Abstract
Schizophrenia (SZ) is a mental illness characterized by psychosis, negative symptoms, and cognitive deficits. The anterior cingulate cortex (ACC), a structurally and functionally diverse region, is one of several brain regions that is abnormal in SZ. The present study compared synaptic organization and mitochondrial number and morphology in postmortem ACC in SZ versus normal control (NC). Total synaptic density in the combined ACC was decreased in SZ, to 72% of normal controls (NCs), due to selective decreases in axospinous synapses, both asymmetric (excitatory) and symmetric (inhibitory). These changes were present in layers 3 and 5/6. The density of mitochondria in all axon terminals combined in SZ was decreased to 64% of NC. In layer 3, mitochondrial density was decreased only in terminals forming asymmetric synapses with spines, while in layers 5/6 mitochondrial density was decreased in terminals forming symmetric synapses with spines and dendrites. The proportion of terminals making symmetric synapses that contained mitochondria was significantly lower in SZ than in NCs, especially for symmetric axospinous synapses. The number of mitochondria per neuronal somata was decreased in the ACC in SZ compared to NCs; this finding was present in layers 5-6. The size of mitochondria in neuronal somata and throughout the neuropil was similar in SZ and NCs. Our results, though preliminary, are well supported by the literature, and support an anatomical substrate for some of the altered executive functions found in SZ.
Keywords: psychosis, electron microscopy, glutamate, dopamine, neuropathology
1. Introduction
The anterior cingulate cortex (ACC) is part of prefrontal cortex and its neuroanatomy has been well described (DeFelipe et al., 2002; Jones, 1998; Lewis at al., 2002; Peters, 2002). The ACC is composed of distinct anatomical subregions, each with different functional properties (Peterson et al., 1999; Vogt et al., 1992). At the microscopic level, each layer of the ACC has characteristic types of neurons, which project to particular targets; each layer is the recipient of inputs from specific regions (DeFelipe et al., 2002; Lewis et al., 2002). Human imaging studies indicate that the dorsal ACC is involved in mediating attention and executive functions, such as task difficulty, remote memory (Koski and Petrides, 2001), conflict (Barch et al., 2001; Kerns et al., 2004), response inhibition and error commission (Braver et al., 2001; Mathalon et al., 2002). The subcallosal ACC is involved in emotional processing (Bush et al., 2000) or internal states (Greicius et al., 2003), the dorsal ACC is involved in cognitive function, and the rostral ACC, located between these two subdivisions, plays an important role in the integration of these functions (Vogt et al., 1992).
The ACC is one of several brain regions that are abnormal in schizophrenia (SZ), as shown in both in vivo imaging and postmortem studies (Fornito et al., 2003). In vivo imaging of people with SZ has shown abnormalities in multiple transmitter systems (Egerton et al., 2012; Kraguljac et al., 2012a,b; Rowland et al., 2012; Theberge et al., 2003), blood flow (Holcomb et al 2000) and metabolism (Nordal et al., 1996; Tamminga et al., 1992). Functional impairments in cognitive interference (Heckers et al., 2004), error or conflict monitoring (Alain et al., 2002; Carter et al., 1997, 2001) and response monitoring (Kopp and Rist, 1999; Mathalon et al., 2002) have also been demonstrated.
Postmortem studies have shown multiple defects in the ACC in SZ (Eastwood and Harrison, 2001; Fornito et al., 2009). Neurochemical and molecular changes include altered distribution or modulation of dopamine (Benes et al., 1997), abnormalities in multiple aspects of the glutamate system (Barksdale et al., 2014; Bauer et al., 2008, 2010; Drummond et al., 2013; Katsel et al., 2011; Oni-Orisan et al., 2008; Woo et al., 2004, 2007) and intracellular signaling abnormalities (Funk et al., 2012, 2014). Mitochondrial pathology has been implicated repeatedly in schizophrenia (Anglin et al., 2012; Manji et al., 2012) and in the ACC, oxidative stress, a result of mitochondrial production of reactive oxygen species, is increased (Wang et al., 2009). Anatomical abnormalities include layer specific alterations in neuronal distribution and density (Benes and Bird, 1987; Brune et al., 2010; Todtenkopt et al., 2005), and increased numbers of glutamatergic (Benes et al., 1987, 1992) and parvalbumin axons (Kalus et al., 1997, 1999). Of note, previous electron microscopic studies in SZ have shown layer specific abnormalities such as fewer synapses in the ACC (Aganova and Uranova, 1990) and alterations in synapses, mitochondria and oligodendrocytes in other areas of prefrontal cortex (Uranova et al., 2004, 2007, 2011).
The purpose of the present study is to compare the synaptic organization and mitochondrial number and morphology in SZ versus normal control postmortem ACC. Several synaptic features were quantified, including morphological features which identify excitatory and inhibitory synapses. This work has been presented in preliminary form (Barksdale et al., 2012a,b; Roberts et al., 2013).
2. Methods
Human brain tissue was obtained with IRB approved protocols from the Maryland Brain Collection and the Alabama Brain Collection. Diagnostic criteria have been described previously (McCollum et al., 2015; Roberts et al., 2008). Demographics are presented in Table I.
Table I.
Demographic information for the cases.
| NCs | source | ARS | PMI | Nicotine | alcoholism | P values |
|---|---|---|---|---|---|---|
| 1 | MBC | 32CF | 7.0 | yes | no | age: 0.311 |
| 2 | MBC | 45AAF | 6.75 | na | yes | race: 0.598 |
| 3 | MBC | 40AAM | 7.0 | yes | no | sex: 1.000 |
| 4 | ABC | 87CM | 7.5 | no | no | PMI: 0.425 |
| 5 | ABC | 32CF | 7.0 | yes | no | nicotine: 0.459 |
| 6 | ABC | 61CM | 4.0 | yes | no | alcoholism:0.747 |
|
| ||||||
| mean | 49.5 | 6.54 | ||||
| SD | 21.3 | 1.27 | ||||
|
| ||||||
| SZ | source | ARS | PMI | Nicotine | EtOH | medication |
| 1 | MBC | 60AAM | 5.0 | na | no | Olanzapine |
| 2 | MBC | 60AAF | 6.0 | na | no | typical |
| 3 | ABC | 56CM | 7.5 | yes | yes | Olanzapine Quetiapine |
| 4 | ABC | 70CF | 5.0 | yes | no | Depakote; Abilify |
|
| ||||||
| mean | 61.5 | 5.88 | ||||
| SD | 6.0 | 1.18 | ||||
NCs, normal controls; ARS, age, race, sex; PMI, postmortem interval in hours; C, Caucasian; AA, African American; M, male; F, female; P values indicate the results of independent t-tests or Pearson Chi-Square tests between groups.
Coronal blocks from the dorsal ACC were preserved in fixative and processed for electron microscopy as previously described (McCollum et al., 2015). One series of sections was stained for Kluver-Barrera stain as previously described (Bolding et al., 2013; Roberts et al., 2014) and used to identify the layers of the cortex (Figure 1).
Figure 1.
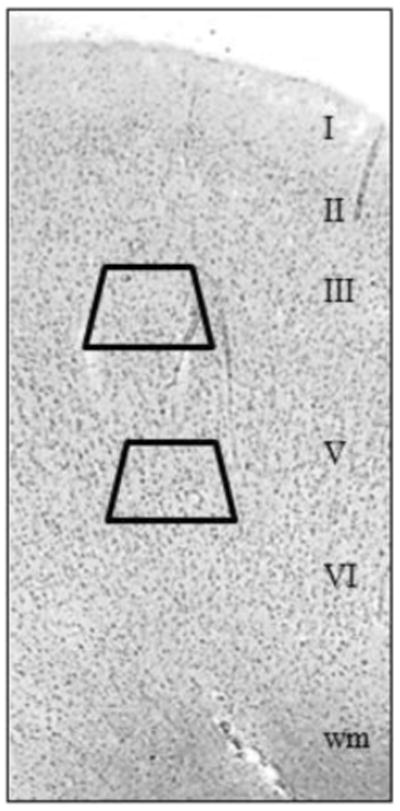
Kluver-Barrera stained section of dorsal ACC. Cortical layers and subcortical white matter (wm) are indicated. Trapezoids show the typical locations of samples taken from layers 3 and 5/6.
A series of tissue adjacent to the ones used for Kluver-Barrera was processed for electron microscopic analysis using standard techniques (McCollum et al., 2015; Roberts et al., 2008). Two to three samples per case, at least 240 μm apart, were used for quantitative analysis. Each sample was cut into an average of 10-11 serial ultrathin sections (90 nm thickness), mounted on Formvar-coated copper grids, and photographed at 80 kV on a Hitachi transmission electron microscope. To determine the number of synapses in the neuropil and mitochondria in terminals, these serial sections were analyzed using the physical disector technique (Geinisman et al., 1996; Perez-Costas et al., 2007) from layers 3 and 5/6 (Figure 2). In each section, eight pictures were photographed at a magnification of 15,000 and stitched together to form a montage. The images are enlarged by 50% for synapse and mitochondria counting. These methods for identifying and quantifying profiles have been described in detail by us (Roberts et al., 2008; Somerville et al., 2001, 2012). Briefly, neuropil only was quantified; cell bodies were not photographed. For controls (combined layer 3 and layers 5/6), a total of 2,313 synapses were counted in a total volume of 11,559 μm3; the average per case was 386 synapses in a volume of 1,926 μm3. For SZ cases (combined layer 3 and layers 5/6), a total of 1,309 synapses were counted in a total volume of 9,248μm3; the average per case was 327 synapses in a volume of 2,320 μm3. A total of 712 and 356 mitochondria were identified in axon terminals in NC and SZ, respectively.
Figure 2.
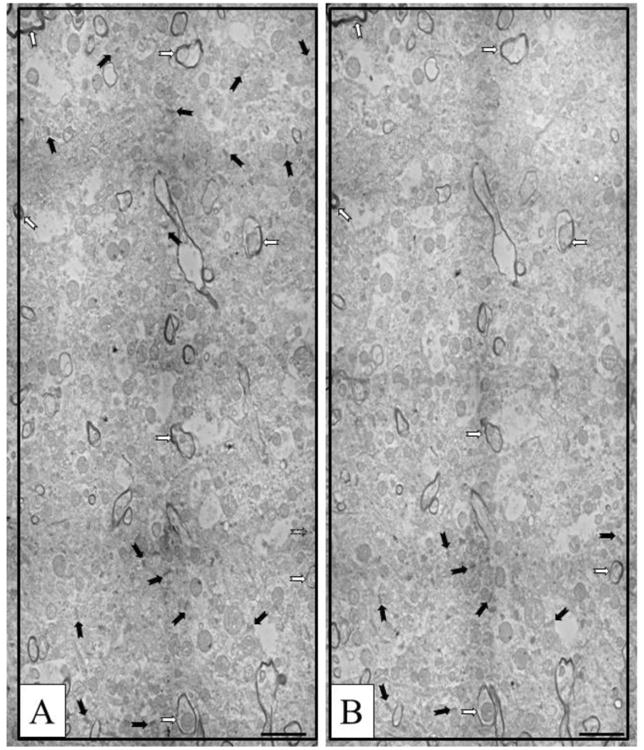
Representative working images used for counting. A and B are taken from serial sections. Black arrows indicate synapses (identified at higher magnification). White arrows with black outlines indicate myelinated axons used as landmarks. Scale bars =2 μm.
In addition, mitochondria throughout the neuropil in 1-2 randomly selected montages per case per layer were counted and their diameters were measured. For this analysis, an average of 1470um2 per case was analyzed and an average of 289 mitochondria per case were counted and measured. Also, neuronal somata near the center of the neuron were photographed at 5,000-8000 magnification and the number of mitochondria present in a single section of each soma was counted. For this analysis a total number of 174 neurons containing a total of 2,881 mitochondria were counted and their diameters measured.
The individuals counting the synapses and mitochondria and measuring mitochondria were blinded to the identity of the groups. The results were compared with unpaired t-tests.
3. Results
3.1. Demographics
Demographics for the groups were similar for age, PMI, sex and race (Table I).
3.2. Synapses
There was no apparent difference in the integrity of the tissue between the NC (Figure 3) and SZ groups (Figure 4). As expected, the majority of synapses in both groups were asymmetric axospinous synapses (Figures 3A,C,D, 4A,D). Asymmetric axodendritic synapses (Figures 3B, 4A), symmetric axospinous synapses (Figures 3C, 4B), symmetric axodendritic synapses (Figures 3E, 4C) were also present in both groups.
Figure 3.
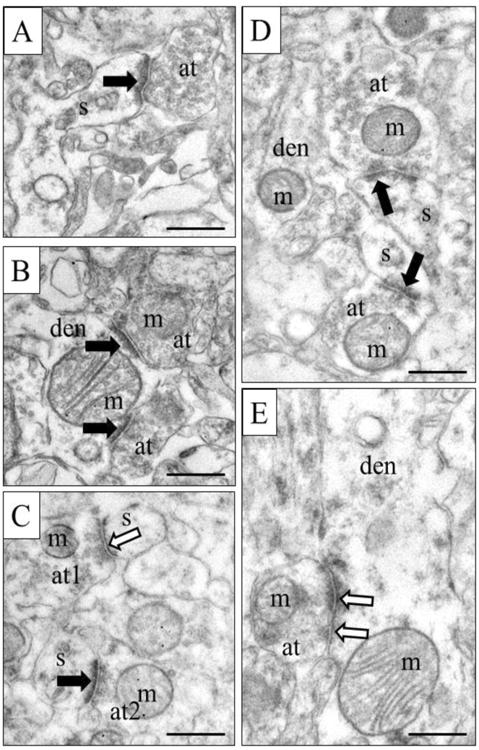
Representative electron micrographs from control tissue. Asymmetric synapses (excitatory) are marked with black arrows, while symmetric synapses (inhibitory) are marked by black-lined white arrows. A) An asymmetric axospinous synapse. B) Two asymmetric axodendritic synapses. C) Two axospinous synapses, one asymmetric and one symmetric. D) Two asymmetric axospinous synapses. E) Symmetric axodendritic synapse. Note that mitochondria are present in all axon terminals except the one in A. S, spine; den, dendrite; m, mitochondrion; at, axon terminal. Scale bars = 500nm.
Figure 4.
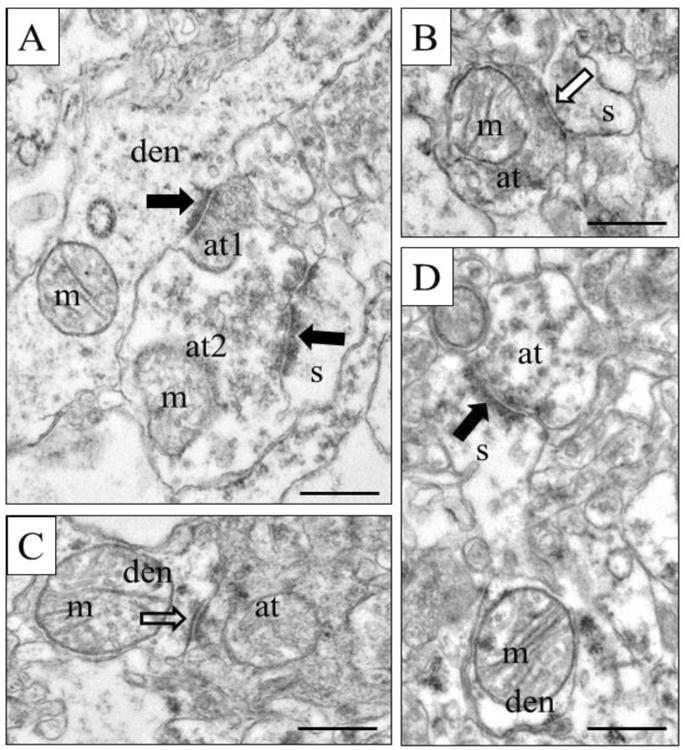
Representative electron micrographs from SZ tissue. Asymmetric synapses are marked with black arrows, while symmetric synapses are marked by black-lined white arrows. A) Two axon terminals form asymmetric synapses, at1 with a dendrite and at2 with a spine. B) A symmetric axospinous synapse. C) A symmetric axodendritic synapse. D) A spine emerges from a dendrite and receives an asymmetric synapse. S, spine; den, dendrite; m, mitochondrion; at, axon terminal. Scale bars = 500nm.
In the ACC (layers 3, 5 and 6 combined) all synapses combined were decreased in density in the SZ cases compared to the NCs (Figure 5A). Both asymmetric and symmetric axospinous synapses were reduced in density in the SZs vs. NCs. Axodendritic synapses, both asymmetric and symmetric types, were similar in density between SZs and NCs. These synaptic density changes were present in layer 3 and layers 5/6 (Figure 5B).
Figure 5.
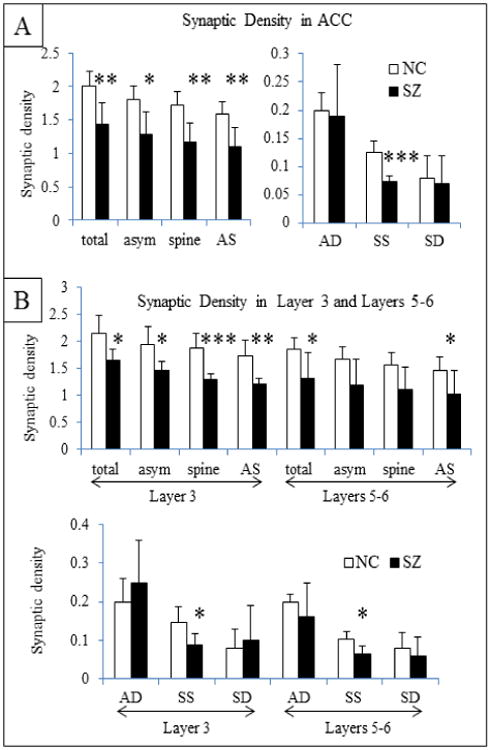
Synaptic density (per 10μm3). A) Density of total synapses and specific types of synapses in the ACC. B) Synaptic density in layers 3 and 5-6 of the ACC. Total (all synapses combined), asym (all asymmetric synapses), spine (all axospinous synapses), AS (asymmetric axospinous), AD (asymmetric axodendritic, SS (symmetric axospinous), and SD (symmetric axodendritic) are shown separately for layer 3, and 5/6 combined. T-test results: *, p<0.05; ** p<0.01; *** p<0.005.
3.3. Mitochondria in the neuropil
The density (Figure 6A) and diameter (Figure 6B) of mitochondria in the neuropil was similar between NCs and the SZ cases in the ACC (layers 3, 5/6 combined) as well as the separate analysis of layer 3 and layers 5/6.
Figure 6.
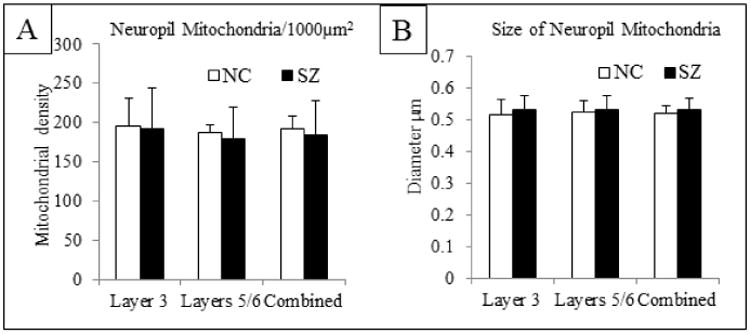
Mitochondria in the neuropil. A) Mitochondrial density per 1000μm2 of neuropil indicates no difference in density between NC and SZ in layer 3, layers 5/6 or the combined value. B) The diameter of mitochondria in the neuropil was similar between NC and SZ in layer 3, layers 5/6 and the combined value.
3.4. Mitochondria in axon terminals
Mitochondria specifically located in axon terminals comprise a small proportion of mitochondria found throughout the neuropil. Therefore, we counted the number of mitochondria found just in axon terminals and their various subtypes. In the ACC (layers 3, 5 and 6 combined), the number of mitochondria in axon terminals forming any type of synapse and those forming asymmetric axospinous synapses was lower in SZ as compared to NCs (Figure 7A). There were also fewer mitochondria in terminals forming symmetric synapses, due to a selective loss of mitochondria in symmetric axospinous synapses. The density of mitochondria in terminals forming synapses with dendrites was similar in SZs and NCs. Layer 3 showed a similar pattern to the combined ACC values for asymmetric synapses, but the number of mitochondria in terminals forming symmetric synapses was similar between SZs and NCs (Figure 7B). Layers 5/6 (Figure 7B) showed a pattern of mitochondrial loss from asymmetric synapses, but it did not reach statistical significance. However, the density of mitochondria in terminals forming symmetric synapses, both with spines and dendrites, was lower in SZs than NCs. The density of mitochondria in terminals forming asymmetric axodendritic synapses was similar in SZ and NCs.
Figure 7.
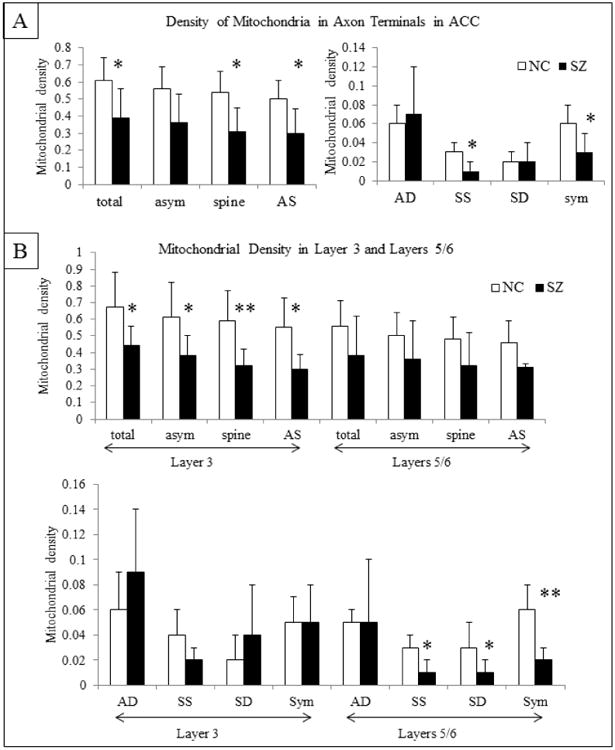
Mitochondria in axon terminals (per 10μm3) in the combined ACC (A) and in Layers 3 and 5/6 (B). Abbreviations: the same as Fig. 5. Sym, symmetric synapses. T-test results: *, p<0.05; ** p<0.01.
A decrease in the density of mitochondria in terminals may be a reflection of fewer terminals rather than fewer mitochondria in existing terminals, so we also examined the proportion of terminals containing at least one mitochondrion (Table 2). The proportion of terminals making symmetric synapses that contained mitochondria was significantly lower in SZ than in NCs. This pattern was significant for symmetric axospinous synapses, with a trend level pattern in symmetric axodendritic synapses. The proportion of terminals in layer 3 (all synapses combined and the various subset of synapses) containing mitochondria was similar between SZs and NCs (Table 2). The proportion of terminals on layers 5/6 containing mitochondria that formed symmetric synapses and symmetric axodendritic synapses was lower in SZs than NCs; the same pattern was observed in symmetric axospinous synapses, albeit non-significant (Table 2).
Table II.
The proportion of axon terminals (AT) containing at least one mitochondrion was 30.5% in NCs and 26% in SZs, for all layers combined. The percentage of symmetric axospinous synapses in all layers combined that contained at least one mitochondrion in SZ was 67% of that of NCs (p<0.03). This change was confined to layers 5/6 where <10% of these terminals contained at least one mitochondrion. Abbreviations are the same as those in Figure 5.
| % of AT w/ ≥ 1 mitochondrion | total | asym | spine | AS | AD | SS | SD | sym | |
|---|---|---|---|---|---|---|---|---|---|
| combo | NC | 30.5 | 30.8 | 30.9 | 31.4 | 26.4 | 29.0 | 34.0 | 27.1 |
| SZ | 26.4 | 27.0 | 25.4 | 25.9 | 32.6 | 17.5* | 15.2 t | 18.3* | |
|
| |||||||||
| L3 | NC | 30.2 | 30.8 | 30.6 | 31.1 | 28.6 | 23.4 | 19.5 | 23.2 |
| SZ | 26.3 | 26.0 | 24.2 | 24.3 | 35.3 | 21.4 | 26.4 | 25.9 | |
|
| |||||||||
| L5/6 | NC | 30.1 | 30.1 | 30.7 | 31.0 | 24.0 | 29.4 | 40.8 | 31.0 |
| SZ | 26.2 | 27.9 | 26.5 | 27.3 | 30.8 | 17.3 | 4.0* | 9.4** | |
p<0.05;
p<0.014.
3.5. Mitochondria in neuronal somata
In the combined ACC, the number of mitochondria per neuronal somata in the SZ cases was decreased by 43% of that of NCs (Figures 8, 9A,B). This was due to a selective loss in layers 5/6. The size of mitochondria in neuronal somata throughout the ACC was similar in SZ cases compared to NCs (Figures 8, 9C).
Figure 8.
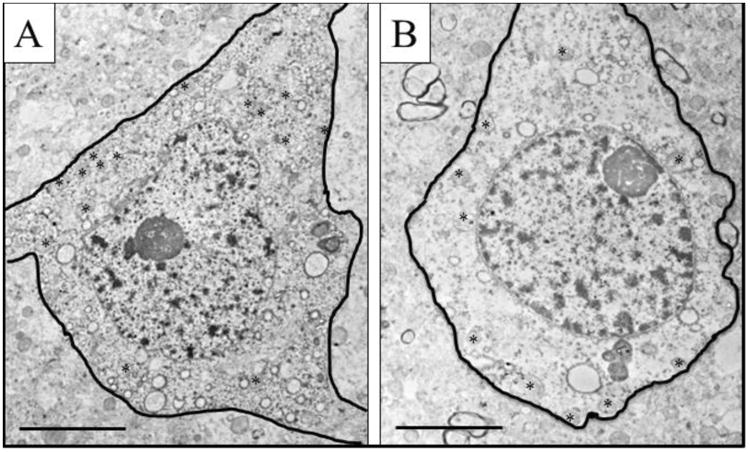
Mitochondria in neuronal cell bodies. Representative electron micrographs of pyramidal cells in NC and SZ. The soma is outlined in black; asterisks indicate mitochondria. Scale bar = 5 um.
Figure 9.
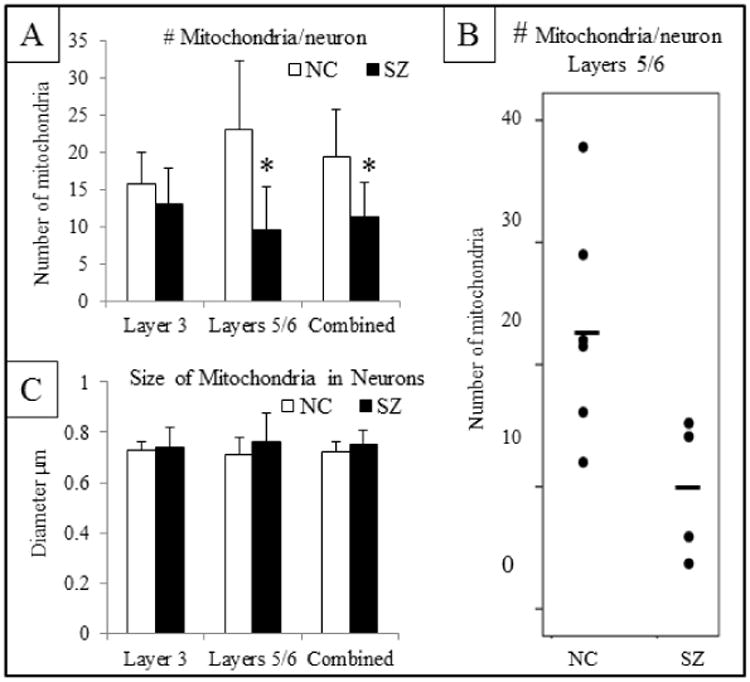
Mitochondrial quantification in neuronal somata for Layer 3, Layers 5/6 and then the combined value. A) Number of mitochondria per soma in a single section. There are significantly fewer mitochondria per neuron in the combined ACC and in layers 5/6 in SZ vs. NCs. B) Scatter plot of the number of mitochondria per neuron in Layers 5/6. C) Diameter of mitochondria indicates no difference in size of mitochondria in SZ.
4. Discussion
Our main results show reduced overall synaptic density in the ACC in SZ compared to NCs. The synaptic loss was confined to axospinous synapses and was present in layers 3 and 5/6. Both asymmetric and symmetric axospinous synapses were affected, indicating abnormalities in both excitatory and inhibitory transmission, respectively. Overall decreases in synaptic density in the SZ group are consistent with the reduced neuropil hypothesis, which posits fewer cortical synaptic connections (Selemon and Goldman-Rakic, 1999). Fewer axospinous synapses in the ACC in SZ are consistent with previously reported spine loss (Glantz and Lewis, 1999). Mitochondria were reduced in number in specific populations of axon terminals and in the somata of neurons in layers 5/6, suggesting abnormal energy levels. Our results complement and extend previous an electron microscopic study of the ACC in SZ, showing synaptic pathology in layers 1-2 (Aganova and Uranova, 1992).
4.1. Limitations of postmortem studies in schizophrenia
Our small sample size, though a limitation, is not unusual for postmortem EM. Postmortem tissue suitable for electron microscopic analysis is very difficult to procure, and though our cohort is small, it is one of only two studies (Aganova and Uranova, 2004) that have been reported in the ACC. Changes reported herein are consistent with abnormalities shown in previous studies. That said if another larger cohort of subjects become available, replication of this pilot study would be prudent. The fact that all of our subjects were treated with medication could complicate the interpretation of our results. However, antipsychotic drugs administered to animals do not affect cortical levels of synaptophysin (Lidow et al., 2001) a general marker of axon terminals (Calhoun et al., 1996), levels of vGLULT1 (Oni-Orisan et al., 2008) a marker of glutamatergic terminals of cortical origin (Fremeau et al., 2004), or the density of tyrosine hydroxylase labeled processes (Akil et al., 1999) which label dopaminergic terminals (Lewis et al., 1988; Sesack et al., 1995; Smiley et al., 1992). Thus, it appears that antipsychotic drugs do not lower overall synaptic density, glutamatergic or dopaminergic terminals in the ACC and changes reported here are probably not due to medication.
4.2. Decreased density of asymmetric axospinous synapses in SZ
Asymmetric axospinous synapses, characteristic of glutamatergic inputs, were decreased in density in layers 3 and 5/6. In layer 3 of prefrontal cortex of non-human primates, asymmetric axospinous synapses are formed on the spines of pyramidal cells by afferents from the medial dorsal thalamus (Jones and Hendry, 1989; Jones 1998; Giguere and Goldman-Rakic, 1988), contralateral ACC (Vogt and Pandya, 1987; Vogt et al., 1987) and intracortical connections (Melchitzky et al., 1998). The loss of axospinous synapses may involve any of these projections and is consistent with reduced spine density of layer 3 pyramidal neurons (Glantz and Lewis, 1999), as the number of spines and axospinous synapse change in tandem (Hensch, 2005). Our results are probably not due to fewer pyramidal neurons, as no difference in pyramidal neuron number has been found in the layers we examined (Benes et al., 2001; Bouras et al., 2001). The thalamocortical projection from mediodorsal nucleus could be involved as it has fewer neurons in SZ (Pakkenberg, 1990; Young et al., 2000) and this may translate to fewer projecting axons.
In the deep layers of prefrontal cortex of non-human primates, asymmetric axospinous synapses are formed mainly by cortical association inputs from the contralateral hemisphere, local collaterals of excitatory neurons (Bannister, 2005; Morecraft et al., 2012; Vogt and Pandya, 1987), and contacts from calretinin-labeled cortical interneurons (del Rio and DeFelipe, 1997; Melchitzky et al., 2005). Thalamic afferents other than the medial dorsal nucleus terminate in the deep layers of the cortex in rat (Shibata, 1993) and in monkey (DeFelipe and Jones 1991) and may also innervate the ACC in human. Another source of excitatory inputs onto spines is from the amygdala (Cunningham et al., 2002) another pathological region in SZ (Benes 2010). Pyramidal neurons in layers 5/6 project to the striatum, brainstem or thalamus (Bannister, 2005; Goldman and Nauta, 1977). Taken together, the ACC in SZ has a decrease in excitatory synaptic connections, possibly from multiple sources, which may have effects on several downstream pathways. Decreased excitatory axospinous synapses have also been reported in the hippocampus of subjects with SZ (Kolomeets et al., 2005, 2007).
4.3. Decreased density of symmetric axospinous synapses in SZ
Symmetric axospinous synapses, characteristic of dopamine inputs and inhibitory interneuron connections, were decreased in density in layers 3 and 5/6. Layers 1, 3 and 6 contain the highest densities of dopaminergic axons in primates (Lewis et al., 1988; Williams and Goldman-Rakic, 1993), including humans (Gaspar et al, 1989; Smiley et al., 1992). Dopaminergic afferents form symmetric synapses with pyramidal cell spines and the dendritic shafts of GABAergic interneurons (Sesack et al., 1995). Thus, the symmetric axospinous synapses that are reduced in density in SZ in both layers 3 and 5/6 may reflect a loss of dopamine input to spiny pyramidal neurons, consistent with the hypodopaminergic cortical tone (Howes and Kapur, 2009). In support of this are previous studies showing a decrease in density of dopaminergic fibers in deep cortical layers in the ACC (Benes et al., 1997) as well as other regions of prefrontal cortex (Akil et al., 1999). Other candidate connections that may be altered include the terminals of local interneurons, which form symmetric synapses onto both spines and dendritic shafts (del Rio and DeFelipe, 1995, 1997; Hendry et al., 1984; Kisvárday et al., 1990). Taken together, the ACC in SZ has a decrease in inhibitory synaptic connections probably from multiple sources.
4.4. Decreased mitochondrial density in SZ
Mitochondria are crucial for cellular functions including energy (Wong-Riley, 1989), calcium buffering (Duchen et al., 2008; Gunter et al., 1994), production of reactive oxygen species (Dugan et al., 1995), regulation of apoptosis (Susin et al., 1999) and modulation of synaptic activity (Duchen et al., 2008; Li et al., 2004; Miller and Sheetz, 2004). The size of mitochondria throughout the neuropil and in neuronal somata was similar in SZ and NCs. This is consistent with our recent finding that levels of Mitofusin 2, a protein responsible for mitochondrial fusion, are normal in the ACC in SZ (Barksdale et al., 2014). However, the density of mitochondria in certain populations of axon terminals and in the somata of neurons in layers 5/6 was decreased in SZ. The proportion of terminals containing mitochondria was lower in inhibitory synapses in layers 5/6. The reduction in number of mitochondria per terminals forming symmetric synapses suggests compromised metabolism in inhibitory synapses. Pyramidal neurons in layers 5/6 project to the striatum or brainstem, or thalamus (Goldman and Nauta, 1977). The reduction of mitochondria per soma in these layers suggests that neurons that project to these subcortical regions may have compromised metabolism. The loss of mitochondria may reflect their death, or abnormalities in mitochondrial trafficking such that mitochondria are transported out of the soma and/or axon terminal at a rate faster than they return (Chang and Reynolds, 2006; Ligon and Steward, 2000). Oxidative stress, an index of mitochondrial dysfunction, is increased in the ACC in SZ (Wang et al., 2009). Thus, remaining mitochondria in the ACC may be functionally compromised.
4.5. Integrating postmortem and imaging studies
Several magnetic resonance spectroscopy studies in SZ have reported decreased N-acetyl-aspartate (NAA) levels in multiple brain regions (Fannon, et al., 2003; Steen et al., 2005), including the ACC (Jessen et al., 2013; Kraguljac et al., 2012; Reid et al., 2010). NAA is an amino acid that is primarily synthesized in neuronal mitochondria (Bates et al., 1996) and is metabolized in oligodendrocytes to acetate, a precursor of myelin lipid synthesis (Chakraborty et al., 2001). Thus the amount of NAA as seen in imaging studies may reflect neuronal integrity, the number of axon terminals and synapses, myelin integrity and/or mitochondrial function (Bates et al., 1996; Chakraborty et al., 2001; Ledeen et al., 2006; Lentz et al., 2005; Moffett et al., 2007; Stork and Renshaw, 2005). Thus, the results of the present study and those of Aganova and Uranova (1992) support that any of these mechanisms could underlie decreased NAA as seen in in vivo imaging studies. The decrease in density of axospinous synapses together with a lower proportion of remaining terminals that contain mitochondria and lower somatic mitochondrial counts, suggests a decrease in cortical synaptic efficiency. Overall, our changes suggest alterations in multiple cortical connections that may impact on cognitive functions subserved by the dorsal ACC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aganova EA, Uranova NA. Morphometric analysis of synaptic contacts in the anterior limbic cortex in the endogenous psychoses. Neurosci Behav Physiol. 1992;22(1):59–65. doi: 10.1007/BF01186670. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psych. 1999;156(10):1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb Cortex. 2002;12(8):840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- Anglin RE, Mazurek MF, Tarnopolsky MA, Rosebush PI. The mitochondrial genome and psychiatric illness. Am J Med Genetics Part B. 2012;159B(7):749–759. doi: 10.1002/ajmg.b.32086. [DOI] [PubMed] [Google Scholar]
- Bannister AP. Inter- and intra-laminar connections of pyramidal cells in the neocortex. Neurosci Res. 2005;53(2):95–103. doi: 10.1016/j.neures.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11(9):837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Barksdale KA, Roche JK, Lahti AC, Roberts RC. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012a. Synaptic and mitochondrial changes in the postmortem anterior cingulate cortex in schizophrenia. Program No. 223.11. [Google Scholar]
- Barksdale KA, Roche JK, Roberts RC, Lahti AC. Synaptic and mitochondrial changes in the anterior cingulate cortex in schizophrenia. Neuropsychopharm. 2012b;38:S314–S446. doi: 10.1016/j.schres.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale KA, Lahti AC, Roberts RC. Synaptic proteins in the postmortem anterior cingulate cortex in schizophrenia: relationship to treatment and treatment response. Neuropsychopharm. 2014;39(9):2095–2103. doi: 10.1038/npp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. NeuroReport. 1996;7(8):1397–1400. [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008;104(1-3):108–120. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117(1):92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropyschopharm. 2010;35:239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Bird ED. An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Arch Gen Psych. 1987;44(7):608–616. doi: 10.1001/archpsyc.1987.01800190024004. [DOI] [PubMed] [Google Scholar]
- Benes FM, Majocha R, Bird ED, Marotta CA. Increased vertical axon numbers in cingulate cortex of schizophrenics. Arch Gen Psych. 1987;44(11):1017–1021. doi: 10.1001/archpsyc.1987.01800230097015. [DOI] [PubMed] [Google Scholar]
- Benes FM, Sorensen I, Vincent SL, Bird ED, Sathi M. Increased density of glutamate-immunoreactive vertical processes in superficial laminae in cingulate cortex of schizophrenic brain. Cereb Cortex. 1992;2(6):503–512. doi: 10.1093/cercor/2.6.503. [DOI] [PubMed] [Google Scholar]
- Benes FM, Todtenkopf MS, Taylor JB. Differential distribution of tyrosine hydroxylase fibers on small and large neurons in layer II of anterior cingulate cortex of schizophrenic brain. Synapse. 1997;25(1):80–92. doi: 10.1002/(SICI)1098-2396(199701)25:1<80::AID-SYN10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psych. 2001;50(6):395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- Bolding M, Reid M, Avsar K, Roberts R, Gamlin P, Gawne T, White D, Lahti A. Magnetic transfer contrast accurately localizes substantia nigra confirmed by histology. Biol Psych. 2013;73(3):289–94. doi: 10.1016/j.biopsych.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras C, Kövari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102(4):373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11(9):825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brune M, Schobel A, Karau R, Benali A, Faustmann PM, Juckel G, Petrasch-Parwez E. Von Economo neuron density in the anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropath. 2010;119(6):771–778. doi: 10.1007/s00401-010-0673-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cog Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;(12):821–828. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psych. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psych. 1997;154(12):1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 2001;78(4):736–745. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80(5):241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol. 2002;31(3-5):299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Parvalbumin immunoreactivity reveals layer IV of monkey cerebral cortex as a mosaic of microzones of thalamic afferent terminations. Brain Res. 1991;562(1):39–47. doi: 10.1016/0006-8993(91)91184-3. [DOI] [PubMed] [Google Scholar]
- Del Rio MR, DeFelipe J. A light and electron microscopic study of calbindin D-28k immunoreactive double bouquet cells in the human temporal cortex. Brain Res. 1995;690:133–140. doi: 10.1016/0006-8993(95)00641-3. [DOI] [PubMed] [Google Scholar]
- Del Rio MR, DeFelipe J. Synaptic connections of calretinin-immunoreactive neurons in the human neocortex. J Neurosci. 1997;17(13):5143–5154. doi: 10.1523/JNEUROSCI.17-13-05143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond JB, Tucholski J, Haroutunian V, Meador-Woodruff JH. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophren Res. 2013;147(1):32–38. doi: 10.1016/j.schres.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44(1):1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;(15):6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55(5):569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, Stone JM. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharm. 2012;37(11):2515–2521. doi: 10.1038/npp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon D, Simmons A, Tennakoon L, O'Ceallaigh S, Sumich A, Doku V, Shew C, Sharma T. Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophrenia. Biol Psych. 2003;54(6):587–598. doi: 10.1016/s0006-3223(03)00185-9. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27(2):98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Funk AJ, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Increased G protein-coupled receptor kinase (GRK) expression in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2014;159(1):130–5. doi: 10.1016/j.schres.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk AJ, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology. 2012;37(4):896–905. doi: 10.1038/npp.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Fabvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Gunderson HJG, Van Der Zee E, West MJ. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996;(12):805–819. doi: 10.1007/BF02284843. [DOI] [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277(2):195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psych. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJ. An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol. 1977;72(3):369–386. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. PNAS. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267(2 Pt 1):C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am J Psych. 2004;161(4):707–715. doi: 10.1176/appi.ajp.161.4.707. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4(10):2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–37. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Weiler M, Dannals RF, Tamminga CA. Brain activation patterns in schizophrenic and comparison volunteers during a matched-performance auditory recognition task. Am J Psych. 2000;157(10):1634–1645. doi: 10.1176/appi.ajp.157.10.1634. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Fingerhut N, Sprinkart AM, Kühn KU, Petrovsky N, Maier W, Schild HH, Block W, Wagner M, Träber F. N-acetylaspartylglutamate (NAAG) and N-acetylaspartate (NAA) in patients with schizophrenia. Schizophren Bull. 2013;39(1):197–205. doi: 10.1093/schbul/sbr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. A new view of specific and nonspecific thalamocortical connections. Adv Neurol. 1998;(77):49–71. discussion 72-73. [PubMed] [Google Scholar]
- Jones EG, Hendry SH. Differential Calcium Binding Protein Immunoreactivity Distinguishes Classes of Relay Neurons in Monkey Thalamic Nuclei. Eur J Neurosci. 1989;1(3):222–246. doi: 10.1111/j.1460-9568.1989.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Kalus P, Senitz D, Beckmann H. Altered distribution of parvalbumin-immunoreactive local circuit neurons in the anterior cingulate cortex of schizophrenic patients. Psych Res. 1997;75(1):49–59. doi: 10.1016/s0925-4927(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Kalus P, Senitz D, Lauer M, Beckmann H. Inhibitory cartridge synapses in the anterior cingulate cortex of schizophrenics. J Neural Transm. 1999;106(7-8):763–771. doi: 10.1007/s007020050197. [DOI] [PubMed] [Google Scholar]
- Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharm. 2011;36(6):1171–1177. doi: 10.1038/npp.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kisvárday ZF, Gulyas A, Beroukas D, North JB, Chubb IW, Somogyi P. Synapses, axonal and dendritic patterns of GABA-immunoreactive neurons in human cerebral cortex. Brain. 1990;113(3):793–812. doi: 10.1093/brain/113.3.793. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61(8):615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57(1):47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol. 1999;108(2):337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- Koski L, Petrides M. Time-related changes in task performance after lesions restricted to the frontal cortex. Neuropsychol. 2001;39(3):268–281. doi: 10.1016/s0028-3932(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Reid MA, White D, Jones R, den Hollander J, Lowman D. Neurometabolites in schizophrenia and bipolar disorder- a systematic review and metaanalysis. Psych Res. 2012;203(2-3):111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen RW, Wang J, Wu G, Lu ZH, Chakraborty G, Meyenhofer M, Tyring SK, Matalon R. Physiological role of N-acetylaspartate: contribution to myelinogenesis. Adv Exp Med Biol. 2006;(76):131–143. doi: 10.1007/0-387-30172-0_9. discussion 361-363. [DOI] [PubMed] [Google Scholar]
- Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern EF, Lackner AA, Masliah E, Gonzalez RG. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235(2):461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Burgos GG. Specificity in the functional architecture of primate prefrontal cortex. J Neurocytol. 2002;31(3-5):265–276. doi: 10.1023/a:1024174026286. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Goldstein M, Morrison JH. The dopaminergic innervation of the monkey prefrontal cortex: a tyrosine hydroxylase immunohistochemical study. Brain Res. 1988;(449):225–243. doi: 10.1016/0006-8993(88)91040-2. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM, Castner SA, Allen PB, Greengard P, Goldman-Rakic PS. Antipsychotic treatment induces alterations in dendrite- and spine-associated proteins in dopamine-rich areas of the primate cerebral cortex. Biol Psych. 2001;49(1):1–12. doi: 10.1016/s0006-3223(00)01058-1. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427(3):351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13(5):293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psych. 2002;111(1):22–41. [PubMed] [Google Scholar]
- Melchitzky DS, Eggan SM, Lewis DA. Synaptic targets of calretinin-containing axon terminals in macaque monkey prefrontal cortex. Neurosci. 2005;13(1):185–195. doi: 10.1016/j.neuroscience.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol. 1998;390(2):211–224. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117(Pt 13):2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull. 2012;87(4-5):457–97. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum LA, Walker CK, Roche JK, Roberts RC. Elevated excitatory input to the nucleus accumbens in schizophrenia: a postmortem ultrastructural study. Schizophren Bull. 2015 doi: 10.1093/schbul/sbv030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negyessy L, Goldman-Rakic PS. Morphometric characterization of synapses in the primate prefrontal cortex formed by afferents from the mediodorsal thalamic nucleus. Exp Brain Res. 2005;164(2):148–154. doi: 10.1007/s00221-005-2237-6. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O'Shora-Celaya L, Eberling J, Robertson L, Huesman RH, Jagust W, Budinger TF. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;6:541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered vesicular glutamate transporter expression in the anterior cingulate cortex in schizophrenia. Biol Psych. 2008;63(8):766–775. doi: 10.1016/j.biopsych.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508(6):906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psych. 1990;(47):1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E, Melendez-Ferro M, Roberts RC. Microscopy techniques and the study of synapses. In: Mendez-Vilas A, Diaz J 3rd, editors. Modern Research and Educational Topics in Microscopy. Formatex; Badajoz, Spain: 2007. pp. 164–170. [Google Scholar]
- Peters A. Examining neocortical circuits: some background and facts. J Neurocytol. 2002;31(3-5):183–193. doi: 10.1023/a:1024157522651. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psych. 1999;45(10):1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psych. 2010;68(7):625–633. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR. Differential synaptic changes in the striatum of subjects with undifferentiated versus paranoid schizophrenia. Synapse. 2008;62(8):616–627. doi: 10.1002/syn.20534. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Barksdale K, Roche JK, Lahti AC. Synaptic and mitochondrial changes in the anterior cingulate cortex in schizophrenia. International Congress on Schizophrenia Research Meeting. Schizophr Bull. 2013;39(Suppl 1)(S203):16. [Google Scholar]
- Roberts RC, Roche JK, McCullumsmith RE. Localization of excitatory amino acid transporters EAAT1 and EAAT2 in human postmortem cortex: a light and electron microscopic study. Neurosci. 2014;(277C):522–540. doi: 10.1016/j.neuroscience.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, Holcomb HH, Barker PB. In Vivo Measurements of Glutamate, GABA and NAAG in Schizophrenia. Schizophr Bull. 2013;39(5):1096–1104. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psych. 1999;45(1):17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363(2):264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol. 1993;330(4):533–42. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol. 1992;321(3):325–335. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Somerville SM, Lahti AC, Conley RR, Roberts RC. Mitochondria in the striatum of subjects with schizophrenia: relationship to treatment response. Synapse. 2011;65(3):215–224. doi: 10.1002/syn.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville SM, Conley RR, Roberts RC. Striatal mitochondria in subjects with chronic undifferentiated vs. chronic paranoid schizophrenia. Synapse. 2012;66(1):29–41. doi: 10.1002/syn.20981. [DOI] [PubMed] [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharm. 2005;30(11):1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psych. 2005;10(10):900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psych. 1992;49(7):522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- Théberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RW, Rajakumar N, Schaefer B, Densmore M, Drost DJ. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psych. 2003;160(12):2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73(1):79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67(2-3):269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treat. 2011;(1155):325789. doi: 10.1155/2011/325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Aganova EA. Ultrastructure of the synapses of the anterior limbic cortex in schizophrenia. Zh Nevropatol Psikhiatr Im S S Korsakova. 1989;89(7):56–59. [PubMed] [Google Scholar]
- Uranova NA, Vikhreva OV, Zimina IS, Rakhmanova VI, Klintsova A, Black J, Greenough WT, Orlovskaia DD. Abnormal patterns of cortical synaptic connectivity in schizophrenia. Vestn Ross Akad Med Nauk. 2007;(3):8–14. [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262(2):256–70. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262(2):271–89. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Wang JF, Shao L, Sun X, Young LT. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disorders. 2009;11(5):523–529. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb Cortex. 1993;(3):199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12(3):94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psych. 2004;(7):649–57. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woo TU, Shrestha K, Amstrong C, Minns MM, Walsh JP, Benes FM. Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr Res. 2007;96(1-3):46–61. doi: 10.1016/j.schres.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psych. 2000;47(11):944–953. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]


