Summary
Within secondary lymphoid tissues, stromal reticular cells support lymphocyte function, and targeting reticular cells is a potential strategy for controlling pathogenic lymphocytes in disease. However, the mechanisms that regulate reticular cell function are not well understood. Here we found that during an immune response in lymph nodes, dendritic cells (DCs) maintain reticular cell survival in multiple compartments. DC-derived lymphotoxin beta receptor (LTβR) ligands were critical mediators, and LTβR signaling on reticular cells mediated cell survival by modulating podoplanin (PDPN). PDPN modulated integrin-mediated cell adhesion, which maintained cell survival. This DC-stromal axis maintained lymphocyte survival and the ongoing immune response. Our findings provide insight into the functions of DCs, LTβR, and PDPN and delineate a DC-stromal axis that can potentially be targeted in autoimmune or lymphoproliferative diseases.
Introduction
Within lymph nodes, lymphocytes are supported by a non-hematopoietic vascular-stromal compartment that modulates lymphocyte survival, localization, and function (Cyster, 2005; Malhotra et al., 2013). Manipulating this compartment may be a means for controlling pathologic lymphocytes in autoimmune or lymphoproliferative diseases. As lymph nodes enlarge with stimulation, stromal reticular cells undergo a proliferative expansion (Chyou et al., 2011; Yang et al., 2014). While initial proliferation and immune activation can potentially be targeted, patients with chronic immune diseases are likely to present with ongoing responses. Understanding how reticular cells are maintained in already-enlarged nodes, then, can lead to the development of more effective therapeutic strategies.
Defined reticular cell populations in lymph nodes share the marker podoplanin (PDPN; also known as gp38) but serve distinct functions in each compartment. These cells are sometimes referred to as “fibroblastic reticular cells” (“FRCs”), although this term has been variably applied to all or different subpopulations (Chyou et al., 2011; Cremasco et al., 2014; Yang et al., 2014). Herein, we will use the descriptive term “PDPN+ reticular cells” and refer to specific subsets when applicable. In the T zone, PDPN+ reticular cells generate and ensheathe a network of collagen-rich fibrils, and the resulting reticular network facilitates T cell-dendritic cell (DC) interactions (Bajenoff et al., 2006; Malhotra et al., 2013). PDPN+ reticular cells also express interleukin-7 (IL-7) required for naïve T cell survival and CCL19 and CCL21 that compartmentalize T cells and DCs in the T zone (Cyster, 2005; Link et al., 2007). In contrast, B follicle reticular cells express CXCL13 required for B cell compartmentalization (Cyster, 2005; Katakai et al., 2008; Mionnet et al., 2013). CXCL13-expressing cells include follicular dendritic cells (FDCs) that present antigen to B cells, PDPN+ marginal reticular cells (MRCs) that extend from the subcapsular sinus, and, in secondary follicles, PDPN+ reticular cells in the mantle zone at the border of the T and B zones. Mantle zone PDPN+ cells express "B-cell activating factor" BAFF (TNFSF13B) that supports naïve B cell survival, and FDCs also express BAFF that can support germinal center responses (Cremasco et al., 2014; Hase et al., 2004; Suzuki et al., 2010). In the medulla, PDPN+ reticular cells presumably express the CCL21 present at low concentrations and the CXCL12 that facilitates accumulation of plasmablasts and plasma cells (herein referred to collectively as “antibody forming cells,” (AFCs)) (Bannard et al., 2013; Braun et al., 2011; Hargreaves et al., 2001; Yang et al., 2014). CXCL12 may also promote AFC survival, and PDPN+ cells can express interleukin-6 (IL-6), "A proliferation-inducing ligand", APRIL (TNFSF13) and other cytokines that may additionally contribute to AFC survival (Malhotra et al., 2013; Mohr et al., 2009). Directly depleting PDPN+ reticular cells disrupts lymphocyte survival and ongoing immune responses (Cremasco et al., 2014; Denton et al., 2014), underscoring the potential utility of delineating reticular cell survival mechanisms.
The regulation of PDPN+ reticular cell survival during ongoing immune responses is poorly understood. Endothelial and reticular cell proliferation begins within 2 days after immunization (Chyou et al., 2011; Yang et al., 2014). After immunization with OVA in CFA or stimulation with bone-marrow-derived dendritic cells, endothelial cell proliferation peaks at day 5 and is subsequently downregulated while endothelial cell numbers are maintained or continue to expand for at least another week (Tzeng et al., 2010). The re-establishment of vascular quiescence is dependent on late-accumulating CD11chi cells presumed to be DCs (Tzeng et al., 2010). CD11chi cells are closely associated with perivascular reticular cells and maintain their tight organization around vessels, suggesting that late-accumulating DCs maintain aspects of reticular cell function. The re-establishment of vascular quiescence after day 5 parallels the development of germinal centers and AFCs, suggesting that understanding how DCs might regulate reticular cells throughout the lymph node may be helpful for manipulating ongoing immune responses.
Here we found that during the re-establishment of quiescence, DCs maintained reticular cell survival in multiple lymph node compartments. DC-derived lymphotoxin β receptor (LTβR) ligands were critical mediators of this effect and the importance of these cell-associated ligands, the DC localization pattern, and the effect of DCs on reticular cell survival in vitro suggested that DCs act directly on reticular cells. LTβR signaling on reticular cells promoted survival by modulating PDPN, and PDPN signaled to modulate integrin-mediated cell adhesion. In vivo, the effects of DCs, PDPN, and cell adhesion were observed during the re-establishment of quiescence but not at homeostasis, suggesting that this DC-stromal axis is specific to an inflamed environment. This axis maintained lymphocyte survival and the ongoing response. Together, our results identify new functions for DCs, LTβR, and PDPN in mediating reticular cell survival and establish a novel DC-stromal axis that can potentially be targeted in chronic immune diseases.
Results
Non-T non-B CD11c+ cells localize with PDPN+ cells in multiple compartments
To examine the effects of DCs on reticular cells in the context of robust B cell responses, we immunized mice with alum-precipitated OVA (OVA-Alum). Lymph node cellularity was increased by day 2 and was maximal by day 9 (Figure S1A). Similar to other immunization strategies (Tzeng et al., 2010), endothelial cells showed peak proliferation at day 5 and relative quiescence at day 9 along with expanded numbers (Figure S1B–D). PDPN+ reticular cells showed similar proliferation and growth dynamics (Figure S1E–F), suggesting that the entire vascular-stromal compartment undergoes re-establishment of quiescence after day 5.
PDPN on reticular cells was upregulated over time (Figure S1G) and CD1 1c+ cells also accumulated. CD11chiMHCIImed (CD11chi) cells are presumed resident DCs, CD11c+MHCIIhi (MHCIIhi) cells include DCs recruited from skin and blood, and CD11cmedMHCIImed (CD11cmed) cells include monocytes, macrophages, plasmacytoid DCs, and inflammatory DCs (Merad et al., 2013; Tzeng et al., 2010). CD11cmed and MHCIIhi cells were highly enriched by day 2 (Figure S1H–I), reflecting the rapid accumulation of interleukin-1β-expressing monocytes and MHCIIhi DCs that can help drive early vascular-stromal proliferation (Benahmed et al., 2014). MHCIIhi DCs remained enriched at day 9 but had a less activated phenotype, with reduced MHCII and CD11b expression (Figure S1H–I)(data not shown). CD11chi cells, in contrast to the other populations, accumulated more slowly, with greatest enrichment at day 9 (Figure S1H–I). Re-establishment of vascular-stromal quiescence, then, was associated with high reticular cell PDPN and maximal accumulation of CD11chi DCs.
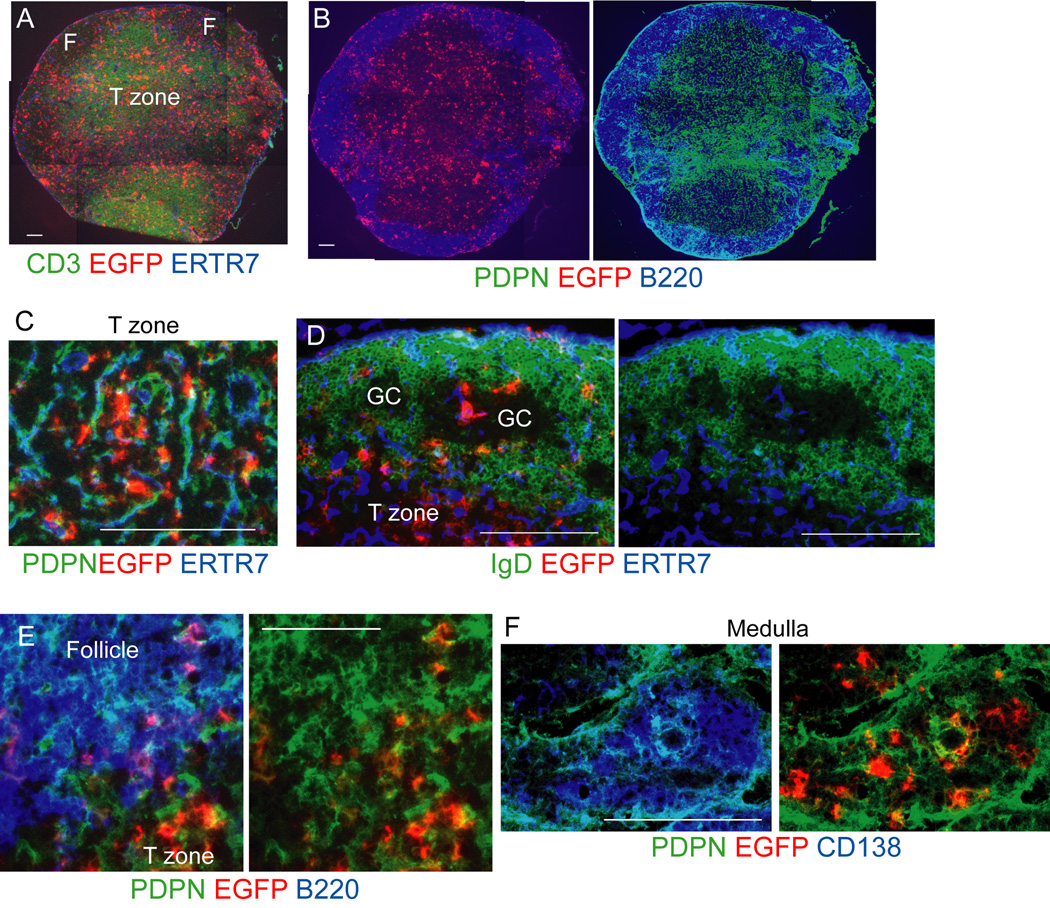
To study the localization and function of non-T non-B CD11c+ cells (Baumjohann et al., 2013; Jung et al., 2002; Tzeng et al., 2010), we generated Cd11c−DTRRag1−/− mixed chimeras whereby lethally irradiated wild-type (WT) recipients were reconstituted with 80% Cd11c−DTRRag1−/− and 20% WT bone marrow (Figure S2). In these chimeras, non-T non-B CD11c+ cells were marked by the expression of the DTR-EGFP fusion protein, At day 9 after immunization, EGFP+ cells were found throughout the T zone and also in B cell areas (Figure 1A–B). In the T zone, EGFP+ cells were closely associated with the network of PDPN+ cells and fibrils marked by ER-TR7 antibody (Figure 1C). In secondary follicles, EGFP+ cells were located within the mantle zone at the T-B border, where they were associated with PDPN+ cells (Figure 1D–E). EGFP+ cells were also located in other areas of the mantle zone and within germinal centers (Figure 1D). Consistent with findings using CD11c staining (Mohr et al., 2009; Tzeng et al., 2010), EGFP+ cells also localized within the medullary cords with CD138+ AFCs (Figure 1F) and were associated with PDPN+ reticular cells (Figure 1F). Together, these chimeras suggested that non-T non-B CD11c+ cells were associated with PDPN+ reticular cells in multiple compartments.
Figure 1. Non-T non-B CD11c+ cells localize with PDPN+ cells in multiple compartments.
Cd11c−DTRRag1−/− mixed chimeras were immunized in footpads with OVA-Alum at day 0 and draining popliteal nodes were taken at day 9. Sections were stained for the DTR-EGFP fusion protein and other indicated markers. (A–B) Nearby sections from the same lymph node showing EGFP+ cell localization relative to (A) T cells and (B) B cells and PDPN+ cells. F=follicle. (C–F) EGFP+ cell localization in the (C) T zone (D) follicles, (GC=germinal center), (E) follicular mantle zone, (F) medulla. Results representative of at least 3 lymph nodes. For (A-B, D, F), bar = 100um. For (C,E), bar=50um. See also Figures S1-S2.
Non-T non-B CD11c+ cells maintain reticular cell numbers and the ongoing immune response
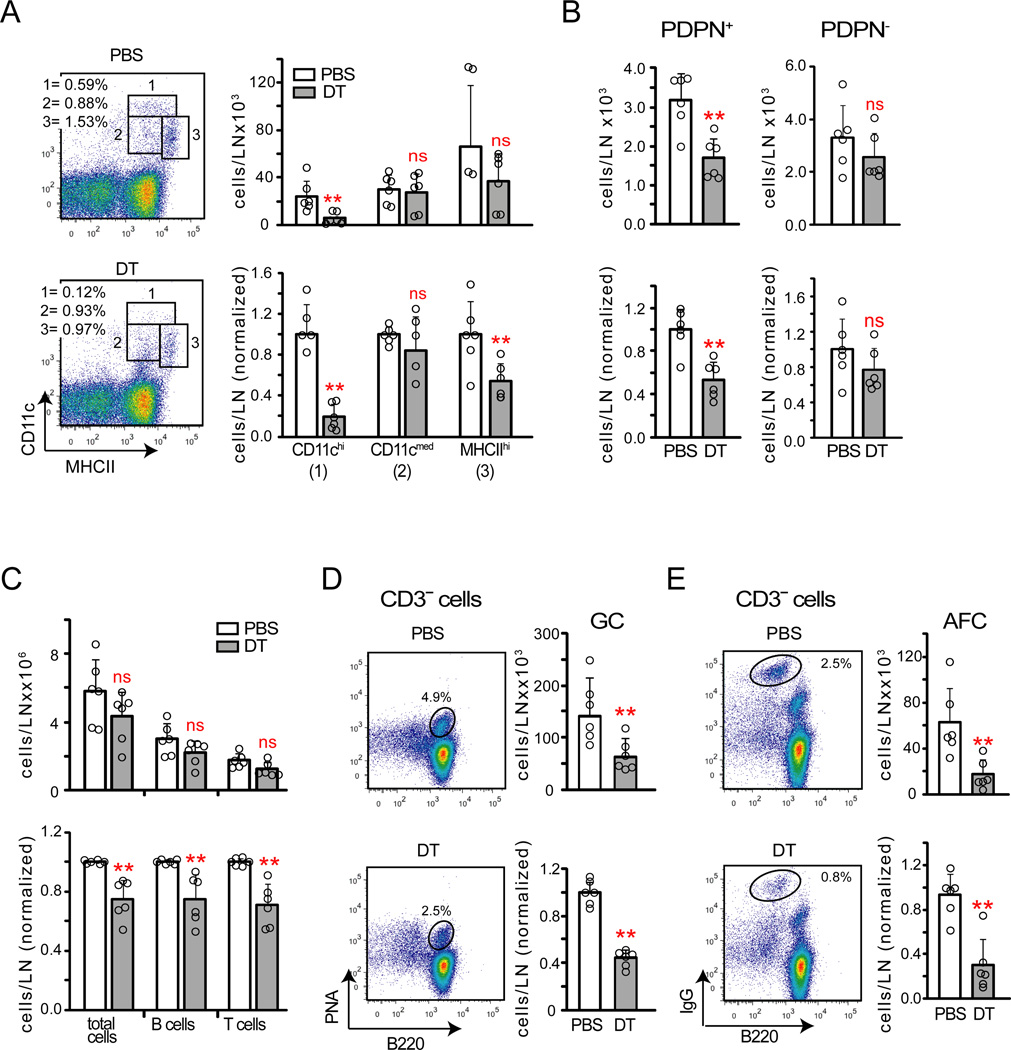
Diphtheria toxin (DT) treatment of the Cd11c−DTRRag1−/− chimeras depleted CD11chi cells by 80% and MHCIIhi DCs by about 50% (Figure 2A). Blood endothelial cell ICAM-1 was upregulated (Figure S3A), consistent with previous findings (Tzeng et al., 2010). PDPN+ reticular cell numbers were partially reduced without a concomitant increase in PDPN− reticular cells (Figure 2B), suggesting the possibility of disrupted PDPN+ reticular cell survival. CD11c+ cell depletion also reduced lymph node cellularity and the numbers of total B and T cells, germinal center B cells, and IgG+ AFCs (Figure 2C -E). Activated CD4+ T cell percentages and regulatory T cell percentages were not altered (Figure S3B–C). CD11c+ cell depletion reduced lymphocyte numbers even upon blockade of lymph node entry and exit (Figure S3D), suggesting that lymphocyte loss was due to compromised survival. We did not detect more AFCs in the blood circulation or bone marrow (Figure S3E), suggesting that the AFC loss was due not to lymph node egress but to disrupted AFC survival. These results together suggested that CD11c+ cells maintain PDPN+ reticular cell numbers and the ongoing immune response.
Figure 2. Non-T non-B CD11c+ cells maintain PDPN+ reticular cells and the ongoing immune response.
Cd11c−DTRRag1−/− mixed chimeras were immunized on day 0, treated with PBS or DT on day 8, and were examined on day 9. (A) CD11c+ cell numbers. Left: Representative flow cytometry plots. Right: Absolute and relative numbers. (B–C) Absolute and relative numbers of (B) PDPN+ and PDPN reticular cells and (C) total, B220+ B, and CD3+ T cells. (D–E) Left: Representative flow cytometry plots. Right: Absolute and relative numbers of (D) germinal center B cells and (E) AFCs. For (A–E), data pooled from 3–4 independent experiments. Each point represents one mouse; error bars represent SD. **=p<.01 in an unpaired t test comparing DT to PBS samples. See also Figure S3.
Classical DCs maintain reticular cell survival and the ongoing immune response
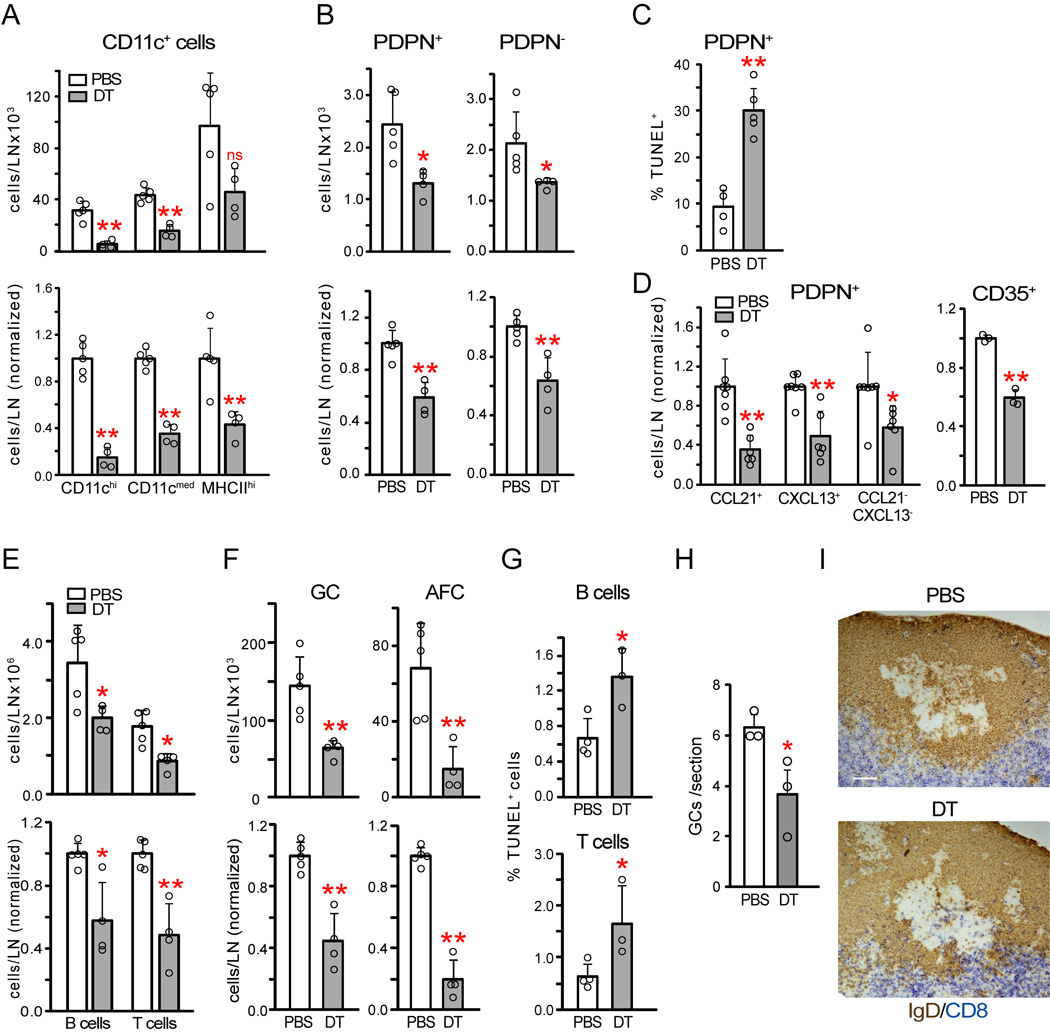
To understand whether the effects of CD11c+ cell depletion reflected primarily depletion of DCs, we used Zbtb46-EGFP reporter mice and zDC-DTR mice that express diphtheria toxin receptor in Zbtb46-expressing cells. Zbtb46 is a transcription factor also expressed by endothelial cells that distinguishes classical Flt3-dependent DCs from monocytes and monocyte-derived cells (Meredith et al., 2012; Satpathy et al., 2012). Zbtb46-EGFP mice (Satpathy et al., 2012) confirmed DC localization in the T zone, within the mantle zone at the T-B boundary, and more sparsely in the rest of the mantle zone and in germinal centers (Figure S4A). To deplete DCs without depleting endothelial cells, we generated zDC-DTR#x02794;WT chimeras. Similar to CD11c+ cell depletion, DT treatment of these chimeras depleted the vast majority of CD11chi cells and partially depleted the MHCIIhi population (Figure 3A). CD11cmed cells were also partially depleted (Figure 3A). As with CD11c+ cell depletion, DC depletion reduced PDPN+ reticular cell numbers without increasing PDPN− cells (Figure 3B). PDPN+ reticular cell loss was accompanied by an increased percentage of TUNEL+ cells (Figure 3C), suggesting that the cell loss was due to disrupted survival. To understand whether DC depletion disrupted reticular cell survival in multiple compartments, we stained reticular cells intracellularly for CCL21 to identify T zone and medullary cells and CXCL13 to identify follicular cells (Figure S4B), respectively. CCL21−CXCL13− cells that likely included medullary cells were calculated by subtracting CCL21+ and CXCL13+ cell numbers from total PDPN+ cell numbers. Such subsetting showed that CCL21+ cells comprised the largest subpopulation at day 9 (Figure S4B). FDCs were identified by CD35 staining and were both PDPN+ and PDPN− (Figure S4C)(Jarjour et al., 2014; Link et al., 2007). PDPN+ FDCs were partially CXCL13+ and partially CCL21+ (Cremasco et al., 2014; Wang et al., 2011) and comprised 20–35% of each PDPN+ subpopulation (Figure S4C). Upon DC depletion, each of the PDPN+ CCL21+, CXCL13+, CCL21−CXCL13− subpopulations was reduced in number (Figure 3D), while the proportions of CD35+ (ie FDCs) and CD35− cells in each subpopulation stayed constant (Figure S4C). This suggested that reticular cells in the T zone, follicular mantle, and medulla along with PDPN+ FDCs were lost with DC depletion. Total (PDPN+ and PDPN−) FDCs were also reduced in number (Figure 3D). These results suggested that DCs maintain the survival of PDPN+ reticular cells in the T zone, follicular mantle, and medulla and of FDCs in germinal centers.
Figure 3. Classical DCs maintain reticular cell survival and the ongoing immune response.
zDC-DTR chimeras were immunized on day 0, injected with PBS or DT on day 8, and examined on day 9. (A) CD11c+ cell numbers. Data pooled from 3 experiments. (B) PDPN+ and PDPN reticular cell numbers. Data pooled from 4 experiments. (C) PDPN+ reticular cell TUNEL staining, expressed as percentage of PDPN+ reticular cells that is TUNEL+. Data pooled from 2 experiments. (D) Reticular cell subpopulation numbers. Left: Normalized numbers of indicated PDPN+ reticular cell subsets. Data from 4 experiments. Right: normalized numbers of total (PDPN+ and PDPN−) CD35+ cells. Data from 2 experiments. (E) B and T cell and (F) germinal center B cell and AFC numbers. Data from 3 experiments. (G) TUNEL staining in B220+ B cells and (CD45+) Thy1+ T cells. Data pooled from 2 experiments. (H) Number of germinal centers per tissue section. Data from 3 experiments. (I) Compartmental integrity. Representative of at least 3 pairs of mice. White bar=100um. For (A–H), each symbol represents 1 mouse; error bars represent SD. *=p<.05 and **=p<.01 when compared to PBS samples using unpaired t-test. See also Figure S4.
DC depletion also reduced B and T cells, germinal center B cells, and AFCs (Figure 3E–F). Lymphocyte staining by TUNEL was increased (Figure 3G), supporting the idea of disrupted lymphocyte survival. Morphologically, follicles with germinal centers were present (Figure S4D), although germinal center numbers were reduced (Figure 3H). Activated caspase 3+ cells were increased (Figure S4E), suggesting disrupted germinal center B cell survival. There was greater mixing of CD8+ T cells with IgD+ B cells at the T-B border (Figures 3I, Figure S4F), suggesting that reticular cells in this region were among the mantle zone cells lost. DC depletion at homeostasis did not affect PDPN+ reticular or lymph node cell numbers (Figure S4G). These results indicated that classical DCs maintain reticular cell survival, integrity of lymph node compartments, and the ongoing immune response in stimulated lymph nodes.
CD11c+ cells maintain stromal-derived lymphocyte survival factor expression
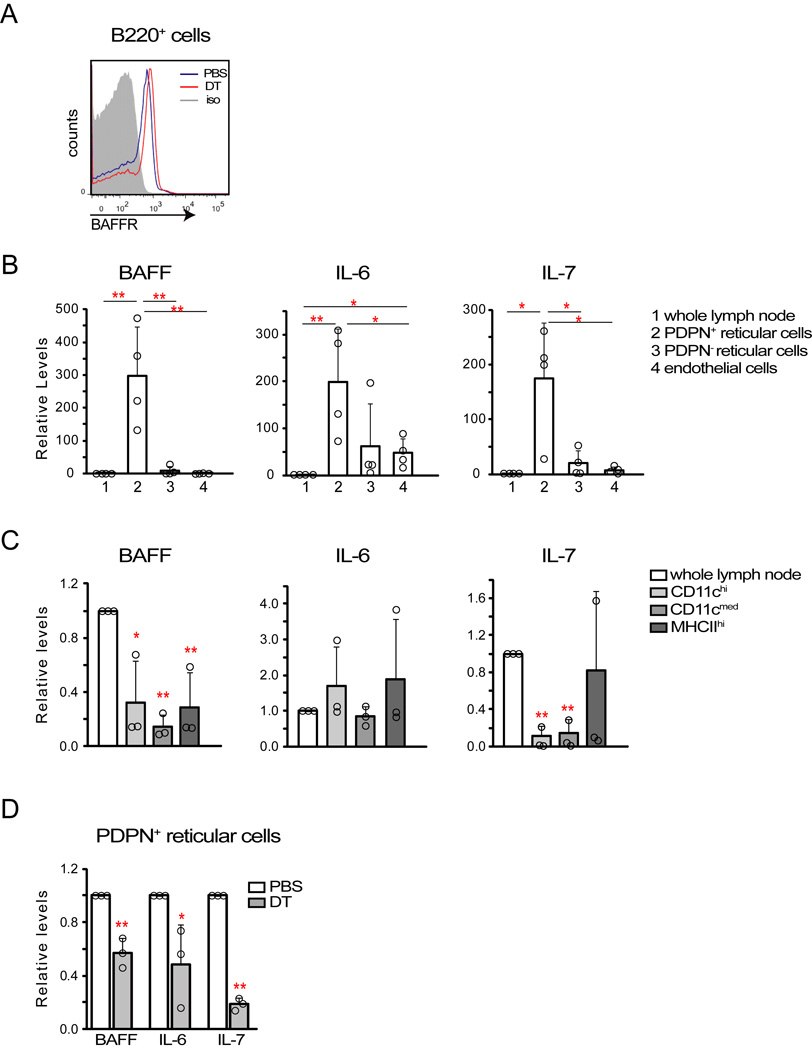
The disrupted lymphocyte survival with DC depletion led us to ask about survival factor expression. B cells in CD11c+ cell-depleted nodes had higher expression of the BAFF receptor (Figure 4A), suggesting reduced BAFF availability (Lesley et al., 2004). PDPN+ reticular cells at day 9 expressed high amounts of BAFF, IL-6, and IL-7 relative to other vascular-stromal cells and to CD11c+ cells (Figure 4B-4C). Upon CD11c+ depletion, amounts of PDPN+ reticular cell BAFF, IL-6, and IL-7 were all reduced (Figure 4D), consistent with the loss of reticular cells in multiple compartments. These results suggested that DC depletion led to reduced stromal expression of lymphocyte survival factors which, along with loss of DC-derived survival factors (Mohr et al., 2009), contributed to disrupting the immune response.
Figure 4. CD11c+ cell depletion reduces stromal expression of lymphocyte survival factors.
Mice were immunized on day 0 and treated or examined at indicated time points after immunization. (A) B cell BAFF receptor expression in Cd11c−DTRRag1−/− chimeras given PBS or DT at day 8 and examined at day 9. Representative of 3 pairs of mice from 2 experiments. (B–C) Expression of BAFF, IL-6, and IL-7 by (B) vascular-stromal and (C) CD11c+ cell subsets. Indicated populations were sorted from (B) day 9 or (C) day 8 draining nodes and cytokine expression evaluated by qPCR. “Whole lymph node” indicates collagenase-digested lymph node cells prior to cell separation. Note differences in scale in (B) and (C). Data pooled from (B) 4 and (C) 3 independent experiments. (D) Cytokine expression by PDPN+ reticular cells at day 9 after CD11c+ cell depletion at day 8 in CD11c-DTR mice. Data pooled from 3 independent experiments. For (B–D), each symbol represents 1 sample sorted from (B, D) 6–8 mice or (C) 4–5 mice. *=p<.05 and **-p<.01 using unpaired t-test compared to controls or as indicated; error bars are SD.
DC-derived LTβR ligands maintain reticular cells
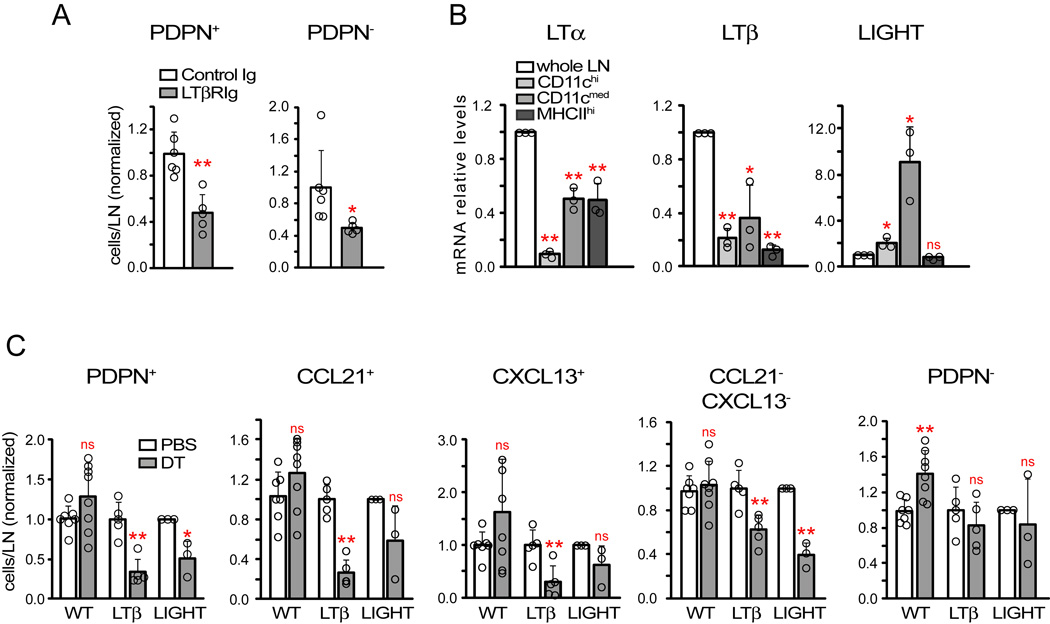
LTβR can modulate stromal function and lymph node CD11c+ cells can express LTβR ligands (Boulianne et al., 2012; Lu and Browning, 2014; Moussion and Girard, 2011; Zhu et al., 2011). LTβR-Ig reduced PDPN+ reticular cell numbers (Figure 5 A), suggesting that DCs could potentially maintain reticular cell survival via LTβR ligands α1β2 and LIGHT (TNFSF14). CD11c+ cells expressed less LTα and LTβ relative to unfractionated lymph node cells (Figure 5B), reinforcing the idea that lymphocytes are major sources of LTα1β2 (Junt et al., 2006) but not excluding the potential importance of DC-derived LTα1β2. CD11c+ cells, on the other hand, expressed more LIGHT than unfractionated lymph node (Figure 5B). We tested the importance of DC-derived LTα1β2 and LIGHT by reconstituting wild-type mice with a mix of zDC-DTR and WT bone marrow or substituted the WT bone marrow with Ltb−/−Rag1−/− or Tnfsf14−/−Rag1−/−bone marrow (Figure S5A). All T and B cells in these chimeras were LTβ- or LIGHT-sufficient, and DT treatment at day 8 after immunization resulted in DCs that had relative LTβ or LIGHT deficiency in the experimental groups (Figure S5B). Lymph node cellularity was similar across groups before depletion and reticular cell numbers were actually greater in LTβ-deficient chimeras (Figure S5C–D), but DT treatment reduced total PDPN+ reticular cells specifically in the LTβ- and LIGHT-deficient chimeras (Figure 5C). LTβ deficiency affected each of the CCL21+, CXCL13+, and CCL21CXCL13- subpopulations (Figure 5C). The reduction in the LIGHT-deficient chimeras was more variable, reaching statistical significance only in the CCL21−CXL13− subset (Figure 5C). These results suggested that both DC-derived LTα1β2 and LIGHT maintain PDPN+ reticular cell survival.
Figure 5. DC-derived LTβR ligands maintain reticular cells.
Mice were immunized on day 0 and treated or examined at indicated time points after immunization. (A) PDPN+ and PDPN− reticular cell numbers in mice treated with LTβR-Ig at day 8 and examined at day 9. Data pooled from 2 experiments. (B) LTβR ligand mRNA expression by “whole” unsorted lymph node cells and indicated CD11c+ cell subsets at day 8. Data from 3 experiments. (C) Role of DC-derived LTβR ligands. zDC-DTR: WT, LTβ-, or LIGHT-deficient mixed chimeras were immunized at day 0, given PBS or DT at day 8, and reticular cell subset numbers were analyzed at day 9. Data from 3 separate experiments for both LTβ- and LIGHT-deficient chimeras. For (A, C), each symbol represents 1 mouse. For (B), each symbol represents 1 sample sorted from 4–5 pooled mice. *=p<.05 and **=p<.01 using unpaired t-test compared to indicated samples; error bars are SD. See also Figure S5.
In vitro, CD11c+ cells maintained the survival of serum-starved PDPN+ reticular cells (Figure S5E, left and middle). This effect was blocked by LTβR-Ig (Figure S5E, left and middle), further supporting the concept that DCs express LTβR ligands to maintain reticular cell survival.
PDPN maintains reticular cell survival and the ongoing immune response
We noted a drop in reticular cell PDPN expression with DC depletion (Figure 6A), and a drop was detectable as early as 5.5–8 hours after DT injection in association with increased apoptosis (Figure S6A). In vitro, CD11c+ cells induced PDPN upregulation, which was blocked by LTβR-Ig (Figure S5E, right). These data suggested the possibility that DC modulated PDPN to maintain reticular cell survival.
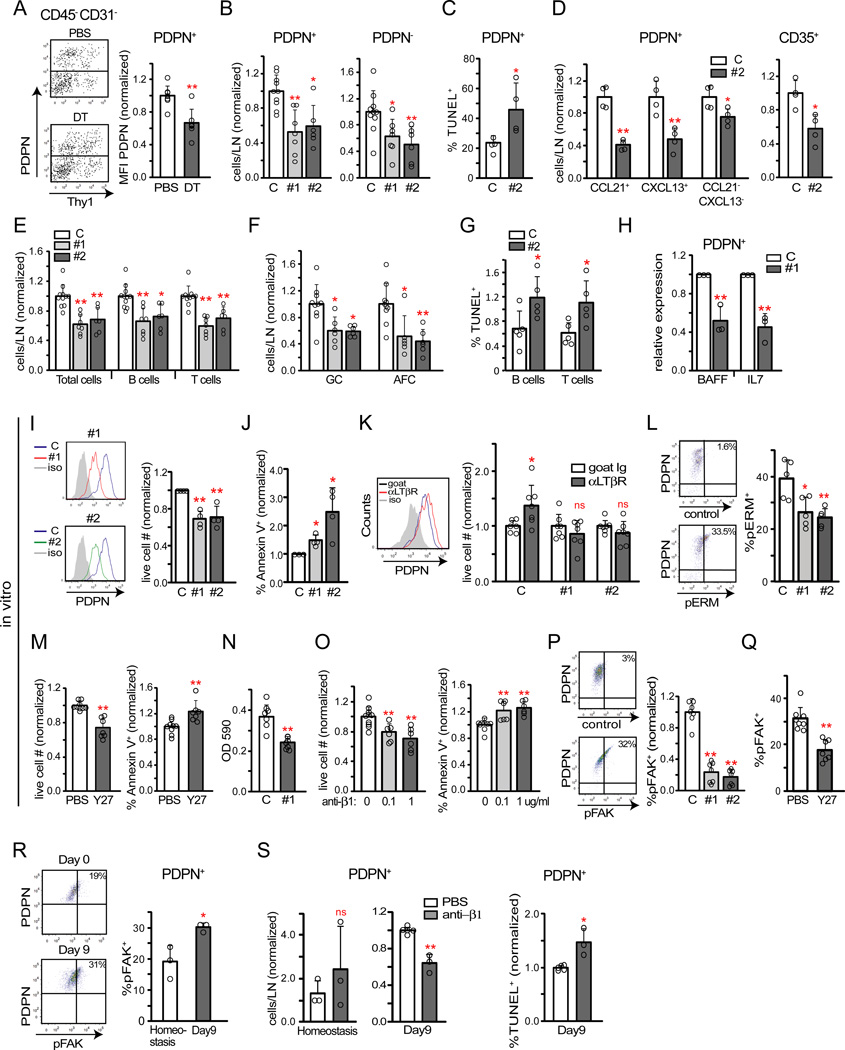
Figure 6. PDPN on reticular cells is regulated by DCs and maintains cell survival.
(A) PDPN expression on PDPN+ reticular cells in zDC-DTR chimeras at 24 hours after DC depletion. Left: representative flow cytometry plots. Right: Pooled analysis of PDPN geometric mean fluorescence intensity from 5 experiments. (B–H) Mice were immunized at day 0, injected with control or indicated PDPN-targeted siRNAs (PDPN #1 or PDPN #2) on days 6 and 7, and draining popliteal nodes examined at day 9. (B) PDPN+ and PDPN reticular cell numbers. Data pooled from at least 3 experiments. (C) PDPN+ reticular cell TUNEL expression. Data pooled from 2 experiments. (D) Relative numbers of indicated PDPN+ reticular cells subsets and of total (PDPN+ and PDPN−) CD35+ cells. Data pooled from 2 experiments. (E–F) Relative numbers of (E) total, B, and T cells and (F) germinal center B cells and AFCs. Data from at least 3 experiments. (G) B and T cell TUNEL staining. Data from 2 experiments. (H) mRNA expression of indicated cytokines by sorted PDPN+ reticular cells. Each symbol represents 1 sample sorted from 8 mice, at 1 sample per condition per experiment. (I–L) Cultured PDPN+ reticular cells were transfected with control, PDPN#1, or PDPN#2 siRNA. (I) Left: Histograms showing extent of PDPN knockdown. Right: Relative cell counts of live gated cells at 48–72 hours after transfection. Data pooled from 4 experiments, at 1 well per condition per experiment. (J) Annexin V binding. Percent of DAPI− or 7AAD− cells that are Annexin V+ was normalized to control siRNA samples. Each symbol represents one well; data pooled from 3 experiments, at 1–2 wells per condition per experiment. (K) Effects of LTβR stimulation. At 48 hours after transfection, media was changed to serum-free media with goat IgG or anti-LTβR. Live-gated cells were analyzed 24 hours later. Left: PDPN expression in control siRNA samples. Right: Cell numbers, normalized to the goat IgG sample. Each symbol represents one well; data pooled from 4 experiments, at 1–3 wells per condition per experiment. (L) pERM expression. Cells were examined 48 hours after transfection. Left: Representative flow cytometry plots of control siRNA cells. Right: pERM levels expressed as a percentage of cells that are pERM+. Each symbol represents one well; data pooled from 2 experiments, at 2–3 samples per condition per experiment. (M) Effects of Y27632 treatment on cell survival. Left: Numbers of DAPI− cells. Right: Annexin V binding on DAPI− cells. Each symbol represents one well; data pooled from 2 of 4 similar experiments, at 3–5 wells per condition per experiment. (N) Cell adhesion upon PDPN knockdown, shown as OD at 590nm. Each symbol represents 1 well; data pooled from 3 experiments, at 2–3 wells per condition per experiment. (O) Effect of β 1 integrin blockade on cell survival. Left: Numbers of DAPI− cells. Right: Annexin V binding on DAPI− cells. Each symbol represents one well; data pooled from 2 experiments, at 3–5 wells per condition per experiment. (P) pFAK (at Y397) expression with PDPN knockdown. Cells were examined at 48 hours after transfection. Left: Representative flow cytometry plot of control siRNA cells. Right: pFAK expression. Percent of cells that are pFAK+ was normalized to control siRNA samples. Each symbol represents 1 well; data pooled from 3 experiments at 2–3 wells per condition per experiment. (Q) Reticular cell pFAK expression with Y27632 treatment. Each symbol represents 1 well; data pooled from 2 experiments, at 2–3 wells per condition per experiment. (R) PDPN+ reticular cell pFAK expression at homeostasis and at day 9 after immunization. Left: Representative flow cytometry plots. Right: pFAK expression. Each symbol represents one mouse; data pooled from 2 experiments. (S) Effect of β1 integrin blockade for 24 hours in vivo. Left: PDPN+ reticular cell numbers at homeostasis and at day 9 after immunization. Right: TUNEL staining in day 9 mice. Each symbol represents one mouse; data shown is from 2 (homeostasis) to 3 (day 9) experiments. For (A–S), *=p<.05 and **=p<.01 when compared to indicated controls using unpaired t-test; error bars are SD. See also Figure S6.
We asked whether PDPN mediated reticular cell survival by treating mice with PDPN-targeted siRNA on days 6 and 7 after immunization and analyzing on day 9. In initial experiments using labeled siRNA, about 30% of PDPN+ reticular cells in draining nodes were labeled, while only 5% of cells were labeled in non-draining nodes (data not shown), potentially reflecting differential blood flow. By day 9, PDPN-targeted siRNA reduced PDPN+ reticular cell numbers (Figure 6B) and increased TUNEL staining (Figure 6C). As with DC depletion, CCL21+, CXCL13+, and CCL21−CXCL13− populations and total FDCs were reduced upon PDPN targeting (Figure 6D). These results suggested that PDPN maintained reticular cell survival in immunized nodes.
PDPN targeting also reduced B and T cell, germinal center B cell, and AFC numbers (Figure 6E–F) and increased lymphocyte TUNEL staining (Figure 6G). Germinal centers were fewer in number and CD8+ T cells and IgD+ B cells mixed at the T-B boundary (Figure S6B–C). PDPN+ reticular cells expressed less BAFF and IL-7 upon PDPN knockdown (Figure 6H). These results suggested that, similar to DC depletion, PDPN knockdown disrupted the ongoing immune response, potentially by disrupting reticular cell survival and reducing lymphocyte survival factor expression.
Because PDPN is also expressed on lymphatic endothelial cells and myeloid cells (Astarita et al., 2012; Schacht et al., 2003), we asked whether PDPN on reticular cells directly modulated cell survival. PDPN knockdown in cultured reticular cells reduced cell numbers (Figure 6I) and increased annexin V staining (Figure 6J), echoing the increased apoptosis seen in vivo. In serum-starved cultures, agonist anti-LTβR treatment increased PDPN expression and cell numbers (Figure 6K). However, PDPN knockdown prevented the increase in cell numbers (Figure 6K), supporting the idea that DC-derived LTβR ligands mediate reticular cell survival via PDPN.
We next examined how PDPN mediated cell survival. PDPN activates Rho GTPases and modulates phosphorylation of the ezrin, radixin, moesin (ERM) family of cytoplasmic signaling proteins that link membrane receptors to the cytoskeleton (Acton et al., 2014; Astarita et al., 2015; Martin-Villar et al., 2006). This signaling was recently identified to mediate cell contractility in lymph node reticular cells (Acton et al., 2014; Astarita et al., 2015). Consistent with this PDPN signaling pathway, PDPN knockdown reduced the amount of phosphoERM (pERM) (Figure 6L). The extracellular domain of PDPN can associate with a number of cell surface molecules and this domain may be key for mediating ERM phosphorylation (Astarita et al., 2012; Astarita et al., 2015); adding PDPN-Fc to disrupt PDPN interactions with other membrane proteins also resulted in reduced cell numbers and pERM (Figure S6D). CLEC-2 on DCs can bind PDPN and act as an antagonist (Acton et al., 2014; Astarita et al., 2015), but CLEC-2-Fc effects can be transient in vitro (Acton et al., 2014) and had not influenced cell numbers by 48 hr after CLEC2-Fe treatment. In vivo, DC depletion reduced reticular cell pERM (Figure S6F). ERM phosphorylation and cell contraction downstream of PDPN are blocked in vitro by the Rho kinase (ROCK) inhibitor Y27632 (Acton et al., 2014; Astarita et al., 2015; Martin-Villar et al., 2006), and Y27632 also disrupted cell survival (Figure 6M). Together, these results suggested that PDPN mediates reticular cell survival via the same Rho-ROCK-ERM pathway that mediates cell contractility.
Cell contractility is linked to cell-matrix adhesion, which can modulate cell survival (Geiger et al., 2009). PDPN is a positive regulator of cell adhesion (Astarita et al., 2015; Schacht et al., 2003), and PDPN knockdown reduced cell adhesion (Figure 6N). Blocking β1 integrins reduced cell survival (Figure 6O), suggesting that cell-matrix adhesion was important for cell survival in our system. Focal adhesion kinase (FAK) phosphorylated at Y397 is a major transducer of integrin-mediated cell survival signals (Mitra and Schlaepfer, 2006), and PDPN knockdown reduced pFAK phosphorylation at Y397 (Figure 6P). Similar to PDPN knockdown, anti-PDPN reduced cell survival and amounts of phosphoFAK (pFAK) (Figure S6G). ROCK inhibition also reduced pFAK amounts, establishing linked regulation of contractility and adhesion in these cells. In vivo, DC depletion reduced amounts of pFAK (Figure S6H). Together, these results suggested that PDPN mediates cell survival at least in part via Rho and modulation of integrin-mediated cell adhesion.
Consistent with the in vivo PDPN-knockdown results, administration of anti-PDPN at day 8 after immunization had reduced reticular cell numbers by day 9 (Figure S6I). In contrast, anti-PDPN in homeostatic mice for 24 hours did not reduce cell numbers (Figure S6I), echoing the recent finding that anti-PDPN for 48 hours at homeostasis increases reticular cell proliferation (Astarita et al., 2015). These results suggested that PDPN plays different roles in homeostatic and stimulated lymph nodes. We asked whether these context-dependent differences could be related to differences in cell adhesion. pFAK was upregulated along with PDPN at day 9 after immunization, suggesting upregulation of cell adhesion. Cell adhesion upregulation can be a survival mechanism to counter the pro-apoptotic effects of TNFα or other insults (Fornaro et al., 2003), and we asked whether adhesion was more important for cell survival in inflamed lymph nodes than in homeostatic ones. Indeed, β1 integrin-blocking antibody reduced PDPN+ reticular cell survival at day 9 but not at homeostasis (Figure 6S). These results suggested that PDPN during the re-establishment of quiescence maintains a level of cell-matrix adhesion that permits reticular cell survival.
Survival factor supplementation or LTβR stimulation rescues the immune response upon DC depletion
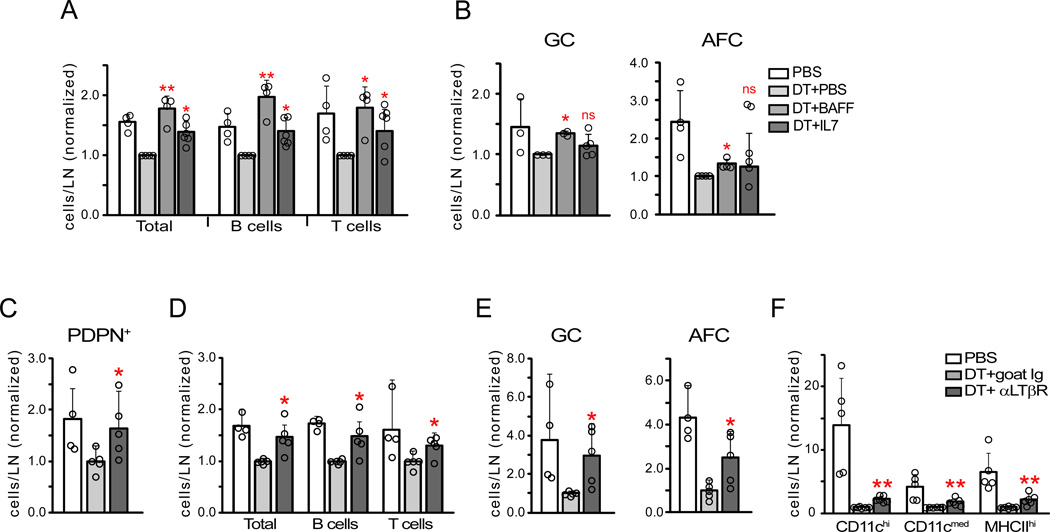
We tested the extent to which loss of survival factors contributed to the lymphocyte loss in DC-depleted mice. BAFF rescued total B and germinal center B cell numbers (Figure 7A), supporting the idea that DC depletion reduced BAFF-expressing mantle zone reticular cells and FDCs. T cell numbers were also rescued (Figure 7A), perhaps in part reflecting T cell responses to BAFF (Mackay and Leung, 2006). AFC numbers were partially rescued (Figure 7B), suggesting roles for additional factors. IL-7 rescued T cell numbers (Figure 7A), consistent with the loss of IL-7-expressing T zone reticular cells. IL-7 also rescued total B cell numbers (Figure 7A), potentially reflecting a role for IL-7 in the medulla or follicles (Link et al., 2007). BAFF and IL-7 supplementation had similar effects when PDPN was knocked down (Figure S7A). These results together supported the idea that reduced stromal-derived survival factor, likely in combination with loss of DC-derived factors (Mohr et al., 2009), contributed to the disrupted immune response in DC-depleted mice.
Figure 7. Disrupted immune responses with DC depletion can be rescued by survival factor administration or LTβR stimulation.
(A–B) BAFF and IL-7 administration. zDC-DTR chimeras were given PBS or DT at day 8 after immunization and indicated cytokine at 5–8 hours after PBS or DT. Mice were examined 20–24 hours after cytokine injection. The numbers of cells in indicated populations were normalized and compared to “DT+PBS” numbers. (A) Total, B, and T cell numbers. (B) Germinal center B cell and AFC numbers. Data from 4 experiments. (C–F) LTβR stimulation. Same experimental setup as in (A–B), except that zDC-DTR chimeras received control goat Ig or agonist anti-LTβR after DT. The numbers of indicated populations were normalized and compared to “DT+goat Ig” numbers. (C) PDPN+ reticular cells. (D) Total, B, and T cells. (E) Germinal center B cells and AFCs. (F) CD11c+ cell subsets. Data from 4 experiments. For (A–F), each symbol represents 1 mouse. *=p<.05 and **=p<.01 when compared to indicated controls using unpaired t-test; error bars are SD. See also Figure S7.
We asked if preventing reticular cell loss despite DC depletion could prevent lymphocyte loss. zDC-DTR chimeras at day 8 after immunization were treated with DT and, 5.5–8 hours later, with control or agonist LTβR antibody. Anti-LTβR prevented the DT-induced reduction of PDPN+ reticular cells, B cells, T cells, and germinal center cells, and partially prevented the loss of AFCs (Figure 7C–E). CD11c+ cell numbers were modestly increased with anti-LTβR but the numbers were still low compared to non-depleted mice (Figure 7F), suggesting that the effects of anti-LTβR reflected effects on the stromal compartment and not on LTβR+ CD11c+ cells. Our results together suggested that the stromal disruption induced by DC depletion played an important role in disrupting the ongoing immune response and supported the idea that a DC-stromal axis maintains immune responses.
Discussion
Our data have suggested a model whereby, upon the re-establishment of vascular-stromal quiescence, DCs maintain stromal integrity and, consequently, the ongoing immune response (Figure S7B). The localization of DCs in all compartments, the CD11c+ cell-reticular cell co-cultures, and the in vivo importance of DC-derived LTβR ligands, which are cell-associated (Boulianne et al., 2012; Lu and Browning, 2014), support the idea that DCs act locally and directly to maintain reticular cell survival. The more numerous T and B cells also express LTβR ligands, suggesting scenarios that argue against DCs as direct mediators of reticular cell survival. One potential scenario is that DC depletion disrupted naïve lymphocyte entry (Moussion and Girard, 2011), and the resulting loss of lymphocyte-derived LTβR ligands primarily disrupted reticular cell survival. However, DC depletion during the re-establishment of quiescence activated endothelial cells and increases lymphocyte entry (data not shown). Additionally, DC depletion reduced lymphocyte numbers despite blockade of entry and exit, suggesting that lymphocyte loss was not primarily due to altered trafficking. Another possible scenario is that DCs act directly on lymphocytes to mediate lymphocyte survival, and the loss of lymphocyte-derived LTβR ligands upon DC depletion was the primary cause of reticular cell loss. While DT treatment reduced lymph node cellularity of LTβ- and LIGHT-deficient but not WT mixed chimeras (data not shown), T and B cells do not express LTβR, suggesting that loss of lymphocytes was secondary to reticular cell loss. The most conservative interpretation of our data, then, is that DCs directly maintain reticular cell survival, and the lymphocyte loss caused by disruption of the DC-stromal axis may potentially amplify reticular cell loss. Resident DCs are relatively non-motile when compared to lymphocytes (Bajenoff et al., 2006), and we speculate that prolonged association with reticular cells might allow more effective delivery of the membrane-bound signals.
Given the density of DCs in the T zone, a role for DCs in maintaining T zone function was less unexpected than the role in supporting follicular function. However, DCs were present in the follicles, and the high density within the mantle zone at the T-B boundary suggests the possibility that reticular cells in this region were among the CXCL13 and BAFF-expressing cells affected upon DC depletion. DCs more sparsely populated germinal centers, and these may directly maintain FDCs, which, in turn, maintain germinal center B cell survival in part via BAFF. That DC depletion reduced FDC numbers at a proportion similar to that of other reticular cell subsets suggests that germinal center DCs are efficient regulators of FDC survival. Alternatively, disruption of the mantle zone with DC depletion may have also contributed to germinal center disruption, as the reticular networks in these adjacent compartments are closely situated (Bannard et al., 2013). In follicles, then, our results suggested that DCs maintain total and germinal center B cell survival at least in part by maintaining mantle zone reticular cells and FDCs.
Our results implicated CD11chi DCs as key mediators of reticular cell survival during the re-establishment of quiescence, as these DCs accumulated during this phase and were the most completely depleted in the zDC-DTR chimeras. This model of late-accumulating CD11chi DCs maintaining PDPN expression and cell survival complements the recently proposed model whereby early-accumulating MHCIIhi DCs express CLEC-2 to inhibit PDPN-mediated cell contractility and allow lymph node growth (Acton et al., 2014; Astarita et al., 2015). However, our experiments do not rule out roles for other DC populations in mediating reticular cell survival, and further studies are needed to understand the roles of each DC subset and the LTβR ligands they use.
In addition to identifying a novel DC-LTβR-PDPN-reticular cell survival axis that supports immune responses, our findings highlighted the idea that reticular cells at homeostasis and in immunized nodes are in different functional states. Reticular cell survival was regulated by DCs, PDPN, and cell adhesion only in stimulated nodes. This raises interesting questions about the drivers and other consequences of this functional change. These results also suggest that targeting a DC-stromal axis might allow specific targeting of inflamed lymph nodes in disease. Lymph nodes in a chronic lupus model appear to be in a state of re-established vascular, and presumably, stromal, quiescence (Chyou et al., 2012), suggesting that a DC-stromal axis could maintain autoantibody generation. Such an axis may also exist in lymphoproliferative diseases and in tertiary lymphoid organs (Lambrecht and Hammad, 2012). Further understanding the role and regulation of the DC-stromal axis has the potential to lead to new therapeutic approaches for immune diseases.
Experimental Procedures
Mice
Mice between 6–14 weeks old were used. C57Bl/6 mice were from The Jackson Laboratory (JAX)(Bar Harbor, ME), Taconic Farms (Hudson, NY), or National Cancer Institute (NCI)(Frederick, MD) or our own breeding colony. Congenic CD45.1+ mice were from NCI or our own breeding colony. Cd11c−DTR mice and Rag1−/− mice originally from JAX were bred at our facility and intercrossed to generate Cd11c−DTR Rag1−/−mice. zDC-DTR mice (Meredith et al., 2012) were bred at our facility. Ltb−/− mice (Koni et al., 1997) were intercrossed with Rag1−/−mice to generate Ltb−/− Rag1−/− mice in our facility. Tnfsf14−/− mice (Zhu et al., 2011) were intercrossed with Rag1−/− mice to generate Tnfsf14−/−Rag1−/− mice at the University of Chicago. All animal procedures were performed in accordance with the regulations of the Institutional Animal Use and Care Committee at the Hospital for Special Surgery (New York, NY).
Flow cytometric staining of lymph node cells and calculations
For flow cytometric staining and analysis, lymph nodes were digested with type II collagenase (Worthington, Lakewood, NJ) prior to staining with antibodies (Chyou et al., 2011).
For calculations of cells per lymph node for indicated cell populations, the % of total of the gated population was multiplied to the total cell count per lymph node. For normalized values, the control sample was set to 1 and the value from the experimental sample was normalized accordingly. For experiments where there was more than one control sample, the control values were averaged and the individual control and experimental samples were calculated relative to this average value.
Enumeration of germinal centers in tissue sections
Seven micron tissue sections were collected every 5 sections through the lymph node and the largest (i.e. middle) 5–10 sections were stained for IgD and either PNA (Vector) or CD21 and CD35. Identifiable germinal centers IgD− area that is PNA+ or (CD21 and CD35)+ in each section were counted and the maximal number of germinal centers per section was recorded.
Reticular cell cultures
PDPN+ reticular cell cultures were generated as described (Benahmed et al., 2014). Briefly, collagenase digested lymph node cells were cultured for 7–8 days, depleted of CD45+ and CD31+ cells via magnetic selection, and used directly or passaged one time before use for experiments. SiRNA transfections were performed following manufacturer’s instructions for the Oligofectamine Reagent kit (Invitrogen). For anti-LTβR experiments, at 48 hours after transfection, the media was changed to serum-free RPMI and 3ug/ml goat IgG or anti-LTβR (R&D Systems) was added. The cells were trypsinized 24 hours later and examined by flow cytometry. For Y27632 and anti-β1 experiments, cells were plated overnight to 80–90% confluency, treated with Y27632 at 10uM (Acton et al., 2014; Astarita et al., 2015) (Calbiochem-EMD Millipore, Billerica, MA) or anti-β1 integrin (HMB1-1) at the indicated concentrations in serum-free media before being harvested at 48 hours for cell numbers and annexin V staining or at 24 hours for pFAK staining.
Cell adhesion assay
To test cell adhesion, 96-well plates were coated with 20% FBS for 1 hour at 37 degrees Celsius. Five thousand reticular cells were added per well in serum-free media and allowed to adhere for 30 minutes before fixation and staining with 0.2% crystal violet in 2% ethanol x 20 minutes. Bound crystal violet was extracted with 50ul of 1%SDS and OD read at 590nm.
Supplementary Material
Acknowledgements
We acknowledge everyone in the Lu lab for helping hands, Alessandra Pernis and Jane Salmon and labs for helpful discussions, Michel Nussenzweig for zDC-DTR mice, and Jeff Browning and Adrian Erlebacher for helpful comments on the manuscript. This work was supported by R01 AI079178 (TL), Alliance for Lupus Research (TL), and the St. Giles Foundation (TL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: VK, DD, SC, TT, CR and YL designed, performed, and interpreted the experiments. YF and NR provided reagents and critical input on the manuscript. TL and WS designed and interpreted experiments. VK and TL wrote the paper.
References
- Acton SE, Farrugia AJ, Astarita JL, Mourao-Sa D, Jenkins RP, Nye E, Hooper S, van Blijswijk J, Rogers NC, Snelgrove KJ, et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514:498–502. doi: 10.1038/nature13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita JL, Acton SE, Turley SJ. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283. doi: 10.3389/fimmu.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR, Nieves-Bonilla JM, Song K, Kondo Y, Woodruff MC, Gogineni A, et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol. 2015;16:75–84. doi: 10.1038/ni.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannard O, Horton RM, Allen CD, An J, Nagasawa T, Cyster JG. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39:912–924. doi: 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Benahmed F, Chyou S, Dasoveanu D, Chen J, Kumar V, Iwakura Y, Lu TT. Multiple CD11c+ cells collaboratively express IL-1beta to modulate stromal vascular endothelial growth factor and lymph node vascular-stromal growth. J Immunol. 2014;192:4153–4163. doi: 10.4049/jimmunol.1301765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulianne B, Porfilio EA, Pikor N, Gommerman JL. Lymphotoxin-sensitive microenvironments in homeostasis and inflammation. Front Immunol. 2012;3:243. doi: 10.3389/fimmu.2012.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M, Lu TT. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J Immunol. 2011;187:5558–5567. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyou S, Tian S, Ekland EH, Lu TT. Normalization of the lymph node T cell stromal microenvironment in lpr/lpr Mice is associated with SU5416-induced reduction in autoantibodies. PLoS ONE. 2012;7:e32828. doi: 10.1371/journal.pone.0032828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol. 2014;15:973–981. doi: 10.1038/ni.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Denton AE, Roberts EW, Linterman MA, Fearon DT. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proc Natl Acad Sci U S A. 2014;111:12139–12144. doi: 10.1073/pnas.1412910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Plescia J, Chheang S, Tallini G, Zhu Y-M, King M, Altieri DC, Languino LR. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. J Biol Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase H, Kanno Y, Kojima M, Hasegawa K, Sakurai D, Kojima H, Tsuchiya N, Tokunaga K, Masawa N, Azuma M, et al. BAFF/BLyS can potentiate B-cell selection with the B-cell coreceptor complex. Blood. 2004;103:2257–2265. doi: 10.1182/blood-2003-08-2694. [DOI] [PubMed] [Google Scholar]
- Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC, Klauschen F, Bajenoff M. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med. 2014;211:1109–1122. doi: 10.1084/jem.20132409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Tumanov AV, Harris N, Heikenwalder M, Zeller N, Kuprash DV, Aguzzi A, Ludewig B, Nedospasov SA, Zinkernagel RM. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur J Immunol. 2006;36:2061–2075. doi: 10.1002/eji.200626255. [DOI] [PubMed] [Google Scholar]
- Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-Like Reticular Stromal Cell Layer Common to Adult Secondary Lymphoid Organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- Lu TT, Browning JL. Role of the lymphotoxin/LIGHT System in the development and maintenance of reticular networks and vasculature in lymphoid tissues. Front Immunol. 2014;5:47. doi: 10.3389/fimmu.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Leung H. The role of the BAFF/APRIL system on T cell function. Semin Immunol. 2006;18:284–289. doi: 10.1016/j.smim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. Journal of Cell Science. 2006;119:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mionnet C, Mondor I, Jorquera A, Loosveld M, Maurizio J, Arcangeli ML, Ruddle NH, Nowak J, Aurrand-Lions M, Luche H, Bajenoff M. Identification of a new stromal cell type involved in the regulation of inflamed B cell follicles. PLoS Biol. 2013;11:e1001672. doi: 10.1371/journal.pbio.1001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, MacLennan ICM. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. Embo J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Tzeng TC, Chyou S, Tian S, Webster B, Carpenter AC, Guaiquil VH, Lu TT. CD11chi dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes. J Immunol. 2010;184:4247–4257. doi: 10.4049/jimmunol.0902914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cho B, Suzuki K, Xu Y, Green JA, An J, Cyster JG. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J Exp Med. 2011;208:2497–2510. doi: 10.1084/jem.20111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-Y, Vogt TK, Favre Sp, Scarpellino L, Huang H-Y, Tacchini-Cottier F, Luther SA. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc Natl Acad Sci U S A. 2014;111:e109–e118. doi: 10.1073/pnas.1312585111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Yang Y, Wang Y, Wang Z, Fu Y-X. LIGHT regulates inflamed draining lymph node hypertrophy. J Immunol. 2011;186:7156–7163. doi: 10.4049/jimmunol.1002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.