Abstract
Obesity in pregnancy is the leading cause of maternal and fetal morbidity, and gestational weight gain (GWG) is one modifiable risk factor that improves pregnancy outcomes. Most pregnant women gain more than the 2009 Institute of Medicine recommendations, particularly overweight and obese women. GWG even less than the 2009 IOM guidelines in obese women may improve pregnancy outcomes and reduce large-for-gestational-age (LGA) infants, an independent risk factor for childhood obesity, without increasing small-for-gestational-age (SGA) infants. Unfortunately, despite the fact that over 50 interventional trials designed to decrease excess GWG have been conducted, these interventions have been only modestly effective, and interventions designed to facilitate weight postpartum weight loss have also been disappointing. Successful interventions are of paramount importance not only to improve pregnancy outcomes but also for the future metabolic health of the mother and her infant, and may be key in attenuating the trans-generational risk on childhood obesity.
Keywords: Obesity, pregnancy, gestational weight gain, pregnancy outcomes, Institute of Medicine guidelines, large for gestational age infants, fetal programming, childhood obesity, postpartum weight loss, diet, physical activity, interventions
Obesity in Pregnancy as Leading Risk Factor for Adverse Maternal and Offspring Outcomes
Obesity in pregnancy is the leading cause of maternal and perinatal morbidity as women who enter pregnancy overweight or obese now represent the majority of pregnant women [1,2]. According to the 2011–2012 National Health and Nutrition Examination survey (NHANES), 58.5% of all US women of childbearing age are overweight or obese. The proportion of women of childbearing age who are overweight or obese varies by ethnic group, from 26% of Asian women, 55% of non-Hispanic white women, 70% of Hispanic women and 80% of non-Hispanic black women [2]. Obesity rates have tripled from 1960 at 9.3% to 32% in 2010,[3] and there has been a marked increase in Class 3 (BMI ≥ 40) obesity rates [4]. Obesity rates in children have paralleled the growth in maternal obesity rates, with about a quarter of 2–5 year olds and one-third of school-age children (including adolescents) now overweight or obese in the U.S. [2]. Furthermore, half of childhood obesity occurs among children who have already become obese by age 5 years [5]. According to World Health Organization (WHO) estimates, there will be 70 million obese children globally by 2025 and the number of overweight or obese infants and children has increased by over 25% from 31 million in 1990 to 44 million in 2012 [6].
Obesity profoundly contributes to the leading causes of mortality including type 2 diabetes and cardiovascular disease (CVD) and the WHO recognizes obesity as the fifth greatest risk factor for global deaths. Obesity-related complications in pregnancy are estimated to increase the cost of prenatal care by 16-fold [1]. Obesity profoundly increases the risk for multiple pregnancy complications, including gestational diabetes (GDM; OR 4.0), preeclampsia or gestational hypertension (OR 3.2), as well as thromboembolic disease, sleep apnea, respiratory complications, cardiomyopathy, and preterm delivery [1,7,8]. Obesity also increases the rate of failed induction of labor, cesarean delivery, post-operative wound disruptions, anesthesia complications, and lactation failure [1,8]. The rate for cesarean delivery approaches 50% with a body mass index (BMI) >35 compared to 21% with a BMI <30 [8].
Maternal obesity also markedly increases the risk of newborn complications. Maternal obesity independently increases the risk of first trimester and recurrent pregnancy losses (OR ~3.0), congenital malformations (OR 2–3.0) including central nervous system, cardiac, and gastrointestinal defects and cleft palate, and perinatal mortality (OR 3–4.0), likely due to tissue hypoxia due to excessively grown fetuses outgrowing their placental blood supply [1]. One study concluded that for every unit increase in BMI the relative risk of a neural tube defect increased 7% [9]. Surprisingly, the majority of large-for-gestational-age (LGA) babies (>90th percentile) are not born to mothers with diabetes or GDM but to obese mothers and the macrosomia risk (>4000 gm) approaches 20% [10–12]. In fact, bariatric surgery has been shown to improve outcomes in subsequent pregnancies in women with severe obesity, including decreasing rates of GDM, preeclampsia, macrosomia, and cesarean delivery [13].
Long-Term Outcomes in Offspring from Obesity in Pregnancy
Even more sobering are the long-term implications of obesity in pregnancy to the offspring [14]. The prevalence of childhood obesity is ~2.5 times higher in offspring of obese women compared to women with normal BMIs [15]. Maternal obesity is also an independent risk factor for excess neonatal fat, a more important predictor of childhood adiposity and a stronger risk factor than GDM in predicting offspring obesity by dual x-ray absorpitometry (DXA) at 9 years of age [11]. The Developmental Origins of Health and Disease (DoHAD) hypothesis, now substantiated by extensive animal and human research, strongly supports that both maternal nutrient deficiency as well as nutrient excess results in an acquired susceptibility to metabolic disease later in life which is programmed in-utero and in early infancy (Figure 1) [16]. Given the strong association between maternal obesity and the risk of childhood obesity and glucose intolerance, the metabolic milieu of the intrauterine environment is now considered to be a key risk factor for the genesis of adult diabetes and CVD [17].
Figure 1.
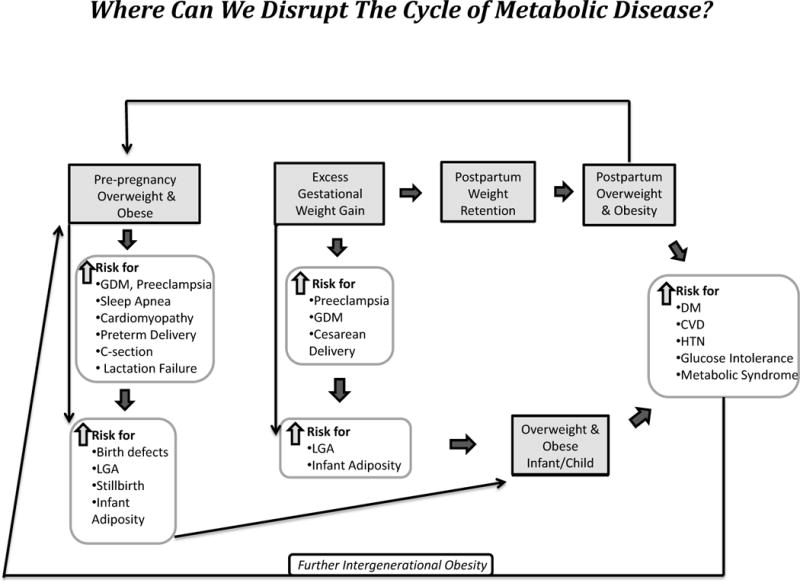
There are several potential areas for intervention that may decrease the intergenerational cycle of obesity and associated disease. Shaded boxes indicate areas for potential intervention.
The field of epigenetics offers a conceptual framework of how intrauterine metabolic factors (glucose, lipids, amino acids, growth factors, cytokines, oxidative stress) could alter DNA methylation and histone modification to change gene expression as a mechanism for this fetal programming influence [18,19]. Epigenetic changes which can be induced by an intrauterine environment characterized by an unhealthy diet, maternal obesity, and excessive weight gain may modify number, growth and function of many cells, promote adipogenesis, and later impact hypothalamic appetite regulation, pancreatic function, and alter mitochondrial and kidney function in the offspring (Figure 1) [20]. There are compelling data in animal and in non-human primate models to support that a maternal high fat diet and obesity can influence offspring mesenchymal stem cells to differentiate along adipocyte rather than osteocyte pathways [21,22] and invoke changes in the serotonergic system resulting in increased anxiety. Human epidemiologic studies suggest a correlation between obesity and a high fat diet and increased rates of anxiety, depression, ADD and autism disorders [23]. In non-human primates and animal studies, a high fat maternal diet also affects neural pathways involved with appetite regulation, promotes lipotoxicity in the fetal liver, and regulates gluconeogenic enzymes generating histology consistent with NAFLD (non-alcoholic fatty liver disease) [24,25]. Similar patterns have been demonstrated with the effect of maternal obesity alone supported by data that siblings born before maternal bariatric surgery are at a much greater risk for obesity than siblings born after weight loss surgery [27]. Recently, Brumbaugh et al. demonstrated that newborns of obese GDM mothers have evidence of increased intrahepatic fat at birth using NMR spectroscopy compared to NW mothers without GDM [28], possibly predisposing offspring to an increased risk for NAFLD. Twenty-five percent of obese 4–10 year olds already have glucose intolerance and as a growing number of these glucose intolerant children eventually become mothers themselves, the cycle is perpetuated (Figure 1).
Although obesity is the leading health risk responsible for maternal and neonatal morbidity, excess gestational weight gain (GWG) is an independent risk factor for childhood obesity [29]. It is also a risk factor that can be potentially modified in pregnancy. As we increasingly recognize that an intrauterine environment characterized by obesity, insulin resistance, nutrient excess, and diabetes may be both contributing to the obesity epidemic in children as well as contributing to increasing rates of pregnancy complications and future cardiometabolic risk, this modifiable risk factor in pregnancy should be carefully scrutinized. For women, we contend that it is time to review the evidence and re-examine our current approaches to GWG and our recommendations for “healthy” weight gain.
Evidence that Gestational Weight Gain Independently Leads to Adverse Short-and Long-Term Outcomes
Studies investigating the association between early GWG and the risk for GDM have demonstrated an increased risk in overweight and obese women [30–32]. In Gibson’s retrospective case control study, early rapid weight gain before 24 weeks of gestation was not associated with increased risk for GDM in normal weight (NW) women but was increased in overweight and obese women [33]. Several studies demonstrate the independent effect of excess GWG on the increased likelihood of cesarean delivery [34–36]. Other studies have shown an association between excess GWG and preeclampsia [7,37].
Excess GWG also increases the risk of LGA as well as infant adiposity, a significant risk factor for childhood obesity [38]. GWG is increasingly associated with risk of childhood obesity and metabolic syndrome. In a study of 4496 children ages 14–22 in the National Longitudinal Survey of Youth [39], GWG was correlated with postpartum weight retention (PPWR), LGA and child obesity. Several epidemiologic studies have found that higher GWG is associated with higher weight for height and fat mass in childhood and adolescence, as well as dysfunctional metabolic and vascular traits [40,41]. In one study of 306 infants [42] in which adiposity was measured by Pea Pod (air displacement plethysmography), overweight mothers who gained excessive weight had the greatest difference in infant fat mass compared to those who gained appropriate weight (484 grams versus 304 grams). Obese mothers gave birth to infants with the highest mean fat mass regardless of weight gain.
In a large retrospective cohort in which birth certificate data were extracted in Florida between 2004–2008 to assess the influence of maternal BMI versus GWG versus GDM on the presence of LGA infants [29], excessive GWG contributed most to LGA, although BMI may have been underestimated in the birth certificate data. Furthermore, excess GWG was associated with increased adiposity using DXA, not only at birth but also at 6 years of age in the Southhampton’s Women’s Study Group [43]. Although pre-pregnancy BMI has been shown to increase the risk of childhood metabolic syndrome at age 6–11 by nearly 2-fold, LGA at birth, in part fueled by GWG, increases the risk more than 2-fold [44].
Gestational Weight Guidelines Proposed by the Institute of Medicine
In 1990, the Institute of Medicine (IOM) published recommendations for GWG based on maternal BMI category. A major priority at that time was preventing small-for-gestational-age (SGA) offspring (weight ≤ 10th percentile for a given gestational age), due to the high costs related to the care of SGA infants and their long term health consequences. The data from the 1980′s that served as the primary basis of the 1990 guidelines demonstrated that nearly as many women were underweight as overweight (20–30% in each group) and that the prevalence of obesity in women 20–39 years was only 12.3% [4]. The 1990 guidelines primarily considered the data to minimize low birth weight or preterm birth [45]. However, by 1994 the prevalence of obesity in women of childbearing age in the US jumped to nearly 21%, and by the year 2006, 33% of women in the U.S. were considered obese, with nearly 2/3 of U.S. women either overweight or obese [4]. At the same time, the rapidly increasing prevalence of childhood obesity was being recognized, with nearly 17% of children and adolescents obese in 2008 compared to only ~5% in 1980. Globally, an increasing trend of LGA infants was being recognized in Denmark, Sweden, Canada and the US, a 20% rise from 1990–2000. In the US, by 2006, nearly 20% of all infants were considered LGA compared to only 7.6% SGA [46]. Importantly, the increase in birthweight (BW) correlated with the increase in maternal weights during the same time period [47]. Further, infants born to obese women were not simply larger in respect to both lean mass and fat mass, but primarily due to an increase in fat mass alone, which accounted for nearly 50% of the variance in body weight [47]. As the prevalence of maternal obesity and LGA infants continued to overwhelm the prevalence of underweight mothers and SGA infants, the IOM reconvened to consider maternal and infant outcome data according to GWG in the face of the adult and childhood obesity epidemic.
The IOM published new GWG guidelines in 2009 given the increasing recognition of the role of obesity on adverse pregnancy outcomes. The IOM did not offer any GWG guidelines specific to women with diabetes or other comorbidities. Surprising to many, the IOM guidelines were minimally modified compared to 1990 guidelines [48]. The guidelines were essentially unchanged for underweight, NW (BMI 18.5–24.9: 25–30 lbs), and overweight (BMI 25–29.9: 15–25 lbs) women. For obese women, they recommended an upper limit to weight gain (BMI ≥30: 11–20 lbs) but provided no distinction for classes of obesity. (Table 1)
Table 1.
2009 IOM Guidelines for GWG
| Pre-pregnancy BMI | Recommended total GWG | Recommended rates of GWG per week in 2nd and 3rd trimester* | ||
|---|---|---|---|---|
| Pounds | Kg | Pounds | Kgs | |
| Underweight < 18.5 kg/m2 | 28–40 | 12.5–18 | 1–1.3 | 0.44–0.58 |
| Normal (18.5–24.9 kg/m2) | 25–35 | 11.5–16 | 0.8–1 | 0.35–0.5 |
| Overweight (25–29.9 kg/m2) | 15–25 | 6.8–11.3 | 0.5–0.7 | 0.23–0.33 |
| Obese (≥ 30 kg/m2) | 11–20 | 5–9.1 | 0.4–0.6 | 0.17–0.27 |
Calculations assume 1.1–4.4 lb (0.5–2.5 kg) weight gain in the 1st trimester
Why were the 2009 IOM guidelines minimally modified from the 1990 guidelines in the face of a rapidly increasing adult and pediatric obesity epidemic? One contention was that women were already exceeding the GWG guidelines and that there should be more emphasis trying to get women to gain within the guidelines [49]. In recent data from 2011, approximately 48% of US women exceeded the 2009 Institute of Medicine guidelines for GWG. The majority of studies suggest that overweight and obese women are in fact more likely to exceed guidelines than NW women but absolute weight gain is less in obese than NW women [46]. Restall’s international cohort of nearly 2000 nulliparous women demonstrated that 74% had excessive GWG gain according to the 2009 IOM guidelines and that women who were overweight and obese had a 2.9 times greater odds (95% CI 2.2–3.8) and 2.5 greater odds (95% CI 1.8–3.5) of exceeding 2009 IOM guidelines, respectively [50]. Similar findings have been demonstrated in the analysis of the HAPO trial cohort of ~5000 women in which 47% of NW, 74% of overweight, and 68% of obese women exceeded the guidelines [51], and in the First Baby cohort of 3006 women in which 42% of NW, 79% of overweight, 65% of obese women exceeded the 2009 guidelines [52]. Furthermore, in a recent retrospective analysis of 466 women with GDM, who presumably received more dietary advice during pregnancy, 29% of NW, 41% of overweight, and 49% of obese women exceeded 2009 IOM guidelines. Exceeding the IOM guidelines was statistically associated with delivering an LGA baby in obese but not in overweight or NW women [53].
In addition to their focus on simply getting women to not exceed the IOM guidelines, another reason cited justifying little change in the guidelines was that the committee felt strongly about trying to balance the risk of low versus high GWG, and a concern that SGA may be more important than LGA. However, there has not been any attempt to distinguish typically defined SGA rates (<10th percentile) from those at <5th percentile, the latter being at a much higher risk for adverse outcomes. They developed their 2009 recommendations primarily on the basis of a primigravida, age 25–29, non-smoker, higher social status, and no exercise. They acknowledged that they did not consider the studies that used preeclampsia and GDM as adverse outcomes because they believed that the data to support these outcomes were weaker [54] and cited that most investigators did not measure weight gain before the diagnosis of GDM. There was not enough new evidence to change previous weight gain recommendations for adolescents, twins, or women carrying higher order multiple fetuses [49].
The committee primarily considered the outcomes of SGA, LGA, unplanned cesarean delivery, and excessive (≥ 5kg) PPWR. They also stated that since maternal BMI appeared to be the most important risk factor for infant adiposity, pre-pregnancy BMI and weight loss before pregnancy should be the primary focus. Lastly, they acknowledged that the committee evaluated data that demonstrated favorable outcomes especially among women with more severe obesity who gained less than 11 lbs or below the obligatory maternal tissue accretion and products of conception requirements (~17 lbs). However, they cautioned that the data were limited, insufficient to support specific weight gain recommendations for women with a BMI ≥ 35, and that they had reservations about recommending weight loss during pregnancy due to concerns about possible ketonemia. The weight gain per week recommendations were simply constructed as linear interpolations between the approximately 1–2 kg weight gain that most women gain in the first trimester and the target total GWG for each BMI category divided by the number of weeks in the remaining two trimesters of pregnancy.
Energy Costs of Pregnancy and Obligate GWG Requirements
If one were to better understand the energy costs of pregnancy, perhaps a more logical basis for weight gain recommendations could be made. The energy requirements of pregnant women have been derived from the incremental increase in basal metabolic rate (BMR) using calorimetry and the energy deposited in maternal and fetal tissues (changes in body protein and fat) (55). Weight gain in pregnancy results from products of conception (fetus, placental, and amniotic fluid), increases in various maternal tissues (uterus, breasts, blood and extracellular fluid), and increases in maternal fat stores. Hytten and Chamberlain developed a theoretical model to estimate energy requirements during pregnancy in a 60–65 kg women assuming an average gestational weight gain of 12.5 kg (~28 lbs) estimating ~0.93 kg for protein, 3.8 kg for fat, and ~7.8 kg for water). This model was the basis of current recommendations for energy intake in pregnant women of an extra ~300 kcal per day. The energy costs of pregnancy are divided into 3 components: 1) energy deposited in the conceptus as new tissue; 2) energy deposited as fat; 3) energy required to maintain the new tissue. However, the energy needs for maintaining a pregnancy is much greater in humans than the cost of synthesizing the products of conception, because the fetus grows slowly and the energy costs of pregnancy, i.e, sustaining and moving the increased mass, is spread over an extended period of time. The rise in maternal metabolic rate during pregnancy is driven by the metabolic cost of maintaining the conceptus and supporting maternal tissue. Because pregnant women gain remarkably different amounts of fat stores, total energy costs of pregnancy correlate with maternal fatness as well as pregnancy weight gain. For example, Swedish women who gain ~40 lbs require ~60,000 calories over the course of pregnancy to increase their fat stores compared to 12,000 kcal for Asian women in the Philippines and Thailand who gain ~ 12 lbs. The result is that true energy costs of pregnancy are driven more by the increase in maternal fat stores and energy required to maintain those fat stores than the energy needed to synthesize a conceptus, resulting in extremely wide estimates of extra calorie recommendations in pregnancy (160–500 kcal/day). Energy requirements are minimal in the 1st trimester compared to 3rd trimester according to the rate of increase in weight gain.
A slightly different approach to consider is the maternal compartmental location of weight gain in pregnancy, i.e. “where weight gain goes,” as noted in the preceding paragraph. The mother who delivers a healthy term infant would typically gain 3400 grams (7.5 lbs) for the fetus, 650 grams for the placenta (1.4 lbs), 970 grams for the increase in uterus size (2.1 lbs), 405 grams for the increase in breast development (0.9 lbs), 800 grams for amniotic fluid (1.8 lbs), and 1450 grams for the increase in blood volume (3.2 lbs), which are all considered obligate weight gain requirements [46]. In addition, most women increase their extravascular water by 1480 grams (3.3 lbs) and fat mass by 3345 grams (7.4 lbs). Thus, the obligate weight gain is ~ 7.5 kg grams (~17 lbs) which would require ~60,000 kcal or 225 kcal on average extra per day to maintain if the mother were not to utilize some of her own fat stores. The obligate weight gain plus the increase in extravascular water and fat drives the caloric requirement to 100,000 kcal, the total weight gain to ~12.5 kg (28 lbs), or an extra 375 kg/day. There is no consensus about optimal fat store increases in pregnant women. However, it would seem reasonable that although undernourished women may benefit from gaining fat stores, well-nourished women may not need to gain more fat tissue which drives the increase in energy requirements to maintain the pregnancy and directly contributes to postpartum weight retention.
The Relationship Between Gestational Weight Gain and Postpartum Weight Retention
Postpartum weight retention is an additional adverse outcome from increased GWG. GWG is the most important predictor of weight retention at one year postpartum [39,56], and predicts future overweight and obesity, and therefore confers increased risk for diabetes and CVD. Studies demonstrate that 14–25% of women retain at least 4.5 kg after pregnancy [57–59]. Studies show significant correlation between excess GWG and weight status at multiple timepoints, including increased PPWR in the early postpartum period, as well as short, medium, and long term overweight and obesity. Excess GWG has been correlated in studies with greater weight retention as early as two days after delivery [60], as well as through six weeks postpartum [61,62]. One study looked at periods of weight gain during pregnancy and noted that high GWG in early pregnancy was highly correlated with later obesity [41]. Multiple studies demonstrate that excess GWG is correlated with greater weight retention [57,58,61–63]. Even in the long term, excess GWG is highly correlated with overweight and obesity. In one cohort study, GWG predicted increased BMI fifteen years later, and weight retained at one year postpartum predicted overweight at 15 years, even in women who were NW before they became pregnant [64]. The Avon Longitudinal Study of Parents and Children (ALSPAC) of 2356 mothers in the UK demonstrated that women with excessive GWG (defined as exceeding the 2009 IOM guidelines) had a three-fold increased odds of overweight 16 years after delivery [65]. In another cohort study with 21 year follow-up, excessive GWG increased odds of overweight by 2.2 (95% CI 1.6, 2.8), and odds of being obese by 4.5 (95% CI 3.4, 5.9) [66]. An additional hazard of PPWR is that women will begin a subsequent pregnancy at a higher BMI, thereby increasing risk of maternal and fetal complications in the next pregnancy. In one retrospective cohort of over 27,000 women, women who had excessive weight gain in their first pregnancy had increased odds of 2.6 (95% CI 2.4, 2.7) of having excessive weight gain in their subsequent pregnancy [67].
Consequences of Inter-pregnancy Weight Gain or Weight Loss
Weight gain (or lack of inter-pregnancy weight loss) is also associated with adverse outcomes in the next pregnancy. Importantly, one study suggested that overweight women may even start gaining weight at 6 weeks postpartum [68] and, in fact, what some studies define as PPWR may be due to weight gain in the postpartum period. A prospective cohort study of 540 women in upstate New York demonstrated that about 25% of women had gained weight by one year postpartum [57]. In one study of over 150,000 Swedish women, an increase in BMI of only 1–2 units between pregnancies was associated with 20–40% increased risk of gestational hypertension, GDM, and LGA deliveries. A gain of 3 or more BMI units was significantly associated with an increased risk for stillbirth. Conversely, interpregnancy weight loss of at least one BMI unit was associated with decreased risk for LGA delivery [69]. An interpregnancy weight loss by 12 lbs decreased risk for GDM in overweight and obese women by approximately 75% [69]. Another recent study demonstrated that interpregnancy weight loss decreased the risk for LGA, preeclampsia, and GDM [70].
Many studies have assessed the impact of bariatric surgery and associated weight loss between pregnancies, demonstrating that bariatric surgery between pregnancies is associated with lower rates of GDM, macrosomia, and cesearean delivery [71–74] and women with Class III obesity are most likely to benefit [75,76]. In one recent meta-analysis of 17 cohort and case-control studies looking at maternal and neonatal outcomes, women who had undergone bariatric surgery had a decreased incidence of preeclampsia (OR 0.45, 95% CI 0.25–0.80), GDM (OR 0.47, 95% CI 0.4–0.56), and LGA babies (0.46, 95% CI 0.34–0.62). However, the women undergoing bariatric surgery had a higher incidence of SGA (OR 1.93, 95% CI 1.52–2.44), preterm birth (OR 1.31, 95% CI 1.08–1.58), NICU admission (OR 1.33, 95% CI 1.02–1.72), and maternal anemia (OR 3.41, 95% CI 1.56–7.44). The authors note that in the subgroup of patients who underwent laparoscopic adjustable gastric banding, the odds of SGA delivery were no longer increased [77]. Another study demonstrated that women had significantly less GWG after bariatric surgery (8.3 vs. 11.3 kg) [78]. To avoid the risk of SGA, it is recommended that women who have had bariatric surgery not be in an active weight loss phase when they become pregnant and it is often advised to wait at least 12–18 months before getting pregnant [75].
Data Supporting Less Weight Gain than IOM Guidelines in Overweight and Obese Women
Data began emerging as early as 2007 that weight gain according to the IOM Guidelines may be too liberal. In the Project Viva study of 1044 mother-child pairs, women who gained within the 2009 IOM guidelines still had a 3.8 fold increased risk of having a 3 year old at or above the 95th percentile for weight compared to those who gained less than the guidelines recommended [79]. This risk increased to a 4.3 fold in women who gained weight above the guidelines. Women who gained less than the recommended guidelines did not increase their risk of having an SGA infant. In the same cohort followed longer [80], it was shown that the lowest predicted prevalence of five adverse outcomes (LGA, SGA, preterm delivery, PPWR, and childhood obesity) was associated with a weight gain of ~11.2 kg (~25 lbs) in NW women, a weight loss of 1.2 kg (~3 lbs) in overweight women, and a weight loss of 7.6 kg (~17 lbs) in obese women. Similarly, Hinkle et al. examined ~122,000 birth records from obese women and found that Class I obese women could gain minimal weight, but that optimal range for Class II and III (BMI >35) obese women was −4.9 to 4.9 kg (−11 to 11 lbs) to avoid both SGA and LGA [81]. Other studies supporting slight weight loss in obese pregnant women include Bodnar et al. who reviewed the Magee Obstetrical and Infant database and concluded that Caucasian obese women who gained only ~50% of the IOM recommendations had a barely higher adjusted odds ratio of SGA (1.1, 1.2, and 1.2 for Class I, II, and III obesity, respectively) but a lower risk of LGA (0.9, 0.9, and 0.8, respectively) [82]. A population cohort study in Sweden found that women with Class II or III obesity who lost weight had either a decreased or unaffected risk for cesarean delivery, preeclampsia, LGA, low Apgar scores, and fetal distress. The SGA risk was unchanged [83].
Data that also considered childhood outcomes included a study with 5000 children ages 14–22 from the 1979 National Longitudinal Survey of Youth. In this cohort, Margerison Zilko et al. determined that GWG clearly increased LGA, PPWR, and child overweight. Further they distinguished that although the SGA rate decreased with sufficient GWG in underweight and NW mothers, overweight and obese mothers did not appear to need to gain “sufficient” weight to decrease the risk of SGA [39]. The investigators recommended an optimal GWG of ~5 kg (11 lbs) for overweight mothers but an optimal GWG of 0–5 kg in obese mothers (0–11 lbs). Lastly, in the 9 year follow-up of the body composition and biomarkers of offspring from ~3500 mothers in the Avon Longitudinal Study Parents/Children in the UK [55,84], the investigators concluded that GWG below the 2009 IOM recommendations reduced offspring adiposity and unfavorable biomarkers for metabolic syndrome.
As early as 1986 and in many subsequent reports, it has been shown that although there is a clear relationship between GWG and birthweight in underweight and NW women, this relationship was not the case for overweight and obese women [7,39,80,85,85–89]. Obese women did not appear to need to gain any significant weight in order to have a normally grown infant (~3500 grams) and even overweight women who did not gain any weight had, on average, an infant weighing at least 3200 grams (~7lbs). Overweight and obese women who have SGA infants often have other morbidities (e.g. chronic hypertension, obstructive sleep apnea, renal or vascular disease) that result in placental insufficiency contributing to the SGA risk [46]. In a systematic review of outcomes of the 35 highest quality studies drawn from the report conducted for the Agency for Healthcare Research and Quality, the authors concluded that there was strong support between excessive weight gain and LGA but only strong support between inadequate weight gain and SGA in NW and underweight women [90]. Further, the AHRQ encouraged the IOM to re-evaluate the GWG guidelines for overweight and obese women given these findings.
Although there have been numerous articles supporting less weight gain for higher BMI groups, [7,39,80, 82–89] a recent retrospective analysis of the data collected in 890 women who had been randomized to treatment or a control arm for mild GDM [91] challenged the conclusion that lower weight gain was optimal in both overweight and obese women [92]. The authors found that the overweight and obese women (both groups combined) who gained ≤5 kg (which included 46/188 women who lost weight) had a SGA rate higher than the women who gained >5 kg. However, the SGA rate in the ≤5 kg group (mean weight gain only 1.1 kg) was only 9.6% and less than the expected SGA rate in this population of 10%. Further, the SGA rate in the >5 kg weight gain group (mean 14.4 kg) was extremely low at 4.9%. Infant body composition was estimated by a single anthropometric flank skinfold and all conclusions regarding fat mass and lean mass were based on this single skin fold rather than more reliable estimates of body composition (Pea pod or DXA) [93]. The women who gained ≤5 kg (n=188) were more likely to be women with GDM requiring insulin and were also more likely to be obese (mean BMI 34.2) than the women who gained >5 kg (n=1053; mean BMI 30.4), which could significantly affect body composition in the infants. The authors did not separate out the women who gained 0–5 kg from those who lost weight in the their analysis of the ≤5 kg group. There was a higher LGA rate in the >5 kg group (13.2% compared to 7.5% in the ≤5 kg group). Furthermore, the mean BW in both groups was normal and >7 lbs (3258 gm in the ≤5kg group and 3466 gm in the >5 kg group) in spite of statistical significance. Although the authors concluded that lean mass was also decreased (in addition to fat mass) in the ≤ 5kg group, this is expected since the infants weighed slightly less. However, only % fat mass (not % lean mass) was actually less in the ≤5 kg group (12% versus 13.2%). Head circumference was different by only 0.3 cm (34.2 versus 34.5 cm) and length by 0.7 cm (49.3 cm versus 50 cm) in the infants born to mothers gaining ≤5 kg compared to those who gained >5 kg. An alternative conclusion supported by the data is that even when overweight and obese women gain ≤5 kg (and a significant number lose weight), the SGA rate is less than the expected rate of 10%, the LGA rate is decreased, there is a slight decrease in infant % body fat, and there are no clinically significant differences in outcomes.
Finally, a recent PRAMs survey using birth certificate data available in the U.S. conducted at a mean of 4 months after birth evaluated the infant death rate according to GWG and concluded that excessive weight gain might be protective against infant death in obese women [94]. However, infant deaths were reported up to >1 year out from delivery (61–450 days), the study did not exclude preterm births (which are higher in obese women but decreases GWG due to an earlier delivery), and neither macrosomia, age >40, or obesity class were reported, all of which increase the risk of perinatal death. Recall bias for reporting GWG is unknown but recall bias likely affected the GDM rate since only 4% of the women reported GDM, lower than expected.
Data on GWG in GDM Women
Data suggest that weight gain prior to 24 weeks is a significant risk for the development of GDM in overweight or obese women [33]. Retrospective data in 215 overweight and obese GDM Korean women demonstrated that women who gained “inadequate” GWG (mean 2.4 kg), i.e. less than the IOM guidelines, had a significantly lower insulin requirement, lower macrosomia risk, and no increase in SGA compared to women who gained according to the IOM guidelines or in excess of the IOM guidelines [95]. In the retrospective analysis of the Sweet Success California GDM program, women who gained less than the IOM guidelines (which included NW women) had a decreased risk of preterm birth, macrosomia, cesarean delivery, and need for insulin with a minimal increase in SGA (1.39, CI 1.01 to 1.9), likely driven by insufficient weight gain in NW women [96]. In a retrospective cohort of 322 GDM overweight and obese women, 19% lost weight (mean 1.4 kg) between their GDM diagnosis and delivery. After adjustment for age, race, parity, A1C, GWG prior to GDM diagnosis and gestational weight at delivery, the BW of these infants was 283 grams lighter in the overweight women with no difference in SGA and with the same trend in the obese women [97]. Lastly in the recent observational cohort study of 802 Irish women (ATLANTIC-DIP) in which 65% had GDM, 23% had T1DM, and 12% had type 2 diabetes, those who gained excessive GWG needed more insulin and were more likely to deliver LGA infants in comparison to women who gained according to the guidelines. However the outcomes of women who actually gained less than the IOM guidelines were not reported [98].
What Can Be Done to Decrease GWG and are Interventions Effective?
While some have suggested that pregnancy may be a “teachable moment,” for overweight and obese women to focus on healthy eating and physical activity habits [56], in practice, effecting behavioral change can be a formidable challenge. Over the past 15 years, there have been over 50 published interventions designed to decrease GWG or to encourage weight gain within established guidelines for pregnant women. Despite the large number of studies that have been conducted, results have been mixed and the overall quality of evidence is low to moderate. In the past five years there have been at least 12 reviews and meta-analyses published summarizing the effects of these studies looking at diet and/or physical activity interventions to reduce GWG. Of these, eight identified a significant reduction in GWG [99–106] and four did not [107–110]. In the positive reviews, the overall reduction in GWG was modest, ranging from 0.4 to 2.2 kg. For example, in the Thangarantinam et al. meta-analysis, of 34 randomized controlled trials through January 2012, there was an overall reduction of 1.42 kg (95% CI 0.95 to 1.89 kg) in GWG compared to controls, as well as a reduction in the incidence of preeclampsia, gestational hypertension, GDM, and preterm delivery. There was no overall impact of interventions on adherence to IOM guidelines (RR 0.85, 0.66 to 1.1). However, when limited to ten studies focusing on dietary changes alone, the effect was larger, a reduction in GWG of 3.84 kg (2.45 to 5.22 kg), and a reduction in risk of GDM and hypertension. This is in contrast to 14 studies focusing on physical activity alone with an overall reduction of only 0.72 kg (1.2 to 0.25 kg), and ten studies with a mixed approach (diet and physical activity) with an overall reduction of 1.06 kg (1.67 to 0.46 kg). When limited to 11 interventions in overweight and obese women only, the overall effect was a 2.41 kg (4.04 to 1.26) reduction in GWG. In their overweight and obese population there was no reduction in BW or significant change in other clinical outcomes. When women with diabetes were excluded from the analysis, all of the effect sizes (i.e. difference between the groups) became larger [99]. The other positive meta-analyses derived modest GWG reductions of similar magnitude, ranging from 1.2 to 2.2 for diet or diet plus physical activity [100–104]. In Choi et al.’s 2013 review of physical activity interventions through December 2011, the overall effect was estimated at 0.91 kg reduction in GWG (1.76 to 0.06) [105] and Sui et al. found a significant reduction of 0.36 kg for five trials antenatal exercise interventions though 2011 for overweight and obese women [106].
In contrast, several meta-analyses and reviews have been published which concluded that the evidence was insufficient and/or there was no significant effect. In the 2012 Cochrane review, the authors included 27 trials and concluded that overall results were not statistically significant and not consistent [107]. Similarly, Campbell’s 2011 review of five controlled trials concluded there was no significant difference in pooled effect between intervention and control [110], and Ronnberg reviewed eight controlled intervention studies (only four randomized) and identified no significant difference between intervention and control groups. They also judged the quality of evidence to be too low to derive any evidence-based recommendations [108]. The 2010 Dodd systematic review of antenatal interventions in overweight and obese women identified seven dietary intervention trials and found no statistically significant differences between interventions and controls for mean GWG, LGA, or any other reported outcomes [109]. A more recent systematic review looking specifically at technology-supported dietary and lifestyle interventions in healthy pregnant women identified just five randomized controlled trials meeting criteria, of which only one was completed. In that trial which included 321 healthy pregnant women, a video-based lifestyle intervention (“Video Doctor”) did not result in statistically significant differences in GWG vs. the control group [111].
Since these reviews were published, several other randomized controlled trials have been published. The ROLO (Randomized cOntrol trial of LOw glycemic index diet) study randomized 800 non-diabetic women with a history of a macrosomic first delivery into a low glycemic diet intervention or control group. Although there was no difference seen in the primary outcome of macrosomic deliveries, women in the intervention group gained significantly less weight (1.3 kg difference between groups) and were more likely to gain within IOM guidelines, although the weight difference was modest [112]. Petrella et al. randomized 61 first trimester overweight or obese women into a Therapeutic Lifestyle Changes (TLC) Program or a control group. The GWG in the TLC group was significantly different only for obese but not overweight (6.7 vs 10.1 kg GWG) [113]. Vesco et al. randomized 114 obese pregnant women to individualized calorie goals and weekly group meetings vs. control, and noted that women in the intervention group gained significantly less weight (5.0 kg vs. 8.4 kg, mean difference −3.4 kg, 95% CI −5.9 to −1.8), and had a significantly lower proportion of LGA babies (9% vs. 26%, OR 0.28, 95% CI 0.09 to 0.84) [114].
Two studies have randomized pregnant women with GDM to metformin or insulin and have looked at GWG as a secondary outcome. In one trial, women randomized to metformin after diagnosis with GDM gained 0.4±2.9 kgs, vs. 2.0±3.3 kgs in women randomized to insulin [115]. In another study, women randomized to metformin after GDM diagnosis gained 3.3±1.4 kgs, vs. 4.5±1.7 kgs in the insulin group [116]. One ongoing study in the UK will examine GWG as a secondary outcome in pregnant women without diabetes (Metformin in Obese Non-diabetic Pregnant Women (MOP).
Evidence for Interventions to Decrease Postpartum Weight Retention
Despite the importance of decreasing PPWR to reduce the risk for future diabetes and CVD, the postpartum period can be a challenging time to intervene in a woman’s life. Women describe multiple barriers to lifestyle change, including time constraints related to taking care of a newborn, responsibilities for other family members, financial constraints, efforts directed to lactation, and sleep deprivation [117–119]. In the past 15 years, over 25 trials of postpartum interventions have been conducted. In the most recent review of these studies, P. van der Pligt et al. reviewed studies through October 2012 and identified eleven controlled trials, with mean weight loss in the studies ranging from 1.9 kg to 7.8 kg, of which seven out of eleven were successful at decreasing PPWR [120]. Similarly, Nascimento et al. identified eleven studies of postpartum physical exercise interventions, with or without diet, with a mean weight loss of 2.57 kg (95% CI 3.66 to 1.47) [121]. They determined that interventions with diet and also with objective targets were the most effective intervention strategies. In Neville et al.’s review of studies through June 2012, specifically looking for weight management interventions in lactating women within two years of delivery, they identified six studies and noted that dietary interventions were most efficacious and yet few studies were tailored to lactating women [122]. A recent Cochrane review looking at diet and/or exercise for postpartum weight loss found 12 studies meeting criteria for inclusion, determining an overall modest effect for diet alone (−1.7 kg, 95% CI −2.1 to −1.3) or diet and exercise (−1.9 kg, −3.0 to −0.9) [123].
Several postpartum intervention studies have recently been published that were not included in these reviews. In the KAN-DO trial, 400 overweight and obese postpartum women were randomized to a family-based intervention with a primary outcome goal to reduce obesity in children; postpartum weight loss was included as a secondary outcome. There was no difference between groups in weight loss or in diet quality [124]. In another recent study, 129 overweight women were randomized into either a Mediterranean diet arm or a normal diet for lactation based on the USDA MyPyramid for Pregnancy and Breastfeeding, and both groups lost a modest amount of weight (−2.3 kg ±3.4 for Mediterranean and −3.1 kg ±3.4 for MyPyramid), with no difference between groups [125]. Wilkinson et al. randomized 71 women into a postpartum weight management correspondence program, finding low engagement and no differences between groups [126].
Recently several investigators have focused on improving engagement using technology, as opposed to primarily group or one-on-one face-to-face interventions, in an attempt to improve reach in the postpartum population. In one recently published study, postpartum Japanese women were randomized into an active video game arm or control and given a Wii Nintendo console and Wii Fit Plus to use for 40 days. Investigators noted increased caloric expenditure and increased weight loss (−2.2 kg ±0.9 intervention vs. −0.5 kg ±0.7 control) [127]. In another pilot study, 18 low-income ethnic minority postpartum women were randomized to the Healthy4Baby intervention arm consisting of text messages and weekly calls with a health coach or a control group. After 14 weeks the intervention group had greater weight loss (−2.9 kg ±3.6 vs. 0.5 kg ±2.3) [128]. A recent pilot study utilizing a phone-based adaptation of the DPP for women with GDM during pregnancy demonstrated a non-significant trend in reaching postpartum weight goals for women, and a larger trial is ongoing [129]. Nicklas et al. demonstrated that women with recent GDM randomized to a web-based intervention, Balance after Baby, lost significantly more weight than controls (−2.8 kg, 95% CI −4.8 to 0.7 vs. +0.5 kg, −1.4 to +2.4). Participants in the Balance after Baby program were closer to their pre-pregnancy weight at 12 months postpartum (−.7 kg, −3.5 to +2.2 intervention vs. +4.0 kg, +1.3 to +6.8 control) [130].
Other factors which may be important in the postpartum period are relatively understudied, including the biological effects of sleep deprivation, appetite changes, postpartum depression, and environmental stressors that may contribute to the difficulties in achieving weight loss during this period. In addition, the relationship between breastfeeding and postpartum weight loss remains unclear. A recent review of 37 prospective studies and eight retrospective studies concluded that the majority of studies (63%) reported little or no association. However, 4/5 high quality studies identified in this review did show a significant relationship [131]. In the infant, weight gain in the first 6 months of life is especially predictive of later obesity [132] and most studies suggest that breastfeeding appears to attenuate the risk of excess infant growth [133]. The majority of studies looking at breastfeeding and later child adiposity, in both glucose tolerant women and in women with recent GDM, suggest that breastfeeding, especially for at least 6 months, is protective [134–138]. A significant body of evidence supports that breastfeeding also has highly beneficial effects in the development of innate immunity and decreasing allergies and that the oligosaccharides in breast milk are likely to favorably affect the infant microbiome [139,140].
On-going Pregnancy and Postpartum Trials
In addition to the recent studies that have been published, there are now multiple large ongoing studies to test interventions in pregnant and postpartum women, which we hope will answer many of these lingering questions. In the randomized controlled multicenter UPBEAT (UK Pregnancies Better Eating and Activity Trial), Briley and colleagues have randomized over 1500 obese pregnant women into a low glycemic diet and physical activity intervention delivered via 9 sessions with a health trainer with the primary objective of decreasing the risk of GDM and LGA deliveries [141,142]. In the multi-center DALI trial (Vitamin D and Lifestyle Intervention for gestational diabetes mellitus prevention), Jelsma and colleagues are planning to enroll 880 pregnant women with a BMI ≥29 kg/m2 into an 8 arm trial, testing diet, physical activity and vitamin D supplementation in various combinations, with a goal of reducing excessive GWG and improving fasting glucose and insulin sensitivity [143]. The HELP (Healthy Eating and Lifestyle in Pregnancy) trial plans to randomize 570 obese pregnant women into a weekly weight management intervention group or control, with the primary goal of reducing BMI at 12 months postpartum [144]. Nagle and colleagues plan to randomize overweight and obese pregnant women in Australia to participate in an intervention they developed called EDGE (Educate, Develop Goals, Engage), using brief telephone, text, and email contacts with the goal of decreasing the incidence of GDM, as well secondary outcomes including restricting GWG to IOM guidelines, decreasing LGA and improving OGTT results [143]. In the GeliS (acronym for “Gesund leben in der Schwangerschaft” /healthy living in pregnancy trial), Rauh and colleagues aim to randomize 2500 pregnant women in Bavaria into an intervention with a primary goal of decreasing the proportion of participants with excessive GWG [146].
In addition to these multiple large trials taking place worldwide, there are seven clinical centers conducting ongoing studies as part of the LIFE-Moms (Lifestyle Interventions in Expectant Moms) consortium. These include Phelan and colleagues’ Healthy Beginnings study, with an intent to randomize 350 obese women into a multi-component lifestyle intervention including partial meal replacements, Pi-Sunyer and colleagues’ LIFT (Lifestyle Intervention for Two) trial, which intends to randomize 210 overweight and obese women into an intensive lifestyle intervention, Joshipura and colleagues’ Pregnancy and Early Lifestyle Improvement Study (PEARLS) intends to randomize 400 overweight and obese women into a group-based lifestyle intervention with the goal of increasing the percent of women gaining within the 2009 IOM guidelines. Other studies in the LIFE-Moms consortium include MOMFIT (Maternal-Offspring Metabolics: Family Intervention Trial), in which Peaceman and colleagues plan to randomize 300 ethnically diverse OW and obese pregnant women into a behavioral intervention using a modified DASH diet as well as monitoring via Smartphone and internet. Additionally, Klein and colleagues’ study intends to randomize 266 overweight and obese African-American women into an intervention adding a diet and exercise lifestyle program to an existing home visit program. Redman and colleagues are conducting the Expecting Success trial, in which they are randomizing 306 overweight and obese women into a personalized gestational weight management program delivered in person or by Smartphone, vs. control; and Knowler and colleagues are randomizing 200 OW and obese American Indian pregnant women into an intensive lifestyle program vs. control [147].
Expert Commentary
The stakes are high for an intergenerational influence of maternal obesity, GWG, and nutrient excess on both short-term as well as long-term adverse outcomes for the mother and her offspring (Figure 1). Given the available data, a multi-pronged approach stressing the critical importance of weight reduction before pregnancy, GWG within or less than guidelines, decreasing PPWR, and avoiding inter-pregnancy weight gain in overweight and obese women is essential given the increased maternal and neonatal morbidity associated with obesity and the strength of pre-gravid BMI in predicting adverse outcomes and infant adiposity. Underscoring the benefit of a healthy diet that is both low in simple carbohydrates and saturated fats is also critical. There are accumulating human data that a high fat diet and maternal triglycerides are also correlated with worsening maternal insulin resistance and excess fetal fat accretion in humans [148–150] and convincing data from non-human primates consuming high fat diets, demonstrating lipid deposition in the liver, changes in appetite regulation, and abnormal behavior in the offspring [16, 24–26].
Most women (>50%) do not gain within the IOM guidelines, and thus delivering a strong message to avoid excessive weight gain and its adverse effects on LGA, preeclampsia, GDM, cesarean delivery, postpartum weight retention, and childhood obesity is an enormously important first step. The IOM has a user-friendly website for both providers and patients with on-line tools to facilitate weight gain according to the guidelines (www.iom.edu/healthypregnancy). However, we believe that the 2009 IOM weight gain recommendations for overweight and obese women may be too liberal given consistent data that although GWG is related to BW and SGA in underweight and NW women, this same relationship does not hold for overweight and obese women. Given the recently cited data, the significant contribution of weight gain to adverse pregnancy outcomes, LGA, PPWR, and the maternal and pediatric obesity epidemic, we recommend that NW and overweight women should strive to gain weight at the lower end of the IOM recommendations (~11 kgs or 25 lbs for NW women and ~7 kg or 15 lbs for overweight women). Obese women with a BMI of 30–34.9 do not appear to be at risk of SGA without any significant weight gain and a GWG not exceeding 5 kg (~10–11 lbs) should be recommended. For women with a BMI of ≥35, increasing data suggest that no weight gain is safe. Although there are limited data in obese women with GDM, they are likely to benefit even more from no significant weight gain. It is certainly feasible that modest weight loss might be of benefit for women with a BMI >35, although caution must be exerted in advocating this until prospective trials are completed.
Although the trials are overall disappointing thus far, the most effective means to achieve lower GWG appears to be through intensive diet strategies, although physical activity interventions may have the potential to decrease LGA. Most studies are limited in their measurement of infant body composition which is likely to be more important to future childhood obesity risk and only measure birthweight or use skinfolds rather than more accurate methods such as Pea Pod or DXA. Long-term prospective longitudinal studies that more carefully tease out the risk of GWG on childhood obesity controlling for maternal BMI, diet, and postnatal feeding practices utilizing better measures for body composition are gravely needed. Capitalizing on the “teachable moment” of pregnancy, [56] and the willingness for a pregnant woman to do whatever it takes to ensure her baby’s health is vital, and should play a major role in the motivational paradigm to design future trials that challenge the current IOM guidelines in overweight and obese women. Postpartum interventional trials that address barriers to participation, and rely less on face-to-face contact using technology may be more likely to be successful. Breastfeeding, especially for at least 6 months, may facilitate postpartum weight loss, and appears to minimize excess infant weight gain, attenuate risk for childhood obesity, and favorably affect the infant microbiome and immune function, and should be strongly encouraged. There is little available research focused on understanding the biologic determinants of weight gain in pregnancy and weight loss in the postpartum period, including the importance of sleep deprivation, appetite regulation, physical activity, and postpartum depression. The window of pregnancy (and preconception) has the capacity to be the ultimate opportunity for primary prevention of CVD and diabetes in both the mothers and their infants. Although effective interventions may be challenging, we cannot afford not to try.
Key Issues.
-
-
Obesity in pregnancy has now been identified as the leading cause of maternal and perinatal morbidity, increasing the health care costs of pregnancy by up to 16-fold due to a striking increase in maternal medical and obstetric complications as well as major malformations, fetal loss, stillbirth, and neonatal complications.
-
-
Extensive animal data and accumulating human data support a “fetal programming” paradigm that maternal obesity, excess GWG and an abnormal intrauterine metabolic environment change organ development (pancreas, kidney, heart, liver), fat and muscle mass, and through epigenetic changes may affect appetite regulation, mitochondrial oxidative capacity, organ function, and offspring behavior resulting in an increased risk for childhood obesity, metabolic syndrome, and diabetes.
-
-
Maternal risks from excess GWG especially in overweight and obese women include a higher risk of GDM, preeclampsia or gestational hypertension, cesarean delivery, and postpartum weight retention which significantly increases the risk of maternal obesity, diabetes, and CVD and entering a subsequent pregnancy with these complications. Infant risks related to GWG include LGA and increases in fat mass and childhood obesity risk.
-
-
The “obligate” amount of GWG needed to minimize maternal fat stores being used as an energy source is ~ 7.5 kg (17 lbs) to account for fetus, placenta, amniotic fluid and increases in breast and uterine tissue and plasma volume.
-
-
Although underweight and NW women need to gain a sufficient amount of weight to minimize the risk of small-for-gestational age (SGA) infants, overweight and obese women who gain no weight on average deliver a normal weight infant. Obese women who deliver SGA infants commonly have other morbidities (e.g. chronic hypertension, renal disease) that place them at risk for placental insufficiency as the cause of the SGA.
-
-
GWG, even when within the 2009 IOM guidelines, has been associated with an increase in the rate of LGA and childhood obesity in overweight and obese women.
-
-
There is increasing evidence that to minimize both SGA and LGA risk and to decrease the risk of GDM, preeclampsia, cesarean delivery, postpartum weight retention, and childhood obesity that NW and overweight women should gain at the lower limit of the IOM guidelines, women with Class 1 obesity < 5kg, and increasing evidence that obese women with a BMI ≥35 do not need to gain any weight to have a normally grown infant.
-
-
Women with GDM who are overweight or obese may benefit from weight gain less than the IOM recommendations because minimal weight gain does not appreciably increase SGA but may decrease insulin requirements and LGA.
-
-
Randomized controlled interventions to minimize excess GWG have been modestly successful with more intensive diet intervention studies tending to be the most successful. Multiple trials are ongoing.
-
-
Postpartum weight loss interventions have been disappointing. Breastfeeding, especially for at least 6 months, may facilitate postpartum weight loss and appears to attenuate the risk for childhood obesity.
-
-
If possible, targeted interventions especially in overweight and obese women should begin preconception due to the powerful influence of pre-pregnancy BMI on adverse pregnancy outcomes, continue during pregnancy to favorably affect diet and GWG, extend postpartum to facilitate weight loss, and follow through the inter-pregnancy period to optimize maternal health.
-
-
Successful interventions to achieve healthy maternal weight antepartum, intrapartum, and postpartum not only have the potential to favorably affect maternal health but also offspring health and decrease the intergenerational risk of obesity and diabetes on subsequent generations.
5 year view.
The adverse effects of maternal overweight and obesity, excessive GWG, and PPWR are well elucidated. However, despite a multitude of intervention studies in pregnancy and postpartum, the most effective interventions as well as the ideal targets for GWG and PPWR are not well understood. Multiple large trials are currently ongoing, especially during pregnancy, which will help answer these questions over the next few years. Interventions that capitalize on the widespread availability and convenience of web-based and mobile strategies may be useful in circumventing barriers to engagement to more effectively promote healthy weight gain during pregnancy and postpartum weight loss. Biologic studies that advance our understanding of the determinants of weight gain or loss in pregnant and postpartum women, including sleep deprivation, appetite regulation, breastfeeding, physical activity, and postpartum depression, may significantly improve the success of interventional strategies. Emerging areas of research that are closely tied with maternal weight gain which demand further investigation include the importance of maternal diet, including potential adverse effects of a high fat or simple carbohydrate diet in humans independent of obesity or GWG; the biologic determinants of breast milk and the ideal duration of breastfeeding to facilitate weight loss, including the impact of breast milk oligosaccharides on the infant microbiome and subsequent health outcomes; and a better understanding of antepartum and postpartum physiology, including contributors to weight gain or loss.
Acknowledgments
This work was supported by the Division of General Internal Medicine and the Department of Medicine at the University of Colorado School of Medicine, and by an NIH BIRCWH K12 HD057022. (JMN)
This work was supported by NIH R01DK078645, NIH R01 DK101659, and an American Diabetes Association/Glaxo Smith Kline Targeted Research Award. (LAB)
Abbreviations
- BMI
body mass index
- BMR
basal metabolic rate
- BW
birthweight
- CVD
cardiovascular disease
- DOHaD
Developmental Origins of Health and Disease
- DXA
dual x-ray absorpitometry
- GWG
gestational weight gain
- GDM
gestational diabetes mellitus
- IOM
Institute of Medicine
- LGA
large-for-gestational-age
- NAFLD
non-alcoholic fatty liver disease
- NW
normal weight
- PPWR
postpartum weight retention
- SGA
small-for-gestational-age
- WHO
World Health Organization
Footnotes
No disclosures
Contributor Information
Jacinda M. Nicklas, Email: Jacinda.Nicklas@ucdenver.edu, Division of General Internal Medicine, University of Colorado School of Medicine, 12348 E. Montview Blvd, C263, Aurora, CO 80045, 303-724-9028 (work phone), 617-510-7273 (cell phone), 303-724-9976 (fax).
Linda A. Barbour, Email: Lynn.barbour@ucdenver.edu, Professor of Medicine and Obstetrics and Gynecology, Divisions of Endocrinology, Metabolism, and Diabetes and Maternal-Fetal Medicine, University of Colorado School of Medicine, Mail Stop 8106, 12801 E. 17th Avenue, Aurora, CO 80045, 303-724-3921 (work phone), 303-594-0474 (cell phone), 303-724-3920 (fax).
References
- 1.American College of Obstetricians and Gynecologists. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol. 2013 Jan;121(1):213–7. doi: 10.1097/01.aog.0000425667.10377.60. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014 Feb 26;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006 Apr 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014 Jan 30;370(5):403–11. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug 30;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 8.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Carter SM, Craigo SD, Carr SR. D’Alton ME; FASTER Research Consortium. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004 Apr;190(4):1091–7. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 9.Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003 May;111:1152–8. [PubMed] [Google Scholar]
- 10.Black MH, Sacks DA, Xiang A, Lawrence JM, et al. The relative contribution of pre-pregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diab Care. 2013;36:56–62. doi: 10.2337/dc12-0741. ** An analysis of the 9835 deliveries in California in which the new IADPSG criteria was used to diagnosed GDM demonstrated that although pre-pregnancy BMI (overweight and obese), GWG and GDM all independently contributed to LGA infants, the attributable risk of overweight and obesity contributed most. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalano PM, McIntyre DH, Cruickshank JK, the HAPO Cooperative Research Group The Hyperglycemia Adverse Pregnancy Outcome Study: Associations of GDM and obesity with pregnancy outcomes. Diab Care. 2012;35:780–86. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrebosch L, Bel S, Vansant G, et al. Maternal and neonatal outcome after laparoscopic adjustable gastric banding : A systematic review. Obes Surg. 2012;22:1568–79. doi: 10.1007/s11695-012-0740-y. [DOI] [PubMed] [Google Scholar]
- 14.Barbour LA. Changing perspectives in pre-exisiting diabetes and obesity in pregnancy: maternal and infant short- and long-term outcomes. Current Opinion Endocrinol, Diabetes, Obes. 2014;21:257–263. doi: 10.1097/MED.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 15.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 16.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299:R711–R722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patti M-E. Intergeneration programming of metabolic disease; evidence from human populations and experimental animal models. Cell Moll Life Sci. 2013;70:1597–16. 8. doi: 10.1007/s00018-013-1298-0. * Excellent discussion reviewing both animal and human data supporting the concept of fetal programming due to exposures in the early life environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillycrop KA, Burdge GC. Epigenetic mechanisms linking early nutrition to long term health. Best Pract Res Clin Endocrinol Metab. 2012 Oct;26(5):667–76. doi: 10.1016/j.beem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Pinney SE, Simmons RA. Metabolic Programming, Epigenetics, and Gestational Diabetes. Curr Diab Repor. 2012;12:67–74. doi: 10.1007/s11892-011-0248-1. *Well written discussion providing a clear explanation as to how metabolism can influence gene expression and phenotype in the offspring of obese and GDM women. [DOI] [PubMed] [Google Scholar]
- 20.Sassone-Corsi P. When metabolism and epigenetics converge. Science. 2013;339:148–9.4. 14–17. doi: 10.1126/science.1233423. [DOI] [PubMed] [Google Scholar]
- 21.Chen JR, Zhang J, Lazarenko OP, et al. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J. 2012;26:1131–41. doi: 10.1096/fj.11-197822. [DOI] [PubMed] [Google Scholar]
- 22.Symonds ME, Pope M, Sharkey D, et al. Adipose tissue and fetal programming. Diabetologia. 2012;55:1597–1606. doi: 10.1007/s00125-012-2505-5. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan L, Nousen EK, Cahmou KA. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiolo Behav. 2014;123:236–42. doi: 10.1016/j.physbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suter MA, Chen A, Burdine MS, et al. A maternal high-fat diet modulates fetal SIRT1 histone and protein deaceylase activity in nonhuman primates. FASEB J. 2012;26(12):5106–14. doi: 10.1096/fj.12-212878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan EL, Grayson B, Takahashi D, et al. Chronic consumption of a high fat diet during pregnancy causes perturbations in the serotinergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neuroscience. 2010;30(10):3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kral JG, Biron S, Simard S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:1644–49. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- 28.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, Reynolds R, Alston M, Hoffman C, Pan Z, Friedman JE, Barbour LA. Intrahepatic Fat Is Increased in the Neonatal Offspring of Obese Women with Gestational Diabetes. J Pediatr. 2012;162(5):930–6. doi: 10.1016/j.jpeds.2012.11.017. **The first human data to show that infants of obese GDM women are born with 68% more intrahepatic fat than offspring of normal weight women using MRI spectroscopy to measure liver fat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SY. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol. 2014;123:737. doi: 10.1097/AOG.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catalano PM, Roman NM, Tyzbir ED, Merritt AO, Driscoll P, Amini SB. Weight gain in women with gestational diabetes. Obstet Gynecol. 1993;81:523–8. [PubMed] [Google Scholar]
- 31.Hackmon R, James R, O’Reilly Green C, Ferber A, Barnhard Y, Divon M. The impact of maternal age, body mass index and maternal weight gain on the glucose challenge test in pregnancy. J Matern Fetal Neonatal Med. 2007;20:253–7. doi: 10.1080/14767050601135220. [DOI] [PubMed] [Google Scholar]
- 32.Saldana TM, Siega-Riz AM, Adair LS, Suchindran C. The relationship between pregnancy weight gain and glucose tolerance status among black and white women in central North Carolina. Am J Obstet Gynecol. 2006;195:1629–35. doi: 10.1016/j.ajog.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Gibson KS, Waters TP, Catalano PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol. 2012 Mar;119(3):560–5. doi: 10.1097/AOG.0b013e31824758e0. [DOI] [PubMed] [Google Scholar]
- 34.Witter FR, Caulfield LE, Stoltzfus RJ. Influence of maternal anthropometric status and birth weight on the risk of cesarean delivery. Obstet Gynecol. 1995 Jun;85(6):947–51. doi: 10.1016/0029-7844(95)00082-3. [DOI] [PubMed] [Google Scholar]
- 35.Young TK, Woodmansee B. Factors that are associated with cesarean delivery in a large private practice: the importance of prepregnancy body mass index and weight gain. Am J Obstet Gynecol. 2002 Aug;187(2):312–8. doi: 10.1067/mob.2002.126200. [DOI] [PubMed] [Google Scholar]
- 36.Cheng YW, Chung JH, Kurbisch-Block I, Inturrisi M, Shafer S, Caughey AB. Gestational weight gain and gestational diabetes mellitus: perinatal outcomes. Obstet Gynecol. 2008 Nov;112(5):1015–22. doi: 10.1097/AOG.0b013e31818b5dd9. [DOI] [PubMed] [Google Scholar]
- 37.DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol. 2007;110:745–51. doi: 10.1097/01.AOG.0000284451.37882.85. [DOI] [PubMed] [Google Scholar]
- 38.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005 Oct 22;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202:574–578. doi: 10.1016/j.ajog.2009.12.007. **Data from 4496 children ages 14–22 in the National Longitudinal Survey of Youth demonstrated that to minimize SGA, LGA, postpartum weight retention, Cesarean delivery and childhood obesity, the optimal gestational weight gain for overweight women was ~11 lbs and for obese women 0–11 lbs. [DOI] [PubMed] [Google Scholar]
- 40.Jedrychowski W, Maugeri U, Kaim I, Budzyn-Mrozek D, Flak E, Mroz E, Sochacka-Tatara E, Sowa A, Musial A. Impact of excessive gestational weight gain in non-smoking mothers on body fatness in infancy and early childhood. Prospective prebirth cohort study in Cracow. J Physiol Pharmacol. 2011;62:55–64. [PubMed] [Google Scholar]
- 41.Andersen CS, Gamborg M, Sorensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring’s body mass index at 7 years of age. Int J Pediatr Obes. 2011;6:e179–e186. doi: 10.3109/17477166.2010.521560. [DOI] [PubMed] [Google Scholar]
- 42.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, Navder K, Yu A, Dorsey K, Gallagher D. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011 Sep;205(3):211.e1–7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, Robinson SM, Southampton Women’s Survey Study Group Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2010 Jun;91(6):1745–51. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005 Mar;115(3):e290–6. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21:521–526. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbour LA. Weight Gain in Pregnancy: Is More Truly Less for Mother and Child? Journal of Obstetric Medicine. 2012 Jun;5:58–64. doi: 10.1258/om.2012.120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catalano PM. Increasing maternal obesity and weight gain during pregnancy: the obstetric problems of plentitude. Obstet Gynecol. 2007;110:743–744. doi: 10.1097/01.AOG.0000284990.84982.ba. [DOI] [PubMed] [Google Scholar]
- 48.Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2009. Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines; pp. 1–250. [PubMed] [Google Scholar]
- 49.Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria Siega-Riz A. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol. 2010;116:1191–1195. doi: 10.1097/AOG.0b013e3181f60da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Restall A, Taylor RS, Thompson JM, Flower D, Dekker GA, Kenny LC, Poston L, McCowan LM. Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. J Obes. 2014;2014:148391. doi: 10.1155/2014/148391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Badon SE, Dyer AR, Josefson JL, HAPO Study Cooperative Research Group Gestational weight gain and neonatal adiposity in the Hyperglycemia and Adverse Pregnancy Outcome study-North American region. Obesity (Silver Spring) 2014 Jul;22(7):1731–8. doi: 10.1002/oby.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraschnewski JL, Chuang CH, Downs DS, Weisman CS, McCamant EL, Baptiste-Roberts K, Zhu J, Kjerulff KH. Association of prenatal physical activity and gestational weight gain: results from the first baby study. Womens Health Issues. 2013 Jul-Aug;23(4):e233–8. doi: 10.1016/j.whi.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berggren EK, Stuebe AM, Boggess KA. Excess Maternal Weight Gain and Large for Gestational Age Risk among Women with Gestational Diabetes. Am J Perinatol. 2014 Jun;27 doi: 10.1055/s-0034-1383848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21:521–526. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- 56.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol. 2010 Feb;202(2):135.e1–8. doi: 10.1016/j.ajog.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord. 2003 Jan;27(1):117–27. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 58.Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. Am J Public Health. 1993 Aug;83(8):1100–3. doi: 10.2105/ajph.83.8.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schauberger CW, Rooney BL, Brimer LM. Factors that influence weight loss in the puerperium. Obstet Gynecol. 1992 Mar;79(3):424–9. doi: 10.1097/00006250-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 60.Luke B, Hediger ML, Scholl TO. Point of diminishing returns: when does gestational weight gain cease benefiting birthweight and begin adding to maternal obesity? J Matern Fetal Med. 1996 Jul-Aug;5(4):168–73. doi: 10.1002/(SICI)1520-6661(199607/08)5:4<168::AID-MFM2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 61.Scholl TO, Hediger ML, Schall JI, Ances IG, Smith WK. Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstet Gynecol. 1995 Sep;86(3):423–7. doi: 10.1016/0029-7844(95)00190-3. [DOI] [PubMed] [Google Scholar]
- 62.Walker LO, Freeland-Graves JH, Milani T, Hanss-Nuss H, George G, Sterling BS, Kim M, Timmerman GM, Wilkinson S, Arheart KL, Stuifbergen A. Weight and behavioral and psychosocial factors among ethnically diverse, low-income women after childbirth: I. Methods and context. Women Health. 2004;40(2):1–17. doi: 10.1300/J013v40n02_01. [DOI] [PubMed] [Google Scholar]
- 63.Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern Child Health J. 2011 Nov;15(8):1166–75. doi: 10.1007/s10995-010-0689-1. [DOI] [PubMed] [Google Scholar]
- 64.Linné Y, Dye L, Barkeling B, Rössner S. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res. 2004 Jul;12(7):1166–78. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- 65.Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM, Lawlor DA. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC) Am J Clin Nutr. 2011 Jun;93(6):1285–92. doi: 10.3945/ajcn.110.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mamun AA, Kinarivala M, O’Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr. 2010 May;91(5):1336–41. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 67.Chin JR, Krause KM, Ostbye T, Chowdhury N, Lovelady CA, Swamy GK. Gestational weight gain in consecutive pregnancies. Am J Obstet Gynecol. 2010 Sep;203(3):279.e1–6. doi: 10.1016/j.ajog.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 68.Gunderson EP, Abrams B, Selvin S. Does the pattern of postpartum weight change differ according to pregravid body size? Int J Obes. 2001;25:853–62. doi: 10.1038/sj.ijo.0801631. [DOI] [PubMed] [Google Scholar]
- 69.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol. 2011 Jun;117(6):1323–30. doi: 10.1097/AOG.0b013e31821aa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA. The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol. 2013 Mar;208(3):205.e1–7. doi: 10.1016/j.ajog.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 71.Dixon JB, Dixon ME, O’Brien PE. Pregnancy after lap-band surgery: management of the band to achieve healthy weight outcomes. Obes Surg. 2001;11:59–65. doi: 10.1381/096089201321454123. [DOI] [PubMed] [Google Scholar]
- 72.Karmon A, Sheiner E. Pregnancy after bariatric surgery: a comprehensive review. Arch Gynecol Obstet. 2008 May;277(5):381–8. doi: 10.1007/s00404-008-0608-5. [DOI] [PubMed] [Google Scholar]
- 73.Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, Suttorp M, Hilton L, Santry HP, Morton JM, Livingston EH, Shekelle PG. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008 Nov 19;300(19):2286–96. doi: 10.1001/jama.2008.641. [DOI] [PubMed] [Google Scholar]
- 74.Wittgrove AC, Jester L, Wittgrove P, Clark GW. Pregnancy following gastric bypass for morbid obesity. Obes Surg. 1998;8:461–4. doi: 10.1381/096089298765554368. [DOI] [PubMed] [Google Scholar]
- 75.Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: Current evidence and recommendations. Obes Surg. 2008;18:1023–27. doi: 10.1007/s11695-007-9389-3. [DOI] [PubMed] [Google Scholar]
- 76.Guelinckx I, Devlieger R, Vansant G. Reproductive outcome after bariatric surgery: a critical review. Hum Repr Update. 2009;15:189–201. doi: 10.1093/humupd/dmn057. [DOI] [PubMed] [Google Scholar]
- 77.Galazis N, Docheva N, Simillis C, Nicolaides KH. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014 Jul 30;181C:45–53. doi: 10.1016/j.ejogrb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 78.Berglind D, Willmer M, Näslund E, Tynelius P, Sørensen TI, Rasmussen F. Differences in gestational weight gain between pregnancies before and after maternal bariatric surgery correlate with differences in birth weight but not with scores on the body mass index in early childhood. Pediatr Obes. 2013 Dec 11; doi: 10.1111/j.2047-6310.2013.00205.x. [DOI] [PubMed] [Google Scholar]
- 79.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322–328. doi: 10.1016/j.ajog.2006.11.027. **Data from 1044 mother child pairs from the Harvard Viva Project in which 51% of mothers gained more than the IOM recommendations and 1/3 were overweight demonstrated that the 3 year old offspring of women who gained according to the IOM guidelines still had nearly a 4-fold higher risk of obesity compared to those offspring of women who gain inadequate weight, however the risk of SGA was not increased in those who gained “inadequate” weight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am. 2009;36:361–369. doi: 10.1016/j.ogc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Hinkle SN, Sharma AJ, Dietz PM. Gestational weight gain in obese mothers and associations with fetal growth. Am J Clin Nutr. 2010;92:644–651. doi: 10.3945/ajcn.2010.29726. [DOI] [PubMed] [Google Scholar]
- 82.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91:1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new Institute of Medicine recommendations. Obstet Gynecol. 2011;117:1065–1070. doi: 10.1097/AOG.0b013e318214f1d1. [DOI] [PubMed] [Google Scholar]
- 84.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, Ness A, Deanfield J, Hingorani A, Nelson SM, Smith GD, Lawlor DA. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abrams BF, Laros RK., Jr Prepregnancy weight, weight gain, and birth weight. Am J Obstet Gynecol. 1986;154:503–509. doi: 10.1016/0002-9378(86)90591-0. [DOI] [PubMed] [Google Scholar]
- 86.Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstet Gynecol. 2007;110:759–764. doi: 10.1097/01.AOG.0000279450.85198.b2. [DOI] [PubMed] [Google Scholar]
- 87.Beyerlein A, Schiessl B, Lack N, von KR. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am J Clin Nutr. 2009;90:1552–1558. doi: 10.3945/ajcn.2009.28026. [DOI] [PubMed] [Google Scholar]
- 88.Han Z, Lutsiv O, Mulla S, Rosen A, Beyene J, McDonald SD. Low gestational weight gain and the risk of preterm birth and low birthweight: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2011;90:935–954. doi: 10.1111/j.1600-0412.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 89.Artal R, Lockwood CJ, Brown HL. Weight gain recommendations in pregnancy and the obesity epidemic. Obstet Gynecol. 2010;115:152–155. doi: 10.1097/AOG.0b013e3181c51908. [DOI] [PubMed] [Google Scholar]
- 90.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201:339–14. doi: 10.1016/j.ajog.2009.07.002. **Systematic review of the 35 highest quality studies concluding that although there was support for inadequate gestational weight gain and SGA in underweight and normal weight women, this is not the case for overweight and obese women. Due to their analysis of the data they encouraged the IOM to reconsider their weight gain recommendations for overweight and obese women. [DOI] [PubMed] [Google Scholar]
- 91.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. NEJM. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Catalano PM, Mele L, Landon MB, Ramin SM, Reddy UM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Jr, Saade G, Sorokin Y, Peaceman AM, Tolosa JE, Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units Network Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014 Aug;211(2):137.e1–7. doi: 10.1016/j.ajog.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.International Atomic Energy Agency. Body composition assessment from birth to two years of age. IAEA Human Health Series No. 22, 2013. http://www.iaea.org/Publications/index.html.
- 94.Davis RR, Hoferth S, Shenassa ED. Gestatonal weight gain and risk of infant death in the United States. Am J Pub Health. 2014;104:S90–96. doi: 10.2105/AJPH.2013.301425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eun Park J, Park S, Daily JW, Sung-Hoon K. Low gestational weight gain improves infant and maternal pregnancy outcomes in overweight and obese Korean women with gestational diabetes mellitus. Gynecological Endocrinology. 2011;27(10):775–81. doi: 10.3109/09513590.2010.540597. [DOI] [PubMed] [Google Scholar]
- 96.Cheng YW, Chung JH, Kurbisch-Block I, Inturrisi M, Shafer S, Caughey AB. Gestational weight gain and gestational diabetes mellitus: perinatal outcomes. Obstet Gynecol. 2008 Nov;112(5):1015–22. doi: 10.1097/AOG.0b013e31818b5dd9. [DOI] [PubMed] [Google Scholar]
- 97.Katon J, Reiber G, Williams MA, Yanez D, Miller E. Weight loss after diagnosis with gestational diabetes and birth weight among overweight and obese women. Matern Child Health J. 2013 Feb;17(2):374–83. doi: 10.1007/s10995-012-1044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Egan AM, Dennedy MC, Al-Ramli W, Heerey A, Avalos G, Dunne F. ATLANTIC-DIP: excessive gestational weight gain and pregnancy outcomes in women with gestational or pregestational diabetes mellitus. J Clin Endocrinol Metab. 2014 Jan;99(1):212–9. doi: 10.1210/jc.2013-2684. [DOI] [PubMed] [Google Scholar]
- 99.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: a meta-analysis of randomized evidence. BMJ. 2012;344:1–15. doi: 10.1136/bmj.e2088. ** One of the most comprehensive and thorough reviews of interventions during pregnancy to decrease excess GWG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012 May 10;10:47. doi: 10.1186/1741-7015-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agha M, Agha RA, Sandell J. Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: a systematic review and meta-analysis. PLoS One. 2014 May 14;9(5):e95132. doi: 10.1371/journal.pone.0095132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gardner B, Wardle J, Poston L, Croker H. Changing diet and physical activity to reduce gestational weight gain: a meta-analysis. Obes Rev. 2011 Jul;12(7):e602–20. doi: 10.1111/j.1467-789X.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 103.Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr. 2010 Oct;92(4):678–87. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- 104.Tanentsapf I, Heitmann BL, Adegboye AR. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011 Oct 26;11:81. doi: 10.1186/1471-2393-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi J, Fukuoka Y, Lee JH. The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum: a systematic review and meta-analysis of randomized controlled trials. Prev Med. 2013 Jun;56(6):351–64. doi: 10.1016/j.ypmed.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sui Z, Grivell RM, Dodd JM. Antenatal exercise to improve outcomes in overweight or obese women: A systematic review. Acta Obstet Gynecol Scand. 2012 May;91(5):538–45. doi: 10.1111/j.1600-0412.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- 107.Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. Interventions for preventing excessive weight gain during pregnancy. Cochrane Database Syst Rev. 2012 Apr 18;4:CD007145. doi: 10.1002/14651858.CD007145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ronnberg A, Nilsson K. Interventions during pregnancy to reduce excessive gestational weight gain: a systematic review assessing current clinical evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. BJOG. 2010;117:1327–1334. doi: 10.1111/j.1471-0528.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- 109.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG. 2010 Oct;117(11):1316–26. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 110.Campbell F, Johnson M, Messina J, Guillaume L, Goyder E. Behavioural interventions for weight management in pregnancy: a systematic review of quantitative and qualitative data. BMC Public Health. 2011 Jun 22;11:491. doi: 10.1186/1471-2458-11-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O’Brien OA, McCarthy M, Gibney ER, McAuliffe FM. Technology-supported dietary and lifestyle interventions in healthy pregnant women: a systematic review. Eur J Clin Nutr. 2014 Jul;68(7):760–6. doi: 10.1038/ejcn.2014.59. [DOI] [PubMed] [Google Scholar]
- 112.Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ. 2012 Aug 30;345:e5605. doi: 10.1136/bmj.e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, Facchinetti F. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med. 2014 Sep;27(13):1348–52. doi: 10.3109/14767058.2013.858318. [DOI] [PubMed] [Google Scholar]
- 114.Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, McEvoy CT, Eckhardt CL, Smith KS, Stevens VJ. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: A randomized trial. Obesity (Silver Spring) 2014 Sep;22(9):1989–96. doi: 10.1002/oby.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008 May 8;358(19):2003–15. doi: 10.1056/NEJMoa0707193. Erratum in: N Engl J Med. 2008 Jul 3;359(1):106. [DOI] [PubMed] [Google Scholar]
- 116.Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract. 2012 Dec;98(3):422–9. doi: 10.1016/j.diabres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 117.Nicklas JM, Zera CA, Seely EW, Abdul-Rahim ZS, Rudloff ND, Levkoff SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy and Childbirth. 2011;11:23. doi: 10.1186/1471-2393-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carter-Edwards L, Østbye T, Bastian LA, Yarnall KS, Krause KM, Simmons TJ. Barriers to adopting a healthy lifestyle: insight from postpartum women. BMC Res Notes. 2009 Aug 17;2:161. doi: 10.1186/1756-0500-2-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swan W, Kilmartin G, Liaw ST. Assessment of readiness to prevent type 2 diabetes in a population of rural women with a history of gestational diabetes. Rural Remote Health. 2007;7(4):802. [PubMed] [Google Scholar]
- 120.van der Pligt P, Willcox J, Hesketh KD, Ball K, Wilkinson S, Crawford D, Campbell K. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev. 2013 Oct;14(10):792–805. doi: 10.1111/obr.12053. [DOI] [PubMed] [Google Scholar]
- 121.Nascimento SL, Pudwell J, Surita FG, Adamo KB, Smith GN. The effect of physical exercise strategies on weight loss in postpartum women: a systematic review and meta-analysis. Int J Obes (Lond) 2014 May;38(5):626–35. doi: 10.1038/ijo.2013.183. [DOI] [PubMed] [Google Scholar]
- 122.Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The effectiveness of weight management interventions in breastfeeding women-a systematic review and critical evaluation. Birth. 2014 Sep;41(3):223–36. doi: 10.1111/birt.12111. [DOI] [PubMed] [Google Scholar]
- 123.Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2013 Jul 23;7:CD005627. doi: 10.1002/14651858.CD005627.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wiltheiss GA, Lovelady CA, West DG, Brouwer RJ, Krause KM, Østbye T. Diet quality and weight change among overweight and obese postpartum women enrolled in a behavioral intervention program. J Acad Nutr Diet. 2013 Jan;113(1):54–62. doi: 10.1016/j.jand.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stendell-Hollis NR, Thompson PA, West JL, Wertheim BC, Thomson CA. A comparison of Mediterranean-style and MyPyramid diets on weight loss and inflammatory biomarkers in postpartum breastfeeding women. J Womens Health (Larchmt) 2013 Jan;22(1):48–57. doi: 10.1089/jwh.2012.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilkinson SA, van der Pligt P, Gibbons KS, McIntyre HD. Trial for Reducing Weight Retention in New Mums: a randomised controlled trial evaluating a low intensity, postpartum weight management programme. J Hum Nutr Diet. 2013 Nov 25; doi: 10.1111/jhn.12193. [DOI] [PubMed] [Google Scholar]
- 127.Tripette J, Murakami H, Gando Y, Kawakami R, Sasaki A, Hanawa S, Hirosako A, Miyachi M. Home-based active video games to promote weight loss during the postpartum period. Med Sci Sports Exerc. 2014 Mar;46(3):472–8. doi: 10.1249/MSS.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 128.Herring SJ, Cruice JF, Bennett GG, Davey A, Foster GD. Using Technology to Promote Postpartum Weight Loss in Urban, Low-Income Mothers: A Pilot Randomized Controlled Trial. J Nutr Educ Behav. 2014 Jul 25; doi: 10.1016/j.jneb.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry CP, Jr, Peng T, Feng J, Ching J, Crites Y. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care. 2011 Jul;34(7):1519–25. doi: 10.2337/dc10-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nicklas JM, Zera CA, England LJ, Rosner BA, Horton E, Levkoff SE, Seely EW. A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2014 Sep;124(3):563–70. doi: 10.1097/AOG.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The relationship between breastfeeding and postpartum weight change-a systematic review and critical evaluation. Int J Obes (Lond) 2014 Apr;38(4):577–90. doi: 10.1038/ijo.2013.132. [DOI] [PubMed] [Google Scholar]
- 132.Young BE, Johnson SL, Krebs NF. Biological determiinants linkinginfant weight gain and child obesity: current knowledge and future directions. Am Soc Nutri. 2012;3:675–86. doi: 10.3945/an.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang J, Himes JH, Guo Y, et al. Birth weight, growth and feeding pattern in early infancy predict overweight/obesity status at two years of age: A birth cohort study of Chinese infants. PLOS One. 2013;8(6):e64542. doi: 10.1371/journal.pone.0064542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arenz S, Rückerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247–1256. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- 135.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162:397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 136.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84:1043–1054. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- 137.Crume TL, Ogden L, Maligie MB, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34:641–45. doi: 10.2337/dc10-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weng SF, Redsell SA, Swift JA. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–26. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Savino F, Benetti S, Liguori SA, et al. Advances on human milk hormones and protection against obesity. Cell mol Biol. 2013;59:89–98. [PubMed] [Google Scholar]
- 140.Guru T. Nature’s first functional food. Science. 2014;345:747–49. doi: 10.1126/science.345.6198.747. [DOI] [PubMed] [Google Scholar]
- 141.Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, Holmes B, Kinnunen TI, Nelson SM, Oteng-Ntim E, Patel N, Robson SC, Sandall J, Sanders T, Sattar N, Seed PT, Wardle J, Poston L. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth. 2014 Feb 18;14:74. doi: 10.1186/1471-2393-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.http://www.medscinet.net/upbeat/, accessed August 20, 2014
- 143.Jelsma JG, van Poppel MN, Galjaard S, Desoye G, Corcoy R, Devlieger R, van Assche A, Timmerman D, Jans Harreiter J, Kautzky-Willer A, Damm P, Mathiesen ER, Jensen DM, Andersen L, Dunne F, Lapolla A, Di Cianni G, Bertolotto A, Wender-Oegowska E, Zawiejska A, Blumska K, Hill D, Rebollo P, Snoek FJ, Simmons D. DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: an European multicentre, randomised trial - study protocol. BMC Pregnancy Childbirth. 2013 Jul 5;13:142. doi: 10.1186/1471-2393-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.John E, Cassidy DM, Playle R, Jewell K, Cohen D, Duncan D, Newcombe RG, Busse M, Owen-Jones E, Williams N, Longo M, Avery A, Simpson SA. Healthy eating and lifestyle in pregnancy (HELP): a protocol for a cluster randomised trial to evaluate the effectiveness of a weight management intervention in pregnancy. BMC Public Health. 2014 May 10;14:439. doi: 10.1186/1471-2458-14-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nagle C, Skouteris H, Morris H, Nankervis A, Rasmussen B, Mayall P, Kennedy RL. Primary prevention of gestational diabetes for women who are overweight and obese: a randomised controlled trial. BMC Pregnancy Childbirth. 2013 Mar 13;13:65. doi: 10.1186/1471-2393-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rauh K, Kunath J, Rosenfeld E, Kick L, Ulm K, Hauner H. Healthy living in pregnancy: a cluster-randomized controlled trial to prevent excessive gestational weight gain - rationale and design of the GeliS study. BMC Pregnancy Childbirth. 2014 Mar 28;14:119. doi: 10.1186/1471-2393-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.(https://portal.bsc.gwu.edu/web/lifemoms, accessed 8/26/14)
- 148.Hernandez TL, Anderson MA, Chartier-Logan C, Friedman JE, Barbour LA. Strategies in the Nutritional Management of Gestational Diabetes. Clin Obstet Gynecol. 2013;56:803–15. doi: 10.1097/GRF.0b013e3182a8e0e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hernandez TL, Van Pelt RE, Anderson MA, Daniels LJ, West NA, Donahoo WT, Friedman JE, Barbour LA. A Higher Complex Carbohydrate Diet in Gestational Diabetes Achieves Glucose Targets and Lowers Postprandial Lipids: A Randomized Crossover Study. Diabetes Care. 2014;37:1254–62. doi: 10.2337/dc13-2411. *A Randomized controlled crossover trial challenging the currently recommended low carbohydrate higher, fat diet conventionally prescribed to GDM women which demonstrated that a diet higher in complex carbohydrates achieved similar glycemic profiles and lower postprandial FFAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011 Jul 9;378(9786):169–81. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]


