Abstract
Background
The eye disorder associated with Graves’ disease, called Graves’ ophthalmopathy (GO), greatly reduces the quality of life in affected patients. Expression of the calsequestrin (CASQ1) protein in thyroid tissue may be the trigger for the development of eye muscle damage in patients with GO. We determined the prevalence of rs74123279, rs3747673, and rs2275703 single-nucleotide polymorphism (SNPs) in patients with autoimmune thyroid disorders, GO, Graves’ hyperthyroidism (GH), or Hashimoto’s thyroiditis (HT) and control subjects with no personal or family history of autoimmune thyroid disorders. Furthermore, we measured the concentration of the CASQ1 protein in normal and Graves’ thyroid tissue, correlating levels with parameters of the eye signs, CASQ1 antibody levels, and the CASQ1 gene polymorphism rs74123279 and rs2275703.
Methods
High-quality genomic DNA was isolated from fresh blood samples, assayed for identification of rs74123279, rs3747673, and rs2275703 SNPs in CASQ1 gene by MassARRAY SNP analysis using iPLEX technology of SEQUENOM.
Results
DNA samples from 300 patients and 106 control subjects (100 males, 306 females) with GO (n=74), GH (n=130), HT (n=96) and control subjects (n=106) were genotyped for the SNPs rs74123279, rs3747673 (n=405), and rs2275703 (n=407). The SNP rs74123279, rs3747673, and rs2275703 were identified as 1) common homozygous or wild type, 2) heterozygote, and 3) rare homozygous. Minor allele frequency for rs74123279, rs3747763, and rs2275703 were 21%, 40%, and 44%, respectively. Multiple comparisons of genotype frequency for rs74123279, rs3747763, and rs2275703 in the GO, GH, HT, and control groups showed P=0.06, 0.641, and 0.189, respectively. These results were substantiated by multiple comparison of alleles frequency for rs74123279, rs3838216, rs3747763, and rs2275703 in the GO, GH, HT, and control groups showed, P=0.36, 0.008, 0.66, and 0.05, respectively. Pairwise analysis of alleles frequency distribution in patients with GO showed significant probability for rs2275703, P=0.008.
Conclusion
Based on their evolutionary conservation and their significant prevalence, we suggest that CASQ1 gene SNPs rs74123279, rs3838216, and rs2275703 may be considered as genetic markers for GO.
Keywords: thyroid eye disease, autoimmune thyroiditis, ophthalmology, orbitopathy, eye muscles antibodies, wild-type, homozygous, heterozygous, genotyping
Introduction
The eye disorder associated with Graves’ disease, called Graves’ ophthalmopathy (GO) or “thyroid-associated ophthalmopathy”, greatly reduces the quality of life in affected patients1,2 and can lead to eye muscle damage and loss of sight. Mild eye signs are also present in approximately 25% of patients with Hashimoto’s thyroiditis (HT),3,4 mainly manifesting as upper eyelid disease (retraction), and in a small proportion of patients, with transient (subacute and silent) thyroiditis.5 Graves’ disease runs in families and is a multifactorial disorder that probably includes a genetic susceptibility6,7 and some subsequent external (environmental) stimulus such as infection, stress, pregnancy, or trauma. The pathogenesis of the GO8 and the mechanism for its link to Graves’ hyperthyroidism (GH), and the more common HT, is poorly understood.4,9–11
Our current research is focused on identifying a genetic marker for this unique association and has addressed the possible role of autoimmunity against the calcium-binding protein calsequestrin (CASQ1) in the pathogenesis of the eye muscle component of GO. We have shown that antibodies against CASQ1 are closely associated with eye muscle damage in patients with GO and appear to be markers for Nunery type 2 ophthalmopathy (ie, with eye muscle involvement).12–16 CASQ1 antibodies are also frequently found in patients with the inflammation of the levator palpebrae superioris muscle that is commonly associated with HT.17
While we have demonstrated that antibodies against CASQ1 are good markers of the eye muscle component of ophthalmopathy, they are not specific to GO, being detected in approximately 10% of apparently normal subjects and in some patients with other forms of skeletal muscle damage or inflammation.18–20 CASQ1 is found in abundance in skeletal muscles, while its isoform, cardiac calsequestrin (CASQ2), is found in heart muscle and, to a lesser degree, skeletal muscle. CASQ1 is expressed 4.7 times more in fast twitch fibers in the extraocular muscles than in other skeletal muscles,21 providing a possible mechanism for the localization of a muscle reaction in the orbit in Graves’ disease. Earlier studies from our laboratory showed that the CASQ2 gene was the most upregulated in thyroid tissue from patients with GO compared with those with no eye signs (odds ratio =2.3).22 We showed that the CASQ1 gene was upregulated (odds ratio =4.1) in the thyroid tissues from patients with GO compared with those patients without eye disease.22
Previously, we studied rs3838216 in patients with thyroid disorders and showed its association with GO and HT.23 Here, we studied three additional informative SNPs in the CASQ1 gene; results show that these rs74123279 and rs2275703 polymorphisms are conceivably genetic markers associated with GO and HT. We showed that CASQ1 protein levels from thyroid tissue extracts of patients having rs74123279 and rs2275703 were reduced in both groups of patients with Graves’ disease compared with normal thyroid tissues.
Material and methods
Clinical subjects
A comprehensive demographic, clinical details and genotypes of SNPs rs74123279, rs3747623, and rs2275703 for patients with GO, GH, HT, and control subjects without autoimmune diseases are summarized in Table 1. Participants were recruited from outpatients’ clinic at Nepean and Royal North Shore Hospitals in New South Wales and Sir Charles Gairdner hospital in Western Australia. Previous treatments for hyperthyroidism (with particular reference to radioactive iodine therapy), sex distribution of patients with Graves’ disease with or without GO, presence or absence of other autoimmune diseases, and presence or absence of ethnic differences in the different groups of patients are shown in Table 1. Patients’ recruitment criteria are described by Walsh et al (2011).24 The ophthalmopathy was assessed as 1) Nunery type 1 (without restrictive myopathy) or type 2 (with restrictive myopathy),14 2) a modified Clinical Activity Score (CAS) (0–12) of Mourits et al25 which is a measure of disease activity, 3) Werner’s NOSPECS classes26 and a NOSPECS score (0–18) derived from scores of 0–3 for each of the main features namely, inflammatory changes, upper eyelid retraction, ocular myopathy, proptosis, corneal involvement, and optic nerve compression, and 4) upper eyelid margin-reflex distance, which is the distance between the center of the papillary light reflex and the upper eyelid margin with the eye in primary gaze, as a measure of eyelid retraction; an margin-reflex distance of >5 mm is taken as significant upper eyelid retraction. The degree of proptosis (mm) was measured using a Hertel exophthalmometer, where a positive reading was defined as >18 mm in either eye or >2 mm difference between the eyes. For the purpose of the study, “ophthalmopathy” was taken as a NOSPECS class ≥2, regardless of the CAS. Ethical approval was obtained from Nepean Blue Mountain Local Health District and Sir Charles Gairdner Group Human Research Ethic Committees.
Table 1.
Demographic and clinical details of patients with thyroid autoimmunity and control subjects without autoimmune disease
| Clinical parameter | Graves’ ophthalmopathy group | Graves’ hyperthyroidism group | Hashimoto’s thyroiditis group |
|---|---|---|---|
| Patients, n (%*) | 74 (18%*) | 131 (32%*) | 92 (23%*) |
| Age (years) | 52.4±14.6 | 48.7±15.8 | 51.8±15.3 |
| Age at diagnosis | 43±14 (n=66) | 40±16 (n=116) | 39±17 (n=99) |
| Other autoimmune disorders | 7 (41%) | 11 (7%) | 17 (15%) |
| Family history of thyroid disorders | 24 (66%) | 77 (54%) | 57 (56%) |
| Isotope scan | 21 (40%) | 52 (71%) | Not available |
| Goiter diffuse with 1–3 nodules | 23 (38%) | 29 (47%) | Not available |
| 1 nodule | 10 (29%) | 19 (27%) | 49 (47%) |
| 2 nodules | 15 (44%) | 39 (56%) | 55 (53%) |
| Medication | 51 (60%) | 88 (61%) | 107 (92%) |
| Radioactive iodine treatment | 12 (14%) | 21 (14%) | Not available |
| Surgery | 22 (26%) | 36 (25%) | Not available |
| TRAB | 46.4±132 (n=30) | 65.5±160 (n=37) | Not available |
| TPO | 136±106 (n=6) | 752±876 (n=10) | 904±899 (n=62) |
| FT4 at diagnosis | 37.7±23.7 (n=40) | 45.2±27.5 (n=74) | 12.2±6.1 (n=47) |
| FT3 at diagnosis | 17.1±11.9 (n=32) | 35.8±124 (n=51) | 17.3±33.2 (n=6) |
| TSH | Not available | Not available | 26.9±39.8 (n=86) |
| TgAB | Not available | Not available | 169±286 (n=35) |
| Non-European | n=7 | n=32 | n=7 |
| Male | n=12 | n=30 | n=12 |
| Female | n=62 | n=101 | n=84 |
| Severity: EUGOGO scale score | 1–3 (n=58) | Not available | Not available |
Notes: Data are presented as mean ± SD, unless otherwise specified.
Number of patients expressed as percentage of total population. Control group (n=108 [26%*]); age: 35.7±12.8 (years).
Abbreviations: TRAB, thyroid receptors antibodies; TPO, thyroid peroxides; TSH, thyroid-stimulating hormone; TgAB, thyroglobulin antibodies; EUGOGO, European Group on Graves’ Orbitopathy; FT4, Free T4; FT3, Free T3.
For CASQ1 protein studies, thyroid tissue was obtained fresh at thyroidectomy from 1) 28 patients with Graves’ disease (8 males and 20 females aged 21–78, [mean age =43.3 years] of whom 2 males and 7 females aged 27–78 [mean age =49.5 years]) had ophthalmopathy, and 2) 22 patients with multinodular goiter or thyroid cancer (3 males and 19 females aged 29–85 [mean age =50.6 years]), and the diagnoses of the various disorders were based on standard clinical criteria and confirmed by thyroid function testing, thyroid ultrasonography, and immunological tests. Local Ethical Committee approval was received for the study and informed consent of participating subjects was obtained.
Methods
MassARRAY SNP analysis using iPLEX technology of SEQUENOM (Agena Bioscience, San Diego, CA, USA) has been described in detail in a previous publication of this laboratory.22 Briefly, genomic DNA were extracted from leucocytes of 300 patients and 106 control subjects, dried in a 96-well microtiter plate for MassARRAY SNP analysis using iPLEX technology of SEQUENOM, tested by Australian Genome Research Facility. All reactions for the iPLEX assay are terminated after a single-base primer extension reactions coupled with MALDI-TOF MS used for multiplexed genotyping of CASQ1 SNPs. Characteristics of four informative polymorphisms identified in CASQ1 gene are summarized in Table 2. Detailed quantitative Western blot method has been described in previous publication27 and detailed enzyme-linked immunosorbent assay have been described in previous publications by this laboratory12,14,18,19 and is standard.
Table 2.
Characteristics of four informative polymorphisms identified in CASQ1 gene
| SNP rs | SNP position | Alleles | Ancestral allele | Gene(s) | Role | Amino acid change | Amino acid position | SNP relativeto chromosome |
|---|---|---|---|---|---|---|---|---|
| rs74123279 | chr1:151389828 | G/A | G | CASQ1 | Promoter | − | − | + |
| rs3838216 | chr1:160161633: 160161634 | –/GGCATTCAGATAGGCCT | Not available | CASQ1 | Intron 1 | − | − | + |
| rs3747623 | chr1:160163509 | C/T | C | CASQ1 | Intron 2 (boundary) | − | − | + |
| rs2275703 | chr1:160165095 | A/C | A | CASQ1 | Intron 4 | − | − | + |
Notes: No change in amino acid sequence or its position is indicated with “−”. Forward or plus (+) strand of DNA 5′----3′ is indicated with “+”.
Abbreviations: CASQ1, calsequestrin; SNP, single-nucleotide polymorphism.
Statistical analyses
Genotype and allelic frequencies of the CASQ1 SNP rs74123279, rs3838216, rs3747763, and rs2275703 in patients with GH, GO, HT, and control subjects were compared using chi-square test or Fisher’s exact test following Hardy–Weinberg equilibrium. Differences in CASQ1 protein concentrations between patient groups, correlations between levels of the CASQ1 protein and parameters of the eye disease, and the presence of the genomic polymorphism rs74123279 and CASQ1 antibody titers, in the three groups, were analyzed using the Mann–Whitney test of GraphPad Prism Version 3.03 (San Diego, CA, USA). P-value of <0.05 was taken as significant in all assessments.
Results
In previous publication,23 we analyzed rs3838216 SNP, across the three genotypes for GO, GH, HT, and control groups, which showed significant probabilities (*P=0.042). In the present study, we analyzed informative SNPs rs74123279, rs3747673, and rs2275703 across the three genotypes for GO, GH, HT, and control groups. rs74123279 (Table 3) failed to reach significant probability (P=0.06), and the other two SNPs rs3747673 in intron 2 and rs2275703 in intron 4 showed no significant probabilities. rs74123279 SNP is in promoter region of CASQ1 gene, and the change in nucleotide is from G > A; therefore, genotypes are wild type (GG), heterozygote (GA), and rare homozygote (AA). rs3747673 is in intron 2 of CASQ1 gene, and the change in nucleotide is from C > T; therefore, genotypes are wild type (CC), heterozygote (CT), and rare homozygote (TT). rs2275703 is in intron 4 of CASQ1 gene, and the change in nucleotide is from A > C; therefore, genotypes are wild type (AA), heterozygote (AC), and rare homozygote (CC).
Table 3.
Genotypes of CASQ1 SNPs: rs74123279, rs3747673, and rs2275703, in patients with autoimmune thyroid disease with and without ophthalmopathy and controls
| rs74123279
|
rs3747673
|
rs2275703
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild-type | Heterozygote | Homozygote | Wild-type | Heterozygote | Homozygote | Wild-type | Heterozygote | Homozygote | |
| Number of patients | 406 | 405 | 407 | ||||||
| GO group | 40 (54%) | 33 (45%) | 1 (1%) | 29 (38%) | 40 (53%) | 7 (9%) | 13 (18%) | 41 (56%) | 19 (26%) |
| GH group | 83 (64%) | 38 (29%) | 9 (7%) | 44 (33%) | 65 (49%) | 22 (18%) | 43 (33%) | 60 (46%) | 27 (21%) |
| HT group | 55 (57%) | 38 (40%) | 3 (3%) | 35 (38%) | 42 (46%) | 15 (16%) | 32 (32%) | 49 (50%) | 18 (18%) |
| Control group | 73 (69%) | 30 (28%) | 3 (3%) | 40 (38%) | 46 (43%) | 20 (19%) | 39 (37%) | 48 (46%) | 18 (17%) |
| Total (all groups) | 251 (62%) | 139 (34%) | 16 (4%) | 148 (37%) | 193 (48%) | 64 (15%) | 127 (30%) | 198 (49%) | 82 (21%) |
| Major allele frequency | 641 (79%) | NA | NA | 489 (60%) | NA | NA | 452 (56%) | NA | NA |
| Minor allele frequency | 171 (21%) | NA | NA | 321 (40%) | NA | NA | 362 (44%) | NA | NA |
| Total number of alleles | 812 (100%) | NA | NA | 810 (100%) | NA | NA | 814 (100%) | NA | NA |
| Chi-square | 11.83 | NA | NA | 4.26 | NA | NA | 8.72 | NA | NA |
| P-value | 0.06 | NA | NA | 0.64 | NA | NA | 0.18 | NA | NA |
Note: Number of patients expressed as percentage of each population.
Abbreviations: CASQ1, calsequestrin; SNP, single-nucleotide polymorphism; GO, Graves’ ophthalmopathy; GH, Graves’ hyperthyroidism; HT, Hashimoto’s thyroiditis; NA, not applicable.
Major allele frequencies for patients and control group shown in Table 3 for rs74123279, rs3747673, and rs2275703 were 79%, 60%, and 56%, respectively, and minor allele frequencies for these SNPs were 21%, 40% and 44%, respectively, and were in Hardy–Weinberg equilibrium. Multiple comparison of alleles frequency for rs74123279, rs3838216, rs3747763, and rs2275703 in the GO, GH, HT, and control groups showed P=0.36, **0.008, 0.66, and *0.05, respectively (Table 4). Pairwise analysis of alleles frequency distribution in GO versus control groups (Table 5) for rs74123279 showed odds ratio =1.51, 95% confidence interval =0.898–2.55, P=0.117 and for rs2275703 showed significant probability, odds ratio =1.77, 95% confidence interval =1.15–2.71, **P=0.008, and for rs3747673, there was no significant probabilities in alleles distribution (Table 5).
Table 4.
Minor and major alleles distribution of CASQ1 SNPs: rs74123279, rs3838216, rs3747673, and rs2275703, in patients with autoimmune thyroid disease with and without ophthalmopathy and controls
| rs74123279
|
rs3838216
|
rs3747673
|
rs2275703
|
|||||
|---|---|---|---|---|---|---|---|---|
| Minor alleles | Major alleles | Minor alleles | Major alleles | Minor alleles | Major alleles | Minor alleles | Major alleles | |
| Alleles population | 812 | 810 | 810 | 814 | ||||
| GO group | 35 (4%) | 113 (14%) | 25 (3%) | 123 (15%) | 54 (7%) | 98 (12%) | 79 (10%) | 67 (8%) |
| GH group | 56 (7%) | 204 (25%) | 64 (8%) | 198 (24%) | 109 (13%) | 153 (19%) | 114 (14%) | 146 (18%) |
| HT group | 44 (6%) | 148 (18%) | 35 (4%) | 149 (19%) | 72 (9%) | 112 (14%) | 85 (10%) | 113 (14%) |
| Control group | 36 (4%) | 176 (22%) | 66 (8%) | 150 (19%) | 86 (11%) | 126 (15%) | 84 (10%) | 126 (16%) |
| Total alleles frequency | 171 (21%) | 641 (79%) | 190 (23%) | 620 (77%) | 321 (40%) | 489 (60%) | 362 (44%) | 452 (56%) |
| Chi-square | 3.15 | NA | 1.77 | NA | 1.59 | NA | 7.42 | NA |
| P | 0.36 | NA | 0.008 | NA | 0.66 | NA | 0.05 | NA |
Note: Number of patients’ alleles expressed as percentage of each population.
Abbreviations: CASQ1, calsequestrin; SNP, single-nucleotide polymorphism; GO, Graves’ ophthalmopathy; GH, Graves’ hyperthyroidism; HT, Hashimoto’s thyroiditis; NA, not applicable.
Table 5.
Minor and major alleles distribution of CASQ1 SNPs rs74123279, rs3747673, rs2275703 in patients with and without ophthalmopathy
| SNP | Graves’ ophthalmopathy group
|
||||
|---|---|---|---|---|---|
| Minor alleles (n) | Major alleles (n) | Chi-square | Odds ratio (95% confidence interval) | P-value | |
| rs74123279 | 35 | 113 | 2.447 | 1.51 (0.898–2.55) | 0.117 |
| Control group | 36 | 176 | |||
| rs3747673 | 54 | 98 | 0.950 | 0.807 (0.525–1.24) | 0.329 |
| Control group | 86 | 126 | |||
| rs2275703 | 79 | 67 | 6.91 | 1.77 (1.15–2.71) | 0.008 |
| Control group | 84 | 126 | |||
Abbreviations: CASQ1, calsequestrin; SNP, single-nucleotide polymorphism.
The differences in alleles frequencies observed for four polymorphism were confirmed by genotypes probabilities and odds ratios in GO versus control and HT versus control. The difference in genotypes frequency for wild type observed in GO versus control were significant for rs74123279 (odds ratio =0.532, 95% confidence interval =0.287–0.984, *P=0.043, data not shown) and for rs2275703 (odds ratio =0.367, confidence interval =0.179–0.752, **P=0.005, data not shown). In heterozygote genotypes, in GO versus control, significant difference in probabilities were observed for rs74123279 (odds ratio =2.039, 95% confidence interval =1.061–2.34, *P=0.024, data not shown). In HT versus control, a difference in wild-type frequency for rs74123279 (odds ratio =0.601, 95% confidence interval =0.341–1.08, P=0.088) failed to reach statistical significance (data not shown). Heterozygote genotype of rs74123279 SNP showed high odds ratio (odds ratio =1.66, 95% confidence interval =0.922–2.99, P=0.090), which failed to reach statistical significance (data not shown).
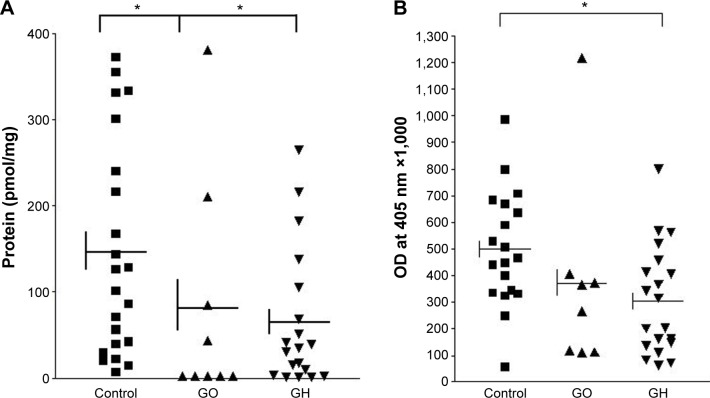
To test for functional significance of rs74123279, we measured CASQ1 protein concentration in thyroid tissues from 19 patients with GH, 9 with GO, and 22 control subjects with nodular goiter or cancer. All these patients and control had rs74123279 polymorphism. Results are summarized in Figure 1A, which shows the mean ± SE (Control =145.6±26.5 pmol/mg, GO =81.4±43.9 pmol/mg, and GH =61.56±18.3 pmol/mg protein) concentrations of the CASQ1 protein in patients and control subject. The mean concentration of CASQ1 was significantly reduced in patients with GO and GH (Mann–Whitney test: *P=0.047 and *P=0.013, respectively) compared with the control subjects. Next, we compared serum levels of CASQ1 antibodies’ titers in 8 patients with GO and 20 patients with GH with 19 control subjects; rs74123279 polymorphism is present in both patients and controls. Results in Figure 1B show mean ± SE (Control =499.2±49.7, GO =370.9±128.8, and GH =305.1±45.6) antibody titers in patients and control subjects. The mean serum CASQ1 antibodies’ titers were significantly reduced in patients with GH (*P=0.011) but failed to reach statistical significance in patients with GO (P=0.084).
Figure 1.
CASQ1 protein concentrations and CASQ1 antibody titer.
Notes: (A) Mean ± standard deviation, CASQ1 protein concentrations in thyroid tissues from patients having rs74123279 SNP with GH (61.56±18.32 pmol/mg), GO (81.44±43.98 pmol/mg) and as normal thyroid, from control patients with multinodular goiter or thyroid cancer (145.6±26.56 pmol/mg) determined from quantitative Western blotting. The difference between GH and controls and GO and controls was highly significant (Mann–Whitney test: *P=0.013 and *P=0.047, respectively). (B) Mean ± standard deviation, CASQ1 antibody titer in serum samples from patients having rs74123279 SNP that thyroid tissues were studied for measurement of CASQ1 protein concentrations with GH (305.1±45.65), GO (370.9±128.8), and as normal thyroid, from control patients with multinodular goiter or thyroid cancer (499.2±49.77) determined by enzyme-linked immunosorbent assay. The difference between GH and Controls was significant (Mann–Whitney test: *P=0.011), and GO and control failed to reach significance (Mann–Whitney test P=0.084).
Abbreviations: CASQ1, calsequestrin; SNP, single-nucleotide polymorphism; GH, Graves’ hyperthyroidism; GO, Graves’ ophthalmopathy; OD, optical density.
When we compared the CASQ1 protein level in thyroid tissues from patients with GO and GH having wild-type genotype of rs74123279 polymorphism with CASQ1 protein level in normal control thyroid tissues having the rs74123279 wild-type genotype polymorphism, we found reduction in thyroid tissues CASQ1 protein level correlated with the wild-type genotype of rs74123279 polymorphism in the CASQ1 gene (Figure 2, Mann–Whitney test for GO versus control, **P=0.0023, and for GH versus control, *P=0.024). When we compared the serum CASQ1 antibodies’ titers from patients with GO and GH having wild-type genotype of CASQ1 rs74123279 with serum CASQ1 antibodies’ titers in controls having the CASQ1 rs74123279 wild-type genotype, we found significant reduction in serum CASQ1 antibodies’ titers in patients with GO and GH having wild-type genotype of rs74123279 polymorphism (Figure 3, Mann–Whitney test for GO versus control, *P=0.023, and for GH versus control *P=0.031). Next, we correlated serum levels of CASQ1 antibodies’ titers in patients with GO and GH against their thyroid tissue CASQ1 protein concentrations. There was significant relationship between the two groups for GO (r =0.958, 95% confidence interval =0.781–0.993, ***P=0.0002).
Figure 2.
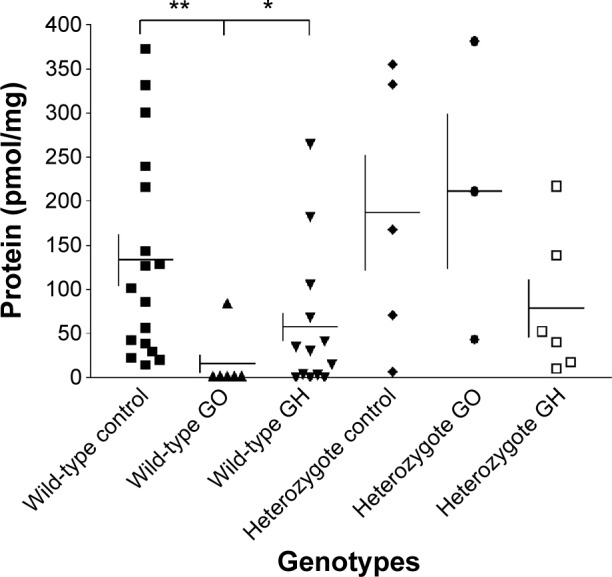
Correlation between mean (± SE) CASQ1 protein levels and SNP rs74123279 genotype of the CASQ1 gene in patients with GO, GH, which were significant (Mann–Whitney test: **P=0.0023 and *P=0.023) for wild-type homozygote genotypes, respectively, but not for the heterozygote genotypes (Mann–Whitney test: P=NS).
Abbreviations: SE, standard error; CASQ1, calsequestrin; SNP, single-nucleotide polymorphism; GO, Graves’ ophthalmopathy; GH, Graves’ hyperthyroidism; NS, not significant.
Figure 3.
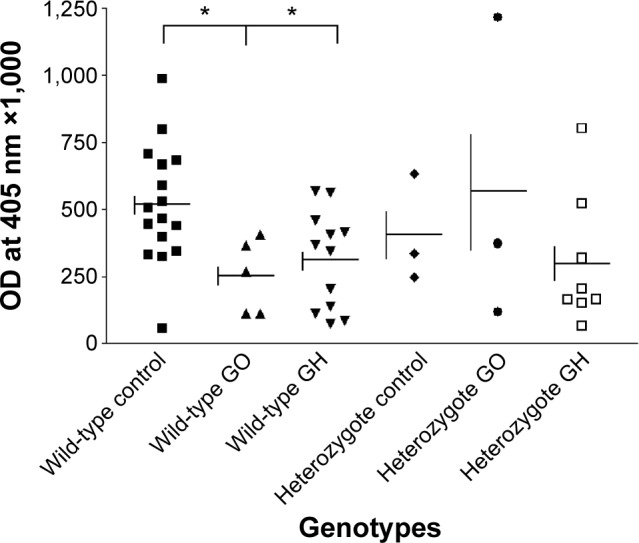
Correlation between mean (± SE) CASQ1 antibody titer and SNP rs74123279 genotype of the CASQ1 gene in patients with GO, GH, which were significant (Mann–Whitney test: *P=0.023 and *P=0.030) for the wild-type homozygote genotypes, respectively, but not for the heterozygote genotypes (Mann–Whitney test: P=NS).
Abbreviations: SE, standard error; CASQ1, calsequestrin; SNP, single-nucleotide polymorphism; GO, Graves’ ophthalmopathy; GH, Graves’ hyperthyroidism; NS, not significant; OD, optical density.
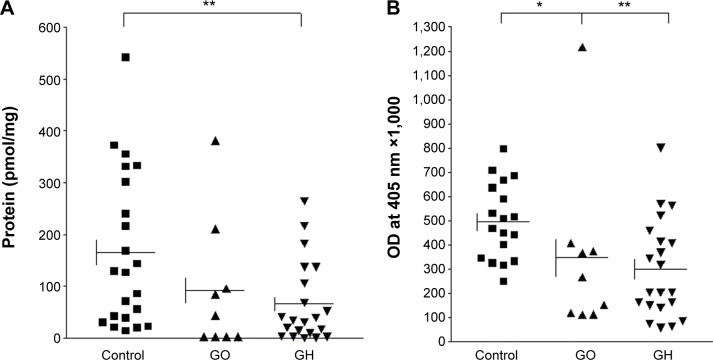
The functional significance of rs2275703 was also studied by measuring CASQ1 protein concentration in thyroid tissues from 21 patients with GH and 9 with GO and in 22 control subjects with nodular goiter or cancer. All these patients and control had rs2275703 polymorphism. Results are summarized in Figure 4A showing mean concentration of CASQ1 protein was significantly reduced in patients with GH (Mann–Whitney test: **P=0.007) compared with the control subjects. Figure 4B showed mean serum CASQ1 antibodies’ titers were significantly reduced in patients with GO (*P=0.019) and in patients with GH (**P=0.003). Figure 5 shows correlation between mean (± SE) CASQ1 protein levels and heterozygote rs2275703 genotypes polymorphism of the CASQ1 gene in patients with GO (Mann–Whitney test: *P=0.050), and in GH, this correlation was significant with homozygote genotypes (Mann–Whitney test: *P=0.031) but not significant for wild-type homozygote genotypes. Figure 6 shows significant correlation between mean (± SE) CASQ1 antibody titer and wild-type rs2275703 homozygote genotypes, in patients with GH (Mann–Whitney test: *P=0.028), but not for the heterozygote or rare homozygote genotypes.
Figure 4.
Concentrations of the CASQ1 protein and serum levels of CASQ1 antibodies titers.
Notes: (A) Mean ± SE (control =166.3±31.8, GO =91.8±42.9, and GH =66.0±17.0 pmol/mg protein) concentrations of the CASQ1 protein in patients and control subject; rs2275703 polymorphism is present in both patients and controls. The mean concentration of CASQ1 was significantly reduced in patients with GH (Mann–Whitney test: **P=0.007) compared with the control subjects. (B) Comparing serum levels of CASQ1 antibodies titers in nine patients with GO and 21 patients with GH with 18 control subjects; rs2275703 polymorphism is present in both patients and controls. Mean ± SE (control =496.4±37.1, GO =346.5±116.2, and GH =297.6±44.3) antibody titers in patients and control subjects. The mean serum CASQ1 antibodies titers was significantly reduced in patients with GO (*P=0.019) and also significantly reduced in patients with GH (**P=0.003)
Abbreviations: SE, standard error; GO, Graves’ ophthalmopathy; GH, Graves’ hyperthyroidism; CASQ1, calsequestrin; OD, optical density.
Figure 5.
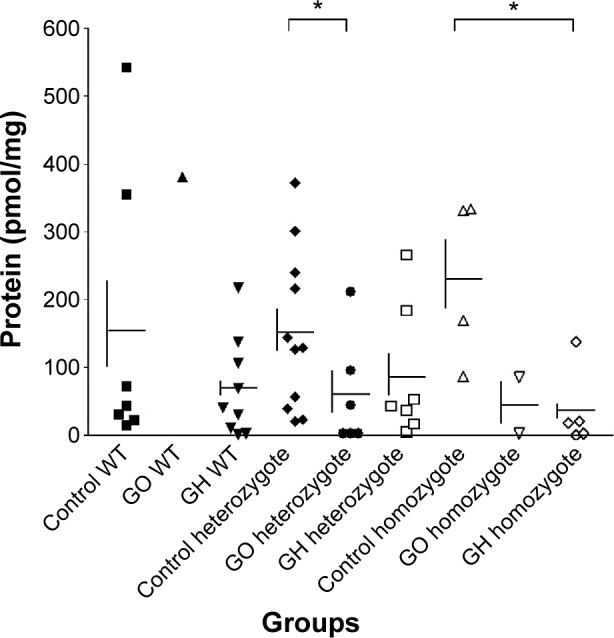
CASQ1 protein levels and heterozygote rs2275703 genotypes polymorphism of CASQ1.
Notes: Correlation between mean (± SE) CASQ1 protein levels and heterozygote rs2275703 genotypes polymorphism of the CASQ1 gene in patients with GO (Mann–Whitney test: *P=0.050), and in GH, this correlation was significant with homozygote genotypes (Mann–Whitney test, *P=0.031) but not significant with the wild-type homozygote genotypes (Mann–Whitney test, P=NS).
Abbreviations: SE, standard error; CASQ1, calsequestrin; GO, Graves’ ophthal-mopathy; GH, Graves’ hyperthyroidism; NS, not significant.
Figure 6.
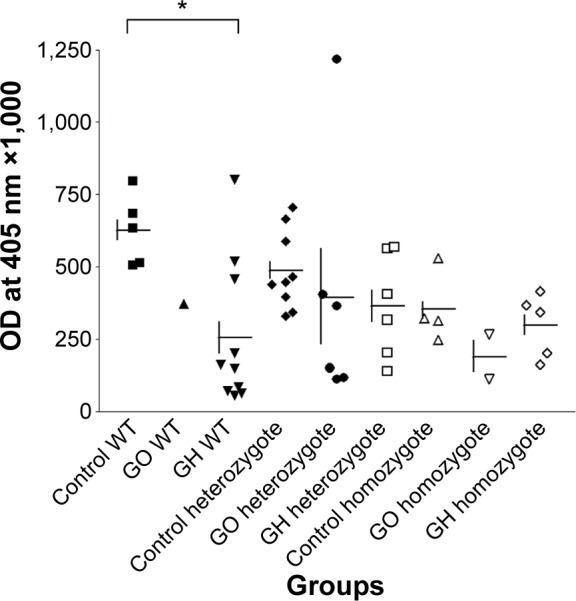
CASQ1 antibody titer and wild-type rs2275703 homozygote genotypes.
Notes: Significant correlation was observed between mean (± SE) CASQ1 antibody titer and wild-type rs2275703 homozygote genotypes, in patients with GH, (Mann–Whitney test: *P=0.028) but not for the heterozygote or rare homozygote genotypes (Mann–Whitney test: P=NS).
Abbreviations: SE, standard error; CASQ1, calsequestrin; GH, Graves’ hyperthy roidism; NS, not significant; WT, wild type; GO, Graves’ ophthalmopathy; OD, optical density.
Discussion
The tentative hypothesis for the development of thyroid ophthalmopathy postulate involvement of antibodies against thyroid-stimulating hormone (TSH)-R that causes fibroblast inflammation, which leads to muscle fiber damage, release of CASQ1 protein, and progression of autoimmunity in those patients with thyroid illnesses that have genetic susceptibility. GO is a complex disease; recent studies have shown a plethora of genes identified to be as risk factors for the development of disease. Many of the genes/loci that are unique to GO and HT identified through small-scale case-controlled association studies were poorly characterized and underpowered.
To characterize patients in this cohort having autoimmune thyroid disease, we included data for various established risk factors such as ethnicity, family history of thyroid disorders, sex differences, other immune disorders, Free T4, Free T3, thyroid receptors antibodies, thyroid peroxidase, thyroglobulin antibodies, drugs treatment, radiation therapy, and surgical interventions.28 In a previous publication,23 we analyzed rs3838216 SNP, across the three genotypes for GO, GH, HT, and control groups, which showed significant probabilities. We showed for first time rs3838216 SNP in the CASQ1 gene at both alleles and genotypic levels is associated with GO and HT but not with GH. Our data showed odds ratio as comparable with other genes suggested to be involved in Graves’ disease23 and equal to those for HLA, thyroid-stimulating receptor, and thyroglobulin gene odds ratio.29 Here, we further analyzed the other three SNPs namely rs74123279, rs3747673, and rs2275703 across the three genotypes for GO, GH, HT, and control groups. rs74123279 SNP is in promoter region of CASQ1 gene, and the change in nucleotide is from G > A; therefore, genotypes are wild-type homozygous (GG), heterozygote (GA), and rare homozygote (AA). This sequence gagcctgggg in promoter region represent YY1 transcription factor binding site, and change of nucleotide from G > A (gagcctggAg) results in loss of YY1 transcription factor binding site (http://www.gene-regulation.com/pub/programs/alibaba2/index.html). YY1 is a ubiquitously distributed transcription factor belonging to the GLI-Kruppel class of zinc finger proteins. The protein is involved in repressing and activating a diverse number of promoters. YY1 may direct histone deacetylases and histone acetyltransferases to a promoter in order to activate or repress the promoter, thus implicating histone modification in the function of YY1 (http://genome.ucsc.edu). We studied rs74123279 across the three genotypes (Table 3), which failed to reach significant probability; however, because it has an odds ratio of 1.51, we further studied this polymorphism at the protein levels to test for functional analysis. Multiple comparison of alleles frequency was significant for rs3838216 and rs2275703. Pairwise analysis of alleles frequency distribution in GO versus control (Table 5) showed high odds ratio for rs74123279 and significant probability for rs2275703. Previously, significant difference in probabilities were observed for HT versus control in heterozygote genotypes of rs3838216.23 These differences in alleles frequency between GO and HT can be utilized for the development of a genetic test to differentiate susceptible patients with autoimmune thyroiditis who are at risk to develop ophthalmopathy. The correlation of alleles frequency with parameters of ophthalmopathy will delineate which polymorphism is involved in various established risk factors such as ethnicity, family history of thyroid disorders, sex differences, other immune disorders, Free T4, Free T3, thyroid receptors antibodies, thyroid peroxidase, thyroglobulin antibodies, drugs treatment, radiation therapy, and surgical interventions.
No significant difference in alleles frequencies observed for GH versus control subjects indicates these polymorphisms may not play a role in the development of GH. Interestingly, the significant differences in frequencies observed for HT versus control mirror that of significant differences in frequencies observed for GO versus control subjects. This phenomenon probably indicates that GO is different from that of GH and has more common characteristics with HT.
The functional significance of these polymorphisms was studied with regard to protein levels of CASQ1 in thyroid tissue extracts, and we found reduction in thyroid tissues CASQ1 protein level correlated with the wild-type genotype of rs74123279 polymorphism in the CASQ1 gene (Figure 2). When we correlated serum levels of CASQ1 antibodies’ titers in patients with GO against their thyroid tissue CASQ1 protein concentrations, there was significant relationship between the two groups. Similar pattern of reduction in protein levels and antibody titers were observed for rs2275703 polymorphism (Figures 4A, B, 5, and 6) with exception that reduction in proteins levels in patients with GO (Figure 5) were correlated significantly to heterozygote genotype and in patients with GH to rare homozygote genotype. This pattern of reduction in protein levels is in contrast to rs7412379, which correlated to wild-type homozygous genotype.
Our future studies will focus on identifying transcriptional regulatory pathways using with and without rs74123279, polymorphisms in CASQ1 gene promoter construct, and translation control mechanisms using with and without rs3838216 in intron 1 and rs2275703 in intron 4 constructs. Equally important is identifying microRNAs regulatory pathways affecting translation of CASQ1 gene constructs with and without rs3838216 and rs2275703 polymorphisms. These CASQ1 gene constructs will also enable us to identify medications that affect the transcriptional activities and open the way for clinical trial studies. Our focus will also be on the putative link between thyroid and orbital reactions and the role of CASQ1 protein in this link. We need to study large cohorts of patients with various phenotypes/severities of disease and correlate their clinical manifestations of thyroid eye disease components with the presence or absence of these two polymorphisms (phenotypes–genotypes studies). This will allow for the development of a genetic test to distinguish between GO and GH and HT. Replication of our results in a separate population of patients (racial and ethnic groups) and controls is required to confirm our findings.
Conclusion
Based on their evolutionary conservation and their significant prevalence, we suggest that CASQ1 gene SNPs rs3838216, rs74123279, and rs2275703 are possible genetic markers in addition to those that are already known for GO and HT. They are potentially pathogenic genetic markers for the eye muscle component of GO. We need to study large populations of patients and controls of different ethnic backgrounds to confirm the values of wild type, heterozygote, and rare homozygote of these three pathogenic CASQ1 gene polymorphisms.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham-Nordling M, Wallin G, Träisk F, et al. Thyroid Study Group of TT 96 Thyroid-associated ophthalmopathy; quality of life follow-up of patients randomized to treatment with antithyroid drugs or radioiodine. Eur J Endocrinol. 2010;163:651–657. doi: 10.1530/EJE-10-0475. [DOI] [PubMed] [Google Scholar]
- 3.Tjiang H, Lahooti H, McCorquodale T, Parmar KR, Wall JR. Eye and eyelid abnormalities are common in patients with Hashimoto’s thyroiditis. Thyroid. 2010;20:287–290. doi: 10.1089/thy.2009.0199. [DOI] [PubMed] [Google Scholar]
- 4.Lahooti H, Parmar KR, Wall JR. Pathogenesis of Thyroid Eye Disease: important role of autoimmunity against calsequestrin and collagen XIII. Pathogenesis of Thyroid Eye Disease: important role of autoimmunity against calsequestrin and collagen XIII. A review. Clin Ophthalmol. 2010;14:417–425. doi: 10.2147/opth.s6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopinath B, Tani J, Bao N, Wall JR. Eye muscle and collagen XIII antibodies and eye signs in patients with transient and chronic thyroiditis. Thyroid. 2007;17:1–7. doi: 10.1089/thy.2007.0054. [DOI] [PubMed] [Google Scholar]
- 6.Khalilzadeh O, Noshad S, Rashidi A, Amirzargar A. Graves’ ophthalmopathy: a review of immunogenetics. Curr Genomics. 2011;12:564–575. doi: 10.2174/138920211798120844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao WL, Wan L, Wang TY, et al. Association of TLR7 and TSHR copy number variation with Graves’ disease and Graves’ ophthalmopathy in Chinese population in Taiwan. BMC Ophthalmol. 2014;14:15. doi: 10.1186/1471-2415-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada M, Wu Li A, Wall J. Thyroid-associated ophthalmopathy: clinical features, pathogenesis, and management. Crit Rev Clin Lab Sci. 2000;37(6):523–549. doi: 10.1080/10408360091174303. [DOI] [PubMed] [Google Scholar]
- 9.Girgis CM, Champion BL, Wall JR. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135–144. doi: 10.1177/2042018811408488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCorquodale T, Lahooti H, Gopinath B, Wall JR. Long-term follow-up of seven patients with ophthalmopathy not associated with thyroid autoimmunity: heterogeneity of autoimmune ophthalmopathy. Clin Ophthalmol. 2012;6:1063–1071. doi: 10.2147/OPTH.S30704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tani J, Gopinath B, Nuygen B, Wall JR. Immunological mechanisms for the eye muscle and orbital connective tissue reactions of thyroid-associated ophthalmopathy. Exp Rev Clin Immunol. 2007;3:299–311. doi: 10.1586/1744666X.3.3.299. [DOI] [PubMed] [Google Scholar]
- 12.Gopinath B, Musselman R, Beard N, Tani J, Adams C, Wall JR. Antibodies targeting the calcium binding skeletal muscle protein calsequestrin are sensitive and specific markers of ocular myopathy in patients with Graves’ disease. Clin Exp Immunol. 2006;145:56–62. doi: 10.1111/j.1365-2249.2006.03110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunery WR, Martin RT, Hinz GW, Gavin TJ. The association of cigarette smoking with clinical subtypes of ophthalmic Graves’ disease. Ophthal Plast Reconstr Surg. 1993;9:77–82. doi: 10.1097/00002341-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gopinath B, Adams CL, Musselman R, Tani J, Wall JR. Antibodies against calsequestrin and type XIII collagen are good markers for chronic upper eyelid lag and retraction. Ocular Immunol Inflammation. 2007;15:81–88. doi: 10.1080/09273940701299362. [DOI] [PubMed] [Google Scholar]
- 15.Wall JR, Lahooti H. Pathogenesis of thyroid eye disease – does autoimmunity against the TSH Receptor explain all cases? Endokrynol Pol. 2010;61(2):222–227. [PubMed] [Google Scholar]
- 16.Gopinath B, Musselman R, Adams C, Tani J, Beard N, Wall JR. Study of serum antibodies against three eye muscle antigens and the connective tissue antigen collagen XIII in patients with Graves’ disease with and without ophthalmopathy – correlation with clinical features. Thyroid. 2006;16:967–974. doi: 10.1089/thy.2006.16.967. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen B, Gopinath B, Tani J, Wescombe L, Wall JR. Peripheral blood T lymphocyte sensitization against calsequestrin and flavoprotein in patients with Graves’ ophthalmopathy. Autoimmunity. 2008;41:372–376. doi: 10.1080/08916930801931142. [DOI] [PubMed] [Google Scholar]
- 18.Gopinath B, Wescombe L, Nguyen B, Wall JR. Can Autoimmunity against calsequestrin explain the eye and eyelid muscle inflammation of thyroid eye disease? Orbit. 2009;28:256–261. [PubMed] [Google Scholar]
- 19.Tani J, Wall JR. Analysis – Can the development of thyroid-associated ophthalmopathy be explained by autoimmunity against eye muscle antigens? Canad Med Assoc. 2006;17:239–241. doi: 10.1503/cmaj.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Haan S, Lahooti H, Morris O, Wall JR. Epitopes, immunoglobulin classes and immunoglobulin G subclasses of calsequestrin antibodies in patients with thyroid eye disease. Autoimmunity. 2010;43(8):698–703. doi: 10.3109/08916931003774954. [DOI] [PubMed] [Google Scholar]
- 21.Porter JD, Khanna S, Kaminski HJ, et al. Extraocular muscle is defined by a fundamentally distinct gene expression profile. PNAS. 2001;98:12062–12067. doi: 10.1073/pnas.211257298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wescombe L, Lahooti H, Gopinath B, Wall JR. The cardiac calsequestrin gene (CASQ2) is up-regulated in the thyroid in patients with Graves’ ophthalmopathy – support for a role of autoimmunity against calsequestrin as the triggering event. Clin Endocrinol (Oxf) 2010;73:522–528. doi: 10.1111/j.1365-2265.2009.03753.x. [DOI] [PubMed] [Google Scholar]
- 23.Lahooti H, Cultrone D, Edirimanne S, et al. Association of the CASQ1 gene SNP rs3838216 with ophthalmopathy in patients with thyroid autoimmunity. Ophthalmol Res. 2014;2(6):281–293. [Google Scholar]
- 24.Walsh JP, Berry J, Liu S, et al. The clinical presentation of autoimmune thyroid disease in men is associated with IL12B genotype. Clin Endocrinol (Oxf) 2011;74(4):508–512. doi: 10.1111/j.1365-2265.2010.03970.x. [DOI] [PubMed] [Google Scholar]
- 25.Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Grave’s ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner SC. Classification of the eye changes of Graves’ disease. Am J Ophthalmol. 1969;68:646–648. doi: 10.1016/0002-9394(69)91246-x. [DOI] [PubMed] [Google Scholar]
- 27.Cultrone D, Lahooti H, Edirimanne S, Delbridge L, Champion B, Wall JR. Calsequestrin is decreased in the thyroid gland of patients with Graves’ disease – further evidence for a role of autoimmunity against this protein in Graves’ ophthalmopathy. Br J Med Med Res. 2014;4(23):4065–4075. [Google Scholar]
- 28.Stan MN, Bahn RS. Risk factors for development or deterioration of Graves’ ophthalmopathy. Thyroid. 2010;20(7):777–783. doi: 10.1089/thy.2010.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies TF, Latif R, Yin X. New genetic insights from autoimmune thyroid disease. J Thyroid Res. 2012;2012:623852. doi: 10.1155/2012/623852. [DOI] [PMC free article] [PubMed] [Google Scholar]