Abstract
Background
Early after renal transplantation it is often challenging to achieve and maintain tacrolimus concentrations within the target range. Computerized dose individualization utilizing population pharmacokinetic models may be helpful. The objective of this study was to prospectively evaluate the target concentration achievement of tacrolimus using computerized dosing compared with conventional dosing performed by experienced transplant physicians.
Methods
A single-center, prospective study was conducted. Renal transplant recipients were randomized to receive either computerized or conventional tacrolimus dosing during the first eight weeks post-transplant. The median proportion of tacrolimus trough concentrations within the target range was compared between the groups. Standard risk (target 3-7 μg/L) and high-risk (8-12 μg/L) recipients were analyzed separately.
Results
Eighty renal transplant recipients were randomized, and seventy-eight were included in the analysis (Computerized dosing (n=39): 32 standard risk/7 high-risk, Conventional dosing (n=39): 35 standard risk/4 high-risk). A total of 1711 tacrolimus whole blood concentrations were evaluated. The proportion of concentrations per patient within the target range was significantly higher with computerized dosing than with conventional dosing, both in standard risk patients (medians 90% [95% confidence interval (CI) 84-95%] vs. 78% [95% CI 76-82%], respectively, p<0.001) and in high-risk patients (medians 77% [95% CI: 71-80%] vs. 59% [95% CI: 40-74%], respectively, p=0.04).
Conclusions
Computerized dose individualization improves target concentration achievement of tacrolimus after renal transplantation. The computer software is applicable as a clinical dosing tool to optimize tacrolimus exposure and may potentially improve long-term outcome.
INTRODUCTION
The calcineurin inhibitor tacrolimus (Tac) is widely used after renal transplantation (1). The dose requirement to obtain Tac target concentrations varies considerably between patients (1,2), as well as within patients over time (3,4). Tac has a narrow therapeutic index, and the consequences of not achieving and maintaining Tac target exposure may be detrimental. Some studies have found that low Tac exposure during the first month post-transplant increases the risk of acute rejection (5-7). In a recent study, Tac trough concentrations below 8 μg/L at discharge (median five days post-transplant) in moderately sensitized recipients were associated with nearly two times greater risk of biopsy-proven acute rejection (6). By contrast, too high exposure increases the risk of serious adverse effects, including nephrotoxicity (8,9). Furthermore, high within-subject variability in Tac exposure has been associated with reduced long-term graft survival (10,11). Applying an effective dose adaption strategy to achieve and maintain Tac concentrations at optimized levels early after transplantation is therefore essential (2).
The conventional therapeutic drug monitoring (TDM) approach to individualize Tac exposure after renal transplantation is to frequently measure whole blood trough concentrations and adjust doses intuitively based on the difference between the measured and desired concentrations. However, due to frequent dose adjustments Tac concentrations are typically not measured at steady state in the early phase after transplantation, and this complicates the intuitive dose adaptation approach. Furthermore, Tac dosing is challenged by its time-varying pharmacokinetics in the early phase after transplantation, which is partly due to systematic changes in hematocrit (12-14) and prednisolone dose (15-17). In previous studies, less than 60% of the Tac concentrations were within the target range in the early post-transplant phase using this approach (13,18,19).
Several computer software packages have been developed to assist physicians in improving dose individualization (20). By implementing a population pharmacokinetic model for the drug in suitable computer software, dosing schemes can be optimized to achieve a selected target concentration for each patient based on their demographic and clinical characteristics and, if available, previously measured drug concentrations (21,22). Such methods are not dependent on steady-state conditions, and they allow quantitative influences of time-varying factors on pharmacokinetic parameters and thereby dose predictions (22). Computer-assisted dose individualization strategies have previously been shown to improve target achievement for several drugs (23-27), but not for Tac after renal transplantation.
We have previously developed population pharmacokinetic models for Tac and retrospectively shown satisfactory predictive performance in an external cohort of renal transplant recipients (28). The objective of this single-center study was to prospectively evaluate the proportion of concentrations within the target range using computerized dosing compared with conventional dosing. De novo renal transplant recipients were randomized to receive Tac doses determined either by the computer or by experienced transplant physicians during the first eight weeks after transplantation.
MATERIALS AND METHODS
Patients
This prospective study was conducted on patients who underwent renal transplantation at Oslo University Hospital Rikshospitalet, Norway. Eighty patients were planned for inclusion. Patients were eligible if they received a kidney as the only transplanted organ, were 18 years or above, were mentally able to comply with study procedures and used Tac-based immunosuppression without concomitant drugs known to interact with Tac pharmacokinetics. On the day of renal transplantation, included patients were consecutively randomized (allocation ratio 1:1 based on a computer-derived, unstratified randomization list with block size=8) to receive either computerized dosing (Computer Group) or conventional dosing (Control Group). The allocation sequence was concealed by using sequentially numbered containers and was generated by a person not involved in enrollment and assignment of participants. All patients gave written, informed consent. The study has been approved by the Norwegian Medicines Agency and the Regional Committee for Medical Research Ethics and is registered at www.ClinicalTrials.gov (NCT02010320).
Immunosuppressive Drugs
Recipients of living and deceased donor kidneys followed the same immunosuppressive protocol. All subjects received oral Tac (Prograf®, Astellas Pharma US Inc.). Our center-standard initial Tac dose regimen of 0.04 mg/kg (0.05 mg/kg in high-risk patients) twice daily was started on the day of transplantation, followed by dose adjustments to reach target whole blood trough concentrations of 3-7 μg/L in standard risk patients (29), and 8-12 μg/L (day 0-30) or 6-10 μg/L (after day 30) in high-risk patients (defined as presence of donor-specific antibodies and/or ABO-incompatibility between donor and recipient).
As induction therapy, all patients received 20 mg basiliximab on day 0 and day 4 after transplantation and 250 mg (standard risk) or 500 mg (high-risk) intravenous methylprednisolone on day 0. High-risk patients also received 500 mg/kg intravenous human immune globulins daily from day 0 to 4 and 375 mg/m2 rituximab on day 0. As maintenance therapy in addition to Tac, patients received 0.75-1 g oral mycophenolate mofetil twice daily and oral prednisolone once daily, initiated at 20 mg (80 mg in high-risk patients) and tapered to 10 mg during the first eight weeks post-transplant.
Pharmacokinetic and Genotyping Assays
Tac concentrations were determined in whole blood using a chemiluminescent microparticle immunoassay (analyzed on the Architect® instrument, Abbott Laboratories, Abbott Park, IL (30)). Concentrations were generally measured four to five days per week the first week, then two to three days per week for the remaining follow-up period. Cytochrome P450 3A4 and 3A5 genotypes (CYP3A4*22; rs35599367 and CYP3A5*3; rs776746) were determined using real-time polymerase chain reaction and melting curve analysis with hybridization probes on the LightCycler 480 instrument® (Roche Applied Science, Penzberg, Germany) as described previously (13).
Tacrolimus Dosing
After the first Tac trough concentration measurement (one or two days after transplantation) Tac dose adjustments were considered following each Tac concentration measurement for the next eight weeks. All patients were informed to take the doses with 12-hour intervals and keep a written journal of the exact times of dose intake.
In the Computer Group, doses were determined by implementing a previously described population pharmacokinetic model for Tac in the dosing software BestDose® (28,31). The model not including CYP3A5 genotype as a covariate was used in the present study, as most patients had not been genotyped at the time of transplantation. The model has previously been externally evaluated with satisfactory predictive performance (28). For each dose prediction, the software required the following updated patient characteristics: fat-free mass (predicted by total bodyweight, height and sex (32)), hematocrit, time post-transplant, Tac dosing history and the patient’s previously measured Tac concentrations (up to the five most recent measurements). The software estimated individual distributions of the pharmacokinetic parameters by balancing the above patient information with information from the population model (22). Next, the software evaluated a range of doses and provided the dose regimen with the highest probability of achieving the middle of the target range for the patient (33). The user rounded the suggested dose to the nearest available dose alternative (each 0.5 mg). One of two trained users operated the software and forwarded the dose suggestions to the physicians. The treating physician was free to accept or reject the dose based on his/her clinical judgment.
In the Control Group, doses were decided by transplant physicians who yearly care for 250-300 de novo renal transplant recipients. All physicians were familiar with the low Tac target in standard risk recipients (3-7 μg/L), which has been used at our center since shortly after the ELiTE-Symphony study was published in 2007 (29). Regardless of induction therapy, the physicians aimed to achieve the Tac target concentration as quickly as possible. The physicians were strictly informed to report if they targeted a different Tac concentration than the middle of the protocol-defined range.
Target Concentrations Achievement and Clinical Outcome
The proportion of Tac trough concentrations within the target range was calculated for each patient, and the median proportion, as well as the median time to reach the target, was compared between the groups. In addition, the intra-subject variability in Tac trough concentrations was assessed by calculating the coefficient of variation (CV%) for each patient, and the median CV% was compared. Tac concentrations unintentionally measured after intake of the morning dose and Tac concentrations measured after aiming for a different target concentration than the protocol-defined ranges were not included in the analysis. Although the software used the first measured Tac concentration to calculate the first dose adjustment, the first concentration was excluded from the final analysis because it was based on standard initial dosing in both groups. Separate analyses were performed on standard risk patients and high-risk patients.
The incidence of biopsy-confirmed acute rejections and infections during follow-up were recorded. Renal function and glucose tolerance was assessed at eight weeks by measured glomerular filtration rate (GFR; iohexol plasma clearance) and an oral glucose tolerance test (OGTT).
Posthoc Analyses
The proportion of concentrations per patient within the target range was evaluated for each independent week during follow-up. The proportions of concentrations below the target concentration range was compared between CYP3A4*22 and CYP3A5*1 allele carriers and the remaining patients in each group for the first post-transplant week and for the entire study period.
Statistical Analyses
Due to lack of available data in the literature the number of patients included in this study was based on convenience rather than a power calculation. Statistical analyses were carried out using R® v. 3.0.2 (34). Each continuous variable was assessed for normality using the Shapiro-Wilk test. Normally distributed variables were compared using the Student’s t-test. Non-normally distributed variables were compared using the Mann-Whitney U test, and confidence intervals (CIs) of the medians were derived from 10,000 bootstrap replicates. These tests were two-tailed, and a p-value below 0.05 was assumed to represent statistical significance. Differences in proportions were analyzed using the χ2-test.
RESULTS
Patients
From 13 January to 9 June 2014 a total of 102 adult renal transplantations were performed at our center. Of these, 22 patients were not included; seven used cyclosporine, six were mentally unfit, four needed translator, two declined to participate, one used a CYP3A-inducing drug, one was previously liver transplanted and one patient was mistakenly not asked to participate. Of the 80 included and randomized recipients, one was excluded after starting on the CYP3A4-inhibitor verapamil (Computer Group) and for one patient the transplantation was cancelled due to a positive cross-match (Control Group). The groups were balanced with respect to demographic, clinical and donor-recipient characteristics, except from a higher number of living donor recipients and ABO mismatches in the Computer Group (Table 1).
TABLE 1.
Baseline demographic and clinical characteristics of participants
| Computer Group (n=39) | Control Group (n=39) | |
|---|---|---|
| Age (years), median (range) | 54 (27-80) | 59 (24-75) |
| Sex (Male / Female) | 29 / 10 | 26 / 13 |
| Total bodyweight (kg)a | 83.9 (21.5) | 79.1 (16.6) |
| Height (cm) | 176 (9) | 174 (10) |
| Predicted fat-free mass (kg)a | 58.7 (11) | 55.7 (11) |
| Hematocrit (%)a | 31 (4) | 31 (4) |
| CYP3A5 genotype (*3/*3, *1/*3, *1/*1) | 31 / 8 / 0 | 31 / 8 / 0 |
| CYP3A4 genotype (*1/*1, *1/*22, *22/*22) | 35 / 4 / 0 | 35 / 4 / 0 |
| Prior transplantations (≥1) | 2 | 2 |
| Delayed graft functionb | 6 | 9 |
| Panel reactive antibodies (≥20%) | 0 | 2 |
| Donor specific antibodies | 2 | 3 |
| Donor age (years), median (range) | 53 (5-77) | 60 (3-78) |
| Donor (Living / Deceased) | 15 / 24 | 7 / 32 |
| ABO mismatches | 5 | 0 |
| HLA mismatches | ||
| A (0 / 1 / 2) | 8 / 20 / 11 | 15 / 18 / 6 |
| B (0 / 1 / 2) | 4 / 21 / 14 | 7 / 23 / 9 |
| DR (0 / 1 / 2) | 12 / 18 / 9 | 13 / 24 / 2 |
Values are shown as numbers or mean (standard deviation) unless stated otherwise
CYP3A5, Cytochrome P450 3A5; CYP3A4, Cytchrome P450 3A4; HLA, human leukocyte antigen
One week post-transplant
Defined by the use of dialysis during the first week post-transplant (47)
The analysis included a total of 1711 Tac concentrations (746 in the Computer Group and 965 in the Control Group). Some concentrations were removed from the final analysis: On 51 occasions the physician chose a different target concentration than the standard targets (e.g. 5-8 μg/L) and on 16 occasions the treating physician over-ruled the computer dose suggestion (feared that the dose would lead to too low concentrations [10 occasions] or too high concentrations [3 occasions] or strongly preferred not changing the current dose for the patient [3 occasions]).
Three patients were lost to follow-up after three weeks of Tac treatment (two in Computer Group, one in Control Group) because they were transferred to local hospitals. One patient (Computer Group) had a graftectomy performed after two weeks. Available concentration measurements from these patients were used in the primary analysis. However, the patients were excluded from the clinical outcome evaluation eight weeks post-transplant. Of the 74 patients who completed the study, GFR was measured in 66 patients and 58 patients performed a glucose tolerance test eight weeks post-transplant (13 did not perform the latter test because they were diabetic).
Target Concentration Achievement and Clinical Outcome
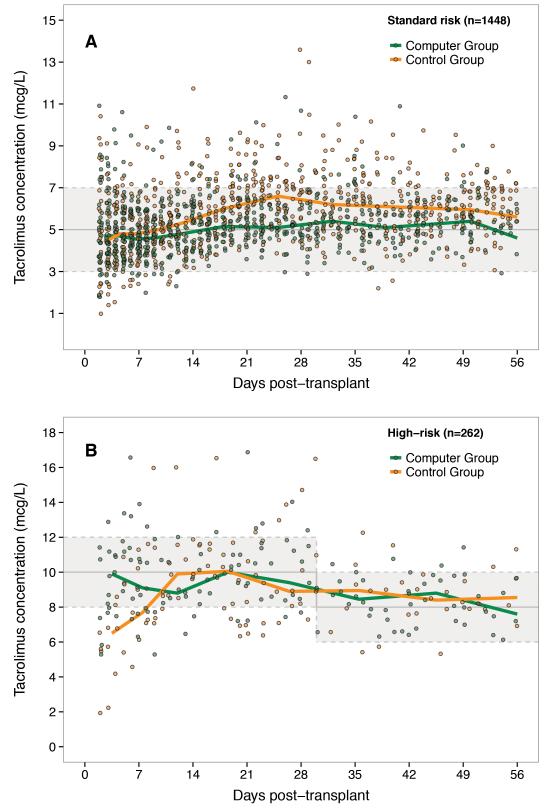
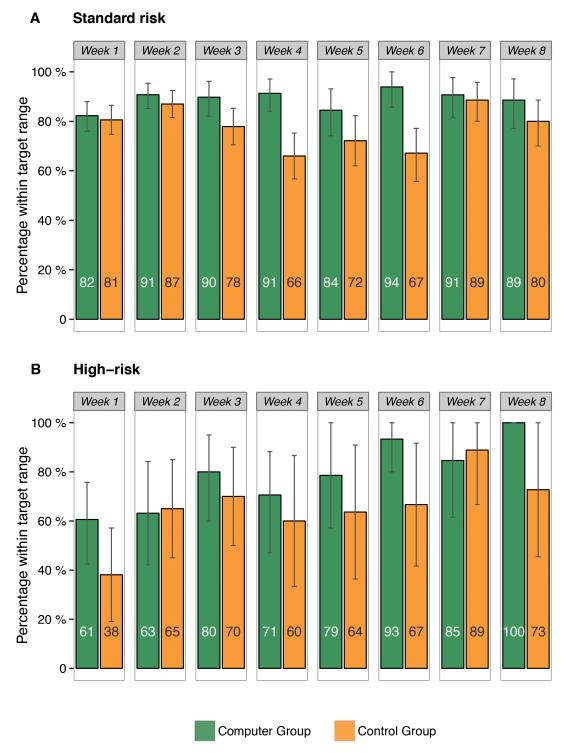
Figure 1 illustrates the Tac concentrations over time after transplantation in each dosing group. Figure 2 shows the percentages of Tac concentrations within the target range for each week. Among standard risk patients, the median proportion of Tac concentrations within the target range was 90% [95% CI: 84-95%] in the Computer Group and 78% [95% CI: 76-82%] in the Control Group (p<0.001) (Table 2). There was no significant difference in median time to achieve the target (2 versus 3 days, respectively, p=0.29). For high-risk patients, the median proportion of concentrations within the target range was 77% [95% CI: 71-80%] for the Computer Group, significantly higher than in the Control Group (59% [95% CI: 40-74%], p=0.04). In these patients, the median time to achieve the target was significantly shorter in the Computer Group (3 versus 5 days, p=0.04). The intra-subject variability was not significantly different between the groups in neither standard nor high-risk patients.
Figure 1.
Observed tacrolimus trough concentrations in the Computer Group and Control Group over time after transplantation in A) standard risk patients and B) high-risk patients. Solid lines show the median concentration. Shaded areas represent the target range. Solid gray line represents the middle of the target range.
Figure 2.
Mean percentages of tacrolimus concentrations within the target range per week after transplantation for the Computer Group and Control Group in A) standard risk patients (n=67) and B) high-risk patients (n=11). The numbers within the bars show the mean percentage within range. Vertical lines represent 95% confidence intervals.
TABLE 2.
Overview of Tac doses and target concentration achievement
| Computer Group | Control Group | ||||
|---|---|---|---|---|---|
| Number / Median [95% CI] |
Range | Number / Median [95% CI] |
Range | p-value | |
| Standard risk (3-7 μg/L) | |||||
| Patients | 32 | 35 | |||
| Tac concentrations | 605 | 844 | |||
| Tac concentrations per patient | 24 | 8-30 | 24 | 12-40 | |
| Tac initial dose (mg/day) | 5.0 | 4.0-8.0 | 5.5 | 4.0-8.0 | 0.96 |
| Tac dose at discharge (mg/day) | 4.0 | 1.0-9.0 | 4.0 | 2.0-13.0 | 0.04 |
| Mean Tac dose during follow-up (mg/day) |
4.1 | 1.3-9.8 | 4.8 | 2.1-11.4 | 0.13 |
| Tac dose, intra-subject variability (CV%) |
29% | 24% | 0.14 | ||
| Tac trough concentration (μg/L) | 5.1 | 1.8-11.3 | 5.8 | 1.0-13.6 | <0.001 |
| Mean Tac trough concentration during follow up (μg/L) |
5.1 | 4.6-6.5 | 5.9 | 4.8-7.1 | <0.001 |
| Tac trough concentration, intra- subject variability (CV%) |
23% | 26% | 0.16 | ||
| Concentrations within target range per patient |
90% [84-95%] | 67-100% | 78% [76-82%] | 58-96% | <0.001 |
| Below target range | 0% [0-0%] | 0-11% | 0% [0-2%] | 0-18% | 0.80 |
| Above target range | 9% [2-11%] | 0-33% | 18% [15-23%] | 4-42% | <0.001 |
| Time to achieve target concentration (days) |
2 | 2-5 | 3 | 2-8 | 0.29 |
|
High-risk (8-12 μg/L day 1-30,
6-10 μg/L day 31+) |
|||||
| Patients | 7 | 4 | |||
| Tac concentrations | 141 | 121 | |||
| Tac concentrations per patient | 22 | 21-25 | 28 | 20-31 | |
| Tac initial dose (mg/day) | 7.0 | 5.0-10.0 | 6.0 | 5.0-6.0 | 0.35 |
| Tac dose at discharge (mg/day) | 6.0 | 2.0-9.0 | 6.0 | 5.0-9.0 | 1.00 |
| Mean Tac dose during follow-up (mg/day) |
7.8 | 3.6-11.5 | 7.4 | 7.7-11.3 | 0.92 |
| Tac dose, intra-subject variability (CV%) |
24% | 22% | |||
| Tac trough concentration (μg/L) | 9.7 | 5.3-16.9 | 9.6 | 1.9-16.5 | 0.46 |
| Mean Tac trough concentration during follow up (μg/L) |
9.8 | 8.9-10.9 | 9.7 | 7.5-10.8 | 0.78 |
| Tac trough concentration, intra- subject variability (CV%) |
22% | 31% | |||
| Concentrations within target range per patient |
77% [71-80%] | 61-84% | 59% [40-74%] | 40 -74% | 0.04 |
| Below target range | 16% [10-17%] | 0-24% | 31% [11-32%] | 11-32% | 0.11 |
| Above target range | 10% [5-22%] | 0-23% | 12% [7-30%] | 7-30% | 0.53 |
| Time to achieve target concentration (days) |
3 | 2-7 | 4.5 | 4-10 | 0.04 |
CI, confidence interval; CV, coefficient of variation; Tac, tacrolimus
p-values were calculated using the Mann-Whitney U test
As shown in Table 3, GFR was higher after eight weeks in the Computer Group as compared with the Control Group (p=0.046). Due to the higher number of living donor recipients in the Computer Group, a sub-analysis was performed in living and deceased donor recipients separately. While the difference in mean GFR was still significant among living donor recipients (Computer Group: 65 [95% CI 59-71] mL/min/1.73 m2, Control Group: 50 [95% CI 42-59] mL/min/1.73 m2, p=0.02), GFR was not significantly different comparing deceased donor recipients only (Computer Group: 55 [95% CI 49-61] mL/min/1.73 m2, Control Group: 53 [95% CI 48-58] mL/min/1.73 m2, p=0.68).
TABLE 3.
Overview of clinical outcome at eight weeks
| Computer Group | Control Group | ||||
|---|---|---|---|---|---|
| Number / Median [95% CI] |
Range | Number / Median [95% CI] |
Range | p-value | |
| Biopsy-proven acute rejections | 3 | 5 | 0.455c | ||
| Recorded infections | 6 | 3 | 0.289c | ||
| Glomerular filtration rate (mL/min/1.73 m2)a, mean |
59 [55-64] | 30-87 | 53 [48-57] | 31-80 | 0.046d |
| Fasting plasma glucose (mmol/L)b | 5.3 [5.1-5.5] | 4.3-6.7 | 5.5 [5.4-5.7] | 4.6-8.5 | 0.058e |
| 2-hour plasma glucose (mmol/L)b | 5.9 [5.6-6.6] | 2.9-9.3 | 6.8 [6.1-8.1] | 4.2-13.5 | 0.008e |
CI, confidence interval
Based on 66 patients (4 lost to follow-up, 8 glomerular filtration rate not measured)
Based on 58 patients (4 lost to follow-up, 13 pre-transplant diabetes, 3 glucose tolerance test not done)
Calculated using the χ2-test
Calculated using the Student’s t-test
Calculated using the Mann-Whitney U test
The OGTT showed lower 2-hour plasma glucose levels (p=0.008) in the Computer Group, but no significant difference in fasting glucose (p=0.058). There were no significant differences in the frequency of acute rejection episodes or infections between the dosing groups.
Post hoc Analyses
The proportion within the target range was significantly higher for the Computer Group at weeks 3 (p=0.027), 4 (p<0.001) and 6 (p=0.004) after transplantation, with smaller, non-significant differences at the remaining weeks.
In the standard risk patients, 81% of the first measured Tac concentrations in each patient (before dose adaptation) were below the target range in CYP3A5 expressers (n=15) versus 24% in CYP3A5 non-expressers (p<0.001). However, after the first concentration, the proportion of Tac concentrations below the target (<3 μg/L) was not higher in CYP3A5 expressers at any time, neither in the Computer Group (p=0.64 for first week post-transplant, p=0.95 for entire period) nor in the Control Group (p=0.07 and p=0.19, respectively). The Tac dose at discharge in CYP3A5 expressers was 65% higher than in non-expressers (median 6.5 and 4.0 mg/day, respectively, p<0.001). There were too few CYP3A4*22 carriers to perform relevant analyses.
DISCUSSION
This is the first prospective study using a population pharmacokinetic model for computerized dosing of Tac after renal transplantation. The results show that computerized dose individualization of Tac in de novo renal transplant recipients significantly increases the proportion of concentrations within the target range as compared with conventional dosing by experienced transplant physicians. In addition, time to achieve target levels was significantly shorter in high-risk patients. The results are in agreement with similar studies on Tac in liver transplant recipients (27) and cyclosporine in renal transplant recipients (26).
The proportion of Tac concentrations above the target range was approximately halved and the median exposure 12% lower (p<0.001) in the Computer Group compared with the Control Group. Tac is known for its exposure-dependent diabetogenic effect (35,36). Two-hour plasma glucose levels were significantly lower in the Computer Group than in the Control Group eight weeks post-transplant, indicating a clinical benefit on glucose metabolism when fine-tuning Tac exposure with less fluctuations to higher concentrations. Other predisposing factors, such as age, bodyweight and use of glucocorticoids, were not significantly different between the groups. A previous study found improved glucose metabolism after slightly lowering Tac exposure, caused by increased secretion capacity of pancreatic β-cells (17), which might also be the mechanism behind our result. In the overall analysis, we also found significantly higher GFR in the Computer Group eight weeks post-transplant. Tac-induced nephrotoxicity has previously been related to Tac exposure (8,9), although at considerably higher concentrations than those observed in this study. When comparing GFR in living donor recipients only, the significant difference remained, whereas in deceased donor recipients, there was not a significant difference. The overall superior renal function in the Computer Group may have been a result of the higher number of living donor recipients (37), hence this result should be interpreted with caution.
In a previous report from our center analyzing clinical TDM data, the proportion of Tac concentrations within the target range was below 60% during the first eight weeks after transplantation (13), considerably lower than the proportions observed in both groups in the present study. One possibility is that the physicians may have considered their dose adjustments more cautiously due to increased awareness being part of this study. In addition, patients kept written records of the exact times of dose intake or any missed doses and were particularly encouraged to take their doses with twelve-hour intervals. This is likely to have improved adherence compared with the non-study patients included in the above-mentioned report, and may also explain why the acute rejection rate in this study (10%) was lower than the approximate 20% usually seen at our center (38,39).
The dose at discharge was 65% higher in CYP3A5 *1/*3 expressers (no *1/*1 expressers present), which is similar to previous observations (14,40). Yet, after the first measured concentration, the proportion of concentrations below the target range and time to reach the target was not different between CYP3A5 expressers and non-expressers for neither of the groups. The number of CYP3A5 expressers in this study was small. Still, the result indicates that both the computer and the physicians increase the dose appropriately after observing the first, low concentration in CYP3A5 expressers without explicit genotype information. This is one of the strengths with the population modeling approach; based on the measured drug concentrations, the software will identify patients belonging to subpopulations with distinct pharmacokinetic properties, such as CYP3A5 expressers, and optimal individual doses can be predicted also for these patients (41). The ideal option for early, adequate immunosuppression would be to involve all potential information, including CYP3A5 genotype. However, one advantage of not basing the dose predictions on genotype in this study is that we have shown that the computerized dosing approach is useful also for centers without the opportunity to perform pre-transplant genotyping.
Theoretically, the benefits of computerized drug dosing strategies are likely to be greatest early after treatment initiation. Surprisingly, the largest differences between the groups were 4-6 weeks post-transplant, without a significant difference at weeks 1 and 2. This may be related to that time-varying factors influencing Tac whole blood concentrations, such as hematocrit and concomitant prednisolone dose, change more noticeably during this later period (13,15). Physicians do not normally consider these factors when dosing Tac. In contrast, the population model that the computer based its predictions on implements hematocrit and time post-transplant and manage to partially predict the time-varying dose requirements. Using individual hematocrit values as predictors of Tac whole blood concentrations is essential because Tac is >90% bound to erythrocytes (3,42). It should however be noted that changes in dose due to changes in hematocrit will alter the unbound, pharmacologically active concentration (3,13,43).
The total number of Tac measurements was higher in the Control Group. There was however no association between number of measurements within range and number of performed Tac measurements during follow-up. The unequal number of measurements may be explained by more early dropouts in the Computer Group or by a higher frequency of acute rejection episodes and delayed graft function in the Control Group, which tend to lead to longer hospitalization and more measurements in general. The median number of measurements per individual was similar between the groups, and the results are not likely to be affected by this imbalance.
Whereas some studies have identified a relationship between Tac exposure and the risk of acute rejection (5-7), others could not confirm such a relationship (44). This questions the clinical relevance of implementing software to improve Tac target achievement. However, the above study investigated Tac concentrations considerably higher than 3-7 μg/L (median trough 9-10 μg/L). Theoretically, there should be a lower threshold at which the acute rejection risk starts to increase. As previously speculated, the slope of the concentration-effect relationship for Tac may reach its maximum at lower concentrations than the currently applied targets (44). With a recent trend towards lower calcineurin inhibitor exposure (29,45), very low Tac targets when combined with everolimus (46) and drug individualization, the need for clinical tools to avoid underexposure will probably increase in the future.
The present study has some limitations. First, the physicians might occasionally have targeted or accepted concentrations at the upper or lower parts of the range without this being recorded. The target of 3-7 μg/L for standard risk recipients is based on the results of the ELiTE-Symphony study (29), which showed superior outcome in patients with this protocol-defined Tac target range. However, the actual Tac concentrations were generally higher than the middle of the range in this group (18), providing little evidence for the safety below 5 μg/L. This knowledge may have influenced the physicians in the present study to aim for higher concentrations (e.g. 6-7 μg/L), although instructed otherwise. The small difference in intra-subject variability in Tac concentrations between the groups supports this hypothesis. This complicates the direct comparison with the computer that consistently aimed for the middle of the range. A second limitation is the small number of high-risk patients. Third, although the software is able to predict starting doses, the initial Tac dose was not computerized due to limited available personnel to perform dose prediction at the time of treatment initiation. On the other hand, a strength of the study was that >99% of the doses after the first concentration measurement were computerized, with exceptions being the 16 occasions where the physician chose to over-rule the computer dose. It should be noted that in six of these occasions, the subsequent concentration was outside the target range.
At our center, more than 250 patients undergo renal transplantation each year, and the physicians have extensive experience with Tac dosing in this patient group. The dosing tool might be of even greater benefit at centers performing fewer transplantations and having less experience with Tac dosing. However, the current version of BestDose® is not yet ready for bedside dosing and should preferably be handled by a person who has experience with population pharmacokinetic modeling and the mathematical processes behind the dose predictions. In 2-3% of the predictions, the standard settings of the program had to be modified for the correct optimal dose to be provided. Moreover, BestDose® is currently run from the statistical software R, which requires some programming knowledge. However, a user-friendly graphical interface is under development (31).
In conclusion, this study showed that utilizing trough concentrations and computerized dose individualization with BestDose® improves target achievement of Tac compared with conventional dosing performed by experienced transplant physicians early after renal transplantation. The computer software is applicable as a clinical dosing tool to optimize Tac exposure and may potentially improve long-term outcome.
ACKNOWLEDGMENTS
The authors wish to thank the medical laboratory technologists, transplant physicians, nurses and patients at Oslo University Hospital for excellent collaboration during the conduction of this study.
Funding
The study was supported by a grant from the South-Eastern Norway Regional Health Authority. MN was supported by National Institutes of Health grants R01 HD070886 and R01 GM068968.
ABBREVIATIONS
- CYP3A4
Cytochrome P450 3A4
- CYP3A5
Cytochrome P450 3A5
- GFR
Glomerular filtration rate
- Tac
Tacrolimus
- TDM
Therapeutic Drug Monitoring
Footnotes
Disclosure
The authors declare no conflicts of interest.
Trial Registry:
The study is registered at www.ClinicalTrials.gov (NCT02010320).
Authors’ specific contributions to the work
ES, AÅ, MS and KM participated in research design and performance of the research.
ES, AÅ and KM participated in data analysis.
MN developed the computer software.
StB and SaB performed pharmacological and genotyping analyses.
All authors participated in writing of the paper.
REFERENCES
- 1.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 2.Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther Drug Monit. 2009;31:139. doi: 10.1097/FTD.0b013e318198d092. [DOI] [PubMed] [Google Scholar]
- 3.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 4.Saint-Marcoux F, Woillard J-B, Jurado C, Marquet P. Lessons from routine dose adjustment of tacrolimus in renal transplant patients based on global exposure. Ther Drug Monit. 2013;35:322. doi: 10.1097/FTD.0b013e318285e779. [DOI] [PubMed] [Google Scholar]
- 5.Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001;16:1905. doi: 10.1093/ndt/16.9.1905. [DOI] [PubMed] [Google Scholar]
- 6.Richards KR, Hager D, Muth B, Astor BC, Kaufman D, Djamali A. Tacrolimus trough level at discharge predicts acute rejection in moderately sensitized renal transplant recipients. Transplantation. 2014;97:986. doi: 10.1097/TP.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 7.Borobia AM, Romero I, Jimenez C, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009;31:436. doi: 10.1097/FTD.0b013e3181a8f02a. [DOI] [PubMed] [Google Scholar]
- 8.Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: A report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900. doi: 10.1097/00007890-199610150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kershner RP, Fitzsimmons WE. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation. 1996;62:920. doi: 10.1097/00007890-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Borra LCP, Roodnat JI, Kal JA, Mathot RAA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant. 2010;25:2757. doi: 10.1093/ndt/gfq096. [DOI] [PubMed] [Google Scholar]
- 11.Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85:1404. doi: 10.1038/ki.2013.465. [DOI] [PubMed] [Google Scholar]
- 12.Woillard JB, de Winter BC, Kamar N, Marquet P, Rostaing L, Rousseau A. Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations - twice daily Prograf and once daily Advagraf. Br J Clin Pharmacol. 2011;71:391. doi: 10.1111/j.1365-2125.2010.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Størset E, Holford N, Midtvedt K, Bremer S, Bergan S, Åsberg A. Importance of hematocrit for a tacrolimus target concentration strategy. Eur J Clin Pharmacol. 2013;70:65. doi: 10.1007/s00228-013-1584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmann TK, Hennig S, Barraclough KA, Isbel NM, Staatz CE. Population pharmacokinetics of tacrolimus in adult kidney transplant patients: Impact of CYP3A5 genotype on starting dose. Ther Drug Monit. 2014;36:62. doi: 10.1097/FTD.0b013e31829f1ab8. [DOI] [PubMed] [Google Scholar]
- 15.Størset E, Holford N, Hennig S, et al. Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling. Br J Clin Pharmacol. 2014;78:509. doi: 10.1111/bcp.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Duijnhoven EM, Boots JMM, Christiaans MHL, Stolk LML, Undre NA, van Hooff JP. Increase in tacrolimus trough levels after steroid withdrawal. Transplant Int. 2003;16:721. doi: 10.1007/s00147-003-0615-1. [DOI] [PubMed] [Google Scholar]
- 17.Boots JMM, van Duijnhoven EM, Christiaans MHL, Wolffenbuttel BHR, van Hooff JP. Glucose metabolism in renal transplant recipients on tacrolimus: the effect of steroid withdrawal and tacrolimus trough level reduction. J Am Soc Nephrol. 2002;13:221. doi: 10.1681/ASN.V131221. [DOI] [PubMed] [Google Scholar]
- 18.Ekberg H, Mamelok RD, Pearson TC, Vincenti F, Tedesco-Silva H, Daloze P. The challenge of achieving target drug concentrations in clinical trials: Experience from the Symphony study. Transplantation. 2009;87:1360. doi: 10.1097/TP.0b013e3181a23cb2. [DOI] [PubMed] [Google Scholar]
- 19.Thervet E, Loriot MA, Barbier S, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2009;87:721. doi: 10.1038/clpt.2010.17. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs A, Csajka C, Thoma Y, Buclin T, Widmer N. Benchmarking therapeutic drug monitoring software: A review of available computer tools. Clin Pharmacokinet. 2013;52:9. doi: 10.1007/s40262-012-0020-y. [DOI] [PubMed] [Google Scholar]
- 21.Sheiner LB, Beal S, Rosenberg B, Marathe VV. Forecasting individual pharmacokinetics. Clin Pharmacol Ther. 1979;26:294. doi: 10.1002/cpt1979263294. [DOI] [PubMed] [Google Scholar]
- 22.Jelliffe RW, Schumitzky A, Bayard D, et al. Model-based, goal-oriented, individualised drug therapy. Clin Pharmacokinet. 1998;34:57. doi: 10.2165/00003088-199834010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338:499. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 24.Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28:743. doi: 10.1038/sj.bmt.1703207. [DOI] [PubMed] [Google Scholar]
- 25.Pea F, Bertolissi M, Di Silvestre A, Poz D, Giordano F, Furlanut M. TDM coupled with Bayesian forecasting should be considered an invaluable tool for optimizing vancomycin daily exposure in unstable critically ill patients. Int J Antimicrob Agents. 2002;20:326. doi: 10.1016/s0924-8579(02)00188-7. [DOI] [PubMed] [Google Scholar]
- 26.Åsberg A, Falck P, Undset LH, et al. Computer-assisted cyclosporine dosing performs better than traditional dosing in renal transplant recipients: Results of a pilot study. Ther Drug Monit. 2010;32:152. doi: 10.1097/FTD.0b013e3181d3f822. [DOI] [PubMed] [Google Scholar]
- 27.Fukudo M, Yano I, Shinsako K, et al. Prospective evaluation of the Bayesian method for individualizing tacrolimus dose early after living-donor liver transplantation. J Clin Pharmacol. 2009;49:789. doi: 10.1177/0091270009333853. [DOI] [PubMed] [Google Scholar]
- 28.Åsberg A, Midtvedt K, van Guilder M, et al. Inclusion of CYP3A5 genotyping in a nonparametric population model improves dosing of tacrolimus early after transplantation. Transplant Int. 2013;26:1198. doi: 10.1111/tri.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 30.Wallemacq P, Goffinet J-S, O’Morchoe S, et al. Multi-site analytical evaluation of the Abbott ARCHITECT tacrolimus assay. Ther Drug Monit. 2009;31:198. doi: 10.1097/FTD.0b013e31819c6a37. [DOI] [PubMed] [Google Scholar]
- 31.Laboratory of Applied Pharmacokinetics [Accessed December 1 2014];BestDose. 2014 Available at: http://www.lapk.org/bestdose.php. [Google Scholar]
- 32.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 33.Hope WW, VanGuilder M, Donnelly JP, et al. Software for dosage individualization of voriconazole for immunocompromised patients. Antimicrob Agents Chemother. 2013;57:1888. doi: 10.1128/AAC.02025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: [Accessed December 1 2014]. 2013. Available at: http://www.R-project.org/ [Google Scholar]
- 35.Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 36.Cotovio P, Neves M, Rodrigues L, et al. New-onset diabetes after transplantation: Assessment of risk factors and clinical outcomes. Transplant Proc. 2013;45:1079. doi: 10.1016/j.transproceed.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transplant Int. 2013;27:19. doi: 10.1111/tri.12217. [DOI] [PubMed] [Google Scholar]
- 38.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: A systematic review. Transplantation. 2004;77:769. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 39.Tielen M, van Exel J, Laging M, et al. Attitudes to medication after kidney transplantation and their association with medication adherence and graft survival: A 2-year follow-up study. J Transplant. 2014;2014:675301. doi: 10.1155/2014/675301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barry A, Levine M. A systematic review of the effect of CYP3A5 genotype on the apparent oral clearance of tacrolimus in renal transplant recipients. Ther Drug Monit. 2010;32:708. doi: 10.1097/FTD.0b013e3181f3c063. [DOI] [PubMed] [Google Scholar]
- 41.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34:467. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jusko WJ, D’Ambrosio R. Monitoring FK 506 concentrations in plasma and whole blood. Transplant Proc. 1991;23:2732. [PubMed] [Google Scholar]
- 43.Hebert MF, Zheng S, Hays K, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95:908. doi: 10.1097/TP.0b013e318278d367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouamar R, Shuker N, Hesselink DA, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: A pooled analysis from three randomized-controlled clinical trials. Am J Transplant. 2013;13:1253. doi: 10.1111/ajt.12191. [DOI] [PubMed] [Google Scholar]
- 45.Helal I, Chan L. Steroid and calcineurin inhibitor-sparing protocols in kidney transplantation. Transplant Proc. 2011;43:472. doi: 10.1016/j.transproceed.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 46.Langer RM, Hené R, Vítko S, et al. Everolimus plus early tacrolimus minimization: A phase III, randomized, open-label, multicentre trial in renal transplantation. Transplant Int. 2012;25:592. doi: 10.1111/j.1432-2277.2012.01465.x. [DOI] [PubMed] [Google Scholar]
- 47.Mallon DH, Summers DM, Bradley JA, Pettigrew GJ. Defining delayed graft function after renal transplantation. Transplantation. 2013;96:885. doi: 10.1097/TP.0b013e3182a19348. [DOI] [PubMed] [Google Scholar]