Abstract
Understanding the host response to influenza A virus infection is essential for developing intervention approaches. We show that infection of human alveolar epithelial cells and human bronchial epithelial cells with influenza A for 3 h resulted in down-regulation of host hsa-miRNA-548an (miRNA-548an) which triggered the overexpression of influenza non-structural-1A binding protein (IVNS1ABP, herein referred to as NS1ABP). Reduced NS1ABP mRNA and NS1ABP protein expression after transfection of miRNA-548an mimic or increased NS1ABP mRNA and NS1ABP protein expression after transfection of miRNA-548an inhibitor provided evidence that miRNA-548an is involved in the regulation of NS1ABP. Transfection of cells with inhibitor led to reduced apoptosis of infected cells while transfection of mimic led to increased apoptosis and reduced influenza copy number suggesting that NS1ABP has a role in viral maintenance. Thus, miRNA-548an may be an important target in controlling the early stage infection of influenza A.
Keywords: miRNA, NS1A binding protein, Influenza virus, Lung epithelial cells, Apoptosis
Introduction
Influenza viruses, which belong to the Orthomyxoviridae family, are human respiratory pathogens that cause both seasonal epidemics and periodic pandemics (Taubenberger and Morens, 2008). Influenza virus pandemics are responsible for substantial mortality and morbidity, particularly in the high-risk groups. Antiviral response factors in host cells significantly down-regulate replication of the virus in host tissues. A significant increase in inflammatory cytokines is documented during influenza infections (Gentile et al., 1998). A recent RNA interference study revealed that the influenza virus relies on specific host factors for replication and at least 295 cellular factors were required for early stage viral replication (Konig et al., 2010). Furthermore, several host cell genes are required for influenza A survival and replication (Karlas et al., 2010).
A critical viral protein-host protein interaction is exemplified in the binding of influenza A non-structural protein-1 (NS1) with the host cell influenza non-structural-1A binding protein (NS1ABP) (Wolff et al., 1998). The influenza A NS1 gene has a significant impact on host cell gene expression and a central role in inhibiting interferon (IFN) and other cytokines, and in activating NF-κB and PI3-K pathways (Geiss et al., 2002; Shin et al., 2007). NS1 protein localizes in the nucleus and to some extent in the cytoplasm of infected cells (Compans, 1973; Krug and Etkind, 1973). NS1 is a powerful translational enhancer that specifically induces viral mRNAs (Das et al., 2008; Fortes et al., 1994). NS1also interacts with cellular β-tubulin and disrupts the microtubule network and induces apoptosis of A549 cells (Han et al., 2012). Another major function of NS1 is the inhibition of pre-mRNA splicing (Fortes et al., 1994). Nemeroff et al. proposed a regulatory action through inhibition of host general gene expression by NS1 (Nemeroff et al., 1998). The NS1 protein inhibits 3′ end processing and the uncleaved pre-mRNA remains in the nucleus, thus NS1 could selectively inhibit the nuclear export of cellular mRNAs (Nemeroff et al., 1998). These actions appear to be mediated by binding with the host cell NS1ABP. NS1ABP plays a key role in cell division and in the dynamic organization of the actin skeleton as a stabilizer of actin filaments, by association with F-actin through Kelch repeats. NS1ABP protects cells from actin filament damage and cell death induced by actin destabilization caused by actin-destabilizing agents (Dunham et al., 2006; Wolff et al., 1998).
Despite the plethora of activities generated by the NS1 protein, there is a lack of understanding of the molecular regulation of its host cell binding protein. miRNAs have been implicated as important regulators of mammalian antiviral responses even though their precise role in the antiviral system has not been reported (Loveday et al., 2012; Russo and Potenza, 2011). miRNAs are 21–23 nucleotide single-stranded RNA molecules that negatively regulate gene expression (Bartel, 2004). Each miRNA can influence hundreds of mRNA targets and regulate cellular processes by binding with the 3′UTRs of their target mRNAs causing translational repression of the target mRNA through degradation or blocking (Selbach et al., 2008). Evidence indicates that target degradation is the predominant form of miRNA mediated translational repression (Lim et al., 2005). miRNAs regulate numerous cellular processes, including development, differentiation, proliferation, apoptosis, stress response, and viral defense (Fabian et al., 2010).
In the present study, we tested the hypothesis that specific miRNA species modulate the expression of NS1ABP in human alveolar epithelial cells (A549 cells), a type II respiratory epithelial model, and in primary human bronchial epithelial cells (HBEpC) during influenza A infection. We show that NS1ABP mRNA is regulated by host-encoded miRNA-548an and assess the functional role of NS1ABP during the early stages of infection.
Results
Influenza A infection deregulates the host miRNA profile
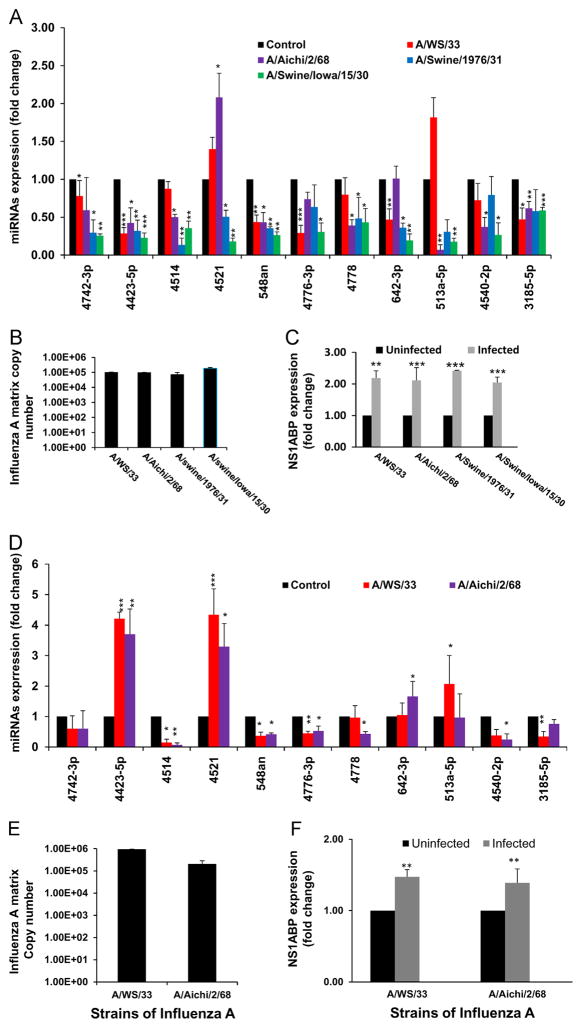
miRNA expression profiling using locked nucleic acid (LNA) based miRNA array on A549 cells infected with influenza A at MOI of 3 showed changes in miRNA expression as compared to that found in uninfected cells. Most of the 2040 human miRNAs included in the array did not show any significant changes during infection. We selected 11 miRNAs that showed differential expression upon microarray analysis and confirmed these results by RT-PCR with different strains of influenza A (Fig. 1A) on A549 cells. A549 cells showed an increase in expression of NS1ABP (Fig. 1C) and also an increase in copy numbers during the 3 h infection with different strains of influenza A virus (Fig. 1B). A similar pattern in miRNA and NS1ABP expression was observed when the primary human bronchial epithelial cells were exposed to H1N1 and H3N2 strains of influenza A (Fig. 1D–F). On exposure to influenza A, miRNA-4423 showed increased expression in HBEpC cells, whereas it significantly decreased in A549 cells. Expression of hsa-miR-548an was significantly downregulated in both cell lines with all strains of influenza A studied.
Fig. 1.
Influenza A alters host miRNA expression. (A) PCR analysis of individual miRNAs that showed differential expression in microarray analysis. miRNA isolated after 3 h of exposure to influenza A (A/WS/33 (H1N1), A/Aichi/2/68 (H3N2), A/Swine/1976/31 (H1N1), and A/Swine/Iowa/15/30 (H1N1)), (MOI of 3). The fold change represents the change in miRNA expression in infected cells relative to that obtained in uninfected cells. (B) A549 cells were infected with 3MOIs of influenza A (A/WS/33 (H1N1), A/Aichi/2/68 (H3N2), A/Swine/1976/31 (H1N1), and A/Swine/Iowa/15/30 (H1N1)), for 3 h and matrix copy numbers were determined by RT-PCR. (C) NS1ABP mRNA expression was normalized to expression of GAPDH mRNA in response to 3MOI of influenza. Data are expressed as ± standard error of the mean (SEM). n=4 (independent experiments) with triplicate replicates (*=p<0.05, **=p<0.01, ***=p<0.001). (D) PCR analysis of individual miRNAs in human bronchial epithelial cells (HBEpC) exposed to influenza A (A/WS/33 (H1N1), and A/Aichi/2/68 (H3N2)). miRNA isolated after 3 h of exposure to influenza A (MOI of 3). The fold change represents the change in miRNA expression in infected cells relative to that of uninfected cells. (E) HBEpC cells were infected with 3 MOIs of influenza A for 3 h and matrix copy numbers were determined by RT-PCR. (F) NS1ABP mRNA expression was normalized to expression of GAPDH mRNA in response to 3MOI of influenza A. p<0.05 was considered significant. The fold change represents the change in miRNA and mRNA expression in infected cells relative to that of uninfected cells. Data are expressed as ± standard error of the mean (SEM). n=4 (independent experiments) with triplicate replicates (*=p<0.05, **=p<0.01, ***=p<0.001).
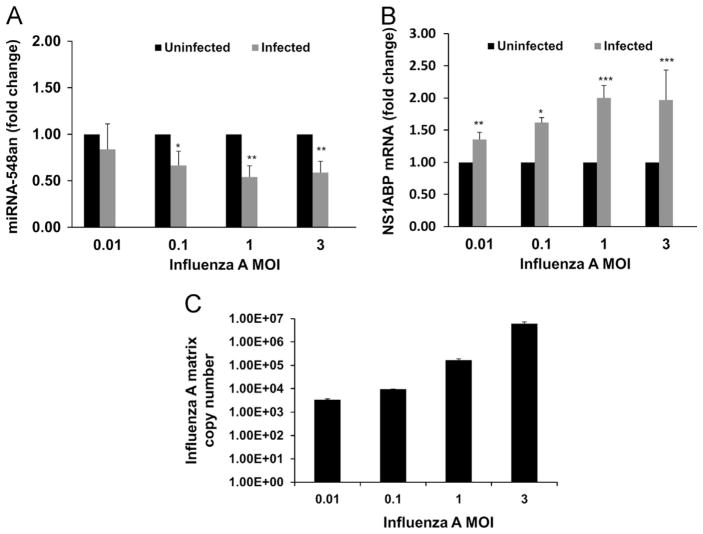
The Target scan database (www.targetscan.org) was used for identifying potential direct miRNA targets. Of the 11 individual miRNAs we examined, hsa-miR548an (miRNA-548an) was identified to have a possible influenza related target, influenza A NS1 binding protein (IVNS1ABP – Gene bank accession NM_006469), therefore further studies focused on miR-548an. The role of influenza on the expression of miR-548an and NS1ABP was assessed by infecting A549 cells with increasing MOIs of influenza A (Fig. 2). At very low MOI’s (≤0.01) where most cells are uninfected, differences in expression of miRNA-548an and NS1ABP mRNA were more difficult to detect during the time points studied. At MOI’s ≥0.1, the results showed a MOI dependent down-regulation of miRNA-548an with the highest down-regulation (p<0.001) at an MOI of 1 (Fig. 2A). An inverse correlation between miR-548an down-regulation and up-regulation of NS1ABP mRNA was seen (Fig. 2B). At MOIs of 1 and 3 there were 2-fold increases (p<0.001) in NS1ABP mRNA expression during influenza A infection which was in agreement with the down-regulation of miR-548an. Changes in NS1ABP expression with lower MOIs were equally comparable to the levels of miRNA-548an expression. The matrix gene copy number increased with increase in MOI (Fig. 2C).
Fig. 2.
miRNA-548an and NS1ABP mRNA expressions in A549 cells infected with different MOIs of influenza A. Differentially expressed miRNAs identified in the microarray analysis were screened for a potential role in influenza propagation and miRNA-548an was selected for further analysis. A549 cells were infected for 3 h with increasing MOIs of influenza. (A) miRNA-548an expression in uninfected and infected A549 cells was normalized to expression of let-7 as a housekeeping miRNA. (B) NS1ABP mRNA expression was normalized to expression of GAPDH mRNA in response to increasing MOIs of influenza. p<0.01 was considered significant. The fold change represents the change in miRNA and mRNA expression in infected cells relative to that obtained in uninfected cells. (C) A549 cells were infected with 3 MOIs of influenza A for 3 h and matrix copy numbers were determined by RT-PCR. Data are expressed as ± SEM. **=p<0.01, ***=p<0.001. n=4 (independent experiments) with triplicate replicates.
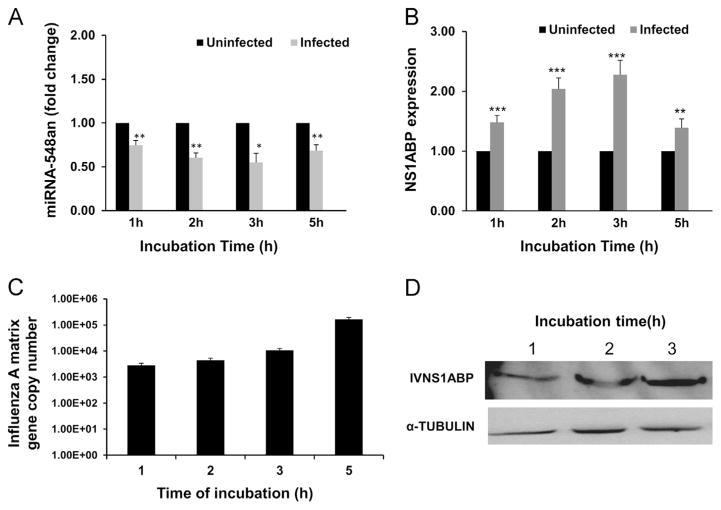
To further understand the regulation of NS1ABP mRNA expression with exposure time to the virus, we examined the kinetics of the NS1ABP mRNA response along with miRNA-548an expression. Down-regulation of miRNA-548an was evident after 1 h of exposure to influenza A and decreased to the lowest point by 3 h (Fig. 3A). In contrast, NS1ABP expression increased 1.5-fold after 1 h of exposure, and the maximal increase of 2.25-fold expression was seen at 3 h (p<0.001) (Fig. 3B). Thereafter, as seen with expression of miRNA-548an, expression of NS1ABP mRNA to the level seen in uninfected cells. An increase in matrix gene copy number with increasing incubation time was observed at all time points studied (Fig. 3C). There was also a 2-fold increase in the level of NS1ABP protein measured by flow cytometry as the mean fluorescent intensity (MFI) of cells exposed to influenza was almost doubled (from 14 to 28, p<0.01) on 3 h of exposure to the virus (data not shown). Western blot analysis showed a similar trend as flow cytometry and was in corroboration with the mRNA expression (Fig. 3D).
Fig. 3.
Time course showing the expression levels of miRNA-548an and NS1ABP mRNA in cells infected with influenza A. (A) A549 cells were infected with 1 MOI of Influenza A and expression of miRNA-548an normalized to let-7 miRNA is shown over 0–6 h of infection. (B) A549 cells were infected with 1 MOI of Influenza A and expression of NS1ABP mRNA normalized to GAPDH mRNA is shown over 0–6 h of infection. The fold change represents the change in miRNA and mRNA expression in infected cells relative to that obtained in uninfected cells. (C) HBEpC cells were infected with 3 MOIs of influenza A for 3 h and matrix copy numbers were determined by RT-PCR. (D) Protein expression by Western blot analysis in cells infected with influenza A for up to 3 h and α-tubulin was used as a loading control. Data are expressed as ± SEM. ***=p<0.001, **=p<0.01, *=p<0.05; n=3 (independent experiments) with triplicate replicates.
NS1ABP expression is regulated by miRNA-548an
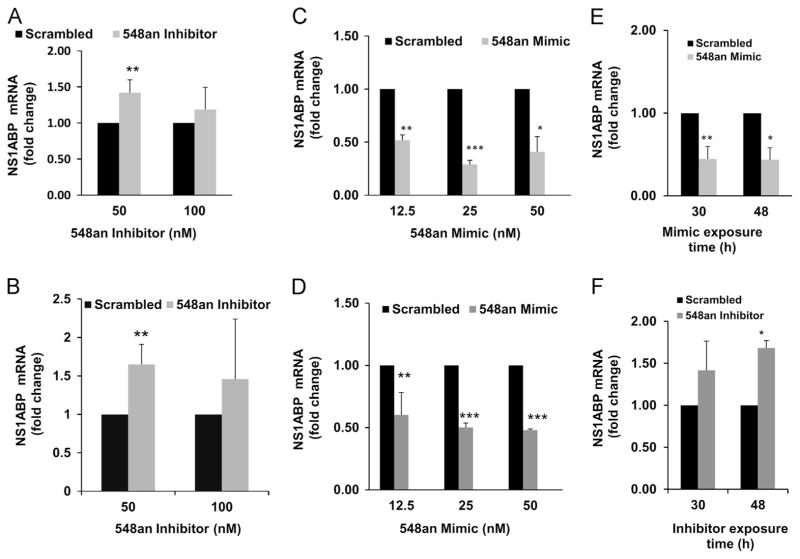
To confirm that miRNA-548an regulates expression of NS1ABP mRNA, A549 cells were transfected with either an inhibitor oligonucleotide specific for miRNA-548an or with a scrambled oligonucleotide control and the expression of NS1ABP mRNA was assessed by RT-PCR. At 50 nM inhibitor, expression of NS1ABP mRNA increased 1.4-fold above that seen with cells exposed to the scrambled oligonucleotide (Fig. 4A). Since cells infected with influenza A have an increased level of NS1ABP mRNA presumably because miRNA-548an decreases (Fig. 2), then infected cells should show a further increase in NS1ABP mRNA when transfected with the inhibitor. As shown, when influenza A was infected into cells exposed to the inhibitor, there was a significant (p<0.001) 1.6-fold further increase in the level of NS1ABP mRNA relative to that seen in infected cells transfected with a scrambled oligonucleotide (Fig. 4B), consistent with the results shown in (Fig. 2). We further confirmed the role of miRNA-548an in regulating the expression of NS1ABP mRNA by transfecting the cells with an oligonucleotide mimic specific for miRNA-548an. Transfection of 25 nM miRNA-548an mimic into uninfected A549 cells significantly (p<0.001) decreased the expression of NS1ABP mRNA by >65% (Fig. 4C). When influenza A was infected into cells transfected with the mimic, there was a significant (p<0.001) further decrease in expression of NS1ABP mRNA by >50% relative to that seen in infected cells transfected with a scrambled oligonucleotide (Fig. 4D). To further evaluate the stability of miRNAs in cells transfected with mimic or inhibitor, we analyzed the expression levels of NS1ABP. Data indicated that the miRNA mimic and inhibitor were stable over a time period of 48 h, as represented by the stability of NS1ABP expression (Fig. 4E and F).
Fig. 4.
Down or up-regulation of miRNA 548an alters the expression of NS1ABP mRNA. (A) Uninfected A549 cells were transfected for 48 h with 50 nM or 100 nM of either miRNA 548an inhibitor or a scrambled oligonucleotide as control. Expression of NS1ABP mRNA normalized to GAPDH mRNA is shown. (B) After 48 h, transfected cells were infected with 1 MOI of influenza A for 3 h. Expression of NS1ABP mRNA normalized to GAPDH mRNA is shown. n=3 (independent experiments) with triplicate replicates. (C) A549 cells were transfected for 48 h with 12.5 nM, 25 nM, or 50 nM of miRNA-548an mimic or scrambled oligonucleotide as control. Expression of NS1ABP mRNA normalized to GAPDH mRNA is shown. (D) A549 cells were transfected for 48 h with miRNA-548an mimic or scrambled oligonucleotide. After 48 h, cells were infected with 1 MOI of influenza A for 3 h. Expression of NS1ABP mRNA normalized to GAPDH mRNA is shown. (E) NS1ABP expression profile after 30 and 48 h of transfection with mimics in A549 cells. (F) NS1ABP expression profile after 30 and 48 h of transfection with inhibitor in A549 cells. The fold change represents the change in NS1ABP mRNA expression and influenza A Matrix copy number in cells transfected with the inhibitor relative to that obtained in cells transfected with the scrambled oligonucleotide. Data are expressed as ± SEM, **=p<0.01 and *=p<0.05. n=4 (independent experiments) with triplicate replicates.
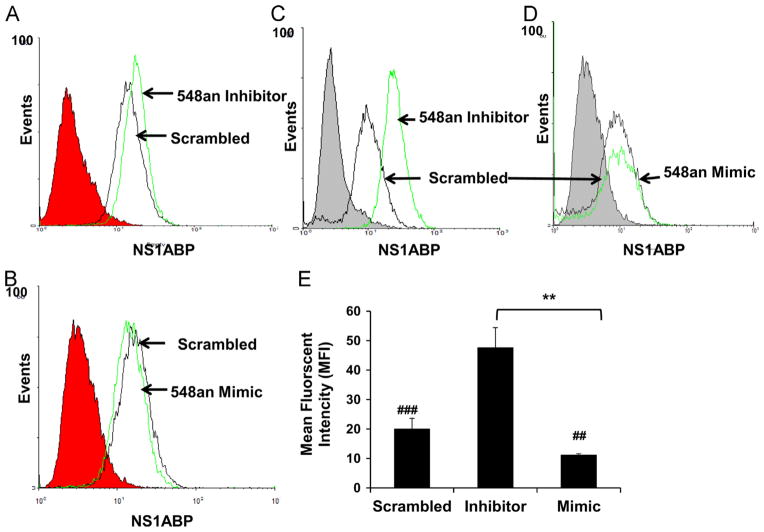
In comparison to the NS1ABP mRNA expression, the flow cytometry data showed a similar effect on NS1ABP protein expression. Uninfected A549 cells transfected with 50 nM miRNA-548an inhibitor showed an up-regulation of NS1ABP protein (Fig. 5A) and transfection with 25 nM of miRNA-548an mimic showed a decrease in the expression of NS1ABP protein (Fig. 5B). A549 cells transfected with miRNA-548an inhibitor and exposed to influenza A showed significantly higher NS1ABP protein expression compared to infected cells transfected with the scrambled oligonucleotide (p<0.001) (Fig. 5C). Further, infected A549 cells transfected with miRNA-548an mimic showed significantly lower (p<0.01) NS1ABP protein expression compared to infected cells transfected with the scrambled oligonucleotide (Fig. 5D). A graphical analysis of these results shows the mean fluorescent intensity (MFI) of cells transfected with inhibitor was 2-fold higher than that of cells transfected with the scrambled oligonucleotide (p<0.001); whereas cells transfected with miRNA-548an mimic had a significant reduction in NS1ABP protein expression (Fig. 5E). Our data demonstrate that miRNA-548an regulates the expression of NS1ABP protein in A549 cells.
Fig. 5.
NS1ABP protein expression is modulated by miRNA 548an. (A) Flow cytometry analysis of A549 cells transfected for 48 h with 50 nM miRNA-548an inhibitor or scrambled oligonucleotide. (B) Flow cytometry analysis of A549 cells transfected for 48 h with 25 nM miRNA-548an mimic or scrambled oligonucleotide. (C) Flow cytometry analysis of A549 cells transfected for 48 h with 50 nM miRNA-548an inhibitor and then infected with 1 MOI of influenza for 3 h. Expression of NS1ABP protein in cells transfected with the miRNA-548an inhibitor (green line), or scrambled oligonucleotide as control (black line) is shown. (D) Flow cytometry analysis in A549 cells transfected for 48 h with miRNA-548an mimic (green line) or scrambled oligonucleotide (black line) and then infected with 1 MOI of influenza for 3 h. The gray shaded or red shaded regions in the graph represent the isotype control. (E) Geometric mean fluorescent intensity (MFI) was measured from 3 independent experiments with triplicate replicates. ###=p<0.001whencomparing cells transfected with inhibitor to cells transfected with the scrambled oligonucleotide; ##=p<0.01when comparing cells transfected with mimic to cells transfected with the scrambled oligonucleotide **=p<0.01 when comparing cells transfected with mimic or inhibitor.
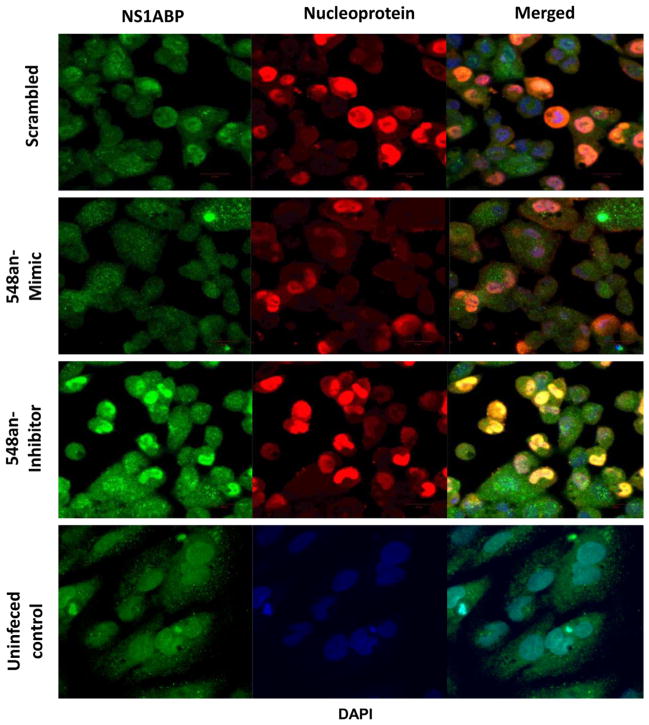
The combined effect of miR-548an on NS1ABP protein expression and virus maintenance in A549 cells infected with influenza A for 3 h was visualized by confocal microscopy (Fig. 6). Most of the A549 cells transfected with the scrambled oligonucleotide miRNA showed intense green fluorescence as a result of active virus infection and intense overlapping red fluorescence generated by concomitant overexpression of virus nucleoprotein (NP) (top panels). In contrast, NS1ABP protein expression was reduced in A549 cells transfected with miR-548an mimic, and this was associated with a sharp reduction in the proportion of infected cells (2nd row panels). Contrary to the results with the mimic, there was higher expression of NS1ABP protein in cells transfected with the miRNA-548an inhibitor that was associated with an increase in the proportion of infected cells with NP now exclusively localized to the nucleus (3rd row panels). The bottom panel represents uninfected cells stained for NS1ABP protein.
Fig. 6.
Visualization of NS1ABP protein expression following modulation of miRNA-548an. A549 cells were transfected for 48 h with either the scrambled oligonucleotide (negative control) (top panel), miRNA-548an mimic (middle panel), or miRNA-548an inhibitor (third panel), and uninfected cells (bottom panel). Transfected cells were infected 1 MOI of influenza A for 3 h and expression of NS1ABP protein (green) and influenza nucleoprotein (red) was determined by immunofluorescence. DAPI (blue) represents the stained nucleus of the cells (not shown separately).
NS1ABP functions as anti-apoptotic protein
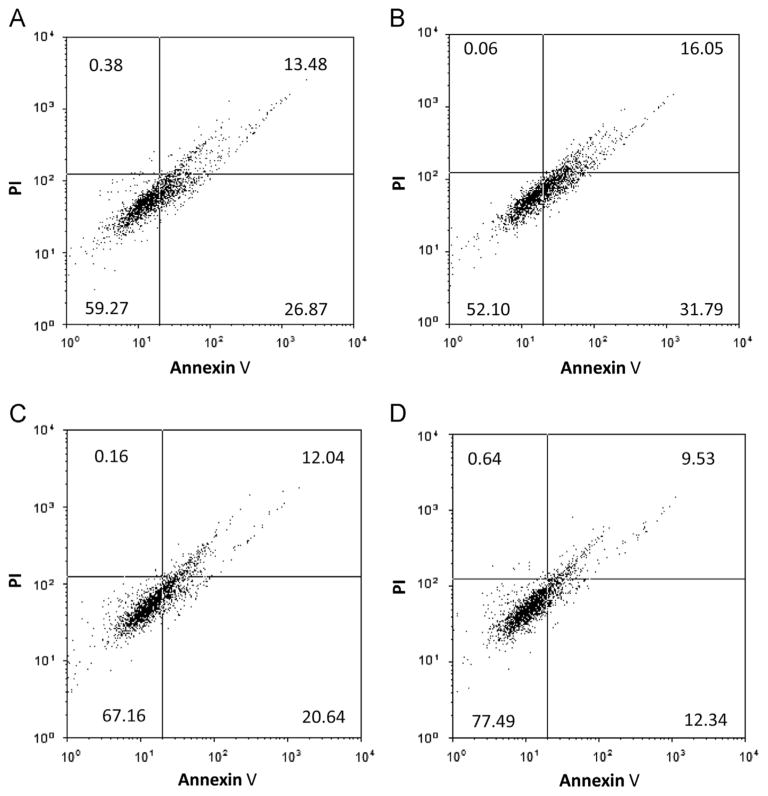
NS1ABP may function as an anti-apoptotic protein during influenza infection to protect the cells from undergoing apoptosis. We verified the functional role of NS1ABP in early stage infection of A549 cells by subjecting the infected cells to apoptosis and necrosis assay. Influenza A infected cells transfected with miR-548an mimic (Fig. 7) were more prone to apoptosis (48%) compared to cells transfected with the scrambled oligonucleotide (41%). Furthermore, 77% of cells transfected with miRNA-548an inhibitor survived compared to 67% of cells transfected with the scrambled oligonucleotide. The increased survival can be attributed to the increased expression of NS1ABP which may function as an anti-apoptotic protein and prevent cells from going into apoptosis. In contrast, the levels of necrotic cells did not change irrespective of transfection with miR-548an mimic or inhibitor. Moreover, the levels of apoptotic cells were comparatively lower in cells transfected with the inhibitor (22%) compared to cells transfected with the scrambled oligonucleotide (33%).
Fig. 7.
Apoptotic pattern of cells transfected with miRNA-548an mimic or inhibitor and infected with influenza A. (A and C) A549 cells were transfected for 48 h with the scrambled oligonucleotide or (B) miRNA-548an mimic, or (D) miRNA-548an inhibitor. Transfected cells were then infected with 1 MOI of influenza A for 3 h. The cells were immune-fluorescently labeled with annexin V and apoptotic cells were analyzed by flow cytometry. Transfection did not change the proportion of necrotic cells detected by propidium iodide (PI) staining. Graph is a representative plot from 3 independent experiments and data presented as percentage of cells in each quadrant. Left upper quadrant=necrotic cells; left lower quadrant=viable cells; right lower quadrant=apoptotic cells; right upper quadrant=late apoptotic cells in necrotic state.
The increase in apoptosis when mimic was used to lower the expression of NS1ABP was accompanied by a reduction in viral copy number (Fig. 8). Cells transfected with 25 nM miRNA-548an mimic and then infected with a low MOI of 0.01 showed 45% reduction in viral copy numbers compared to infected cells transfected with the scrambled oligonucleotide. The effect of the mimic was effectively reduced by increasing the MOI (Fig. 8). There was no significant difference in viral copy number between infected cells transfected with scrambled oligonucleotide and infected cells that were not transfected with miRNA-548an or scrambled oligonucleotide (data not shown).
Fig. 8.
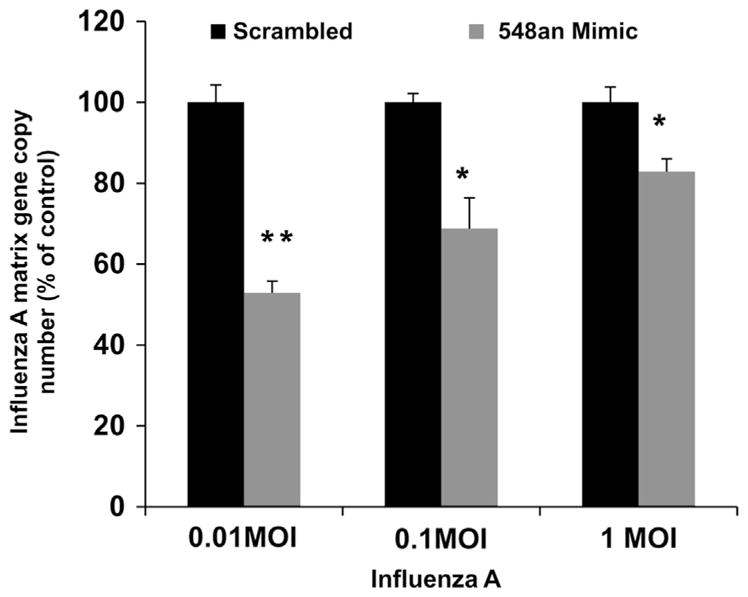
Effect of miRNA 548an mimic on viral replication. (A) A549 cells were transfected for 48 h with 25 nM miRNA-548an mimic or a scrambled oligonucleotide. After 48 h, the cells were infected with increasing MOIs of influenza A for 3 h and matrix copy numbers were determined by RT-PCR. Expression of influenza Matrix gene copies in cells transfected with the mimic is expressed relative to that obtained in cells transfected with the scrambled oligonucleotide. Data are expressed as ± SEM, **=p<0.01, and *=p<0.05. n=3 (independent experiments) performed in triplicate.
Discussion
Although the biological functions of most miRNAs are unknown, their importance for molecular regulation of differentiation, cell proliferation, and cell death has been well documented (Callis et al., 2007). Influenza virus regulates the functions of certain genes in host organisms by high-jacking the protein synthesis machinery, which is essential for viral propagation. In addition to modifying host mRNA expression, the virus also alters its protein profile. We focused on a host protein that is required for viral function and the miRNA that controls its expression. Down-regulation of miRNA-548an, a miRNA not well studied, had a significant role in regulating viral copy number in early stages of infection. MiRNA-548an has been predicted as a target for the degradation of NS1ABP mRNA. Our data demonstrate that lower levels of miR-548an increase the expression of NS1ABP and protect the cells from apoptotic cell death in the early stages of infection (3 h), which is essential for viral particle assembly and propagation. Our data suggests that the decreased expression of miR-548an was not influenza A strains or cell line dependent. We showed that decreased apoptosis in the early stages of infection enhanced infectivity and/or viral maintenance. A recent study implicated influenza A nucleoprotein and its interaction with host clusterin protein in the induction of early apoptosis (Tripathi et al., 2013).
NS1ABP has multiple functions in the cells, and although its ligand, NS1, has been well characterized, the literature lacks studies on NS1ABP which could represent a good target to control infection of the virus. The miRNA548an mimic, which decreases NS1ABP mRNA and protein levels, was found to significantly reduce the maintenance of influenza A virus in A549 cells suggesting its use as a therapeutic intervention in the early stages of infection. With antigenic drift and draft of the viral genes each season, a solution to control viral infection may be through modulation of host resistance by decreasing the possibility of viral multiplication in the early stages of infection through exogenous supplementation of miRNAs. The role of NS1ABP as an anti-apoptotic protein in infected cells was demonstrated following its down-regulation by exogenous supplementation of miRNA-548an (transfection of mimic) which led to enhanced apoptosis. In the presence of miRNA 548an inhibitor, the expression of NS1ABP was significantly increased and a concordant increase in cell survival was observed. Interestingly, the relocation of NS1ABP throughout the cytoplasm was proposed to result in functional changes (Wolff et al., 1998), which was very similar to our findings (Fig. 6). The extended lifespan of cells due to decreased apoptosis would allow the virus to propagate for a longer period of time. The anti-apoptotic properties are favorable for the early life-cycle of the virus, which is reminiscent of the effect of anti-apoptotic proteins on respiratory syncytial virus infection (Othumpangat et al., 2012). Induction of apoptosis by influenza virus has been reported in numerous cells both in vitro (Roulston et al., 1999) and in vivo (Yang et al., 2011). Interaction of the NS1 protein with β-tubulin in cells infected with influenza disrupted cell division and induced apoptosis in A549 cells (Han et al., 2012). One scenario is that an increased expression of NS1ABP and its subsequent binding to NS1 may decrease the availability of NS1 to induce apoptosis.
MiRNAs affect a multitude of genes and regulate cellular physiology through different mechanisms (Ambros, 2003; Bartel, 2004; Lai, 2003). Each miRNA is potentially able to bind up to hundreds of partially complimentary mRNA transcripts and target them for degradation (He and Hannon, 2004). Studies on the functions of miR-548an are sparse in the literature, though the Target Scan database showed hundreds of targets for this miRNA. We focused on miR-548an as it was significantly down-regulated during the early stages of infection. Moreover, its overexpression (by transfecting with its mimic) showed less susceptibility to viral attack while the reverse (by transfecting with the inhibitor) increased influenza maintenance and demonstrated that decreased expression of NS1ABP enables cells to block propagation at least in the early stages of an infection. The exogenous administration of synthetic miRNAs in the form of mimics may antagonize influenza replication in airway epithelium, and provide a novel strategy for therapy for the flu. Based on our findings, we speculate that miR-548an may play a vital role in reducing the symptoms of influenza infection such as acute bronchiolitis and possibly its chronic sequelae including post-severe bronchitis. There are several other miRNAs that are reported to have a role in the life cycle of the influenza virus, and these or other unidentified miRNAs may also affect programmed cell death and viral replication. Deciphering the regulation of miRNA expression may be important not only for diagnostic but also for therapeutic purposes (Janssen et al., 2013; Kasinski and Slack, 2011).
During viral replication, miRNA patterns expressed by infected cells can influence the ability of the invading virus to propagate and survive (Triboulet et al., 2007). Several DNA viruses including herpes viruses encode their own miRNAs that can alter or saturate the miRNA composition of host cells (Ghosh et al., 2009). The host-cellular miRNAs modulate the expression of various viral genes and play a pivotal role in the host-pathogen interaction network. Thus, both the virus and the host are able to manipulate the miRNAs as part of their evolutionary strategies for survival, and in fact both virus-encoded and cell encoded miRNAs are key for prolonging host cell survival. Influenza does not encode any miRNAs but is capable of inducing the expression of 18–27 nucleotide viral leader RNAs, which are incapable of functioning as miRNAs (Umbach et al., 2010). But influenza virus can alter the miRNA profile of the host which, in turn, can directly alter the virus life cycle (Gottwein and Cullen, 2008). A recent clinical study using small inhibitory RNA complimentary to the mRNA encoding respiratory syncytial virus (RSV) protein demonstrated feasibility and potential efficacy of delivering small RNAs directly to the airway mucosa (Zamora et al., 2011). Human miRNAs with antiviral effects thus have significant potential to use as new strategies for antiviral intervention.
In normal cells NS1ABP is concentrated in the intra-nuclear domain, an area enriched with multiple splicing factors. During influenza infection NS1ABP is re-localized which may alter its functional role. Wolff et al. (1998) have proposed that NS1 may down-regulate NS1ABP activity directly by blocking its normal association with spliceosomes. We overexpressed NS1 by transfecting A549 cells with an NS1 expression plasmid and also showed that NS1ABP was significantly down-regulated (data not shown). Wolff et al. (1998) showed that expression of NS1 increases in the later stages of infection but whether NS1 acts by inducing miRNA548an expression is unclear.
In conclusion, our data show that influenza infection modifies miRNA expression patterns in the human lung epithelium. Down-regulation of one miRNA, miRNA-548an, led to increased expression of NS1ABP which favors anti-apoptosis of host cells and increased viral maintenance. That miRNAs are involved in regulating viral replication which has been shown in other viral systems including Hepatitis C and HIV-1 (Jopling et al., 2006; Roberts et al., 2011; Triboulet et al., 2007). Our findings provide a better understanding of the regulatory mechanisms that contribute to the host response to viral infection and disease pathogenesis, and may provide a basis for the development of safe and effective intervention strategies to block influenza infection through the delivery of synthetic miRNAs in influenza infections.
Material and methods
Cell culture
Human alveolar epithelial A549 cells (CCL-34), American Type Culture Collection [ATCC, Manassas, VA] were cultured in standard F12K medium containing 10% heat-inactivated fetal bovine serum (FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate. Madin-Darby canine kidney (MDCK) cells were used for the propagation of influenza virus. MDCK cells were cultivated in MEM (ATCC) supplemented with 10% FBS and100 IU/ml penicillin and 100 μg/ml streptomycin sulfate.
Human bronchial epithelial cells (HBEpC) were purchased from Promo Cell (Heidelberg, Germany) and were maintained in the recommended media and supplements provided by the vendor. Confluent plate of human bronchial epithelial cells was incubated with 3 MOI (multiplicity of infection) of influenza viruses (A/WS/33 and A/Aichi/2/68).
Influenza A virus
Influenza strain A/WS/33 (H1N1, ATCC VR-825), lot 58023547 at 1.58 ×108 50% chicken embryo infectious dose 50% endpoint (CEID50)/ml and lot 58772128 at 2.8 ×106 [CEID]50/ml, A/Aichi/2/68 (H3N2, ATCC-1680), A/Swine/1976/31 (H1N1, ATCC 1682), and A/Swine/Iowa/15/30 (H1N1, ATCC 1683) were purchased from the ATCC and maintained as described (Blachere et al., 2009, 2011).
miRNA array profiling
miRNA microarray analysis of the A549 cells infected with influenza virus (A/WS/33) was performed by Exiqon (Vedbaek, Denmark). Briefly, A549 cells in 6 well plates were infected with influenza A at a multiplicity of infection (MOI) of 3 for 3 h and RNA was extracted from the infected and control cells using the Exiqon miRCURY LNA miRNA extraction kit (Exiqon). The quality of the total RNA was verified by an Agilent 2100 Bioanalyzer profile. 750 ng of total RNA from both sample and reference was labeled with Hy3™ and Hy5™ fluorescent label, respectively, using the miRCURY LNA™ microRNA Hi-Power Labeling Kit, Hy3™/Hy5™ (Exiqon) following the procedure described by the manufacturer. The Hy3™-labeled samples and a Hy5™-labeled reference RNA sample were mixed pair-wise and hybridized to the miRCURY LNA™ microRNA Array 7th gen (Exiqon), which contains capture probes targeting all microRNAs for human, mouse or rat registered in the miRBASE 16.0. The hybridization was performed according to the miRCURY LNA™ microRNA Array instruction manual using a Tecan HS4800™ hybridization station (Tecan, Mannedorf, Switzerland). After hybridization the microarray slides were scanned and stored in an ozone free environment (ozone level below 2.0 ppb) in order to prevent potential bleaching of the fluorescent dyes. The miRCURY LNA™ microRNA Array slides were scanned using the Agilent G2565BA Microarray Scanner System (Agilent Technologies, Inc., Santa Clara, CA) and the image analysis was carried out using the ImaGene® 9 (miRCURY LNA™ microRNA Array Analysis Software, Exiqon). The quantified signals were background corrected (Norm expressed with offset value 10) and normalized using the global Lowess (Locally Weighted Scatterplot Smoothing) regression algorithm. The data obtained were subjected to statistical analysis and differentially regulated miRNAs in infected and uninfected cells were identified and reported.
RNA isolation for miRNA studies
Total RNA (including miRNA) was extracted from the control and infected cells (performed in triplicate) using the miRCURY™ RNA extraction kit (Exiqon). cDNA was synthesized using the universal cDNA kit from Exiqon, diluted and used for real time SYBR green (Exiqon) PCR as instructed by the supplier. Forward and reverse primers for miRNA analysis were purchased from Exiqon. Real time quantitation of miRNAs were done using power SYBR green PCR master mix on a real time PCR 7500 ABI (Applied Biosystems, Foster City, CA). miRNA let-7 was used as the internal control. The fold change in expression of miRNA was calculated as described below.
Quantitative real time (RT) PCR analysis
Total RNA was isolated from A549 and HBEpC cells using the RNeasy kit (Qiagen, Valencia, CA) and cDNA synthesized using the ABI-RT PCR kit (Applied Biosystems) were used for mRNA expression studies. RT-PCR reactions were carried out using the TaqMan FAST PCR reagent (Applied Biosystems) and the ABI 7500 FAST real-time cycler (Applied Biosystems). TaqMan primers for NS1ABP and glyceraldehyde phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. Transcript expression was normalized using GAPDH as the housekeeping gene. The relative change in gene expression was calculated using the formula: fold change=2^(ddCt)=2−dCt (treated samples)2dCt (control samples), where dCt=Ct (detected gene)−Ct (GAPDH) and Ct is the threshold number. Cycle threshold (Ct) values were calculated with the SDS software using automatic baseline settings with assigned minimum threshold.
Influenza copy number was calculated based on the matrix gene copy number, using the following matrix-specific primers: Forward 5′AGATGAGTCTTCTAACCGAGGTCG3′, Reverse 5′TGCAA-AAACATCTTCA AGTCT CTG3′ and probe: 6FAM-TCAGGCCCC-CTCAAAGCCGA-MGBNFQ (Spackman et al., 2002). All primers and probes were synthesized by Applied Biosystems and used at a final concentration of 0.8 μM and 0.2 μM, respectively. Reactions were performed and analyzed using the Applied Biosystems 7500 Fast Real-Time PCR. To determine the relative gene copy number from strains A/WS/33-infected A549 cells, a standard curve was generated from the cloned influenza H1N1 matrix gene and analyzed concurrently with RT-PCR reactions (Blachere et al., 2009).
Transfection studies
A549 cells were transfected with a miRNA-548an inhibitor oligonucleotide (complementary strand to miRNA-548an) or a miRNA-548an mimic oligonucleotide (corresponding to the miRNA-548an sequence) (Life Technologies, Carlsbad, CA) using the lipid-based Lipofectamine 2000 reagent diluted in Opti-MEM-I reduced serum medium (Life Technologies) according to the protocol provided by the supplier. Briefly, A549 cells were grown to 80% confluence in 6-well plates. Transfection complexes were directly applied to the cells (final concentration of 0–100 nM) and the plates were incubated in a humidified chamber with 5% CO2 at 37 °C. After 6 h of transfection, the medium was replaced with fresh complete MEM medium. As negative control, cells were transfected with a scrambled oligonucleotide (Life Technologies). To evaluate the effects of miRNA-548an in the context of viral infection, cells after 48 h of transfection were infected with influenza A at an MOI of 1. Virus were allowed to attach to the cells for 45 min at 37 °C, then the excess virus was washed off and fresh MEM medium containing 1 μg/mL of TPCK trypsin (Sigma, St. Louis, MO) was added. Cells were then incubated for another 3 h. Following the incubation, cells were harvested by trypsinization, washed with phosphate buffered saline (PBS) and used for RNA extraction or flow cytometry.
Flow cytometric analysis
A549 cells were transfected for 48 h with mimic, inhibitor or the scrambled control oligonucleotide and, in some experiments, transfected cells were then infected for 3 h with influenza A. Cells were isolated by trypsinization and stained with specific antibodies for NS1ABP (Abcam, Cambridge, MA). Rabbit IgG (Sigma, St Louis, MO) was used as isotype control for detection of non-specific binding. Secondary antibody labeled with Alexaflour-488 was purchased from Life Technologies. Briefly, 5 × 106 cells infected with influenza A were washed in 2 ml PBS, pelleted by centrifugation, resuspended in 10% formaldehyde, and held for 30 min in ice. Cells were permeabilized using 70% cold ethanol for 30 min, washed with PBS, and then blocked with 0.1% BSA for 15 min. Following blocking, primary antibody was added diluted in PBS-BSA solution. After 20 min of incubation on ice, cells were washed with PBS containing 0.2% tween20. The cells were treated with fluorescently-labeled secondary antibody and held in the dark for 20 min. Unbound antibody was washed with PBS-tween and the resulting cell pellet was reconstituted in 300 uL of PBS. Data were acquired using a FACSCalibur instrument (BD Biosciences, San Jose, CA). A minimum of 10,000 events were collected per sample. Non-infected cells were used to establish the background level of fluorescence in the FL-1 channel (488 nm). Data were analyzed with Windows Multiple Document Interface version 2.9 software for flow cytometry (Win MIDI, La Jolla, CA), and the mean fluorescent intensity was calculated using the same software.
Western blotting
IVNS1ABP protein in infected A549 cells was analyzed by Western blot under reducing conditions using goat anti-rabbit anti-IVNS1ABP antibody (Abcam, MA). NS1ABP protein levels were examined using 30 μg of whole cell homogenates. α-Tubulin or GAPDH (SantaCruz, CA) served as a loading controls. The immunoreactive bands were detected using enhanced chemiluminescence (ECL, Thermoscientific).
Imaging with confocal microscopy
A549 cells were grown on chamber slides (Chamber slide™, Lab-TekII, Thermo Fisher Scientific, Rochester, NY) and transfected for 48 h with the miRNA-548an inhibitor, mimic or scrambled oligonucleotide. The transfected cells were then infected with influenza A for 3 h. After washing with PBS, the cells were fixed with 4% methanol-free formaldehyde (Polysciences Inc., Warrington, PA), permeabilized with 0.5% Triton × 100 (Sigma) followed by blocking with 5% bovine serum albumin (BSA). Slides were washed twice with cold PBS after each treatment. Cells were stained for 1 h with rabbit anti-human NS1ABP antibody (Abcam), and the anti-mouse anti-influenza A nucleoprotein (Millipore, Billerica, MA) followed by a secondary Alexa-488 conjugated anti-rabbit antibody and Alexa-546 conjugated anti-mouse antibody (Invitrogen). The nuclei were stained with DAPI (Invitrogen). The glass slides were mounted with Prolong Gold anti-fade reagent (Invitrogen) and protected with cover slips. Images were obtained using a Zeiss LSM510 confocal microscope with the AxioImager system (Carl Zeiss, Obertochen, AG Germany).
Apoptotic death of cells infected with virus
A549 cells (1 × 106) were transfected with for 48 h with 25–100 nM of miRNA-548an inhibitor, mimic or scrambled oligonucleotide. The transfected cells were then infected with influenza A at MOI of 1 virus for 3 h. Cells were harvested, resuspended in 100 μl of annexin 1 × binding buffer, and stained for 15 min at room temperature with 5 μl of annexin V-FITC (BD Biosciences) and 5 μl of propidium iodide (BD Biosciences) to detect apoptosis and necrosis, respectively. After adding 300 μl of 1 × binding buffer to each sample, flow cytometry analysis was performed using a FACSCalibur. For gating purposes we have used unstained cells, cells stained with only Annexin V, and cells stained only with PI. Data were collected using Cellquest software (BD biosciences) and analysis done with Win MIDI software.
Statistical analysis
Data from RT-PCR and flow cytometry analysis of geometric mean fluorescent intensity (MFI) experiments are the average of 3–4 independent experiments with triplicate replicates. One-way analysis of variance was used to analyze all data and post-hoc pairwise multiple comparisons between means were performed using the Holm-Sidak method with the statistical significance of p<0.05. Statistical analysis was performed using the software Sigma Plot version 11.0 for Windows (Systat Software, Chicago, IL).
Acknowledgments
We thank Prof. Eleanor N. Fish, Ph.D., Dept. of Immunology, University of Toronto for providing the NS1 plasmid construct.
Footnotes
The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
The authors declare no conflict of interest.
References
- Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Blachere FM, Cao G, Lindsley WG, Noti JD, Beezhold DH. Enhanced detection of infectious airborne influenza virus. J Virol Methods. 2011;176:120–124. doi: 10.1016/j.jviromet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Blachere FM, Lindsley WG, Pearce TA, Anderson SE, Fisher M, Khakoo R, Meade BJ, Lander O, Davis S, Thewlis RE, Celik I, Chen BT, Beezhold DH. Measurement of airborne influenza virus in a hospital emergency department. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- Callis TE, Chen JF, Wang DZ. MicroRNAs in skeletal and cardiac muscle development. DNA Cell Biol. 2007;26:219–225. doi: 10.1089/dna.2006.0556. [DOI] [PubMed] [Google Scholar]
- Compans RW. Influenza virus proteins. II Association with components of the cytoplasm. Virology. 1973;51:56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Das K, Ma LC, Xiao R, Radvansky B, Aramini J, Zhao L, Marklund J, Kuo RL, Twu KY, Arnold E, Krug RM, Montelione GT. Structural basis for suppression of a host antiviral response by influenza A virus. Proc Natl Acad Sci USA. 2008;105:13093–13098. doi: 10.1073/pnas.0805213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham EE, Stevens EA, Glover E, Bradfield CA. The aryl hydrocarbon receptor signaling pathway is modified through interactions with a Kelch protein. Mol Pharmacol. 2006;70:8–15. doi: 10.1124/mol.106.024380. [DOI] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Fortes P, Beloso A, Ortin J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 1994;13:704–712. doi: 10.1002/j.1460-2075.1994.tb06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss GK, Salvatore M, Tumpey TM, Carter VS, Wang X, Basler CF, Taubenberger JK, Bumgarner RE, Palese P, Katze MG, García-Sastre A. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: The role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc Natl Acad Sci. 2002;99:10736–10741. doi: 10.1073/pnas.112338099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile D, Doyle W, Whiteside T, Fireman P, Hayden FG, Skoner D. Increased interleukin-6 levels in nasal lavage samples following experimental influenza A virus infection. Clin Diagn Lab Immunol. 1998;5:604–608. doi: 10.1128/cdli.5.5.604-608.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh Z, Mallick B, Chakrabarti J. Cellular versus viral microRNAs in host-virus interaction. Nucleic Acids Res. 2009;37:1035–1048. doi: 10.1093/nar/gkn1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Cullen BR. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe. 2008;3:375–387. doi: 10.1016/j.chom.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Li Z, Chen H, Wang H, Mei L, Wu S, Zhang T, Liu B, Lin X. Influenza virus A/Beijing/501/2009(H1N1) NS1 interacts with beta-tubulin and induces disruption of the microtubule network and apoptosis on A549 cells. PLOS ONE. 2012;7:e48340. doi: 10.1371/journal.pone.0048340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Norman KL, Sarnow P. Positive and negative modulation of viral and cellular mRNAs by liver-specific microRNA miR-122. Cold Spring Harbour Symp Quant Biol. 2006;71:369–376. doi: 10.1101/sqb.2006.71.022. [DOI] [PubMed] [Google Scholar]
- Karlas A, Machuy N, Shin Y, Pleissner KP, Artarini A, Heuer D, Becker D, Khalil H, Ogilvie LA, Hess S, Maurer AP, Muller E, Wolff T, Rudel T, Meyer TF. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463:818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, Gao Q, Andrews SE, Bandyopadhyay MR, De Jesus P, Tu BP, Pache L, Shih C, Orth A, Bonamy G, Miraglia L, Ideker T, Garcia-Sastre A, Young JA, Palese P, Shaw ML, Chanda SK. Human host factors required for influenza virus replication. Nature. 2010;463:813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug RM, Etkind PR. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973;56:334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Lai EC. MicroRNAs: runts of the genome assert themselves. Curr Biol. 2003;13:R925–R936. doi: 10.1016/j.cub.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Loveday EK, Svinti V, Diederich S, Pasick J, Jean F. Temporal- and strain-specific host microRNA molecular signatures associated with swine-origin H1N1 and avian-origin H7N7 influenza A virus infection. J Virol. 2012;86:6109–6122. doi: 10.1128/JVI.06892-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- Othumpangat S, Walton C, Piedimonte G. MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression. PLOS ONE. 2012;7:e30030. doi: 10.1371/journal.pone.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AP, Lewis AP, Jopling CL. miR-122 activates hepatitis C virus translation by a specialized mechanism requiring particular RNA components. Nucleic Acids Res. 2011;39:7716–7729. doi: 10.1093/nar/gkr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- Russo A, Potenza N. Antiviral effects of human microRNAs and conservation of their target sites. FEBS Lett. 2011;585:2551–2555. doi: 10.1016/j.febslet.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Shin YK, Liu Q, Tikoo SK, Babiuk LA, Zhou Y. Effect of the phosphatidylinositol 3-kinase/Akt pathway on influenza A virus propagation. J Gen Virol. 2007;88:942–950. doi: 10.1099/vir.0.82483-0. [DOI] [PubMed] [Google Scholar]
- Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A Influenza virus and the Avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triboulet R, Mari B, Lin YL, Chable-Bessia C, Bennasser Y, Lebrigand K, Cardinaud B, Maurin T, Barbry P, Baillat V, Reynes J, Corbeau P, Jeang KT, Benkirane M. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science. 2007;315:1579–1582. doi: 10.1126/science.1136319. [DOI] [PubMed] [Google Scholar]
- Tripathi S, Batra J, Cao W, Sharma K, Patel JR, Ranjan P, Kumar A, Katz JM, Cox NJ, Lal RB, Sambhara S, Lal SK. Influenza A virus nucleoprotein induces apoptosis in human airway epithelial cells: implications of a novel interaction between nucleoprotein and host protein Clusterin. Cell Death and Dis. 2013;4:e562. doi: 10.1038/cddis.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JL, Yen HL, Poon LL, Cullen BR. Influenza A virus expresses high levels of an unusual class of small viral leader RNAs in infected cells. mBio. 2010;1(4) doi: 10.1128/mBio.00204-10. http://dx.doi.org/10.1128/mBio.00204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, O’Neill RE, Palese P. NS1-Binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J Virol. 1998;72:7170–7180. doi: 10.1128/jvi.72.9.7170-7180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Hong X, Yang P, Ju X, Wang Y, Tang J, Li C, Fan Q, Zhang F, Chen Z, Xing L, Zhao Z, Gao X, Liao G, Li Q, Wang X, Li D, Jiang C. The 2009 pandemic A/Wenshan/01/2009 H1N1 induces apoptotic cell death in human airway epithelial cells. J Mol Cell Biol. 2011;3:221–229. doi: 10.1093/jmcb/mjr017. [DOI] [PubMed] [Google Scholar]
- Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J, Vaishnaw A, Cehelsky J, Albert G, Nochur S, Gollob JA, Glanville AR. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2011;183:531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]