Abstract
Despite tremendous research efforts, hypertension remains an epidemic health concern, leading often to the development of cardiovascular disease. It is well established that in many instances, the brain plays an important role in the onset and progression of hypertension via activation of the sympathetic nervous system. Further, the activity of the renin-angiotensin system (RAS) and of glial cell-mediated proinflammatory processes have independently been linked to this neural control and are, as a consequence, both attractive targets for the development of antihypertensive therapeutics. Although it is clear that the predominant effector peptide of the RAS, ANG II, activates its type-1 receptor on neurons to mediate some of its hypertensive actions, additional nuances of this brain RAS control of blood pressure are constantly being uncovered. One of these complexities is that the RAS is now thought to impact cardiovascular control, in part, via facilitating a glial cell-dependent proinflammatory milieu within cardiovascular control centers. Another complexity is that the newly characterized antihypertensive limbs of the RAS are now recognized to, in many cases, antagonize the prohypertensive ANG II type 1 receptor (AT1R)-mediated effects. That being said, the mechanism by which the RAS, glia, and neurons interact to regulate blood pressure is an active area of ongoing research. Here, we review the current understanding of these interactions and present a hypothetical model of how these exchanges may ultimately regulate cardiovascular function.
Keywords: neurogenic hypertension, astrocyte, microglia, renin, angiotensin II
diseases of the cardiovascular system are the predominant causes of mortality in the United States (128), and it is imperative that new and innovative strategies to combat these pathologies are developed. This can only be achieved with a thorough understanding of the systems that regulate cardiovascular control under normal and pathological conditions. The central nervous system (CNS) is crucial to maintaining cardiovascular homeostasis. Dysregulation of the neural systems controlling cardiovascular function can lead to chronic sympathoexcitation and so-called “neurogenic hypertension”, a key contributor to the development of cardiovascular disease (72, 128). Despite the known risks of chronically elevated blood pressure, therapeutics that effectively and consistently normalize high blood pressure in hypertensive patients remain elusive; this is particularly so for neurogenic hypertension. As a consequence, many hypertensive patients exhibit resistance to current antihypertensive medications (128, 159). Two important aspects of the CNS control of blood pressure that are promising areas of focus for the enhancement of therapeutic interventions are 1) the newly recognized complexity of the influence of the renin-angiotensin system (RAS) over cardiovascular control (37, 44, 98, 111, 164) and 2) the impact of neuronal-glial interactions within cardiovascular control centers of the brain on these processes (19, 39, 104, 167, 168, 170, 179). The RAS and neuronal-glial interactions become altered in disease states and are intimately interrelated (39, 41, 192, 194). That is, the RAS influences neuronal-glial interactions, while communication between glial and neural cells impacts brain RAS signaling (39, 41, 168, 169).
To further illustrate the interactions between the RAS and neuron-glia communication, glial cells are often recognized as the innate immune cells of the brain (53, 59, 99, 133), and ANG II has long been recognized to exert immunomodulatory effects within various tissues by activating its type-1 receptor (AT1R) (11, 155). Additionally, other, more recently identified, components of the RAS, such as (pro)renin and its (pro)renin receptor (PRR), are thought to impact the neural control of cardiovascular function, potentially by impacting neuron-glial interactions and inflammatory pathways within the brain (110, 111, 131, 132, 164).
Here, we review the role of neural and glial cells in the brain regulation of cardiovascular function by the RAS. To accomplish this, we first provide a brief overview of what is known regarding the localization of the RAS components to specific cell types within the brain. We then review the well-characterized impact of ANG II activation of neuronal AT1R in the brain regulation of cardiovascular function. Next, the role of communication between neurons and glia in the control of cardiovascular function is discussed, as is the impact that ANG II has on these interactions. The complementary influence of PRR signaling and the counterregulatory impact of other components of the RAS [e.g., angiotensin-converting enzyme 2, and angiotensin type 2 receptors (AT2R)] on these processes are also considered. Last, we present a hypothetical model of how the RAS may impact neuronal-glial interactions during cardiovascular pathology and how elucidating these interactions may lead to the development of novel antihypertensive therapeutics.
Cellular Localization of the Renin-Angiotensin System Components Within the Brain
Countless efforts have been made to provide a thorough characterization of the cellular localization of components of the RAS within the brain. These efforts have been somewhat complicated by the unreliability of angiotensin receptor antibodies (9, 73, 79) and the poor cellular resolution of angiotensin receptor autoradiography techniques. That being said, decades of research have led to a tremendous literature base that collectively localizes all of the integral components of the RAS to the various cell populations within the brain (36, 37, 42, 62, 83, 86, 105, 123, 151, 157, 180).
Localization of components for ANG II synthesis.
The inability of circulating ANG II to effectively cross the blood-brain barrier (BBB) under normal, healthy conditions coupled with the dense localization of ANG II receptors to brain structures that are protected by the BBB has led to the prediction that ANG II can be synthesized within the brain to act in an autocrine or paracrine fashion at its CNS receptors. There are studies that have suggested that the production of ANG II occurs within the neurons themselves and that ANG II is generated intracellularly and on demand to act as a peptide neurotransmitter within neural circuits that regulate cardiovascular function (7, 68, 112). However, additional intricacies involved in CNS ANG II synthesis may be inferred by the localization of the components required for its production to multiple cell types within the brain, rather than exclusively to one cellular phenotype.
The ANG II precursor, angiotensinogen, is densely expressed within the CNS, and, in particular, within astrocytes (85, 151, 162, 180). Renin, which converts angiotensinogen to ANG I, is also localized to neurons and astrocytes; however, the low expression of this enzyme within the CNS has led to reservations in regard to its functional significance (83, 86, 87, 114, 142). On the other hand, the PRR, which binds (pro)renin, thereby sequestering and rendering it active to convert angiotensinogen to ANG I, is highly expressed in the brain, perhaps providing a mechanism by which ANG II may be synthesized within the CNS (37, 131, 132). Along these lines, this receptor is thought to be involved in the autocrine/paracrine actions of the RAS within several specific tissues, including the brain (37, 131, 132). Furthermore, PRR is associated with intracellular signaling cascades that act independently of ANG II receptor signaling to facilitate the development of neurogenic hypertension (37, 164). PRRs are particularly abundant on neurons (111, 164, 188) and are also localized to microglia (169) and astrocytes (152). An additional mechanism by which ANG II may be generated within the brain, despite the low levels of renin observed within this tissue, involves another peptide derived from angiotensinogen, proangiotensin-12, or ANG 1–12 (129). This peptide is also present in the brain and may allow for ANG II generation independent of renin (129).
Angiotensin-converting enzyme (ACE) is the next step in the generation of ANG II, and this enzyme is also present in the brain (36, 62, 157), with a particular abundance in the choroid plexus and in the capillary endothelial cells (5, 23) and also to a lesser extent in neurons within a few regions that are involved in the neural regulation of blood pressure, such as the subfornical organ (SFO) and paraventricular nucleus of the hypothalamus (PVN) (156, 182). Collectively, the localization of the RAS components required for the synthesis of ANG II within diverse cell types of the brain implies that a network of neuronal-glial interactions may be required for the synthesis of ANG II within the brain. Once ANG II is synthesized within the brain, it likely activates its AT1R and AT2R in BBB-protected nuclei to influence cardiovascular function and hydromineral balance.
Cellular localization of receptors mediating ANG II action.
ANG II binds to either the AT1R or the AT2R to exert its physiological actions, and these receptors are found within the CNS (42, 67, 77, 92–94, 105, 124, 195). Whereas humans contain only AT1R and AT2R, the AT1R subtype within rodents can be further subdivided into the angiotensin type 1a receptor (AT1aR) and type 1b receptor (AT1bR), whose genes are present on separate chromosomes but are highly homologous (119, 160). Within the brain of rodents, AT1R is primarily present in its AT1aR form; however, in some cases, AT1bR has been detected in the brain, and it is also abundant in the anterior pituitary and adrenal cortex (27, 95, 105).
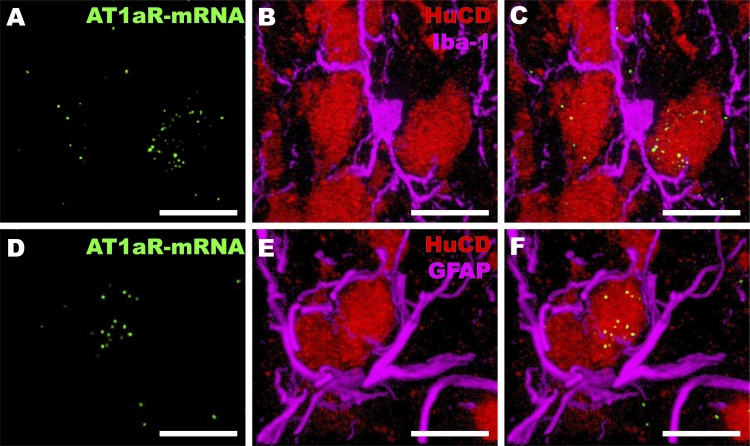
In rodents, AT1Rs (primarily AT1aR) are abundant in regions that are traditionally known to regulate blood pressure and hydromineral balance, including the PVN and SFO (105), while AT2R are densely expressed in a number limbic and thalamic regions that indirectly regulate cardiovascular function, as well as some regions that play a more direct role in cardiovascular homeostasis, such as the nucleus of the solitary tract (NTS) (42, 77, 92, 93). Despite the seeming consensus regarding the localization of these receptors to specific brain nuclei, the cellular localization of the angiotensin receptors within the brain has been a matter of debate. In contrast to immunohistochemistry (IHC) and in vitro studies that have localized AT1R and AT2R to non-neuronal cells in the brain (108, 127, 207), genetic mouse models and advances in in situ hybridization provide evidence that, at least under normal conditions, these receptor subtypes are predominantly neuronal and are absent from glia (33, 42, 67). In support of a predominantly neuronal localization of AT1aR within a cardiovascular control center, Fig. 1 depicts dual in situ hybridization and immunohistochemistry studies localizing this receptor to HuC/D-positive neurons in normotensive rat PVN, but not to Iba-1-positive microglia or GFAP-positive astrocytes. As can be observed in this figure, although microglia and astrocytes themselves lack AT1aR expression, they are situated in close proximity to AT1aR-containing neurons. These cells are, therefore, positioned to interact intimately with one another.
Fig. 1.
AT1R localizes to neurons but not astrocytes and microglia in the paraventricular nucleus (PVN). Representative images through the PVN of normotensive Sprague-Dawley rats depicting AT1aR mRNA as punctate green dots (in situ hybridization via RNAscope) combined with dual-label immunohistochemistry for the neuronal marker HuC/D, shown in red, and either the microglial marker Iba-1 (A–C) or the astrocytic marker GFAP (D–F), both shown in magenta. Combined in situ hybridization and immunohistochemistry were conducted as described in detail in de Kloet et al. (42). Scale = 10 μm.
Importantly these highly specific and sensitive in situ hybridization and genetic techniques for the localization of ANG II receptors have not yet been extended to models of cardiovascular pathophysiology, in which it is possible that AT1R and/or AT2R may become expressed on the non-neuronal cell types of the brain. However, it is probable that these technological advancements may be utilized in future studies to definitively ascertain the impact of cardiovascular pathology on the cellular localization of ANG II receptors in the brain. Along these lines, there are a number of previous in vitro and some in vivo studies that have suggested a role for angiotensin receptors on non-neuronal brain cells in the development and progression of hypertension, and these studies will be discussed in subsequent sections.
Cellular localization of the ANG 1–7 receptor, Mas.
Other nontraditional components of the RAS, such as the ANG 1–7 receptor, Mas, are thought to counter-regulate ANG II's actions at its type-1 receptor and are detected within the brain (75, 91, 212). Like the AT1R and AT2R, the Mas is a G protein-coupled receptor that is found in various areas of the brain, including the hippocampus, amygdala, forebrain, piriform cortex, olfactory bulb, thalamus, and portions of the hypothalamus (25, 60, 125, 212). Although these previous studies revealed that the Mas was primarily localized to neurons within these regions (60), others have observed Mas localization to glial cells as well (150), implying that this axis may directly and/or indirectly impact numerous cell types in the brain to ultimately influence physiology.
Renin-Angiotensin System Actions Mediated via Neurons to Regulate Cardiovascular Function
Because of the overwhelming acceptance that receptors for RAS-mediated actions are localized to neurons, much research has focused on the effects of their activation on the neuronal regulation of cardiovascular function, and these neuronal actions have been extensively reviewed elsewhere (54, 58, 135, 141) and will, therefore, only briefly be discussed here. Importantly, and as described above, the prohypertensive AT1R and PRR and the antihypertensive AT2R and Mas are localized to neurons within brain regions both directly and indirectly involved in cardiovascular function. These regions include those that are protected by the BBB (e.g., the NTS), as well as those that are termed circumventricular organs (CVOs), which serve as an interface between the systemic circulation and the brain.
The endocrine RAS is traditionally known to impact the neural control of cardiovascular function via the activation of AT1R in CVOs, such as the SFO (54–56, 101, 136). These CVO neurons then, in turn, send projections to and influence the activity of numerous cardiovascular control centers that are generally protected by the BBB, including the PVN (55, 101). Upon activation of these CVO projections, the release of traditional neurotransmitters (e.g., glutamate) from their nerve terminals and onto neurons situated in downstream cardiovascular control nuclei is modulated, and sympathetic outflow and blood pressure become elevated (161, 190). That being said, many of these downstream brain nuclei also densely express AT1R. As discussed in detail in Localization of components for ANG II synthesis, it is recognized that the RAS acts as an autocrine or paracrine system within a number of tissues, including the brain (37, 105, 123), where it has been speculated that brain-derived ANG II serves as a neurotransmitter within circuits that regulate cardiovascular function (7, 112). Although, as described in detail above, the mechanism by which ANG II would be synthesized within the brain and then released from nerve terminals is a topic of ongoing research, the putative ANG II neurotransmitter action is an intriguing method by which BBB-protected AT1R may impact physiology, and it likely involves coordination among numerous cell types in the brain.
Another mode by which ANG II has been proposed to activate AT1R on neurons within cardiovascular control centers that are normally protected by the BBB is via passive transport of circulating ANG II across a compromised (leaky) BBB. To this point, there are various pathological conditions that are accompanied by increased BBB permeability (16, 78, 122, 126), and it is possible that regions that are highly vascularized, such as the PVN (174, 186), are particularly susceptible to such a mechanism by which circulating ANG II can access CNS receptors. In support of this notion, hypertension causes an ANG II-dependent increase in BBB permeability that then leads to the facilitated access of endocrine ANG II to brain regions typically considered to be protected from circulating factors (16). The interpretation is that there exists an ANG II-contingent feed-forward mechanism potentiating hypertensive actions by facilitating the access of ANG II to cardiovascular control centers of the brain.
In any event, regardless of the origin of ANG II within the brain, once levels of this peptide become elevated within cardiovascular control centers and neuronal AT1R are activated, the outcome is in many instances an increase in sympathetic outflow and blood pressure. As a consequence, neuronal ANG II/AT1R actions are by and large thought to facilitate neurogenic hypertension; granted, there is also evidence for ANG II/AT1R-dependent antihypertensive mechanisms within distinct brain nuclei such as the NTS (165) and the caudal ventrolateral medulla (3). Further, many currently prescribed ANG II receptor blockers cross the blood-brain barrier and are thought to exert some of their antihypertensive actions via the blockade of AT1R within the brain (18).
In regard to the neural circuitry of RAS-mediated cardiovascular actions, the PVN is one brain region containing AT1Rs that are thought to facilitate neurogenic hypertension. Neurons of the PVN can be divided into a number of functionally distinct subgroups based on their anatomical location and neurochemical phenotype. Parvocellular PVN neurons, many of which express AT1R (105), are classified as neurosecretory or preautonomic. The neurosecretory neurons project to the median eminence and, upon stimulation, release factors such as corticotropin-releasing hormone and thyrotropin-releasing hormone, which are integral components of the hypothalamic-pituitary-adrenal axis and the hypothalamic-pituitary-thyroid axis, respectively. Preautonomic neurons influence cardiovascular function by controlling sympathetic nervous system activity via projections to the hindbrain [i.e., rostral ventrolateral medulla (RVLM)] and spinal cord (31, 35, 196). Magnocellular PVN neurons express vasopressin (AVP) or oxytocin (187), and their activation results in the release of AVP and oxytocin from the posterior pituitary and into the circulation. Although the specific predominating roles for the subgroups of neurons within the PVN are established, there is some overlap and crosstalk between the subtypes of neurons of the PVN. For example, preautonomic neurons can also express markers that are specific to the other subregions of the PVN, such as AVP. As a consequence, AVP neurons in the PVN may impact blood pressure via effects on sympathetic outflow, as well as their effects on AVP secretion. Moreover, there is a growing body of evidence that indicates that various cell types of the PVN communicate with one another via the dendritic release of neuropeptides (51, 117, 172, 178). This phenomenon allows for the effective integration and coordination of responses that are mediated by the PVN, and this area is reviewed in detail elsewhere (117, 178).
In regard to ANG II's actions at preautonomic neurons of the PVN, two potential mechanisms have been proposed for the neuronal AT1R-mediated excitation of these neurons and consequent increases blood pressure: 1) ANG II stimulation of AT1R on RVLM-projecting PVN neurons to cause their depolarization (31) and 2) presynaptic disinhibition of preautonomic neurons via the activation of AT1R on GABAergic nerve terminals that contact them (106, 107). Although, there is some evidence to support both of these mechanisms, the above-mentioned impediments to definitively localizing AT1R to specific neuronal phenotypes have cast uncertainty upon these propositions, as it is not entirely clear from anatomical studies that the AT1R are localized to the correct neuronal phenotypes to substantiate the involvement of these mechanisms. Further, it is also unclear as to whether these ANG II actions are direct neuronal effects or are mediated via actions of ANG II at other cell elements. That said, whether one or both of the above-described mechanisms is, indeed, at play can perhaps be definitively ascertained by the utilization of advanced genetic techniques, such as optogenetics and AT1R reporter mice. Regardless, the end result of either of these proposed mechanisms of ANG II actions within the PVN would be an excitation of the preautonomic PVN neurons and subsequent increase in blood pressure via projections onto neurons of the RVLM and the intermediolateral cell column of the spinal cord, and the available literature is supportive of an excitatory role for AT1R activation on preautonomic neurons (32, 35, 148, 163, 185).
Another important point is that the central RAS has been implicated in the regulation of the magnocellular AVP system, which likely also contributes to ANG II's hypertensive effects (115, 199, 213). Elevations in brain ANG II (or ANG III) lead to activation of AVP neurons causing a rise in systemic AVP levels that can be attenuated by administration of AT1R blockers either via the intracerebroventricular route or specifically within magnocellular neuron-containing areas of the brain (199, 213, 214). Further, several recent studies have indicated that AVP may play an important role in RAS-dependent hypertension. For example, the hypertension observed in mice with transgenic activation of the brain RAS is AVP-dependent (115). On the basis of data from BAC transgenic reporter mice, it is unlikely that magnocellular neurons of the PVN (or supraoptic nucleus) contain AT1R or AT2R (42, 67), and the impact of ANG II on AVP secretion may be mediated by ANG II receptor-positive neural connections arising from upstream brain nuclei, such as the organum vasculosum of the lamina terminalis or SFO (57, 193). Alternatively, it may also involve non-neuronal cell types in the brain, and this notion will be discussed in the subsequent sections. While these stimulatory effects of ANG II on AVP secretion are likely mediated by the AT1R (34, 199, 213), several lines of evidence support the notion that activation of AT2R acts in opposition to these hypertensive and AVP secretion stimulatory effects. For example, whole body genetic deletion or pharmacological blockade of AT2R (using PD123,219) in mice leads to increased pressor responses to centrally administered ANG II. These experimental manipulations also augment ANG II-induced AVP release, reflected by an increase of plasma AVP levels (80, 113) and decreased pituitary AVP content (118). That being said, the therapeutic utility of AT2R agonists has only recently begun to be evaluated in this regard.
The projections from the preautonomic portion of the PVN to the hindbrain and spinal cord are generally glutamatergic, and the release of glutamate from their synapses onto RVLM neurons and preganglionic neurons in the intermediolateral cell column of the spinal cord leads to increased sympathetic outflow to cardiovascular tissues and a rise in blood pressure (32, 43, 48, 120, 148, 163, 185). Furthermore, the RVLM contributes to the regulation of barosensitive sympathetic efferents (38), and hyperactivity of RVLM-barosensitive neurons controlling renal sympathetic nerve activity exerts similar pressor effects. AT1Rs are also localized to the RVLM (2), as well as other hindbrain cardiovascular control brain nuclei, such as the NTS (another important integrative site for many homeostatic systems). Activation of AT1R within these hindbrain regions has been thought to contribute to neurogenic hypertension, via mechanisms that involve abnormalities in the receipt, processing, or integration of information concerning sympathetic afferents, arterial baroreceptors, chemoreceptors, and volume receptors (1, 140, 145, 211).
There is evidence that the PRR is localized to neurons and that its activation influences cardiovascular homeostasis, ultimately contributing to neurogenic hypertension (110, 111, 164). Administration of (pro)renin into the brain of mice increases blood pressure, an effect that is reversed by the deletion of the PRR specifically from neurons (111). Further, neuronal PRR deletion prevents DOCA-salt hypertension in mice (111). Again, the implication of these previous studies is that neuronal PRR are critical for the development of hypertension. On the other hand, much like AT1R activation, PRR can also have diverse effects on blood pressure, depending on the brain nuclei in which PRR becomes activated. In other words, PRR stimulation can be pressor or depressor, depending on the specific population of neuronal PRR that are impacted. For example, PRR deletion in all neurons and in specific forebrain nuclei (e.g., supraoptic nucleus) is anti-hypertensive, suggesting that the PRR within the entire brain and within these specific areas is pressor (110, 111, 164). On the other hand, activation of this receptor specifically within the NTS has also been associated with an antihypertensive response that is nuclear factor-κB (NF-κB)-dependent, but AT1R-independent (215). Additionally, there is also evidence for (pro)renin/PRR actions within non-neuronal cells of the brain, which will be discussed in subsequent sections.
It has been suggested that AT1R activation can be opposed by stimulation of AT2R (20, 82, 177) and by the ACE2-(ANG 1–7)-Mas axis (44, 47, 50, 98, 158). At least under normal, nonhypertensive conditions, AT2Rs within or adjacent to cardiovascular control nuclei, are exclusively neuronal (42), and Mas have similarly been localized, in large part to neurons (60). As would then be expected, these putative cardioprotective limbs of the RAS directly impact the neuronal control of cardiovascular function. Consistent with this, AT2R activation via the administration of the selective agonist Compound 21 or Mas activation via the administration of ANG 1–7 within the brain reduces blood pressure (20, 22, 64, 69, 208). Conversely, blockade of either AT2R or Mas specifically in the brain via the delivery of the AT2R antagonist (PD123,319) or the Mas antagonist (A-779) elevates blood pressure, as well as sympathetic nerve activity to specific tissues (63, 64, 137). Furthermore, additional studies have extended these findings by determining specific brain nuclei that are involved in the antihypertensive actions of the Mas and AT2R. For example, overexpression of ACE2, an enzyme required for ANG 1–7 formation, within the NTS (209) or PVN (176) leads to similar antihypertensive responses. In regard to AT2R, its antihypertensive actions have been postulated to be mediated by the RVLM (65) and the NTS (17), among other brain areas containing AT2R (42). Despite these lines of evidence, suggesting that AT2R and Mas-mediated mechanisms are antihypertensive, there are also studies that have revealed that ANG 1–7 administered into specific brain regions (e.g., the RVLM or PVN) enhances cardiac sympathetic afferent reflex and increases sympathetic outflow and blood pressure via Mas activation (76, 109, 171).
Important questions are whether these countering effects of AT2R and Mas over AT1R-mediated cardiovascular effects are physiologically relevant, and under what physiological conditions might they occur. These questions are relevant because, thus far, actions of AT2R and Mas have been uncovered under pharmacological conditions. With regard to AT2R, it is known that AT1R and AT2R are present at different levels of expression within various cardiovascular control centers. It is generally accepted that AT1R are more abundant than AT2R within many of the cardiovascular control centers of the brain, and, therefore, AT1R actions, in general, are thought to predominate. Nonetheless, AT2R actions are potentially dampening these AT1R-mediated responses, such that if AT2R is blocked pharmacologically or deleted genetically, AT1R actions are often augmented (21, 84, 113). It is also important to note that although these receptors are present within the same brain nuclei, they are primarily localized to separate cells within these sites (42). Thus, antagonistic actions of these receptors within or near cardiovascular control centers are likely opposite or offsetting, rather than mediated through the same neuron. Although brain AT1R and AT2R may have opposite effects on cardiovascular control and there is anatomical evidence that shows they are found in close proximity to each other, the physiological conditions that might precipitate predominance of one receptor subtype over the other, or increased expression of one versus the other, are not established. Nonetheless, we feel that the AT2R does, indeed, play a physiological role in countering AT1R effects. Perhaps the ratio of AT1R to AT2R is important in determining the extent of ANG II's hypertensive actions. Further, we hypothesize that AT2R can be exploited pharmacologically to also counter AT1R-mediated hypertension.
It is clear from the reviewed studies, as well as numerous other reports, that the RAS can exert powerful cardiovascular effects via direct actions on neurons. On the other hand, AT1R-neuronal-glial interactions are an emerging area of interest, as is the impact of PRR activation and the counter-regulatory AT2R and ACE2/ANG 1–7/Mas axis on neuronal-glial communications, and consequently, on cardiovascular function. These concepts are discussed in the subsequent sections.
Neuron and Glia Interactions in the Regulation of Cardiovascular Function by the RAS
In addition to direct effects of ANG II and other RAS components on neurons, the localization of the RAS elements to diverse cell types within the CNS brings to light the possibility that communication between neurons and glia mediates CNS RAS actions. It is a fact that neurons and glial cells interact with one another on many levels and thereby impact neurophysiology. Glial cells influence neurotransmitter release and metabolism, while neurotransmitters and other factors released by neurons impact the activity of glial cells. Furthermore, since both microglia and astrocytes are considered the brain's resident innate immune cells (149), there is potential for the RAS to initiate inflammatory responses within cardiovascular control centers of the brain via the activation of these non-neuronal cells, possibly modulating cardiovascular homeostasis.
Cross-talk between neurons, astrocytes, and microglia.
Over recent years, the understanding of brain function has shifted from a predominant focus on neuron-neuron communication, to the reinforced appreciation that active cross-talk between the various cell types within the brain is an imperative component of signaling within the tissue. There are extensive communications between microglia, astrocytes, and neurons that facilitate the ability of microglia and astrocytes to sense disturbances to the nervous system and to regulate the development, structure, and function of neural connections (99). The impact of these glial-neuronal interactions on brain function have been extensively reviewed elsewhere (4, 179, 191).
Astrocytes have traditionally been considered the support cells or “glue” of the brain, and this accepted, but passive, role has often overshadowed the appreciation of their many essential active roles in brain function (153, 203). That being said, it is currently understood that astrocytes and neurons participate in a bidirectional communication that is essential for the coordination of synaptic transmission and the function of neuronal networks (4). Individual astrocytes contact and oversee hundreds to thousands of dendrites and synapses within various brain areas and are situated such that they can integrate a tremendous amount of synaptic activity (28, 74). This positioning allows them to regulate neurotransmitter diffusion into the extracellular space not only via the formation of a physical barrier to diffusion, but also via effects on neurotransmitter uptake (46, 144, 154, 191). For example, they are known to modulate GABA tone via its removal by the GABA transporter 3 within various neural circuits, including those that regulate sympathetic outflow and blood pressure (138). It is also established that astrocytes regulate the release of glutamate, ATP, and other signaling molecules from nerve terminals (139). Further, astrocytes buffer CNS potassium, remove excess cytotoxic glutamate, and modulate blood flow (8, 71, 149). Finally, astrocytes impact synaptic activity by themselves releasing “gliotransmitters” (121). Of particular relevance, there is accumulating evidence for a potential role of astrocyte-neuronal interactions in the regulation of cardiovascular function, in particular, and this is discussed in greater depth below.
Microglial cells similarly influence neuronal signaling. They make contact with synaptic structures and are, thereby, positioned to sense activity within neuronal circuits (200), and gap junctions between microglia and neurons allow the exchange of ions and small molecules between these cell types (49, 52). Further, microglia facilitate synaptogenesis subsequent to brain injury (13). Additionally, astrocytes and microglia interact with each other to regulate neuronal function (8, 14, 15, 100, 116). That is, astrocytes release factors that modulate microglial activity and vice versa (14, 45, 210).
It is well known that these two glial cell types respond to severe threats to the internal milieu of the brain, such as injury subsequent to stroke, by undergoing phenotypic transformations. On one hand, astrocytes are known to retract their processes in response to certain threats, thereby, in a sense, withdrawing their influence. Of particular relevance, this phenotypic shift can be observed within the magnocellular PVN during hyperosmotic challenges (197). On the other hand, in response to other threats (e.g., obesity and injury) astrocytes undergo a phenomenon that is often referred to as astrogliosis, which is characterized by hyperplasia and hypertrophy of these cells and can be observed via the upregulation of glial fibrillary acidic protein (GFAP) and an increased number of GFAP-positive cells within the site of injury (24, 26, 41, 143). Similarly, the microglial response to injury is characterized by a phenotypic shift from a “resting state” appearance, in which they contain long processes that are dynamically surveying the microenvironment for potential threats, to an “activated state” in which they respond to a potential threat by transforming into cells with enlarged cell bodies and ramified processes (102, 130, 167). Upon inury, they also migrate to and infiltrate the damaged tissue (133, 167, 192). Activated microglial cells then facilitate neuroprotection by removing debris, clearing dead cells, and secreting neurotrophic factors. However, chronic recruitment and activation of microglia can be maladaptive by contributing to impaired neuronal function or inducing neuronal death, as reviewed by several authors (6, 99, 130, 192). Of relevance, the “protective” microglia are often designated as M2 microglia, while the “maladaptive microglia” are often referred to as M1 microglia. Furthermore, once activated, both microglia and astrocytes perpetuate a proinflammatory milieu, and are, thereby, also thought to impact neuronal signaling.
Proinflammatory and immune involvement in communication between neurons and glia.
One mechanism by which the various neuronal and glial cell types of the brain communicate with one another is via the release and detection of proinflammatory or immune factors (39). Consistent with their roles in the innate immune system, they express the pattern recognition Toll-like receptors (TLRs; pattern recognition receptors) that identify molecules conserved among many pathogens [e.g., TLR-4 is well known to recognize lipopolysaccharides (LPS)], and they are capable of expressing and synthesizing numerous cytokines and chemokines, as well as their receptors (53, 90). As a consequence, microglia and astrocytes both sense and perpetuate a proinflammatory milieu within the CNS, and this glial-mediated rise in inflammation has a profound impact on neuronal activity.
Importantly, astrocytes and microglia are both localized to cardiovascular control centers of the brain, and both lie in close proximity to preautonomic (39) and to AT1R-containing PVN neurons. Within these areas, they are capable of sensing and responding to hypertensive stimuli to impact cardiovascular function (24, 66, 192). Consequently, it is possible that astrocyte-neuron and/or astrocyte-microglia interactions contribute to the inflammation within cardiovascular control centers of the brain that ultimately elevates blood pressure. During experimental hypertension, the PVN, as well as other brain regions important for regulating sympathetic outflow, such as the NTS, are infiltrated with additional microglia and astrocytes, the levels of proinflammatory factors and reactive oxygen species within these regions rise, and these events have the potential to influence neuronal activity within these regions (29, 167, 201, 202, 216). This neuroimmune communication between microglia, astrocytes, and neurons has then been suggested to facilitate neurogenic hypertension (30, 39).
In regard to cardiovascular function, increases in proinflammatory cytokines within brain nuclei that regulate cardiovascular homeostasis are generally considered to promote sympathetic nervous system activity and increase blood pressure. For instance, such is the case upon an elevation in proinflammatory cytokines specifically within the PVN. Blockade of proinflammatory cytokines within the PVN, for example, via the administration of a TNF-α receptor antagonist, etanercept, has the opposite impact on blood pressure (175). Furthermore, elevations in the levels of the anti-inflammatory cytokine, IL-10, within the PVN similarly reduce blood pressure (167). The implication is that the proinflammatory actions of ANG II within the brain may also contribute to the hypertensive actions of this peptide.
Evidence for astrocyte and microglial involvement in the neural regulation of cardiovascular function by ANG II and (pro)renin.
Although it is recognized that ANG II can have a direct impact on neurons to regulate cardiovascular function, several lines of evidence indicate that the RAS also impacts cardiovascular function by facilitating the communication between neurons and glia (39). Along these lines, since astrocytes represent the key cell type within the nervous system that synthesize angiotensinogen (88, 123, 134, 166, 180), it is intriguing to hypothesize that astrocyte-generated angiotensinogen itself acts as a signaling moiety to facilitate this communication. Similarly, as it is evident that astrocytic angiotensinogen is critical for the integrity of the BBB (96), it is possible that the brain RAS plays a “gatekeeping” role, whereby it maintains and/or potentiates the access of endocrine ANG II and other circulating factors to the brain. In this regard, ANG II derived from astrocytic angiotensinogen has been found to activate AT1R on endothelial cells to maintain BBB function (206).
Also of particular relevance, astrocytes and the RAS have independently been acknowledged to impact the magnocellular AVP system to influence blood pressure, and it is possible that an angiotensinergic-neuronal-glial communication impacts AVP signaling to ultimately regulate cardiovascular function (34, 46, 115, 191). As discussed above, astrocytes regulate neuronal function, in part, by influencing neurotransmitter diffusion into the extracellular space through a variety of mechanisms involving the formation of a physical barrier to diffusion and via neurotransmitter uptake (46, 144, 154, 191). Within the magnocellular region, in particular, astrocytes are involved in regulating excitatory/inhibitory neurotransmitter balance through such mechanisms, and the disruption of this balance is associated with disease conditions that are known to impact the RAS, such as heart failure (146, 147). For example, they influence crosstalk between NMDA and GABAA postsynaptic receptors in magnocellular neurons (147). Moreover, dehydration, which is known to impact the RAS and blood pressure, is associated with a structural remodeling of the neuronal-glial interactions within the magnocellular portion of the PVN, as well as the SON (197), and it is possible that a modulation of the neuronal-glial interactions within the magnocellular hypothalamus is integral to the RAS regulation of blood pressure. Furthermore, it is also possible that hypertensive stimuli induce such a remodeling of the astrocyte-neuron interactions within the preautonomic portion of the PVN and, thereby, also allows for altered angiotensinergic-neuronal-glial interactions [e.g., obesity (41)]. Along these lines, there is evidence that astrocytes do impact preautonomic neurons within the PVN in that they regulate the inhibitory GABA tone onto these neurons, thereby influencing renal sympathetic nerve activity, an important component of blood pressure regulation (138).
Another mechanism by which the RAS may impact neuronal-glial interactions is via an escalation of the proinflammatory milieu within cardiovascular control centers (10, 41, 167). Hypertension produced by chronic infusion of ANG II is associated with AT1R-mediated increases in microglial activation and cytokine levels in the PVN (167). Furthermore, accumulating evidence has indicated that the beneficial properties of RAS blockade are attributable, in part, to anti-inflammatory actions (11, 155). That being said, unlike the general acceptance that ANG II receptors are localized to neurons, whether or not non-neuronal cells of the brain also express ANG II receptors under normal conditions in vivo is less clear. Consequently, whether ANG II impacts astrocytes and microglia directly via receptors expressed on these cell-types or indirectly via its effects on neuronal receptor activation is a subject of debate.
In support of direct ANG II actions on non-neuronal cells, there are studies that have localized AT1R to cultured microglia that were exposed to LPS (127) and to astrocytes both in culture and in vivo (61, 183, 184). However, as some of these previous localization studies (61, 183) were conducted using ANG II receptor antibodies, the presence of the ANG II receptors on these non-neuronal cell types should be verified using alternate methodologies (e.g., reporter mice or in situ hybridization). Further, there is evidence that ANG II can elicit immune responses via activation of these AT1R (59, 61, 97). For example, in vitro, ANG II administration impacts cultured microglia and astrocytes by increasing transforming growth factor-β (TGF-β) expression, while angiotensin receptor blockers reduce inflammatory responses to ANG II and other stimuli (e.g., LPS) (103, 127). Moreover, in vivo, AT1R blockade using candesartan, an angiotensin receptor blocker that crosses the BBB, leads to reduced inflammatory responses during immune challenges or hypertensive stimuli within specific brain regions that is often accompanied by decreased microglial activation (10, 103). However, these in vivo actions may or may not be due to direct actions on glia.
As discussed in detail above, there are studies that have determined that ANG II receptors are likely not localized to glia and are instead exclusively localized to neurons in the brain (42, 67, 134). Characterization of recently generated transgenic AT1R and AT2R reporter mice indicate that, under normotensive in vivo conditions, brain AT1R and AT2R expression is almost solely neuronal (42, 67). Although a lack of ANG II receptor expression on non-neuronal cells of the brain may rule out direct effects of ANG II on glial cells, it would not per se rule out a key role of ANG II in glial cell influence over cardiovascular homeostasis. It is possible that microglia (and astrocytes) sense neuronal AT1R stimulation and that this then triggers their activation [i.e., an indirect mechanism by which ANG II influences glial cells via secretion of chemotactic factors from neurons (173, 204, 205)]. The prediction is that this feeds forward to further enhance hypertension, by promoting the above-described inflammatory response in glia that then further enhances sympathetic outflow. In this regard, there is some evidence for such a role for chemotactic proteins such as CC-chemokine ligand-2 (CCL2), as well as stromal cell-derived factor 1 and high-mobility group box 1 in these processes (168, 173, 204, 205).
There are several studies that would be congruent with either a direct or indirect influence of AT1R activation on microglia or astrocytes (40–42, 167, 192). For example, a study conducted by our research group revealed that the central administration of minocycline, an anti-inflammatory antibiotic that reduces microglial activation also decreases ANG II-induced hypertension (167). Furthermore, deletion of AT1R from neurons within the PVN of mice, leads to reduced microglial and inflammatory responses that occur subsequent to long-term high-fat diet consumption (40, 41, 192).
It is also possible that during hypertension, the expression pattern of AT1R changes and that microglia and/or astrocytes begin to express the receptor when challenged. Although, as described above, ANG II receptors are not detected in microglia or astrocytes of normotensive rodents under baseline conditions in situ (42, 67), some immunohistochemical studies have revealed localization of AT1R, AT2R, and ANG II labeling in microglia or astrocytes of the ischemic brain or in brains collected from rodents during other pathological conditions (89, 207). As mentioned throughout this review, however, the unreliability of many ANG II receptor antibodies (9, 79) has led to a hesitance to rely solely on immunohistochemical data to conclude that ANG II receptors are localized to non-neuronal cells of the brain. Therefore, it will be necessary to verify these results using alternative localization techniques. In this regard, using another approach to determine the role of a potential glial AT1R receptor population in cardiovascular function, Isegawa et al. (89) recently determined that mice that have undergone Cre/Lox-mediated deletion of AT1R from GFAP-containing cells have an improved prognosis when subjected to myocardial infarction-induced heart failure. In particular, they found that this “knockout” mouse did not exhibit the increase in AT1aR mRNA or protein (IHC and Western blot analysis) within the brain stem that wild-type mice exhibit in response to MI-induced heart failure. Importantly, under baseline, non-MI-induced heart failure, conditions, there was no difference in AT1aR. The implication is that astrocytic AT1aR is increased in response to heart failure and that this increase contributes to heart failure-induced mortality. Future studies need to be conducted to definitively determine the localization of ANG II receptors during such pathological conditions. Nonetheless, the plasticity of ANG II receptor expression in non-neuronal cell-types of the brain during disease states is an interesting potential mechanism by which ANG II/glial interactions may impact cardiovascular function. Such plasticity of ANG II receptor expression in non-neuronal cells may also be induced during the production of cell cultures, potentially explaining the observations that have demonstrated the presence of AT1R on microglia and astrocytes within in vitro models (127, 189).
As with ANG II, there is also evidence suggesting that (pro)renin impacts the non-neuronal cells in the brain, either directly or indirectly, to ultimately influence neuronal function. In this regard, (pro)renin elicits a direct proinflammatory action on microglial cells, mediated through NF-κB and potentiated by pretreatment with ANG II (169). Furthermore, we have found that (pro)renin exerts proinflammatory actions in cultured astrocytes of rats, an effect that is potentiated in astrocyte cultures generated from hypertensive rats (152).
On the other hand, there are several lines of evidence that have linked the neuroprotective effects of AT2R activation and of the ACE2-(ANG 1–7)-Mas axis to their ability to alter the interactions between neurons and non-neuronal cells in the brain (12, 198). For example, AT2R activation using the selective AT2R agonist Compound 21 attenuates microglial responses during autoimmune encephalomyelitis (81), and there is some evidence that astrocytic AT2R correlates with BBB breakdown during CNS inflammation (181), implying that AT2R may also play a role in the access of the peripheral RAS to the CNS. In regard to the ACE2-(ANG 1–7)-Mas axis, in addition to Mas's documented expression in neurons, there is also evidence that this receptor is localized to microglia (150) and that its activation impacts microglial responses to stroke, implying that activation of this protective RAS either directly or indirectly influences these non-neuronal cells. It is possible that the putative protective effects of these systems in the CNS control of cardiovascular function similarly involve astrocytes and microglia (70).
Conclusions and Hypothesis
Although it has been suggested that ANG II receptors located on glial cells mediate certain cardiovascular actions of ANG II, we propose rather that cooperation between neurons and glia is integral to the RAS influence over the brain control of cardiovascular function under normal and hypertensive conditions. As discussed throughout this review, this premise stems, in part, from the realization that the components required for ANG II synthesis and action are not confined to a single cellular compartment within the CNS and are instead localized to diverse cell types throughout the tissue. Further, despite numerous research efforts to localize ANG II receptors to non-neuronal cells in the brain, their presence on cells other than neurons of the CNS in vivo is not definitively supported by the literature, particularly under normotensive conditions. In spite of this, there are numerous lines of evidence indicating that AT1R activation does, indeed, impact microglial activation and astrogliosis and that this is associated with cardiovascular dysfunction.
This profound impact of the ANG II on microglia and astrocytes coupled with the apparent lack of AT1R expression within these cell types under normal baseline conditions, further reinforces the appreciation of a probable intricate relationship between microglia, astrocytes, and ANG II receptor-containing neurons. Along these lines, we have previously determined, that both astrocytes and microglia lie in close proximity to preautonomic PVN neurons (39) and are, therefore, positioned to regulate sympathetic outflow. Here, we further determined that in normotensive Sprague-Dawley rats, microglia and astrocytes, although they themselves lack AT1aR mRNA under normotensive conditions, are localized in close proximity to AT1aR-containing neurons within the PVN. Consequently, these AT1aR-containing neurons within the PVN are similarly positioned to interact closely with glial cells and perhaps respond to glial cell-generated or circulating RAS peptides, and it is possible that similar interactions are present in other cardiovascular control nuclei, as well.
Another important component of the RAS-neuron-glia interactions in cardiovascular control is that during hypertension, the activity of the RAS actions within the CNS rises. This elevation in central RAS actions is due to an increase in ANG II synthesis within the CNS and/or an increase in peripheral RAS access to the CNS. In regard to the CNS synthesis of ANG II, the PRR plays a key role in this and then in the subsequent activation of CNS AT1R. That being said, PRR's influence over the neural control of blood pressure extends beyond its ability to increase ANG II levels locally, within cardiovascular control nuclei. That is, PRR activation is also linked to independent intracellular signaling cascades that facilitate neurogenic hypertension. Furthermore, PRR is likely expressed on neurons, microglia, and astrocytes during both normotensive and hypertensive conditions and, therefore, also has the potential to both directly and indirectly influence the neuronal-glial influence over cardiovascular function.
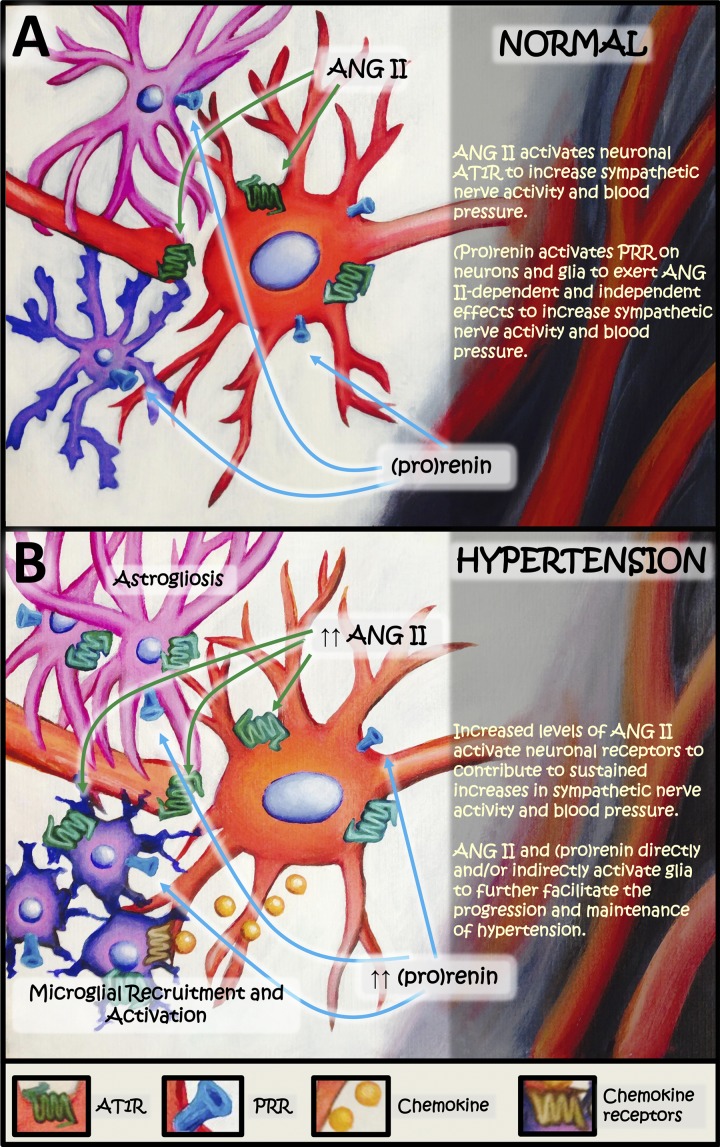
Regardless of the source of the ANG II within the brain, we propose the following model to help explain the respective roles of neurons and glia in the cardiovascular actions of RAS components under normal and hypertensive conditions. First, under normotensive conditions ANG II activates neuronal AT1R to increase sympathetic nerve activity and blood pressure (Fig. 2A). We further propose that during the normotensive state, (pro)renin can influence neuronal PRR to exert ANG II-dependent and -independent effects and ultimately increase sympathetic nerve activity and blood pressure (Fig. 2A). This action of (pro)renin may involve glial effects on RAS components to assist in the generation of ANG II. During hypertension (Fig. 2B), we expect that the elevation in the CNS ANG II leads to increased activation of neuronal AT1R in BBB-protected brain nuclei, with subsequent activation of preautonomic neurons to augment sympathetic outflow and blood pressure. Furthermore, we postulate that during hypertension, activation of neuronal AT1R leads to the secretion of certain factors (e.g., the chemotactic protein CCL2), which then bind to their receptors (e.g., CCR2) on microglia and stimulate their migration toward these neurons. This hypothesis has been described in detail in a previous review (39). Our model also incorporates the possibility that a certain plasticity exists in regard to the cellular localization of the AT1R in the brain, whereby under cardiovascular pathologies, AT1Rs come to be expressed on astrocytes and microglia (Fig. 2B). The hypothesis is then that activation of these non-neuronal AT1R directly facilitates astrogliosis and microglial activation, leading eventually to a perpetuation of neurally mediated cardiovascular dysfunction. Finally, we predict that in addition to enhanced neuronal effects during hypertension, (pro)renin acts via PRR on astrocytes and microglia to elicit astrogliosis and microglial activation, and subsequent neuronal activation via paracrine interactions.
Fig. 2.
Hypothetical model for the roles of neurons and glia in the central nervous system's actions of the renin-angiotensin system in normotensive (A) and hypertensive (B) conditions.
Perspectives and Significance
Collectively, we believe that these RAS-mediated increases in neuronal, microglial, and astrocyte activity contribute to the perpetuation of neurogenic hypertension. Despite tremendous research efforts, hypertension and the often consequential morbidity or mortality associated with cardiovascular pathologies remain an immense problem for our society. Taken together, the reviewed studies highlight a promising avenue for the development of antihypertensive therapeutics. These studies suggest that one potential contributing mechanism to these pathologies is the RAS-mediated interactions between neurons and glia within cardiovascular control centers of the brain. These interactions are now an area of active research that may lead to new and innovative ways to combat treatment-resistant hypertension.
GRANTS
This work was supported by National Institutes of Health Grants HL-033610 (to C. Sumners), HL-076803 (to C. Sumners), HL-093186 (to C. Sumners), HL-096830 (to E. G. Krause), F32-HL-116074 (to A. D. de Kloet), and K99-HL-125805 (to A. D. de Kloet).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.D.d.K. and C.S. conception and design of research; A.D.d.K. and M.L. performed experiments; A.D.d.K. analyzed data; A.D.d.K. interpreted results of experiments; A.D.d.K. prepared figures; A.D.d.K. drafted manuscript; A.D.d.K., M.L., V.R., E.G.K., and C.S. edited and revised manuscript; A.D.d.K., M.L., V.R., E.G.K., and C.S. approved final version of manuscript.
REFERENCES
- 1.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 52: 932–937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen AM, Dampney RA, Mendelsohn FA. Angiotensin receptor binding and pressor effects in cat subretrofacial nucleus. Am J Physiol Heart Circ Physiol 255: H1011–H1017, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Allen AM, Mendelsohn FA, Gierobat ZJ, Blessing WW. Vasopressin release following microinjection of angiotensin II into the caudal ventrolateral medulla oblongata in the anaesthetized rabbit. J Neuroendocrinol 2: 867–873, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 81: 728–739, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arregui A, Iversen LL. Angiotensin-converting enzyme: presence of high activity in choroid plexus of mammalian brain. Eur J Pharmacol 52: 147–150, 1978. [DOI] [PubMed] [Google Scholar]
- 6.Badoer E. Microglia: activation in acute and chronic inflammatory states and in response to cardiovascular dysfunction. Int J Biochem Cell Biol 42: 1580–1585, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res 599: 223–229, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14: 724–738, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Benicky J, Hafko R, Sanchez-Lemus E, Aguilera G, Saavedra JM. Six commercially available angiotensin II AT1 receptor antibodies are non-specific. Cell Mol Neurobiol 32: 1353–1365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36: 857–870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benicky J, Sanchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol 29: 781–792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennion DM, Haltigan E, Regenhardt RW, Steckelings UM, Sumners C. Neuroprotective mechanisms of the ACE2-angiotensin-(1–7)-Mas axis in stroke. Curr Hypertens Rep 17: 512, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia 55: 233–238, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nat Neurosci 4: 702–710, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Bezzi P, Domercq M, Vesce S, Volterra A. Neuron-astrocyte cross-talk during synaptic transmission: physiological and neuropathological implications. Prog Brain Res 132: 255–265, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63: 572–579, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanch GT, Freiria-Oliveira AH, Speretta GF, Carrera EJ, Li H, Speth RC, Colombari E, Sumners C, Colombari DS. Increased expression of angiotensin II type 2 receptors in the solitary-vagal complex blunts renovascular hypertension. Hypertension 64: 777–783, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology 94: 12–20, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des 12: 1623–1635, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the nonpeptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol 159: 709–716, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemsen R. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology 33: 863–873, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwers S, Smolders I, Wainford RD, Dupont AG. Hypotensive and sympathoinhibitory responses to selective central AT2 receptor stimulation in spontaneously hypertensive rats. Clin Sci (Lond) 129: 81–92, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brownfield MS, Reid IA, Ganten D, Ganong WF. Differential distribution of immunoreactive angiotensin and angiotensin-converting enzyme in rat brain. Neuroscience 7: 1759–1769, 1982. [DOI] [PubMed] [Google Scholar]
- 24.Buckman LB, Thompson MM, Moreno HN, Ellacott KLJ. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol n/a-n/a, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunnemann B, Fuxe K, Metzger R, Mullins J, Jackson TR, Hanley MR, Ganten D. Autoradiographic localization of mas proto-oncogene mRNA in adult rat brain using in situ hybridization. Neurosci Lett 114: 147–153, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol Endocrinol Metab 267: E260–E267, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension 59: 113–121, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, Kodzius R, Shimokawa K, Bajic VB, Brenner SE, Batalov S, Forrest AR, Zavolan M, Davis MJ, Wilming LG, Aidinis V, Allen JE, Ambesi-Impiombato A, Apweiler R, Aturaliya RN, Bailey TL, Bansal M, Baxter L, Beisel KW, Bersano T, Bono H, Chalk AM, Chiu KP, Choudhary V, Christoffels A, Clutterbuck DR, Crowe ML, Dalla E, Dalrymple BP, de Bono B, Della Gatta G, di Bernardo D, Down T, Engstrom P, Fagiolini M, Faulkner G, Fletcher CF, Fukushima T, Furuno M, Futaki S, Gariboldi M, Georgii-Hemming P, Gingeras TR, Gojobori T, Green RE, Gustincich S, Harbers M, Hayashi Y, Hensch TK, Hirokawa N, Hill D, Huminiecki L, Iacono M, Ikeo K, Iwama A, Ishikawa T, Jakt M, Kanapin A, Katoh M, Kawasawa Y, Kelso J, Kitamura H, Kitano H, Kollias G, Krishnan SP, Kruger A, Kummerfeld SK, Kurochkin IV, Lareau LF, Lazarevic D, Lipovich L, Liu J, Liuni S, McWilliam S, Madan Babu M, Madera M, Marchionni L, Matsuda H, Matsuzawa S, Miki H, Mignone F, Miyake S, Morris K, Mottagui-Tabar S, Mulder N, Nakano N, Nakauchi H, Ng P, Nilsson R, Nishiguchi S, Nishikawa S, Nori F, Ohara O, Okazaki Y, Orlando V, Pang KC, Pavan WJ, Pavesi G, Pesole G, Petrovsky N, Piazza S, Reed J, Reid JF, Ring BZ, Ringwald M, Rost B, Ruan Y, Salzberg SL, Sandelin A, Schneider C, Schonbach C, Sekiguchi K, Semple CA, Seno S, Sessa L, Sheng Y, Shibata Y, Shimada H, Shimada K, Silva D, Sinclair B, Sperling S, Stupka E, Sugiura K, Sultana R, Takenaka Y, Taki K, Tammoja K, Tan SL, Tang S, Taylor MS, Tegner J, Teichmann SA, Ueda HR, van Nimwegen E, Verardo R, Wei CL, Yagi K, Yamanishi H, Zabarovsky E, Zhu S, Zimmer A, Hide W, Bult C, Grimmond SM, Teasdale RD, Liu ET, Brusic V, Quackenbush J, Wahlestedt C, Mattick JS, Hume DA, Kai C, Sasaki D, Tomaru Y, Fukuda S, Kanamori-Katayama M, Suzuki M, Aoki J, Arakawa T, Iida J, Imamura K, Itoh M, Kato T, Kawaji H, Kawagashira N, Kawashima T, Kojima M, Kondo S, Konno H, Nakano K, Ninomiya N, Nishio T, Okada M, Plessy C, Shibata K, Shiraki T, Suzuki S, Tagami M, Waki K, Watahiki A, Okamura-Oho Y, Suzuki H, Kawai J, Hayashizaki Y. The transcriptional landscape of the mammalian genome. Science 309: 1559–1563, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol 272: 579–604, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Chen D, Jancovski N, Bassi JK, Nguyen-Huu TP, Choong YT, Palma-Rigo K, Davern PJ, Gurley SB, Thomas WG, Head GA, Allen AM. Angiotensin type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. J Neurosci 32: 2051–2061, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiodera P, Volpi R, Caiazza A, Giuliani N, Magotti MG, Coiro V. Arginine vasopressin and oxytocin responses to angiotensin II are mediated by AT1 receptor subtype in normal men. Metabolism 47: 893–896, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol 25: 461–463, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Correa FM, Plunkett LM, Saavedra JM. Quantitative distribution of angiotensin-converting enzyme (kininase II) in discrete areas of the rat brain by autoradiography with computerized microdensitometry. Brain Res 375: 259–266, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Cuadra AE, Shan Z, Sumners C, Raizada MK. A current view of brain renin-angiotensin system: Is the (pro)renin receptor the missing link? Pharmacol Ther 125: 27–38, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol 29: 261–268, 2002. [DOI] [PubMed] [Google Scholar]
- 39.de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C. Neuroimmune communication in hypertension and obesity: A new therapeutic angle? Pharmacol Ther 138: 428–440, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33: 4825–4833, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav 136: 31–38, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Function In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dean C, Seagard JL, Hopp FA, Kampine JP. Differential control of sympathetic activity to kidney and skeletal muscle by ventral medullary neurons. J Auton Nerv Syst 37: 1–10, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol 91: 163–198, 2006. [DOI] [PubMed] [Google Scholar]
- 45.DeWitt DA, Perry G, Cohen M, Doller C, Silver J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Exp Neurol 149: 329–340, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Di S, Popescu IR, Tasker JG. Glial control of endocannabinoid heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. J Neurosci 33: 18,331–18,342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MF, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 27: 12–19, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Ding ZQ, Li YW, Wesselingh SL, Blessing WW. Transneuronal labelling of neurons in rabbit brain after injection of Herpes simplex virus type 1 into the renal nerve. J Auton Nerv Syst 42: 23–31, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Dobrenis K, Chang HY, Pina-Benabou MH, Woodroffe A, Lee SC, Rozental R, Spray DC, Scemes E. Human and mouse microglia express connexin36, and functional gap junctions are formed between rodent microglia and neurons. J Neurosci Res 82: 306–315, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–E9, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Du W, Stern JE, Filosa JA. Neuronal-derived nitric oxide and somatodendritically released vasopressin regulate neurovascular coupling in the rat hypothalamic supraoptic nucleus. J Neurosci 35: 5330–5341, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eugenin EA, Eckardt D, Theis M, Willecke K, Bennett MV, Saez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc Natl Acad Sci USA 98: 4190–4195, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol 28: 138–145, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 89: 370–376, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson AV. Systemic angiotensin acts at the subfornical organ to control the activity of paraventricular nucleus neurons with identified projections to the median eminence. Neuroendocrinology 47: 489–497, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24: 96–101, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Ferguson AV, Renaud LP. Systemic angiotensin acts at subfornical organ to facilitate activity of neurohypophysial neurons. Am J Physiol Regul Integr Comp Physiol 251: R712–R717, 1986. [DOI] [PubMed] [Google Scholar]
- 58.Fink GD. Long-term sympatho-excitatory effect of angiotensin II: a mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol 24: 91–95, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Finsen B, Owens T. Innate immune responses in central nervous system inflammation. FEBS Lett 585: 3806–3812, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Freund M, Walther T, von Bohlen und Halbach O. Immunohistochemical localization of the angiotensin-(1–7) receptor Mas in the murine forebrain. Cell Tissue Res 348: 29–35, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Fuchtbauer L, Groth-Rasmussen M, Holm TH, Lobner M, Toft-Hansen H, Khorooshi R, Owens T. Angiotensin II Type 1 receptor (AT1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav Immun 25: 897–904, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Ganten D, Minnich JL, Granger P, Hayduk K, Brecht HM, Barbeau A, Boucher R, Genest J. Angiotensin-forming enzyme in brain tissue. Science 173: 64–65, 1971. [DOI] [PubMed] [Google Scholar]
- 63.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens 24: 724–730, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am J Hypertens 27: 1248–1256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao L, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension 51: 521–527, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Gerber AR, Bale TL. Antiinflammatory treatment ameliorates HPA stress axis dysfunction in a mouse model of stress sensitivity. Endocrinology 153: 4830–4837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, Marques-Lopes J, Young CN, Butler SD, Davisson RL, Iadecola C, Pickel VM, Pierce JP, Milner TA. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 226: 489–509, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 23: 187–193, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guimaraes PS, Santiago NM, Xavier CH, Velloso EP, Fontes MA, Santos RA, Campagnole-Santos MJ. Chronic infusion of angiotensin-(1–7) into the lateral ventricle of the brain attenuates hypertension in DOCA-salt rats. Am J Physiol Heart Circ Physiol 303: H393–H400, 2012. [DOI] [PubMed] [Google Scholar]
- 70.Guo F, Liu B, Tang F, Lane S, Souslova EA, Chudakov DM, Paton JF, Kasparov S. Astroglia are a possible cellular substrate of angiotensin(1–7) effects in the rostral ventrolateral medulla. Cardiovasc Res 87: 578–584, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurden H, Uchida N, Mainen ZF. Sensory-evoked intrinsic optical signals in the olfactory bulb are coupled to glutamate release and uptake. Neuron 52: 335–345, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Hafko R, Villapol S, Nostramo R, Symes A, Sabban EL, Inagami T, Saavedra JM. Commercially available angiotensin II At2 receptor antibodies are nonspecific. PLos One 8: e69234, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13: 54–63, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han Y, Sun HJ, Li P, Gao Q, Zhou YB, Zhang F, Gao XY, Zhu GQ. Angiotensin-(1–7) in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLos One 7: e48966, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hauser W, Johren O, Saavedra JM. Characterization and distribution of angiotensin II receptor subtypes in the mouse brain. Eur J Pharmacol 348: 101–114, 1998. [DOI] [PubMed] [Google Scholar]
- 78.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 50: 202–211, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 61: 253–258, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hohle S, Spitznagel H, Rascher W, Culman J, Unger T. Angiotensin AT1 receptor-mediated vasopressin release and drinking are potentiated by an AT2 receptor antagonist. Eur J Pharmacol 275: 277–282, 1995. [DOI] [PubMed] [Google Scholar]
- 81.Horiuchi M, Cui TX, Li Z, Li JM, Nakagami H, Iwai M. Fluvastatin enhances the inhibitory effects of a selective angiotensin II type 1 receptor blocker, valsartan, on vascular neointimal formation. Circulation 107: 106–112, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Huang XC, Richards EM, Sumners C. Mitogen-activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J Biol Chem 271: 15,635–15,641, 1996. [DOI] [PubMed] [Google Scholar]
- 83.Husain A, Smeby RR, Krontiris-Litowitz J, Speth RC. Brain renin: localization in rat brain synaptosomal fractions. Brain Res 222: 182–186, 1981. [DOI] [PubMed] [Google Scholar]
- 84.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 377: 748–750, 1995. [DOI] [PubMed] [Google Scholar]
- 85.Imboden H, Harding JW, Hilgenfeldt U, Celio MR, Felix D. Localization of angiotensinogen in multiple cell types of rat brain. Brain Res 410: 74–77, 1987. [DOI] [PubMed] [Google Scholar]
- 86.Inagami T, Celio MR, Clemens DL, Lau D, Takii Y, Kasselberg AG, Hirose S. Renin in rat and mouse brain: immunohistochemical identification and localization. Clin Sci (Lond) 59 Suppl 6: 49s–51s, 1980. [DOI] [PubMed] [Google Scholar]
- 87.Inagami T, Clemens DL, Celio MR, Brown A, Sandru L, Herschkowitz N, Hoffman LH, Kasselberg AG. Immunohistochemical localization of renin in mouse brain. Neurosci Lett 18: 91–98, 1980. [DOI] [PubMed] [Google Scholar]
- 88.Intebi AD, Flaxman MS, Ganong WF, Deschepper CF. Angiotensinogen production by rat astroglial cells in vitro and in vivo. Neuroscience 34: 545–554, 1990. [DOI] [PubMed] [Google Scholar]
- 89.Isegawa K, Hirooka Y, Katsuki M, Kishi T, Sunagawa K. Angiotensin II type 1 receptor expression in astrocytes is upregulated leading to increased mortality in mice with myocardial infarction-induced heart failure. Am J Physiol Heart Circ Physiol 307: H1448–H1455, 2014. [DOI] [PubMed] [Google Scholar]
- 90.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175: 4320–4330, 2005. [DOI] [PubMed] [Google Scholar]
- 91.Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR. The mas oncogene encodes an angiotensin receptor. Nature 335: 437–440, 1988. [DOI] [PubMed] [Google Scholar]
- 92.Johren O, Inagami T, Saavedra JM. AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. Neuroreport 6: 2549–2552, 1995. [DOI] [PubMed] [Google Scholar]
- 93.Johren O, Inagami T, Saavedra JM. Localization of AT2 angiotensin II receptor gene expression in rat brain by in situ hybridization histochemistry. Brain Res Mol Brain Res 37: 192–200, 1996. [DOI] [PubMed] [Google Scholar]
- 94.Johren O, Saavedra JM. Expression of AT1A and AT1B angiotensin II receptor messenger RNA in forebrain of 2-wk-old rats. Am J Physiol Endocrinol Metab 271: E104–E112, 1996. [DOI] [PubMed] [Google Scholar]
- 95.Kakar SS, Riel KK, Neill JD. Differential expression of angiotensin II receptor subtype mRNAs (AT1A and AT1B) in the brain. Biochem Biophys Res Commun 185: 688–692, 1992. [DOI] [PubMed] [Google Scholar]
- 96.Kakinuma Y, Hama H, Sugiyama F, Yagami K, Goto K, Murakami K, Fukamizu A. Impaired blood-brain barrier function in angiotensinogen-deficient mice. Nat Med 4: 1078–1080, 1998. [DOI] [PubMed] [Google Scholar]
- 97.Kandalam U, Clark MA. Angiotensin II activates JAK2/STAT3 pathway and induces interleukin-6 production in cultured rat brainstem astrocytes. Regul Pept 159: 110–116, 2010. [DOI] [PubMed] [Google Scholar]
- 98.Katovich MJ, Grobe JL, Huentelman M, Raizada MK. Angiotensin-converting enzyme 2 as a novel target for gene therapy for hypertension. Exp Physiol 90: 299–305, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 91: 461–553, 2011. [DOI] [PubMed] [Google Scholar]
- 100.Kloss CU, Kreutzberg GW, Raivich G. Proliferation of ramified microglia on an astrocyte monolayer: characterization of stimulatory and inhibitory cytokines. J Neurosci Res 49: 248–254, 1997. [PubMed] [Google Scholar]
- 101.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci 31: 15,009–15,015, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19: 312–318, 1996. [DOI] [PubMed] [Google Scholar]
- 103.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-β. J Clin Invest 120: 2782–2794, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lazartigues E. Inflammation and neurogenic hypertension: a new role for the circumventricular organs? Circ Res 107: 166–167, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: A functional neuroanatomical review. Front Neuroendocrinol 18: 383, 1997. [DOI] [PubMed] [Google Scholar]
- 106.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther 313: 1035–1045, 2005. [DOI] [PubMed] [Google Scholar]
- 108.Li JJ, Lu J, Kaur C, Sivakumar V, Wu CY, Ling EA. Expression of angiotensin II and its receptors in the normal and hypoxic amoeboid microglial cells and murine BV-2 cells. Neuroscience 158: 1488–1499, 2009. [DOI] [PubMed] [Google Scholar]
- 109.Li P, Sun HJ, Cui BP, Zhou YB, Han Y. Angiotensin-(1–7) in the rostral ventrolateral medulla modulates enhanced cardiac sympathetic afferent reflex and sympathetic activation in renovascular hypertensive rats. Hypertension 61: 820–827, 2013. [DOI] [PubMed] [Google Scholar]
- 110.Li W, Peng H, Cao T, Sato R, McDaniels SJ, Kobori H, Navar LG, Feng Y. Brain-targeted (pro)renin receptor knockdown attenuates angiotensin II-dependent hypertension. Hypertension 59: 1188–1194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li W, Peng H, Mehaffey EP, Kimball CD, Grobe JL, van Gool JM, Sullivan MN, Earley S, Danser AH, Ichihara A, Feng Y. Neuron-specific (pro)renin receptor knockout prevents the development of salt-sensitive hypertension. Hypertension 63: 316–323, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol 265: R302–R309, 1993. [DOI] [PubMed] [Google Scholar]
- 113.Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui TX, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol 284: H116–H121, 2003. [DOI] [PubMed] [Google Scholar]
- 114.Lippoldt A, Fuxe K, Luft FC. A view of renin in the brain. J Mol Med 79: 71–73, 2001. [DOI] [PubMed] [Google Scholar]
- 115.Littlejohn NK, Siel RB Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol 304: R818–R828, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci 89: 141–146, 2011. [DOI] [PubMed] [Google Scholar]
- 117.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7: 126–136, 2006. [DOI] [PubMed] [Google Scholar]
- 118.Macova M, Pavel J, Saavedra JM. A peripherally administered, centrally acting angiotensin II AT2 antagonist selectively increases brain AT1 receptors and decreases brain tyrosine hydroxylase transcription, pituitary vasopressin and ACTH. Brain Res 1250: 130–140, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacTaggart TE, Ito M, Smithies O, John SW. Mouse angiotensin receptor genes Agtr1a and Agtr1b map to chromosomes 13 and 3. Mamm Genome 8: 294–295, 1997. [DOI] [PubMed] [Google Scholar]
- 120.Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am J Physiol Heart Circ Physiol 285: H2734–H2748, 2003. [DOI] [PubMed] [Google Scholar]
- 121.Martineau M. Gliotransmission: focus on exocytotic release of l-glutamate and d-serine from astrocytes. Biochem Soc Trans 41: 1557–1561, 2013. [DOI] [PubMed] [Google Scholar]
- 122.Mauro C, De Rosa V, Marelli-Berg F, Solito E. Metabolic syndrome and the immunological affair with the blood-brain barrier. Front Immunol 5: 677, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. [DOI] [PubMed] [Google Scholar]
- 124.Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA 81: 1575–1579, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D. Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett 357: 27–32, 1995. [DOI] [PubMed] [Google Scholar]
- 126.Min LJ, Mogi M, Shudou M, Jing F, Tsukuda K, Ohshima K, Iwanami J, Horiuchi M. Peroxisome proliferator-activated receptor-gamma activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood-brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension 59: 1079–1088, 2012. [DOI] [PubMed] [Google Scholar]
- 127.Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor κB and activator protein-1 activation. Eur J Neurosci 27: 343–351, 2008. [DOI] [PubMed] [Google Scholar]
- 128.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the american heart association. Circulation 131: e29–e322, 2015. [DOI] [PubMed] [Google Scholar]
- 129.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350: 1026–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 130.Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem 130: 169–175, 2001. [DOI] [PubMed] [Google Scholar]
- 131.Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Curr Opin Pharmacol 8: 127–132, 2008. [DOI] [PubMed] [Google Scholar]
- 132.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 3: 216–227, 2002. [DOI] [PubMed] [Google Scholar]
- 134.O'Callaghan EL, Bassi JK, Porrello ER, Delbridge LM, Thomas WG, Allen AM. Regulation of angiotensinogen by angiotensin II in mouse primary astrocyte cultures. J Neurochem 119: 18–26, 2011. [DOI] [PubMed] [Google Scholar]
- 135.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007. [DOI] [PubMed] [Google Scholar]
- 136.Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin II-salt hypertension in the rat. Exp Physiol 97: 80–88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oscar CG, Muller-Ribeiro FC, de Castro LG, Martins Lima A, Campagnole-Santos MJ, Santos RA, Xavier CH, Fontes MA. Angiotensin-(1–7) in the basolateral amygdala attenuates the cardiovascular response evoked by acute emotional stress. Brain Res 1594: 183–189, 2015. [DOI] [PubMed] [Google Scholar]
- 138.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol 587: 4645–4660, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev 63: 83–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paton JF, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii—a microinjection study in the rat. J Physiol 521: 213–225, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood-brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med (Berl) 86: 705–710, 2008. [DOI] [PubMed] [Google Scholar]
- 142.Paul M, Hermann K, Printz M, Lang RE, Unger T, Ganten D. The brain angiotensin system: subcellular localization and interferences with converting enzyme inhibitors. J Hypertens Suppl 1: 9–15, 1983. [PubMed] [Google Scholar]
- 143.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94: 1077–1098, 2014. [DOI] [PubMed] [Google Scholar]
- 144.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci USA 101: 2151–2155, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Polson JW, Dampney RA, Boscan P, Pickering AE, Paton JF. Differential baroreflex control of sympathetic drive by angiotensin II in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol 293: R1954–R1960, 2007. [DOI] [PubMed] [Google Scholar]
- 146.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol 303: R291–R300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci 33: 631–640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000. [DOI] [PubMed] [Google Scholar]
- 149.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest 122: 1164–1171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, Sumners C. Anti-inflammatory effects of angiotensin-(1–7) in ischemic stroke. Neuropharmacology 71: 154–163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Richoux JP, Bouhnik J, Clauser E, Corvol P. The renin-angiotensin system in the rat brain. Immunocytochemical localization of angiotensinogen in glial cells and neurons. Histochemistry 89: 323–331, 1988. [DOI] [PubMed] [Google Scholar]
- 152.Rodriguez V, deKloet AD, Llerena V, Kitchen-Pareja MC, Sumners C. Prorenin-induced pro-inflammatory effect in hypothalamic astrocytes from spontaneously hypertensive rats. Hypertension 62 (High Blood Pressure Research Scientific Sessions Meeting Abstracts): A163, 2013. [Google Scholar]
- 153.Rossi D. Astrocyte physiopathology: At the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol 130: 86–120, 2015. [DOI] [PubMed] [Google Scholar]
- 154.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16: 675–686, 1996. [DOI] [PubMed] [Google Scholar]
- 155.Saavedra JM. Angiotensin II AT1 receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond) 123: 567–590, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saavedra JM, Chevillard C. Angiotensin-converting enzyme is present in the subfornical organ and other circumventricular organs of the rat. Neurosci Lett 29: 123–127, 1982. [DOI] [PubMed] [Google Scholar]
- 157.Saavedra JM, Fernandez-Pardal J, Chevillard C. Angiotensin-converting enzyme in discrete areas of the rat forebrain and pituitary gland. Brain Res 245: 317–325, 1982. [DOI] [PubMed] [Google Scholar]
- 158.Santos RA, Ferreira AJ, Simoes ESAC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp Physiol 93: 519–527, 2008. [DOI] [PubMed] [Google Scholar]
- 159.Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension—its identification and epidemiology. Nat Rev Nephrol 9: 51–58, 2013. [DOI] [PubMed] [Google Scholar]
- 160.Sasamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, Dzau VJ. Cloning, characterization, and expression of two angiotensin receptor (AT1) isoforms from the mouse genome. Biochem Biophys Res Commun 185: 253–259, 1992. [DOI] [PubMed] [Google Scholar]
- 161.Saxena A, Little JT, Nedungadi TP, Cunningham JT. Angiotensin II type 1A receptors in subfornical organ contributes towards chronic intermittent hypoxia associated sustained increase in mean arterial pressure. Am J Physiol Heart Circ Physiol 308: H435–H446, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sernia C. Location and secretion of brain angiotensinogen. Regul Pept 57: 1–18, 1995. [DOI] [PubMed] [Google Scholar]
- 163.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998. [DOI] [PubMed] [Google Scholar]
- 164.Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, Seth DM, Navar LG, Katovich MJ, Sumners C, Raizada MK. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res 107: 934–938, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shan Z, Zubcevic J, Shi P, Jun JY, Dong Y, Murça TM, Lamont GJ, Cuadra A, Yuan W, Qi Y, Li Q, Paton JFR, Katovich MJ, Sumners C, Raizada MK. Chronic knockdown of the nucleus of the solitary tract AT1 receptors increases blood inflammatory-endothelial progenitor cell ratio and exacerbates hypertension in the spontaneously hypertensive rat. Hypertension 61: 1328–1333, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sherrod M, Davis DR, Zhou X, Cassell MD, Sigmund CD. Glial-specific ablation of angiotensinogen lowers arterial pressure in renin and angiotensinogen transgenic mice. Am J Physiol Regul Integr Comp Physiol 289: R1763–R1769, 2005. [DOI] [PubMed] [Google Scholar]
- 167.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension 56: 297–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shi P, Grobe JL, Desland FA, Raizada MK, Sumners C. Microglial activation by the brain renin-angiotensin system. FASEB J 25: 661–662, 2011. [Google Scholar]
- 169.Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, Liu M, Raizada MK, Sumners C. Direct pro-inflammatory effects of prorenin on microglia. PLos One 9: e92937, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol 37: e52–e57, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Silva AQ, Santos RA, Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension 46: 341–348, 2005. [DOI] [PubMed] [Google Scholar]
- 172.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron 78: 1036–1049, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Song C, Santisteban M, Zubcevic J, Raizada M. Involvement of neuronal high mobility group box 1 (HMGB1) in Angiotensin II-mediated neuronal-microglial interaction (Abstract). Hypertension 64: A202, 2014. [Google Scholar]
- 174.Sposito NM, Gross PM. Morphometry of individual capillary beds in the hypothalamo-neurohypophysial system of rats. Brain Res 403: 375–379, 1987. [DOI] [PubMed] [Google Scholar]
- 175.Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLos One 8: e63847, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res 92: 401–408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Steckelings UM, Paulis L, Namsolleck P, Unger T. AT2 receptor agonists: hypertension and beyond. Curr Opin Nephrol Hypertens 21: 142–146, 2012. [DOI] [PubMed] [Google Scholar]
- 178.Stern JE. Neuroendocrine-autonomic integration in the PVN: novel roles for dendritically released neuropeptides. J Neuroendocrinol 27: 487–497, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stern JE, Filosa JA. Bidirectional neuro-glial signaling modalities in the hypothalamus: Role in neurohumoral regulation. Auton Neurosci 175: 51–60, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science 242: 1444–1446, 1988. [DOI] [PubMed] [Google Scholar]
- 181.Strittmatter G, Chua NH. Artificial combination of two cis-regulatory elements generates a unique pattern of expression in transgenic plants. Proc Natl Acad Sci USA 84: 8986–8990, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Strittmatter SM, Snyder SH. Angiotensin converting enzyme immunohistochemistry in rat brain and pituitary gland: correlation of isozyme type with cellular localization. Neuroscience 21: 407–420, 1987. [DOI] [PubMed] [Google Scholar]
- 183.Sumners C, Tang W, Paulding W, Raizada MK. Peptide receptors in astroglia: focus on angiotensin II and atrial natriuretic peptide. Glia 11: 110–116, 1994. [DOI] [PubMed] [Google Scholar]
- 184.Sumners C, Tang W, Zelezna B, Raizada MK. Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proc Natl Acad Sci USA 88: 7567–7571, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980. [DOI] [PubMed] [Google Scholar]
- 186.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 269–324, 1983. [DOI] [PubMed] [Google Scholar]
- 187.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980. [DOI] [PubMed] [Google Scholar]
- 188.Takahashi K, Hiraishi K, Hirose T, Kato I, Yamamoto H, Shoji I, Shibasaki A, Kaneko K, Satoh F, Totsune K. Expression of (pro)renin receptor in the human brain and pituitary, and co-localisation with arginine vasopressin and oxytocin in the hypothalamus. J Neuroendocrinol 22: 453–459, 2010. [DOI] [PubMed] [Google Scholar]
- 189.Tallant EA, Diz DI, Ferrario CM. Identification of AT1 receptors on cultured astrocytes. Adv Exp Med Biol 396: 121–129, 1996. [DOI] [PubMed] [Google Scholar]
- 190.Tanaka J, Hayashi Y, Sakamaki K, Okumura T, Nomura M. Activation of the subfornical organ enhances extracellular noradrenaline concentrations in the hypothalamic paraventricular nucleus in the rat. Brain Res Bull 54: 421–425, 2001. [DOI] [PubMed] [Google Scholar]
- 191.Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol 24: 566–576, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thrasher TN, Keil LC. Regulation of drinking and vasopressin secretion: role of organum vasculosum laminae terminalis. Am J Physiol Regul Integr Comp Physiol 253: R108–R120, 1987. [DOI] [PubMed] [Google Scholar]
- 194.Tomassoni D, Nwankwo IE, Gabrielli MG, Bhatt S, Muhammad AB, Lokhandwala MF, Tayebati SK, Amenta F. Astrogliosis in the brain of obese Zucker rat: a model of metabolic syndrome. Neurosci Lett 543: 136–141, 2013. [DOI] [PubMed] [Google Scholar]
- 195.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol Regul Integr Comp Physiol 261: R209–R216, 1991. [DOI] [PubMed] [Google Scholar]
- 196.Tucker DC, Saper CB. Specificity of spinal projections from hypothalamic and brainstem areas which innervate sympathetic preganglionic neurons. Brain Res 360: 159–164, 1985. [DOI] [PubMed] [Google Scholar]
- 197.Tweedle CD, Hatton GI. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res 181: 59–72, 1977. [DOI] [PubMed] [Google Scholar]
- 198.Valero-Esquitino V, Lucht K, Namsolleck P, Monnet-Tschudi F, Stubbe T, Lucht F, Liu M, Ebner F, Brandt C, Danyel LA, Villela DC, Paulis L, Thoene-Reineke C, Dahlof B, Hallberg A, Unger T, Sumners C, Steckelings UM. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin Sci (Lond) 128: 95–109, 2015. [DOI] [PubMed] [Google Scholar]
- 199.Veltmar A, Culman J, Qadri F, Rascher W, Unger T. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II-induced vasopressin release. J Pharmacol Exp Ther 263: 1253–1260, 1992. [PubMed] [Google Scholar]
- 200.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29: 3974–3980, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Waki H, Gouraud SS, Maeda M, Paton JF. Gene expression profiles of major cytokines in the nucleus tractus solitarii of the spontaneously hypertensive rat. Auton Neurosci 142: 40–44, 2008. [DOI] [PubMed] [Google Scholar]
- 202.Waki H, Gouraud SS, Maeda M, Paton JF. Specific inflammatory condition in nucleus tractus solitarii of the SHR: novel insight for neurogenic hypertension? Auton Neurosci 142: 25–31, 2008. [DOI] [PubMed] [Google Scholar]
- 203.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol 86: 342–367, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wei SG, Zhang ZH, Yu Y, Felder RB. Central SDF-1/CXCL12 expression and its cardiovascular and sympathetic effects: the role of angiotensin II, TNF-α, and MAP kinase signaling. Am J Physiol Heart Circ Physiol 307: H1643–H1654, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wei SG, Zhang ZH, Yu Y, Weiss RM, Felder RB. Central actions of the chemokine stromal cell-derived factor 1 contribute to neurohumoral excitation in heart failure rats. Hypertension 59: 991–998, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci 27: 9032–9042, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wu CY, Zha H, Xia QQ, Yuan Y, Liang XY, Li JH, Guo ZY, Li JJ. Expression of angiotensin II and its receptors in activated microglia in experimentally induced cerebral ischemia in the adult rats. Mol Cell Biochem 382: 47–58, 2013. [DOI] [PubMed] [Google Scholar]
- 208.Xue B, Zhang Z, Johnson RF, Guo F, Hay M, Johnson AK. Central endogenous angiotensin-(1–7) protects against aldosterone/NaCl-induced hypertension in female rats. Am J Physiol Heart Circ Physiol 305: H699–H705, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yamazato M, Ferreira AJ, Yamazato Y, Diez-Freire C, Yuan L, Gillies R, Raizada MK. Gene transfer of angiotensin-converting enzyme 2 in the nucleus tractus solitarius improves baroreceptor heart rate reflex in spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst 12: 456–461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang L, Tanaka J, Zhang B, Sakanaka M, Maeda N. Astrocytes modulate nitric oxide production by microglial cells through secretion of serine and glycine. Biochem Biophys Res Commun 251: 277–282, 1998. [DOI] [PubMed] [Google Scholar]
- 211.Yao F, Sumners C, O'Rourke ST, Sun C. Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol 294: H2712–H2720, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Young D, O'Neill K, Jessell T, Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci USA 85: 5339–5342, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhu Q, Guo SY, Gong S, Yin QZ, Hisamitsu T, Jiang XH. Losartan blocks the excitatory effect of peripheral hypertonic stimulation on vasopressinergic neurons in hypothalamic paraventricular nucleus in rats: electrophysiological and immunocytochemical evidence. Neurosci Lett 380: 12–16, 2005. [DOI] [PubMed] [Google Scholar]
- 214.Zini S, Fournie-Zaluski MC, Chauvel E, Roques BP, Corvol P, Llorens-Cortes C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci USA 93: 11,968–11,973, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zubcevic J, Jun JY, Lamont G, Murca TM, Shi P, Yuan W, Lin F, Carvajal JM, Li Q, Sumners C, Raizada MK, Shan Z. Nucleus of the solitary tract (pro)renin receptor-mediated antihypertensive effect involves nuclear factor-κB-cytokine signaling in the spontaneously hypertensive rat. Hypertension 61: 622–627, 2013. [DOI] [PubMed] [Google Scholar]
- 216.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension 57: 1026–1033, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]