Abstract
Adrenocortical carcinoma (ACC) is a rare malignancy with poor prognosis and limited response to chemotherapy. Hepatocyte growth factor (HGF) and its receptor cMET augment cancer growth and resistance to chemotherapy, but their role in ACC has not been examined. In this study, we investigated the association between HGF/cMET expression and cancer hallmarks of ACC. Transcriptomic and immunohistochemical analyses indicated that increased HGF/cMET expression in human ACC samples was positively associated with cancer-related biological processes including proliferation and angiogenesis, and negatively correlated with apoptosis. Accordingly, treatment of ACC cells with exogenous HCG resulted in increased cell proliferation in vitro and in vivo while short hairpin RNA-mediated knockdown or pharmacological inhibition of cMET suppressed cell proliferation and tumor growth. Moreover, exposure of cells to mitotane, cisplatin, or radiation rapidly induced pro-cMET expression and was associated with an enrichment of genes (e.g., CYP450 family) related to therapy resistance further implicating cMET in the anticancer drug response. Together, these data suggest an important role for HGF/cMET signaling in ACC growth and resistance to commonly used treatments. Targeting cMET, alone or in combination with other drugs, could provide a breakthrough in the management of this aggressive cancer.
INTRODUCTION
ACC is a rare endocrine malignancy that originates in the adrenal cortex. ACC has poor prognosis with an estimated recurrence rate is 60–70% after resection of tumors confined to the adrenal gland and the 5-year survival rate for patients presenting with stage IV disease of about 15% (1–3). While surgery remains the best option for ACC patients presenting with localized disease, surgical resection is often not feasible in patients with advanced/recurrent disease, and systemic chemotherapy is often used. The response rate with the current first-line chemotherapy regimen (etoposide, doxorubicin and cisplatin with mitotane) is only 23%, and median time to disease progression is about 6 months (4). Unfortunately, there are no approved second-line regimens, and patients are often referred to clinical trials using agents or regimens with unproven efficacy after failing first-line treatment. Similarly, ACC has limited response to external beam radiation, and radiotherapy is mostly used as a palliative measure (5, 6). Thus, there is an urgent need to identify clinically actionable molecular pathways driving ACC tumorigenesis and tumor progression.
To develop more effective and less toxic treatments for ACC, much of the research over the past two decades has focused on understanding the molecular pathways involved in ACC. It is well accepted that insulin-like growth factor-2 (IGF2) is overexpressed in most cases of ACC. IGF2 promotes tumor cell growth through IGF1 receptor-mediated downstream activation of the protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway, but does not seem to be the major driver of adrenocortical carcinogenesis (7). Clinical studies using inhibitors of IGF1R/mTOR signaling have revealed minimal tumor responses (8). We hypothesized that other regulatory pathways are simultaneously active in ACC, leading to invasive behavior and treatment resistance. cMET has been reported to be expressed in normal adrenal tissue (9), and the cMET signaling pathway (Figure 1A), which is activated through binding to HGF, is critical in tumor progression/invasiveness and therapy resistance in multiple malignancies (10–13). However, the roles of HGF and cMET in ACC have not been evaluated. We therefore examined the potential contribution of the HGF/cMET pathway to cancer hallmarks in ACC as an essential step towards exploration of the utility of drugs targeting this pathway.
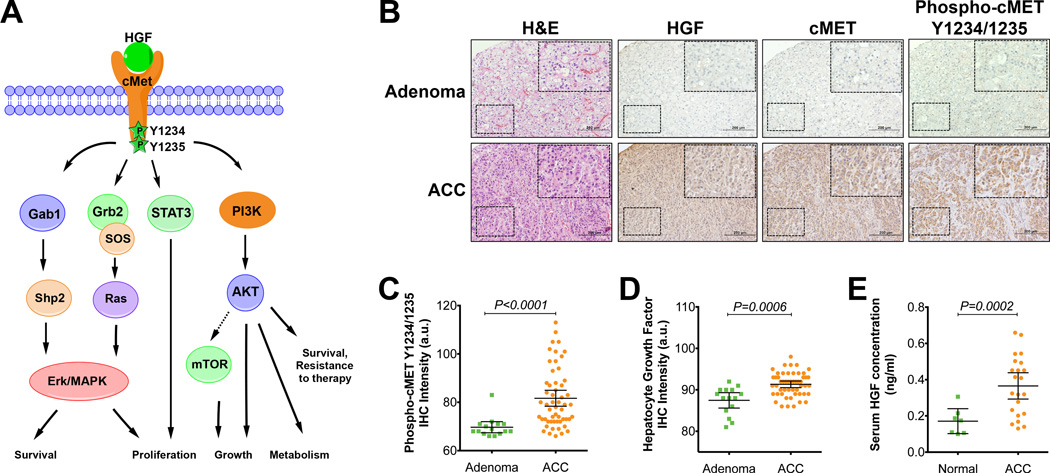
Figure 1.
High expression and activation of HGF/cMET signaling in ACC patients.
(A) HGF/cMET signaling pathway activation triggers a number of downstream oncogenic signaling cascades, leading to cell proliferation and tumor growth.
(B) Representative hematoxylin and eosin (H&E) staining and HGF, cMET and phospho-cMET immunohistochemical analyses of tissue microarray samples from 55 ACC and 15 adrenal adenoma tissue samples (derived from 28 chemotherapy naïve ACCs and 15 patients with adrenal adenomas).
(C,D) Immunohistochemical (IHC) analysis results for phosphorylated cMET Y1234/1235 (C) or HGF (D) in ACC tumors (n=55) compared with data for adrenal adenoma samples (n=15). a.u., arbitrary units.
(E) Serum HGF concentration levels for ACC patients (n=22) compared to samples obtained from controls (n=7). The error bars represent 95% confidence intervals.
MATERIALS AND METHODS
External ACC databases
Transcriptomic profiles of ACC datasets GSE10927 and GSE49278 were downloaded from the Gene Expression Omnibus databases (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE10927; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE49278). The GSE10927 dataset included 10 normal adrenal cortex samples, 22 adrenocortical adenoma samples, and 33 ACC samples (14), and the GSE49278 dataset included 44 ACC samples (15). The downloaded GSE10927 raw data from Affymetrix (Santa Clara, CA) HG U133 plus 2 arrays (with 54,675 probe sets) and the GSE49278 raw data from Affymetrix Human Gene 2.0 ST arrays (with 53,617 probe sets) were analyzed using Nexus Expression 3.0 software (BioDiscovery, Hawthorne, CA) and gene set enrichment analysis comparing the MET mRNA gene expression profiles of the highest MET expression quartile with those of the two lowest MET expression quartile. The widths of the links and the relationship of the biological processes to cancer hallmarks were determined using Z scores and illustrated in a Circos plot (16, 17). Genes from the GSE10927 dataset with significant changes in expression relative to that in noncancerous adrenocortical tissue (P ≤ 0.01, absolute value of log ratio >0.1, pool size for intensity-based pooling 100,000) are presented as heat maps (Supplemental Figure 1) and are listed in Supplemental Table 3.
Patient tissue samples
Adrenocortical tissue samples were collected from available specimens in our pathology department and analyzed according to a protocol approved by the institutional review board (IRB) of MD Anderson Cancer Center. We measured serum HGF in 22 ACC patients and 7 healthy controls (Supplemental Table 1). Two tissue microarrays were constructed from core samples in duplicates and prepared by the Biospecimens Core Facility at MD Anderson. The first tissue microarray (TMA) contained duplicate cores from 13 ACC patients and 7 adrenal adenoma samples. The second TMA included 55 evaluable ACC cores (from 28 chemotherapy naïve ACC patients and constructed as duplicate cores from each subject) and 15 adrenal adenoma samples (from 15 patients with adrenal adenomas that were constructed as single core from each patient to serve as control) (Supplemental table 2). Tissues and serum samples were collected and frozen prospectively, after we had obtained patients’ written informed consent to participate in our research according to a protocol approved by our IRB, or were obtained retrospectively from our institutional tissue bank. A waiver of the requirement for informed consent was granted by the IRB for inclusion of specimens that were retrospectively obtained. In all cases, the diagnosis of ACC was confirmed by board-certified pathologists based on Weiss scores ≥3 (18).
Cell lines and reagents
The NCI-H295R human ACC cell line was obtained from the American Type Culture Collection (Manassas, VA; catalog no. CRL-2128). NCI-H295R cells were grown in Dulbecco’s modified Eagle’s medium–Ham’s F12 medium supplemented with 5% Nu-Serum I (BD Biosciences, San Jose, CA), ITS (BD Biosciences; 0.00625 mg/ml insulin, 0.00625 mg/ml transferrin, 6.25 ng/ml selenium, 1.25 mg/ml bovine serum albumin and 0.00535 mg/ml linoleic acid) and antibiotic-antimycotic solution (Corning Cellgro; 100 IU/ml penicillin, 100 µg/ml streptomycin and 250 ng/ml amphotericin B). Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously (19). The total number of live cells was determined by multiplying the number of cells counted with a Coulter counter by the percentage of live cells in the cell population as determined by Trypan blue dye exclusion. For radiation experiments H295R cells were irradiated at room temperature with a Mark I 137Cs irradiator (JL Shepherd & Associates, San Fernando, CA) at a dose rate of 3.5 Gy/minute (8Gy). Protein lysates were collected at different time points after irradiation (1, 3, 6, 12 and 30 hours). Protein level of cMET and phospho-cMET was evaluated by Western blot analysis.
Immunohistochemical analysis
Tissue microarray slides were then stained with antibodies against cMET (Cell Signaling [Danvers, MA] #8198; 1:200 dilution), anti-cMET phosphorylated at Y1234/1235 (Cell Signaling #3077; 1:150 dilution) and anti-HGF antibodies (Abgent #AP1724b; 1:100 dilution) according to a standard immunohistochemistry (IHC) protocol. Slides were also stained for markers of cell proliferation (Ki-67), tumor vascularity (CD34), and apoptosis (cleaved caspase 3) at the core laboratory of the MD Anderson Department of Pathology. After staining, the IHC slides were analyzed and quantified objectively using an ACIS III Image Analysis System (Dako Corporation, Carpinteria, CA). The intensity of IHC staining within tumor areas was determined by the ACIS III Image Analysis System. IHC staining intensity values were used for statistical analyses and graph preparation. IHC staining was additionally analyzed by pathologists at MD Anderson Cancer Center. Representative photomicrographs were obtained using Dako ACIS and Olympus microscopes.
Serum HGF measurement
Patients’ serum samples were obtained by centrifuging blood samples at 900g for 15 minutes at 4°C. Human HGF levels were measured by enzyme-linked immunosorbent assay according to the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO).
Protein analysis
All protein analyses were performed using lysates from whole-cell pellets or human tumor samples in radioimmunoprecipitation assay buffer, as previously described (19). Protein level of cMET and phospho-cMET was evaluated by Western blot analysis. Protein level of phospho-STAT3, phospho-ATF2 and phospho-cJUN was evaluated by ELISA-based xMAP multiplex immunoanalysis as described by the manufacturer (EMD Millipore, Billerica, MA). Antibodies against cMET and phospho-cMET (Y1234/1235) were obtained from Cell Signaling. Secondary antibodies goat anti-mouse IgG (1:10000 dilution in 1X TBST solution containing 3% BSA) and goat anti-rabbit IgG (1:10000 dilution in 1X TBST solution containing 3% BSA) were obtained from Sigma-Aldrich.
RNA analysis
Total RNA was isolated from treated cells using TRIzol reagent (Invitrogen Life Technologies, Grand Island, NY) by following the manufacturer’s protocol, as previously described (19). The Qiagen RNeasy Mini Kit was used to increase RNA purity and remove residual genomic DNA. Single-stranded complementary DNA from RNA samples (1µg of total RNA) was generated using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Relative gene expression was determined by real-time quantitative PCR using an Applied Biosystems StepOnePlus real-time PCR system (Invitrogen) and iQ SYBR Green Supermix reagents (Bio-Rad). The sequences of the primers used for the relative gene expression analysis of MET (long isoform a, accession number NM_001127500) were 5’ caggcagtgcagcatgtagt 3’ (forward) and 5’ gatgattccctcggtcagaa 3’ (reverse). The expression level of the β-actin housekeeping gene, ACTB, was used as the internal control and analyzed in each experiment for normalization. The sequences of the primers used for ACTB (accession number NM_001101.3) were 5’ ggacttcgagcaagagatgg 3’ (forward) and 5’agcactgtgttggcgtacag 3’ (reverse). Relative changes were calculated using the ΔΔCt formula.
Animal models
For xenografting in vivo experiments we generated H295R-cMET-KD cells with decreased cMET expression by lentiviral infection with cMET shRNA. As control cells we generated H295R-GFP-KD cells by lentiviral infection with GFP (green fluorescent protein) shRNA. H295R-cMET-KD or H295R-GFP-KD cells (4 × 106 cells) in 100µl with 50% Reduce Growth Hormone Matrigel (BD Bioscience) were injected into the right flank of male Nu/Nu mice (n=5 mice per group). XL-184 (cabozantinib, a small molecule tyrosine kinase inhibitor including VEGFR and cMET), which is property to the National Cancer Institute (NCI) Collaborator Exelixis, Inc., was provided through the Cancer Therapy Evaluation Program. For in vivo experiments with cabozantinib, NCI-H295R cells (6 × 106 cells) in 100µl with 50% Reduce Growth Hormone Matrigel (BD Bioscience) were injected into the right flank of male Nu/Nu mice. After one to two weeks of cells inoculation when the tumors reached 5 mm in diameter, xenografted mice were randomized into cabozantinib treatment (30mg/Kg/day)(20) and placebo groups (n=6 mice per group). The selected dose of cabozantinib experiments is in line of similar published data in mice experiments (20). Tumor growth was measured by means of tumor volume. Measurements of tumor volume were taken every week or two weeks, and volumes were estimated with this formula: Length × Width2/2. All animal experiments were conducted in accordance with AAALAS regulations and the approval of The University of Texas MD Anderson Cancer Center Institutional Animal Care & Use Committee.
Statistical analysis
Statistical differences were assessed with Student’s t-test or the Mann-Whitney U test, as appropriate. For experiments involving more than two groups, we used the one-way analysis of variance; post hoc intergroup comparisons were performed using the Kruskal-Wallis test, and the Bonferroni correction was used to account for false discovery. All data are reported as means ± 95% confidence intervals. All results were considered statistically significant when P value was <0.05 except when the Bonferroni correction was applied. GraphPad Prism version 5.0d software was used for the statistical analysis and data presentation.
RESULTS
High expression and activation of HGF/cMET signaling in ACC patients
Our analysis of transcriptomic profiles of an ACC patient cohort (dataset GSE10927, National Center for Biotechnology Information) (14) revealed significant up-regulation of MET mRNA in ACC samples compared with adrenal adenoma and normal adrenocortical tissue samples (Supplemental Figure 1, Supplemental Table 3). This finding was confirmed using real-time PCR analysis of an independent set of ACC and adrenal adenoma samples obtained at our institute following IRB approval (Supplemental Figure 2). Immunohistochemical analysis of two independent tissue microarrays and Western blot analysis results further demonstrated a significant ACC-specific elevations of HGF and total cMET protein levels, and activation of cMET signaling, as seen by phosphorylation at the Y1234/1235 sites (Figure 1 and 2, Supplemental Figure 3). The mean concentration of serum HGF in ACC patients was 365pg/ml that is 2.1 fold (p=0.0002) higher than that in control subjects (Figure 1E, Supplemental Table 1). We were also able to detect HGF in the culture medium of human ACC cell line NCI-H295R at a concentration of 497.2 pg/ml. This suggests a potential autocrine loop in ACC. The HGF/cMET pathway interacts in complex ways with other important signaling pathways. HGF is known to stimulate tumor angiogenesis by increasing the production of angiogenic cytokines and by direct cMET activation, enhancing endothelial cell proliferation and motility (21, 22).
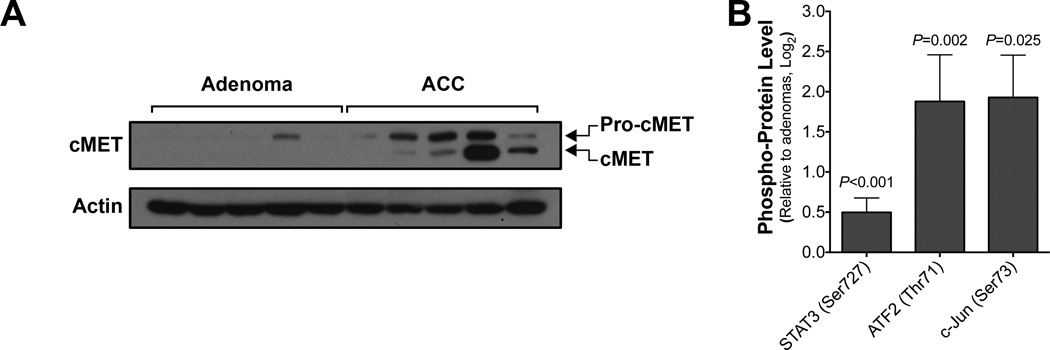
Figure 2.
cMET signaling is activated in ACC.
(A) Western blot analysis of frozen tumor tissues revealed higher levels of cMET in ACC (n=5) than in adrenocortical adenomas (n=5).
(B) Multiplex immunoanalysis analysis of the cMET signaling downstream effectors phospho-STAT3, phospho-ATF2 and phospho-cJUN revealed activation of the cMET signaling in frozen tumor tissues from ACC (n=5) than in adrenocortical adenomas (n=5). The error bars represent 95% confidence intervals.
cMET is associated with enhancement of cancer hallmarks in ACC
Functional genomic analysis identified 63 biological processes that were significantly different (P<0.05) between the high and low MET expression ACC patients (Supplemental Table 4). These data provide evidence that MET expression is associated with biological processes related to cancer hallmarks in ACC, as visualized in a Circos plot (16, 17) (Figure 3A). Biological processes related to sustained proliferation, increased tumor metabolism, resistance to cell death, chemotherapy resistance, and activation of metastasis were among the cancer hallmarks most enhanced in ACC associated with the high MET phenotype. Bioinformatics analysis revealed marked overexpression of oncogenes while down-regulation of tumor suppressor genes in association with high expression of MET (Figure 3B, Supplemental Table 5). Gene set enrichment analysis (23) of transcriptomic profiles of two independent ACC patient cohorts (GSE10927 and GSE49278) (14, 15) showed that high MET expression was associated with a collective up-regulation of genes involved in cell proliferation, as well as genes involved in negative regulation of apoptosis (Figure 4, Supplemental Tables 6–9).
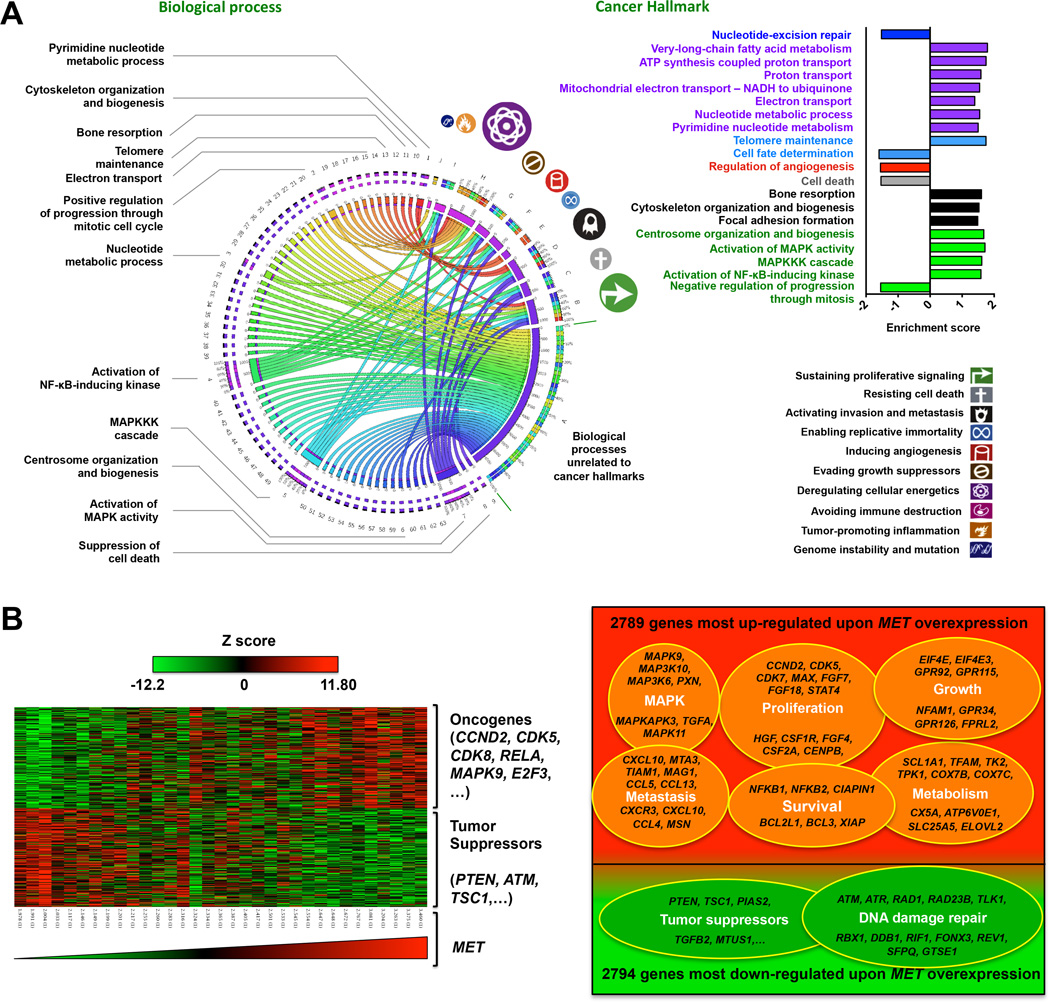
Figure 3.
cMET is associated with enhancement of cancer hallmarks in ACC.
(A,) Circos plot of the association between significantly increased biological processes (P<0.05) (see also Supplemental Table 2) and cancer hallmarks (symbols and color-coded labels are indicated on the right) upon MET overexpression. The widths of the connectors represent the absolute values of the Z scores of the biological processes. Bar graphs on the right illustrate the enrichment of some important biological processes.
(B,) The left panel shows a heat map of changes in gene expression associated with high MET expression. The right panel shows a Venn diagram of dataset GSE10927 microarray data from pretreatment tumor biopsy samples of ACC patients; representative genes that were significantly up-regulated or down-regulated upon MET overexpression (P≤0.05, log ratio > 0.1) are indicated.
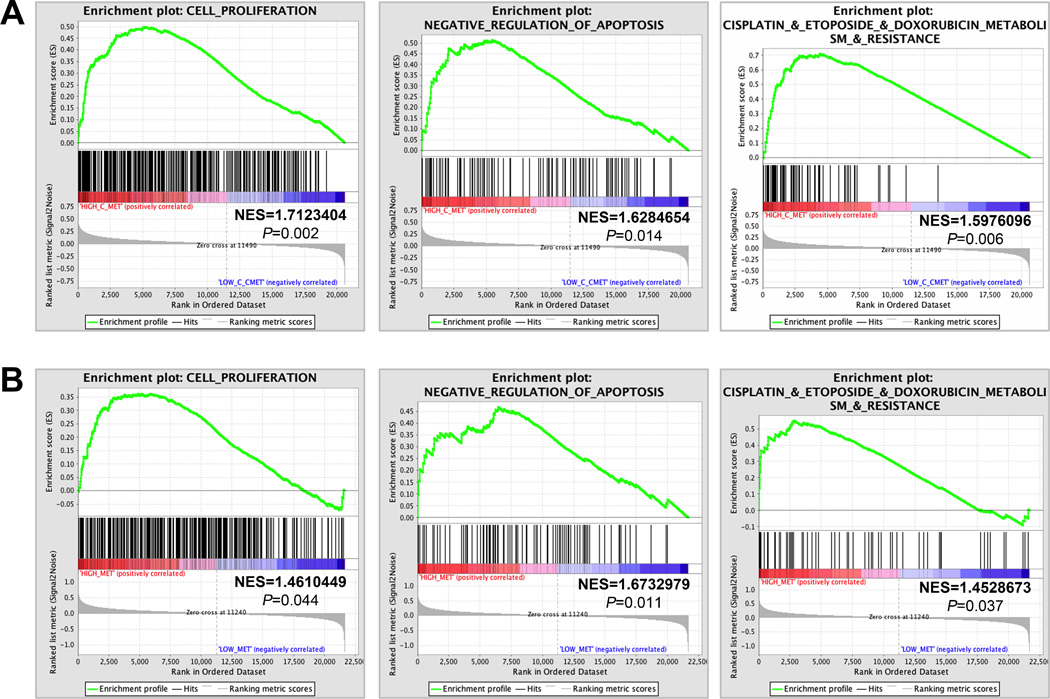
Figure 4.
cMET is associated with enhancement of cell proliferation, negative regulation of apoptosis, and drug resistance in ACC.
(A) Gene set enrichment analyses of ACC patient dataset GSE10927 for genes involved in cell proliferation (left panel), negative regulation of apoptosis (middle panel) or metabolism of and resistance to cisplatin, etoposide and doxorubicin (right panel). Each bar corresponds to one gene. Gene enrichment scores of all genes in each gene set are listed in Supplemental Tables 4, 5 and 9. (B) Gene set enrichment analyses of dataset GSE49278 showed that high cMET expression is correlated with increased proliferation (upper panel), negative regulation of apoptosis (middle panel) and metabolism of and resistance to cisplatin, etoposide and doxorubicin (bottom panel). Gene enrichment scores of all genes in each gene set are listed in Supplementary Tables 6, 7 and 10. NES, normalized enrichment score.
Increased HGF/cMET signaling is associated with enhanced proliferation, angiogenesis, tumor growth and reduced apoptosis in ACC
To validate the significance of HGF/cMET activation in ACC, we evaluated the correlation between HGF/cMET activation and proliferation, promotion of angiogenesis and apoptosis. Tumor tissue microarray sections (55 ACCs cores from 28 therapy naïve patients and 15 adenoma cores from 15 individual patients) stained for HGF, cMET and phosphorylated cMET antibodies are positively correlated with cell proliferation marker (Ki-67) and tumor vascularity (CD34 staining) (Figure 5, Supplemental Figure 4) and negatively correlated with apoptosis (cleaved caspase 3 staining) (Supplemental Figure 4). To evaluate HGF’s effect on ACC growth and viability, we added recombinant human HGF into the culture medium of NCI-H295R ACC cells. Recombinant HGF significantly stimulated in vitro NCI-H295R cell viability (Figure 7A) and proliferation (Supplemental Figure 5). These findings are in concordance with the fact that HGF activates cMET leading to enhanced cancer cell proliferation and metastatic potential, and HGF activation of cMET is associated with poor prognosis in a variety of malignancies (10–12, 24–28).
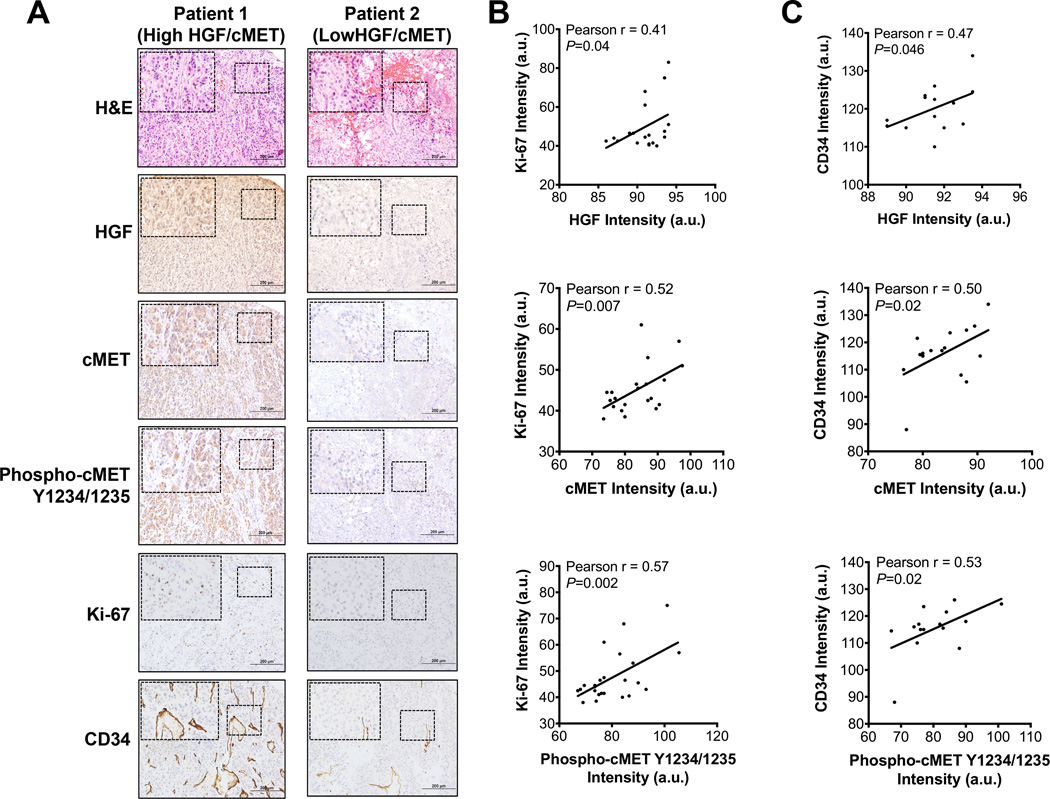
Figure 5.
Increased HGF/cMET signaling is associated with enhanced proliferation and angiogenesis in tumors from ACC patients.
(A) Representative images of immunohistochemistry staining of a tissue microarray of samples from two ACC patients showed that high HGF/cMET signaling was accompanied by elevated biomarkers of cellular proliferation (Ki-67) and angiogenesis (CD34) in ACC tumors.
(B,C) Pearson correlation analyses indicated an association of HGF/cMET signaling with markers of tumor proliferation (Ki-67) (b) and angiogenesis (CD34) (c). a.u., arbitrary units.
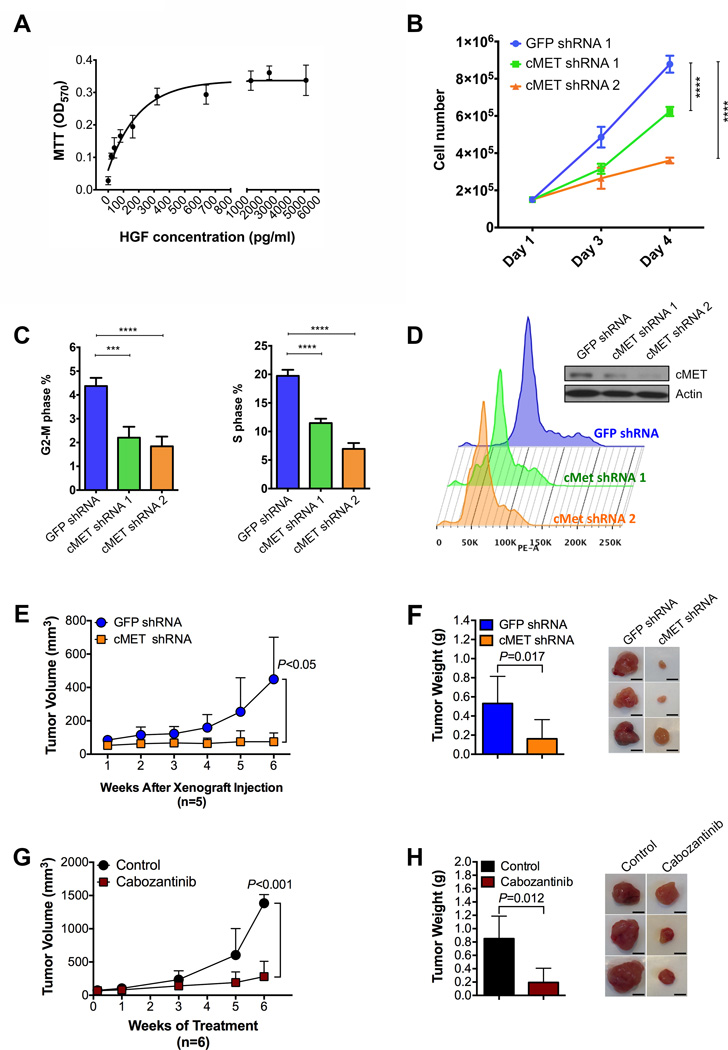
Figure 7.
Increased HGF/cMET signaling is associated with enhanced proliferation, tumor growth and reduced apoptosis in ACC.
(A) Cell viability measured by MTT assay of NCI-H295R cells cultured at different concentrations of recombinant human HGF for 7 days.
(B) Knockdown of MET expression by lentiviral shRNAs decreases ACC cell proliferation.
(C) Knockdown of MET expression by lentiviral shRNAs decreases percentages of ACC cells in G2-M or S phase.
(D) Cell cycle progression analysis showing the important role of cMET in ACC cell proliferation.
(E) Mean tumor volume in mice at different weeks after xenografting of H295R–GFP (green fluorescent protein)–shRNA or H295R-cMET-shRNA cells (5 mice per group).
(F) Mean tumor weights in mice 6 weeks after xenografting of H295R-GFP-shRNA or H295R-cMET-shRNA cells (5 mice per group; left panel) and representative images of xenografted tumors harvested from the mice (right panel; scale bars represent 5mm).
(G) Mean volumes of tumors formed from xenografted H295R cells at different weeks after treatment of randomized control and cabozantinib-treated mice (6 mice per group).
(H) Mean weights of tumors from randomized control and cabozantinib-treated mice after 6 weeks of treatment (6 mice per group; left panel) and representative images of xenografted tumors harvested from the mice (right panel; scale bars represent 5mm) Statistical significance of data in panels f and h was calculated by one-way analysis of variance. The error bars represent 95% confidence intervals; *** P<0.001, and **** P<0.0001.
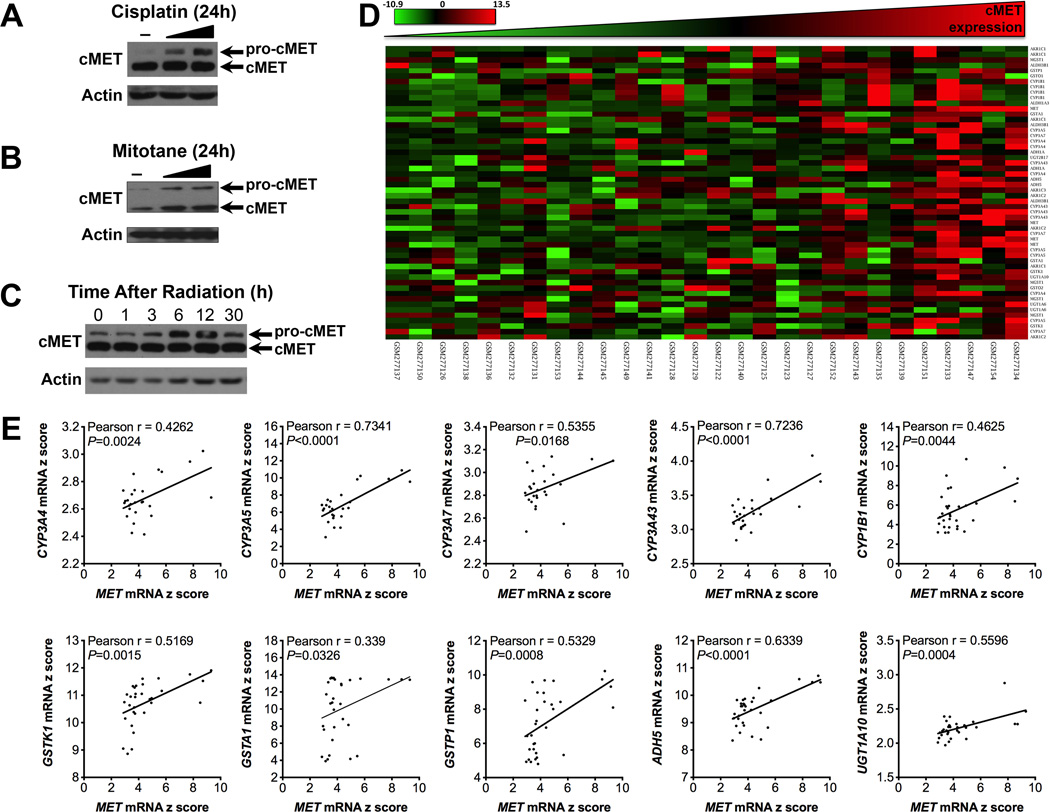
Only a small percentage of ACC patients respond to currently available systemic therapy (4–6). Genes associated with resistance to or metabolism of cisplatin, etoposide and doxorubicin were significantly enriched in ACC patients’ tumor tissues with high MET expression (Figure 4, Supplemental Tables 11,12). Interestingly, we found that cisplatin and mitotane (two key components of first line chemo therapy for advanced ACC), and radiation treatment induce cMET expression in NCI-H295R cells, as manifested by a rise in pro-cMET (Figure 6, A-C). Further bioinformatics analysis demonstrated overexpression of genes related to drug metabolism (Figure 6, C and D, and Supplemental Table 10) in patients with high MET expression.
Figure 6.
cMET expression is correlated with increased gene expression of enzymes involved in drug metabolism in ACC.
(A) NCI-H295R cells were treated with cisplatin (50ng/ml and 500ng/ml), and cMET protein levels were measured by Western blot analysis after 24 h of treatment.
(B) NCI-H295R cells were treated with mitotane (5µM and 10µM), and cMET protein levels were measured by Western blot analysis after 24 h of treatment.
(C) NCI-H295R cells were treated with radiation (8Gy), and cMET protein levels were measured by Western blot analysis after 0, 1, 3, 6, 12 and 30 h of treatment.
(D) Heat map of the genes related to drug metabolism.
(E) Pearson correlation analysis of elevated cMET expression and expression of genes related to drug metabolism.
To further investigate the functional role of cMET in ACC cell biology, we generated a NCI-H295R ACC cell line with decreased cMET expression by knockdown using cMET-targeted shRNA (H295R-cMET-KD). Knockdown of cMET mRNA significantly decreased in vitro cell proliferation (Figure 7B) and induced cell cycle arrest (Figure 7, C and D). To further validate the role of cMET signaling in ACC tumor growth and progression, we established an ACC in vivo xenograft mouse model using H295R-cMET-KD cells. Our results confirmed that cMET knockdown significantly decreased (P<0.05) tumor growth (Figure 7, E and F). Moreover, in vivo inhibition of cMET by cabozantinib (a commercially available small molecule tyrosine kinase inhibitor with potent activity toward cMET)(29) significantly (P<0.001) reduced tumor growth (Figure 7, G and H). Our results also show that stable knockdown of cMET mRNA significantly reduced both mitochondrial respiration and glycolytic metabolism (Supplemental Figure 6). Thus, ACC tumor growth is dependent, at least in part, on cMET signaling, and cMET inhibition is likely to have a role in treatment of advanced ACC.
DISCUSSION
We found that HGF/cMET are expressed at a higher level in ACC than in adrenal adenomas and normal cortex. Moreover, activation of HGF/cMET appears to enhance ACC growth, tumor-related angiogenesis, chemotherapy resistance and cell survival. Therefore, our data suggest that cMET may be a valuable therapeutic target for ACC.
The field of adrenal neoplasia has achieved important milestones during the past 25 years, including the discovery of major genetic alterations and molecularly characterizing adrenal cortical cancer genomic profiles (15, 30). However, ACC is considered an aggressive malignancy with limited response to chemotherapy. (1, 4–6, 31, 32). Therefore, deciphering the mechanisms driving adrenal cortical tumorigenesis as well as identifying the vulnerabilities of this aggressive type of cancer remains a challenge in this field (15, 30, 33). Most ACCs show IGF2 overexpression with the possible role of the AKT/mTOR pathway as a downstream effect promoting tumor cell growth, but with minimal effect in adrenocortical carcinogenesis (7). Clinical studies to determine the effect of blocking mTOR signaling have revealed minimal tumor responses (8). We hypothesized that other signaling pathways are simultaneously active in ACC, leading to invasiveness and treatment resistance. HGF activates cMET in an autocrine and paracrine fashion, leading to enhanced of cancer cell proliferation and metastatic potential and associated with poor prognosis in a variety of malignancies (10–12, 24–27). For the first time, we report that the expression of HGF/cMET is high in ACC and that cMET activation is associated with ACC growth. These data raise the possibility that cMET is a potential therapeutic target for ACC. In addition, the activation of HGF/cMET pathway was associated with increased cell proliferation and reduced apoptosis based on our immunohistochemistry analysis of the tissue microarray. We also found that HGF promotes H295R cell growth in vitro. In other cancer models, HGF secretion was reported to be produced by tumor-derived fibroblasts and to have a paracrine role in stimulating tumor growth (28).
Serum HGF is elevated in different malignancies; it has prognostic value, is correlated with disease burden, and can be used to identify responders to systemic therapy in a variety of solid and hematological malignancies (34–38). However, circulating HGF can be non-specific and transiently elevated in other non-neoplastic disease processes (39–41). We identified HGF-induced cell growth in the H295R ACC cell line. A complex interaction exists between HGF/cMET pathway and other important signaling pathways. HGF stimulates tumor angiogenesis via enhancing endothelial cell proliferation and motility. These pro-angiogenic effects are mediated by increasing the production of angiogenic cytokines, such as vascular endothelial growth factor and interleukin-8, and by direct cMET activation (21, 22).
The molecular mechanisms responsible for cMET and HGF overexpression in ACC remain unclear and warrant investigation. Somatic mutations of MET are rare in ACC (42), however, the genome area where MET is located is commonly amplified in ACC (15). We found somatic MET alteration in only one out of 14 ACC specimen and this opens the door for other mechanisms to be responsible for cMET activation such as gene amplification as reported in other solid malignancies (43). The adrenocortical carcinoma genomic atlas (ACC TCGA) data shows genomic amplification on MET in ACC patients compared to normal adrenal cortex. Thus, both MET amplification and transcriptional induction after exposure to radiation or chemotherapy are likely responsible for cMET overexpression.
In this study, we have identified activation of HGF/cMET signaling pathway as a driver of ACC tumorigenesis and at the same time a potential Achilles’ heel of this malignancy. In fact, by combining multiple functional omics screenings with tissue microarray analysis, in vitro assays, animal modeling, and pharmaceutical intervention, we demonstrated for the first time that HGF/cMET signaling pathway played a central role in ACC tumorigenesis, discovering a previously unknown therapeutic opportunity for clinical management of this aggressive malignancy. Therefore, emerging cMET inhibitors hold promise as a potential breakthrough in ACC treatment.
Moreover, the findings that ACC cells rapidly upregulated cMET expression as an emergency response to radiation and chemotherapy, and that MET upregulation was associated with enrichment of major genes responsible for anti-cancer therapy resistance, survival, and drug metabolism, could be of significant interest for the field of cancer therapy innovation. In fact, these findings may establish a scientific foundation for using cMET inhibitors to overcome drug resistance in ACC, which is currently a major challenge in ACC treatment (1, 4, 31, 32). Besides, whether the fast increase in MET expression after anti-cancer treatments is a particular feature of ACC cells or a common phenomenon across many types of cancers remain to be explored. In addition, how ACC cells induce MET expression after anti-cancer therapies and what is the mechanism for crosstalk between MET and CYP450 family are interesting questions that warrant further study. We think that a complete understanding about the contribution of HGF/cMET signaling in ACC could perhaps establish activation of HGF/cMET pathway as a predictive marker for tumor progression and response to therapies, which may better stratification of ACC patients, optimize treatment plans and ameliorate therapeutic outcomes.
In addition, despite remarkable progress in the field of ACC, the process and mechanisms of ACC evolution still remain largely unclear (30, 33, 44–47). It is also undetermined whether ACC evolutionary process originates from adrenal adenoma (30, 45, 47). There are several evidences supporting the adenoma-carcinoma sequential tumorigenesis but more direct proofs are needed to elucidate this mysterious evolution. Two studies performed by Bernard et al. and Trezzi et al. found the presence of malignant components within adrenal adenomas in occasional cases (44, 45). Moreover, Heaton et al. has recently developed a mouse model with enhanced IGF2 expression and increased β-catenin stability to temporarily promote adrenal cortical hyperplasia progression to the formation of adenomas and seldom carcinomas (46). In addition, high-resolution genomic analyses performed by Ronchi et al. pointed out several common molecular genetic signatures and some shared signaling transduction pathways (i.e., Notch and Wnt/ β-catenin pathways) in adrenal adenomas and carcinomas (33), which suggests a possibly common origin. In our study, we observed a seemingly gradual increase in HGF/cMET pathway upregulation and activation from normal adrenal cortex to adrenal adenoma and then carcinoma. Furthermore, when the HGF/cMET pathway is highly activated in ACC, their whole gene expression landscapes are reprogrammed toward promoting cancer hallmarks deregulation, accelerating tumorigenesis, enabling drug resistance while inhibiting tumor-suppressing activities. These findings suggest that HGF/cMET signaling activation could be a landmark in the evolutionary process and tumorigenesis sequence of adrenal cortical carcinoma.
The relatively small number of studied specimens in our report is a common shortcoming in studies seeking to find new signaling pathways in ACC, but highlights the need for collaborative work to establish the prognostic value of serum HGF as well as ACC cMET expression (48–50). The paucity of ACC cell lines is another limitation in ACC research. However, we share the view of other groups that the H295R cell line is the closest model to corticosteroid-producing ACC (51). Considering the heterogeneity of ACC, there is a need to validate our findings in a large cohort of patients via a multi-institutional collaboration, and to incorporate HGF measurements in future prospective studies to assess its role as a prognostic marker in ACC. Future work is also necessary to clarify the effect of cMET signaling on ACC resistance to currently used chemotherapy strategies in ACC as well as exploring the effect of HGF/cMET inhibition on ACC and determining the mechanisms involved in cMET upregulation in ACC. A better understanding of this relationship may provide a rationale for combination therapy.
In summary, we found that HGF/cMET are expressed at a higher level in ACC than in adrenal adenomas and normal cortex. We have also shown that ACC cells produce HGF, leading to positive autocrine feedback, which promotes ACC cell growth and proliferation. Moreover, activation of HGF/cMET appears to enhance ACC proliferation/growth, tumor-related angiogenesis, chemotherapy resistance and cell survival. Furthermore, commonly used chemotherapeutic agents and radiation increased cMET expression in vitro and cMET inhibition reduced ACC growth in vitro and in vivo. Therefore, our data suggest that cMET may be a valuable therapeutic target for ACC, and further investigation of combinations of new cMET inhibitors alone or in combination with current therapies may lead to clinical breakthroughs in management of this disease. This study provides preliminary data about HGF/cMET activation as a possible predictive marker for ACC progression and response to therapies, which may improve stratification of ACC patients and clinical outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the “Run for Rivenes” and the Beverlin Fund for Adrenal Cancer Research. L.M.P. was supported by the Vietnam Education Foundation, the Rosalie B. Hite Foundation, and the U.S. Department of Defense Breast Cancer Research Program (W81XWH-10-0171). E.F.-M. was supported by the National Cancer Institute Training Grant Program in Molecular Genetics (T32-CA009299) and National Institutes of Health Loan Repayment Program. W.W. was supported by a Health Professional Training Grant from the Department of Health of Fujian Province, China, and a grant from the Xiamen Public Health Bureau of Science and Technology Project (3502z20077042 and WQK0605). G.V.-T. was supported by a National Institutes of Health cancer prevention fellowship (National Cancer Institute fellowship R25T CA57730) and by a National Institutes of Health minority supplement (3-R01CA089266-08S1, 3-R01CA089266-09S1 and 3-R01CA089266-10S1; principal investigator M.-H.L.). M.-H.L. and S.-C.J.Y. were supported in part by a Susan G. Komen for the Cure Promise Grant (KG081048). M.-H.L. was also supported by a National Cancer Institute grant (R01-CA089266). The University of Texas MD Anderson Cancer Center is supported by the NIH/NCI under award number P30CA016672. XL-184 (cabozantinib), which is property to the National Cancer Institute (NCI) Collaborator Exelixis, Inc., was provided through the Cancer Therapy Evaluation Program.
Footnotes
Conflict of interest statement: The authors have no potential conflicts of interest to disclose.
AUTHORS CONTRIBUTION
L.M.P., E.F.-M., M.-H.L. , S.-C.J.Y. and M.A.H. designed the experiments of the project. L.M.P., E.F.-M., W.W., G.V.-T., T.H., S.Z. and M.A.C. performed the research. L.M.P., E.F.-M., G.J.C., L.O., R.V., M.-H.L., S.-C.J.Y. and M.A.H. analyzed the data; W.W., K.S., C.G.W., C.J., S.-C.J.Y. and M.A.H. provided clinical and pathological assessments. R.V. and S.-C.J.Y. contributed to the statistical analysis. L.M.P., E.F.-M. and M.A.H. wrote the paper with input from all authors.
REFERENCES
- 1.Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 2.Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocrine reviews. 2014;35:282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. European journal of endocrinology / European Federation of Endocrine Societies. 2013;169:891–899. doi: 10.1530/EJE-13-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 5.Fassnacht M, Hahner S, Polat B, et al. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91:4501–4504. doi: 10.1210/jc.2006-1007. [DOI] [PubMed] [Google Scholar]
- 6.Habra MA, Ejaz S, Feng L, et al. A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 98:192–197. doi: 10.1210/jc.2012-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drelon C, Berthon A, Ragazzon B, et al. Analysis of the role of Igf2 in adrenal tumour development in transgenic mouse models. PLoS One. 2012;7:44171. doi: 10.1371/journal.pone.0044171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naing A, Lorusso P, Fu S, et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108:826–830. doi: 10.1038/bjc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajima H, Higuchi O, Mizuno K, Nakamura T. Tissue distribution of hepatocyte growth factor receptor and its exclusive down-regulation in a regenerating organ after injury. J Biochem. 1992;111:401–406. doi: 10.1093/oxfordjournals.jbchem.a123769. [DOI] [PubMed] [Google Scholar]
- 10.Forte G, Minieri M, Cossa P, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 11.Neaud V, Faouzi S, Guirouilh J, et al. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: evidence for a role of hepatocyte growth factor. Hepatology. 1997;26:1458–1466. doi: 10.1053/jhep.1997.v26.pm0009397985. [DOI] [PubMed] [Google Scholar]
- 12.Zeng ZS, Weiser MR, Kuntz E, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265:258–269. doi: 10.1016/j.canlet.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma PC, Tretiakova MS, MacKinnon AC, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer. 2008;47:1025–1037. doi: 10.1002/gcc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano TJ, Kuick R, Else T, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–676. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assie G, Letouze E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46:607–612. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 16.Krzywinski M, Schein J, Birol I, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8:163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes-Mattei E, Velazquez-Torres G, Phan L, et al. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres KE, Zhu QS, Bill K, et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res. 2011;17:3943–3955. doi: 10.1158/1078-0432.CCR-11-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911–5918. [PubMed] [Google Scholar]
- 22.Saucier C, Khoury H, Lai KM, et al. The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis. Proc Natl Acad Sci U S A. 2004;101:2345–2350. doi: 10.1073/pnas.0308065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 25.Takami T, Kaposi-Novak P, Uchida K, et al. Loss of hepatocyte growth factor/c-Met signaling pathway accelerates early stages of N-nitrosodiethylamine induced hepatocarcinogenesis. Cancer Res. 2007;67:9844–9851. doi: 10.1158/0008-5472.CAN-07-1905. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–1595. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–3750. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 30.Papotti M, Duregon E, Volante M, McNicol AM. Pathology of the adrenal cortex: a reappraisal of the past 25 years focusing on adrenal cortical tumors. Endocrine pathology. 2014;25:35–48. doi: 10.1007/s12022-013-9291-6. [DOI] [PubMed] [Google Scholar]
- 31.Dackiw AP, Lee JE, Gagel RF, Evans DB. Adrenal cortical carcinoma. World journal of surgery. 2001;25:914–926. doi: 10.1007/s00268-001-0030-7. [DOI] [PubMed] [Google Scholar]
- 32.Fassnacht M, Libe R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7:323–335. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- 33.Ronchi CL, Sbiera S, Leich E, et al. Single nucleotide polymorphism array profiling of adrenocortical tumors--evidence for an adenoma carcinoma sequence? PLoS One. 2013;8:73959. doi: 10.1371/journal.pone.0073959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seidel C, Borset M, Hjorth-Hansen H, Sundan A, Waage A. Role of hepatocyte growth factor and its receptor c-met in multiple myeloma. Med Oncol. 1998;15:145–153. doi: 10.1007/BF02821933. [DOI] [PubMed] [Google Scholar]
- 35.Toi M, Taniguchi T, Ueno T, et al. Significance of circulating hepatocyte growth factor level as a prognostic indicator in primary breast cancer. Clin Cancer Res. 1998;4:659–664. [PubMed] [Google Scholar]
- 36.Hsiao LT, Lin JT, Yu IT, et al. High serum hepatocyte growth factor level in patients with non-Hodgkin’s lymphoma. Eur J Haematol. 2003;70:282–289. doi: 10.1034/j.1600-0609.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 37.Seidel C, Lenhoff S, Brabrand S, et al. Hepatocyte growth factor in myeloma patients treated with high-dose chemotherapy. Br J Haematol. 2002;119:672–676. doi: 10.1046/j.1365-2141.2002.03898.x. [DOI] [PubMed] [Google Scholar]
- 38.Toiyama Y, Miki C, Inoue Y, Okugawa Y, Tanaka K, Kusunoki M. Serum hepatocyte growth factor as a prognostic marker for stage II or III colorectal cancer patients. Int J Cancer. 2009;125:1657–1662. doi: 10.1002/ijc.24554. [DOI] [PubMed] [Google Scholar]
- 39.Ueda T, Takeyama Y, Toyokawa A, Kishida S, Yamamoto M, Saitoh Y. Significant elevation of serum human hepatocyte growth factor levels in patients with acute pancreatitis. Pancreas. 1996;12:76–83. doi: 10.1097/00006676-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Maeda J, Ueki N, Hada T, Higashino K. Elevated serum hepatocyte growth factor/scatter factor levels in inflammatory lung disease. Am J Respir Crit Care Med. 1995;152:1587–1591. doi: 10.1164/ajrccm.152.5.7582299. [DOI] [PubMed] [Google Scholar]
- 41.Heeschen C, Dimmeler S, Hamm CW, Boersma E, Zeiher AM, Simoons ML. Prognostic significance of angiogenic growth factor serum levels in patients with acute coronary syndromes. Circulation. 2003;107:524–530. doi: 10.1161/01.cir.0000048183.37648.1a. [DOI] [PubMed] [Google Scholar]
- 42.Jardim DL, de Melo Gagliato D, Falchook G, et al. MET Abnormalities in Patients With Genitourinary Malignancies and Outcomes With c-MET Inhibitors. Clinical genitourinary cancer. 2014 doi: 10.1016/j.clgc.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Renzo MF, Olivero M, Giacomini A, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1:147–154. [PubMed] [Google Scholar]
- 44.Bernard MH, Sidhu S, Berger N, et al. A case report in favor of a multistep adrenocortical tumorigenesis. The Journal of clinical endocrinology and metabolism. 2003;88:998–1001. doi: 10.1210/jc.2002-021117. [DOI] [PubMed] [Google Scholar]
- 45.Trezzi R, Poli F, Fellegara G. “Dedifferentiated” adrenal cortical neoplasm. International journal of surgical pathology. 2009;17:343–344. doi: 10.1177/1066896909335155. [DOI] [PubMed] [Google Scholar]
- 46.Heaton JH, Wood MA, Kim AC, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and beta-catenin. The American journal of pathology. 2012;181:1017–1033. doi: 10.1016/j.ajpath.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. European journal of endocrinology / European Federation of Endocrine Societies. 2003;149:273–285. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 48.Durand J, Lampron A, Mazzuco TL, Chapman A, Bourdeau I. Characterization of differential gene expression in adrenocortical tumors harboring beta-catenin (CTNNB1) mutations. J Clin Endocrinol Metab. 2011;96:1206–1211. doi: 10.1210/jc.2010-2143. [DOI] [PubMed] [Google Scholar]
- 49.Hermsen IG, Haak HR, de Krijger RR, et al. Mutational analyses of epidermal growth factor receptor and downstream pathways in adrenocortical carcinoma. Eur J Endocrinol. 2013;169:51–58. doi: 10.1530/EJE-13-0093. [DOI] [PubMed] [Google Scholar]
- 50.Mariniello B, Rosato A, Zuccolotto G, et al. Combination of sorafenib and everolimus impacts therapeutically on adrenocortical tumor models. Endocr Relat Cancer. 2012;19:527–539. doi: 10.1530/ERC-11-0337. [DOI] [PubMed] [Google Scholar]
- 51.Lichtenauer UD, Shapiro I, Osswald A, et al. Characterization of NCI-H295R cells as an in vitro model of hyperaldosteronism. Horm Metab Res. 2013;45:124–129. doi: 10.1055/s-0032-1323810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.