.67
It is still unclear how GBA mutations lead to PD, and several hypotheses have been raised, including mechanisms involving loss-of-function or gain-of-function mutations.68 First, it was demonstrated that chemical inhibition of GBA can lead to accumulation of SNCA,69 a finding that was later replicated in other models with GBA mutations.70-73 In brains of PD patients without GBA mutations, reduction of GBA enzymatic activity is associated with increased SNCA.74 It was also demonstrated that GBA dysfunction leads to increased cell-to-cell transmission of SNCA.75 A possible mechanism suggested to explain the accumulation of SNCA and PD development is a positive feedback loop, in which GBA depletion increases SNCA accumulation, which in turn inhibits the function of normal GBA, causing additional aggregation of SNCA.71 Alternatively, toxic gain-of-function mechanisms were suggested,68,76 for example by causing ER stress.76 However, GBA toxic gain of function, although it can contribute to PD development, is less likely to have an essential role in the disease development. This can be explained by the fact that null GBA mutations, which result in lack of expression of GBA, also cause PD.67 Hence, if no protein is produced, it cannot cause a toxic effect, and loss of function is more likely to be the cause of increased susceptibility for PD.
Several studies have also suggested that GBA dysfunction may lead to general lysosomal dysfunction and disruption of autophagy.74,75,77 Moreover, it was suggested that in PD patients without GBA mutations, there is a reduced function of GBA. For example, a study on brains of PD patients with wild-type GBA, the GBA levels and its enzymatic activity were reduced, which led to reduced lysosomal chaperone-mediated autophagy, increased SNCA levels and reduced ceramide levels.74 This observation implies that factors other than GBA mutations, whether genetic or environmental, can affect the activity of GBA. Identifying these factors could be of great importance for the understanding of PD pathogenesis. In a cellular model with GBA null mutations, there is a general lysosomal impairment, demonstrated by the accumulation of SQSTM1 and polyubiquitinated proteins, as well as increased LysoTracker-positive structures, reduced degradation of dextran and accumulation of vacuoles.75
Interestingly, one of the genes that is repeatedly implicated in GWASs, the SCARB2 gene, associated with a 10–20% reduced risk for PD (p < 8 × 10−10).4,7 SCARB2 encodes the scavenger receptor class B, member 2, which is responsible for the transport of GBA to the lysosome.19 SCARB2 deficiency can lead to reduced GBA activity and SNCA accumulation.20 Mutations in SCARB2 cause autosomal recessive progressive myoclonus epilepsy, in which reduced GBA activity is also noted.78 Both SCARB2 and GBA are also strongly associated with another synucleinopathy, Lewy body dementia,79-81 further supporting the effect of these genes on SNCA aggregation, and suggesting that SCARB2 variants may affect GBA and lysosomal function. It will be important to examine the effects of SCARB2 variants on transport of GBA to the lysosome and on its function in PD-relevant models. At the end of this review, we propose a novel mechanism that can explain how GBA mutations impair lysosomal function and increase susceptibility to PD.
SMPD1—Niemann-Pick type A and B
The SMPD1 gene codes for the lysosomal enzyme sphingomyelin phosphodiesterase 1, acid lysosomal, and carriage of homozygous or compound heterozygous mutations in this gene may lead to the lysosomal storage disorder Niemann-Pick type A or B. Since the end product of both GBA and SMPD1 is ceramide, which was previously implicated in PD,82 and since both are causing closely related lysosomal storage disorders, the potential role of the SMPD1 gene in PD was also examined. Two studies identified rare SMPD1 mutations that are associated with increased risk for PD;49,50 however, more studies are needed to determine the significance of the SMPD1 gene in PD.
Similar to GBA, it is possible that SMPD1 deficiency may lead to more general disruption of the lysosome and autophagy. For example, fibroblasts from patients from Niemann-Pick type A show accumulation of autolysosomes, and an excess of sphingomyelin, caused by SMPD1 deficiency, which affects autophagy regulation.83 In addition, SMPD1 also regulates autophagy in smooth muscle of mice.84 Therefore, it will be of interest to examine the effects of SMPD1 mutations on lysosomal activity in general and on the internalization and processing of SNCA specifically.
Other lysosomal storage disorder genes
Several other lysosomal storage disorders may be associated with PD. Limited evidence exists suggesting that the Fabry disease gene GLA, encoding the lysosomal enzyme galactosidase, α, may also have a role in PD. One case study of a patient with Fabry disease presenting with parkinsonism was described.85 Reduced activity of GLA is observed in leukocytes of PD patients,86 and a subsequent study showed a reduced expression of GLA both in the mRNA level and in the protein level in PD patients.87 However, there is little evidence that GLA genetic variants can be associated with PD,88 therefore the role of GLA in PD is still to be determined.
A possible association of Niemann-Pick type C with PD, caused by mutations in the NPC1 or NPC2 gene, was also suggested, although the evidence for this association is also not strong. In a study of 563 PD patients, 1.1% had NPC1 mutations, which was only slightly higher than controls (0.8%) and not statistically significant.89 A case series of 3 heterozygous carriers90 and a case report of an additional carrier91 of NPC1 disease-causing mutations with parkinsonism, further support a potential role for NPC1 in PD. Some pathological evidence from autopsies of Niemann-Pick type C patients' brains, suggest that SNCA may be aberrantly phosphorylated and aggregate in Lewy bodies.92,93 Although none of these genes was implicated in human genetic studies, it is possible that rare mutations in these genes can be found in PD; therefore, sequencing of GLA and NPC1 in different PD cohorts is important for determining if they have a role in PD.
Autosomal Dominant PD Genes and Autophagy
LRRK2
LRRK2 mutations, together with GBA, are the 2 most common genetic risk factors for PD. Both are dominant with reduced penetrance, i.e., heterozygous carriage of a mutation increases the risk for the disease, but other genetic or environmental factors are required for PD to develop. Although LRRK2 was initially identified in several familial PD cases,45,94 it is a common cause of sporadic PD, found in more than 30% of Arab-Berber PD patients,95,96 more than 12% of Ashkenazi-Jews with PD,97 and in many other patient populations worldwide with various frequencies, mostly ranging between 1–5%.98
Evidence from recent years suggests that LRRK2 has an important role in autophagy as a part of the pathogenic mechanism leading to PD. While the wild-type LRRK2 protein is degraded by CMA, the common LRRK2G2019S mutation impairs this degradation.99 Moreover, it was demonstrated that the interaction between the mutated LRRK2 and the CMA receptor, LAMP2A, disturbed the multimerization of the receptor, resulting in accumulation of its substrates, including SNCA.99 These findings suggest a possible mechanism linking LRRK2 mutations to defective autophagy and subsequent accumulation of SNCA. Other studies suggest a role for LRRK2 also in general lysosomal function and in macroautophagy. In a cellular model transfected with the gene encoding the LRRK2G2019S mutation, an accumulation of autophagic vacuoles is observed.100 Similar responses are noted in cells with another common mutation, LRRK2R1441C,101 in mice with the LRRK2G2019S mutation,102 and in Drosophila with Lrrk2 loss of function.103 Knockdown of LRRK2 increases autophagic activity and reduces cell death;101 however, independent studies demonstrated that either Lrrk2 mutations or knockout of Lrrk2 result in the reduction of the autophagic marker LC3-II (although this could be attributed to accelerated autophagic flux).104,105 In addition, increased GTPase activity of the LRRK2 protein impairs endocytic vesicular trafficking and autophagy.106 Collectively, these results raise the issue of gain vs. loss of function in LRRK2-associated PD. While it was demonstrated that the mutations that occur in PD patients can cause gain of the kinase or GTPase functions,54 the above experiments demonstrate that both loss and gain-of function have similar deleterious effects on autophagy. The reduction in the autophagic marker LC3-II occurs in both gain- (Lrrk2 mutations)104 and loss-of-function (knockout) models of LRRK2-associated PD. Likewise, accumulation of autophagic vacuoles is observed in both gain- (mutated mice)102 and loss-of-function (Drosophila)103 models. This could be explained by differences between species and models, but it is also possible that while LRRK2 mutations cause a gain of kinase/GTPase activity, they also cause loss of another function at the same time, and therefore this issue should be further studied.
A recent study also suggested an effect of the LRRK2G2019S mutation on induction of mitophagy by interacting with the mitochondrial membrane protein BCL2.107 LRRK2 also interacts with RAB29 and affects PD risk and lysosomal dysfunction; these interactions will be discussed in the RAB29 section. While there is clear evidence that LRRK2 is involved in various forms of autophagy and lysosomal functions, the exact mechanisms are not fully understood and necessitate more study. Interestingly, LRRK2-associated PD is not always characterized by accumulation of SNCA, and it can be associated with deposition of MAPT/tau protein or ubiquitin-positive inclusions.108 It was suggested that LRRK2 dysfunction may be upstream to the accumulation of SNCA and MAPT, and that other genetic or environmental factors determine which pathology will develop;109 however, more evidence is needed to support this hypothesis.
SNCA and autophagy
SNCA is the main component of Lewy bodies. Duplications, triplications,110,111 and point mutations 33,112-114 in the SNCA gene cause PD or parkinsonian syndromes, and genetic markers in its locus are associated with PD in various populations.4,7,8,13,115 Since SNCA oligomers are toxic in neurons, a leading paradigm in PD research suggests that SNCA accumulation, resulting from its overexpression or lack of degradation, is one of the important mechanisms causing degeneration of dopaminergic neurons.116
Early reports suggested that both ALP and the ubiquitin-proteasome system (UPS) are responsible for SNCA degradation,117 but it seems that the main cellular mechanism responsible for its degradation is the ALP, as SNCA accumulation results from inhibition of the ALP, but not the UPS.118 CMA internalizes SNCA into the lysosome,14 but macroautophagy also takes part in SNCA clearance.118,119
Chaperone-mediated autophagy and SNCA degradation
CMA of wild-type SNCA is mediated by the LAMP2A lysosomal receptor and the chaperone molecule HSPA8.14,118,120 Interestingly, the mutant forms of SNCA that cause PD, SNCAA53T and SNCAA30P, are poorly degraded by CMA,14,121 although they have high affinity to LAMP2A.14 This observation may suggest that these mutant forms attach to the receptor and prevent it from internalizing wild-type or mutant SNCA, resulting in SNCA accumulation and cellular toxicity. Furthermore, dopamine can modify SNCA, and as a result the CMA of SNCA is blocked by the dopamine-modified form of the protein.122 This finding may explain the higher sensitivity of dopaminergic neurons to SNCA accumulation, as observed in PD. Additional effects of SNCA on CMA and neurodegeneration may be mediated by the neuronal survival factor MEF2D. Both wild-type and mutant SNCA inhibit the CMA of MEF2D, accompanied by a decline of MEF2D function and survival of neuronal cells.123 Of note, the UCHL1 gene product, whose role in PD is still under debate, interacts with LAMP2 and increases SNCA levels, suggesting that it affects the CMA of SNCA.124
Wild-type SNCA is also degraded by macroautophagy, and inhibition of this process causes SNCA accumulation, in contrast to inhibition of the UPS.118 In addition, following dysfunction of the CMA pathway in cells with the SNCAA53T mutation, a subsequent activation of the macroautophagy pathway occurs.121 The same phenomenon follows inhibition of CMA with dopamine,122 or overexpression of wild-type SNCA or SNCAA53T,125 suggesting that macroautophagy may be a compensatory mechanism for dysfunctional CMA of SNCA. It was further demonstrated that induction of autophagy can induce degradation of SNCA and rescue neurons from cell death. For example, trehalose, a dissacharide that induces macroautophagy, accelerates the degradation of the SNCAA30P and SNCAA53T mutant forms of SNCA,126 and induction of autophagy by the transcription regulator of the ALP, TFEB,127 rescues dopaminergic neurons from SNCA toxicity.128
VPS35 and other genes in the lysosomal-endosomal pathway
Mutations in VPS35 (VPS35 retromer complex component), are a rare but well validated cause of autosomal dominant PD, the most common mutation being VPS35D620N.47,48,129-131 In a meta-analysis of mRNA expression in the substantia nigra (SN), there was a highly significant decrease in VPS35 levels, which was further replicated in dopaminergic neurons that were laser-microdissected from the SN of PD patients.17 VPS35 is a component of the retromer complex, which mediates endosome-to-Golgi transport of proteins, for recycling and reuse or for further degradation. One of these proteins is M6PR (mannose 6-phosphate receptor [cation dependent]), which is responsible for transporting many of the lysosomal hydrolases to the lysosome.132 Therefore, retromer dysfunction may lead to lysosomal dysfunction. It was recently shown that the VPS35D620N mutation leads to reduced association with the WASH1/Wiskott–Aldrich syndrome homolog complex,133 which is a complex of proteins essential for the process of endosomal actin polymerization and facilitation of protein sorting.134 The reduced association of mutated VPS35 with the WASH complex, further reduce its recruitment to endosomes, which leads to impairment of autophagy.133 In addition, knockdown or mutated VPS35 in neuronal cell cultures leads to reduced colocalization of the M6PR with the Golgi apparatus and with late endosome/lysosome markers.17 Interestingly, 2 additional genes that cause rare autosomal recessive atypical parkinsonism, ATP6AP2,38 and SYNJ1,39,40 and the dominant PD gene DNAJC13,41 are also involved in the endosomal pathway. Similar to VPS35, DNAJC13 is also associated with the WASH complex by binding to the FAM21 protein.135 Atp6ap2 regulates endolysosomal trafficking in Drosophila136 and it is essential for the acidification of lysosomes.137 SYNJ1 also has an important role in endolysosonal trafficking of synaptic proteins,138 together with the involvement of the other PD-related genes in this pathway, including LRRK2 and RAB29 (discussed separately), emphasizing its important role in PD.
Autosomal Recessive PD Genes, Autophagy, and Mitophagy
Carriage of homozygous or compound heterozygous mutations in 3 genes: PARK2,34 PINK1 35 and PARK7,36 causes young or early onset PD, with motor symptoms usually occurring between the first and sixth decades of life. PARK2 is the most common autosomal recessive PD-causing gene, accounting for most cases of young onset PD, followed by PINK1.108 Interestingly, these genes are all involved in the process of selective mitochondria engulfment by autophagosomes and their degradation within the lysosome, termed mitophagy. Pathologically, PARK2-associated PD is often limited to the substantia nigra, mostly without Lewy bodies,108 suggesting that the pathogenic mechanism is downstream of the accumulation of SNCA. This could mean that ALP dysfunction that leads only to mitophagy dysfunction may be sufficient for the development of PD, or a sub-type of PD.
PARK2 and PINK1 in mitophagy
Initially, PARK2 was identified as a cytosolic E3 ubiquitin ligase, but it was later shown that when mitochondria are depolarized, PARK2 is selectively recruited to their surface and ubiqitinates other proteins on the outer mitochondrial membrane, such as MFN1 and MFN2.139,140 The translocation of PARK2 to the mitochondrial membrane and the recruitment and ubiqitination of mitochondrial proteins initiates mitophagy, and this process requires PINK1 expression and kinase activity. PINK1 has low levels of expression on healthy mitochondria, whereas on dysfunctional mitochondria it rapidly accumulates and recruits PARK2 to induce mitophagy.141-143 Furthermore, PD-causing mutations in PINK1144 and PARK2145 disrupt PARK2 recruitment to the mitochondria and the induction of mitophagy. Overexpression of PARK2 has a protective effect in cells deficient for PINK1, and overexpression of PINK1 suppresses autophagy or mitophagy induced by toxins.146 It is possible that lysosomal dysfunction in lysosomal storage disorders can also affect PARK2-mediated mitophagy.147 Studies of other genetic conditions in which parkinsonism is a part of the disorder may also shed more light on the importance of mitophagy in PD. For example, mutations in the VCP gene cause a multisystem degenerative disorder in which one of its many clinical features is parkinsonism.148 A recent study demonstrated that the pathogenic mutations in VCP also impair the clearance of damaged mitochondria via the PINK1/PARK2 pathway.149
PARK7 and mitophagy
Interestingly, PARK7 acts under conditions of oxidative stress in a parallel pathway to that of PINK1 and PARK2 to sustain mitochondrial function and mitophagy, and overexpression of PARK2 protects against PARK7 loss and prevents mitochondrial damage.150 In another study, Park7 overexpression rescues the phenotype of Pink1 loss of function in Drosophila.151 Altogether, these studies demonstrated that the PD-related genes PARK2, PINK1, and PARK7 have an important role in mitophagy, suggesting that impairment of this mechanism has a role in PD pathogenesis. Impaired mitochondria processing by mitophagy due to mutations in any of these genes, may lead to excess production of reactive oxygen species, cause excessive oxidative stress, and contribute to cell death and neurodegeneration.152
ATP13A2 lysosomal function and autophagy/mitophagy
ATP13A2 codes for a lysosomal transmembrane protein that functions as P-type ATPase on the lysosome and late endosome, and is highly expressed in the substantia nigra, as well as in other parts of the brain.153 Mutations in ATP13A2 cause Kufor-Rakeb syndrome, which is a rare autosomal recessive disorder characterized by early-onset parkinsonism with pyramidal degeneration and dementia.37 Studies on fibroblasts from these patients demonstrated that, as compared to controls, there are high rates of mitochondrial DNA damage, decreased ATP synthesis rates and increased fragmentation of the mitochondrial network, all suggesting an effect of mitochondrial quality control. When wild-type ATP13A2 is overexpressed, this mitochondrial phenotype is rescued.154 Similarly, silencing of ATP13A2 induces fragmentation of mitochondria in a neuronal cell model, and its overexpression delays mitochondrial fragmentation.155 These and similar findings156 suggest a role for ATP13A2 in quality control of mitochondria, probably through mitophagy. A more general role in lysosomal function and autophagy was suggested for ATP13A2, as fibroblasts from patients with Kufor-Rakeb syndrome as well as mouse primary neurons with ATP13A2 deficiency lead to reduced capacity of lysosomal degradation, resulting in SNCA accumulation and neurotoxicity.157
FBXO7 and mitophagy
Homozygous and compound heterozygous mutations in the FBXO7 gene can cause a parkinsonism disorder that can be clinically similar to PARK2-associated PD,158,159 or to typical PD.160 Interestingly, just like the other autosomal recessive forms of PD, it was suggested that FBXO7 is involved in mitophagy.161 Given its similar phenotype to PARK2-associated PD, the interaction of FBXO7 with PARK2 was examined, and it was shown that reduced expression of FBXO7 leads to reduced translocation of PARK2 to the mitochondria. FBXO7 directly interacts with PARK2 and recruits it to the mitochondria, and it is also involved in mitophagy of mitochondria treated with carbonyl cyanide m-chlorophenylhydrazone.161 Overall, it seems that the PD autosomal recessive genes may all converge into a subpathway of the ALP, mitophagy. It may suggest that severe impairment of mitophagy can elicit PD or a similar disease. Why this impairment affects mainly dopaminergic neurons at the substantia nigra and the exact mechanism by which this impairment occurs is still to be determined.
Genes Identified in GWAS and their Potential role in the Autophagy-Lysosome Pathway
RAB29
Two GWASs identified a region on chromosome 1, termed PARK16, which includes 5 genes: PM20D1, SLC41A1, RAB29/RAB7L1, NUCKS1, and SLC45A3.12,13 Minor alleles in this region are associated with a 30–40% reduced risk for PD, a finding that was later replicated by several other studies in different populations.162-166 A particularly reduced risk was demonstrated in the Ashkenazi Jewish population, demonstrating that a haplotype that included SNPs in the promoter of RAB29 is associated with a 10-fold reduced risk. Furthermore, one of the promoter SNPs, rs1572931, has the strongest association and is found in both haplotypes with a protective effect.162 Subsequently, it was demonstrated that the same SNP is associated with alternative splicing of RAB29, where the protective allele is associated with increased inclusion of exon 2, while the risk allele is associated with exon skipping.17 A second SNP within the promoter region of RAB29, rs823114, has the strongest epistasis with LRRK2, jointly affecting the risk for PD.
These 2 proteins, RAB29 and LRRK2, not only genetically interact, they also physically interact, as shown in an unbiased screen using protein-protein interaction arrays.17,22 This interaction was further supported in a study where RAB29 overexpression inhibits the known effect of LRRK2 mutations on neurite length.17 In relation to the ALP, knockdown of RAB29 leads to swelling of lysosomes and to reduction of M6PR accumulation in the lysosome, and it was suggested that these changes may be secondary to altered retromer-mediated trafficking between the lysosome and the Golgi apparatus.17 It was further confirmed that RAB29 has an important role in the retrograde trafficking of M6PRs to the Golgi apparatus.23 M6PRs are needed for recruitment and transport of lysosomal hydrolases to the lysosome; therefore, disruption of this function may lead to lysosomal dysfunction.17 Identifying the specific mechanism underlying the protective effect of RAB29, as well as other protective genetic factors, should be a priority, since they may provide clinicians with crucial information for treatment or prevention of PD.
SREBF1
The association between the SREBF1 gene, encoding the sterol regulatory element binding transcription factor 1, and PD was initially identified in a GWAS,4 and it was recently replicated in the largest GWAS performed to date.7 In both GWASs, the minor allele is associated with a reduced risk for PD, with an estimated effect of 5–15% reduced risk. Recently, in a whole genome RNAi screen that aimed to identify factors that promote mitophagy mediated by PARK2, the lipogenesis pathway was implicated with 4 genes of this pathway, SREBF1, SREBF2, FBXW7 and GSK3A, among the top hits.29,167 Knockdown of SREBF1 blocks both the translocation of PARK2 to the mitochondria and the consequent mitophagy, an effect that is independent of PINK1 expression levels.167 Therefore it is hypothesized that reduced SREBF1 expression my lead to reduced mitophagy and risk for PD.
SREBF1 also regulates the expression levels of the NPC1 gene (which is associated with the lysosomal storage disorder Niemann-Pick Type C), by binding to its promoter and increasing its transcription. Downregulation of this pathway may induce the sequestration of cholesterol within late endosomes and lysosomes,168 and the transcriptional activity of SREBF1 is enhanced in models of lysosomal storage disorders, leading to increased transcription of LDLR (low density lipoprotein receptor).169 These findings suggest that SREBF1 has a role in mitophagy, as well as in regulation of lysosomal lipid accumulation, and more studies of its potential interactions with PARK2, PARK7, and PINK1 are necessary.
MAPT
MAPT is one of the most intriguing genes in neurogenetics, as it is associated with different neurological conditions in different ways. MAPT codes for the microtubule-associated protein tau, which aggregates in Alzheimer and other diseases, generally termed tauopathies. In some of these diseases, such as progressive supranuclear palsy,170 corticobasal degeneration,171 and argyrophilic grain disease,172 MAPT variations play an important role. Additionally, MAPT mutations may cause fronto-temporal dementia with parkinsonism173 and lower motor neuron disease.174 However, the association between MAPT variations and the most common tauopathy, Alzheimer disease, is still not clear, although several studies suggest such an association.175-177 MAPT haplotypes, such as the H1 haplotype, were identified in various studies as important risk factors for PD,4,7-9,12,13 in which tau does not accumulate. Furthermore, MAPT-associated SNP is the second strongest risk factor in PD GWAS, with an OR of 0.77 and p = 2 × 10−48.7 Considering all these findings, it is clear that MAPT plays a critical role in the nervous system.
MAPT is both affected by and affects the function of the lysosome, since it is being degraded by it and at the same time is important to its function.178-181 It is possible that the H1 haplotype is associated with impaired autophagy, or with dysfunction of MAPT, which may lead to autophagy impairment.26 However, this hypothesis still needs to be supported by future research.
Other GWAS hits with a possible role in the autophagy-lysosome pathway
A locus that contains 2 genes, LAMP3 and MCCC1, was repeatedly identified in GWASs of PD, with an estimated risk reduction of 13–20%.4,7, 8 LAMP3 encodes the lysosomal-associated membrane protein 3, which has a role in the unfolded protein response, and is expressed in immune system cells, mainly dendritic cells.182 Since the immune system is also suggested to be involved in PD pathogenesis,183 it is possible that the role of LAMP3 in PD is related to the lysosomal function in these cells. It was recently shown that LAMP3 is involved in autophagy, as its knockdown reduces the ability of cells to complete the autophagic process, and cells with high LAMP3 expression show increased basal autophagy levels.21 However, not much is known about this potential function, and since the risk locus for PD includes both LAMP3 and MCCC1, it is possible that it is MCCC1 that is involved in PD. Although MCCC1 functions at the mitochondria as a subunit of the 3-methylcrotonyl-CoA carboxylase enzyme,184 it is currently not known if it has any role in mitophagy or mitochondrial quality control. Studying the potential relationship of MCCC1 with other mitochondrial-related genes that are involved in PD, such as PARK2, PARK7, PINK1 and SREBF1 may help determining if it is the LAMP3 gene or MCCC1 that affects the risk for PD in this locus.
GAK is another gene that was identified in the largest GWASs, with an estimated effect of 14–30% on risk for PD.4,7,8 GAK encodes the cyclin G associated kinase, and RNAi-mediated depletion of GAK results in diminished sorting of CTSD (cathepsin D),27 an important lysosomal hydrolase that is also responsible for the degradation of SNCA.185,186 Introducing RNAi-resistant GAK following RNAi restores the proper lysosomal sorting of CTSD.27 Therefore, it is possible that GAK is involved in PD through its effect on the lysosomal activity of CTSD and subsequent SNCA degradation, and more studies are necessary to examine this hypothesis. In another model, it was demonstrated that knockout of GAK results in destabilization of the lysosomal membrane and leakage of iron, causing DNA damage.28
Genetic markers around GPNMB were identified and replicated in several GWASs, with approximately 10% risk reduction.7,9 Interestingly, GPNMB is an important gene in melanoma,187 a skin malignancy that is associated with PD.188 The GPNMB protein localizes mainly to melanosomes and to a lesser extent to lysosomes in melanoma cells; however, in other cells it localizes to lysosomes.31 Furthermore, GPNMB is involved in phagocytosis, and is essential for recruitment of the autophagy protein LC3-II to the phagosome. In addition, GPNMB is necessary for the fusion of the lysosome with the phagosome leading to the degradation of the phagosome content.30 Another gene associated with PD in GWAS, TMEM175 (same locus and effects on risk as GAK), is found in the lysosomal membrane, but its function is unknown.32
Lysosomal Membrane Properties, Autophagy, and Parkinson Disease: A Novel Hypothesis
SNCA is the major constituent of Lewy bodies, the neuropathological hallmark of PD, and mutations and gene dosage variations in SNCA cause PD.33,110-114 It was suggested that overexpression or accumulation of SNCA is toxic to cells, contributing to the development of PD.189 The association between GBA mutations or dysfunction with SNCA accumulation,46,69-73 in addition to other findings, prompted us to hypothesize a novel mechanism that may explain GBA-associated PD, but may also explain sporadic PD (Fig. 2).
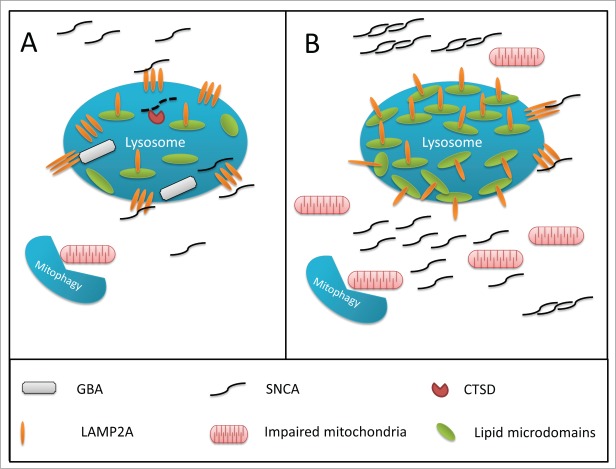
Figure 2.
Hypothesized mechanism for GBA-associated lysosomal dysfunction in Parkinson disease. (A) In a normally functioning lysosome, GBA is associated with the inner part of the lysosomal membrane, and degrades glucocerebrosides to glucose and ceramide, thus controlling the proper composition of the membrane. The chaperone-mediated autophagy receptor, LAMP2A, is able to freely move out of lipid rafts, create complexes, and internalize SNCA into the lysosome for degradation. (B) Impaired GBA activity can affect the composition of the membrane, leading to an increased density of lipid rafts on the lysosomal membrane. In this scenario, it is more difficult for LAMP2A to create the complexes required for the internalization of SNCA into the lysosome, leading to SNCA accumulation. This effect of GBA impairment on the lysosomal membrane may interrupt other pathways, and affect macroautophagy and mitophagy, which will lead to the accumulation of damaged mitochondria and increased oxidative stress.
The receptor for CMA of SNCA is LAMP2A, a lysosomal transmembrane protein.14 In order to transport SNCA into the lysosome for further degradation by CTSD,186 the LAMP2A protein must form complexes on the lysosomal membrane.190 In its monomeric state, LAMP2A is found in specific membrane lipid microdomains, and the protein-complex formation process requires LAMP2A to leave these domains.191 Retention of LAMP2A within the lipid microdomains may not allow protein-complex formation, which in turn will prevent the autophagy of SNCA, resulting in SNCA accumulation.
GBA is located on the surface of the inner membrane of the lysosome, where it cleaves membrane glucocerebrosides into ceramide and glucose. In a cellular model of Gaucher disease, when GBA is inhibited, the composition of the lysosomal membrane changes, including increased concentration of glucocerebrosides.192 Similar results were observed in a mouse model of Gaucher disease, demonstrating highly increased concentrations of glucocerebrosides in lipid rafts.193 This process may prevent or reduce the formation of LAMP2A protein-complexes, which will then reduce the autophagy of SNCA, and result in its accumulation. It is possible that alterations in the composition of the lysosomal membrane may affect other forms of autophagy, such as microautophagy, macroautophagy and mitophagy, contributing to PD development. It was already demonstrated, for example, that GBA deficiency may lead to defective mitophagy and mitochondrial damage.194,195
Is it possible that this model can be relevant not only for GBA-associated PD, but for other forms of PD as well? Is it possible that other events, such as oxidative stress or aging affect the composition of the lysosomal membrane and therefore affect its ability to internalize SNCA for degradation? It seems that the answer for both questions may be yes. There is a growing body of evidence that GBA activity is reduced not only in GBA-associated PD, but in sporadic PD as well,74,196,197 suggesting that other genetic or environmental factors may lead to GBA impairment and to the subsequent pathological effect. One possible factor is SNCA itself, which may interact with and inhibit wild-type GBA.71,198 An interesting observation suggests that in the normal process of aging, the mobilization of LAMP2A to the lysosome membrane is altered.199 More research is needed to answer these questions and to determine the exact mechanisms by which dysfunction of the ALP affects SNCA accumulation and increases the risk for PD.
Therefore, this hypothesis may be valid mainly to GBA-associated PD and to other forms of PD in which GBA activity is reduced, or to late onset PD in which aging has a similar effect on the lysosomal membrane. While these patients could be the majority of patients, it is possible that different ALP subpathways are involved in other forms of PD. Furthermore, it is possible that what we call PD, is a group of phenotypically similar yet pathogenically somewhat different diseases. In PARK2-associated PD, in which the pathology is limited in most cases to nigral degeneration only, and in LRRK2-associated PD in which there is no SNCA accumulation in some cases, a different ALP dysfunctional mechanism may be involved. This could be true as well for PINK1- and PARK7-associated PD, in which mitophagy is impaired in a similar way to PARK2-associated PD. Accordingly, future treatment of these forms of PD could be different, and it is already hypothesized that LRRK2-associated PD may benefit from kinase inhibitors 200,201 whereas GBA-associated PD may benefit from enzymatic therapy, once it can cross the blood-brain barrier.
Conclusion
This review summarizes the knowledge arising from genetic studies, emphasizing the central role of the ALP and endolysosomal trafficking in PD. Given the presented evidence, it is likely that lysosomal dysfunction results in a reduced ability to degrade SNCA and/or defective mitochondria, leading to the loss of dopaminergic neurons seen in PD. The exact mechanism and why it happens in dopaminergic neurons rather than others is still to be explained, and several hypotheses have been raised, such as specific calcium-related properties of dopaminergic neurons,202 or blockage of CMA by modified dopamine.122 Whether the ALP can serve as a cell death-signaling pathway, or whether it is a more pro-survival mechanism, is still under debate.203-205 Nevertheless, the contribution of the ALP to the normal function of cells is clear, and therefore impairment of the ALP may lead to reduced ability of cells to survive. It is also likely, however, that the ALP participates in the cell death that occurs in PD. It is important to note that although this review focuses on the ALP, it is clear that other pathways and mechanisms are also involved in PD pathogenesis. Such mechanisms include, for example, prion-like propagation of SNCA,206 mitochondrial dysfunction, neuro-inflammation,207 and calcium regulation,208 and efforts to find therapeutic interventions targeting these and other pathways are of great importance as well.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ms. Stephanie Strong for her assistance.
Funding
Ziv Gan-Or and Guy A. Rouleau received research grants from the Michael J. Fox Foundation to study the genetics of Parkinson disease and REM Sleep Behavior disorder. Ziv Gan-Or is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR). Guy A. Rouleau holds a Canada Research Chair in Genetics of the Nervous System and the Wilder Pennfield Chair in Neurosciences.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson disease. Lancet 2009; 373:2055-66; PMID:19524782; http://dx.doi.org/ 10.1016/S0140-6736(09)60492-X [DOI] [PubMed] [Google Scholar]
- 2.Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Risk tables for parkinsonism and Parkinson disease. J Clin Epidemiol 2002; 55:25-31; PMID:11781119; http://dx.doi.org/ 10.1016/S0895-4356(01)00425-5 [DOI] [PubMed] [Google Scholar]
- 3.Kopin IJ, Markey SP. MPTP toxicity: implications for research in Parkinson disease. Annu Rev Neurosci 1988; 11:81-96; PMID:3129982; http://dx.doi.org/ 10.1146/annurev.ne.11.030188.000501 [DOI] [PubMed] [Google Scholar]
- 4.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, et al.. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson disease. PLoS Genet 2011; 7:e1002141; PMID:21738487; http://dx.doi.org/ 10.1371/journal.pgen.1002141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamza TH, Payami H. The heritability of risk and age at onset of Parkinson disease after accounting for known genetic risk factors. J Hum Genet 2010; 55:241-3; PMID:20203693; http://dx.doi.org/ 10.1038/jhg.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller MF, Saad M, Bras J, Bettella F, Nicolaou N, Simon-Sanchez J, Mittag F, Buchel F, Sharma M, Gibbs JR, et al.. Using genome-wide complex trait analysis to quantify ‘;missing heritability’ in Parkinson disease. Hum Mol Genet 2012; 21:4996-5009; PMID:22892372; http://dx.doi.org/ 10.1093/hmg/dds335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, et al.. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson disease. Nat Genet 2014; 46:989-93; PMID:25064009; http://dx.doi.org/ 10.1038/ng.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Parkinson Disease Genomics C, Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, et al.. Imputation of sequence variants for identification of genetic risks for Parkinson disease: a meta-analysis of genome-wide association studies. Lancet 2011; 377:641-9; PMID:21292315; http://dx.doi.org/ 10.1016/S0140-6736(10)62345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Parkinson Disease Genomics C, Wellcome Trust Case Control C . A two-stage meta-analysis identifies several new loci for Parkinson disease. PLoS Genet 2011; 7:e1002142; PMID:21738488; http://dx.doi.org/ 10.1371/journal.pgen.1002142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide BM, Schjeide LM, Meissner E, Zauft U, Allen NC, et al.. Comprehensive research synopsis and systematic meta-analyses in Parkinson disease genetics: The PDGene database. PLoS Genet 2012; 8:e1002548; PMID:22438815; http://dx.doi.org/ 10.1371/journal.pgen.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pankratz N, Beecham GW, DeStefano AL, Dawson TM, Doheny KF, Factor SA, Hamza TH, Hung AY, Hyman BT, Ivinson AJ, et al.. Meta-analysis of Parkinson disease: identification of a novel locus, RIT2. Ann Neurol 2012; 71:370-84; PMID:22451204; http://dx.doi.org/ 10.1002/ana.22687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, et al.. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson disease. Nat Genet 2009; 41:1303-7; PMID:19915576; http://dx.doi.org/ 10.1038/ng.485 [DOI] [PubMed] [Google Scholar]
- 13.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, et al.. Genome-wide association study reveals genetic risk underlying Parkinson disease. Nat Genet 2009; 41:1308-12; PMID:19915575; http://dx.doi.org/ 10.1038/ng.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 2004; 305:1292-5; PMID:15333840; http://dx.doi.org/ 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- 15.Osellame LD, Duchen MR. Defective quality control mechanisms and accumulation of damaged mitochondria link Gaucher and Parkinson diseases. Autophagy 2013; 9:1633-5; PMID:23989665; http://dx.doi.org/ 10.4161/auto.25878 [DOI] [PubMed] [Google Scholar]
- 16.Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain 2014; 137:1304-22; PMID:24531622; http://dx.doi.org/ 10.1093/brain/awu002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, et al.. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson disease risk. Neuron 2013; 77:425-39; PMID:23395371; http://dx.doi.org/ 10.1016/j.neuron.2012.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schapansky J, Nardozzi JD, Felizia F, LaVoie MJ. Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum Mol Genet 2014; 23:4201-14; PMID:24682598; http://dx.doi.org/ 10.1093/hmg/ddu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T, Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of β-glucocerebrosidase. Cell 2007; 131:770-83; PMID:18022370; http://dx.doi.org/ 10.1016/j.cell.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 20.Rothaug M, Zunke F, Mazzulli JR, Schweizer M, Altmeppen H, Lullmann-Rauch R, Kallemeijn WW, Gaspar P, Aerts JM, Glatzel M, et al.. LIMP-2 expression is critical for β-glucocerebrosidase activity and α-synuclein clearance. Proc Natl Acad Sci U S A 2014; 111:15573-8; PMID:25316793; http://dx.doi.org/ 10.1073/pnas.1405700111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagelkerke A, Sieuwerts AM, Bussink J, Sweep FC, Look MP, Foekens JA, Martens JW, Span PN. LAMP3 is involved in tamoxifen resistance in breast cancer cells through the modulation of autophagy. Endocr Relat Cancer 2014; 21:101-12; PMID:24434718; http://dx.doi.org/ 10.1530/ERC-13-0183 [DOI] [PubMed] [Google Scholar]
- 22.Beilina A, Rudenko IN, Kaganovich A, Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe K, et al.. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci U S A 2014; 111:2626-31; PMID:24510904; http://dx.doi.org/ 10.1073/pnas.1318306111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Ma Z, Xu X, Wang Z, Sun L, Zhou Y, Lin X, Hong W, Wang T. A role of Rab29 in the integrity of the trans-Golgi network and retrograde trafficking of mannose-6-phosphate receptor. PLoS One 2014; 9:e96242; PMID:24788816; http://dx.doi.org/ 10.1371/journal.pone.0096242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambegaokar SS, Jackson GR. The downward spiral of tau and autolysosomes: a new hypothesis in neurodegeneration. Autophagy 2012; 8:1144-5; PMID:22635052; http://dx.doi.org/ 10.4161/auto.20515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonskaya I, Hebron M, Chen W, Schachter J, Moussa C. Tau deletion impairs intracellular β-amyloid-42 clearance and leads to more extracellular plaque deposition in gene transfer models. Mol Neurodegener 2014; 9:46; PMID:25384392; http://dx.doi.org/ 10.1186/1750-1326-9-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco CD, Elrick MJ, Lieberman AP. Tau deletion exacerbates the phenotype of Niemann-Pick type C mice and implicates autophagy in pathogenesis. Hum Mol Genet 2009; 18:956-65; PMID:19074461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kametaka S, Moriyama K, Burgos PV, Eisenberg E, Greene LE, Mattera R, Bonifacino JS. Canonical interaction of cyclin G associated kinase with adaptor protein 1 regulates lysosomal enzyme sorting. Mol Biol Cell 2007; 18:2991-3001; PMID:17538018; http://dx.doi.org/ 10.1091/mbc.E06-12-1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olszewski MB, Chandris P, Park BC, Eisenberg E, Greene LE. Disruption of clathrin-mediated trafficking causes centrosome overduplication and senescence. Traffic 2014; 15:60-77; PMID:24138026; http://dx.doi.org/ 10.1111/tra.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivatt RM, Whitworth AJ. SREBF1 links lipogenesis to mitophagy and sporadic Parkinson disease. Autophagy 2014; 10:1476-7; PMID:24991824; http://dx.doi.org/ 10.4161/auto.29642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Castano AP, Hudson TE, Nowlin BT, Lin SL, Bonventre JV, Swanson KD, Duffield JS. The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J 2010; 24:4767-81; PMID:20709912; http://dx.doi.org/ 10.1096/fj.10-154757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomihari M, Hwang SH, Chung JS, Cruz PD Jr., Ariizumi K. Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion. Exp Dermatol 2009; 18:586-95; PMID:19320736; http://dx.doi.org/ 10.1111/j.1600-0625.2008.00830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapel A, Kieffer-Jaquinod S, Sagne C, Verdon Q, Ivaldi C, Mellal M, Thirion J, Jadot M, Bruley C, Garin J, et al.. An extended proteome map of the lysosomal membrane reveals novel potential transporters. Mol Cell Proteomics 2013; 12:1572-88; PMID:23436907; http://dx.doi.org/ 10.1074/mcp.M112.021980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al.. Mutation in the α-synuclein gene identified in families with Parkinson disease. Science 1997; 276:2045-7; PMID:9197268; http://dx.doi.org/ 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- 34.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998; 392:605-8; PMID:9560156; http://dx.doi.org/ 10.1038/33416 [DOI] [PubMed] [Google Scholar]
- 35.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, et al.. Hereditary early-onset Parkinson disease caused by mutations in PINK1. Science 2004; 304:1158-60; PMID:15087508; http://dx.doi.org/ 10.1126/science.1096284 [DOI] [PubMed] [Google Scholar]
- 36.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, et al.. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003; 299:256-9; PMID:12446870; http://dx.doi.org/ 10.1126/science.1077209 [DOI] [PubMed] [Google Scholar]
- 37.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, et al.. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 2006; 38:1184-91; PMID:16964263; http://dx.doi.org/ 10.1038/ng1884 [DOI] [PubMed] [Google Scholar]
- 38.Korvatska O, Strand NS, Berndt JD, Strovas T, Chen DH, Leverenz JB, Kiianitsa K, Mata IF, Karakoc E, Greenup JL, et al.. Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum Mol Genet 2013; 22:3259-68; PMID:23595882; http://dx.doi.org/ 10.1093/hmg/ddt180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krebs CE, Karkheiran S, Powell JC, Cao M, Makarov V, Darvish H, Di Paolo G, Walker RH, Shahidi GA, Buxbaum JD, et al.. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum Mutat 2013; 34:1200-7; PMID:23804563; http://dx.doi.org/ 10.1002/humu.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, Wu B, Xu F, Erro R, Amboni M, et al.. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum Mutat 2013; 34:1208-15; PMID:23804577; http://dx.doi.org/ 10.1002/humu.22373 [DOI] [PubMed] [Google Scholar]
- 41.Vilarino-Guell C, Rajput A, Milnerwood AJ, Shah B, Szu-Tu C, Trinh J, Yu I, Encarnacion M, Munsie LN, Tapia L, et al.. DNAJC13 mutations in Parkinson disease. Hum Mol Genet 2014; 23:1794-801; PMID:24218364; http://dx.doi.org/ 10.1093/hmg/ddt570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Fonzo A, Rohe CF, Ferreira J, Chien HF, Vacca L, Stocchi F, Guedes L, Fabrizio E, Manfredi M, Vanacore N, et al.. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson disease. Lancet 2005; 365:412-5; PMID:15680456; http://dx.doi.org/ 10.1016/S0140-6736(05)17829-5 [DOI] [PubMed] [Google Scholar]
- 43.Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, et al.. A common LRRK2 mutation in idiopathic Parkinson disease. Lancet 2005; 365:415-6; PMID:15680457 [DOI] [PubMed] [Google Scholar]
- 44.Nichols WC, Pankratz N, Hernandez D, Paisan-Ruiz C, Jain S, Halter CA, Michaels VE, Reed T, Rudolph A, Shults CW, et al.. Genetic screening for a single common LRRK2 mutation in familial Parkinson disease. Lancet 2005; 365:410-2; PMID:15680455 [DOI] [PubMed] [Google Scholar]
- 45.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, et al.. Cloning of the gene containing mutations that cause PARK8-linked Parkinson disease. Neuron 2004; 44:595-600; PMID:15541308; http://dx.doi.org/ 10.1016/j.neuron.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 46.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, et al.. Multicenter analysis of glucocerebrosidase mutations in Parkinson disease. N Engl J Med 2009; 361:1651-61; PMID:19846850; http://dx.doi.org/ 10.1056/NEJMoa0901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, et al.. VPS35 mutations in Parkinson disease. Am J Hum Genet 2011; 89:162-7; PMID:21763482; http://dx.doi.org/ 10.1016/j.ajhg.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, et al.. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 2011; 89:168-75; PMID:21763483; http://dx.doi.org/ 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foo JN, Liany H, Bei JX, Yu XQ, Liu J, Au WL, Prakash KM, Tan LC, Tan EK. Rare lysosomal enzyme gene SMPD1 variant (p.R591C) associates with Parkinson disease. Neurobiol Aging 2013; 34:2890 e13-5; PMID:23871123; http://dx.doi.org/ 10.1016/j.neurobiolaging.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 50.Gan-Or Z, Ozelius LJ, Bar-Shira A, Saunders-Pullman R, Mirelman A, Kornreich R, Gana-Weisz M, Raymond D, Rozenkrantz L, Deik A, et al.. The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology 2013; 80:1606-10; PMID:23535491; http://dx.doi.org/ 10.1212/WNL.0b013e31828f180e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069-75; PMID:18305538; http://dx.doi.org/ 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al.. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 2005; 169:425-34; PMID:15866887; http://dx.doi.org/ 10.1083/jcb.200412022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature 2004; 432:1032-6; PMID:15525940; http://dx.doi.org/ 10.1038/nature03029 [DOI] [PubMed] [Google Scholar]
- 54.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al.. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885-9; PMID:16625204; http://dx.doi.org/ 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- 55.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al.. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441:880-4; PMID:16625205; http://dx.doi.org/ 10.1038/nature04723 [DOI] [PubMed] [Google Scholar]
- 56.Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, Komatsu M, Holstein GR, Yue Z. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of α-synuclein and LRRK2 in the brain. J Neurosci 2012; 32:7585-93; PMID:22649237; http://dx.doi.org/ 10.1523/JNEUROSCI.5809-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, et al.. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007; 131:1149-63; PMID:18083104; http://dx.doi.org/ 10.1016/j.cell.2007.10.035 [DOI] [PubMed] [Google Scholar]
- 58.Lim J, Kim HW, Youdim MB, Rhyu IJ, Choe KM, Oh YJ. Binding preference of p62 towards LC3-ll during dopaminergic neurotoxin-induced impairment of autophagic flux. Autophagy 2011; 7:51-60; PMID:21045561; http://dx.doi.org/ 10.4161/auto.7.1.13909 [DOI] [PubMed] [Google Scholar]
- 59.Ramesh Babu J, Lamar Seibenhener M, Peng J, Strom AL, Kemppainen R, Cox N, Zhu H, Wooten MC, Diaz-Meco MT, Moscat J, et al.. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem 2008; 106:107-20; PMID:18346206; http://dx.doi.org/ 10.1111/j.1471-4159.2008.05340.x [DOI] [PubMed] [Google Scholar]
- 60.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 2014; 15:634-46; PMID:25237825; http://dx.doi.org/ 10.1038/nrm3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat 2008; 29:567-83; PMID:18338393; http://dx.doi.org/ 10.1002/humu.20676 [DOI] [PubMed] [Google Scholar]
- 62.Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, Reches A, Bembi B, Zimran A. Occurrence of Parkinson syndrome in type I Gaucher disease. QJM 1996; 89:691-4; PMID:8917744; http://dx.doi.org/ 10.1093/qjmed/89.9.691 [DOI] [PubMed] [Google Scholar]
- 63.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson disease in Ashkenazi Jews. N Engl J Med 2004; 351:1972-7; PMID:15525722; http://dx.doi.org/ 10.1056/NEJMoa033277 [DOI] [PubMed] [Google Scholar]
- 64.Zimran A, Neudorfer O, Elstein D. The glucocerebrosidase gene and Parkinson disease in Ashkenazi Jews. N Engl J Med 2005; 352:728-31; author reply -31; PMID:15719452; http://dx.doi.org/ 10.1056/NEJM200502173520719 [DOI] [PubMed] [Google Scholar]
- 65.Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, Bar-Shira A, Orr-Urtreger A. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology 2008; 70:2277-83; PMID:18434642; http://dx.doi.org/ 10.1212/01.wnl.0000304039.11891.29 [DOI] [PubMed] [Google Scholar]
- 66.Beutler E, Gelbart T, Scott CR. Hematologically important mutations: Gaucher disease. Blood Cells Mol Dis 2005; 35:355-64; PMID:16185900; http://dx.doi.org/ 10.1016/j.bcmd.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 67.Gan-Or Z, Amshalom I, Kilarski LL, Bar-Shira A, Gana-Weisz M, Mirelman A, Marder K, Bressman S, Giladi N, Orr-Urtreger A. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology 2015; 84(9):880-7; PMID:25653295; http://dx.doi.org/10.1212/WNL.0000000000001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Velayati A, Yu WH, Sidransky E. The role of glucocerebrosidase mutations in Parkinson disease and Lewy body disorders. Curr Neurol Neurosci Rep 2010; 10:190-8; PMID:20425034; http://dx.doi.org/ 10.1007/s11910-010-0102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manning-Bog AB, Schule B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology 2009; 30:1127-32; PMID:19576930; http://dx.doi.org/ 10.1016/j.neuro.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 70.Argyriou A, Dermentzaki G, Papasilekas T, Moraitou M, Stamboulis E, Vekrellis K, Michelakakis H, Stefanis L. Increased dimerization of α-synuclein in erythrocytes in Gaucher disease and aging. Neurosci Lett 2012; 528:205-9; PMID:22981881; http://dx.doi.org/ 10.1016/j.neulet.2012.08.069 [DOI] [PubMed] [Google Scholar]
- 71.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 2011; 146:37-52; PMID:21700325; http://dx.doi.org/ 10.1016/j.cell.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, Stanek LM, Passini MA, Grabowski GA, Schlossmacher MG, Sidman RL, et al.. CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci U S A 2011; 108:12101-6; PMID:21730160; http://dx.doi.org/ 10.1073/pnas.1108197108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cullen V, Sardi SP, Ng J, Xu YH, Sun Y, Tomlinson JJ, Kolodziej P, Kahn I, Saftig P, Woulfe J, et al.. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann Neurol 2011; 69:940-53; PMID:21472771; http://dx.doi.org/ 10.1002/ana.22400 [DOI] [PubMed] [Google Scholar]
- 74.Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B, Halliday GM. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson disease. Brain 2014; 137:834-48; PMID:24477431; http://dx.doi.org/ 10.1093/brain/awt367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bae EJ, Yang NY, Song M, Lee CS, Lee JS, Jung BC, Lee HJ, Kim S, Masliah E, Sardi SP, et al.. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat Commun 2014; 5:4755; PMID:25156829; http://dx.doi.org/ 10.1038/ncomms5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ron I, Rapaport D, Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet 2010; 19:3771-81; PMID:20643691; http://dx.doi.org/ 10.1093/hmg/ddq292 [DOI] [PubMed] [Google Scholar]
- 77.Sun Y, Liou B, Ran H, Skelton MR, Williams MT, Vorhees CV, Kitatani K, Hannun YA, Witte DP, Xu YH, et al.. Neuronopathic Gaucher disease in the mouse: viable combined selective saposin C deficiency and mutant glucocerebrosidase (V394L) mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits. Hum Mol Genet 2010; 19:1088-97; PMID:20047948; http://dx.doi.org/ 10.1093/hmg/ddp580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeigler M, Meiner V, Newman JP, Steiner-Birmanns B, Bargal R, Sury V, Mengistu G, Kakhlon O, Leykin I, Argov Z, et al.. A novel SCARB2 mutation in progressive myoclonus epilepsy indicated by reduced β-glucocerebrosidase activity. J Neurol Sci 2014; 339:210-3; PMID:24485911; http://dx.doi.org/ 10.1016/j.jns.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 79.Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, Hernandez DG, Nalls MA, Clark LN, Honig LS, et al.. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet 2014; 23:6139-46; PMID:24973356; http://dx.doi.org/ 10.1093/hmg/ddu334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clark LN, Kartsaklis LA, Wolf Gilbert R, Dorado B, Ross BM, Kisselev S, Verbitsky M, Mejia-Santana H, Cote LJ, Andrews H, et al.. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol 2009; 66:578-83; PMID:19433657; http://dx.doi.org/ 10.1001/archneurol.2009.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, et al.. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 2012; 79:1944-50; PMID:23035075; http://dx.doi.org/ 10.1212/WNL.0b013e3182735e9a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson disease: Potential role of ceramide metabolism in Lewy body disease. FEBS J 2008; 275:5767-73; PMID:19021754; http://dx.doi.org/ 10.1111/j.1742-4658.2008.06709.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabande-Rodriguez E, Boya P, Labrador V, Dotti CG, Ledesma MD. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ 2014; 21:864-75; PMID:24488099; http://dx.doi.org/ 10.1038/cdd.2014.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X, Xu M, Pitzer AL, Xia M, Boini KM, Li PL, Zhang Y. Control of autophagy maturation by acid sphingomyelinase in mouse coronary arterial smooth muscle cells: protective role in atherosclerosis. J Mol Med (Berl) 2014; 92:473-85; PMID:24463558; http://dx.doi.org/ 10.1007/s00109-014-1120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buechner S, De Cristofaro MT, Ramat S, Borsini W. Parkinsonism and Anderson Fabry's disease: a case report. Mov Disord 2006; 21:103-7; PMID:16149089; http://dx.doi.org/ 10.1002/mds.20675 [DOI] [PubMed] [Google Scholar]
- 86.Wu G, Yan B, Wang X, Feng X, Zhang A, Xu X, Dong H. Decreased activities of lysosomal acid α-D-galactosidase A in the leukocytes of sporadic Parkinson disease. J Neurol Sci 2008; 271:168-73; PMID:18495164; http://dx.doi.org/ 10.1016/j.jns.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 87.Wu G, Huang J, Feng X, Zhang A, Li J, Pang S, Gu K, Dong H, Zhang J, Gao H, et al.. Decreased expression of lysosomal α-galactosiase A gene in sporadic Parkinson disease. Neurochem Res 2011; 36:1939-44; PMID:21643977; http://dx.doi.org/ 10.1007/s11064-011-0516-0 [DOI] [PubMed] [Google Scholar]
- 88.Wu G, Pang S, Feng X, Zhang A, Li J, Gu K, Huang J, Dong H, Yan B. Genetic analysis of lysosomal α-galactosidase A gene in sporadic Parkinson disease. Neurosci Lett 2011; 500:31-5; PMID:21683120; http://dx.doi.org/ 10.1016/j.neulet.2011.05.238 [DOI] [PubMed] [Google Scholar]
- 89.Zech M, Nubling G, Castrop F, Jochim A, Schulte EC, Mollenhauer B, Lichtner P, Peters A, Gieger C, Marquardt T, et al.. Niemann-Pick C disease gene mutations and age-related neurodegenerative disorders. PLoS One 2013; 8:e82879; PMID:24386122; http://dx.doi.org/ 10.1371/journal.pone.0082879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kluenemann HH, Nutt JG, Davis MY, Bird TD. Parkinsonism syndrome in heterozygotes for Niemann-Pick C1. J Neurol Sci 2013; 335:219-20; PMID:24035292; http://dx.doi.org/ 10.1016/j.jns.2013.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Josephs KA, Matsumoto JY, Lindor NM. Heterozygous Niemann-Pick disease type C presenting with tremor. Neurology 2004; 63:2189-90; PMID:15596783; http://dx.doi.org/ 10.1212/01.WNL.0000145710.25588.2F [DOI] [PubMed] [Google Scholar]
- 92.Chiba Y, Komori H, Takei S, Hasegawa-Ishii S, Kawamura N, Adachi K, Nanba E, Hosokawa M, Enokido Y, Kouchi Z, et al.. Niemann-Pick disease type C1 predominantly involving the frontotemporal region, with cortical and brainstem Lewy bodies: an autopsy case. Neuropathology 2014; 34:49-57; PMID:23711246; http://dx.doi.org/ 10.1111/neup.12047 [DOI] [PubMed] [Google Scholar]
- 93.Saito Y, Suzuki K, Hulette CM, Murayama S. Aberrant phosphorylation of α-synuclein in human Niemann-Pick type C1 disease. J Neuropathol Exp Neurol 2004; 63:323-8; PMID:15099022 [DOI] [PubMed] [Google Scholar]
- 94.Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol 2002; 51:296-301; http://dx.doi.org/ 10.1002/ana.10113 [DOI] [PubMed] [Google Scholar]
- 95.Hulihan MM, Ishihara-Paul L, Kachergus J, Warren L, Amouri R, Elango R, Prinjha RK, Upmanyu R, Kefi M, Zouari M, et al.. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a case-control genetic study. Lancet Neurol 2008; 7:591-4; PMID:18539535; http://dx.doi.org/ 10.1016/S1474-4422(08)70116-9 [DOI] [PubMed] [Google Scholar]
- 96.Lesage S, Durr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, Pollak P, Brice A, French Parkinson Disease Genetics Study G . LRRK2 G2019S as a cause of Parkinson disease in North African Arabs. N Engl J Med 2006; 354:422-3; PMID:16436781; http://dx.doi.org/ 10.1056/NEJMc055540 [DOI] [PubMed] [Google Scholar]
- 97.Gan-Or Z, Bar-Shira A, Mirelman A, Gurevich T, Kedmi M, Giladi N, Orr-Urtreger A. LRRK2 and GBA mutations differentially affect the initial presentation of Parkinson disease. Neurogenetics 2010; 11:121-5; PMID:19458969; http://dx.doi.org/ 10.1007/s10048-009-0198-9 [DOI] [PubMed] [Google Scholar]
- 98.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, et al.. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson disease: a case-control study. Lancet Neurol 2008; 7:583-90; PMID:18539534; http://dx.doi.org/ 10.1016/S1474-4422(08)70117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, et al.. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 2013; 16:394-406; PMID:23455607; http://dx.doi.org/ 10.1038/nn.3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plowey ED, Cherra SJ 3rd, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem 2008; 105:1048-56; PMID:18182054; http://dx.doi.org/ 10.1111/j.1471-4159.2008.05217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet 2009; 18:4022-34; PMID:19640926; http://dx.doi.org/ 10.1093/hmg/ddp346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramonet D, Daher JP, Lin BM, Stafa K, Kim J, Banerjee R, Westerlund M, Pletnikova O, Glauser L, Yang L, et al.. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One 2011; 6:e18568; PMID:21494637; http://dx.doi.org/ 10.1371/journal.pone.0018568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dodson MW, Leung LK, Lone M, Lizzio MA, Guo M. Novel ethyl methanesulfonate (EMS)-induced null alleles of the Drosophila homolog of LRRK2 reveal a crucial role in endolysosomal functions and autophagy in vivo. Dis Model Mech 2014; 7:1351-63; PMID:25288684; http://dx.doi.org/ 10.1242/dmm.017020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hakimi M, Selvanantham T, Swinton E, Padmore RF, Tong Y, Kabbach G, Venderova K, Girardin SE, Bulman DE, Scherzer CR, et al.. Parkinson disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J Neural Transm 2011; 118:795-808; PMID:21552986; http://dx.doi.org/ 10.1007/s00702-011-0653-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of α-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A 2010; 107:9879-84; PMID:20457918; http://dx.doi.org/ 10.1073/pnas.1004676107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiong Y, Coombes CE, Kilaru A, Li X, Gitler AD, Bowers WJ, Dawson VL, Dawson TM, Moore DJ. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet 2010; 6:e1000902; PMID:20386743; http://dx.doi.org/ 10.1371/journal.pgen.1000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Su YC, Guo X, Qi X. Threonine 56 phosphorylation of Bcl−2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy. Biochim Biophys Acta 2015; 1852:12-21; PMID:25446991; http://dx.doi.org/ 10.1016/j.bbadis.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson disease. Acta Neuropathol 2012; 124:325-38; PMID:22806825; http://dx.doi.org/ 10.1007/s00401-012-1013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson disease. Nat Rev Neurosci 2010; 11:791-7; PMID:21088684; http://dx.doi.org/ 10.1038/nrn2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schule B, Langston JW, Middleton FA, Ross OA, Hulihan M, et al.. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology 2007; 68:916-22; PMID:17251522; http://dx.doi.org/ 10.1212/01.wnl.0000254458.17630.c5 [DOI] [PubMed] [Google Scholar]
- 111.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al.. α-Synuclein locus triplication causes Parkinson disease. Science 2003; 302:841; PMID:14593171; http://dx.doi.org/ 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- 112.Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, Pieri L, Madiona K, Durr A, Melki R, et al.. G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol 2013; 73:459-71; PMID:23526723; http://dx.doi.org/ 10.1002/ana.23894 [DOI] [PubMed] [Google Scholar]
- 113.Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel α-synuclein missense mutation in Parkinson disease. Neurology 2013; 80:1062-4; PMID:23427326; http://dx.doi.org/ 10.1212/WNL.0b013e31828727ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, et al.. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 2004; 55:164-73; PMID:14755719; http://dx.doi.org/ 10.1002/ana.10795 [DOI] [PubMed] [Google Scholar]
- 115.Sardi SP, Clarke J, Viel C, Chan M, Tamsett TJ, Treleaven CM, Bu J, Sweet L, Passini MA, Dodge JC, et al.. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci U S A 2013; 110:3537-42; PMID:23297226; http://dx.doi.org/ 10.1073/pnas.1220464110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Auluck PK, Caraveo G, Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson disease. Annu Rev Cell Dev Biol 2010; 26:211-33; PMID:20500090; http://dx.doi.org/ 10.1146/annurev.cellbio.042308.113313 [DOI] [PubMed] [Google Scholar]
- 117.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 2003; 278:25009-13; PMID:12719433; http://dx.doi.org/ 10.1074/jbc.M300227200 [DOI] [PubMed] [Google Scholar]
- 118.Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 2008; 283:23542-56; PMID:18566453; http://dx.doi.org/ 10.1074/jbc.M801992200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu WH, Dorado B, Figueroa HY, Wang L, Planel E, Cookson MR, Clark LN, Duff KE. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric α-synuclein. Am J Pathol 2009; 175:736-47; PMID:19628769; http://dx.doi.org/ 10.2353/ajpath.2009.080928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal degradation of α-synuclein in vivo. J Biol Chem 2010; 285:13621-9; PMID:20200163; http://dx.doi.org/ 10.1074/jbc.M109.074617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Abberant α-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One 2009; 4:e5515; PMID:19436756; http://dx.doi.org/ 10.1371/journal.pone.0005515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, et al.. Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J Clin Invest 2008; 118:777-88; PMID:18172548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Q, She H, Gearing M, Colla E, Lee M, Shacka JJ, Mao Z. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 2009; 323:124-7; PMID:19119233; http://dx.doi.org/ 10.1126/science.1166088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kabuta T, Furuta A, Aoki S, Furuta K, Wada K. Aberrant interaction between Parkinson disease-associated mutant UCH-L1 and the lysosomal receptor for chaperone-mediated autophagy. J Biol Chem 2008; 283:23731-8; PMID:18550537; http://dx.doi.org/ 10.1074/jbc.M801918200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang Y, Chegini F, Chua G, Murphy K, Gai W, Halliday GM. Macroautophagy in sporadic and the genetic form of Parkinson disease with the A53T α-synuclein mutation. Transl Neurodegener 2012; 1:2; PMID:23210740; http://dx.doi.org/ 10.1186/2047-9158-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J Biol Chem 2007; 282:5641-52; PMID:17182613; http://dx.doi.org/ 10.1074/jbc.M609532200 [DOI] [PubMed] [Google Scholar]
- 127.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al.. TFEB links autophagy to lysosomal biogenesis. Science 2011; 332:1429-33; PMID:21617040; http://dx.doi.org/ 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci U S A 2013; 110:E1817-26; PMID:23610405; http://dx.doi.org/ 10.1073/pnas.1305623110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar KR, Weissbach A, Heldmann M, Kasten M, Tunc S, Sue CM, Svetel M, Kostic VS, Segura-Aguilar J, Ramirez A, et al.. Frequency of the D620N mutation in VPS35 in Parkinson disease. Arch Neurol 2012; 69:1360-4; PMID:22801713; http://dx.doi.org/ 10.1001/archneurol.2011.3367 [DOI] [PubMed] [Google Scholar]
- 130.Lesage S, Condroyer C, Klebe S, Honore A, Tison F, Brefel-Courbon C, Durr A, Brice A, French Parkinson Disease Genetics Study G . Identification of VPS35 mutations replicated in French families with Parkinson disease. Neurology 2012; 78:1449-50; PMID:22517097; http://dx.doi.org/ 10.1212/WNL.0b013e318253d5f2 [DOI] [PubMed] [Google Scholar]
- 131.Sheerin UM, Charlesworth G, Bras J, Guerreiro R, Bhatia K, Foltynie T, Limousin P, Silveira-Moriyama L, Lees A, Wood N. Screening for VPS35 mutations in Parkinson disease. Neurobiol Aging 2012; 33:838 e1-5; PMID:22154191; http://dx.doi.org/ 10.1016/j.neurobiolaging.2011.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 2006; 7:568-79; PMID:16936697; http://dx.doi.org/ 10.1038/nrm1985 [DOI] [PubMed] [Google Scholar]
- 133.Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson disease impairs WASH complex association and inhibits autophagy. Nat Commun 2014; 5:3828; PMID:24819384; http://dx.doi.org/ 10.1038/ncomms4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seaman MN, Gautreau A, Billadeau DD. Retromer-mediated endosomal protein sorting: all WASHed up! Trends Cell Biol 2013; 23:522-8; PMID:23721880; http://dx.doi.org/ 10.1016/j.tcb.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Freeman CL, Hesketh G, Seaman MN. RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J Cell Sci 2014; 127:2053-70; PMID:24643499; http://dx.doi.org/ 10.1242/jcs.144659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hermle T, Guida MC, Beck S, Helmstadter S, Simons M. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J 2013; 32:245-59; PMID:23292348; http://dx.doi.org/ 10.1038/emboj.2012.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 2007; 8:917-29; PMID:17912264; http://dx.doi.org/ 10.1038/nrm2272 [DOI] [PubMed] [Google Scholar]
- 138.George AA, Hayden S, Holzhausen LC, Ma EY, Suzuki SC, Brockerhoff SE. Synaptojanin 1 is required for endolysosomal trafficking of synaptic proteins in cone photoreceptor inner segments. PLoS One 2014; 9:e84394; PMID:24392132; http://dx.doi.org/ 10.1371/journal.pone.0084394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Y, Dorn GW. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013; 340:471-5; PMID:23620051; http://dx.doi.org/ 10.1126/science.1231031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 2010; 19:4861-70; PMID:20871098; http://dx.doi.org/ 10.1093/hmg/ddq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Narendra D, Walker JE, Youle R. Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb Perspect Biol 2012; 4; PMID:23125018; http://dx.doi.org/ 10.1101/cshperspect.a011338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8:e1000298; PMID:20126261; http://dx.doi.org/ 10.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al.. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 2010; 107:378-83; PMID:19966284; http://dx.doi.org/ 10.1073/pnas.0911187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Geisler S, Holmstrom KM, Treis A, Skujat D, Weber SS, Fiesel FC, Kahle PJ, Springer W. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy 2010; 6:871-8; PMID:20798600; http://dx.doi.org/ 10.4161/auto.6.7.13286 [DOI] [PubMed] [Google Scholar]
- 145.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010; 12:119-31; PMID:20098416; http://dx.doi.org/ 10.1038/ncb2012 [DOI] [PubMed] [Google Scholar]
- 146.Dagda RK, Cherra SJ 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem 2009; 284:13843-55; PMID:19279012; http://dx.doi.org/ 10.1074/jbc.M808515200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.de Pablo-Latorre R, Saide A, Polishhuck EV, Nusco E, Fraldi A, Ballabio A. Impaired parkin-mediated mitochondrial targeting to autophagosomes differentially contributes to tissue pathology in lysosomal storage diseases. Hum Mol Genet 2012; 21:1770-81; PMID:22215441; http://dx.doi.org/ 10.1093/hmg/ddr610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spina S, Van Laar AD, Murrell JR, Hamilton RL, Kofler JK, Epperson F, Farlow MR, Lopez OL, Quinlan J, DeKosky ST, et al.. Phenotypic variability in three families with valosin-containing protein mutation. Eur J Neurol 2013; 20:251-8; PMID:22900631; http://dx.doi.org/ 10.1111/j.1468-1331.2012.03831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim NC, Tresse E, Kolaitis RM, Molliex A, Thomas RE, Alami NH, Wang B, Joshi A, Smith RB, Ritson GP, et al.. VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron 2013; 78:65-80; PMID:23498974; http://dx.doi.org/ 10.1016/j.neuron.2013.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet 2011; 20:40-50; PMID:20940149; http://dx.doi.org/ 10.1093/hmg/ddq430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci U S A 2010; 107:9747-52; PMID:20457924; http://dx.doi.org/ 10.1073/pnas.0911175107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Joselin AP, Hewitt SJ, Callaghan SM, Kim RH, Chung YH, Mak TW, Shen J, Slack RS, Park DS. ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Hum Mol Genet 2012; 21:4888-903; PMID:22872702; http://dx.doi.org/ 10.1093/hmg/dds325 [DOI] [PubMed] [Google Scholar]
- 153.Lopes da Fonseca T, Outeiro TF. ATP13A2 and Alpha-synuclein: a Metal Taste in Autophagy. Exp Neurobiol 2014; 23:314-23; PMID:25548531; http://dx.doi.org/ 10.5607/en.2014.23.4.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grunewald A, Arns B, Seibler P, Rakovic A, Munchau A, Ramirez A, Sue CM, Klein C. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome. Neurobiol Aging 2012; 33:1843 e1-7; PMID:22296644; http://dx.doi.org/ 10.1016/j.neurobiolaging.2011.12.035 [DOI] [PubMed] [Google Scholar]
- 155.Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, Tsika E, Pletnikova O, Troncoso JC, Glauser L, Moore DJ. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet 2012; 21:1725-43; PMID:22186024; http://dx.doi.org/ 10.1093/hmg/ddr606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gusdon AM, Zhu J, Van Houten B, Chu CT. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis 2012; 45:962-72; PMID:22198378; http://dx.doi.org/ 10.1016/j.nbd.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J Neurosci 2012; 32:4240-6; PMID:22442086; http://dx.doi.org/ 10.1523/JNEUROSCI.5575-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Di Fonzo A, Dekker MC, Montagna P, Baruzzi A, Yonova EH, Correia Guedes L, Szczerbinska A, Zhao T, Dubbel-Hulsman LO, Wouters CH, et al.. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology 2009; 72:240-5; PMID:19038853; http://dx.doi.org/ 10.1212/01.wnl.0000338144.10967.2b [DOI] [PubMed] [Google Scholar]
- 159.Paisan-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, Schneider SA, Schwingenschuh P, Bajaj N, Emre M, et al.. Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord 2010; 25:1791-800; PMID:20669327; http://dx.doi.org/ 10.1002/mds.23221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lohmann E, Coquel AS, Honore A, Gurvit H, Hanagasi H, Emre M, Leutenegger AL, Drouet V, Sahbatou M, Guven G, et al.. A new F-box protein 7 gene mutation causing typical Parkinson disease. Mov Disord 2015; 30(8):1130-3; http://dx.doi.org/10.1002/mds.26266 [DOI] [PubMed] [Google Scholar]
- 161.Burchell VS, Nelson DE, Sanchez-Martinez A, Delgado-Camprubi M, Ivatt RM, Pogson JH, Randle SJ, Wray S, Lewis PA, Houlden H, et al.. The Parkinson disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat Neurosci 2013; 16:1257-65; PMID:23933751; http://dx.doi.org/ 10.1038/nn.3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gan-Or Z, Bar-Shira A, Dahary D, Mirelman A, Kedmi M, Gurevich T, Giladi N, Orr-Urtreger A. Association of sequence alterations in the putative promoter of RAB7L1 with a reduced parkinson disease risk. Arch Neurol 2012; 69:105-10; PMID:22232350; http://dx.doi.org/ 10.1001/archneurol.2011.924 [DOI] [PubMed] [Google Scholar]
- 163.Ramirez A, Ziegler A, Winkler S, Kottwitz J, Giesen R, Diaz-Grez F, Miranda M, Venegas P, Godoy OT, Avello R, et al.. Association of Parkinson disease to PARK16 in a Chilean sample. Parkinsonism Relat Disord 2011; 17:70-1; PMID:20934365; http://dx.doi.org/ 10.1016/j.parkreldis.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 164.Tan EK, Kwok HH, Tan LC, Zhao WT, Prakash KM, Au WL, Pavanni R, Ng YY, Satake W, Zhao Y, et al.. Analysis of GWAS-linked loci in Parkinson disease reaffirms PARK16 as a susceptibility locus. Neurology 2010; 75:508-12; PMID:20697102; http://dx.doi.org/ 10.1212/WNL.0b013e3181eccfcd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vilarino-Guell C, Ross OA, Aasly JO, White LR, Rajput A, Rajput AH, Lynch T, Krygowska-Wajs A, Jasinska-Myga B, Opala G, et al.. An independent replication of PARK16 in Asian samples. Neurology 2010; 75:2248-9; PMID:21172849; http://dx.doi.org/ 10.1212/WNL.0b013e318202031f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yan YP, Mo XY, Tian J, Zhao GH, Yin XZ, Jin FY, Zhang BR. An association between the PARK16 locus and Parkinson disease in a cohort from eastern China. Parkinsonism Relat Disord 2011; 17:737-9; PMID:21840748; http://dx.doi.org/ 10.1016/j.parkreldis.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 167.Ivatt RM, Sanchez-Martinez A, Godena VK, Brown S, Ziviani E, Whitworth AJ. Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proc Natl Acad Sci U S A 2014; 111:8494-9; PMID:24912190; http://dx.doi.org/ 10.1073/pnas.1321207111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garver WS, Jelinek D, Francis GA, Murphy BD. The Niemann-Pick C1 gene is downregulated by feedback inhibition of the SREBP pathway in human fibroblasts. J Lipid Res 2008; 49:1090-102; PMID:18272927; http://dx.doi.org/ 10.1194/jlr.M700555-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Puri V, Jefferson JR, Singh RD, Wheatley CL, Marks DL, Pagano RE. Sphingolipid storage induces accumulation of intracellular cholesterol by stimulating SREBP-1 cleavage. J Biol Chem 2003; 278:20961-70; PMID:12657626; http://dx.doi.org/ 10.1074/jbc.M300304200 [DOI] [PubMed] [Google Scholar]
- 170.Conrad C, Andreadis A, Trojanowski JQ, Dickson DW, Kang D, Chen X, Wiederholt W, Hansen L, Masliah E, Thal LJ, et al.. Genetic evidence for the involvement of tau in progressive supranuclear palsy. Ann Neurol 1997; 41:277-81; PMID:9029080; http://dx.doi.org/ 10.1002/ana.410410222 [DOI] [PubMed] [Google Scholar]
- 171.Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A, et al.. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 2001; 56:1702-6; PMID:11425937; http://dx.doi.org/ 10.1212/WNL.56.12.1702 [DOI] [PubMed] [Google Scholar]
- 172.Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, de Silva R, Lees A, Dickson DW. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 2002; 61:547-56; PMID:12071638 [DOI] [PubMed] [Google Scholar]
- 173.Dumanchin C, Camuzat A, Campion D, Verpillat P, Hannequin D, Dubois B, Saugier-Veber P, Martin C, Penet C, Charbonnier F, et al.. Segregation of a missense mutation in the microtubule-associated protein tau gene with familial frontotemporal dementia and parkinsonism. Hum Mol Genet 1998; 7:1825-9; PMID:9736786; http://dx.doi.org/ 10.1093/hmg/7.11.1825 [DOI] [PubMed] [Google Scholar]
- 174.Di Fonzo A, Ronchi D, Gallia F, Cribiu FM, Trezzi I, Vetro A, Della Mina E, Limongelli I, Bellazzi R, Ricca I, et al.. Lower motor neuron disease with respiratory failure caused by a novel MAPT mutation. Neurology 2014; 82:1990-8; PMID:24808015; http://dx.doi.org/ 10.1212/WNL.0000000000000476 [DOI] [PubMed] [Google Scholar]
- 175.Allen M, Kachadoorian M, Quicksall Z, Zou F, Chai HS, Younkin C, Crook JE, Pankratz VS, Carrasquillo MM, Krishnan S, et al.. Association of MAPT haplotypes with Alzheimer disease risk and MAPT brain gene expression levels. Alzheimers Res Ther 2014; 6:39; PMID:25324900; http://dx.doi.org/ 10.1186/alzrt268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Desikan RS, Schork AJ, Wang Y, Witoelar A, Sharma M, McEvoy LK, Holland D, Brewer JB, Chen CH, Thompson WK, et al.. Genetic overlap between Alzheimer disease and Parkinson disease at the MAPT locus. Mol Psychiatry 2015; http://dx.doi.org/10.1038/mp.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gerrish A, Russo G, Richards A, Moskvina V, Ivanov D, Harold D, Sims R, Abraham R, Hollingworth P, Chapman J, et al.. The role of variation at AbetaPP, PSEN1, PSEN2, and MAPT in late onset Alzheimer disease. J Alzheimers Dis 2012; 28:377-87; PMID:22027014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cardenas AM, Ardiles AO, Barraza N, Baez-Matus X, Caviedes P. Role of tau protein in neuronal damage in Alzheimer disease and Down syndrome. Arch Med Res 2012; 43:645-54; PMID:23142525; http://dx.doi.org/ 10.1016/j.arcmed.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 179.Correia SC, Resende R, Moreira PI, Pereira CM. Alzheimer Disease-Related Misfolded Proteins and Dysfunctional Organelles on Autophagy Menu. DNA Cell Biol 2015; 34(4):261-73; PMID:25664381; http://dx.doi.org/10.1089/dna.2014.2757 [DOI] [PubMed] [Google Scholar]
- 180.Peric A, Annaert W. Early etiology of Alzheimer disease: tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol 2015; 129:363-81; PMID:25556159; http://dx.doi.org/ 10.1007/s00401-014-1379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet 2009; 18:4153-70; PMID:19654187; http://dx.doi.org/ 10.1093/hmg/ddp367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mujcic H, Rzymski T, Rouschop KM, Koritzinsky M, Milani M, Harris AL, Wouters BG. Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol 2009; 92:450-9; PMID:19726095; http://dx.doi.org/ 10.1016/j.radonc.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 183.Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson disease. J Parkinsons Dis 2013; 3:493-514; PMID:24275605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Morscher RJ, Grunert SC, Burer C, Burda P, Suormala T, Fowler B, Baumgartner MR. A single mutation in MCCC1 or MCCC2 as a potential cause of positive screening for 3-methylcrotonyl-CoA carboxylase deficiency. Mol Genet Metab 2012; 105:602-6; PMID:22264772; http://dx.doi.org/ 10.1016/j.ymgme.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 185.Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, Xie ZL, Speake LD, Parks R, Crabtree D, et al.. Lysosomal enzyme cathepsin D protects against α-synuclein aggregation and toxicity. Mol Brain 2008; 1:17; PMID:19021916; http://dx.doi.org/ 10.1186/1756-6606-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of α-synuclein and generation of its carboxy-terminally truncated species. Biochemistry 2008; 47:9678-87; PMID:18702517; http://dx.doi.org/ 10.1021/bi800699v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Okamoto I, Pirker C, Bilban M, Berger W, Losert D, Marosi C, Haas OA, Wolff K, Pehamberger H. Seven novel and stable translocations associated with oncogenic gene expression in malignant melanoma. Neoplasia 2005; 7:303-11; PMID:15967107; http://dx.doi.org/ 10.1593/neo.04514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu R, Gao X, Lu Y, Chen H. Meta-analysis of the relationship between Parkinson disease and melanoma. Neurology 2011; 76:2002-9; PMID:21646627; http://dx.doi.org/ 10.1212/WNL.0b013e31821e554e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu W, Tan L, Yu JT. The link between the SNCA gene and parkinsonism. Neurobiol Aging 2015; 36(3):1505-18; http://dx.doi.org/10.1016/j.neurobiolaging.2014.10.042 [DOI] [PubMed] [Google Scholar]
- 190.Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol 2008; 28:5747-63; PMID:18644871; http://dx.doi.org/ 10.1128/MCB.02070-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kaushik S, Massey AC, Cuervo AM. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J 2006; 25:3921-33; PMID:16917501; http://dx.doi.org/ 10.1038/sj.emboj.7601283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hein LK, Duplock S, Hopwood JJ, Fuller M. Lipid composition of microdomains is altered in a cell model of Gaucher disease. J Lipid Res 2008; 49:1725-34; PMID:18427156; http://dx.doi.org/ 10.1194/jlr.M800092-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hattersley KJ, Hein LK, Fuller M. Lipid composition of membrane rafts, isolated with and without detergent, from the spleen of a mouse model of Gaucher disease. Biochem Biophys Res Commun 2013; 442:62-7; PMID:24220330; http://dx.doi.org/ 10.1016/j.bbrc.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 194.Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, Wood NW, Hardy J, Mark Cooper J, Schapira AH. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int 2013; 62:1-7; PMID:23099359; http://dx.doi.org/ 10.1016/j.neuint.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AH, Duchen MR. Mitochondria and quality control defects in a mouse model of Gaucher disease-links to Parkinson disease. Cell Metab 2013; 17:941-53; PMID:23707074; http://dx.doi.org/ 10.1016/j.cmet.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, Schapira AH. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol 2012; 72:455-63; PMID:23034917; http://dx.doi.org/ 10.1002/ana.23614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parnetti L, Chiasserini D, Persichetti E, Eusebi P, Varghese S, Qureshi MM, Dardis A, Deganuto M, De Carlo C, Castrioto A, et al.. Cerebrospinal fluid lysosomal enzymes and α-synuclein in Parkinson disease. Mov Disord 2014; 29:1019-27; PMID:24436092; http://dx.doi.org/ 10.1002/mds.25772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound α-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol Genet Metab 2013; 108:56-64; PMID:23266198; http://dx.doi.org/ 10.1016/j.ymgme.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC, Martinez-Vicente M, Cuervo AM. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci 2007; 120:782-91; PMID:17284523; http://dx.doi.org/ 10.1242/jcs.001073 [DOI] [PubMed] [Google Scholar]
- 200.Lee BD, Shin JH, VanKampen J, Petrucelli L, West AB, Ko HS, Lee YI, Maguire-Zeiss KA, Bowers WJ, Federoff HJ, et al.. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson disease. Nat Med 2010; 16:998-1000; PMID:20729864; http://dx.doi.org/ 10.1038/nm.2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu Z, Hamamichi S, Lee BD, Yang D, Ray A, Caldwell GA, Caldwell KA, Dawson TM, Smith WW, Dawson VL. Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson disease models. Hum Mol Genet 2011; 20:3933-42; PMID:21768216; http://dx.doi.org/ 10.1093/hmg/ddr312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Surmeier DJ, Guzman JN, Sanchez-Padilla J, Schumacker PT. The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson disease. Neuroscience 2011; 198:221-31; PMID:21884755; http://dx.doi.org/ 10.1016/j.neuroscience.2011.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 2008; 9:1004-10; PMID:18971948; http://dx.doi.org/ 10.1038/nrm2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 2005; 115:2679-88; PMID:16200202; http://dx.doi.org/ 10.1172/JCI26390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 2004; 304:1500-2; PMID:15131264; http://dx.doi.org/ 10.1126/science.1096645 [DOI] [PubMed] [Google Scholar]
- 206.Kovacs GG, Breydo L, Green R, Kis V, Puska G, Lorincz P, Perju-Dumbrava L, Giera R, Pirker W, Lutz M, et al.. Intracellular processing of disease-associated α-synuclein in the human brain suggests prion-like cell-to-cell spread. Neurobiol Dis 2014; 69:76-92; PMID:24878508; http://dx.doi.org/ 10.1016/j.nbd.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 207.Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson disease and Huntington's disease: future therapeutic perspectives. Lancet 2014; 384:545-55; PMID:24954676; http://dx.doi.org/ 10.1016/S0140-6736(14)61010-2 [DOI] [PubMed] [Google Scholar]
- 208.Rivero-Rios P, Gomez-Suaga P, Fdez E, Hilfiker S. Upstream deregulation of calcium signaling in Parkinson disease. Front Mol Neurosci 2014; 7:53; PMID:24987329 [DOI] [PMC free article] [PubMed] [Google Scholar]