Abstract
Catecholamines and adipokines function as hormones; catecholamines as neurotransmitters in the sympathetic nervous system, and adipokines as mediators of metabolic processes. It has become increasingly clear, however, that both also function as immunomodulators of innate and adaptive immune cells, including macrophages. Macrophages can respond to, as well as produce their own catecholamines. Dopamine, noradrenaline, and adrenaline are the most abundant catecholamines in the body, and can induce both pro-inflammatory and anti-inflammatory immune responses in macrophages, as well as non-immune processes such as thermogenesis. Though they are responsive to adipokines, particularly lipoproteins, leptin, and adiponectin, macrophages generally do synthesize their own adipokines, with the exception being resistin-like molecules. Adipokines contribute to adverse metabolic and immune response by stimulating lipid accumulation, foam cell formation and pro-inflammatory cytokine production in macrophages. Adipokines can also promote balance or resolution during metabolic and immune processes by promoting reverse lipid transport and expression of Th2 cytokines. This review will explore the mechanisms by which catecholamines and adipokines influence macrophage function in neural pathways, immunity and metabolism.
Keywords: Adipokine, dopamine, macrophage, resistin-like molecules, atherosclerosis, sepsis
1. Introduction
Macrophages are essential components of the innate immune system. First identified by Metchnikoff for their potent phagocytic capabilities, which explains their name “big eater” in Greek, their function in engulfing and eliminating microbial pathogens is well-recognized. The importance of macrophages in other immune contexts, such as influencing adaptive immunity, mediating wound healing and downregulating inflammation is also appreciated. New studies, however, have revealed that the macrophage function extends beyond the immunological realm, affecting both the central nervous system and metabolism. First, macrophages respond to and can produce catecholamines, which are neurotransmitters that signal through the sympathetic nervous pathway. Second, macrophages make and respond to adipokines that influence the outcome of several metabolic diseases such as atherosclerosis. This suggests the requirement for multidisciplinary research spanning immunology, neuroscience and metabolism for the improved understanding of these critical cell-types. Here we review the main mediators of these neural-immune or metabolic-immune circuits, which are either synthesized by macrophages or that influence their function, and discuss their function in neural pathways, immunity and metabolism.
2. Catecholamines
Catecholamines are hormones produced in both the adrenal medulla and the central nervous system. As neurotransmitters, catecholamines are an integral part of the sympathetic nervous pathway, also known as the “fight-or-flight response”, which mediates essential physiologic responses including increased heart rate and blood pressure, mobilization of energy stores and control of core body temperature [1]. In addition to their hormonal and neurotransmitter roles, catecholamines also influence immune responses, and the importance of this neural-immune cross-talk via neurotransmitters and cytokines has been increasingly recognized [2]. For instance, stimulation of the vagus nerve can regulate inflammatory cytokine production, and conversely, macrophages and lymphocytes are able to synthesize catecholamines that influence the central nervous system (CNS) [3–5]. Additionally, immune cells express adrenergic receptors and are therefore responsive to catecholamines [6]. Catecholamine signaling in immune cells exerts a number of effects including cell activation, proliferation and apoptosis [7, 8]. Furthermore, catecholamines can be locally produced by immune cells and act in both autocrine and paracrine ways [6]. Here, we focus on the macrophage-specific modulatory effects of catecholamines.
The most abundant catecholamines in the human body are dopamine, adrenaline and noradrenaline. Catecholamines are synthesized from the non-essential amino acid tyrosine by a series of enzymatic pathways [9]. First, tyrosine hydroxylase removes a hydroxyl group from tyrosine to produce the dopamine precursor L-DOPA. L-DOPA is decarboxylated to form dopamine, which is then catabolized to noradrenaline and adrenaline by hydroxylases. Dopamine binds dopamine receptors, while noradrenaline and adrenaline bind α and β-adrenergic receptors, all of which belong to a family of G protein-coupled receptors that signal through phospholipase C and cAMP/protein kinase A pathways [10, 11]. In the immune system, myeloid cells express α and β-adrenergic receptors, while lymphocytes primarily express β-adrenergic receptors [1].
Functionally, catecholamine receptor signaling in macrophages has significant effects on the inflammatory response. Inhibition of the β-adrenergic receptor with the β-blocker propranolol, or depletion of adrenal catecholamines by adrenalectomy, led to increased LPS-induced tumor necrosis factor (TNF) α production in peritoneal macrophages [12]. Alveolar macrophages recovered from mice chronically treated with β-blockers produced more noradrenaline, interleukin (IL) 6 and TNFα following LPS treatment ex vivo [13]. Conversely, adrenaline, noradrenaline and dopamine treatment of RAW 264.7 macrophages inhibited LPS-induced production of nitric oxide [14]. Finally, treatment of RAW cells with dopamine or noradrenaline decreased proliferation and increased apoptosis [8]. Taken together, these studies suggest that macrophage responsiveness to catecholamines via the β-adrenergic receptor exerts an important immunoregulatory mechanism to reduce inflammation. Supportive of this, treatment of mice with β2-adrenergic agonists ameliorated LPS-induced endotoxemia and acute lung inflammation [15]. This was associated with alternatively activated macrophage (AAM) polarization, characterized by increased IL-4, IL-10 and Arginase-1 expression, and decreased expression of iNOS and IL-12 [16].
Recent data suggest that catecholamines can auto-regulate their levels and function by controlling expression of both tyrosine hydroxylase as well as catecholamine receptors [12]. For instance, adrenal catecholamines contribute to the paracrine regulation of macrophage synthesis of catecholamines and expression of the β-adrenergic receptor. Adrenalectomy resulted in decreased expression of β2-adrenergic receptor and increased expression of tyrosine hydroxylase by peritoneal macrophages presumably as a compensatory mechanism to increase catecholamine levels. Consistent with this, treatment with the β-blocker propanonol increased macrophage expression of tyrosine hydroxylase.
In contrast to the anti-inflammatory effect of β-adrenergic receptor signaling, stimulation of the α-adrenergic receptor of murine peritoneal macrophages in combination with LPS treatment led to increased TNFα and IL-1β expression compared with LPS alone [17]. Additionally, treatment of human monocytes with the α1-adrenergic receptor agonist phenylephrine hydrochloride promoted LPS-induced IL-1β [18]. Use of protein kinase C and MAP kinase inhibitors demonstrated that these signaling pathways were downstream of the α-adrenergic receptor-induced inflammatory response. Together, these observations suggest that the differential roles of catecholamines on macrophages may depend on the adrenergic receptor. Specifically in the context of LPS-induced inflammation, β-adrenergic receptors agonists inhibit inflammation, while α-adrenergic receptor signaling or β-adrenergic receptor blockers promote pro-inflammatory responses. The differential responses between α-adrenergic and β-adrenergic receptors is likely due to variance in G protein pairings with the receptors [11]. Briefly, α1 preferentially binds noradrenaline and signals via the PKC-activating Gq subunit, while α2 preferentially binds adrenaline and stimulates Gi, thereby decreasing cAMP. β1-adrenergic receptor equivalently binds noradrenaline and adrenaline, which leads to Gs subunit-mediated increase of cAMP. Though the β2 receptor also couples with the Gs subunit, its preferential binding partner is adrenaline. Influencing differential adrenergic receptor expression and G protein pairing on macrophages could therefore have therapeutic potential in dictating the inflammatory outcome of several disease conditions such as endotoxemia or acute respiratory disease.
In addition to regulation of inflammation by the sympathetic nervous system via catecholamine-adrenergic receptor signaling, macrophages are also influenced by the parasympathetic/cholinergic nervous system, through recognition of acetylcholine by nicotinic receptors. In this neural immune circuit, termed the inflammatory reflex, stimulation of the vagus nerve leads to the release of acetylcholine that acts on macrophages to downregulate expression of inflammatory cytokines such as TNFα. In a mouse model of sepsis, this pathway was critical in limiting inflammation, and was dependent on acetylcholine production by a small subset of memory T cells [19]. In more recent studies, Ulloa and colleagues utilized electroacupuncture at the sciatic nerve to protect mice from fatal sepsis induced by LPS treatment [4]. This protective mechanism was associated with decreased levels of TNFα, CCL2, IL-6, and IFN-γ in the serum, and dependent on vagal nerve stimulation and adrenal-derived catecholamines. Specifically, vagotomy or adrenalectomy abolished the production of catecholamines, and treatment with dopamine receptor agonists could rescue the adrenalectomized mice from fatal sepsis. Together, these studies demonstrate the importance of both dopaminergic and cholinergic nervous pathways in the regulation of the inflammatory immune response during sepsis.
In contrast to its role in preventing sepsis, macrophage exposure to dopamine may increase susceptibility to HIV [20, 21]. Macrophages are the main cell type in the CNS that are infected with HIV, and recent studies showed that dopamine treatment of human peripheral blood monocyte-derived macrophages led to a two-fold increase in CCR5-mediated HIV entry and increased HIV replication. Supportive of these studies, another group reported a positive correlation between dopamine levels and CNS viral loads in SIV-infected macaques [22]. These studies implicate catecholamines as immunomodulatory molecules and elucidate a potential role for these neurotransmitters in HIV-associated neurocognitive disorders. Since therapeutic drugs, such as ritalin and some antidepressants, and illicit drugs, such as cocaine, can lead to increased CNS dopamine, these drugs may contribute to increased HIV virulence.
Catecholamine signaling also negatively impacts the rate of wound repair. The stress induced by injury can lead to a surge in catecholamines, with 10-fold increases in circulating adrenaline in severe burn injuries [23]. Macrophages and neutrophils that are recruited to the injury respond to and produce catecholamines. Wounding studies in mice and in skin biopsies have allowed evaluation of the effects of systemic and local elevation in catecholamines in wound healing. Burn wounds generated in excised human skin exhibited delayed re-epithelialization when treated with high levels of adrenaline [24]. This was due to the effects of adrenaline on inhibiting the migration of keratinocytes, which express the β2-adrenergic receptor. Treatment with β2-adrenergic receptor antagonists rescued the wound healing process. α2-adrenergic receptor−/− mice had accelerated wound closure [25], supporting the negative effect of both α and β adrenergic receptor signaling in wound healing. In another study, mice that were chronically delivered adrenaline via an osmotic pump, exhibited impaired wound healing associated with persistent neutrophil trafficking. Interestingly, the chronic inflammation was mediated by β2-adrenergic receptor signaling in macrophages that promoted IL-6 production. Therefore, while β2-adrenergic receptor signaling is protective in downregulating excessive inflammation during endotoxemia, in response to persistent exposure to adrenaline, it can have detrimental effects by promoting inflammation and impairing keratinocyte responses that are necessary for wound healing [26].
In addition to the effects of catecholamines in modulating macrophage immune responses, recent studies have shown that macrophages can potently affect the central nervous system (CNS), demonstrating that bi-directional communication exists in these neural immune circuits [27, 28]. Like all tissues in the body, the CNS has a resident population of macrophages. Referred to as microglia, these cells play essential roles promoting optimal brain function by editing neuronal synapses and providing growth factors promoting neuroprotection during health, injury or infection. Chronic activation of microglia as can lead to dysregulated and/or neurotoxic functions contributing to neurodegeneration, neuropathic pain and/or decreased cognitive ability. Most studies have focused on the role of pathogen associated molecular patterns (PAMP) or danger associated molecular pattern (DAMP) molecules as the primary signals triggering maladaptive microglial activation in CNS injury and disease function [27, 28]. However, in vitro and in vivo studies now reveal that norephinephrine (NE) plays a non-redundant and complementary role to DAMP and PAMP signals. For example, ATP acting via P2 purinergic receptors potently promotes microglial process extension, phagocytosis and inflammasome activation [29, 30]. Using both in vitro and in vivo approaches, Gyoneva and Traynelis [31] demonstrated that activation of microglial β2 adrenergic decreased their base line rate as well as the higher ATP induced rate of process extension and migration. These data suggest that the decreasing levels of NE observed in progressive degenerative disorders such as Alzheimer’s disease directly contributes to decreased ability to inhibit microglial activation by classic DAMPs, Conversely, drugs of abuse associated with activation of microglial adrenergic receptors will lead to altered microglial surveillance, decreased responses to CNS DAMP signals with likely alterations in microglial regulations of neuronal synapses.
Macrophages can also serve as a source of catecholamines and serve essential roles in maintaining physiologic homeostasis. As previously mentioned, macrophages express tyrosine hydroxylase in response to numerous stimuli including LPS but also as a compensatory mechanism when local catecholamine levels are low [8]. More recently, it was shown that IL-4/IL-13-induced AAM were a critical extraneuronal source of catecholamines in thermogenesis. Thermogenesis is an essential physiologic response in mammals that maintains constant body temperature in response to temperature changes [32, 33]. In a mouse model of adaptive thermogenesis, where mice were exposed to cold temperatures, maintenance of body temperature in wild-type mice was associated with catecholamine production by AAM in the brown adipose tissue. In contrast, macrophage-specific STAT6−/− mice, which lack alternatively activated macrophages, had decreased catecholamine levels and were unable to maintain body temperature homeostasis following thermogenic stress. Conversely, wild-type mice treated with IL-4 exhibited increased AAM-derived tyrosine hydroxylase and noradrenaline. Mechanistically, the catecholamine-producing AAM that infiltrated white adipose tissue spurred the development of thermogenic beige adipose tissue. AAM polarization, and subsequent development of beige adipose tissue, led to increased energy expenditure, mediated by uncoupling protein 1 and fatty acid metabolism, and the generation of non-shivering thermogenesis. This previously unrecognized function of macrophages in instructing beige adipose tissue development and subsequent energy expenditure has significant implications for the role of these cells in metabolic disorders.
These studies dramatically demonstrate the potent effects of catecholamines in regulating and mediating macrophage contributions to immunity and physiology. However, conclusions from these studies are often cited and applied to contexts quite different from those tested in the original experiments as if macrophage populations are homogenous and essentially identical throughout development and between tissues. Macrophages are highly plastic cells and multiple different types of activation states can be observed within a tissue dependent on local environmental cues [27].
Three studies of macrophages acutely isolated from the spleen and brain reveal the effects of development, activation state and local tissue environment [30]. For example, careful characterization of signal transduction revealed that only a subset of microglia are responsive to catecholamines, with only 7% of adult cortical microglia demonstrating responsiveness to dopamine [30]. Cytokine activation of microglia regulated dopamine responsiveness but the regulation was developmentally regulated. While IFNg tripled the number of neonatal microglia responsive to dopamine, IFNg had no effect on adult microglial responsiveness. Conversely, IL-4 had no effect on dopamine responsiveness of neonatal microglia, but did decrease dopamine responsiveness of adult microglia. While most microglia appear to express adrenergic receptors, the ratio of B2 to alpha adrenergic receptor expression dramatically increases upon activation by B-amyloid or PAMP signals. Thus activated microglia are preferentially inhibited by NE as compared to homeostatic microglia. Even within the spleen, there is substantial heterogeneity in macrophage responsiveness to catecholamines. Clenbuterol is a β2 agonist with some structural and pharmacological similarities to epinephrine and salbutamol. Within the spleen, Shirato and colleagues compared three macrophage populations and found that only the bacterial phagocytosis by the small but MARCOhi expressing macrophages was inhibited by Clenbuterol.
Taken together, these observations illustrate the diverse, context-dependent, complex roles of catecholamines and acetylcholine in macrophage responses, and reveal that non-traditional therapeutic strategies, such as acupuncture, that target neural-immune circuits could provide new effective treatments for infection, inflammation and metabolism (Figure 1).
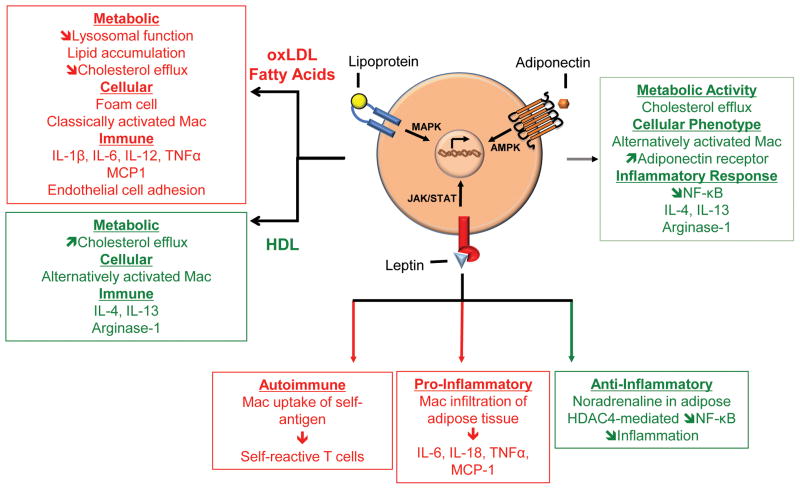
Figure 1. Catecholamine signaling in macrophage function.
A. Catecholamines are recognized by α and β-adrenergic receptors (1). Signaling through α-adrenergic receptors is pro-inflammatory and promotes LPS-induced gene expression whereas β-adrenergic receptor signaling inhibits this and induces expression of anti-inflammatory cytokines (2). Cold temperature induces macrophage synthesis of catecholamines which act to increase white to beige adipose tissue conversion and energy expenditure (3). B. Sciatic and vagus nerve stimulation promotes dopamine synthesis (1). Dopamine receptor signaling enhances GPCR activity leading to increased viral entry and replication (2). Dopamine signaling also inhibits pro-inflammatory cytokine production (3).
3. Adipokines
Adipose-derived hormones, also known as adipokines, are molecules that exhibit hormone characteristics during metabolic processes as well as cytokine functions in modulating the immune response [34, 35]. Their metabolic functions range from stopping hunger signals to promoting lipid and glucose uptake and metabolism [34, 36]. Adipokines act on both innate and adaptive immune cells to increase cell activation, survival and chemotaxis, Also, depending on the particular adipokine, they can increase pro-inflammatory or anti-inflammatory cytokine production [37]. Here, we discuss the lipoproteins, leptin, and adiponectin due to their profound influence on macrophages during metabolic and immune processes.
3.1 Lipoproteins
An amalgamation of lipids and proteins, lipoproteins provide hydrophilic properties to lipids, allowing them to be transported within aqueous environments inside and outside of cells. Some well-studied lipoproteins include Apolipoprotein (Apo) A and E that bind lipids reversibly to form high density lipoprotein (HDL) and Apo B that binds lipids irreversibly to form low density lipoprotein (LDL) [38, 39]. One of the main functions of HDL is to promote cholesterol efflux from cells, such as foam cells that contribute to arterial plaques. As such, decreased HDL levels are indicative of increased atherosclerosis and cardiovascular events. In addition to being a fat molecule transporter, HDL also has a number of anti-inflammatory properties including decreasing expression of adhesion molecules, TNF and CCL2 in endothelial cells. LDL is also a fat molecule transporter; it differs from HDL in that it contains higher proportions of fat molecules. In conditions of oxidative stress, LDL is susceptible to oxidation, and can form aggregates. These oxLDL aggregates form fat droplets that are recognized by scavenger receptors on macrophages and lead to macrophage development into foam cells. Together, the accumulation of oxLDL aggregates and foam cell activation contribute to plaque formation in artery walls that precipitate atherosclerotic events.
One mechanism by which oxidized LDL (oxLDL), as well as cholesterol, may promote atherosclerosis is by causing dysfunction in macrophage lysosomal activity that contributes to processing of lipids [40]. Peritoneal macrophages treated in vitro with oxLDL or cholesterol exhibited altered lysosomal function and morphology. Furthermore, macrophages from cardiovascular plaques displayed similar lysosomal dysfunction. Lysosomal biogenesis is controlled by transcription factor EB; in the presence of pro-atherosclerotic lipids, TFEB was less able to translocate to the nucleus to turn on protective autophagy genes. Overexpressing TFEB rescued lysosomal function, enhanced cholesterol efflux and decreased lipid-mediated inflammation by reducing inflammasome activation and IL-1β production.
In addition to directly modulating macrophage activity, oxLDLs can indirectly influence macrophages during atherogenesis by promoting expression of adhesion molecules on endothelial cells [41]. OxLDLs increased expression of vascular cell adhesion molecule (VCAM) 1 and intercellular adhesion molecule (ICAM) 1, subsequently promoting macrophage adhesion to endothelial cells. OxLDLs, the glycoprotein fibronectin, and its receptor, integrin α5, form a pro-atherogenic network that contributes to the formation of aortic plaques. Treatment of atherosclerosis-prone mice with integrin α5 inhibitor led to decreased lipid accumulation, VCAM-1 expression, and macrophage infiltration, which ultimately led to reduced plaque formation. Another important therapeutic strategy to reduce the pathogenic effects of oxLDL is treatment with lipoprotein mimetic molecules. These are synthetic peptides that mimic the ApoA and ApoE, which are components of HDL, the protective cholesterol. Treatment with mimetic peptides can counteract the pro-atherogenic and pro-inflammatory functions of LDLs, and human clinical trials testing these peptides are underway [42]. RAW 264.7 macrophages treated with mimetic peptides neutralized negatively charged LDLs and, prevented LDL uptake and foam cell formation [43]. Furthermore, production of pro-inflammatory cytokines IL-1α, IL-6, and chemokine CCL2, were decreased in macrophages after treatment. In vivo challenge with oxLDL led to increased IL-6 secretion into plasma, while pre-treatment of the oxLDL molecules with mimetic peptides decreased inflammation.
Other indirect mechanisms that impact macrophage biology include lipoprotein enzymes that catalyze the formation of immune-modulating metabolites. Lipoprotein lipase (LPL), a lipoprotein hydrolyzing enzyme, contributes to atherogenesis by liberating free fatty acids from lipoproteins [44]. Exposing THP-1 macrophages to LPL-hydrolyzed lipoproteins products led to decreased expression of cholesterol transporter genes including ATP-binding cassette transporters, peroxisome proliferator-activated receptors (PPARs), HDL scavenger receptor and liver x receptor. Treatment of macrophages with free fatty acids isolated via LPL hydrolysis caused decreased expression of transporter genes and impaired reverse transport of cholesterol from cells.
Finally, lipoproteins modulate the functions of macrophages by influencing their polarization into classically activated macrophages, which are associated with exacerbated disease progression in atherosclerosis or AAM, which are considered atheroprotective. Phosphatidylcholine is a major component of oxLDL that forms pro-inflammatory lysophosphotydalcholine (lysoPC) when metabolized. In human macrophage differentiation cultures, lysoPC promoted production of conventional classically activated macrophage cytokines IL-1β, IL-12, IL-6 and TNFα [45]. This stimulatory effect was dependent on the G protein-coupled receptor G2A. In contrast, the HDL-associated lipid, sphingosine-1-phosphate (S1P) was atheroprotective and promoted AAM polarization [46]. S1P exposure in macrophages reduced expression of pro-inflammatory cytokines, but stimulated production and secretion of prototypical AAM cytokine IL-4. In conjunction with increased macrophage-derived IL-4, macrophages exhibited augmented production of other AAM proteins including IL-13, arginse-1, and IL-4 receptor. S1P-mediated macrophage polarization resulted in attenuated expression of CD36, a scavenger receptor that recognizes oxLDL, and increased expression of ATP-binding cassette transporter, suggesting that S1P prevents lipid accumulation in macrophages. Indeed, macrophages treated with S1P exhibited decreased lipid storage in an IL-4 dependent manner. These data provide insights into opposing roles for LDL and HDL in macrophage polarization and the subsequent effects in exacerbating or inhibiting atherosclerosis.
3.2 Leptin
Leptin is a hormone produced in the adipose tissue that was discovered by studies of ob/ob mice that have a spontaneous mutation in the leptin gene, leading to obese and developed diabetes [47]. Functionally, leptin affects the hypothalamus region of the brain, where it triggers satiety signals and helps regulate food intake by counter-acting ghrelin, the hunger hormone, but also functions to promote energy expenditure in peripheral tissues [48]. Leptin expression is directly related to the amount of adipose tissue a person has, with increased adipose tissue leading to greater expression of leptin. Chronically high leptin levels can lead to leptin resistance and changes in the dynamics of fat storage, glucose metabolism and insulin signaling.
In contrast to its metabolic function in reducing obesity, leptin also acts as an immune mediator where it promotes activation, chemotaxis and survival of both innate and adaptive immune cells [49]. Leptin shares structural similarity with IL-6 and acts on immune cells via the leptin receptor, which belongs to the cytokine receptor family. Stimulation of the leptin receptor activates JAK-STAT signal transduction, utilizing JAK2 and STAT3 to relay its signals [50]. Because it shares a similar signal transduction mechanism as cytokines, leptin signaling can promote obesity-associated induction of pro-inflammatory mediators [51]. Leptin receptor deficient bone marrow cells were transferred into irradiated wild-type mice. Deficiency of leptin receptor led to decreased adipose tissue infiltration of inflammatory macrophages and reduced formation of crown-like structures, foci of macrophages that contribute to disease pathogenesis. In agreement with decreased inflammatory macrophages, expression of pro-inflammatory cytokines including TNFα, IL-6 and CCL2 were decreased in adipose tissue. Furthermore, leptin also stimulated IL-18 secretion from THP-1 macrophages. Increased IL-18 release from leptin-stimulated cells was not dependent upon increasing IL-18 transcription, suggesting leptin promotes IL-18 release via activation the inflammasome/caspase-1 to cleave pro-IL-18. Indeed, inhibiting caspase-1 activity abolished leptin-stimulated IL-18 secretion. Since both leptin and IL-18 are increased during obesity, these data provide further insight into potential pathogenic mechanism of obesity-associated inflammation [52].
In addition to inflammation, zinc deficiency is another potential consequence of obesity observed in humans. Mice that were fed a zinc deficient high fat diet exhibited enhanced alterations in adipose tissue expression of zinc transporters compared to mice that were fed zinc sufficient high fat diet [53]. Zinc deficiency also augments leptin production, increases leptin receptor expression, and increased infiltration of macrophages and formation of crown-like structures in adipose tissue. The mechanism by which zinc deficiency contributes to leptin-mediated inflammation during obesity remains elusive. However, the authors speculate that because zinc can exhibit antioxidant properties and leptin production can be augmented by pro-inflammatory cytokines, altered zinc metabolism and oxidative stress resultant of zinc deficiency contributes to leptin production and inflammation.
Leptin may also contribute to Systemic Lupus Erythematosus, an autoimmune disorder. Leptin promotes uptake of apoptotic self-antigen in peritoneal macrophages [54]. Macrophages then transfer antigen to self-reactive T cells. These data indicate leptin in promoting crosstalk between innate and adaptive immune cells, and suggest the inhibiting leptin signaling could alleviate SLE.
Contrary to its pro-inflammatory effects, leptin can also reduce adipose tissue inflammation by enabling a leptin-catecholamine signaling axis [55]. Mice challenged with LPS exhibited induction of pro-inflammatory cytokines, which was attenuated with prostaglandin E2, a hormone that spurs production of cAMP. PGE2-mediated suppression of inflammation occurred via HDAC4, a histone deacetylase that can inhibit NF-κB-mediated inflammation, dephosphorylation, nuclear translocation, and association with genes that transcribe pro-inflammatory cytokines, namely TNFα and IL-12. Administration of exogenous leptin increased expression of noradrenaline in adipose tissue, which increased cAMP production, ultimately leading to dephosphorylation and nuclear translocation of HDAC4 in bone marrow-derived macrophages during short-term high fat diet feeding to mice. Loss of HDAC4 promoted increased expression of pro-inflammatory cytokines in macrophages, as well as increased crown-like structure formation in adipose tissue. These effects were more modest during long-term feeding. As mice become leptin resistant, HDAC4 function decreased and contributed to metabolic dysfunction. These data support an earlier study that showed decreased HDAC4 expression in obese individuals [56].
3.3 Adiponectin
Initially discovered as hormone produced exclusively in adipose, adiponectin was first described as a modulator of glucose levels; adiponectin stimulates a decrease in gluconeogenesis, while increasing glucose uptake [57]. Adiponectin also regulates fat metabolism by promoting β-oxidation of lipids. Though adiponectin is primarily expressed in adipose tissue, it is also produced in endothelial cells, as well as skeletal and cardiac myocytes [37]. Expression of adiponectin can be enhanced by PPARs, contrary to catecholamines, which inhibit its expression. Pro-inflammatory cytokines, including TNFα and IL-6, also suppress expression of adiponectin. Given the inflammatory nature of obesity-related diseases, this offers one potential explanation for decreased adiponectin expression during insulin resistance, metabolic syndrome, etc. Outside of its metabolic functions, adiponectin also exerts anti-inflammatory effects on macrophages. Adiponectin stimulates production of IL-10 and IL-1R antagonist, decreases phagocytic activity, and suppresses pro-inflammatory cytokine production by inhibiting NF-κB [58–60]. Below, we discuss some of the mechanisms by which adiponectin protects against cardiovascular and metabolic dysfunction.
Adiponectin has been proposed as a protective mediator against obesity-related atherogenesis. Rosiglitazone, a PPARγ agonist, stimulated adiponectin production in adipose tissue and was associated with decreased inflammatory cytokine production, as well as decreased macrophage infiltration [61]. In addition, rosiglitazone decreased aortic inflammation and plaque formation. Increased adiponectin led to an induction of Irak3, a negative regulator of NF-κB-mediated inflammation. Increased Irak3 expression in bone marrow-derived macrophages, and led to a reduction in CCL2. The protective role of adiponectin/Irak3 in obesity-related atherogenesis was supported in high fat diet mouse studies. HFD-fed mice exhibited decreased PPARγ, adiponectin and Irak3 expression, but augmented plaque formation and inflammation.
Furthermore, foam cell formation can be reduced by exposure to adiponectin [62]. Adiponectin treatment of primary macrophages from diabetic patients lead in increased cholesterol efflux in an adiponectin-receptor dependent manner. Signaling via adiponectin receptor increased expression of ATP-binding cassette transporter and liver x receptor α, both of which are important in mediating cholesterol efflux.
In a model of alcoholic liver disease, which can lead to inflammation and metabolic dysfunction, adiponectin suppresses both MyD88 dependent and independent TLR4 signaling [63, 64]. LPS stimulation of rat liver macrophages, or Kupffer cells, leads to MyD88-dependent production of TNFα. Adiponectin treatment reduced LPS-induced TNFα in rat Kupffer cells by decreasing ERK signaling and increasing IκB stability. In contrast, LPS-induced MyD88-independent TLR4 signaling leads to production of immune mediators CXCL10 and IFNβ. Adiponectin reduced CXCL10 and IFNβ production in LPS treated, ethanol exposed macrophages. Inflammation was reduced by adiponectin-mediated expression of heme oxygenase 1, a potent anti-inflammatory molecule. In accordance with its ability to decrease inflammation, adiponectin also promotes polarization of RAW 264.7 macrophages from the classically activated phenotype to anti-inflammatory alternatively activated phenotype [65]. Macrophages treated with adiponectin exhibited increased IL-4 production, together with increased expression of alternatively activated macrophage markers including Arginase 1, mannose receptors and IL-1 receptor antagonist. Adiponectin-mediated alternatively activated macrophage polarization was partially dependent upon the adiponectin-heme oxygenase 1 axis. Finally, macrophage polarization differentially affects adiponectin receptor expression [66]. Classically activated macrophages, stimulated by IFN-γ-LPS, decreased expression of adiponectin receptors, while anti-inflammatory cytokines IL-4 and IL-10 stimulated adiponectin receptor expression in peritoneal and bone marrow-derived macrophages.
Collectively, these data point to important roles for lipoproteins, leptin, and adiponectin in promoting or ameliorating diseases associated with metabolic dysfunction, including atherosclerosis and alcoholic liver disease, by altering lipid storage and metabolism, and shifting the balance of immune responses to pro-inflammaory or anti-inflammatory (Figure 2).
Figure 2. Adipokine influence on macrophage function.
In addition to influencing the metabolic status of macrophages, adipokines and lipoproteins also exert both pro-inflammatory and anti-inflammatory effects on macrophages.
4. Resistin and Resistin-like molecules
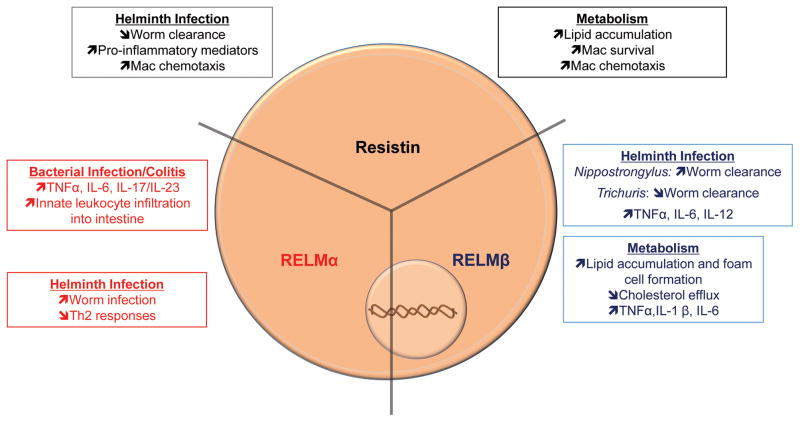
The Resistin-Like Molecules (RELM) are a family of secreted mammalian proteins that have both hormonal and immune functions. In mice, resistin was first described as a gene expressed by adipocytes that caused resistance to insulin thereby leading to the protein family’s name [67]. In a screen for adipocyte genes that were sensitive thiazolidinedione (TZD), a PPARγ ligand that improves insulin sensitivity in diabetic patients, resistin was identified as gene that was profoundly inhibited by TZD. Subsequent studies in both mouse models and clinical studies of obese or diabetic individuals have implicated resistin in mediating obesity-induced diabetes [68]. Interestingly, in humans, resistin is expressed by immune cells, where it promotes inflammatory cytokine production. Additionally, the related proteins RELMα, RELMβ and RELMγ are highly expressed in infection, inflammatory diseases and metabolic disorders [69]. Here, we will discuss the recent studies demonstrating the complexity in function of these proteins in modulating macrophage function.
In metabolic studies, resistin contributes to insulin resistance by increasing production of hepatic glucose, while impairing insulin-mediated glucose metabolism [70]. Research studies using mice support the involvement of resistin in promoting obesity-related pathologies, however, resistin studies with human subjects are controversial. Though increased resistin levels are correlated with obesity, and is predictive of adverse cardiovascular events by promoting vascular inflammation and lipid uptake [71], other studies have not seen a significant correlation between resistin and adiposity or insulin resistance [72]. Another difference in physiology of resistin between mice and humans relates to the cellular source; mouse resistin is expressed primarily in adipose, while human resistin is produced by macrophages, and to a lesser extent, adipocytes. Increased resistin expression observed during obesity-related pathologies could be related to increased infiltration of macrophages into the adipose tissue. In a model of atherosclerosis using rabbits, resistin-expressing macrophages infiltrated aortic plaques after cholesterol feeding or surgical injury [73]. Adenoviral expression of human resistin induced macrophage migration to the plaque. This process was mediated by integrins; resistin induced macrophage expression of integrins and expression of VCAM-1 and ICAM-1 by vascular endothelial cells, which led to increased macrophage-endothelial cell adhesion. In addition, resistin promoted macrophage survival, and chemotaxis both directly and indirectly. Macrophages migrated toward resistin in the absence of other chemokines, while migration was enhanced in the presence of resistin and CCL2. Macrophage infiltration was associated with increase lipid accumulation and decreased plaque stability. Resistin also promotes chemotaxis of primary human macrophages by inducing expression of fractalkine (FKN) [74]. Using an endothelial cell-smooth muscle cell co-culture system to mimic cell interactions within vessel walls, the presence of resistin in conjunction with smooth muscle cells in the sub-endothelial space promoted macrophage transmigration. Resistin augmented production of FKN and CCL2 in endothelium, and this response was enhanced in the presence of smooth muscle cells. Resistin-mediated increases in CCL2 was also shown to be partially dependent upon FKN up-regulation, however, macrophage transmigration could be reduced by inhibiting FKN or CCL2. Additionally, inhibiting both abolished macrophage transmigration, pointing to a compensatory role for FKN and CCL2 in promoting macrophage transmigration. Finally, resistin-mediated macrophage transmigration was dependent upon expression of FKN receptor, CX3CR1, and CCL2 receptor, CCR2. These data suggest that resistin contributes to promotion and sustainment of adverse cardiovascular events by stimulating macrophage chemotaxis directly, or indirectly via modulation of other chemokines.
Resistin is also a key immune mediator. Resistin directly stimulates NF-κB-mediated inflammation, including the promoting expression and secretion of TNFα, IL-1β, IL-6 and IL-12 [71]. Recent data from our lab indicate that the immune stimulatory effect of human resistin is detrimental in helminth infection and impairs worm expulsion [75]. Transgenic mice expressing human resistin exhibited increased expression of resistin and infiltration of pro-inflammatory monocytes following infection with the helminth Nippostrongylus. Mechanistically, human resistin promoted a pro-inflammatory environment, including increased expression of Toll-like receptor 4, IL-1β, and CCL2, without influencing the type 2 T helper cytokine immune response. These observations were confirmed in helminth-infected humans, who exhibited increased serum levels of resistin that was associated with increased parasite burden and circulating levels of CCL2 and TNFα.
The related murine protein RELMα is also expressed by immune cells and is immunomodulatory [69]. RELMα is a prototypical marker for AAMs, and its expression is spurred by stimulants that induce Th2 immune responses such as allergens and helminths. Although RELMα is a marker for AAMs, it acts as a negative regulator of Th2 immune responses during helminth infection [76]. RELMα−/− mice challenged with Schistosoma eggs exhibited increased lung granuloma formation and exacerbated production of IL-4, IL-13 and IL-5, and circulating IgE. RELMα−/− AAMs co-cultured with CD4+ T cells promoted increased proliferation and Th2 cytokine production. These data illustrate a role for AAM-derived RELMα in regulating Th2 responses during helminth infection. RELMα−/− mice also showed enhanced immunity to Nippostrongylus infection, associated with increased Th2 immune responses [77]. RELMα is also expressed by dendritic cells [78], and in contrast to AAM-derived RELMα, dendritic cell-derived RELMα was important in T cell priming and production of IL-13 and IL-10 [79]. In non-infection Th2 inflammatory settings such as murine asthma models, the function of RELMα is controversial. Delivery of RELMα into the lungs promoted Th2 cytokine-mediated fibrosis by the DNA damaging agent bleomycin [80]. Conversely, RELMα−/− mice exhibited reduced bleomycin-induced fibrosis. In contrast, transgenic mice that overexpressed RELMα were protected from ova-induced allergic inflammation and exhibited reduced Th2 cytokines [81]. These studies suggest that the immune function of RELMα is complex and may depend on which cell-type expresses RELMα, the RELMα levels and the type of inflammatory environment.
In a model of bacterial-induced colitis with gram negative bacterium Citrobacter, we showed that RELMα exhibited a pro-inflammatory role [82]. Citrobacter infection led to colitis and increased RELMα expression by intestinal epithelial cells and infiltrating macrophages and eosinophils. RELMα−/− mice were protected from Citrobacter-induced colitis; however, treatment with exogenous RELMα restored Citrobacter-related pathologies in RELMα−/− mice in an IL-17A dependent manner. These results suggest that RELMα contributes to intestinal inflammation following bacterial infection by promoting a Th17 inflammatory environment. RELMα is also involved in pathogenesis of non-bacterial colitis [83]. RELMα stimulated intestinal production of IL-6 in response to DSS-induced colitis. Additionally, LPS and RELMα acted synergistically to induce IL-6 and TNF-α expression following ex vivo stimulation of bone marrow-derived macrophages.
New studies have identified a critical metabolic function for RELMα in protection against atherosclerosis in both high fat diet fed mice and LDL receptor deficient mice [84]. Mice lacking the LDL receptor (ldlr−/−) cannot efficiently remove circulating LDL, leading to increased formation of atherosclerotic plaques in the context of high fat diet. However, ldlr−/− mice that were deficient in RELMα suffered from exacerbated atheroscleoric disease compared to RELMα sufficient ldlr−/− mice, evidenced by increased circulating cholesterol, and increased number and size of aortic plaques. Additionally, overexpression of RELMα in high fat diet fed mice was protective and reduced circulating cholesterol levels. This atheroprotective function for RELMα is conflicting with the pathogenic role for human resistin in related metabolic disease, suggesting that although related in protein structure, these proteins may have opposing functions. Interestingly, in an inflammatory environment mediated by DSS, a compound that is toxic to intestinal epithelial cells, RELMα−/− mice showed ameliorated metabolic function compared to wild-type mice and were protected from hyperglycemia induced by glucose challenge [83]. This suggests that RELMα promotes metabolic dysfunction in the context of ongoing inflammation. Similar to resistin, the effects of RELMα may depend on the inflammatory and metabolic environment.
Similar to RELMα, RELMβ is induced following helminth-induced Th2 immune responses. Their expression pattern, however, varies. RELMβ is primarily produced by mucus-producing goblet cells, as opposed to hematopoietic cells that are a main cellular source for RELMα [69]. Following helminth infection with Nippostronglus and Heligmosomoides, RELMβ−/− mice exhibited impaired worm expulsion [85]. In vitro studies showed that RELMβ could bind to the helminths and decrease their fecundity and viability. In contrast to this host protective role by directly acting on the worm, RELMβ also had an immunostimulatory function following Trichuris infection where it promoted activation of splenic and bone marrow-derived macrophages, and production of inflammatory cytokines, analogous to the function of human resistin [86]. While RELMβ has been shown to be almost exclusively expressed in goblet cells in helminth infection, foam cells also express RELMβ in atherosclerotic plaques [87]. RELMβ was expressed in human aortic lesions, and expression was co-localized with macrophage marker CD68. ApoE−/− mice, which are susceptible atherosclerosis, were bred with RELMβ−/− mice to determine its role in aortic lesions. Presence of RELMβ augmented aortic lipid accumulation and macrophage infiltration in ApoE−/− mice. Additionally, RELMβ supported lipid uptake and the formation of foam cells by down-regulating cholesterol efflux mediators. Similar to the Trichuris infection studies, RELMβ promoted expression of pro-inflammatory molecules TNFα, IL-1β, and IL-6 in macrophages, which likely contributes to RELMβ-mediated atherosclerotic pathogenesis.
The function of RELMγ, which is expressed by haematopoietic cells, is less clear. In high fat fed diet mice and obese leptin receptor deficient mice, both RELMγ and RELMβ serum levels were significantly upregulated [88], suggesting that analogous to the other RELM proteins, RELMγ is also induced in metabolic dysfunction. In conclusion, these multiple studies on RELM proteins highlight the complexity in function of this protein family as important adipokines that regulate metabolism, immunity and inflammation (Figure 3).
Figure 3. Resistin-like molecules influence macrophage physiology during in infection and metabolic disease.
In general, RELM proteins promote pro-inflammatory responses during infection and metabolic dysfunction, leading to detrimental effects on the host. In some cases, however, the presence of RELM proteins can be beneficial, such as RELMβ promoting resolution of Nippostrongylus infection.
5. Conclusion
Macrophage phenotypes are as diverse as the stimuli that activate them [89]. In both in vitro and ex vivo experiments, culture conditions such as media, growth factors and the type of culture dish may affect the physiological readouts. Additionally, the tissue source of macrophages can account for differences in macrophage responses. Investigators should therefore consider how using immortal or primary cells, bone marrow derived or tissue resident macrophages, and mouse or human macrophages, could influence the experimental outcome. In this review, we have summarized recent studies that encompass many of these different sources of macrophages to highlight the significance of catecholamines, adipokines and RELM proteins in macrophage function.
Catecholamines and adipokines have long been recognized as hormone signaling molecules, but recent studies have elucidated previously unrecognized functions for these proteins in modulating the immune system specifically through effects on macrophages. These advances in knowledge of neuro-immune and metabolism-immune interactions offer valuable perspective when considering human health and physiology. For example, while the health benefits of exercise are well known from the metabolic perspective, these studies provide insight into new immune mechanisms that influence positive health outcomes via the CNS and metabolic processes. With the discovery that thermostress promotes an immune response that mediates adipogenesis of energy-burning beige fat, while electroacupuncture triggers CNS-mediated anti-inflammatory pathways, exploring these non-traditional macrophage-mediated pathways may identify innovative treatments for metabolic and inflammatory diseases.
Highlights.
Macrophages modulate immunity in response to, and by producing, catecholamines.
Adipokines influence metabolic outcomes by modulating macrophage phenotype.
RELM proteins promote adverse metabolic outcomes such as lipid accumulation.
RELM proteins can both promote and decrease susceptibility to infection.
Abbreviations
- AAM
alternatively activated macrophage
- Apo
apolipoprotein
- CNS
central nervous system
- FKN
fractalkine
- HDL
high density lipoprotien
- ICAM
intercellular adhesion molecule
- IL
interleukin
- LDL
low density lipoprotein
- LPL
Lipoprotein lipase
- lysoPC
lysophosphatidylcholine
- MCP
monocyte chemoattractant protein
- oxLDL
oxidized LDL
- PPAR
peroxisome proliferator-activated receptor
- RELM
resistin-like molecule
- S1P
sphingosine-1-phosphate
- TNF
tumor necrosis factor
- TZD
thiazolidinedione
- VCAM
vascular cell adhesion molecule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schulze J, Vogelgesang A, Dressel A. Catecholamines, steroids and immune alterations in ischemic stroke and other acute diseases. Aging Dis. 2014;5:327–39. doi: 10.14336/AD.2014.0500327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. The Journal of Experimental Medicine. 2012;209:1057–68. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso NR, Brenci S, Setti M, Indiveri F, Lotti G. Catecholamine content and in vitro catecholamine synthesis in peripheral human lymphocytes. J Clin Endocrinol Metab. 1996;81:3553–7. doi: 10.1210/jcem.81.10.8855800. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–5. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 6.Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proceedings of the National Academy of Sciences. 1994;91:12912–6. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offen D, Ziv I, Gorodin S, Barzilai A, Malik Z, Melamed E. Dopamine-induced programmed cell death in mouse thymocytes. Biochim Biophys Acta. 1995;31:171–7. doi: 10.1016/0167-4889(95)00075-4. [DOI] [PubMed] [Google Scholar]
- 8.Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, et al. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- 9.Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–41. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- 10.Perreault ML, Hasbi A, O’Dowd BF, George SR. Heteromeric Dopamine Receptor Signaling Complexes: Emerging Neurobiology and Disease Relevance. Neuropsychopharmacology. 2014;39:156–68. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorn GW. Adrenergic Signaling Polymorphisms and Their Impact on Cardiovascular Disease. 2010 doi: 10.1152/physrev.00001.2010. [DOI] [PubMed] [Google Scholar]
- 12.Stanojevic S, Dimitrijevic M, Kustrimovic N, Mitic K, Vujic V, Leposavic G. Adrenal hormone deprivation affects macrophage catecholamine metabolism and beta2-adrenoceptor density, but not propranolol stimulation of tumour necrosis factor-alpha production. Exp Physiol. 2013;98:665–78. doi: 10.1113/expphysiol.2012.070524. [DOI] [PubMed] [Google Scholar]
- 13.Guo YP, Liu Y, Li JB, Huang Y, Qi HP, Xie J, et al. Chronic beta-adrenoceptor antagonists upregulate the rat alveolar macrophage adrenergic system through the beta1-subtype. Cell Physiol Biochem. 2011;28:315–22. doi: 10.1159/000331747. [DOI] [PubMed] [Google Scholar]
- 14.Pekarova M, Kralova J, Kubala L, Ciz M, Papezikova I, Macickova T, et al. Carvedilol and adrenergic agonists suppress the lipopolysaccharide-induced NO production in RAW 264.7 macrophages via the adrenergic receptors. J Physiol Pharmacol. 2009;60:143–50. [PubMed] [Google Scholar]
- 15.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, et al. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. Faseb J. 2012;26:2137–44. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS, Ward PA. Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun. 2014;6:607–18. doi: 10.1159/000358524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol. 1994;152:3024–31. [PubMed] [Google Scholar]
- 18.Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE. alpha1-adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J Pharmacol Exp Ther. 2011;338:648–57. doi: 10.1124/jpet.110.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW. Dopamine receptor activation increases HIV entry into primary human macrophages. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175:1148–59. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller JG, et al. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol. 2001;101:85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- 23.Sedowofia K, Barclay C, Quaba A, Smith A, Stephen R, Thomson M, et al. The systemic stress response to thermal injury in children. Clin Endocrinol. 1998;49:335–41. doi: 10.1046/j.1365-2265.1998.00553.x. [DOI] [PubMed] [Google Scholar]
- 24.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. Stress-Mediated Increases in Systemic and Local Epinephrine Impair Skin Wound Healing: Potential New Indication for Beta Blockers. PLoS Med. 2009;6:e1000012. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romana-Souza B, Nascimento AP, Brum PC, Monte-Alto-Costa A. Deletion of the alpha2A/alpha2C-adrenoceptors accelerates cutaneous wound healing in mice. Int J Exp Pathol. 2014;95:330–41. doi: 10.1111/iep.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MH, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol. 2014;134:809–17. doi: 10.1038/jid.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis D, Carson M. An Introduction to CNS-Resident Microglia: Definitions, Assays, and Functional Roles in Health and Disease. In: Cui C, Grandison L, Noronha A, editors. Neural-Immune Interactions in Brain Function and Alcohol Related Disorders. Springer; US: 2013. pp. 3–29. [Google Scholar]
- 28.Carson MJ, Crane J, Xie AX. Modeling CNS microglia: the quest to identify predictive models. Drug Discov Today Dis Models. 2008;5:19–25. doi: 10.1016/j.ddmod.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gyoneva S, Shapiro L, Lazo C, Garnier-Amblard E, Smith Y, Miller GW, et al. Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinson’s disease. Neurobiol Dis. 2014;67:191–202. doi: 10.1016/j.nbd.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannell M, Meier MA, Szulzewsky F, Matyash V, Endres M, Kronenberg G, et al. The subpopulation of microglia expressing functional muscarinic acetylcholine receptors expands in stroke and Alzheimer’s disease. Brain Struct Funct. 2014;19:19. doi: 10.1007/s00429-014-0962-y. [DOI] [PubMed] [Google Scholar]
- 31.Gyoneva S, Traynelis SF. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem. 2013;288:15291–302. doi: 10.1074/jbc.M113.458901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abella V, Scotece M, Conde J, López V, et al. Adipokines, Metabolic Syndrome and Rheumatic Diseases. Journal of Immunology Research. 2014;2014:14. doi: 10.1155/2014/343746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010:10. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 38.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–32. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 39.Lu M, Gursky O. Aggregation and fusion of low-density lipoproteins and. Biomol Concepts. 2013;4:501–18. doi: 10.1515/bmc-2013-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emanuel R, Sergin I, Bhattacharya S, Turner JN, Epelman S, Settembre C, et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol. 2014;34:1942–52. doi: 10.1161/ATVBAHA.114.303342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yurdagul A, Jr, Green J, Albert P, McInnis MC, Mazar AP, Orr AW. alpha5beta1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1362–73. doi: 10.1161/ATVBAHA.114.303863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman CB, Peterson SJ, Frishman WH. Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis. Cardiol Rev. 2010;18:141–7. doi: 10.1097/CRD.0b013e3181c4b508. [DOI] [PubMed] [Google Scholar]
- 43.Aluganti Narasimhulu C, Selvarajan K, Brown M, Parthasarathy S. Cationic peptides neutralize Ox-LDL, prevent its uptake by macrophages, and attenuate inflammatory response. Atherosclerosis. 2014;236:133–41. doi: 10.1016/j.atherosclerosis.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Thyagarajan N, Coady BM, Brown RJ. Cholesterol efflux from THP-1 macrophages is impaired by the fatty acid component from lipoprotein hydrolysis by lipoprotein lipase. Biochem Biophys Res Commun. 2014;451:632–6. doi: 10.1016/j.bbrc.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 45.Qin X, Qiu C, Zhao L. Lysophosphatidylcholine perpetuates macrophage polarization toward classically activated phenotype in inflammation. Cell Immunol. 2014;289:185–90. doi: 10.1016/j.cellimm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Park SJ, Lee KP, Kang S, Lee J, Sato K, Chung HY, et al. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell Signal. 2014;26:2249–58. doi: 10.1016/j.cellsig.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–8. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 48.Park HK, Ahima RS. Leptin signaling. F1000Prime Rep. 1000;6:6–73. doi: 10.12703/P6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fern #225 ndez-Riejos P, Najib S, Santos-Alvarez J, Mart, et al. Role of Leptin in the Activation of Immune Cells. Mediators of Inflammation. 2010 doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cava AL, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–9. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 51.Dib LH, Ortega MT, Fleming SD, Chapes SK, Melgarejo T. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology. 2014;155:40–6. doi: 10.1210/en.2013-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jitprasertwong P, Jaedicke KM, Nile CJ, Preshaw PM, Taylor JJ. Leptin enhances the secretion of interleukin (IL)-18, but not IL-1beta, from human monocytes via activation of caspase-1. Cytokine. 2014;65:222–30. doi: 10.1016/j.cyto.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Liu MJ, Bao S, Bolin ER, Burris DL, Xu X, Sun Q, et al. Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. J Nutr. 2013;143:1036–45. doi: 10.3945/jn.113.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amarilyo G, Iikuni N, Liu A, Matarese G, La Cava A. Leptin enhances availability of apoptotic cell-derived self-antigen in systemic lupus erythematosus. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0112826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luan B, Goodarzi MO, Phillips NG, Guo X, Chen YD, Yao J, et al. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 2014;19:1058–65. doi: 10.1016/j.cmet.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abu-Farha M, Tiss A, Abubaker J, Khadir A, Al-Ghimlas F, Al-Khairi I, et al. Proteomics Analysis of Human Obesity Reveals the Epigenetic Factor HDAC4 as a Potential Target for Obesity. PLoS ONE. 2013;8:e75342. doi: 10.1371/journal.pone.0075342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nedvidkova J, Smitka K, Kopsky V, Hainer V. Adiponectin, an adipocyte-derived protein. Physiol Res. 2005;54:133–40. [PubMed] [Google Scholar]
- 58.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 61.Hulsmans M, Geeraert B, Arnould T, Tsatsanis C, Holvoet P. PPAR Agonist-Induced Reduction of Mcp1 in Atherosclerotic Plaques of Obese, Insulin-Resistant Mice Depends on Adiponectin-Induced Irak3 Expression. PLoS ONE. 2013;8:e62253. doi: 10.1371/journal.pone.0062253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M, Wang D, Zhang Y, Wang X, Liu Y, Xia M. Adiponectin increases macrophages cholesterol efflux and suppresses foam cell formation in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;229:62–70. doi: 10.1016/j.atherosclerosis.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185:4928–37. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:12. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular Mechanism for Adiponectin-dependent M2 Macrophage Polarization: LINK BETWEEN THE METABOLIC AND INNATE IMMUNE ACTIVITY OF FULL-LENGTH ADIPONECTIN. Journal of Biological Chemistry. 2011;286:13460–9. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Stijn CMW, Kim J, Lusis AJ, Barish GD, Tangirala RK. Macrophage polarization phenotype regulates adiponectin receptor expression and adiponectin anti-inflammatory response. The FASEB Journal. 2014 doi: 10.1096/fj.14-253831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz DR, Lazar MA. Human Resistin: Found in Translation From Mouse to Man. Trends in endocrinology and metabolism: TEM. 2011;22:259–65. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair MG, Guild KJ, Artis D. Novel Effector Molecules in Type 2 Inflammation: Lessons Drawn from Helminth Infection and Allergy. The Journal of Immunology. 2006;177:1393–9. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McTernan PG, Kusminski CM, Kumar S. Resistin. Curr Opin Lipidol. 2006;17:170–5. doi: 10.1097/01.mol.0000217899.59820.9a. [DOI] [PubMed] [Google Scholar]
- 71.Park HK, Ahima RS. Resistin in rodents and humans. Diabetes Metab J. 2013;37:404–14. doi: 10.4093/dmj.2013.37.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hasegawa G, Ohta M, Ichida Y, Obayashi H, Shigeta M, Yamasaki M, et al. Increased serum resistin levels in patients with type 2 diabetes are not linked with markers of insulin resistance and adiposity. Acta Diabetol. 2005;42:104–9. doi: 10.1007/s00592-005-0187-x. [DOI] [PubMed] [Google Scholar]
- 73.Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 74.Pirvulescu MM, Gan AM, Stan D, Simion V, Calin M, Butoi E, et al. Subendothelial resistin enhances monocyte transmigration in a co-culture of human endothelial and smooth muscle cells by mechanisms involving fractalkine, MCP-1 and activation of TLR4 and Gi/o proteins signaling. Int J Biochem Cell Biol. 2014;50:29–37. doi: 10.1016/j.biocel.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 75.Jang JC, Chen G, Wang SH, Barnes MA, Chung JI, Camberis M, et al. Macrophage-derived human resistin is induced in multiple helminth infections and promotes inflammatory monocytes and increased parasite burden. PLoS Pathog. 2015:11. doi: 10.1371/journal.ppat.1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, et al. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. The Journal of Experimental Medicine. 2009;206:937–52. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, et al. Retnla (Relmα/Fizz1) Suppresses Helminth-Induced Th2-Type Immunity. PLoS Pathogens. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nair MG, Gallagher IJ, Taylor MD, Loke Pn, Coulson PS, Wilson RA, et al. Chitinase and Fizz Family Members Are a Generalized Feature of Nematode Infection with Selective Upregulation of Ym1 and Fizz1 by Antigen-Presenting Cells. Infection and Immunity. 2005;73:385–94. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109:9977–82. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu T, Yu H, Ullenbruch M, Jin H, Ito T, Wu Z, et al. The In Vivo Fibrotic Role of FIZZ1 in Pulmonary Fibrosis. PLoS ONE. 2014;9:e88362. doi: 10.1371/journal.pone.0088362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee M-R, Shim D, Yoon J, Jang HS, Oh S-W, Suh SH, et al. Retnla Overexpression Attenuates Allergic Inflammation of the Airway. PLoS ONE. 2014;9:e112666. doi: 10.1371/journal.pone.0112666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osborne LC, Joyce KL, Alenghat T, Sonnenberg GF, Giacomin PR, Du Y, et al. Resistin-like molecule alpha promotes pathogenic Th17 cell responses and bacterial-induced intestinal inflammation. J Immunol. 2013;190:2292–300. doi: 10.4049/jimmunol.1200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, et al. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–7. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee M-R, Lim C-j, Lee Y-H, Park J-G, Sonn SK, Lee M-N, et al. The adipokine Retnla modulates cholesterol homeostasis in hyperlipidemic mice. Nat Commun. 2014:5. doi: 10.1038/ncomms5410. [DOI] [PubMed] [Google Scholar]
- 85.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–57. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, et al. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181:4709–15. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kushiyama A, Sakoda H, Oue N, Okubo M, Nakatsu Y, Ono H, et al. Resistin-like molecule beta is abundantly expressed in foam cells and is involved in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2013;33:1986–93. doi: 10.1161/ATVBAHA.113.301546. [DOI] [PubMed] [Google Scholar]
- 88.Shojima N, Ogihara T, Inukai K, Fujishiro M, Sakoda H, Kushiyama A, et al. Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia. 2005;48:984–92. doi: 10.1007/s00125-005-1735-1. [DOI] [PubMed] [Google Scholar]
- 89.Murray Peter J, Allen Judith E, Biswas Subhra K, Fisher Edward A, Gilroy Derek W, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]