Abstract
Gamma-butyric acid (GABA) dysfunction has been implicated in the pathophysiology of schizophrenia and its cognitive deficits. Proton magnetic resonance spectroscopy (MRS) was used to test the hypothesis that older participants with schizophrenia have lower anterior cingulate GABA levels compared to older control participants. One-hundred and forty-five participants completed this study. For detection of GABA, spectra were acquired from the medial frontal/anterior cingulate cortex using a macromolecule-suppressed MEGA-PRESS sequence. Patients were evaluated for psychopathology and all participants completed neuropsychological tests of working memory, processing speed, and functional capacity. GABA levels were significantly lower in the older participants with schizophrenia(n=31) compared to the older control(n=37) group (p=0.003) but not between the younger control(n=40) and schizophrenia (n=29) groups (p=0.994). Age strongly predicted GABA levels in the schizophrenia group accounting for 42% of the variance, but the effect of age was less in the control group accounting for 5.7% of the variance. GABA levels were specifically related to working memory but not processing speed performance, functional capacity, or positive or negative symptom severity. This is the largest MRS study of GABA in schizophrenia and the first to examine GABA without macromolecule contamination, a potentially significant issue in previous studies. GABA levels more rapidly declined with advancing age in the schizophrenia compared to the control group. Interventions targeted at halting the decline or increasing GABA levels may improve functional outcomes and quality of life as patients with schizophrenia age.
Introduction
Gamma-butyric acid (GABA) is the primary inhibitory neurotransmitter in the mammalian brain1. GABA dysfunction has been implicated in the pathophysiology and cognitive deficits of schizophrenia, mainly based on post-mortem and preclinical research2, 3. Reduced expression of GAD67, a GABA synthesis enzyme, is a well-replicated molecular finding in schizophrenia4–7. GABAergic interneurons are thought to facilitate the rhythmic entrainment of pyramidal cell discharge and their abnormalities may lead to cognitive dysfunctions, especially working memory impairments, in schizophrenia2, 8–11. Because GABA plays a critical role in brain functions in general and in schizophrenia in particular, emerging techniques to achieve more accurate measurements of GABA concentrations in patients with schizophrenia in vivo will play a critical role in advancing pathophysiological and pharmacological research in schizophrenia.
Proton magnetic resonance spectroscopy (1H-MRS) techniques such as spectral editing, also widely known as “MEGA-PRESS,” (MEGA-Point REsolved Spectroscopy Sequence) have enabled quantification of brain GABA concentrations in vivo12. With the traditional MEGA-PRESS, the frequency selective pulses are placed at 1.9 and 7.5ppm, and macromolecules coedit with the GABA signal. As a result, macromolecules contribute up to 50% of the GABA signal13. This measurement is typically denoted as “GABA+”.
Several MRS studies of schizophrenia have reported abnormal but inconsistent findings, including increased14, 15 or reduced GABA+ levels16–19 in schizophrenia, depending upon antipsychotic and concomitant medication status, illness duration, and brain region. There is currently no consensus in the field as to whether the differences between schizophrenia and normal control participants are present or due to differences in macromolecules, GABA, or a combination. The inconsistency is reminiscent of earlier MRS findings in glutamate, although subsequent evidence suggests that glutamate was elevated in younger schizophrenia patients20–22, but reduced in chronic, older patients18, 23. Interestingly, our preliminary analysis of GABA+ aging trend, using the traditional MEGA-PRESS method, showed a similar trend of age-related reduction of GABA levels in patients with schizophrenia18. Motivated by these prior observations, our study included an adequately powered sample to examine the schizophrenia diagnosis by age cohort effect on GABA concentration, and utilized a critically improved GABA assessment technique that removes the macromolecule contamination.
To address this, we employed MEGA-PRESS with frequency selective pulses at 1.9 and 1.5ppm24 to assess macromolecule-suppressed GABA in a cohort of participants with schizophrenia and psychiatrically healthy controls. We tested the hypothesis that older participants with schizophrenia would have lower anterior cingulate GABA levels compared to older control participants. We predicted that there would be no significant difference between younger participants with schizophrenia and controls.
This study also examined the relationships between GABA and psychiatric symptoms severity in positive and negative symptoms, and cognitive functions in working memory, processing speed, functional capacity. Based on the strong evidence supporting GABAergic function in working memory2, 25, we predicted that GABA level would play a stronger role in working memory compared with other clinical, cognitive, and functional measures.
Methods
Participants
One-hundred and forty-five participants completed this study but 8 were excluded due to poor data quality, leaving a total of 29 younger and 31 older in the schizophrenia group and 40 younger and 37 older in the control group. Participants with schizophrenia had diagnosis of schizophrenia (n=50) or schizoaffective disorder (n=10) as determined with the Structured Clinical Interview for DSM-IV-TR, Patient Version. Normal controls had no past or present Axis I psychiatric disorder as determined with the Structured Clinical Interview for DSM-IV-TR, Non-Patient Version. All participants were 18–65 years old, with no current or past neurological condition and major medical conditions, and no DSM-IV-TR substance abuse in the last 6 months or substance dependence in lifetime or 1 year or more (except nicotine). We made attempt to frequency-match the smoking status between patients and controls (see Table 1). Participants with schizophrenia were evaluated for their ability to provide informed consent before signing consent documents. All participants gave written informed consent prior to participation in the study. This study was approved by the University of Maryland Baltimore Institutional Review Board.
Table 1.
Participant Characteristics. Mean (SD)
| Schizophrenia Younger (n=29) |
Control Younger (n=40) |
Schizophrenia Older (n=31) |
Control Older (n=37) |
|
|---|---|---|---|---|
| Gender Male/Female |
20/9 |
20/20 |
19/12 |
25/12 |
| Age (years) | 25.7 (4.3) | 25.3 (4.6) | 48.3 (5.8) | 51.0 (6.0) |
| 1Education (years) | 13.3 (2.1) | 14.4 (2.2) | 12.7(2.0) | 14.9 (2.4) |
| 2Smoking status (yes/no) | 12/17 | 10/30 | 12/19 | 9/28 |
| 1.3Processing speed (digit sequencing test) | 63.7 (20.6) | 72.0 (19.3) | 50.4(15.3) | 64.4 (19.5) |
| 1,3 Functional Capacity (UPSA) | 94. 7 (11.5) | 104.2 (7.4) | 85.43 (14.8) | 101.06 (10.4) |
| 1Working memory (digit symbol coding test) | 42.9 (12.1) | 46.1 (13.0) | 35.6 (13.6) | 47.3 (11.2) |
| Duration of illness (years) | 5.6 (4.6) | 24.0 (9.8) | ||
| Psychiatric Ratings BNSS |
15.6 (12.1) |
17.6 (15.4) |
||
| BPRS (total) | 39.2 (10.5) | 38.3 (11.5) | ||
| BPRS (+ subscale) | 8.4 (4.4) | 8.6 (4.6) | ||
| BPRS (− subscale) | 6.6 (2.7) | 6.2 (2.4) | ||
| Chlorpromazine units (CPZ) | 562.0 (511.4) | 668.9 (643.9) | ||
| Antipsychotic Medication Typical Atypical Both typical and atypical None |
1 25 1 2 |
4 18 6 3 |
Main effect of diagnostic group (schizophrenia < control; p < 0.05)
Chi square (schizophrenia > control; p < 0.05)
Main effect of age group (older < younger; p < 0.05)
Patients were evaluated for psychopathology with the Brief Psychiatric Rating Scale (BPRS) and the Brief Negative Symptom Scale (BNSS)26. Five patients were not on antipsychotics (2 in the younger vs. 3 in the older group), 5 only on typical (1 vs. 4), and 50 on atypical antipsychotics (26 vs. 24) (χ2 = 2.02, p=0.36). Seven of those on atypicals were also on typical antipsychotics (1 vs. 6). Of the atypical antipsychotics, 15 were on clozapine (7 vs. 8), 14 on risperidone (8 vs. 6), 7 on olanzapine (3 vs. 4), 2 on paliperidone (1 vs. 1), 6 on quetiapine (3 vs. 3), 5 on aripiprazole (4 vs. 1), and 1 on ziprasidone (0 vs. 1). Patients on daily use of benzodiazepines or nonbenzodiazepine GABAergic hypnotics were excluded. Three patients (1 vs. 2) were on the anticholinergic drug benztropine; patients with clinically uncontrolled extrapyramidal symptoms or tardive dyskinesia were excluded. Participants completed a targeted cognitive/functional capacity battery of three measures: processing speed by the digit symbol coding test27, working memory by the digit sequencing test28, and functional capacity was assessed with the UPSA-229. While cognitive deficits in schizophrenia encompass multiple domains, deficits in working memory30–32 and processing speed33–35 are particularly severe. The UCSD Performance-based Skills Assessment (UPSA-2) was used to measure functional capacity. UPSA-2 uses role-play to assess functioning across five domains: organization/planning, financial skills, communication skills, transportation, and household skills, and is a validated tool to assess community functional capacity in schizophrenia patients36. Nine controls and 3 participants with schizophrenia did not complete UPSA or processing speed testing and 12 controls and 4 participants with schizophrenia did not complete digit span testing. Participants were monetarily compensated for their time.
MRS Acquisition and Analyses
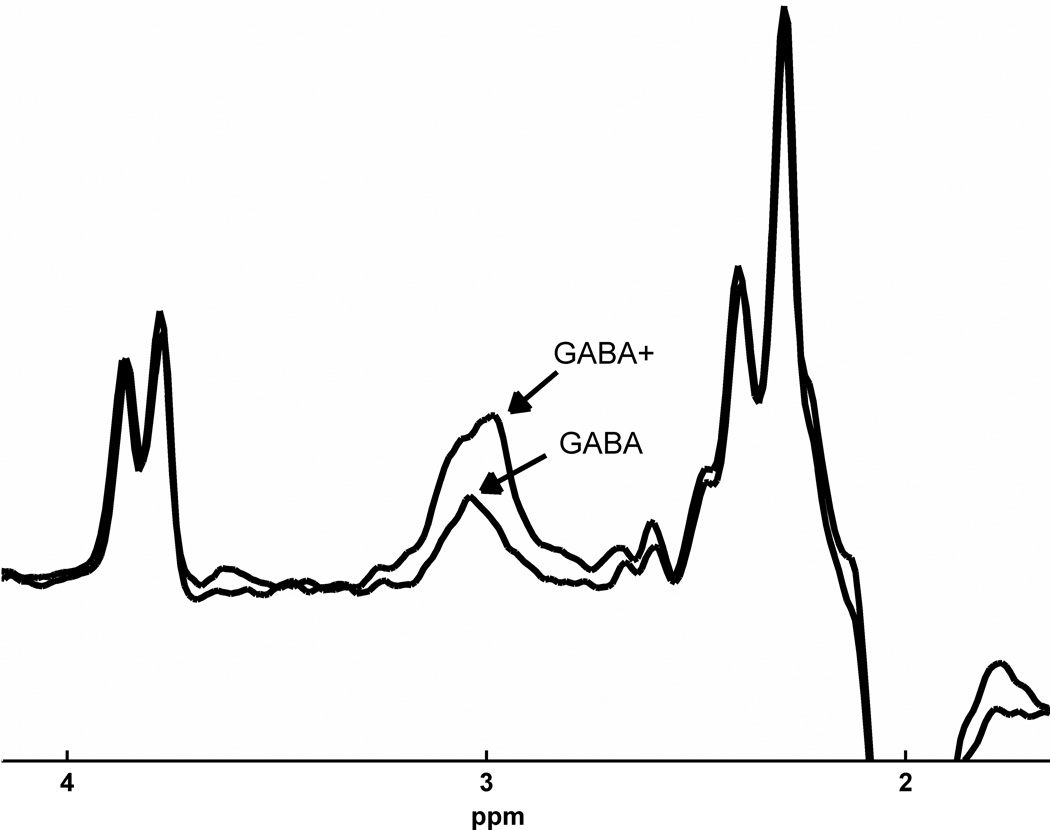
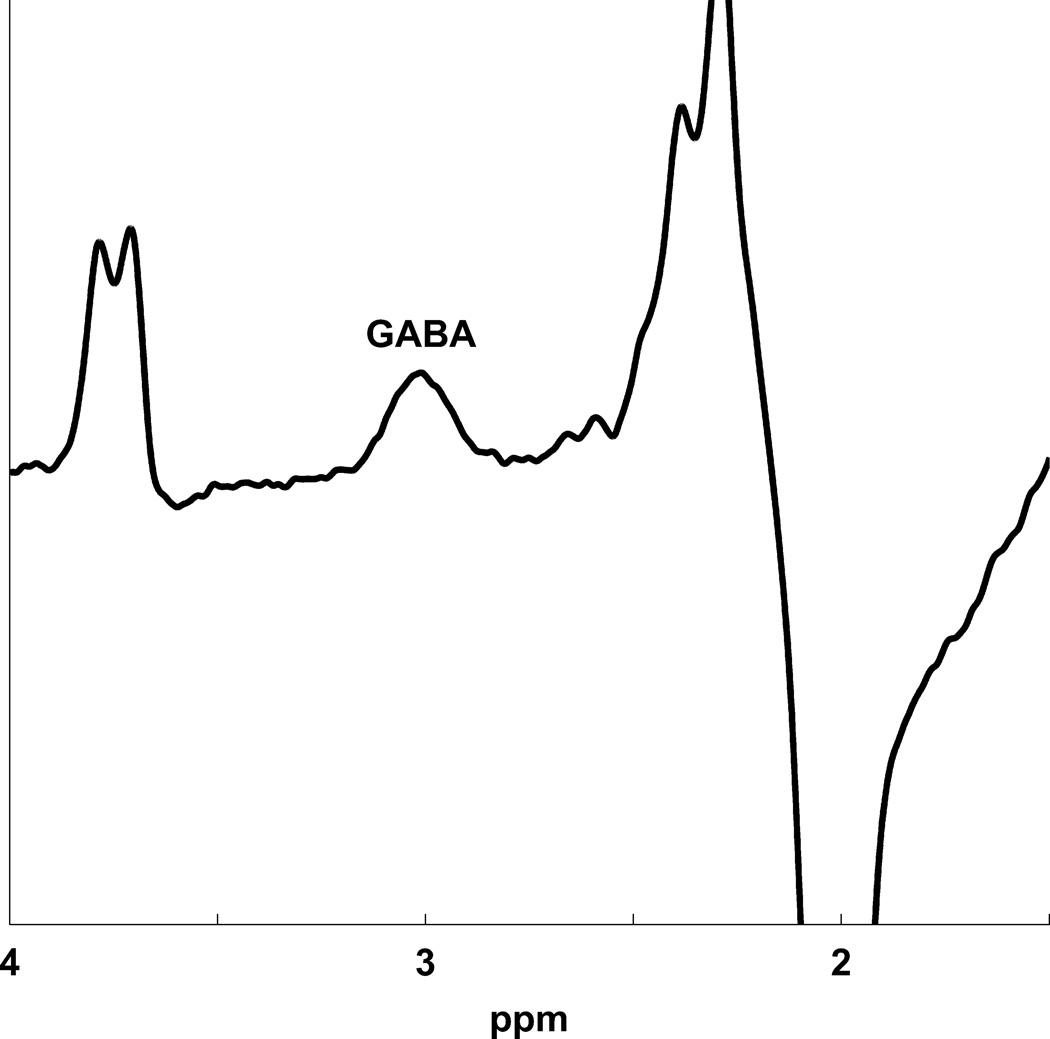
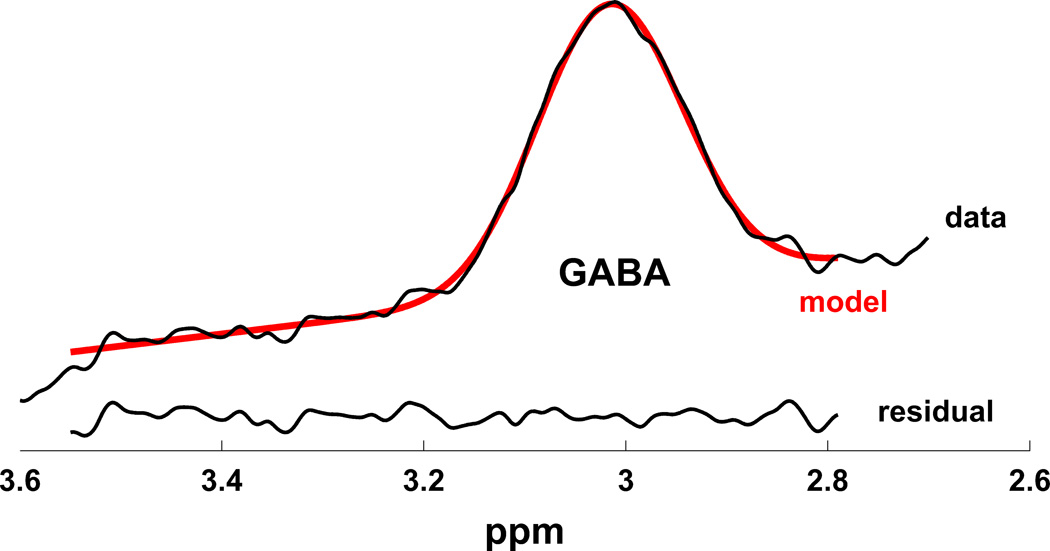
MR scanning was conducted on a 3T Siemens Tim Trio equipped with a 32-channel head coil. Head position was fixed with foam padding to minimize movement. Anatomical T1-weighted images were acquired for spectroscopic voxel placement with a ‘MP-RAGE’ sequence37. For detection of GABA, spectra were acquired from a medial frontal region that included the anterior cingulate (Brodmann areas 24 and 32) using a macromolecule-suppressed MEGA-PRESS sequence: TR=2000, TE=68 ms, 20.36 ms length and 44 Hz bandwidth full width at half maximum (FWHM) editing pulses applied at 1.9 (ON) and 1.5 (OFF) ppm, and 256 averages (128 ON and 128 OFF); water unsuppressed 16 averages. Water suppression was achieved using Siemens modified WET water suppression technique. Automated shimming followed by manual shimming was conducted to achieve approximately 12Hz water linewidth. Spectral acquisition methodology was similar to Aufhaus et al13 including the sequence, voxel and saturation bands placement. The 40 × 30 × 20 mm voxel was prescribed on the midsagittal slice and positioned parallel to the genu of the corpus callosum and scalp with the midline of the voxel corresponding to the middle of the genu of the corpus callosum. Figure 1 illustrates comparison of conventional and macromolecule-suppressed spectra.
Figure 1.
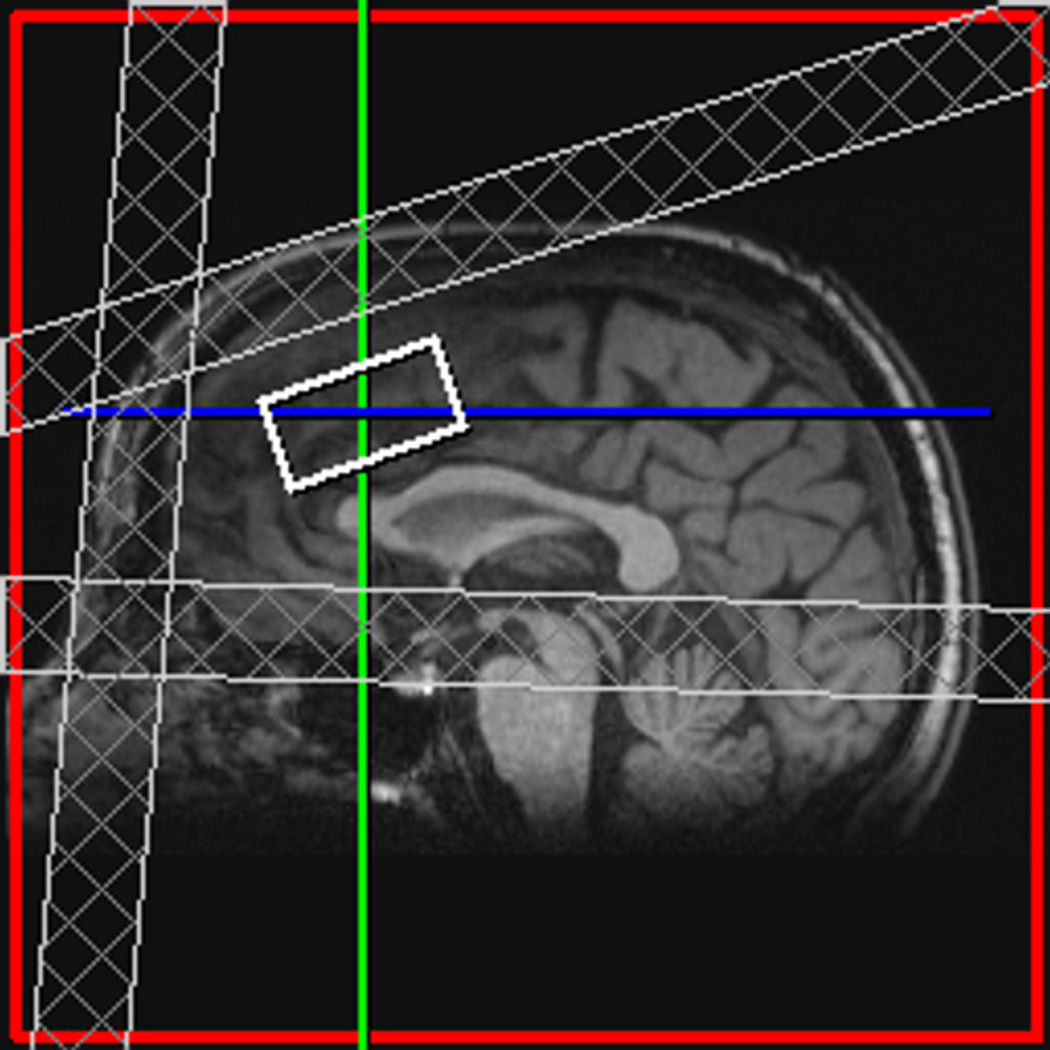
(a) Conventional (GABA+) and macromolecule suppressed (GABA) spectra obtained from same individual, (b) Anterior cingulate voxel location with saturation bands (c) representative GABA spectrum, and (d) GABA peak fit in red
Quantification was conducted with GANNET 2.0 toolkit, a Matlab program specifically developed for analysis of GABA MEGA-PRESS spectra38. Individual spectra were frequency and phase corrected, and then “ON” and “OFF” subtracted resulting in the edited spectrum. The edited GABA peak was modeled as a single-Gaussian and values of GABA relative to water (modeled as a mixed Gaussian-Lorentzian) in institutional units were produced. The normalized fitting residual was calculated by dividing the standard deviation of the fitting residual by the amplitude of the fitted peak. Spectra were included if the normalized fitting residual of GABA was below 15%. The spectroscopic voxel was segmented into gray, white, and CSF tissues using SPM8 and in house MATLAB code, and GABA concentrations were corrected for the proportion of gray, white, and CSF tissue proportions39 (See Supplementary Materials). See Figure 1 for illustration of voxel location, representative spectrum, and GABA peak fit.
Statistical Analysis
Demographic variables were analyzed with chi-square or Fisher’s exact tests for categorical data and ANOVAs for continuous variables. Participant assignment to age group was determined by median split of age (35 years old). GABA levels were analyzed with a 2(diagnosis) × 2 (age group) ANOVA. Gender and smoking status were included as covariates since previous literature reported GABA+ age related decline differed between males and females40 and GABA+ may be impacted by smoking and sex41. Also, smoking status was different between diagnostic groups (see below). Significant effects (p < 0.05) were followed-up with post-hoc tests when appropriate. Given the strong interest in GABA and age, the relationship between age and GABA levels per diagnostic group was investigated with linear regressions, as well as by LOESS42, a nonparametric smoothing method where the predicted value for each data point is estimated from a polynomial fitted on nearby data points. LOESS is useful for exploratory analysis of relationships between two variables, such as developmental changes with age, in which a simple global regression function (such as a straight line) cannot be assumed to apply throughout the range of the independent variable. Applications of both linear and LOESS models for the relationship of GABA and age by diagnostic group were conducted. The differences in regression slopes (based on linear regression) between groups were examined by analysis of covariance (ANCOVA) where we tested the effect of group on GABA level while controlling for age. The regression lines were compared by testing the interaction. A significant interaction indicates that the effect of age on GABA level depends on group43. Finally, we also compared the correlation coefficients between groups using Fisher's z-transform44.
The relationships between GABA levels and BPRS total and positive symptoms and BNSS negative symptoms in patients with schizophrenia were analyzed using Pearson product moment correlation with Bonferroni correction for three comparisons (p ≤ 0.017). Linear regression analyses were used to test whether GABA levels predict working memory, processing speed, and/or functional capacity after covarying out effects of diagnosis and age that are known to impact cognition and functional capacity. The three regression models tested were: working memory (then replaced by processing speed, and then functional capacity) = b0 + b1*diagnosis + b2* age + b3*GABA level, with significance level set at p ≤ 0.017 after Bonferroni correction for three comparisons. Based on extensive evidence of GABAergic signaling on working memory, we predicted that GABA could have a more robust contribution to working memory. Analyses were conducted with the Statistical Package for Social Sciences (SPSS) version 22.0 software package.
Results
Participant characteristics
Participant demographic, clinical, and cognitive characteristics by diagnostic and age group are provided in Table 1. As expected, the duration of illness was significantly shorter for the younger (5.6 years) compared to the older (24 years) participants with schizophrenia (p < 0.001). There were no significant differences in psychiatric symptom severity (all p’s > 0.4) or antipsychotic medication status (Fisher’s exact test, p > 0.1) between the younger and older schizophrenia groups. Participants with schizophrenia had significantly fewer years of education (average of 1.7yrs) compared to the control group as illustrated by a main effect of diagnostic group (F(1, 134)=18.7, p < 0.001).
There were main effects of diagnostic group such that the schizophrenia group had lower working memory (F(1,109)=11.1, p =0.001), processing speed (F(1,120=10.3, p =0.002), and functional capacity UPSA scores (F(1,121) = 39.6, p < 0.001) compared to the control group. Age group main effects also emerged such that the older groups performed worse in processing speed (F1,121)= 10.4, p = 0.002) and functional capacity (F(1,121)=9.1, p = 0.003). There were no significant diagnostic group by age group interactions for either of the cognitive measures. There were no significant differences in age, gender, smoking status among diagnostic by age groups. There was a trend for more smokers in the schizophrenia group collapsed across age group (Chi square = 3.7, p = 0.055).
GABA levels
Eight spectra from five control and three schizophrenia participants were discarded due to excessive movement resulting in normalized fitting residual > 15%. All included spectra had a normalized fitting residual of 12% or lower, which did not differ between diagnostic by age groups (p=0.2) but did significantly differ between diagnostic group (p = 0.03; control mean: 6.7% (1.8), schizophrenia mean: 7.4% (2.0). However, inclusion of the normalized fitting residual as a covariate did not change the statistical results. There were no significant differences in the proportion of voxel gray, white, and CSF tissues between diagnostic group by age group, but both older groups had less gray matter (p=0.004). The inclusion of gray matter as a covariate did not alter the results.
Results of age group × diagnostic group ANOVA with gender and smoking as covariates revealed a significant diagnosis by age interaction (F(1,132) = 5.9, p = 0.017) indicating that GABA levels declined from younger to older groups in schizophrenia but less so in the control sample. Results of analysis of simple effects supported our a priori hypothesis that GABA levels were significantly lower in the older participants with schizophrenia compared to older controls (t (66)= 3.1, p =0.003) but not between the younger control and schizophrenia groups (t (67)=0.25, p=0.994). See Figure 2 that illustrates GABA levels by age and diagnostic group.
Figure 2.
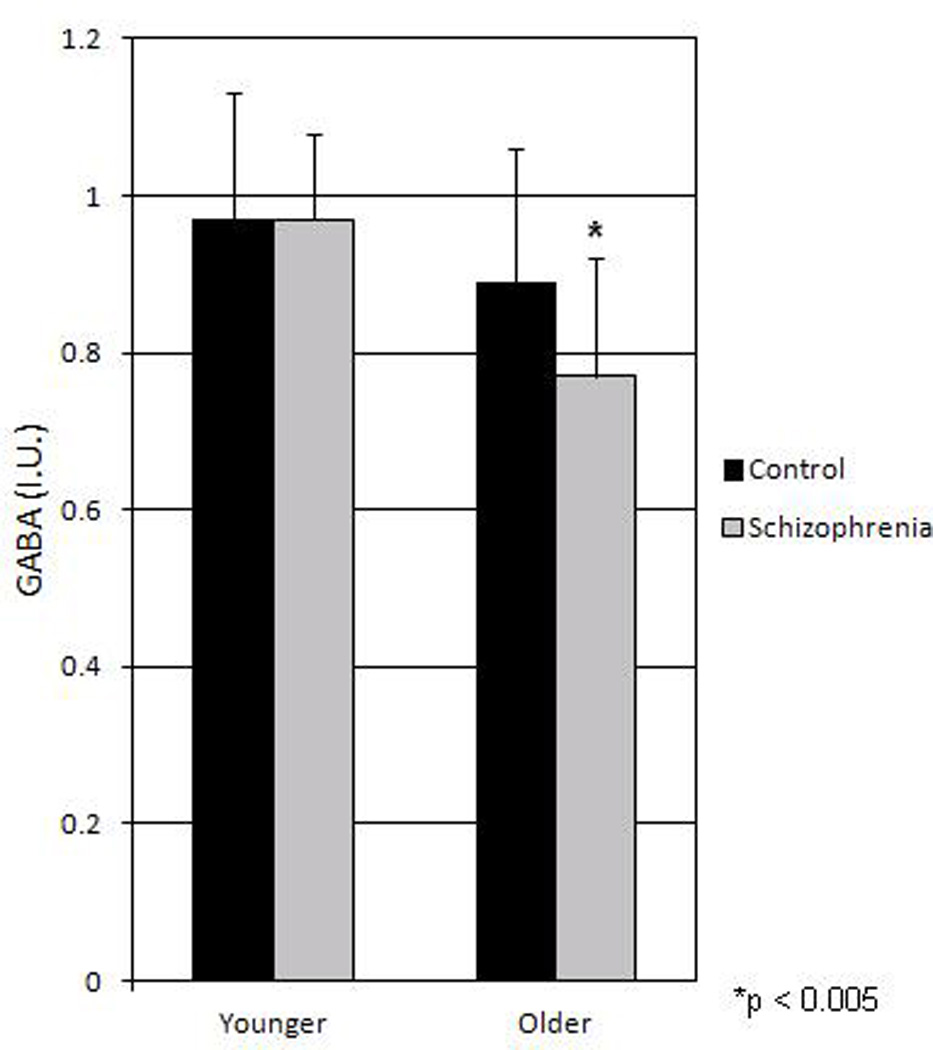
Mean (SD bars) GABA levels by diagnostic and age groups. There was a significant diagnosis by age interaction (p = 0.017) with lower GABA levels in older schizophrenia compared to older control group (p < 0.005).
The relationship between GABA and age
Linear regression analyses revealed that age strongly predicted GABA levels in the schizophrenia group (B = −0.6, t(59) = −6.42, p < 0.001, R2=0.42) accounting for 42% of variance, but the effect of age was less in the control group (B = −0.24, t(75) = −2.1, p = 0.036, R2= 0.057) accounting for 5.7% of the variance (See Figure 3). The slope of the age effect was significantly different (t = −2.86, df = 132, p = 0.005) and the correlation coefficients of the age vs. GABA effect were also significantly different between groups (z = 2.97, p = 0.0015). This suggests there was a significantly steeper age-related decline in GABA level in schizophrenia compared with the age-related decline in controls, and that age explained significantly more of the individual variance in GABA level in schizophrenia compared with controls. Application of LOESS model to data suggested similar, gradually increasing GABA levels among schizophrenia and controls groups until around 33 years old after which GABA declined in both groups, with a steeper, continuous decline (See Figure 3) in the schizophrenia group, and a tendency for average decline to level off after age 50 in the control group.
Figure 3.
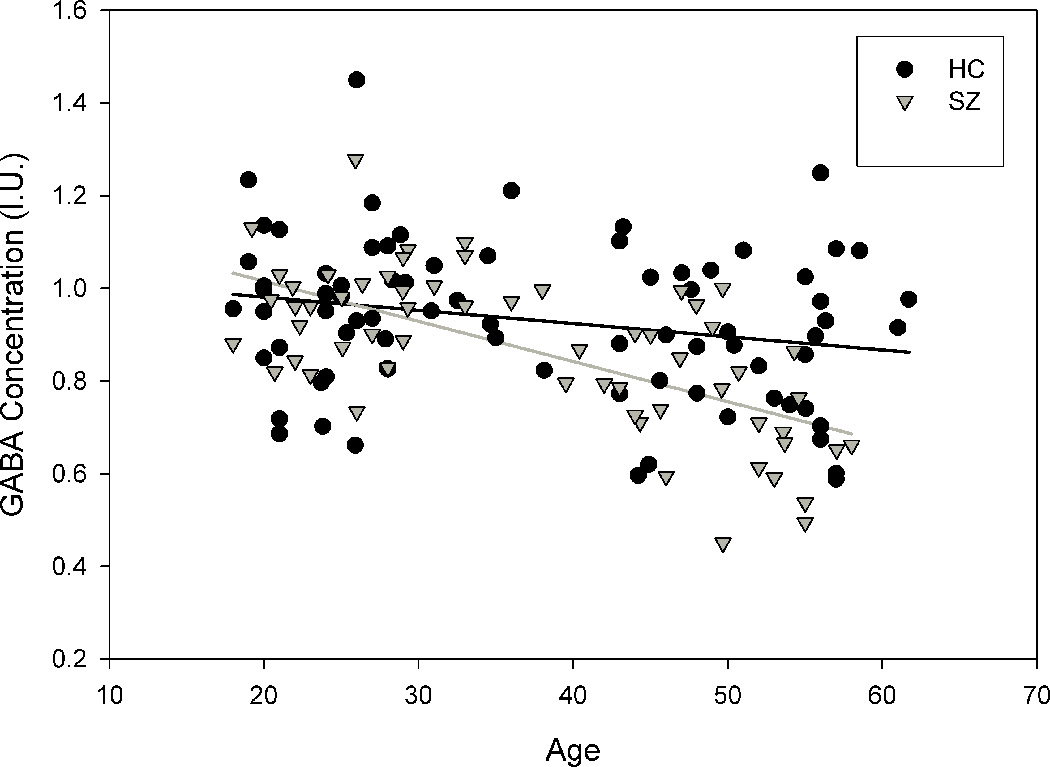
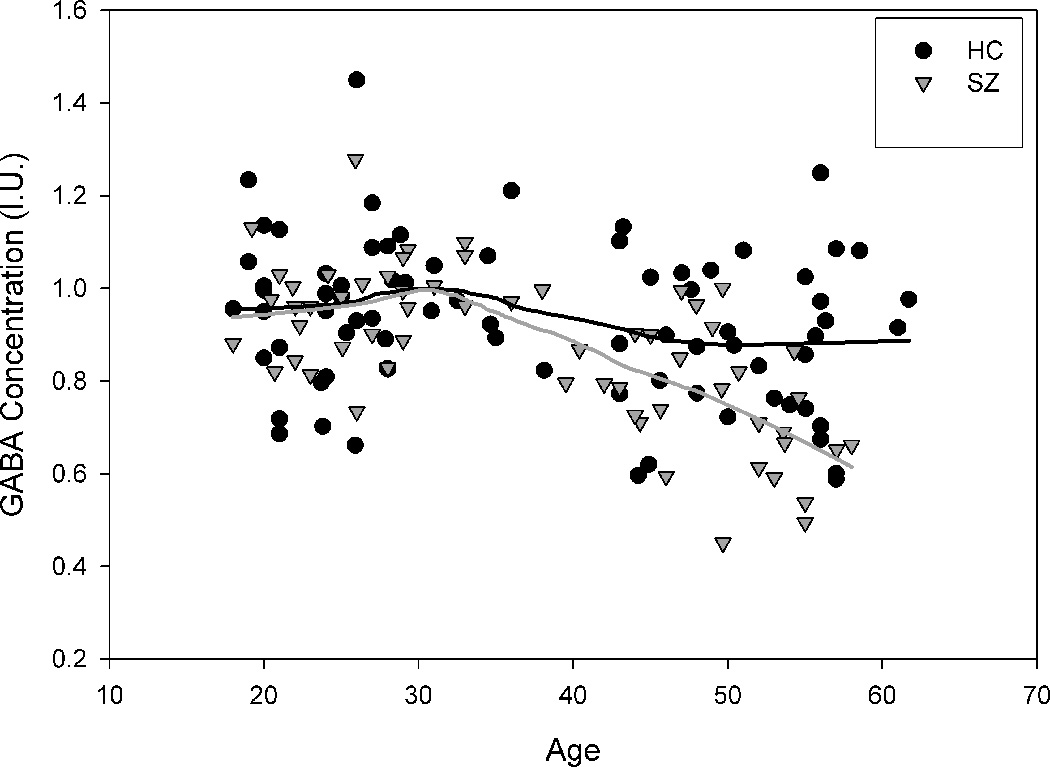
The relationship between GABA and age by diagnostic group. Both (a) Linear regression and (b) Loess regression show greater decline in schizophrenia compared to control group.
Illness duration was significantly related to GABA levels (r=−0.63, p<0.001) in the patients. This was to be expected since age is strongly related to illness duration. However, using age as the control variable, the partial correlation of GABA level and illness duration was no longer significant (r=−0.13, p=0.35). In comparison, when illness duration is used as the control variable, age remains significantly correlated with GABA level (r=−0.37, p=0.007). Age plays a significant role even when illness duration is taken into account.
GABA and symptoms and cognition
There were no statistically significant relationships between GABA and BPRS positive and total scores or negative symptom score measured by BNSS (all p’s > 0.8).
Linear regression analysis revealed that GABA level significantly predicted working memory (F=7.5, p <0.001, R2=0.17) in the overall model. GABA level was positively and significantly associated with working memory (t=2.96, p=0.004) when diagnosis and age were controlled. Diagnosis (t=2.7, p=0.008) but importantly not age (t=0.31, p=0.76) predicted working memory. Repeating the above analysis for functional capacity, the model was again significant (F=15.6, p <0.001, R2=0.28,), although only diagnosis (t=5.7, p<0.001) but not age (t=1.7, p = 0.10) or GABA (t=1.2, p=0.23) was a significant contributor to functional capacity. Finally, the model for processing speed was also significant (F=5.0, p =0.003, R2=0.13), although only age (t=2.0, p = 0.047) and diagnosis (t=3.0, p=0.003), but not GABA (t=0.19, p=0.85) were significant contributors to processing speed. Therefore, medial frontal/anterior cingulate GABA level was a significant predictor for working memory; while for processing speed or functional capacity, only diagnosis and/or age but not GABA level were significant predictors.
GABA and antipsychotic medication
Chlorpromazine (CPZ) equivalent units45 did not significantly differ between the younger and older groups (p= 0.50) and were not significantly related to GABA levels (p = 0.90). Moreover, the inclusion of CPZ as a covariate in the omnibus test did not change the interaction results (p = 0.012 vs 0.017).
Participants with schizophrenia were assigned to one of four antipsychotic treatment groups: typical only (n=5), atypical only (n=44), both typical and atypical (n=7), and no medication (n=5). Visual inspection of individual GABA values indicates that GABA levels did not differ between the four antipsychotic drug groups (Figure 4). It is also important to note that the groups did not significantly differ in psychiatric symptom severity or cognitive measures (all p>0.05). Patients on one vs. two antipsychotics were also not significantly different in positive symptom severity (p=0.81). Removing those patients on two antipsychotics, results of the significant age × diagnostic group interaction remained significant (p values changed from 0.017 to 0.015). Visual inspection of the GABA levels of participants taking 2 antipsychotics were no different from those taking 1 antipsychotic, as illustrated in Figure 4. Six patients were also on a mood stabilizer (5 on valproic acid and 1 on lamotrigine), which could raise GABA levels15. However, all patients were in the older age group and removing them did not change the significant group differences or the age-related correlation in GABA levels. Finally, different antipsychotic medication may affect the GABAergic system differently due to different neurotransmitter binding profiles. Comparing patients on clozapine (n=15) vs. risperidone (n=14), the two medications with the largest participant number and different binding profiles, we found no evidence of a significant difference in GABA levels (0.83±0.18 vs. 0.81±0.16, respectively, F(1,28)=0.11, p=0.78).
Figure 4.
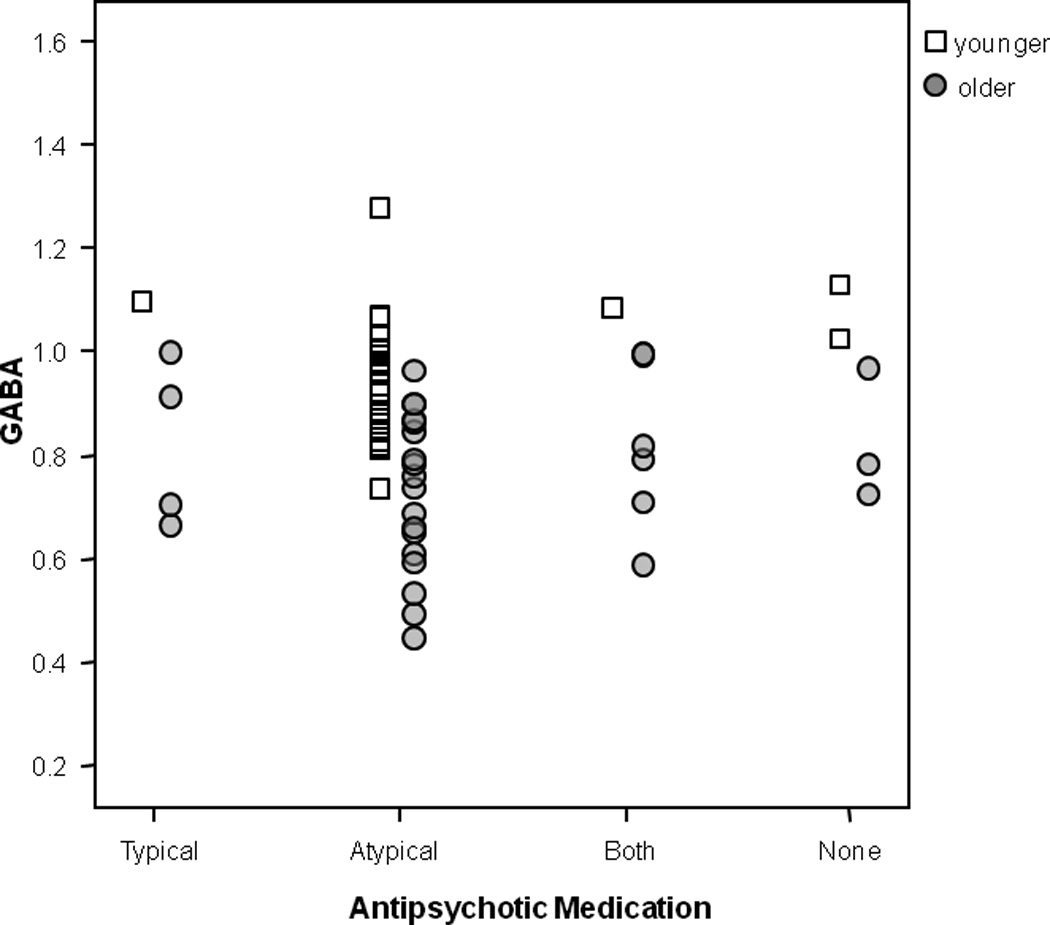
Individual GABA values by antipsychotic drug group.
Discussion
To our knowledge, this is the first study to investigate in vivo medial frontal/anterior cingulate GABA levels in schizophrenia using MEGA-PRESS with macromolecule suppression. This is also the largest study of GABA levels in schizophrenia measured with MRS to date. We confirm our previously reported trend finding of reduced medial frontal GABA+ in older schizophrenia using traditional MEGA-PRESS18. It is important to note that this previous work was conducted on a different 3T MR scanner and platform (Philips). The participants in the current study also had no overlap with the previous sample.
Our findings add to the growing literature of GABA+ level alterations measures with MRS in schizophrenia14, 15, 18. The result of lower GABA in older participants with schizophrenia may have reconciled the seemingly contradictory observations in several previous reports in schizophrenia. Anterior cingulate GABA+ levels assessed with MRS have been shown to be higher in participants with schizophrenia who were younger and not taking antipsychotic medication (including 9 who were off medication for at least two weeks and 7 were medication-naive)14, and during exacerbation of psychosis15 compared to control participants. The participants assessed here and in our previous work18 were mostly medicated patients across a large age span. The five participants with schizophrenia off medication (for one month or more) did not have higher GABA levels and had a similar relationship between age and GABA to the medicated schizophrenia groups. When considering this and previous studies, it is likely that GABA is higher in younger off-medicated and actively psychotic patients but decreases in older, antipsychotic- medicated patients.
It remains to be determined if GABA levels are greater in a larger cohort of older participants with schizophrenia who are off antipsychotic medication, as perhaps suggested by a GABA+ study14. It is likely that higher GABA levels are restricted to just a small subset of off medication people with schizophrenia since plots of individual subject values in Kegeles et al14 reveal 4 extreme values possibly driving the group effect of higher GABA+ in the off medicated group, reflective of the heterogeneity of the schizophrenia illness. It also remains to be determined if GABA levels are higher in older persons experiencing an exacerbation in psychotic symptoms as suggested by a GABA+/Cr study15. There are substantial methodological differences between the current study and the other studies14, 15, including the suppression of macromolecules, fitting algorithms used to quantify GABA, thorough correction of GABA levels based on gray, white, and CSF spectroscopic tissue proportions, and GABA relaxation times implemented in this study that deserve mentioning (see Methods).
GABA levels were not related to positive or negative symptoms but were related to cognition, more specifically in working memory, as hypothesized. GABA levels significantly predicted working memory but not functional capacity or processing speed when controlling for age and diagnosis effects. As anticipated, diagnosis strongly contributed to working memory, processing speed, and functional capacity, all of which were significantly impaired in schizophrenia. Functional capacity encompasses multiple cognitive domains and therefore relies on multiple neural networks and mechanisms. Processing speed likely depends on rapid communication among multiple sensory - motor cortical regions, a hypothesis strongly supported by its correlation with widespread white matter integrity46, 47. In contrast, strong a priori evidence suggests that working memory deficits in schizophrenia are related to frontal GABAergic interneuron dysfunction2, 25. The results of this study are thus consistent with animal models that show working memory deficits induced by disruption of frontal cortex GABA receptors48, as well as preliminary results from an MRS studies suggesting an important role for GABA+ in working memory49. Therefore, treatments targeted at increasing GABA levels may prove beneficial for working memory impairments in schizophrenia50.
There are several outstanding questions regarding interpretation meaning of in vivo GABA tissue levels assessed with 1H-MRS. The MRS GABA signal presumably reflects total tissue levels in the voxel of interest. Additional basic science research is needed to determine if the 1H-MRS signal is derived from GABA in all cell types, synapses, extra-synaptic extracellular space, and/or synaptic vesicles. Hence, altered GABA levels in clinical samples cannot be conclusively attributed to alterations in synthesis or specific neuronal mechanisms. Despite these caveats, the finding of the seemingly precipitous decline of GABA levels in schizophrenia over the course of their adult life versus controls could be compatible with post-mortem studies of reduced GAD67, the major enzyme involved in GABA synthesis7 and altered GABAergic interneuron morphology3.
It remains to be determined if lower GABA levels are due to longer antipsychotic medication exposure, illness phase or aging. While we observed an age related decline in GABA within the 5 unmedicated participants, a larger unmedicated sample would be needed to confirm that lower GABA levels are still present without prolonged antipsychotic medication exposure. In psychiatrically normal subjects, one study showed anterior cingulate GABA+/Cr to decline with age40 but another failed to show anterior GABA (with macromolecule suppression) to decline with age13. Our data on the normal controls were consistent with the small decline of GABA over time. The much greater age-related decline in GABA in schizophrenia is thus striking. We propose that this finding is consistent with other neuroimaging studies showing accelerated age-related decline in the brains of schizophrenia patients compared to controls51, 52. We believe that these data support the hypothesis of accelerated aging in schizophrenia53, 54.
There are several additional limitations to this study that deserve mentioning. The spectroscopic voxel was large and only one region was assessed. Consequently anatomical specificity was not considered in these results. One previous study suggests that GABA+ differences occur in medial frontal (including ACC) but not dorsolateral prefrontal region14, while another study suggests GABA+ differences in both anterior and posterior cingulate regions15. Therefore, ACC provides a key region for the current study. Future studies should survey multiple brain regions to discern anatomic specificity. Substance use was determined by clinical interview (SCID) and information from the treating clinicians when available, and as such cannot be definitely ruled out as impacting GABA levels. Adherence to antipsychotic medication was based on careful clinical evaluation although not determined through blood testing, which is another limitation. There is the potential confound of antipsychotic medication, which is inherent to the majority of schizophrenia studies, but accounting for CPZ units did not impact results. The heterogeneity of antipsychotic medication is another study consideration since some antipsychotic medications may affect the GABAergic system, and future studies should examine the differential effects of specific antipsychotic medications on GABA levels. The medication subgroups were small and so results should be interpreted with caution.
In conclusion, these data suggest that medial frontal/anterior cingulate GABA levels appear to become lower with advancing age in the schizophrenia group compared to the control group. Developing interventions targeted at increasing GABA levels may improve functional outcomes especially working memory in adult patients with schizophrenia.
Supplementary Material
Acknowledgements
We thank the volunteers, especially the participants with schizophrenia, for participating in the study. This work is supported by National Institutes of Health (T32MH067533, R01MH094520, R01MH085646, R01DA027680, and P50MH103222). We thank Dr. Richard Edden and colleagues who kindly provided GANNET funded through NIH R01 EB016089 and P41 EB015909. We kindly thank Drs. William Carpenter and Juan Bustillo for their comments on this manuscript.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Curtis DR, Duggan AW, Felix D, Johnston GA. GABA, bicuculline and central inhibition. Nature. 1970;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- 2.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 3.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 4.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of general psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 5.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of general psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Current psychiatry reports. 2010;12(4):335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent SL, Sorensen I, Benes FM. Localization and high-resolution imaging of cortical neurotransmitter compartments using confocal laser scanning microscopy: GABA and glutamate interactions in rat cortex. BioTechniques. 1991;11(5):628–634. [PubMed] [Google Scholar]
- 9.Volk DW, Lewis DA. GABA Targets for the Treatment of Cognitive Dysfunction in Schizophrenia. Current neuropharmacology. 2005;3(1):45–62. doi: 10.2174/1570159052773396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa E, Guidotti A, Veldic M. Should allosteric positive modulators of GABA(A) receptors be tested in the treatment of schizophrenia? Schizophr Res. 2005;73(2–3):367–368. doi: 10.1016/j.schres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Molecular psychiatry. 2008;13(2):147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, et al. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013;69(2):317–320. doi: 10.1002/mrm.24257. [DOI] [PubMed] [Google Scholar]
- 14.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Archives of general psychiatry. 2012;69(5):449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 15.Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biological psychiatry. 2010;68(7):667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto N, Yoshimura R, Kakeda S, Moriya J, Hori H, Hayashi K, et al. No alterations of brain GABA after 6 months of treatment with atypical antipsychotic drugs in early-stage first-episode schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34(8):1480–1483. doi: 10.1016/j.pnpbp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Kelemen O, Kiss I, Benedek G, Keri S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Progress in neuro-psychopharmacology & biological psychiatry. 2013;47:13–19. doi: 10.1016/j.pnpbp.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophrenia bulletin. 2013;39(5):1096–1104. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(10):3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff-Guerrero A. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16(2):471–475. doi: 10.1017/S1461145712000314. [DOI] [PubMed] [Google Scholar]
- 21.de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA psychiatry. 2013;70(10):1057–1066. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(9):1781–1791. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophrenia bulletin. 2013;39(1):120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2001;45(3):517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends in cognitive sciences. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, et al. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res. 2012;142(1–3):88–92. doi: 10.1016/j.schres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. Psychological Corporation; 1997. [Google Scholar]
- 28.Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr Res. 2008;102(1–3):108–115. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Mausbach BT, Bowie CR, Harvey PD, Twamley EW, Goldman SR, Jeste DV, et al. Usefulness of the UCSD performance-based skills assessment (UPSA) for predicting residential independence in patients with chronic schizophrenia. J Psychiatr Res. 2008;42(4):320–327. doi: 10.1016/j.jpsychires.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychological medicine. 2009;39(6):889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of abnormal psychology. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 32.Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biological psychiatry. 2008;64(1):11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knowles EE, Weiser M, David AS, Dickinson D, Glahn D, Gold J, et al. Dedifferentiation and substitute strategy: deconstructing the processing-speed impairment in schizophrenia. Schizophr Res. 2012;142(1–3):129–136. doi: 10.1016/j.schres.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of general psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson D. Digit symbol coding and general cognitive ability in schizophrenia: worth another look? The British journal of psychiatry : the journal of mental science. 2008;193(5):354–356. doi: 10.1192/bjp.bp.108.049387. [DOI] [PubMed] [Google Scholar]
- 36.Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. The American journal of psychiatry. 2006;163(3):418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- 37.Mugler JP, 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1990;15(1):152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 38.Edden RA, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A Batch-Processing Tool for the Quantitative Analysis of Gamma-Aminobutyric Acid–Edited MR Spectroscopy Spectra. JOURNAL OF MAGNETIC RESONANCE IMAGING. doi: 10.1002/jmri.24478. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 40.Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. NeuroImage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epperson CN, O'Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biological psychiatry. 2005;57(1):44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cleveland WS, Devlin SJ. Locally Weighted Regression - an Approach to Regression-Analysis by Local Fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 43.Armitage PBG. Statistical methods in medical research. 2nd edn. Oxford: Blackwell Scientific; 1987. [Google Scholar]
- 44.Fisher RA. On the "Probable Error" of a Coefficient of Correlation Deduced from a Small Sample. Metron. 1921;1:3–32. [Google Scholar]
- 45.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of clinical psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 46.Salami A, Eriksson J, Nilsson LG, Nyberg L. Age-related white matter microstructural differences partly mediate age-related decline in processing speed but not cognition. Biochimica et biophysica acta. 2012;1822(3):408–415. doi: 10.1016/j.bbadis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Karbasforoushan H, Duffy B, Blackford JU, Woodward ND. Processing speed impairment in schizophrenia is mediated by white matter integrity. Psychological medicine. 2014:1–12. doi: 10.1017/S0033291714001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hines RM, Hines DJ, Houston CM, Mukherjee J, Haydon PG, Tretter V, et al. Disrupting the clustering of GABAA receptor alpha2 subunits in the frontal cortex leads to reduced gamma-power and cognitive deficits. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(41):16628–16633. doi: 10.1073/pnas.1308706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CM, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, et al. GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage Clinical. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stan AD, Lewis DA. Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Current pharmaceutical biotechnology. 2012;13(8):1557–1562. doi: 10.2174/138920112800784925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kochunov P, Chiappelli J, Wright SN, Rowland LM, Patel B, Wijtenburg SA, et al. Multimodal white matter imaging to investigate reduced fractional anisotropy and its age-related decline in schizophrenia. Psychiatry research. 2014;223(2):148–156. doi: 10.1016/j.pscychresns.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright SN, Kochunov P, Chiappelli J, McMahon RP, Muellerklein F, Wijtenburg SA, et al. Accelerated white matter aging in schizophrenia: role of white matter blood perfusion. Neurobiology of aging. 2014;35(10):2411–2418. doi: 10.1016/j.neurobiolaging.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophrenia bulletin. 2011;37(3):451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophrenia bulletin. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.