Summary
Changes in DNA methylation are required for the formation of germinal centers (GC), but the mechanisms of such changes are poorly understood. Activation-induced cytidine deaminase (AID) has been recently implicated recently in DNA demethylation through its deaminase activity coupled with DNA repair. We investigated the epigenetic function of AID in vivo in germinal center B cells (GCB) isolated from wild type (WT) and AID-deficient (Aicda-/-) mice. We determined that the transit of B cells through the GC is associated with marked locus-specific loss of methylation and increased methylation diversity, both of which are lost in Aicda-/- animals. Differentially methylated cytosines (DMCs) between GCB and naïve B cells (NB) are enriched in genes that are targeted for somatic hypermutation (SHM) by AID and these genes form networks required for B cell development and proliferation. Finally, we observed significant conservation of AID-dependent epigenetic reprogramming between mouse and human B cells.
Keywords: lymphocytes, B cells, germinal centers, AID, DNA methylation, epigenetic plasticity
Graphical abstract

Introduction
DNA methylation is an epigenetic modification that regulates genomic imprinting, X chromosome inactivation and cell fates during development (Smith and Meissner, 2013); and chromosomal stability, repression of transposable elements and gene expression during the lifetime of the organism (Bird, 2002; Jones and Takai, 2001). The majority of the DNA methylation in mammalian genomes takes place within a CpG dinucleotide context and 5-methyl-C has been called a fifth base in the genome (Novik et al., 2002).
B cell development is associated with significant plasticity and changes of the DNA methylome, occurring from lymphoid commitment of hematopoietic stem cells in the bone marrow to peripheral B cell maturation in the secondary lymphoid organs (Hodges et al., 2011; Ji et al., 2010; Zilbauer et al., 2013). The transition from NB to GCB, critical for affinity maturation and generation of an improved, long-lasting immune response, is accompanied by marked demethylation of the genome and more heterogeneous DNA methylation patterning (Lai et al., 2013; Shaknovich et al., 2011). The mechanisms of these modifications of the methylome remain poorly understood and, therefore, we set out to understand how these changes arise in B cells.
In contrast to DNA methylation gain, the identities of the enzymes that catalyze DNA demethylation have largely remained elusive. AID is highly expressed in GCB and is necessary for the antigen-dependent activation process by which NB transition through the GC of the secondary lymphoid organs (Bunting and Melnick, 2013; Klein and Dalla-Favera, 2008). AID converts deoxycytosines (dC) into deoxyuracils (dU), producing dU:dG mismatches that are removed by both mismatch repair and base excision repair (Honjo et al., 2005; Zan and Casali, 2013). These repair processes are required for SHM and class switch recombination (CSR) of immunoglobulin (Ig) genes, necessary steps of affinity maturation within the GC (Conticello, 2008; Muramatsu et al., 2000; Zan and Casali, 2013). Interestingly, AID function is not restricted to Ig loci and 25% of highly expressed genes in GCB are targeted by AID (Liu et al., 2008). Moreover, anti-AID ChIP experiments showed a genome-wide recruitment of AID in ex vivo activated B cells (Yamane et al., 2011).
Several studies in non-lymphoid tissues have demonstrated that AID can also participate in loss of methylation. AID has been implicated in DNA demethylation during zebrafish development (Rai et al., 2008), reprogramming in heterokaryons (Bhutani et al., 2010) and pluripotent germ cells (Popp et al., 2010); and late reprogramming of induced pluripotent stem cells in mice (Kumar et al., 2013). The mechanism by which AID demethylates is not completely elucidated, although it is thought to occur via deaminase activity followed by base excision DNA repair and replacement with unmethylated C (Klein and Dalla-Favera, 2008; Kuppers, 2005).
No DNA demethylation role for AID has yet been uncovered in vivo in B cells, although several lines of evidence point to such function. We previously demonstrated that hypomethylated regions in human GCB were enriched for the putative AID binding site RGYW (Shaknovich et al., 2011) and that hypomethylation in GCB-derived lymphomas correlated with AID expression (De et al., 2013). Considering these observations in light of the AID demethylation role in other cell types, we examined the epigenetic function of AID in GCB. In this study we establish that AID functions as an epigenetic modifier by promoting loss of DNA methylation and increasing methylation diversity during the GC stage of B cell maturation in vivo in human and murine B cells.
Results
Loss of AID abrogates CpG methylation changes during GC transition
We previously observed significant loss of DNA methylation in human GCB (Lai et al., 2013; Shaknovich et al., 2011). We hypothesized that AID would be at least partly responsible for this decrease. To investigate the role of AID in the genome-wide methylation changes occurring during NB to GCB transition, we induced T cell-dependent GC formation with 4-NP-Chicken Gamma Globulin (NP-CGG) in WT (7 replicates) and Aicda-/- mice (6 replicates). Mice were sacrificed at day 10 post-injection and splenic NB (B220+ GL7- CD95-) and GCB (B220+ GL7+ CD95+) were isolated. To profile the methylome of NB and GCB cells, we performed Enhanced Reduced Representation Sequencing (ERRBS), an efficient single-nucleotide resolution high-throughput technique that interrogates 2-4 million distinct CpGs (Akalin et al., 2012). Upon rigorous quality control of bisulfite conversion (>99.5% in all samples) and read mapping frequency (>70%), we called differentially methylated CpGs (DMCs) between NB and GCB using a combination of statistical difference (FDR<0.001 using Fisher exact test) and methylation level difference greater than 20% (see Experimental Procedures). We observed that NB to GCB transition in WT mice was accompanied by significant changes in DNA methylation, including 8,308 hypomethylated DMCs (hypoDMCs) and 3,390 hypermethylated DMCs (hyperDMCs) (Figures 1A and 1B). These changes were independent of class-switched B cell receptors, since unswitched (IgM+) GCB and total GCB presented similar patterns of methylation (data not shown). This is consistent with our previous results showing a genome-wide loss of methylation in primary human GCB samples compared to NB (Shaknovich et al., 2011). On the contrary, our profiling of Aicda-/- animals resulted in minimal observed changes in DNA methylation during the transition from NB to GCB: only 703 of CpGs revealed hypomethylation and 172 CpGs revealed hypermethylation (Figures 1A and 1B). We also found that Aicda-/- mice had reduced global methylation differences during the NB to GCB transition, indicating that loss of AID also resulted in less methylome plasticity at non-differentially methylated CpGs (Figure 1C). This occurred despite comparable ERRBS coverage in WT and Aicda-/- cells and similar global methylation levels, as measured using LC-MS, in NB from WT and Aicda-/- mice (Figure S1). LC-MS analysis also revealed higher genome-wide levels of 5mC in Aicda-/- GCB compared to WT GCB (Figure S1). Our results indicate that AID is responsible for the majority of the methylome changes that B cells undergo during their transit through the GC.
Figure 1. Loss of AID abrogates CpG methylation changes during GC transition.
(A) Combined CpG methylation of NB and GCB from WT mice (7 replicates; left) and Aicda-/- mice (6 replicates; right), determined by ERRBS using a 20% methylation difference threshold and FDR<0.001, Fisher's exact test. HypoDMCs are indicated in blue and hyperDMCs are indicated in yellow. (B) Number of DMCs between GCB and NB from WT and Aicda-/- mice determined by ERRBS using a 20% methylation difference threshold and FDR<0.001, Fisher's exact test. HypoDMCs are indicated in blue and hyperDMCs are indicated in yellow. A and B show that the DNA methylation changes after GC transition in WT mice are abrogated in Aicda-/- mice (C) Density plot showing delta methylation values (GCB % - NB %) determined by ERRBS in WT and Aicda-/- mice. Aicda-/- delta methylation values are decreased compared to WT, indicating less changes in methylation during GC transition. See also Figure S1.
AID contributes to epigenetic diversity within GCB
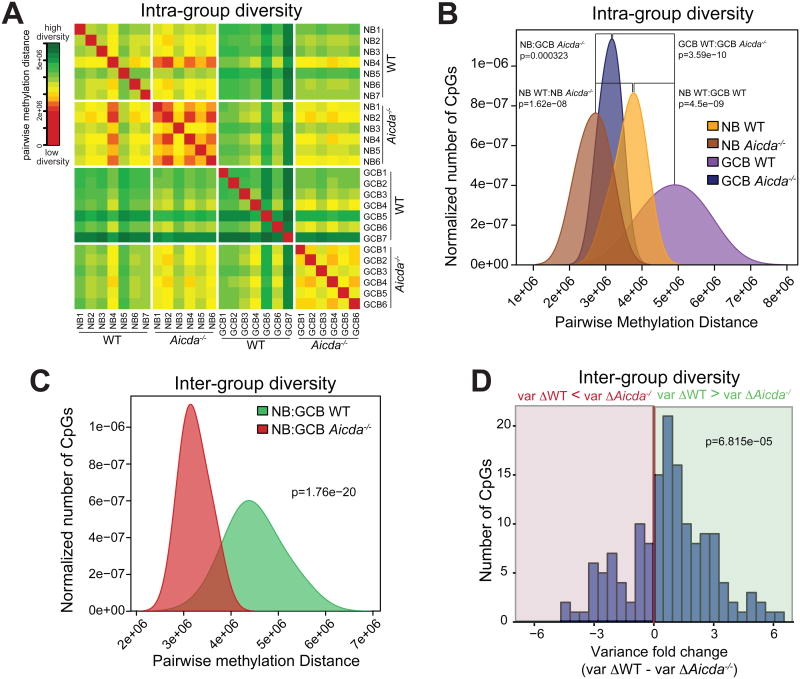
We hypothesized that AID might also be responsible for the increased methylation diversity in GCB compared to NB, previously described (De et al., 2013). To that end, we evaluated the epigenetic diversity within the NB group and GCB group from WT and Aicda-/- mice, calculating all pairwise distances between ERRBS profiles (see Experimental Procedures). We found that WT GCB replicates had greater pairwise methylation distance to each other than WT NB samples had to each other, corresponding to higher average methylation diversity (Figures 2A and 2B NB WT:GCB WT, Wilcoxon p=4.5e-09). This is consistent with epigenetic diversification of B cells during their passage through the GC. Importantly, Aicda-/- GCB replicates displayed significantly lower intra-group methylation distance than WT GCB replicates (Figures 2A and 2B GCB WT:GCB Aicda-/-, Wilcoxon p=3.59e-10), indicating that loss of AID results in a more homogenous GCB methylome, closer to the methylome of Aicda-/- NB (Figure 2A and 2B NB Aicda-/-:GCB Aicda-/-, Wilcoxon p=0.000323). We also found lower pairwise methylation distance during transition from Aicda-/- NB to GCB than from WT NB to GCB (Figure 2C Wilcoxon p=1.76e-20). This decreased diversity is consistent with the abrogation of methylation changes during the NB to GCB transition observed in AID-deficient mice (see Figure 1C).
Figure 2. AID contributes to epigenetic diversity within GCB.
(A) Heat map and (B) density plot showing the intra-group pairwise methylation distance between ERRBS profiles of NB and GCB replicates from WT and Aicda-/- mice. WT GCB have greater pairwise distance than WT NB, indicating increased diversity among methylation profiles. On the contrary, Aicda-/- GCB replicates have lower pairwise distance than their WT counterparts, closer to Aicda-/- NB samples. (C) Density plot comparing pairwise methylation distance between NB and GCB from WT and Aicda-/- mice. NB to GCB transition in Aicda-/- mice is associated with lower pairwise distance than in WT mice, indicating less diversity between Aicda-/- NB and GCB profiles. (D) CpG methylation variance from Mass Array data. The histogram shows the difference in variance between WT and Aicda-/- mice (varゔWT - varゔAicda-/-), being varゔ the log-fold change in methylation variance during NB to GCB transition in WT or Aicda-/- cells. Variance in WT animals is statistically greater than in AID-deficient animals (positive values). See also Figures S2 and S3.
To further validate these findings, we utilized an orthogonal DNA methylation quantification approach based on Sequenom Mass Array Epityping, which detects the mass difference between methylated and unmethylated CpG using MALDI-TOF mass spectrometer. We performed Mass Array on 10 randomly selected genomic loci spanning 30 kbs in 3 replicate pairs of NB and GCB from WT and Aicda-/- mice. We then calculated log-fold change in methylation variance to determine whether methylation diversity increased during NB to GCB transition in WT (varΔWT) and Aicda-/-cells (varΔAicda-/-). Most studied CpGs had greater methylation variance augmentation in WT as compared to Aicda-/- cells (positive values) during GC transition (Figures 2D and S2). These results altogether strongly suggest that, in addition to its established role in SHM and CSR, AID contributes to the diversification of DNA methylation in the GCB genome.
Previous experiments with AID depletion within an ex vivo system showed no methylation changes (Fritz et al., 2013). In order to reconcile our in vivo AID-dependent methylation changes with these earlier observations, we activated CD43- splenic WT cells (NB) in the presence of LPS, IL4 and anti-CD40 and infected them with either empty vector (EV) or a vector expressing the full-length Aicda cDNA (AID). We confirmed by qPCR and Western blot that the AID-overexpressing cells expressed higher levels of AID than EV-infected cells (Figure S3A). Moreover, we detected a higher percentage of class-switched splenocytes when AID was overexpressed (Figures S3B and S3C). We performed ERRBS profiling on sorted GFP+IgG1+ cells (class-switched infected cells) and we observed few differences in methylation between AID-overexpressing and EV-infected B cells, with less than 1000 hyper- and hypoDMCs (Figure S3D). We also calculated the pairwise methylation distance and found a high degree of homogeneity among all sample ERRBS profiles, suggesting that methylation diversity between AID-overexpressing and EV-infected B cells was similar, and comparable to NB diversity (Figure S3E). These results support the previous findings obtained by Fritz et al. (Fritz et al., 2013). The ex vivo stimulated B cells differ from GCB, in that they present significantly reduced levels of SHM -targeting predominantly the Sμ region- compared to GCB induced in vivo (Liu et al., 2008; McKean et al., 1984; Robbiani et al., 2009). Reduced rate of SHM and the absence of methylome modifications in ex vivo activated B cells support the hypothesis of convergence of the mechanisms for SHM and methylome editing.
Methylation changes in Aicda-/- GCB are not due to changes in the cellular composition or clonality within the GC
In order to rule out the possibility that abrogation of methylation changes in Aicda-/- GCB arises due to changes in the cellular composition within the GC (content of centroblasts, CB, vs centrocytes, CC) or clonal diversity, we carried out detailed analysis of the GCB from WT and Aicda-/- animals. Flow cytometry analysis of the spleen confirmed hyperplasia of the GC in Aicda-/- mice (Figure S3F), in agreement with previous reports (Muramatsu et al., 2000; Robbiani et al., 2009). Both WT and Aicda-/- animals had the same proportion of CB (CXCR4high) and CC (CXCR4low) within the GC (Figure S3G). To confirm this result, we performed RNA sequencing (RNAseq) on GCB isolated from WT and Aicda-/- mice and compared their expression profiles for the genes that constitute the CB (dark zone, DZ) and CC (light zone, LZ) signatures, identified by Victora et al. (Victora et al., 2010) (Figure S3H). The expression for these CB- and CC-specific genes was highly correlated between WT and Aicda-/- cells (Pearson correlation coefficient=0.984 for DZ genes and 0.989 for LZ genes), indicating that both genotypes had comparable gene expression profiles. In addition, we investigated whether there were any differences in clonal complexity in the GC between WT and Aicda-/- mice. For that purpose, we amplified rearranged IgH, Igκ and Igλ regions using primers capturing the most abundant families of Ig rearrangements (Chang et al., 1992; Cobaleda et al., 2007; Schlissel et al., 1991) and performed high-throughput sequencing using the Illumina MiSeq (PE2×150). Statistical analysis of the Ig rearrangements (see Experimental Procedures) revealed no significant difference in clonal complexity and or composition of VH regions between WT GCB and Aicda-/- GCB (p=0.8571 Wilcoxon rank sum test) (Figure S3I). Altogether, these findings indicate that the composition and the clonality of WT and AID-deficient GCB are equivalent.
AID-dependent hypoDMCs are enriched at SHM hotspot genes and dsDNA breaks
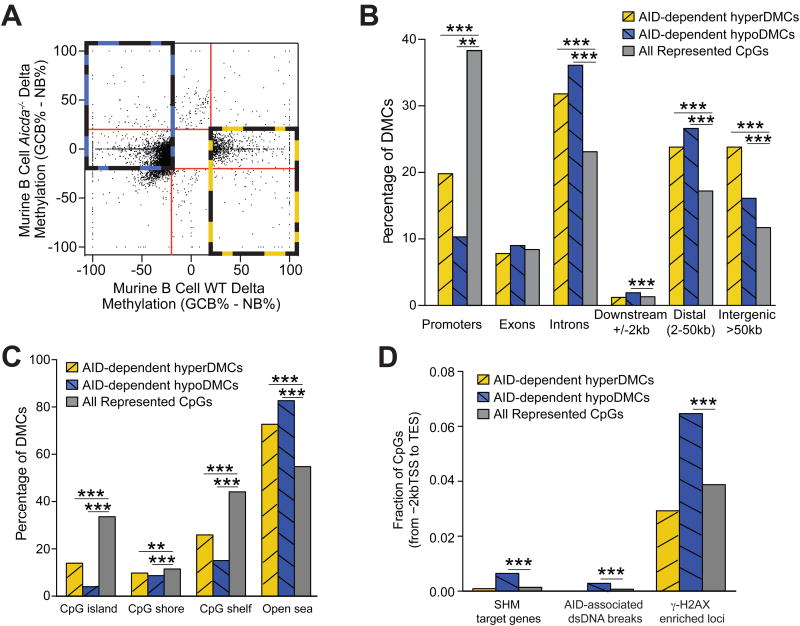
To investigate the genomic distribution of AID-dependent methylation changes, we defined AID-dependent hypo- and hyperDMCs as CpGs that are hypomethylated (blue rectangle, Figure 3A) or hypermethylated (yellow rectangle, Figure 3A) during NB to GCB transition in WT animals, but show no respective differential methylation changes in Aicda-/- animals. We found that these AID-dependent hypo- and hyperDMCs were significantly depleted in promoters of genes and enriched in introns and intergenic areas (binomial test, p< 0.001) (Figure 3B). AID-dependent hypo- and hyperDMCs were also depleted in CpG islands, shores and shelves and enriched in open sea (binomial test, p <0.001) (Figure 3C). Since AID-dependent DNA demethylation is thought to be carried out via deamination and subsequent DNA repair, similar to SHM, we investigated whether AID-dependent DMCs were enriched in genes reported to be targets of SHM in the GCB (Liu et al., 2008). Interestingly, AID-dependent hypoDMCs were enriched in SHM hotspot genes (binomial test, p <0.001) (Figure 3D). As SHM occurs at highly expressed genes, we also tested if AID-dependent DMCs were enriched in genes highly expressed in WT GCB (FPKM>20). We found no enrichment for hypoDMCs (data not shown), suggesting that hypomethylation results from AID targeting specific genomic loci, not simply as a consequence of open chromatin structure or regions with high transcriptional activity. We also found enrichment of AID-dependent hypoDMCs in AID-associated dsDNA breaks identified using high throughput genomic translocation sequencing (Meng et al., 2014) (Fisher exact test, p<0.001) (Figure 3D) and in loci associated with double strand breaks defined through γ-H2AX occupancy (Barlow et al., 2013) (Fisher exact test, p<0.001) (Figure 3D), suggesting an association between AID-dependent hypomethylation and DNA breaks.
Figure 3. AID-dependent DNA methylation changes show characteristic distribution.
(A) Scatterplot comparing delta methylation (GCB % - NB %) of WT versus Aicda-/- mice. AID-dependent hypoDMCs are indicated by yellow dash box and hyperDMCs by blue dash box. (B) Bar plot showing genomic distribution of AID-dependent hypoDMCs and hyperDMCs as well as all CpGs represented within ERRBS experiments. AID-dependent DMCs are depleted within promoters and enriched for presence in introns, distal and intergenic regions. (C) Bar plot showing the distribution of AID-dependent hypoDMCs and hyperDMCs within CpG islands, shores, shelves and open sea. AID-dependent DMCs are depleted in CpG islands, shores and shelves, and are enriched in CpG open sea. (D) Fraction of AID-dependent DMCs enriched in SHM hotspot genes, AID-mediated dsDNA breaks and loci with γH2AX occupancy (*p<0.05, **p<0.01, ***p<0.001). See also Figure S4.
The AID/APOBEC family of proteins has been known to contribute to intrinsic immunity against retrotransposition of endogenous and exogenous retroviruses (Goodier and Kazazian, 2008). Endogenous retroviruses are present in multiple copies in mammalian genomes and constitute up to a staggering 8% of human and mouse genomic DNA (Ryan, 2004; Stocking and Kozak, 2008). As AID-dependent DMCs are enriched in introns and intergenic regions (Figure 3B), we investigated whether AID targets repetitive elements present in those regions of the genome. We annotated our AID-dependent DMCs according to RepeatMasker (see Experimental Procedures) and we identified six intergenic repetitive elements that were significantly enriched for the presence of AID-dependent DMCs, including L1 repeat element, IAPEY3_LTR, and MLT1J1 (Figure S4A). We also found two intragenic repetitive elements that were significantly enriched to contain AID-dependent DMCs (Figure S4B). In summary, although we do find cases of enrichment at specific repetitive elements, AID acts mostly upon non-repetitive DNA sequences.
Epigenetic hotspots and effect on gene expression
We next investigated if, similar to SHM targets, there were hotspots of AID epigenetic activity in GCB. In order to identify such “epigenetic hotspots” we looked for differentially methylated regions (DMRs) based on the presence of at least five DMCs, a maximum distance of 250 kb between DMCs, and at least 10% difference between average methylation within the region. We identified 119 DMRs between NB and GCB from WT mice, distributed throughout all chromosomes and consisting predominantly of hypoDMRs (Figure 4A, left). We observed that these DMRs were mostly AID-dependent and 88 hypoDMRs and 16 hyperDMRs were lost in Aicda-/- animals (Figure 4A, right, and Table S1), in agreement with our results from the analysis of DMCs (Figure 1). We validated these findings using Mass Array Epityper on several loci and on replicates of WT and AID-deficient NB and GCB. Figure S5A shows that the transition from NB to GCB in Aicda-/- mice is accompanied by lower demethylation in AID-dependent DMRs compared to WT mice. As a result, the percentage of methylation in Aicda-/- GCB is statistically higher than in WT GCB- although still lower than in Aicda-/- NB due to AID-independent demethylation mechanisms (Figure S5A).
Figure 4. AID epigenetic hotspots and effect on gene expression.
(A) Ideogram of DMRs in WT (left) and Aicda-/- (right) cells, showing depletion of DMRs in Aicda-/- cells. HypoDMRs are indicated in blue and hyperDMRs are indicated in yellow. (B) Genes overlapping AID-dependent hypoDMRs with >1.2-fold GCB/NB expression ratio between WT and Aicda-/- cells. Up-regulated genes in Aicda-/-cells are shown in red and down-regulated genes in Aicda-/- cells in are shown in blue. (C) Pathway analysis (IPA) of the genes overlapping AID-dependent hypoDMRs. The graph shows the name and the functional annotations of the genes with hypoDMRs in WT cells that are absent in Aicda-/- cells. (D) Heat map showing significance of enrichment of the pathways identified by IPA, using hypergeometric p values. (E) Density plot showing expression correlation distance for genes overlapping AID-dependent hypoDMRs between WT and Aicda-/- GCB replicates. Variability of gene expression is lower in Aicda-/- GCB. See also Figure S5.
In order to understand the significance of AID-dependent methylation changes, we evaluated the effect on gene expression in NB and GCB using RNAseq data for all RefSeq genes. We found that, although there is a clear difference in expression profiles according to cell type, there is no significant difference in profiles according to AID genotype (Figure S5B and S5C). However, we did identify several genes containing AID-dependent hypoDMRs, including B cell relevant genes (e.g. Cdk6, Abcc4, and Fanca) and known targets of SHM (e.g. Pa×5 and Cd83), that exhibit small gene expression differences between the respective genotypes (Figure 4B). These genes represent epigenetic targets of AID, which may be deregulated not just by SHM or translocations, but also via changes in DNA methylation. We also analyzed the expression of genes that have been implicated in DNA methylation (DNMTs, TETs and IDHs) and found no significant difference between WT and Aicda-/- cells (data not shown). An unsupervised pathway analysis revealed that AID-dependent hypomethylated genes are involved in pathways critical for B lymphocyte development, such as proliferation, B cell commitment, lymphocyte arrest of differentiation, and antibody response (Figures 4C and 4D). To assess whether DMR-associated genes have decreased diversity of expression in Aicda-/- GCB, we calculated inter-sample pairwise correlation distances based on the expression of genes associated with AID-dependent hypoDMRs. We observed that AID-dependent hypoDMR-associated genes show a trend toward decreased heterogeneity of expression in the Aicda-/- GCB compared to WT GCB (Figure 4E). Even though this change is not statistically significant, the potential biological consequence of epigenetic diversification may be deregulated gene expression and predisposition toward neoplastic transformation (Hansen et al., 2011).
We further explored the phenotypical consequences of the AID-dependent DNA methylation diversity. We hypothesized that if CpG methylation diversity was functional, it would not be randomly distributed throughout the genome but rather associated with specific gene expression patterns and with specific gene function. To address this, we investigated whether the GCB methylome contained any hotspots for CpGs with high methylation variability. We defined “divergent CpG hotspots” as contiguous 1kb regions enriched for divergent CpGs (methylation interquartile range >25% among replicates; (Kemp et al., 2014)) using a hypergeometric mean distribution test. We found 6,952 divergent CpG hotspots in WT GCB replicates and only 788 hotspots in Aicda-/- GCB (Figure S5D). We also observed that 96.7% (n=6,725) of these divergent CpG hotspots were AID-dependent, as they were lost in the Aicda-/- animals (Figure S5D). Interestingly, AID-dependent divergent CpG hotspots were located mostly in introns, distal regions and intergenic areas (Figure S5E), similarly to AID-dependent DMCs (Figure 3B). A gene expression analysis revealed that Aicda-/- GCB had higher expression in genes overlapping divergent CpG hotspots (n=3,055) than WT GCB (gene set enrichment analysis (GSEA) false discovery rate (FDR) q < 0.001; Figure S5F). Pathway analysis of these genes showed enrichment in pathways regulating caspase activity and innate and adaptive immune responses (Figure S5G).
To further elucidate the biological consequences of the AID-mediated epigenetic changes in the GCB genome, we investigated whether the methylome differences in WT and Aicda-/- GCB affected the proliferation and/or the differentiation capacity of these populations. We observed no differences in the cell cycle distribution based on BrdU incorporation and 7-AAD staining of GCBs from WT and Aicda-/- mice (Figure S5H). Although there was an increase in the percentage of GCB in the Aicda-/- mice, we observed a reduction in the NP-specific population, indicating a defect in the formation of antigen-specific GCB in the absence of AID (Figure S5I). We also found that Aicda-/- GCB presented lower expression of the genes that are normally expressed upon terminal differentiation into plasma and memory cells when compared to WT GCB (Figure S5J). Even though the cause of such effect on plasmacytic differentiation in Aicda-/- mice is rooted in the absence of AID-dependent affinity maturation, further investigation of the contribution of DNA methylation to this process is warranted.
Conserved epigenetic function of AID between human and mouse B cells
To investigate whether the AID epigenetic program in mouse GC was conserved in human GC, we sorted NB (CD20+IgD+CD77-) and GCB (CD20+IgD-CD77+) from reactive human tonsils (see Experimental Procedures) and profiled their methylome using ERRBS. Using the same criteria applied to mouse data to call DMCs, we confirmed that human GCB also underwent extensive hypomethylation compared to NB and displayed greater epigenetic diversity than NB (Figures 5A and 5B). Although we identified a greater number of DMCs in the human NB to GCB transition (69,277 hypoDMCs and 5,991 hyperDMCs), the genome-wide distribution of human DMCs was very similar to the distribution of murine DMCs, with enrichment in introns and depletion in CpG islands, CpG shores and CpG shelves (Figures 5C and 5D). To address whether the methylation changes underlying the GC transition in human cells affected the same AID-epigenetic targets, we characterized DMRs between human NB and GCB, applying the same criteria than in mouse samples, and assessed the significance of overlap between orthologs of murine genes associated with AID-dependent DMRs and human genes associated with DMRs. Remarkably, we observed a significant overlap between murine AID-dependent hypoDMR-associated gene orthologs and human genes containing hypoDMRs (p=5.73 e-08) (Figure 5E). The comparability of epigenetic reprogramming between mouse and human GC suggests that epigenetic changes associated with the NB to GCB transition are conserved between species, similar to conservation of the transcriptional programming.
Figure 5. Conserved epigenetic function of AID between human and mouse B cells.
(A) Combined CpG methylation values (top) and DMCs (bottom) between NB and GCB from human tonsils (4 replicates), determined by ERRBS using a 20% methylation difference threshold and FDR<0.001, Fisher's exact test. Hypomethylated CpGs are indicated in blue and hypermethylated CpGs are indicated in yellow. (B) Heat map showing pairwise methylation distance between ERRBS profiles of NB and GCB replicates from human tonsils with GCBs showing greater intra-sample methylation distance (green vs yellow color). (C) Bar plot showing genomic distribution of hypoDMCs and hyperDMCs as well as all CpGs represented within ERRBS experiments. DMCs show depletion in promoters and enrichment in introns, distal and intergenic regions. (D) Bar plot showing distribution of hypoDMCs and hyperDMCs within CpG islands, shores, shelves and open sea. DMCs are depleted within CpG islands and shelves and enriched within CpG open sea. (E) Heatmap showing significance of overlap between murine AID-dependent hypoDMR-associated genes and human hypoDMR-associated genes (*p<0.05, **p<0.01, ***p<0.001).
Discussion
In the present study, we have demonstrated using a genome-wide approach that B cell transit through the GC is accompanied by locus-specific hypomethylation and minor gains of methylation, along with a substantial increase in DNA methylation diversity. More importantly, our results indicate that such changes are largely mediated by AID. In the last decade, we have gained in-depth knowledge regarding the function of epigenetic alterations in normal development and cancer biology. DNA methylation is understood to play a key role in gene imprinting, X chromosome inactivation, and regulation of gene expression specific to tissue identity, developmental stage and cell lineage (Bird, 2002; Jones and Takai, 2001). Changes in the DNA methylome mark specific stages of B cell ontogeny and play an important role in B cell lymphomagenesis (De et al., 2013; Jeong et al., 2014; Kulis et al., 2012; Lai et al., 2013; Mayle et al., 2014; Shaknovich et al., 2010; Zhang et al., 2014). The GC stage of B cell development is associated with a proliferative burst, affinity maturation of B cells with associated SHM and CSR, all of which contribute to adaptive immune response and determine antibody diversity (Klein and Dalla-Favera, 2008), but the contribution of the methylome to these processes is not clearly defined. Previously, we showed that changes in methylation are required for the successful formation of the GC and that such modifications are dependent on DNMT1, a methyltransferase highly expressed in the GCB (Shaknovich et al., 2011). While the mechanism of DNA methylation gain is well understood, the mechanism of demethylation, the factors responsible for the loss of methylation in GCB, and its biological significance remain almost completely unknown. Since AID is highly expressed in the GCB and has been implicated in DNA demethylation during embryonic development and epigenetic reprogramming (Bhutani et al., 2010; Kumar et al., 2013; Popp et al., 2010; Rai et al., 2008), we hypothesized that AID is involved in the active demethylation of B cells during GC transition.
To prove this hypothesis, we isolated NB and GCB from in vivo WT and Aicda-/- mice and profiled their methylome using ERRBS, a genome-wide approach capable of interrogating three million CpGs. We observed that over 90% of methylome alterations characterizing the transition from NB to GCB were lost in Aicda-/- animals, confirming the role of AID in the DNA demethylation of the GCB genome. We also found that AID-depletion caused loss of hypermethylation in GCB. We suspect this to be a result of reduced recruitment of DNMT1 to double strand breaks (Ha et al., 2011), putatively generated as a consequence of the AID deamination activity (Zan and Casali, 2008). Several prior attempts to link AID to demethylation in GCB were made before. Fritz et al. addressed this same question using an ex vivo system, activating primary splenocytes in the presence of anti-CD40, LPS and IL4 (Fritz et al., 2013). The authors could not detect AID-induced changes in the B cell methylome, consistent with our results with ex vivo stimulated B cells. This suggests that AID-dependent demethylation is coupled to the rate of SHM, which is much lower in the ex vivo system than in GCB (McKean et al., 1984; Robbiani et al., 2008). In this regard, it has recently been demonstrated that ex vivo stimulated B cells are defective in SHM because the initiating form of RNA polymerase II is not retained in the variable regions of the Ig genes, hampering the recruitment of the cofactor Spt5 and AID (Maul et al., 2014). Another attempt to delineate the demethylation function of AID in GCB was made by Hogenbirk et al. using Methyl-Cap-Seq and failed to find any AID-dependent changes (Hogenbirk et al., 2013). Methyl-Cap-Seq is an affinity purification-based technique, which is likely not to be sufficiently sensitive to detect variable methylation changes in CpGs scattered throughout genome. Here we have used ERRBS, a genome-wide technique with higher coverage compared to Methyl-cap-Seq and single nucleotide level resolution (Rodriguez et al., 2012). We think that above differences are due to the experimental system and the techniques used in earlier studies. Confirmation of AID-dependent changes that we identified using Mass Array Epityping validates ERRBS-based findings.
Importantly, we have demonstrated that the epigenetic diversification of the B cell methylome during GC transition is dependent on AID activity. It is tempting to speculate that this methylation diversification may contribute, along with SHM, to clonal evolution among normal GCB. We show here that the genomic distribution of hypoDMRs in GCB is similar to the distribution of AID binding sites revealed by Liu et al. (Liu et al., 2008). We also provide circumstantial evidence that the demethylase function of AID may arise from its deaminase activity, showing that AID-dependent hypoDMCs are enriched within known AID target genes for SHM. Despite expectation that AID-dependent differential methylation would be concentrated around TSS of genes, similar to SHM hotspots, our data reveals that DMCs are enriched in gene introns and intergenic regions. This is consistent with the location of AID-dependent demethylation observed in other systems (Kumar et al., 2013; Popp et al., 2010). We also found enrichment of AID-dependent hypoDMCs at loci associated with dsDNA breaks. It is possible that, despite the intense focus on SHM target genes, AID may bind genome-wide, with the majority of binding similarly distributed outside of TSS and gene bodies. This would suggest AID deamination activity to have more far-reaching consequences than we have yet appreciated. It has been proposed that AID-dependent regions of demethylation may extend beyond the deamination sites as a result of the activity of processive DNA repair pathways (mismatch repair or long-patch base excision repair). These pathways can result in the replacement of long stretches of DNA (up to 2 kb) with concomitant possible repair of all somatic mutations (Franchini et al., 2014). Such broad extension of hypomethylation could, in turn, have various consequences, including instability of transposable elements, chromosomal translocations and gene deregulation, as suggested by the Jaenisch's group (Gaudet et al., 2003).
In the present study, we were able to detect subtle effects of AID on genes associated with AID-dependent DMRs, including greater variability of gene expression of these epigenetic targets and at the same time consistent changes in a handful of epigenetic hotspots that include genes also targeted by SHM, such as Pa×5 and Cd83. The up- and downregulation of gene expression between WT and Aicda-/- cells may be explained by either the DMR location in relation to the transcription start site (TSS): promoter versus gene body methylation may have opposite effects on gene expression (Jaenisch and Bird, 2003), or as a secondary effect of a permissive environment for gene regulation through binding of transcription factors or through histone modifications. There is a significant effect on expression in genes overlapping divergent CpG hotspots -loci with high methylation variability-, indicating that AID-dependent methylation diversity is not random and affects specific functions. There is also a detectable statistically significant hypomethylation of repetitive elements, with still uncertain consequences to be investigated in future work.
Although AID loss abrogates the majority of the methylation changes experienced by GCB, we observe residual hypomethylation in Aicda-/- GCB, suggesting that other demethylation mechanisms are likely to exist in these cells. The most plausible scenario is TET-dependent oxidative demethylation, which has been proposed as an alternative to AID deamination-dependent demethylation (Kohli and Zhang, 2013). Another source of demethylation may be passive loss of methylation in highly proliferative GCB. The fact that Aicda-/- GCB are hyperplastic and highly proliferative, and nevertheless have minimal loss of methylation argues against this theory. Moreover, passive stochastic loss of methylation would likely be randomly distributed throughout the genome and our results show preferential genomic distribution of the methylation changes. All mechanisms will need more formal examination before the final model of DNA demethylation in GCB is formulated, but our results indicate for the fist time a clear epigenetic role for AID in B cell maturation during GC transition.
Experimental Procedures
Mouse and human B-cell isolation
Aicda-/- mice were a generous gift from T. Honjo. WT (BALB/c) mice were from The Jackson Laboratory. All animals were maintained according to the guidelines of the Research Animal Resource Center of the Weill Cornell Medical College, which approved all mouse procedures. Ten-to 12-week-old WT or Aicda-/- mice were immunized intraperitoneally with NP-CGG ratio 20-25 (Biosearch Technologies) in alum (1:1) to induce GC formation. Mice were sacrificed at day 10 after immunization, spleens were dissected and mononuclear cells were purified using Histopaque (Sigma) gradient centrifugation. Cell suspensions were enriched in B cells by positive selection with anti-B220 magnetic microbeads (Miltenyi Biotech). B cells were separated in NB (B220+GL7-FAS-DAPI-) and GCB (B220+GL7+FAS+DAPI-) using a BD FACSAria II sorter.
Leftover human tonsils were obtained after routine tonsillectomies, performed at New York Presbyterian Hospital. All tissue collection was approved by the Weill Cornell Medical College Institutional Review Board. Tonsils were minced and mononuclear cells were isolated using Histopaque density centrifugation. NB were separated by positive selection using AUTOMACS system (Miltenyi Biotech) after incubation with anti-IgD-FITC (BD Pharmingen) followed by anti-FITC microbeads (Miltenyi Biotec). GCB were separated by positive selection with anti-CD77 (AbD Serotec) followed by mouse anti-IgM, IgG1 isotype (BD Pharmingen) and anti-mouse-IgG1 microbeads (Miltenyi Biotec).
Ex vivo activated B-cell cultures
Activation of B cells and infection with EV or AID-expressing vector was performed as described in Supplemental Experimental Procedures.
Flow cytometry analysis. Antibodies
Flow cytometry analysis of mouse NB and GCB was performed using the following fluorescent-labeled anti–mouse antibodies: APC-conjugated anti-B220 (BD Pharmingen), PE-Cy7-conjugated anti-CD95 (BD Pharmingen), FITC-conjugated anti-GL7 (BD Pharmingen) and PE-conjugated CXCR4 (eBioscience). Cell cycle analysis was performed using the BrdU Flow Kit (BD Pharmingen) and antigen-specific GCB (NP+GL7+CD95+B220+) were detected using PE-conjugated NP (biosearch Technologies, Inc.). Ex vivo stimulated B cells were stained with PE-Cy7-conjugated anti-B220 (eBioscience), PE-conjugated anti-IgD (BD Pharmingen) and APC-conjugated anti-IgG1 (BD Pharmingen). DAPI was used for the exclusion of dead cells. Data was acquired on a MACSQuant Analyzer (Miltenyi Biotec) and analyzed using FlowJo 7.6.4 software (TreeStar).
Enhanced reduced representation bisulfite sequencing (ERRBS)
50 ng of genomic DNA were bisulfite converted using the EZ DNA Methylation kit (Zymo Research). Base-pair resolution DNA methylation analysis was performed on WT mice (n=7, 3 male, 4 female) and Aicda-/- mice (n=6, 3 male, 3 female), following the ERRBS protocol previously described(Akalin et al., 2012). DMCs were identified by using a 20% methylation difference threshold and Benjamini-Hochberg adjusted Fisher Exact Test p-value<0.001 on sum of all methylated and unmethyated CpGs among replicate samples. Delta methylation was calculated by subtracting the combined NB methylation percent from the combined GCB methylation percent. DMRs were detected using RRBSseeqer with parameters of 250bp maximum distance between DMCs, minimum of 5 DMCs per region and 10% total methylation difference for region. Ideogram was generated using the ggbio package (Yin et al., 2012) and UCSC GRCm38 CytoBand data from the RCircos package (Zhang et al., 2013). Epigenetic diversity and diverse CpG hotspots were calculated as described in Supplemental Experimental Procedures.
Single locus DNA methylation assays
EpiTYPER assays were performed on bisulfite-converted gDNA. For the biologic validation of the AID-dependent hypoDMRs in mouse B cells, primers were designed to cover CpG islands associated with the respective DMRs. All primers were designed using Sequenom EpiDesigner BETA software (http://www.epidesigner.com/). Primer sequences are shown in Supplemental Experimental Procedures.
Mass array based variance comparisons
We performed 3 pairwise comparisons of methylation variances: 1) AID_KO_NB vs AID_KO_GCB, 2) AID_WT_GCB vs AID_KO_GCB, and 3) AID_WT_NB vs AID_WT_GCB. For each pairwise comparison we calculated the sample variances in each of the two groups across all evaluated CpG sites as described in Supplemental Experimental Procedures.
Cytosine methylation mass spectrometry
1ug of genomic DNA was denatured by heating at 100°C. 5 U of Nuclease P1 (Sigma) were added and the mixture was incubated at 37°C for 2 hours. A 1/10 volume of 1 M Ammonium bicarbonate and 0.002 units of venom phosphodiesterase 1 (Sigma) were added to the mixture and the incubation continued for 2 hours at 37°C. Then, 0.5 U of Alkaline phosphatase (Roche) were added and the mixture was incubated for 1 h at 37°C. Quantification was done using a LC-ESI-MS/MS system (Agilent 1200 Series liquid chromatography machine in tandem with the Agilent 6410 Triple Quad Mass Spectrometer) in multiple reaction monitoring (MRM) mode as described (Song et al., 2005). Chromatographic separation was performed at a flow rate of 220 L/min.
V(D)J rearrangement analysis
Ig rearrangement analysis was performed on gDNA of mouse WT GCB (n=3) and Aicda-/- mice GCB (n=4), amplifying IgH, Igκ and Igλ regions by PCR. Primers annealed to the framework region of the most abundant families of Ig rearrangements, as described previously (Chang et al., 1992; Cobaleda et al., 2007; Schlissel et al., 1991). Primer sequences have been described previously (Hanna et al., 2008). Ig rearrangement analysis was performed as described in Supplemental Experimental Procedures.
RNA sequencing
RNA-seq was carried out as described in Supplemental Experimental Procedures.
Pathway enrichment analysis
Ingenuity Pathway Analysis (IPA, QIAGEN) was used for the functional analysis of the genes containing AID-dependent hypoDMRs. For each pathway, we determined the extent to which the pathway was over-represented in the target gene group, using the hypergeometric distribution test. Pathway enrichment for AID-dependent divergent CpG hotspot genes was performed using the iPAGE program (Goodarzi et al., 2009) and gene sets from the Gene Ontology (electronic annotations were not included).
Quantitative real-time PCR
cDNA synthesis from RNA was performed using the Verso cDNA Synthesis kit (Thermo Scientific). The expression was detected using the Green FastMix kit (Applied Biosystems) on a 7900HT Fast RT-PCR System (Applied Biosystems). Gene expression was normalized to RPL13 using the ΔΔC(t) method and results were represented as fold expression compared to NB. Primer sequences are shown in Supplemental Experimental Procedures.
Immunoblotting
Total cells extracts were prepared after treatment with lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 1% Triton X-100, 0.1% SDS and 1mM EDTA), supplemented with PMSF (Sigma) and a protease inhibitor cocktail (Roche). Lysates were subjected to SDS-PAGE, transferred to a PVDF membrane (Bio-Rad Laboratories) and blotted with anti-AID (L7E7, Cell Signaling Technology) or anti-actin (A5441, Sigma). Signals were detected with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) using the ECL system (Thermo Scientific).
Supplementary Material
Figure S1. Global cytosine methylation levels of WT and Aicda-/- NB and GCB, related to Figure 1. Liquid chromatography-mass spectrometry (LC-MS) was performed in 2 WT NB, 2 Aicda-/- NB, 2 WT GCB and 2 Aicda-/- GCB replicates. The Y-axis depicts the percentage of 5mdC in total cytosines in each condition (*p<0.05, t-test).
Figure S2. Mass Array analysis of methylation variance confirms the results obtained by ERRBS, related to Figure 2. We performed 3 pairwise comparisons of methylation variances: WT_NB vs WT_GCB (A), Aicda-/-_NB vs Aicda-/-_GCB (B) and WT_GCB vs Aicda-/-_GCB (C). For each pairwise comparison we calculated the sample variances in each of the two groups across all evaluated CpGs. The log-ratio of methylation variance in the two groups was calculated and compared using the Wilcoxon signed-rank test to test whether the median log-ratios were equally distributed. We concluded that the methylation variances were different in the two groups for all three comparisons, that is: higher variance in WT GCB compared to WT NB (A), in Aicda-/-_GCB compared to Aicda-/-_NB (B) and in WT_GCB compared to Aicda-/-_GCB (C) (p-values = 0.0002681, 1.503e-06, and 9.969e-12 respectively).
Figure S3. Characterization of AID-overexpressing ex vivo stimulated splenic B cells and AID-deficient in vivo GCB populations, related to Figure 2. (A) AID mRNA (top) and protein (bottom) expression in unstimulated CD43- splenic cells (NB) or stimulated splenic B cells (activated B cells). Activated B cells were stimulated with LPS, IL-4 and anti-CD40 for 96 h and left uninfected (uninf) or were simultaneously infected with either empty vector (EV) or AID-expressing pMIG vector (AID). (B) Flow cytometry analysis of activated B cells. NB were stimulated for 96 h with LPS, IL-4 and anti-CD40 and simultaneously infected with either EV or AID-expressing pMIG vector. Infected cells were GFP+ and the class-switched cells among the GFP+ fraction were identified by IgG1 expression. (C) Percentage of GFP+ IgG1+ splenic B cells stimulated with LPS, IL-4 and anti-CD40 for 96 h and infected with EV or AID-expressing pMIG vector. (D) DMCs between GFP+IgG1+ splenic B cells infected with AID-expressing vector and EV, sorted after 96 h stimulation with LPS, IL-4 and anti-CD40. DMCs were determined by ERRBS using a 20% methylation difference threshold and FDR<0.001, Fisher's exact test. HypoDMCs are indicated in blue and hyperDMCs are indicated in yellow. (E) Heat map showing pairwise methylation distance between ERRBS profiles from NB and GFP+IgG1+ splenic B cells infected with AID-expressing vector or EV, sorted after 96 h stimulation with LPS, IL-4 and anti-CD40. (F) Sorting strategy for NB (B220+GL-7-FAS-) and GCB (B220+GL-7+FAS+) (left) and percentage of GCB (right) from 3 WT and 3 Aicda-/- mice. (G) Percentage of CB and CC according to CXCR4 expression levels in GCB from 3 WT and 3 Aicda-/- mice. Expression of CXCR4 in NB is shown as negative control. (H) Scatterplot comparing the mean expression of CB and CC gene signatures between GCB replicates of WT and Aicda-/- mice. (I) Boxplot showing similarity of clonal composition based on VH rearrangements in WT and Aicda-/- GCB.
Figure S4. AID-dependent changes in DNA methylation targeting repetitive elements, related to Figure 3. (A) Volcano plot identifying intergenic repeats enriched for AID-dependent DMCs. Red lines indicate thresholds for two-fold enrichment versus all represented CpGs and Bonferroni-corrected p<0.05. (B) Volcano plot identifying intragenic repeats enriched for AID-dependent DMCs.
Figure S5. Further evaluation and validation of AID-dependent changes in DNA methylation and its biological consequences, related to Figure 4. (A) Mass Array validation of AID-dependent hypoDMRs. Genes containing AID-dependent DMRs were selected for validation by Mass Array. The results are represented as box plots in which the Y-axis corresponds to methylation value and X-axis corresponds to individual samples of NB and GCB from WT and Aicda-/- mice (Abcc4 2/5 CpGs *p<0.05, Camk2a 5/7 CpGs **p<0.01). (B) Relative expression heatmap showing gene expression of all RefSeq genes between NB and GCB replicates of WT and Aicda-/- mice. Hierarchical clustering using Euclidean distance and Ward's method shows that samples cluster according to cell type, but not according to genotype. (C) Expression correlation distance of all RefSeq genes between GCB replicates from WT and Aicda-/- mice. There is no difference in diversity in gene expression between the genotypes. (D) Bar plot showing number of divergent CpG hotspots in WT GCB and Aicda-/- GCB. (E) Genomic distribution of AID-dependent divergent CpG hotspots. (F) GSEA plot showing that AID-dependent divergent CpG hotspot genes are enriched for higher expression in Aicda-/- GCB compared to WT GCB. (G) Heatmap showing pathway enrichment for AID-dependent divergent CpG hotspot genes. Pathway analysis was performed using the iPAGE program (Goodarzi et al., 2009) and gene sets from the Gene Ontology (electronic annotations were not included). (H) The percentages of cells in G0/G1, S and G2/M phases -detected by BrdU incorporation and 7-AAD staining- are similar in WT GCB (n=5) and Aicda-/- GCB (n=5). (I) The percentage of GCB (B220+GL7+CD95+) in Aicda-/- mice is higher than in WT mice (left) (***p<0.001, t-test), but the percentage of antigen-specific GCB (NP+ GCB) is lower in Aicda-/- mice. (J) GSEA showing lower expression of the plasma/memory cell signature genes in Aicda-/- GCB than in WT GCB.
Table S1. List of AID-dependent DMRs, related to Figure 4.
Acknowledgments
We would like to thank T. Honjo (Kyoto University Graduate School of Medicine) for Aicda-/- mice, members of Melnick laboratory (WCMC) for useful discussions and suggestions, H. Geng (UCSF) for helping with human data analysis, Epigenomics Core of WCMC for ERRBS and RNAseq and J. McCormick (Cell Sorting Core of WCMC) for cell sorting. P.M.D. is supported by STARR Cancer Consortium grant; R.S. is supported by STARR Cancer Consortium grant and LLS TRP grant 6304-11. O. E. is supported by NSF CAREER, LLS SCOR, Hirschl Trust Award, Starr Cancer Consortium I6-A618, NIH 1R01CA194547.
Footnotes
Accession Numbers: The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE71702.
Supplemental Information: Supplemental Information includes five figures and one table and can be found with this article online
Author Contributions: P.M.D. and M. T. performed the experiments, analyzed the data and wrote the manuscript; N.C., D.R. and M.K. contributed to the bioinformatics analysis; J.I. prepared the RNA samples and generated the RNAseq libraries; B.V. and J. C. provided reagents and contributed to the design of the experiments; A.M. provided materials and tools and contributed to the design of the experiments; A.V. and L.G. performed the LC-MS experiments; N. P. provided data and contributed to the design of the experiments; O.E and R.S. conceived the experiments and wrote the manuscript.
Competing Financial Interests: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akalin A, Garrett-Bakelman FE, Kormaksson M, Busuttil J, Zhang L, Khrebtukova I, Milne TA, Huang Y, Biswas D, Hess JL, et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet. 2012;8:e1002781. doi: 10.1371/journal.pgen.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JH, Faryabi RB, Callen E, Wong N, Malhowski A, Chen HT, Gutierrez-Cruz G, Sun HW, McKinnon P, Wright G, et al. Identification of early replicating fragile sites that contribute to genome instability. Cell. 2013;152:620–632. doi: 10.1016/j.cell.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes & development. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Bunting KL, Melnick AM. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Curr Opin Immunol. 2013;25:339–346. doi: 10.1016/j.coi.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Paige CJ, Wu GE. Enumeration and characterization of DJH structures in mouse fetal liver. EMBO J. 1992;11:1891–1899. doi: 10.1002/j.1460-2075.1992.tb05241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- Conticello SG. The AID/APOBEC family of nucleic acid mutators. Genome biology. 2008;9:229. doi: 10.1186/gb-2008-9-6-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Shaknovich R, Riester M, Elemento O, Geng H, Kormaksson M, Jiang Y, Woolcock B, Johnson N, Polo JM, et al. Aberration in DNA methylation in B-cell lymphomas has a complex origin and increases with disease severity. PLoS Genet. 2013;9:e1003137. doi: 10.1371/journal.pgen.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini DM, Chan CF, Morgan H, Incorvaia E, Rangam G, Dean W, Santos F, Reik W, Petersen-Mahrt SK. Processive DNA demethylation via DNA deaminase-induced lesion resolution. PloS one. 2014;9:e97754. doi: 10.1371/journal.pone.0097754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz EL, Rosenberg BR, Lay K, Mihailovic A, Tuschl T, Papavasiliou FN. A comprehensive analysis of the effects of the deaminase AID on the transcriptome and methylome of activated B cells. Nature immunology. 2013;14:749–755. doi: 10.1038/ni.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- Goodarzi H, Elemento O, Tavazoie S. Revealing global regulatory perturbations across human cancers. Mol Cell. 2009;36:900–911. doi: 10.1016/j.molcel.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodier JL, Kazazian HH., Jr Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell. 2008;135:23–35. doi: 10.1016/j.cell.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Ha K, Lee GE, Palii SS, Brown KD, Takeda Y, Liu K, Bhalla KN, Robertson KD. Rapid and transient recruitment of DNMT1 to DNA double-strand breaks is mediated by its interaction with multiple components of the DNA damage response machinery. Human molecular genetics. 2011;20:126–140. doi: 10.1093/hmg/ddq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, McDonald OG, Wen B, Wu H, Liu Y, Diep D, et al. Increased methylation variation in epigenetic domains across cancer types. Nature genetics. 2011;43:768–775. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges E, Molaro A, Dos Santos CO, Thekkat P, Song Q, Uren PJ, Park J, Butler J, Rafii S, McCombie WR, et al. Directional DNA methylation changes and complex intermediate states accompany lineage specificity in the adult hematopoietic compartment. Mol Cell. 2011;44:17–28. doi: 10.1016/j.molcel.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenbirk MA, Heideman MR, Velds A, van den Berk PC, Kerkhoven RM, van Steensel B, Jacobs H. Differential programming of B cells in AID deficient mice. PloS one. 2013;8:e69815. doi: 10.1371/journal.pone.0069815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T, Nagaoka H, Shinkura R, Muramatsu M. AID to overcome the limitations of genomic information. Nature immunology. 2005;6:655–661. doi: 10.1038/ni1218. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33 Suppl:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jeong M, Sun D, Luo M, Huang Y, Challen GA, Rodriguez B, Zhang X, Chavez L, Wang H, Hannah R, et al. Large conserved domains of low DNA methylation maintained by Dnmt3a. Nature genetics. 2014;46:17–23. doi: 10.1038/ng.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ehrlich LI, Seita J, Murakami P, Doi A, Lindau P, Lee H, Aryee MJ, Irizarry RA, Kim K, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Kemp CJ, Moore JM, Moser R, Bernard B, Teater M, Smith LE, Rabaia NA, Gurley KE, Guinney J, Busch SE, et al. CTCF haploinsufficiency destabilizes DNA methylation and predisposes to cancer. Cell reports. 2014;7:1020–1029. doi: 10.1016/j.celrep.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulis M, Heath S, Bibikova M, Queiros AC, Navarro A, Clot G, Martinez-Trillos A, Castellano G, Brun-Heath I, Pinyol M, et al. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nature genetics. 2012;44:1236–1242. doi: 10.1038/ng.2443. [DOI] [PubMed] [Google Scholar]
- Kumar R, DiMenna L, Schrode N, Liu TC, Franck P, Munoz-Descalzo S, Hadjantonakis AK, Zarrin AA, Chaudhuri J, Elemento O, et al. AID stabilizes stem-cell phenotype by removing epigenetic memory of pluripotency genes. Nature. 2013;500:89–92. doi: 10.1038/nature12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- Lai AY, Mav D, Shah R, Grimm SA, Phadke D, Hatzi K, Melnick A, Geigerman C, Sobol SE, Jaye DL, et al. DNA methylation profiling in human B cells reveals immune regulatory elements and epigenetic plasticity at Alu elements during B-cell activation. Genome research. 2013;23:2030–2041. doi: 10.1101/gr.155473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, Schatz DG. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- Maul RW, Cao Z, Venkataraman L, Giorgetti CA, Press JL, Denizot Y, Du H, Sen R, Gearhart PJ. Spt5 accumulation at variable genes distinguishes somatic hypermutation in germinal center B cells from ex vivo-activated cells. The Journal of experimental medicine. 2014;211:2297–2306. doi: 10.1084/jem.20131512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV, Challen GA, Li W, Wheeler D, Rebel VI, et al. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood. 2014 doi: 10.1182/blood-2014-08-594648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean D, Huppi K, Bell M, Staudt L, Gerhard W, Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng FL, Du Z, Federation A, Hu J, Wang Q, Kieffer-Kwon KR, Meyers RM, Amor C, Wasserman CR, Neuberg D, et al. Convergent transcription at intragenic super-enhancers targets AID-initiated genomic instability. Cell. 2014;159:1538–1548. doi: 10.1016/j.cell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Novik KL, Nimmrich I, Genc B, Maier S, Piepenbrock C, Olek A, Beck S. Epigenomics: genome-wide study of methylation phenomena. Current issues in molecular biology. 2002;4:111–128. [PubMed] [Google Scholar]
- Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–1212. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-San-Martin B, Difilippantonio S, Hanitsch L, Masilamani RF, Nussenzweig A, Nussenzweig MC. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. The Journal of experimental medicine. 2003;197:1767–1778. doi: 10.1084/jem.20030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, Bolland DJ, Chen HT, Corcoran AE, Nussenzweig A, et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–1038. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Bunting S, Feldhahn N, Bothmer A, Camps J, Deroubaix S, McBride KM, Klein IA, Stone G, Eisenreich TR, et al. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol Cell. 2009;36:631–641. doi: 10.1016/j.molcel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez BA, Frankhouser D, Murphy M, Trimarchi M, Tam HH, Curfman J, Huang R, Chan MW, Lai HC, Parikh D, et al. Methods for high-throughput MethylCap-Seq data analysis. BMC genomics. 2012;13 Suppl 6:S14. doi: 10.1186/1471-2164-13-S6-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan FP. Human endogenous retroviruses in health and disease: a symbiotic perspective. Journal of the Royal Society of Medicine. 2004;97:560–565. doi: 10.1258/jrsm.97.12.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel MS, Corcoran LM, Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. The Journal of experimental medicine. 1991;173:711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaknovich R, Cerchietti L, Tsikitas L, Kormaksson M, De S, Figueroa ME, Ballon G, Yang SN, Weinhold N, Reimers M, et al. DNA methyltransferase 1 and DNA methylation patterning contribute to germinal center B-cell differentiation. Blood. 2011;118:3559–3569. doi: 10.1182/blood-2011-06-357996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaknovich R, Geng H, Johnson NA, Tsikitas L, Cerchietti L, Greally JM, Gascoyne RD, Elemento O, Melnick A. DNA methylation signatures define molecular subtypes of diffuse large B-cell lymphoma. Blood. 2010;116:e81–89. doi: 10.1182/blood-2010-05-285320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nature reviews Genetics. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- Song L, James SR, Kazim L, Karpf AR. Specific method for the determination of genomic DNA methylation by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Chem. 2005;77:504–510. doi: 10.1021/ac0489420. [DOI] [PubMed] [Google Scholar]
- Stocking C, Kozak CA. Murine endogenous retroviruses. Cellular and molecular life sciences : CMLS. 2008;65:3383–3398. doi: 10.1007/s00018-008-8497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane A, Resch W, Kuo N, Kuchen S, Li Z, Sun HW, Robbiani DF, McBride K, Nussenzweig MC, Casellas R. Deep-sequencing identification of the genomic targets of the cytidine deaminase AID and its cofactor RPA in B lymphocytes. Nature immunology. 2011;12:62–69. doi: 10.1038/ni.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Cook D, Lawrence M. ggbio: an R package for extending the grammar of graphics for genomic data. Genome biology. 2012;13:R77. doi: 10.1186/gb-2012-13-8-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan H, Casali P. AID- and Ung-dependent generation of staggered double-strand DNA breaks in immunoglobulin class switch DNA recombination: a post-cleavage role for AID. Molecular immunology. 2008;46:45–61. doi: 10.1016/j.molimm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan H, Casali P. Regulation of Aicda expression and AID activity. Autoimmunity. 2013;46:83–101. doi: 10.3109/08916934.2012.749244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Meltzer P, Davis S. RCircos: an R package for Circos 2D track plots. BMC bioinformatics. 2013;14:244. doi: 10.1186/1471-2105-14-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jima D, Moffitt AB, Liu Q, Czader M, Hsi ED, Fedoriw Y, Dunphy CH, Richards KL, Gill JI, et al. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood. 2014;123:2988–2996. doi: 10.1182/blood-2013-07-517177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Vuong BQ, Vaidyanathan B, Lin JY, Huang FT, Chaudhuri J. Non-coding RNA Generated following Lariat Debranching Mediates Targeting of AID to DNA. Cell. 2015;161:762–773. doi: 10.1016/j.cell.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbauer M, Rayner TF, Clark C, Coffey AJ, Joyce CJ, Palta P, Palotie A, Lyons PA, Smith KG. Genome-wide methylation analyses of primary human leukocyte subsets identifies functionally important cell-type-specific hypomethylated regions. Blood. 2013;122:e52–60. doi: 10.1182/blood-2013-05-503201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Global cytosine methylation levels of WT and Aicda-/- NB and GCB, related to Figure 1. Liquid chromatography-mass spectrometry (LC-MS) was performed in 2 WT NB, 2 Aicda-/- NB, 2 WT GCB and 2 Aicda-/- GCB replicates. The Y-axis depicts the percentage of 5mdC in total cytosines in each condition (*p<0.05, t-test).
Figure S2. Mass Array analysis of methylation variance confirms the results obtained by ERRBS, related to Figure 2. We performed 3 pairwise comparisons of methylation variances: WT_NB vs WT_GCB (A), Aicda-/-_NB vs Aicda-/-_GCB (B) and WT_GCB vs Aicda-/-_GCB (C). For each pairwise comparison we calculated the sample variances in each of the two groups across all evaluated CpGs. The log-ratio of methylation variance in the two groups was calculated and compared using the Wilcoxon signed-rank test to test whether the median log-ratios were equally distributed. We concluded that the methylation variances were different in the two groups for all three comparisons, that is: higher variance in WT GCB compared to WT NB (A), in Aicda-/-_GCB compared to Aicda-/-_NB (B) and in WT_GCB compared to Aicda-/-_GCB (C) (p-values = 0.0002681, 1.503e-06, and 9.969e-12 respectively).
Figure S3. Characterization of AID-overexpressing ex vivo stimulated splenic B cells and AID-deficient in vivo GCB populations, related to Figure 2. (A) AID mRNA (top) and protein (bottom) expression in unstimulated CD43- splenic cells (NB) or stimulated splenic B cells (activated B cells). Activated B cells were stimulated with LPS, IL-4 and anti-CD40 for 96 h and left uninfected (uninf) or were simultaneously infected with either empty vector (EV) or AID-expressing pMIG vector (AID). (B) Flow cytometry analysis of activated B cells. NB were stimulated for 96 h with LPS, IL-4 and anti-CD40 and simultaneously infected with either EV or AID-expressing pMIG vector. Infected cells were GFP+ and the class-switched cells among the GFP+ fraction were identified by IgG1 expression. (C) Percentage of GFP+ IgG1+ splenic B cells stimulated with LPS, IL-4 and anti-CD40 for 96 h and infected with EV or AID-expressing pMIG vector. (D) DMCs between GFP+IgG1+ splenic B cells infected with AID-expressing vector and EV, sorted after 96 h stimulation with LPS, IL-4 and anti-CD40. DMCs were determined by ERRBS using a 20% methylation difference threshold and FDR<0.001, Fisher's exact test. HypoDMCs are indicated in blue and hyperDMCs are indicated in yellow. (E) Heat map showing pairwise methylation distance between ERRBS profiles from NB and GFP+IgG1+ splenic B cells infected with AID-expressing vector or EV, sorted after 96 h stimulation with LPS, IL-4 and anti-CD40. (F) Sorting strategy for NB (B220+GL-7-FAS-) and GCB (B220+GL-7+FAS+) (left) and percentage of GCB (right) from 3 WT and 3 Aicda-/- mice. (G) Percentage of CB and CC according to CXCR4 expression levels in GCB from 3 WT and 3 Aicda-/- mice. Expression of CXCR4 in NB is shown as negative control. (H) Scatterplot comparing the mean expression of CB and CC gene signatures between GCB replicates of WT and Aicda-/- mice. (I) Boxplot showing similarity of clonal composition based on VH rearrangements in WT and Aicda-/- GCB.
Figure S4. AID-dependent changes in DNA methylation targeting repetitive elements, related to Figure 3. (A) Volcano plot identifying intergenic repeats enriched for AID-dependent DMCs. Red lines indicate thresholds for two-fold enrichment versus all represented CpGs and Bonferroni-corrected p<0.05. (B) Volcano plot identifying intragenic repeats enriched for AID-dependent DMCs.
Figure S5. Further evaluation and validation of AID-dependent changes in DNA methylation and its biological consequences, related to Figure 4. (A) Mass Array validation of AID-dependent hypoDMRs. Genes containing AID-dependent DMRs were selected for validation by Mass Array. The results are represented as box plots in which the Y-axis corresponds to methylation value and X-axis corresponds to individual samples of NB and GCB from WT and Aicda-/- mice (Abcc4 2/5 CpGs *p<0.05, Camk2a 5/7 CpGs **p<0.01). (B) Relative expression heatmap showing gene expression of all RefSeq genes between NB and GCB replicates of WT and Aicda-/- mice. Hierarchical clustering using Euclidean distance and Ward's method shows that samples cluster according to cell type, but not according to genotype. (C) Expression correlation distance of all RefSeq genes between GCB replicates from WT and Aicda-/- mice. There is no difference in diversity in gene expression between the genotypes. (D) Bar plot showing number of divergent CpG hotspots in WT GCB and Aicda-/- GCB. (E) Genomic distribution of AID-dependent divergent CpG hotspots. (F) GSEA plot showing that AID-dependent divergent CpG hotspot genes are enriched for higher expression in Aicda-/- GCB compared to WT GCB. (G) Heatmap showing pathway enrichment for AID-dependent divergent CpG hotspot genes. Pathway analysis was performed using the iPAGE program (Goodarzi et al., 2009) and gene sets from the Gene Ontology (electronic annotations were not included). (H) The percentages of cells in G0/G1, S and G2/M phases -detected by BrdU incorporation and 7-AAD staining- are similar in WT GCB (n=5) and Aicda-/- GCB (n=5). (I) The percentage of GCB (B220+GL7+CD95+) in Aicda-/- mice is higher than in WT mice (left) (***p<0.001, t-test), but the percentage of antigen-specific GCB (NP+ GCB) is lower in Aicda-/- mice. (J) GSEA showing lower expression of the plasma/memory cell signature genes in Aicda-/- GCB than in WT GCB.
Table S1. List of AID-dependent DMRs, related to Figure 4.