Abstract
Background
Chronic inflammation plays a key role in cancer etiology. DNA methylation modification, one of the epigenetic mechanisms regulating gene expression, is considered a hallmark of cancer. Human and animal models have identified numerous links between DNA methylation and inflammatory biomarkers. Our objective was to prospectively and longitudinally examine associations between methylation of four inflammatory genes and cancer risk.
Methods
We included 795 Normative Aging Study participants with blood drawn 1-4 times from 1999-2012 (median follow up 10.6 years). Promoter DNA methylation of IL-6, ICAM-1, IFN, and TLR2 in blood leukocytes was measured using pyrosequencing at multiple CpG sites and averaged by gene for data analysis. We used Cox regression models to examine prospective associations of baseline and time-dependent methylation with cancer risk, and compared mean methylation differences over time between cancer cases and cancer-free participants.
Results
Baseline IFN hypermethylation was associated with all-cancer (HR=1.49, p=0.04) and prostate cancer incidence (HR=1.69, p=0.02). Baseline ICAM-1 and IL-6 hypermethylation were associated with prostate cancer incidence (HR=1.43, p=0.02; HR=0.70, p=0.03 respectively). In our time-dependent analyses, IFN hypermethylation was associated with all-cancer (HR=1.79, p=0.007) and prostate cancer (HR=1.57, p=0.03) incidence; and ICAM-1 and IL-6 hypermethylation were associated with prostate cancer incidence (HR=1.39, p=0.02; HR=0.69, p=0.03 respectively). We detected significant ICAM-1 hypermethylation in cancer cases (p=0.0003) 10-13 years pre-diagnosis.
Conclusion
Hypermethylation of IFN and ICAM-1 may play important roles in early carcinogenesis, particularly that of prostate cancer.
Impact
These methylation changes could inform the development of early detection biomarkers and potential treatments of inflammation-related carcinogenesis.
Keywords: DNA Methylation, cancer incidence, longitudinal studies
Introduction
Chronic inflammation is a significant contributor to carcinogenesis. Drivers of inflammation are diverse, such as chronic or recurrent infection, autoimmune disease, obesity, and toxic exposures. Recent estimates suggest that inflammatory mechanisms directly contribute to roughly 25% of all cancers.(1) Large-scale studies have found significant associations between circulating inflammatory factors (IF) and risk of multiple types of cancer,(2, 3) as well as environmental and behavioral exposures previously linked to cancer.(4-6) Dysregulation in the inflammatory immune response can potentially facilitate carcinogenesis through a number of mechanisms. Other studies have also suggested a potential field effect whereby chronic inflammation can induce epigenetic changes in blood leukocytes, which play a pivotal role in inflammation-related carcinogenesis.(7-9) For example, inflammation induces characteristic aberrant methylation patterns associated with colorectal cancer,(10-12) and inflammation-mediated formation of DNA damage by-products have been linked to aberrant hypermethylation associated with glioblastoma.(13) In prostate cancer, pro-inflammatory cytokines are reportedly susceptible to altered expression via aberrant DNA hypermethylation, and in turn alter the regulation of other genes involved in cancer development.(14-16) Genetic and epigenetic changes affecting genes regulating inflammation have been singled out as a potential cause of prostate cancer.(17)
Aberrant methylation of DNA in inflammatory genes can be induced by environmental carcinogens,(18-21) and this aberrant methylation is an important predictor of cancer incidence.(22-24) This suggests DNA methylation as a promising candidate for specific mechanisms by which environmental carcinogens and chronic inflammation can contribute to cancer development. Methylation of blood leukocytes is a critical component governing immune response and inflammatory processes in the body,(25-28) both of which have been linked to a wide variety of cancers(29) (often through the induction of additional methylation aberrations).(26, 30) In addition, the accessibility of blood leukocytes has led to increased interest in their use as potential epigenetic biomarkers for a variety of cancers.(31)
However, most published human subjects studies in this area are limited by their case-control design, preventing researchers from establishing a temporal relationship between cancer and IF expression. As blood samples taken post-diagnosis may be affected by the disease, or by treatment, this is an important issue for studies of cancer epigenetics. Longitudinal data to prospectively explore the viability of methylation measures as a biomarker of cancer are also greatly needed.(32) Furthermore, most prospective studies of IF methylation have methylation measurements at a single time point only. This means the relationship over time between changes in IF methylation and carcinogenesis has yet to be established. The objective of our present study is to better understand this relationship by exploring the prospective associations between pre-diagnostic blood leukocyte DNA methylation measured at multiple time points, methylation rates of change over time, and risk of developing cancer.
Materials and Methods
Study population
The Normative Aging Study (NAS) was established by the US Department of Veteran Affairs in 1963. The initial enrollment of the cohort consisted of 2280 healthy men. Eligibility criteria included veteran status; living in or around Boston, Massachusetts; age 21-80; and no history of hypertension or other chronic conditions including heart disease, cancer, and diabetes. Since then, participants have been recalled periodically for clinical exams every 3-5 years. From 1963-1999, 981 participants died and 470 were lost to follow up. Statistical comparisons between the remaining 829 participants and those lost to follow up revealed no significant differences in subject characteristics (age, BMI, etc.). Beginning in 1999 follow-up exams included a 7-mL blood sample for genetic and epigenetic analysis. From 1999 through 2012, 802/829 (96.7%) NAS participants who had been regularly attending study follow-up visits consented to the blood donation. This analysis focuses on 795 participants who had at least one methylation measurement of one or more of the following inflammatory genes (selected by the NAS for sequencing based on a literature review): Interleukin-6 (IL-6), Intercellular Adhesion Molecule-1 (ICAM-1), Interferon-gamma (IFN), and Toll-like Receptor-2 (TLR2). Circulating levels of IL-6 and ICAM-1 proteins were measured from the same blood samples. Of the 795 total participants 221 (28%) had one visit that included blood collection, 208 (26%) had two visits, 233 (29%) had three visits, and 133 (17%) had four visits. This study was approved by the Institutional Review Boards of all participating institutions, and written consent forms obtained from all participants. Study baseline (defined as date of the first collection of a blood sample) ranged from 1999 to 2010 (median baseline 2000, IQR 1999-2001). In total, 303/582 (52%) subjects had their baseline visit in 1999 or 2000, 245/582 (42%) from 2001-2003, and 34/582 (6%) from 2003-2010.
In addition to blood samples, the NAS consists of anthropometric measurements, standardized medical exams, and questionnaires about medical history and lifestyle. For purposes of this analysis, we controlled for the following potential confounders: Race (dichotomized as white or nonwhite), education (<13 years, 13-16 years, >16 years), two cigarette smoking variables (status of never/former/current, and estimated cumulative pack-years), whether the respondent reported consuming two or more alcoholic drinks per day on average, body mass index (calculated from weight and height measurements), and age. Because our methylation measures were obtained from blood DNA, we also adjusted for white blood cell count and proportion of neutrophils in the blood samples, to account for the possibility that our results might reflect disease-related changes in the white blood cells rather than epigenetic alteration of their DNA.
Cancer diagnosis
NAS investigators obtained cancer diagnoses from questionnaires and confirmed them via medical records and histological reports. Among the 795 participants included in the present study, a total of 213 participants had been diagnosed with cancer (64 prostate cancers, 95 skin cancers, 54 other) as of the study baseline. These participants were excluded. Among the 582 participants free of cancer at baseline, 137 (23.5%) developed cancer during a median of 10.6 years of follow up including: 47 prostate cancers, 43 skin cancers, and 47 other cancers. These 582 participants’ median methylation levels were also determined at baseline to define cut-offs for variable categorization (see below). Initial analytical results of incident skin cancer as an outcome were not significant, therefore it was dropped from our study and the incidence of all cancers and prostate cancers used as our primary outcomes of interest.
Methylation measurement
The full procedure for blood leukocyte DNA extraction and measurement has been reported previously.(21) In order to maximize methylation measurement accuracy, we used a pyrosequencing-based assay to measure CpG sites at two positions each on IFN and IL-6, three positions on ICAM-1, and five positions on TLR2. The CpG sites measured were selected to maximize assay coverage of the promoter region in each target gene, so as to provide the most accurate data on regional methylation. All methylation loci were selected as described in a previously-reported protocol,(33) based on the reproducibility of primer sets and PCR products. All assays used built-in controls. Methylation measurements from each position were averaged via a simple mean (by gene), then standardized by processing batch number to have a mean value of 0 and a standard deviation of 1. In light of previous NAS findings(34) that gene-specific methylation can be affected by point mutations at the site of measurement, we searched for SNPs in these genes using the University of California Santa Cruz (UCSC) genome browser (genome.ucsc.edu). Out of all 12 CpG sites in the four inflammatory genes examined in our study, we only observed one SNP (C/T), for IL-6 position 1 (rs2069831).
To determine whether to categorize methylation variables for purposes of our analysis, we performed simple scatter plots and fit trend lines (with R2 statistics) to assess the nature of the relationship between cancer incidence and the methylation measures present in the dataset in both continuous and categorical form. For all cancers, methylation dichotomized about the median (as measured among all subjects free of cancer at baseline) fit the data better. For prostate cancer incidence, continuous methylation resulted in better fit with the data.
Statistical Analysis
After descriptive analysis, correlations between mean DNA methylation level of IL-6 and ICAM-1 and each of their corresponding blood protein levels were evaluated via Spearman’s rank correlation coefficients. To capture dynamic changes in DNA methylation we used Cox proportional hazards models of time to cancer diagnosis on mean methylation as a time-dependent independent variable, the first using methylation data from baseline (first blood sample) only and the second using all available follow-up visits. For circulating proteins with significant Spearman’s rank correlations, we compared these model results to those using the corresponding protein level instead of methylation.
In order to examine the potential effects of the above-mentioned SNP and other potential variability by CpG position on our results, we also conducted a sensitivity analysis of standardized measurements of IF methylation at each position separately, to look for significant departures from our findings with mean methylation. We also conducted a second sensitivity analysis examining short-term time trends by creating variables for methylation values measured at 3-, 4-, and 5-year intervals from baseline minus baseline methylation values and using multiple Cox proportional hazards models to estimate prospective associations between these interval variables and risk of developing cancer.
We obtained change rate (in standardized units/year) as the slope of the repeated measures of DNA methylation to examine the relationship between increasing methylation change rate and cancer incidence. This involved using a linear regression model to estimate changes in methylation over time (slope) for all participants with more than one measurement, and subsequently treating the slope value from this model as an independent variable in additional Cox regression models.
Finally, we compared the mean difference in methylation between cancer cases and cancer-free participants each year prior to cancer diagnosis to examine the difference in methylation trajectory between two groups. Due to low sample size, these one-year intervals were collapsed into categories based on five-year intervals (<5 years, 5 to less than 10 years, and 10+ years). Individual methylation measures were plotted, and statistical significance of the mean between-group difference between subjects who later developed cancer and those who did not was assessed via linear mixed-effects regression models of methylation on cancer status, time interval, and other independent variables as above. All analyses were performed using SAS version 9.3, with p≤0.05 set as our threshold for statistical significance.
Results
Participant characteristics by cancer status are similar to those reported previously for this cohort,(35) although the number of incident cancer cases slightly increased as of the most recent follow up. Overall, participants were older (mean age 72 years), overwhelmingly Caucasian (96%), mostly (72%) college educated or more, and the majority (71%) were current or former smokers. Table 1 shows the results of our descriptive analysis of baseline IF methylation by participant characteristics. Briefly, IFN methylation varied across both smoking variables (p=0.05 for smoking status, and p=0.01 for pack-years of smoking), white blood cell count (p=0.006), and percent neutrophils (p<0.001). IL-6 methylation varied across race (p=0.01), and was significantly correlated with circulating IL-6 protein level (ρ= −0.08, p=0.02).
Table 1. Participant Characteristics and Cancer Risk Factors by IF Methylation Among Cancer-Free Participants at Baseline.
| Mean ± SD / n(%) |
IFN | ICAM-1 | IL-6 | TLR2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | |||
| Age | 72.26 ± 6.84 | 72.1 ± 6.6 | 71.4 ± 6.8 | 71.6 ± 6.4 | 71.9 ± 7.1 | 71.8 ± 7.2 | 71.69 ± 6.3 | 71.5 ± 6.2 | 72.1 ± 7.0 | |
| p=0.17 | p=0.67 | p=0.80 | p=0.33 | |||||||
| Race | ||||||||||
| White | 766 (96%) | 271 (96.8%) | 266 (94.7%) | 208 (97.2%) | 204 (95.8%) | 273 (97.9%) | 262 (93.2%) | 226 (95.8%) | 212 (95.9%) | |
| Non-white | 29 (4%) | 9 (3.2%) | 15 (5.3%) | 6 (2.8%) | 9 (4.2%) | 6 (2.1%) | 19 (6.8%) | 10 (4.2%) | 9 (4.1%) | |
| p=0.30 | p=0.45 | p=0.01* | p=1.00 | |||||||
| Education | ||||||||||
| HS Grad or Less | 224 (28%) | 77 (27.5%) | 84 (29.9%) | 58 (27.1%) | 63 (29.6%) | 76 (27.2%) | 83 (29.5%) | 74 (31.4%) | 65 (29.4%) | |
| Some College/College Grad | 387 (49%) | 135 (48.2%) | 139 (49.5%) | 111 (51.9%) | 99 (46.5%) | 144 (51.6%) | 133 (47%) | 109 (43.2%) | 108 (48.9%) | |
| Any professional/graduate school | 184 (23%) | 68 (24.3%) | 58 (20.6%) | 45 (21.0%) | 51 (23.9%) | 59 (21.1%) | 65 (23.13%) | 53 (22.5%) | 48 (21.7%) | |
| p=0.56 | p=0.54 | p=0.61 | p=0.85 | |||||||
| BMI | 28.3 ± 4.1 | 28.3 ± 3.9 | 28.3 ± 4.4 | 28.5 ± 4.1 | 28.3 ± 4.4 | 28.6 ± 4.3 | 28.1 ± 3.9 | 28.5 ± 4.4 | 28.2 ± 4.0 | |
| p=0.92 | p=0.57 | p=0.15 | p=0.52 | |||||||
| Smoking Status | ||||||||||
| Never smoked | 227 (28.55%) | 90 (32.1%) | 66 (23.5%) | 58 (27.1%) | 60 (28.2%) | 74 (26.5%) | 80 (28.5%) | 60 (25.4%) | 65 (29.4%) | |
| Current Smoker | 33 (4%) | 10 (3.6%) | 16 (5.7%) | 10 (4.7%) | 11 (5.2%) | 12 (4.3%) | 13 (4.6%) | 12 (5.1%) | 8 (3.6%) | |
| Former Smoker | 535 (67%) | 180 (64.3%) | 199 (70.8%) | 146 (68.2%) | 142 (66.7%) | 193 (69.2%) | 188 (66.9%) | 164 (69.5%) | 148 (67.0%) | |
| p=0.05* | p=0.93 | p=0.87 | p=0.54 | |||||||
| Average Pack-Years | 21.5 ± 26.7 | 18.2 ± 24.2 | 23.4 ± 24.2 | 21.87 ± 24.9 | 22.05 ± 24.4 | 21.6 ± 24.5 | 19.7 ± 23.8 | 21.9 ± 24.3 | 20.4 ± 25.2 | |
| p=0.01* | p=0.94 | p=0.35 | p=0.51 | |||||||
| Alcohol Consumption | ||||||||||
| 0-1 average drinks/day | 644 (81%) | 237 (84.6%) | 224 (79.7%) | 170 (79.4%) | 172 (80.8%) | 232 (83.2%) | 228 (81.1%) | 195 (82.6%) | 179 (81.0%) | |
| 2+ average drinks/day | 151 (19%) | 43 (15.4%) | 57 (20.3%) | 44 (20.6%) | 41 (19.3%) | 47 (16.9%) | 53 (18.9%) | 41 (17.4%) | 42 (19.0%) | |
| p=0.15 | p=0.81 | p=0.58 | p=0.72 | |||||||
| White Blood Cell Count | 6.5 ± 3.25 | 6.1 ± 1.4 | 6.7 ± 2.8 | 6.5 ± 1.7 | 6.4 ± 2.9 | 6.5 ± 1.7 | 5.2 ± 1.5 | 6.5 ± 2.8 | 6.2 ± 1.6 | |
| p=0.006* | p=0.78 | p=0.07 | p=0.12 | |||||||
| Proportion Neutrophils | 61.9 ± 8.7 | 59.3 ± 6.9 | 64.9 ± 8.3 | 62.5 ± 8.2 | 62.1 ± 8.6 | 62.3 ± 8.1 | 61.9 ± 7.9 | 62.8 ± 8.7 | 61.3 ± 7.7 | |
| p<0.001* | p=0.64 | p=0.54 | p=0.06 | |||||||
= Statistically significant at p<0.05; p-values shown for Student’s t-test and Fischer’s Exact test for continuous and categorical characteristics, respectively
Table 2 shows the results of our analyses of baseline and time-dependent IF methylation with risk of developing cancer. For methylation measured at baseline only, high IFN methylation was associated with all-cancer (HR: 1.49, 95% CI: 1.01-2.20) and prostate cancer incidence (HR: 1.69, 95% CI: 1.10-2.60). High baseline ICAM-1 (HR: 1.43, 95% CI: 1.07-1.92) and IL-6 (HR: 0.70, 95% CI: 0.51-0.97) methylation were associated with prostate cancer incidence as well. For the time-dependent analyses, participants with high IFN methylation were significantly more likely to develop any cancer (HR: 1.71, 95% CI: 1.16-2.51), prostate cancer (HR: 1.57, 95% CI: 1.04-2.37), and other cancers (HR: 1.85, 95% CI: 1.15-2.99; data not shown). Participants with high time-dependent ICAM-1 methylation were more likely to develop prostate cancer (HR: 1.39, 95% CI: 1.02-1.89), while participants with high time-dependent methylation of IL-6 were significantly less likely to develop prostate cancer (HR: 0.69, 95% CI: 0.50-0.95). There were no significant associations between TLR2 methylation and risk of developing cancer. When rerunning the above models using IL-6 protein level instead of IL-6 methylation level we found no significant associations (data available upon request). Examining incident skin cancer as the outcome of interest likewise produced no noteworthy results (data available upon request).
Table 2. Multivariable Model Results.
| Baseline Methylation Measure |
Time-Dependent Methylation |
||||||
|---|---|---|---|---|---|---|---|
| Cancer Dx (n) | |||||||
| No | Yes | HR (95% CI) | p | HR (95% CI) | p | ||
| IFN: | |||||||
| All Cancer | |||||||
| Low | 222 | 58 | Ref. | Ref. | |||
| High | 209 | 72 | 1.49 (1.01, 2.20) | 0.04* | 1.71 (1.16, 2.51) | 0.007* | |
| Prostate Cancer | 431 | 43 | 1.69 (1.10, 2.60) | 0.02* | 1.57 (1.04, 2.37) | 0.03* | |
|
| |||||||
| ICAM-1: | |||||||
| All Cancer | |||||||
| Low | 169 | 44 | Ref. | Ref. | |||
| High | 160 | 54 | 1.25 (0.83, 1.88) | 0.29 | 1.17 (0.79, 1.74) | 0.42 | |
| Prostate Cancer | 329 | 33 | 1.43 (1.07, 1.92) | 0.02* | 1.39 (1.02, 1.89) | 0.04* | |
|
| |||||||
| IL-6: | |||||||
| All Cancer | |||||||
| Low | 213 | 66 | Ref. | Ref. | |||
| High | 219 | 62 | 0.93 (0.65, 1.34) | 0.7 | 0.87 (0.60, 1.24) | 0.43 | |
| Prostate Cancer | 432 | 44 | 0.70 (0.51, 0.97) | 0.03* | 0.69 (0.50, 0.95) | 0.02* | |
|
| |||||||
| TLR2: | |||||||
| All Cancer | |||||||
| Low | 178 | 49 | Ref. | Ref. | |||
| High | 179 | 51 | 0.96(0.63, 1.44) | 0.83 | 0.88 (0.59, 1.31) | 0.53 | |
| Prostate Cancer | 357 | 35 | 0.88 (0.60, 1.28) | 0.49 | 0.95 (0.66, 1.37) | 0.77 | |
= Statistically significant at p<0.05.
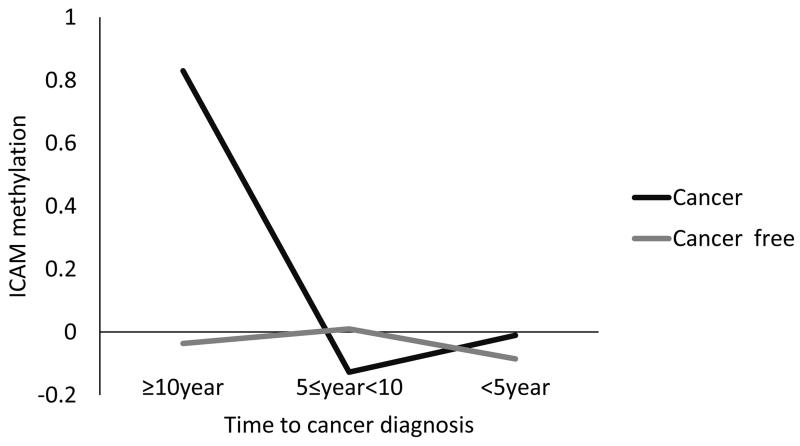
Reanalyzing significant results by individual CpG position revealed no substantive deviations from mean methylation in directionality, magnitude, or statistical significance of the above associations (data available upon request). We also did not observe any significant associations between methylation change values over 3-, 4-, or 5-year intervals and risk of developing cancer. Increased rate of change of ICAM-1 methylation was significantly associated with prostate cancer incidence (HR: 25.1, 95% CI: 1.05-596, data not shown), while increased rate of IFN methylation was inversely associated with cancer incidence (HR: 0.58, 95% CI: 0.34-0.99, data not shown). Figure 1 plots the mean difference in ICAM-1 methylation between participants who ultimately developed cancer and cancer-free participants by time intervals prior to diagnosis. Notably, ICAM-1 methylation was significantly higher in participants who ultimately developed cancer than those who did not 10 or more years prior to cancer diagnosis (p=0.0003).
Figure 1. Mean ICAM-1 Methylation in Participants with and without an Eventual Cancer Diagnosis by 5-Year Interval.
Discussion
Our results show prospective relationships between IFN methylation levels over time and elevated risk of developing cancer. We also found a number of associations between risk of developing prostate cancer and three of the IF genes studied (IFN, ICAM-1, and IL-6). The consistent associations of both methylation at baseline and time-dependent IF methylation (incorporating all follow-up visits) suggest that methylation of IFN, ICAM-1, and IL-6 are epigenetic changes that occur early in prostate cancer development (and possibly other cancers as well, in the case of IFN), pointing to the involvement of IF methylation in carcinogenesis. The methylation change rate analysis suggests that more rapid increases in ICAM-1 methylation are associated with risk of developing cancer, while a high rate of increase in IFN methylation is protective. Finally, a temporal relationship emerged with ICAM-1 methylation in cancer cases being significantly higher a decade or more prior to diagnosis. To our knowledge, this is the first study finding detectable differences in mean methylation level in cancer cases compared to cancer-free participants so many years prior to cancer diagnosis.
The positive association between IFN methylation and risk of developing cancer is consistent with its previously described role in an apoptosis pathway via DAP-kinase.(36) The role of IFN in promoting cell apoptosis can lead to it serving a tumor suppressive function, one that has been previously shown to be reduced in colon cancer.(37) IFN also serves to stimulate IFN-8, which can exert tumor suppressive effects on a wide range of carcinomas.(38) IFN methylation has been specifically shown to be a mechanism used by infiltrating tumor cells to induce immunosuppression.(39) In our study, IFN methylation varied across both smoking variables (p=0.05 for smoking status, and p=0.01 for pack-years of smoking), with heavier smokers tending to have higher methylation of IFN. Studies suggest that exposure to chemicals in cigarettes can affect gene-specific DNA methylation levels through pathways similar to those through which smoking can induce genetic changes.(32) Animal studies have found that one mechanism through which smoking depresses the immune response is through reduced expression of IFN.(40) Ouyang, et al. reported the hypermethylation of the IFN promoter among workers with diisocyanate-induced occupational asthma due to exposure to inhaled carcinogens.(41) This evidence suggests that hypermethylation of IFN may be one of the epigenetic mechanisms through which inhaled carcinogens induce carcinogenesis, and if confirmed may lead to new interventions to reduce the impact of smoking, a significant public health concern worldwide.
In contrast to the results of our time-dependent analysis, where higher IFN methylation was associated with increased risk of developing cancer, we found that the speed of increase over time of IFN methylation was inversely associated with risk of developing prostate cancer. One possible explanation for this apparent contradiction with our baseline and time-dependent analyses (both of which found a significant, positive association), is that the mechanism by which IFN methylation influences the risk of developing prostate cancer may be cumulative in nature. In other words, early low-intensity IFN hypermethylation has a stronger cancer-promoting effect than later, high-intensity IFN hypermethylation. This may be a reflection of the indirect nature of the causal pathway, e.g., a reduction in normal cellular apoptosis achieves an elevated risk of cancer that can only be fully realized over time, or it may be related to other involved epigenetic mechanisms that we were unable to incorporate into our analysis. Although the tumor suppressive functions of IFN have been found in blood and tissue of cancer patients,(42, 43) the possibility that accumulative IFN methylation over time helps drive cancer development has not been established. Under this theory, long-term IFN methylation aberration is necessary for prostate carcinogenesis, rather than severe, short-term methylation. If accurate, then epigenetic targets involved in the IFN pathway may be effective therapeutic or preventive targets for prostate cancer. One study already suggests that this may be the case in MYC-driven prostate cancer.(44) Given the absence of genetic data from tumor tissue in our study, confirmation of our finding with regard to IFN methylation (particularly in conjunction with MYC expression in prostate cancer cells) is necessary. Future research incorporating IFN expression (e.g., through circulating protein levels unavailable in our data) can explore these hypotheses and help explain our complex findings for IFN methylation.
Similarly, ICAM-1 acts as a tumor suppressor primarily by modulating anti-tumor immunity.(45) A previous study has detected reduced ICAM-1 expression in ovarian cancer cell lines,(46) as well as some malignant melanomas.(47) Recent work on targeted demethylation of genes in the ICAM-1 pathway(48) and up-regulation of ICAM-1 expression(49) are promising avenues for enhancing the immune response to cancer. Our finding that ICAM-1 methylation level is, on average, significantly higher many years prior to conventional diagnosis of cancer is intriguing, and may be reflected in our finding of elevated risk of developing cancer with greater ICAM-1 methylation rate of change. If replicated, this finding of hypermethylated ICAM-1 in cancer patients many years before diagnosis may assist in the development, already underway,(48) of enhanced diagnostic and therapeutic techniques to improve outcomes of a variety of cancers. The large lead time found in our analysis may explain why other studies with less pre-diagnostic follow up do not consistently find associations between ICAM-1 methylation and cancer,(47, 50) as well as the absence of other published findings relating ICAM-1 methylation to prostate cancer. Alternate methods of epigenetic silencing, such as histone modification,(45) may also be involved in suppressing the expression of ICAM-1 (and through it the immune response) on tumor-conditioned endothelial cells in the time period closer to diagnosis. This is a plausible mechanism through which ICAM-1 methylation can affect cancer development independently of circulating ICAM protein levels, and may be an explanation for the lack of any statistical associations between ICAM protein level and cancer incidence found in our study. As methylation is only one factor affecting gene transcription/expression as it relates to carcinogenesis,(51) this may also explain the lack of a significant correlation between ICAM-1 methylation and circulating ICAM protein.
Elevated levels of the IL-6 cytokine have been found repeatedly in both serum and local tissue samples taken from prostate cancer patients,(52, 53) and suppression of SOCS3 via expression of IL-6 has been implicated in the development(54) and aggressiveness(55) of prostate cancer. These findings may point to an important causal factor for prostate cancer. Alternatively, IL-6 hypomethylation in these cases may simply be a marker for more widespread (possibly even genome-wide) methylation in prostate cancer, a possibility that can be confirmed in other studies of the relationship between methylation and prostate cancer. Our significant finding despite the lack of an association between circulating IL-6 and cancer incidence suggests that methylation of IL-6 may potentially exert effects contributing to prostate carcinogenesis through mechanisms other than circulating protein expression (e.g., by affecting SOCS3 expression(56)), and adds further evidence of its importance in that process and potential usefulness for further study. The significant inverse correlation between IL-6 methylation and IL-6 circulating protein found in our study may also facilitate the development of IL-6 as a biomarker of prostate cancer.
DNA methylation is dynamic. However, it is still largely unknown whether the magnitude of methylation changes plays a role in cancer etiologically and, if they do, how much change is necessary to facilitate cancer initiation/development. The lack of significant findings for our interval analysis comparing the risk of developing cancer by 3-, 4-, and 5-year differences in IF methylation may be due to an insufficiently large effect to be detectable across the interval chosen, if there is such an effect. Alternatively, it may be that lengthier follow up or a larger sample than was available in this study is necessary to detect differences in IF methylation related to early carcinogenesis.
This analysis has limitations. We were unable to consider other potential epigenetic mechanisms affecting IF genes, such as histone modification or microRNAs. We were also unable to examine expression (e.g., circulating protein levels) for two of the IF genes studied, preventing direct causal inference for these factors. Other data potentially relevant to the study of cancer (e.g., family history of cancer) was also unavailable in the source dataset, making unmeasured confounding of our results a potential concern. A strength of our study was the large quantity of data and multiple follow-up measurements available, offset by a relatively low sample size. This is particularly true for some of our subgroup analyses, such as those with large (>10 years) follow up. Sample size also prevented us from examining many subtypes of cancer, and even our prostate cancer sample was limited, resulting in wide confidence intervals for our rate-of-change analysis that should be confirmed in future studies with a larger sample size. This forced the use of all-cancer incidence as our outcome of interest. Given the heterogeneity of cancers and their biological diversity, this aggregation may not reflect the biological reality of the development and progression of all cancers. However, given that the majority of diagnoses in our study population were prostate (n=47) or skin (n=43) cancers, our findings suggest that IF gene methylation may be an important factor involved in the development of these specific diseases. Our results will need to be verified for other specific cancer subtypes before viable interventions based on them can be developed. This also affected our interval analysis, as it was similarly restricted to participants whose visits were 3, 4, or 5 years apart. As our time-dependent analysis could utilize the full sample it may have had sufficient statistical power to capture false negatives missed in the interval analyses due to the lack of a specific short-term temporal mechanism relating IF methylation and cancer. The variable number of follow-up visits also introduces potential information bias, as individuals who are less healthy (e.g., diagnosed with more aggressive metastatic disease) are less likely to be able to participate, and study participants are more likely to be cancer survivors. This combined with the fact that our sample was overwhelmingly older, Caucasian, and male warrants further studies in larger, more representative populations.
In conclusion, our results suggest several relationships between methylation of various IF genes and cancer. The methylation of both IFN and ICAM-1 appears to play a role in the development of cancer, potentially during early stages of carcinogenesis, and these pathways should be investigated in larger studies with other populations including women, younger adults, and racial/ethnic minorities to confirm the mechanistic hypotheses discussed above. Our finding regarding ICAM-1 methylation and time to diagnosis in particular is novel and also warrants further investigation, but if true provides novel insight into when carcinogenetic methylation aberrations may occur relative to diagnosis, leading to new developments in the detection of a variety of cancers. The associations between prostate cancer and methylation in all three significant IFs also warrant further study, in particular with larger populations of African-Americans due to the well-documented racial disparities in prostate cancer incidence(57) and mortality(58) in the US. Such studies can help elucidate the causal paths involved in prostate cancer and potentially explain part of this health disparity.
Acknowledgements
The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC).
Funding Support: The Normative Aging Study is supported by the Epidemiology Research and Information Center of U.S. Department of Veterans Affairs; NIEHS R01-ES015172. L. Hou received additional support from the Northwestern University Robert H. Lurie Comprehensive Cancer Center Rosenberg Research Fund. A. Baccarelli and J. Schwartz received additional support from the National Institute of Environmental Health Sciences; NIEHS R01-ES021733, NIEHS R01-ES015172, and NIEHS P30-ES00002.
Footnotes
Conflicts of Interest: Dr. Lei Liu reports support from Celladon, Outcome Research Solutions, and Zensun. The authors have no other conflicts of interest to report.
REFERENCES
- 1.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–80. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 2.Xu B, Niu XB, Wang ZD, Cheng W, Tong N, Mi YY, et al. IL-6 -174G>C polymorphism and cancer risk: a meta-analysis involving 29,377 cases and 37,739 controls. Mol Biol Rep. 2011;38:2589–96. doi: 10.1007/s11033-010-0399-1. [DOI] [PubMed] [Google Scholar]
- 3.Borges AH, Silverberg MJ, Wentworth D, Grulich AE, Fatkenheuer G, Mitsuyasu R, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. Aids. 2013;27:1433–41. doi: 10.1097/QAD.0b013e32835f6b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang FF, Santella RM, Wolff M, Kappil MA, Markowitz SB, Morabia A. White blood cell global methylation and IL-6 promoter methylation in association with diet and lifestyle risk factors in a cancer-free population. Epigenetics. 2012;7:606–14. doi: 10.4161/epi.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata M, Thanan R, Ma N, Kawanishi S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol. 2012;2012:623019. doi: 10.1155/2012/623019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res. 2011;90:9–17. doi: 10.1177/0022034510378683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–37. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberyszyn TM, Conti CJ, Ross MS, Oberyszyn AS, Tober KL, Rackoff AI, et al. Beta2 integrin/ICAM-1 adhesion molecule interactions in cutaneous inflammation and tumor promotion. Carcinogenesis. 1998;19:445–55. doi: 10.1093/carcin/19.3.445. [DOI] [PubMed] [Google Scholar]
- 9.Yongvanit P, Thanan R, Pinlaor S, Sithithaworn P, Loilome W, Namwat N, et al. Increased expression of TLR-2, COX-2, and SOD-2 genes in the peripheral blood leukocytes of opisthorchiasis patients induced by Opisthorchis viverrini antigen. Parasitol Res. 2012;110:1969–77. doi: 10.1007/s00436-011-2725-5. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Remaileh M, Bender S, Raddatz G, Ansari I, Cohen D, Gutekunst J, et al. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-14-3295. [DOI] [PubMed] [Google Scholar]
- 11.Hahn MA, Hahn T, Lee DH, Esworthy RS, Kim BW, Riggs AD, et al. Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 2008;68:10280–9. doi: 10.1158/0008-5472.CAN-08-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda Y, Ando T, Nanjo S, Ushijima T, Sugiyama T. DNA methylation of microRNA-124a is a potential risk marker of colitis-associated cancer in patients with ulcerative colitis. Dig Dis Sci. 2014;59:2444–51. doi: 10.1007/s10620-014-3193-4. [DOI] [PubMed] [Google Scholar]
- 13.Sowers JL, Johnson KM, Conrad C, Patterson JT, Sowers LC. The role of inflammation in brain cancer. Adv Exp Med Biol. 2014;816:75–105. doi: 10.1007/978-3-0348-0837-8_4. [DOI] [PubMed] [Google Scholar]
- 14.Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–11. doi: 10.1097/01.ju.0000142058.99614.ff. discussion S-2. [DOI] [PubMed] [Google Scholar]
- 15.Song LN, Silva J, Koller A, Rosenthal A, Chen EI, Gelmann EP. The Tumor Suppressor NKX3.1 is Targeted for Degradation by DYRK1B Kinase. Mol Cancer Res. 2015 doi: 10.1158/1541-7786.MCR-14-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasmin R, Siraj S, Hassan A, Khan AR, Abbasi R, Ahmad N. Epigenetic Regulation of Inflammatory Cytokines and Associated Genes in Human Malignancies. Mediators Inflamm. 2015;2015:201703. doi: 10.1155/2015/201703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ianni M, Porcellini E, Carbone I, Potenzoni M, Pieri AM, Pastizzaro CD, et al. Genetic factors regulating inflammation and DNA methylation associated with prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:56–61. doi: 10.1038/pcan.2012.30. [DOI] [PubMed] [Google Scholar]
- 18.Stoccoro A, Karlsson HL, Coppede F, Migliore L. Epigenetic effects of nano-sized materials. Toxicology. 2013;313:3–14. doi: 10.1016/j.tox.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Garza O, Baccarelli A, Byun HM, Battista Bartolucci G, Carrieri M. Gene-specific DNA methylation as a valuable tool for risk assessment: the case of occupational exposure to different VOC’s in Mexican workers. Occup Environ Med. 2014;71(Suppl 1):A36. [Google Scholar]
- 20.Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–80. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 21.Hou L, Zhang X, Tarantini L, Nordio F, Bonzini M, Angelici L, et al. Ambient PM exposure and DNA methylation in tumor suppressor genes: a cross-sectional study. Part Fibre Toxicol. 2011;8:25. doi: 10.1186/1743-8977-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubois RN. Role of inflammation and inflammatory mediators in colorectal cancer. Trans Am Clin Climatol Assoc. 2014;125:358–72. discussion 72–3. [PMC free article] [PubMed] [Google Scholar]
- 23.Patel SA, Bhambra U, Charalambous MP, David RM, Edwards RJ, Lightfoot T, et al. Interleukin-6 mediated upregulation of CYP1B1 and CYP2E1 in colorectal cancer involves DNA methylation, miR27b and STAT3. Br J Cancer. 2014;111:2287–96. doi: 10.1038/bjc.2014.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song TY, Lim J, Kim B, Han JW, Youn HD, Cho EJ. The role of tumor suppressor menin in IL-6 regulation in mouse islet tumor cells. Biochem Biophys Res Commun. 2014;451:308–13. doi: 10.1016/j.bbrc.2014.07.113. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AG. Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J Periodontol. 2008;79:1514–9. doi: 10.1902/jop.2008.080172. [DOI] [PubMed] [Google Scholar]
- 26.Backdahl L, Bushell A, Beck S. Inflammatory signalling as mediator of epigenetic modulation in tissue-specific chronic inflammation. Int J Biochem Cell Biol. 2009;41:176–84. doi: 10.1016/j.biocel.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Campion J, Milagro FI, Goyenechea E, Martinez JA. TNF-alpha promoter methylation as a predictive biomarker for weight-loss response. Obesity (Silver Spring) 2009;17:1293–7. doi: 10.1038/oby.2008.679. [DOI] [PubMed] [Google Scholar]
- 28.Nile CJ, Read RC, Akil M, Duff GW, Wilson AG. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008;58:2686–93. doi: 10.1002/art.23758. [DOI] [PubMed] [Google Scholar]
- 29.Richardson BC. Role of DNA methylation in the regulation of cell function: autoimmunity, aging and cancer. J Nutr. 2002;132:2401s–5s. doi: 10.1093/jn/132.8.2401S. [DOI] [PubMed] [Google Scholar]
- 30.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Hou L, Zhang X, Gawron AJ, Liu J. Surrogate tissue telomere length and cancer risk: shorter or longer? Cancer letters. 2012;319:130–5. doi: 10.1016/j.canlet.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics. 2011;6:828–37. doi: 10.4161/epi.6.7.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madrigano J, Baccarelli A, Mittleman MA, Sparrow D, Vokonas PS, Tarantini L, et al. Aging and epigenetics: longitudinal changes in gene-specific DNA methylation. Epigenetics. 2012;7:63–70. doi: 10.4161/epi.7.1.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepeule J, Baccarelli A, Motta V, Cantone L, Litonjua A, Sparrow D, et al. Gene promoter methylation is associated with lung function in the elderly: the Normative Aging Study. Epigenetics. 2012;7:261–9. doi: 10.4161/epi.7.3.19216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu ZZ, Sparrow D, Hou L, Tarantini L, Bollati V, Litonjua AA, et al. Repetitive element hypomethylation in blood leukocyte DNA and cancer incidence, prevalence, and mortality in elderly individuals: the Normative Aging Study. Cancer Causes Control. 2011;22:437–47. doi: 10.1007/s10552-010-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen O, Kimchi A. DAP-kinase: from functional gene cloning to establishment of its role in apoptosis and cancer. Cell Death Differ. 2001;8:6–15. doi: 10.1038/sj.cdd.4400794. [DOI] [PubMed] [Google Scholar]
- 37.McGough JM, Yang D, Huang S, Georgi D, Hewitt SM, Rocken C, et al. DNA methylation represses IFN-gamma-induced and signal transducer and activator of transcription 1-mediated IFN regulatory factor 8 activation in colon carcinoma cells. Mol Cancer Res. 2008;6:1841–51. doi: 10.1158/1541-7786.MCR-08-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KY, Geng H, Ng KM, Yu J, van Hasselt A, Cao Y, et al. Epigenetic disruption of interferon-gamma response through silencing the tumor suppressor interferon regulatory factor 8 in nasopharyngeal, esophageal and multiple other carcinomas. Oncogene. 2008;27:5267–76. doi: 10.1038/onc.2008.147. [DOI] [PubMed] [Google Scholar]
- 39.Janson PC, Marits P, Thorn M, Ohlsson R, Winqvist O. CpG methylation of the IFNG gene as a mechanism to induce immunosuppression [correction of immunosupression] in tumor-infiltrating lymphocytes. J Immunol. 2008;181:2878–86. doi: 10.4049/jimmunol.181.4.2878. [DOI] [PubMed] [Google Scholar]
- 40.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol. 2014;192:5226–35. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang B, Bernstein DI, Lummus ZL, Ying J, Boulet LP, Cartier A, et al. Interferon-gamma promoter is hypermethylated in blood DNA from workers with confirmed diisocyanate asthma. Toxicol Sci. 2013;133:218–24. doi: 10.1093/toxsci/kft079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganapathi SK, Beggs AD, Hodgson SV, Kumar D. Expression and DNA methylation of TNF, IFNG and FOXP3 in colorectal cancer and their prognostic significance. Br J Cancer. 2014;111:1581–9. doi: 10.1038/bjc.2014.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma D, Jiang C, Hu X, Li Q, Li T, Yang Y, et al. Methylation patterns of the IFN-gamma gene in cervical cancer tissues. Sci Rep. 2014;4:6331. doi: 10.1038/srep06331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wee ZN, Li Z, Lee PL, Lee ST, Lim YP, Yu Q. EZH2-mediated inactivation of IFN-gamma-JAK-STAT1 signaling is an effective therapeutic target in MYC-driven prostate cancer. Cell Rep. 2014;8:204–16. doi: 10.1016/j.celrep.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 45.Hellebrekers DM, Castermans K, Vire E, Dings RP, Hoebers NT, Mayo KH, et al. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res. 2006;66:10770–7. doi: 10.1158/0008-5472.CAN-06-1609. [DOI] [PubMed] [Google Scholar]
- 46.Arnold JM, Cummings M, Purdie D, Chenevix-Trench G. Reduced expression of intercellular adhesion molecule-1 in ovarian adenocarcinomas. Br J Cancer. 2001;85:1351–8. doi: 10.1054/bjoc.2001.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pergoli L, Favero C, Pfeiffer RM, Tarantini L, Calista D, Cavalleri T, et al. Blood DNA methylation, nevi number, and the risk of melanoma. Melanoma Res. 2014;24:480–7. doi: 10.1097/CMR.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Kazemier HG, de Groote ML, Ruiters MH, Xu GL, Rots MG. Induced DNA demethylation by targeting Ten-Eleven Translocation 2 to the human ICAM-1 promoter. Nucleic Acids Res. 2014;42:1563–74. doi: 10.1093/nar/gkt1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coral S, Parisi G, Nicolay HJ, Colizzi F, Danielli R, Fratta E, et al. Immunomodulatory activity of SGI-110, a 5-aza-2′-deoxycytidine-containing demethylating dinucleotide. Cancer Immunol Immunother. 2013;62:605–14. doi: 10.1007/s00262-012-1365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedrich MG, Chandrasoma S, Siegmund KD, Weisenberger DJ, Cheng JC, Toma MI, et al. Prognostic relevance of methylation markers in patients with non-muscle invasive bladder carcinoma. Eur J Cancer. 2005;41:2769–78. doi: 10.1016/j.ejca.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Wallace AD, Du P, Lin S, Baccarelli AA, Jiang H, et al. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ Toxicol Pharmacol. 2012;34:959–68. doi: 10.1016/j.etap.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001;159:2159–65. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–5. [PubMed] [Google Scholar]
- 54.Calarco A, Pinto F, Pierconti F, Sacco E, Marrucci E, Totaro A, et al. Role of SOCS3 evaluated by immunohistochemical analysis in a cohort of patients affected by prostate cancer: preliminary results. Urologia. 2012;79(Suppl 19):4–8. doi: 10.5301/RU.2012.9392. [DOI] [PubMed] [Google Scholar]
- 55.Pierconti F, Martini M, Pinto F, Cenci T, Capodimonti S, Calarco A, et al. Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate. 2011;71:318–25. doi: 10.1002/pros.21245. [DOI] [PubMed] [Google Scholar]
- 56.Sommer U, Schmid C, Sobota RM, Lehmann U, Stevenson NJ, Johnston JA, et al. Mechanisms of SOCS3 phosphorylation upon interleukin-6 stimulation. Contributions of Src- and receptor-tyrosine kinases. J Biol Chem. 2005;280:31478–88. doi: 10.1074/jbc.M506008200. [DOI] [PubMed] [Google Scholar]
- 57.Mordukhovich I, Reiter PL, Backes DM, Family L, McCullough LE, O’Brien KM, et al. A review of African American-white differences in risk factors for cancer: prostate cancer. Cancer Causes Control. 2011;22:341–57. doi: 10.1007/s10552-010-9712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham-Steed T, Uchio E, Wells CK, Aslan M, Ko J, Concato J. ‘Race’ and prostate cancer mortality in equal-access healthcare systems. Am J Med. 2013;126:1084–8. doi: 10.1016/j.amjmed.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]