Abstract
Bacillus thuringiensis (Bt) has been used successfully as a biopesticide for more than 60 years. More recently, genes encoding their toxins have been used to transform plants and other organisms. Despite the large amount of research on this bacterium, its true ecology is still a matter of debate, with two major viewpoints dominating: while some understand Bt as an insect pathogen, others see it as a saprophytic bacteria from soil. In this context, Bt’s pathogenicity to other taxa and the possibility that insects may not be the primary targets of Bt are also ideas that further complicate this scenario. The existence of conflicting research results, the difficulty in developing broader ecological and genetics studies, and the great genetic plasticity of this species has cluttered a definitive concept. In this review, we gathered information on the aspects of Bt ecology that are often ignored, in the attempt to clarify the lifestyle, mechanisms of transmission and target host range of this bacterial species. As a result, we propose an integrated view to account for Bt ecology. Although Bt is indeed a pathogenic bacterium that possesses a broad arsenal for virulence and defense mechanisms, as well as a wide range of target hosts, this seems to be an adaptation to specific ecological changes acting on a versatile and cosmopolitan environmental bacterium. Bt pathogenicity and host-specificity was favored evolutionarily by increased populations of certain insect species (or other host animals), whose availability for colonization were mostly caused by anthropogenic activities. These have generated the conditions for ecological imbalances that favored dominance of specific populations of insects, arachnids, nematodes, etc., in certain areas, with narrower genetic backgrounds. These conditions provided the selective pressure for development of new hosts for pathogenic interactions, and so, host specificity of certain strains.
Keywords: Bacillus ecology, multiple hosts, virulence factors, pathogenicity
1. Introduction
Bacillus thuringiensis (Bt) is a widespread endospore-forming bacteria with a complex life cycle, which has been commonly found in soil, water, plants, stored cereals and dead insects. It is classified as a Gram-positive, facultative anaerobic and its main feature is the production of crystalline proteinaceous inclusions during the stationary and sporulation growth phases [1,2,3]. According to Lecadet et al. [4] and Xu and Côté [5], the Bt classification based upon biochemical properties and composition of the flagellar antigen ‘H’ contains 69 serotypes, 82 antigenic serovars and 13 antigenic subgroups, thereby demonstrating the high degree of genetic variability presented by this species. This classification, however, does not take into account the toxicity profile presented by the diverse array of Bt lineages isolated wordlwide, which is defined by the type(s) of toxin(s) produced.
Due to economic necessity and ease of manipulation, the majority of Bt-related research has focused on direct effects against insect-pests relevant to agriculture. However, studies focusing purely on the ecology of Bt, including its interactions with a diverse range of organisms that occupy the same niches are scarce. The discussion about the real ecology of Bt has heated up in the last few years, with various researchers now referring to Bt as an obligate pathogen [1,2,6,7]. To shed more light on this matter, some studies have demonstrated that, as long as the Bt metabolic requirements are met, this bacterium grows vegetatively in a variety of environments, such as soil, leaf surfaces and other internal plant tissues, although this growth occurs in much lower levels when compared with that inside insect cadavers [8,9]. It is important to realize that we cannot consider the soil only as a general deposit for Bt, since it is from such environment that Bt can colonize rhizospheres, germinating plants and invertebrates, so that it can multiply in a sufficient level and reach appropriate places that allow proper infection of bonafide hosts [6,8,10]. Taking this into consideration, the concept of ‘environmental pathogen’ has emerged, referring to microbes that spend a significant portion of their lifecycle outside hosts, but can cause disease when in coming into contact with them [11]. The ability to survive and grow outside a host, a large dispersal capacity in an array of different environments, the recognized genetic plasticity that provides an adaptive arsenal to infect multiple hosts, all help explain why it is not trivial to monitor, assess and understand the ecology of Bt. In this review we addressed some concepts that are seldom used in Bt context, placing them into perspective with other common views in the attempt to stimulate critical discussion and aid in the understanding of Bt’s role in nature.
2. Niches Occupied by Bt
The ecology and lifestyle of Bt have been a target of many yet unanswered questions. Some defends that Bt is a soil-dwelling microorganism that obtains nutrition for its survival and reproduction in nature from decaying organic matter or roots exudates, reaching the aerial parts of plants when these germinate and emerge from soil. An opposed view states that Bt is a specialized pathogen that, by colonizing and killing its hosts, and multiplying in their cadavers, is then deposited in soil and plants, which thereby become natural reservoirs. In fact, according to Bt’s capability to survive and colonize various niches (see more below), it is reasonable to classify it as a ‘copiotrophic’ microorganism.
2.1. Soil Environment
Many authors classify Bt as a soil microorganism due to the fact that a great deal of its isolation throughout the years has been obtained from this environment (e.g., [12,13,14,15]). Others, however, consider the soil only as a storage milieu for Bt spores, as they barely germinate in these places, requiring specific nutrients and pH conditions for that [16,17,18,19]. The indigenous microbiota, as well as soil properties, such as pH, humidity, mineral and organic matter concentrations have a direct effect on Bt’s survival, acting positively or negatively on germination, growth, sporulation, and production of proteins [18,20,21,22,23]. These conditions, on another hand, tend to have a much lesser effect on Bacillus cereus (a taxon very closely related to Bt), which has shown the ability to multiply in non-sterilized soils, increasing its population up to 20% [17]. Nevertheless, under specific conditions, Bt spores can germinate and grow well, such as in humid, nutrient-rich soils, with pH near neutrality, even in the presence of other microbial populations [18]. Moreover, Bt toxins can find protection against precocious degradation in the soil, as they associate with humic acids and/or clay particles, which can help keep their toxicity for soil microbes [24,25].
Bt has been found as a natural soil inhabitant from several regions in the world. Areas with no history of Bt-based products application have shown a great diversity of H-serotype isolates with variable levels of toxicity, and persistence of Bt for many years after its application in areas of environmental conservation has been reported as well [12,26,27,28,29]. However, they noticed a reduction in the number of spores through time, which agrees with the idea that Bt hardly multiplies in this environment. Petras and Casida [30] have also observed a reduction in the initial population of spores, although it has tended to stabilization. On the other hand, the presence of insects does not guarantee the presence of Bt in the soil. Bt has been found in soils with little or no insect activity, whereas it was not found in soils with high insect activity [12]. Moreover, Bt apparently does not have to be an obligate microbe in its association with insects, as it grows in regular media in vitro [12]. Nevertheless, the chemical properties of the assessed soils, which are widely known to interphere in Bt’s survival [18,20,21,22,23], have not been analyzed.
It is yet unclear which is the main form of Bt dispersion in the soil. It is known that its soil dispersal is limited, as it appears that very little of Bt populations in soil is altered by the action of rain, invertebrates or by plant growth [8,29,31,32]. However, it has been found that Bt spores can pass through the digestive tract of vertebrates and invertebrate organisms, reaching their feces. In these circumstances, they find favorable conditions for germination, and so, become capable of being dispersed in the environment through migration of these animals [8,33,34,35,36].
The researchers who argue that Bt is a soil microorganism generally believe in its saprophytic lifestyle. However, research focusing on the colonization of insects cadavers has shown that Bt is in fact necrotrophic, which appears to be an important feature to ensure bacterial proliferation and dispersion in the environment. Naryanan [37] demonstrated that an infected dead insect on a leaf surface caused the death of one third of the larvae around. Dubois et al. [38] showed that the necrotrophic stage is part of a complex life cycle of Bt that involves the activation and regulation of several genes that alter its metabolism after the host death. Such changes are required for survival and colonization of the host cadaver, involving the production of enzymes (proteases, lipases, esterases and chitinases) that allow not only the use of host contents, but also the breakage of cuticle for release of toxins and spores. However, the main survival factor for Bt seems to be the ‘kurstakin’, a lipopeptide with biosurfactant and antifungal activity that grants mobility, ability to form biofilms and improved microbial competition. Outside the concept of necrotrophism, few studies have demonstrated a saprotrophic lifestyle for Bt, such as growth on feces and wastewater sludge [39,40,41].
2.2. Epiphytic Environment—Phylloplane
Bt has been isolated from the phylloplane in natural or artificial ways [34,42,43,44]. It has been shown that Bt can reach this niche by rain splash from the soil to lower leaves [31], from soil as a result of being carried by germinating seeds [43], from animal feces such as those from insects or birds [34,36], and from dead insects [34]. There is a variation in Bt’s survival rates in the phylloplane that seems to be linked to the plant species. As an example of this effect, the strain HD1 was able to grow vegetatively and persist in Trifolium hybridum leaves [34], whereas in cotton leaves, its population disappeared entirely in few days [45]. In addition, there are some evidences indicating Bt as a poor leaves colonizer, being found mostly as spores in these habitats. Even with nutritionally rich leaf surface that leads to spore germination, vegetative Bt cells sporulates again after a few rounds of division, which confers a survival ability for long periods, even under stressing conditions, such as desiccation [42]. Despite serving as nutrient sources, leaf exudates can also affect Bt survival negatively; for instance, organic acids that decrease surface pH can increase mortality rates of Bt in this environment [18,46]. Because Bt demonstrates a better survival and persistence in soil than in leaves, these have been suggested to work as a secondary reservoire that aid in the recycling process of the bacterium by returning cells and spores to the soil through rain, falling leaves, feces from phytophagous and dead insects bodies [8,31,42]. Alternatively, epiphytic Bt may well be a transient condition between endophytic environment and insect host (see below).
2.3. Epiphytic Environment—Rhizosphere
Few studies have demonstrated rhizosphere colonization by Bt. Rabinovitch et al. [47] isolated a Bt strain from the rhizosphere of a Ficus doliaria tree highly toxic against the Blackfly larvae (Simuliidae), a vector of human diseases, which was not present in the area. Interestingly, the region where the tree was found had not received any prior application of a Bt-based product. The closely related B. cereus was also shown to germinate and grow in the rhizosphere of plants [48]. Hendricksen and Hansen [8] found a Bt population that was 260 times higher in the rhizosphere than in the phylloplane of the same plants. It is assumed that such a more effective colonization of Bt in the rhizosphere and roots surfaces might be likely due to a richer nutrient availability in these environments [49], as carbohydrates and amino acids are more abundantly released in the form root exudates [50], thereby favoring microbial growth. Nevertheless, a simply better adapted genetic set up for this particular population cannot be ruled out as a reason for this successful rhizosphere colonization.
2.4. Endophytic Environment
Generally, endophytic microorganisms have the ability to colonize internal plant tissues without damaging the physiological processes and the morphology of the plant, in a mutually beneficial symbiotic relationship [51], or in a neutral non-damaging form. It has been reported that bacterial endophytes, after entering and colonizing plant tissues, can reach the seeds systemically and be vertically transmitted [52,53]. Another form of transmission is the horizontal, in which environmental microbes can enter the plant body mainly through the roots [54], the epidermis (passive absorption during transpiration—Quadt-Hallmann et al. [53]) and stomata [55].
In an endophytic relationship, microbes (including Bt) take up necessary nutrients from the plant to survive, but compensate such activity by promoting protection of the plant against parasite attacks (by stimulation of, and direct production of toxins), and emergence of diseases (by production of antimicrobial agents and enzymes, or induction of plant immunity system [56,57]. With all these functionalities under play, the plant becomes well assisted in its growth and development [51,58,59]. Some studies in cotton, soybean, corn, sugar cane, cabbage, ricebean, gahat and lentil [50,60,61,62] have reported that Bt was successful in endophytic colonization, even with concomitant production of Cry toxins; the efficient Bt colonization of cabbage seedlings roots suggests this might be in fact the main route of its penetration in the plant. After this event, vegetative cells, spores and crystals were found in several parts of the seedlings, which characterized a complete Bt colonization [60]. Similarly, Bt was able to colonize the roots of certain legumes, which resulted in an increase of nodulation and growth of the plants [49,63]. Recent work of our group added passionfruit and cacao to this list of plants with rhizospheric/endophytic Bt [64,65]. Even in its vegetative stage, Bt produces toxins that can reduce pests or diseases attacks [66]. Bt can reach the interior of the plant through the roots, stomata and wounds, or through the action of hydrolytic enzymes [53,67]. It is known that Cry toxins are inactivated by UV radiation and can be washed out from the leaves by rain or irrigation [6,68,69,70]; when Bt grows endophytically, these adverse conditions do not occur. Recent studies, however, have evidenced that biocontrol strategies employing direct endophytic capabilities of Bt have been much less explored than transformation of other endophytic bacteria to express Bt toxins, which removes the requirements for sporulation [71,72,73,74,75].
2.5. Aquatic Environment
Although few studies have focused on the survival in water, they have shown such a capability for Bt. Ichimatsu et al. [39] isolated a great variety of Bt serovars from 50% of running (river, stream, and ditch) and still water (pond) samples in Japan. They found that 26.7% of isolates exhibited larvicidal activities against Diptera Culex pipiens molestus and Clogmia albipunctata. Such an action in aquatic organisms can be viewed as a way of promoting recycling and dispersal of Bt in a wider variety of environments. By assessing the survival rates of Btk in water samples, Menon and Mestral [76] have shown that its viability remains for large periods: around 40 days in sea water, and over 70 days in lake water, likely due to a greater nutrients availability in this case. Konecka et al. [27] isolated a Bt strain from a forest creek sample that appeared to be 24× more toxic than the Btk HD1 against Cydia pomonella (Lepidoptera) larvae. This isolate carried the cry1B and cry15 genes that encode toxins against coleopteran, dipteran, and lepidopteran insects; the Cry15 is a binary toxin that has been rarely found. Further work has also demonstrated the presence of Bt in water samples subjected to hypochloride treatment [77,78]. Bacterial viability in aquatic environments is influenced by biological, chemical and physical factors (e.g., microbial competitors, bacteriophages, pH, aeration and nutrients); for instance, it was shown that Bt is capable of growth in aquatic environments that are rich in nutrients and oxigen, such as those from sewage treatment stations [39,79]. Another interesting possibility found was the use of vacuoles excreted by aquatic protozoans, such as Tetrahymena pyriformis [80], as nutritional source for Bt growth in natural environments. Taken together, these data suggest that Bt is also ubiquitous in aquatic environments. Since most part of water-isolated serovars were also isolated from soil and phylloplane, it is likely that they reach the water bodies through rain, percolation, floodings, wind, animal excrements, etc.
2.6. Paratenic Behavior
As it could be seen thus far, Bt is a versatile bacterium that occupies various niches, becoming readily accessible to a great variety of organisms. Digestion is the main access route of Bt to invertebrate or vertebrate animals [36]. When a microorganism colonizes a certain host in a way that it does not cause damage (disease), and this host is not indispensable to the microbe’s life, this organism is known as ‘paratenic host’. An array of vertebrates and invertebrates has shown to be colonized by Bt, although only few studies have gone deeper in the understanding of the ecological relationships among Bt and these organisms, both in the pathogenic and non-pathogenic way. For instance, Wilcks et al. [33] demonstrated Bt’s capability of colonizing the whole intestinal tract of germ-free rats, in high concentrations and in a stable form; Bt grew vegetatively for various generations (>90% of Bt population) before sporulation and elimination through their feces. Ammons et al. [35] reported the presence of Bt in rectal samples from milk cows, with indication that multiplication of Bt cells had occurred in the digestive tract of these animals. Zhang et al. [36] showed the presence of Bt in the intestinal tract of chickens, with the duodenum being the main portion colonized; moreover, it was verified that these animals kept releasing the bacterium through their feces for a certain time, even after removal of Bt from the diet. Similarly to vertebrates, Bt has been also isolated from fecal pellets of non-susceptible caterpillars from forests of conservation areas [44]. In another report, Bt germination in the alimentary tract was demonstrated in different invertebrates (e.g., earthworms), with sporulation occurring after defecation [8]. Even assuming that intestinal colonization does not occur, the fact that the Bt spores are able to cross the digestive tract of these animals and reach their feces does provide a nutrient-rich environment for their multiplication [34,39,41]. This ecological feature of survival, proliferation and sporulation in paratenic hosts warrants to Bt a wide dispersion in the environment through animals migration and defecation; such a dispersion mode is not only critical in the process of Bt populations recycling and multiplication in nature, but it also may help explain, at least in part, the presence of Bt in regions where specific host insects were not apparently found [12].
Kweon et al. [81] isolated a Bt strain from fresh milk samples obtained from healthy cows udders, which were likely colonized by Bt from the external environment. Interestingly, this isolate was capable of working as an oral probiotic, preventing fatal pathogenic infections by bacteria in mice; the authors suggest that antibiotic production, tissue colonization, and host immune system stimulation by Bt turn it a strong candidate for development of an efficient probiotic product. These results are suggestive of a possible symbiotic behavior between Bt and other organisms [82].
2.7. Pathogenic Behavior
The microorganisms can be classified according to their specific abilities to parasitically colonize and promote disease in other susceptible host organisms. When a microbe never or rarely causes disease, it is considered as being non-pathogenic. When it only causes diseases in situations in which the hosts have their defenses suppressed by various other stress agents, it is then called ‘opportunistic pathogen’. When it has a genetic make-up that serve to specifically facilitate the infection (virulence factors), even of healthy organisms not under any sort of stress, it is then considered a true pathogen [83]. An alternative classification though is ‘environmental pathogen’, which refers to those microorganisms that can spend part of its life cycle out of its host, but causes disease when coming into contact with it, without the need of specific conditions as in the opportunistic pathogens [11].
For a microorganism to be considered pathogenic, it must display some specific features and abilities such as (i) occupy the same host’s niche; (ii) persist in it; (iii) overcome the host defenses; and (iv) colonize its tissues and/or impair its physiology [83]. For this, the pathogens have the so called virulence factors, which provide the mechanisms by which they can overcome the host resistance and cause disease. The life cycle of an environmental pathogen may involve different habitats, hosts and niches with different availability of nutrients, which makes it metabolically versatile [11]. This versatility is required for the movement of the pathogen through different niches and different hosts [84]. In this regard, Bt has several factors (such as enzymes, toxins, antimicrobial compounds and structural proteins) that allow dwelling in a diverse array of environments and reaching various target organisms/hosts (Figure 1), turning this bacterium into a well-succeeded environmental pathogen [1,6,18,34,36].
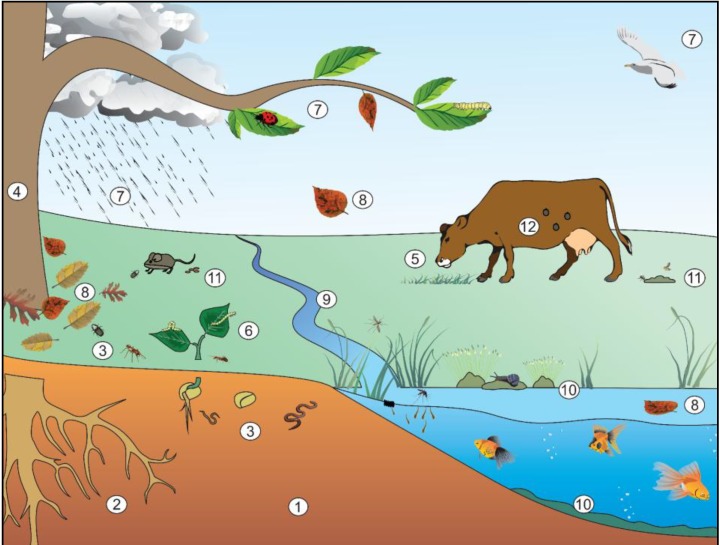
Figure 1.
Simplified view of complex lifestyle of Bacillus thuringiensis (Bt) in different environmental niches. Vertebrates and invertebrates are only examples of a much wider range of possible hosts. The soil (1) is usually the largest reservoir of Bt, because it receives the highest amount of propagules from other environments. From it, Bt can colonize the rhizosphere (2) feeding on roots exudates. If eaten by soil invertebrates such as worms, insects and nematodes (3), Bt can infect in a paratenic way, colonizing the gut and feces, or in a pathogenic way, killing the host and growing in the cadaver. Thus Bt is re-introduced into the environment through these two ways. Rhizosphere colonization favors endophytic colonization (4) which protects the plant from some herbivores, while helps Bt to proliferate in plant tissues and infecting herbivores in paratenic (5) or pathogenic (6) ways. Besides endophytism, Bt can reach the surface of the plants from the soil due to the germination process, by splashes of rain water, and through the feces of animals that carry it, such as insects and birds (7). The infected fallen leaves can re-introduce Bt in soil and water (8). The rain may also carry the Bt to water bodies from soil and plants (9). In water the Bt can infect and proliferate in vertebrates or invertebrates and persiste in this environment by associating with substrates as aquatic plants and sediments (10). Faeces from animals that feed on contaminated plants or insects can serve as a source of nutrients for Bt growth, and they can act as a source of infection for coprophagous (11). It is known that ticks and mites are also Bt hosts (12), but the natural mechanism of infection is unknown. It is possible to observe a wide range of strategies for Bt occupy different niches and disperse in environment with or without causing disease.
On another hand, septicemia (blood infection) usually kills the host, and the ability of a microbe to reach, grow and produce toxins in the hemolymph, causing host poisoning and death, is often associated with pathogenic organisms, not with opportunists [85]; these characteristics are widely recognized in Bt [2,86,87] thereby strongly pointing to it as bona fide insect pathogen [6]. However, another important aspect to consider in this scenario is that host specificity (due to different gut-membrane receptors, pH levels, protease activities, etc.), growth physiology, and secreted toxin types and amounts in Bt can vary substantially among strains [1,3,88], thereby interfering with a clearer understanding of its action. Moreover, Bt collections are frequently not properly assessed, with isolates being misclassified with regards to their toxicity when inappropriate hosts and/or toxin-secretion times for each isolate are used in the screening procedures. Methodologies have been developed to attempt screening large collections [89,90], but the lack of knowledge of possible targets, as well as suitable rearing techniques and bioassays hinder even further more appropriate assessments of Bt ecology concerning its pathogenicity. For instance, some studies have reported isolation of non-insecticidal Bt strains [26,91], but only a few species representing most relevant taxonomic orders of insects were used for the toxicity assays. Given the very wide range of possibilities for novel or different hosts for the Bt isolates (insects or other taxa), such a statement of non-pathogenicity might be premature and uncertain. In addition, most studies base their assessments only on the variable ‘mortality rate’ to define pathogenicity; other toxi-infection pathogenic symptoms, such as delays in development, lack of pupation, body deformations in adults etc., can also be used to evaluate different types and/or intensities of pathogenic effects under play. Therefore, it appears to be very difficult to be absolutely certain about a Bt strain not being pathogenic in all circumstances.
2.7.1. Pathogenic Arsenal of Bt Cells towards Insects
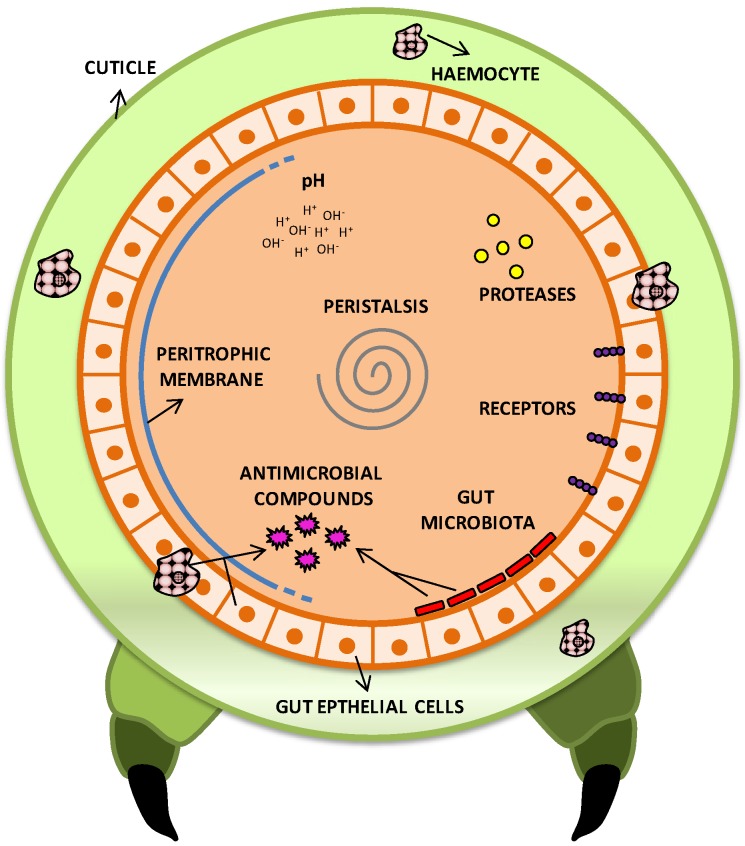
The insects are well studied target hosts and possess a wide range of defensive systems against pathogens. For instance, physical and chemical defenses/barriers help prevent pathogens to invade or cause damage to the insect body. The external cuticle, intestinal microbiota, intestinal peristalsis and peritrophic membrane (the inner layer of the insect midgut) are examples of physical barriers. As chemical defenses, the pH of the gastro-intestinal tract, proteases, antimicrobial peptides, cellular receptors and the immune system (Figure 2) are the main ones [6,92]. Therefore, for a microbe to cause disease, it must overcome host defenses, colonize its body (totally or partially) and elicit some physiological injury. To achieve this, it must possess molecular and biochemical resources and tools, which are usually known as ‘virulence factors’. Virulence is the measure of pathogenicity of a microorganism and reflects its ability to cause disease, even in the presence of host defense mechanisms [83]. The Bt species has a large variety of factors that guarantee its success as a pathogen (Table 1).
Figure 2.
Schematic diagram of local defense mechanisms of an insect model against pathogenic bacteria. The cuticle is a first barrier, which can be overcome through spiracles or injury. To cause infection, ingested pathogens must overcome various physical barriers, such as peritrophic membrane, epithelium, peristalsis and commensal microbiota, as well as chemical defenses present in the digestive system as pH, proteases, cell receptors and antimicrobial compounds. In addition, the commensal microbiota provides a competitive environment for the pathogen establishment, and also produces antimicrobial compounds that hinder the pathogenic action. Much of the toxins secreted by pathogens require specific receptors to perform their functions; changes in these receptors allow development of insect resistance to pathogens. Finally, after overcoming all these defenses, the pathogen must still deal with the innate immune system and circulating haemocytes to succeed with an infection.
Table 1.
Virulence strategies found in Bacillus thuringiensis and their roles.
| Virulence strategies | Function | References |
|---|---|---|
| Toxins production | Degradation of intestinal membrane and release of nutrients, favoring spore germination and colonization. Gate opening to reach hemolymph and cause septicemia. | [1,2,6,66,93] |
| Antimicrobial resistance | Resistance to antimicrobials produced by the host and midgut resident microbiota, allowing colonization. | [94] |
| Antimicrobial production | Decrease of competition for space and nutrients, and reduction of antimicrobial compounds production by midgut microbiota. | [95,96] |
| Peristalsis and feeding reduction | Decrease of toxins and Bt cells elimination from intestine. | [6,97] |
| Production of degrading enzymes | Degradation of antimicrobial agents from host and facilitation of intestinal colonization. Direct toxicity in some cases. | [92,98,99,100,101,102,103] |
| Imune system resistance | Prevention of phagocytosis and resistance to antimicrobial compounds, digestive enzymes, pH, reactive oxygen species. | [92,104] |
In a balanced symbiotic condition, microorganisms and their hosts live harmoniously, with the latter ensuring food and shelter and the former helping with protection against other pathogenic microbes [105]. However, any stress factor can initiate a pathogenic action from certain symbionts, which for example, reach alternative habitats such as migrating from intestine to the haemolymph [106]. In this scenario, Bt toxins can work as such stressing factors, decreasing the action of host’s natural defenses, and so, providing access of Bt cells and other commensal microbes from intestine to other body parts where they can cause disease. In this colonization event, the most adapted microorganisms tend to carry specific virulence factors that furnish a selective advantage towards higher proliferation rates. This establishes complex microbial interactions that can help explain more or less contribution of the resident microbiota in the mortality of organisms infected by Bt. These interactions, defined by host-specific composition of the intestinal microbiota, can determine the predominance of Bt as the main pathogen or only as a facilitator of infections by other species [95,107,108,109]. In this case, for its own proliferation, Bt can benefit from an eventual host death caused mainly by another microbe. Table 1 displays some strategies found for Bt to cope with these scenarios.
In evolutionary terms, nonpathogenic microbes do not have to develop so many mechanisms to overcome defense systems of other organisms, since they do not belong to the same natural niche [92]. Moreover, it is of general ‘interest’ for pathogens not to kill their hosts [83], so that biotrophic parasitic activities can be maintained. In this sense, host death seems to provide the main way for Bt reproduction, since the host cadaver is where the greatest multiplication rate of the bacterium occurs [9,110]. However, a confounding aspect when thinking on Bt’s ecology is that it apparently does not have to be an obligate microbe in its association with insects, as most of pathogenic Bt isolates characterized thus far are capable of growing in regular media in vitro [12]. Moreover, Bt can reproduce well in other hosts (and some insects) without having to kill them (see Section 2.7.2. below). Part of the difficulty in a better understanding of Bt’s life style appears to be due to the fact that the vast majority of related research has focused on insect-pest species, using mortality as the single parameter of pathogenicity. More studies outside this utilitarian view are required to unravel possible alternative targets and niches occupied by Bt.
In the next sub-sections, the large variety of virulence factors that compose Bt’s pathogenic arsenal is discussed, focusing on the available knowledge about their structure, action mechanisms, molecular interactions and practical implications on biocontrol strategies and/or ecological attributes.
2.7.1.1. Protein Toxins that Need Receptors in Host-Cells Membranes
The main insecticidal activity of Bt comes from endospore inclusions of proteinaceous crystals. These are composed of a class of proteins called δ-endotoxins, which are divided into two families, the Cry (crystal) and Cyt (cytolytic) proteins encoded by the respective cry and cyt genes [2,111,112]. Their ability to rapidly crystallize helps decreasing its susceptibility to premature proteolytic degradation, although there are active Cry proteins that do not crystallize, such as the Cry1I (formerly CryV) [113,114]. Cry proteins generally have three domains. Domain I is responsible for (i) the toxin insertion into the insect host’s midgut membrane; (ii) lytic pore formation; and (iii) maintenance of the toxin binding to receptors in the intestinal epithelium. Domain II is the least conserved among δ-endotoxins and is responsible for toxin binding to specific membrane receptors, playing a key role in toxin selectivity. Domain III is responsible for preserving the structural integrity of the toxin, and also for assisting in binding, penetration and pores formation in cells of the intestinal epithelium [2,115].
One of the greatest advantages of Cry toxins for development of biological control approaches is their high specificity to target host, with no significant effect to invertebrate or vertebrate non-target organisms [2]. The classical and intensively researched general mechanism for insecticidal activity as a consequence of Cry toxins action is (i) spore + crystals ingestion by a susceptible insect; (ii) crystals solubilization in the intestinal alkaline environment; (iii) action of specific proteases that cleave the protoxin into smaller active peptides—the δ-endotoxins; (iv) toxin binding to specific receptors of midgut cells; (v) membrane insertion of toxin; (vii) formation of permeable ionic pores leading to cell destruction by colloid osmotic lysis; (viii) paralysis of the insect gut and mouthparts, resulting in cessation of feeding; (ix) intestinal rupture that favors germination of the ingested Bt endospores; and (x) septicemia caused by the consequent bacterial proliferation. This sequence of steps and combination of factors lead to insect death in a few days [2,3]. Variations of sensitivity to intestinal pHs, types of gut proteases, and different intestinal receptors help to explain differences in the toxicity degrees among Cry proteins. Moreover, these characteristics are also considered to be the basis upon which insect resistance mechanisms can develop [2,3]. Based on all information currently available, two recently proposed theories are attempting to account for the mechanistic details of Cry toxins’ action. The first one is the Sequential Binding Model, which postulates that the Cry toxin must undergo proteolytic cleavages to sequentially bind to at least three different receptors; then, changes in 3-D conformation allow its oligomerization to form a pore in the cell membrane [116]. However, it is not yet fully understood whether there is a need for different receptors, and/or whether oligomeric ‘pre-pore’ structures are formed, being (or not) more active than the monomers. There is some evidence suggesting that pores are assembled only after insertion of the monomers into the membrane [112,113,114,115,117,118]. The second is the Signaling Pathway Model, which postulates that a signaling pathway starts in cell after Cry binding, leading to its necrotic death without necessarily forming lytic pores in the gut membranes. This model has not been easily accepted, as it does not attempt to reconcile with all research evidence strongly pointing to the formation of pores and to a direct toxicity of Cry proteins [119,120]. Further studies are yet needed to confirm, refute, or at least try to reconcile these two models, which may co-exist and complement each other (for reviews, see [6,116]).
Another type of δ-endotoxin that needs a receptor in the host gut membrane is the Cyt protein, which has a molecular weight between 25 and 28 kDa [121]. It is a cytolytic and hemolytic toxin, with primary toxic effects on dipteran insects [112,122]. These toxins have a high specificity to target hosts, being harmless to humans, other vertebrates and plants [112]. They are hydrophobic, show no homology to Cry sequences, and have different membrane receptors in the insects [2]. Their possible mechanisms of action have not been completely understood; they appear to operate in two different ways, depending on the toxin concentrations. At low levels, this toxin can develop oligomeric pores in the cell membranes of the insect gut by forming β-barrel structures [122,123]. At high concentrations, due to their high affinity to lipids, these toxins can act similarly to detergents by rupturing the cell membrane [122]. Moreover, several authors have reported a synergistic effect of Cyt with Cry toxins [19,124,125,126]. However, antagonistic effects have also been observed between Cry and Cyt proteins, especially when using toxins of different strains [127].
The Vegetative Insecticidal Proteins (Vip) are other promising toxins in biological control of insect-pests that require receptors in insect-gut cell membranes for their action [66,128,129]. Vips were named as such because, unlike the Cry and Cyt proteins, they are mainly produced during the vegetative growth phase of Bt cultures, although their secretion can also be extended into the sporulation stage [66,130]. Vip genes have shown no DNA sequences homology to δ-endotoxins, suggesting they bind to different receptors [66,130]. A practical advantage of this difference is that cross-resistance of insects to different toxins are unlikely to occur, so that Vips can be used together with Cry and Cyt proteins in biological control programs [129]. The genes encoding Vips are located in plasmids that also encode Cry proteins [131]. Four basic types of Vip toxins have been described, although they can be present in a variety of forms within each class [128,132]. Vip1 and Vip2 types have 100 and 52 kDa, respectively [133], and form a pair of binary toxins, where Vip1 binds to specific receptors on the intestinal membrane, creating pores through which Vip2 penetrates and causes inhibition of the polymerization of monomeric actin (G-actin) [133,134]. Its main effect is against insects of the Coleoptera order [133]. Vip3 toxin has 88.5 kDa and is effective primarily against lepidopteran insects [66,133], with a mechanism of action apparently similar to that of Cry toxins, by forming pores in midgut cell membranes of the target-pest that cause osmotic imbalance and degeneration of the epithelial layer [128,129]. Vip3 also appears to do not show deleterious effects to non-target organisms [135]. Vip4 was not characterized until now.
2.7.1.2. Proteins and Toxins that Do Not Need Receptors in Host-Cell Membranes
The α-exotoxins (a.k.a. phospholipase-C) have been purified from culture supernatant of certain Bt strains [98]. They are thermolabile proteins classified according to the types of phospholipids onto which they act; for example, phosphatidylcholine-specific phospholipase-C (PC-PLC) [136], phosphatidyinositol-specific phospholipase C (PI-PLC) [137], etc. Their toxicity can be explained by the hydrolysis of various phospholipids on cell membranes [138], being highly toxic to insects by oral or intra-haemocoelic administration. They cause degeneration and lysis of insect cells such as the haemocytes [139] and, at high doses, can also cause toxicity against vertebrates [140].
Chitin is a long-chain polymer of N-acetylglucosamine (a glucose derivative), and is the main component in the cell walls of fungi, exoskeletons of arthropods, and midgut peritrophic membrane of many insects. Endochitinases are enzymes that degrade chitin by randomly cleaving within its chain, and exochitinases hydrolyze diacetylchitobiose units from the chain’s end [99,141]. Pathogens that produce chitinases have a great advantage over host defenses, with some Bt strains showing to produce both forms of chitinase simultaneously [99]. It has been proposed that Bt can degrade the perithrophic membrane to facilitate binding of other toxins to their receptors in midgut epthelium [142]. To support this view, some researchers have shown that the use of both exogenous [99,143] and endogenous [99] chitinases has increased the efficiency of Vip and Cry toxins. It appears, therefore, that the combined use of usual Bt toxins and chitinases can increase success in pests biocontrol programs.
Further types of toxins have also been described for Bt. For instance, hemolysins are also produced during Bt vegetative growth phase, especially when iron is depleted. Its mechanism of action is based on the lysis of insect haemocytes and macrophages by forming pores in the cell membranes [102]. Essentially, they allow the Bt cells to obtain nutrients during the infection, while repressing the host immune system.
Li and Yousten [144] first described the production of a Bt protease, specifically the metal chelator-sensitive protease (metalloprotease), which was shown to be secreted near the stationary phase of growth. The required proteolytic cleavage of Cry toxins for their activation [2,3] depends on not only the action of exogenous proteases, present in the host midgut, but also on the action of various Bt-synthesized endogenous proteases [103,144,145]. A metalloprotease named InhA was shown to be involved in the toxicity of Bt against Lepidoptera [100], while the Bmp1 showed activity against Nematoda [103]. In addition to participating in the activation of Cry toxins, the metalloproteases can also protect Bt from the innate insect immune system through cleavage of antimicrobial peptides, or by facilitation of the bacterial cells’ escape from the haemocytes [104,146]. The destruction of cells and tissues to facilitate Bt colonization of the host body is another form of proteases action [92,103].
Finally, secreted insecticidal proteins (Sip) were discovered and characterized by Donovan et al. [147]. This protein is secreted during the vegetative phase of Bt growth and was identified as toxic to coleopteran larvae. Its basic mechanism of action is similar to Cry and Vip toxins, i.e., by forming pores in cell membranes that disrupt the regular physiology of the insect.
2.7.1.3. Non-Proteinaceous Toxins
The type I β-exotoxin (a.k.a. ‘thuringiensin’) is a thermostable toxin of a low molecular weight (701 Da), composed of adenosine, glucose and alaric acid. It is an adenine nucleotide (ATP) analog [148] that acts by inhibiting eukaryotic DNA-dependent RNA polymerases, thereby affecting the normal development of the organism [149]. Due to this general mechanism of action, a very high toxicity was observed against a diverse array of taxa, including mammals and other vertebrates, so that the World Health Organization has recommended that Bt strains producing these toxins must not be used for insect control [150]. Some countries, however, still use products based on β-exotoxins, mainly in specific control programs of dipteran pests that are resistant to other insecticides [151]. Ohba et al. [152], studying other Bacillus species (B. subtilis, B. megaterium and B. natto) have not identified the β-exotoxin production, suggesting this feature is strain-specific to B. thuringiensis and B. cereus. It is known that the phosphate group present in the β-exotoxin’s structure is essential to its toxicity. Thus, the cleavage of the phosphate ester when treating with phosphatase [149], or heating at a pH < 3 [153,154], causes phosphate removal, and so, the loss of the toxic activity. Genes that control the synthesis of β-exotoxin seem to be located in plasmids that also encode Cry proteins [153,155]. This may explain why it is secreted near and during the sporulation phase, along with the Cry toxins. Another form of β-exotoxin molecule identified (type II) is analogous to the uracil nucleotide (UTP) and seems to be the most toxic, being particularly effective against insects of the order Coleoptera [153]. Its whole structure, however, is not yet fully known.
Taking together all virulence features described above, it becomes readily apparent the extraordinary genetic plasticity found among specimens of this bacterial species, being clear-cut evidence towards a characteristic fast pace for its evolutionary path. However, the existence of a toxin with lack of host-specificity, but not present in all studied strains, poses a further puzzle to be solved in the understanding of Bt’s ecology related to pathogenicity: why evolving such a sophisticated mechanism of overcoming defenses to kill the host (through Cry, Cyt, Vip and Sip toxins, as well as other proteinaceous enzymes that aid in the infection), if non-specific action in killing any type of host would be apparently more advantageous for occupation of various niches by Bt? Perhaps it may represent ancient steps in the co-evolution of insects’ specificities; its presence in some strains may represent ‘live fossils’ of primitive mechanisms of host killing. Alternative, it may have simply been acquired through horizontal transfer and being maintained in some strains as a way of helping the bacterium with some levels of proliferation and dispersion through indistinct transfer among a variety of hosts. Further studies based on these hypothesis need to be devised to answer this question.
2.7.2. Pathogenicity to Other Taxa
The fact that Bt has been found in places apparently free from insects [12] is likely related to its dispersion capacity in the environment (wind, rain, paratenic hosts feces, etc.—Figure 1), or yet, to the presence of other host taxa that were not yet identified and/or assessed. Besides insects, other taxa have been described as being targets for Bt. Thus far, high levels of Bt toxicity have also been found against ticks, mites, nematodes and protozoans, mostly from studies aiming at searching for appropriate biocontrol agents.
The tick (Arachnida: Acari) is a major ectoparasite that affects animals, causing several zoonoses of economic relevance. Its control is achieved by synthetic acaricides. Although research on the biological control of ticks has been mainly focused on fungal agents, some work has been done with bacteria; B. thuringiensis subspecies kurstaki, israelensis and thuringiensis have shown mortality and development retardation of soft and hard ticks, including their eggs [156]. Bt kurstaki’s toxins were effective against hard ticks by spraying [157,158] and immersion [159]. It has also been found that toxic effects are the greatest during critical physiological stages such as egg, pupation and metamorphosis. These data also suggest that there might be another form of infection beyond the intake, perhaps through other natural openings in the body (e.g., genitals and spiracles) [6], thereby pointing to the possibility of alternative mechanisms of pathogenicity. Bt has been isolated from the hemolymph of dead ticks, proving the existence of a systemic infection [156]. It was demonstrated that some Bt toxins are lethal to hemoplast cells and that Cry4Ba was capable of binding receptors, forming pores and lysing the cells [160]. Besides ticks, pathogenicity of Bt has also been reported against storage mites (Arachnida: Acari), particularly by intake [161,162].
The Nematoda species are cylindrical and elongated multicellular animals that can be either free-living or animal and plant parasites. Because of its economic importance, its biological control has been investigated. Some studies involving Bt toxins have demonstrated their effectiveness against this parasite, including both Cry [163,164] and vegetative proteins from the culture supernatant [164]. The Cry5, Cry6, Cry12, Cry13, Cry14 and Cry21 proteins were described as nematicidal or developmental retardant [3,163]. The mechanism of Cry toxins action in nematodes is similar to that on insects, correlating with damage to the intestine. Symptoms of nematode poisoning include lethargy, reduced size, pale coloration and contraction, vacuolation and degeneration of the intestine [163].
Protozoa are unicellular eukaryotic microorganisms present preferably in moist environments. They may be free-living or animal parasites, including humans. The first report of a Bt strain able to control infections by a protozoan was made by Thompson and Gaertner [165]; subsequently, other strains were identified as effective in the control of Trichomonas vaginalis [166]. It has also been reported that Bt, after ingested by Tetrahymena pyriformis and encapsulated in food vacuoles, was able to multiply and sporulate, producing δ-endotoxins that remained protected, stable and active. This behavior demonstrates that protozoans can act at least as an intermediate host, being possibly part of the natural recycling and transmission of Bt [80].
In our view, two main lessons can be learned from these research: the first is that conclusions about the lack of hosts for Bt isolates in given areas must be always considered with care, as potential true hosts may simply be there but not aware of; the second is that mortality should not be the only (or main) parameter to use for assessment of pathogenic effects of Bt, as different forms or levels of toxicity that affects development and/or reproduction of hosts can be both ecologically informative and agronomically useful.
3. Reconciling Alternative Ecological Views
To achieve this task, it seems necessary to take some distance from the utilitarian view of Bt as a bioinsecticide/biocontrol agent, as this tends to make us overlook the behavioral and functional complexity of this bacterial species. A multidisciplinary and integrated view involving concepts from microbiology, epidemiology, infectology, toxicology, development, ecology, genetics, genomics and evolution is required to provide a more suitable framework for the debate on Bt’s role in nature. This approach inevitably leads us to recognize that perhaps the choice of a single line of thoughts—“saprophytic Bt”, “pathogenic Bt”, “free-living Bt”, etc.—is likely insufficient to account for all related aspects already found and reported in the literature. We argue that all the ideas and concepts currently available about Bt’s ecology actually complement each other and points to an extreme versatility of this bacterial species in occupy an array of different niches. The bona fide pathogenicity of Bt on various insect taxa has already been more than sufficiently proved, as it is a fact that host-specificity is achieved by different combinations of elements from a multifaceted arsenal of toxins and strategies to overcome host defenses. In evolutionary terms, this integrated view of a functionally versatile microbe implies that Bt should have a fast rate of changes in its genome, both through accumulated mutations (reasonable to be expected for a short living-cycle organism) and incorporation of new genes (through conjugation, transformation and transduction). Fang et al. [15] have recently demonstrated that Bt shows an ‘open pangenome’ as a key evolutionary characteristic, which favors its adaptation through the mechanisms just mentioned, as opposed to another closely related Bacillus species, the B. anthracis, which appears to have a ‘closed pangenome’ that tends to restrict its ecological niches. Conjugation of cry genes are thought to be responsible for the high degree of diversity in Cry proteins; such an ever increasing diversity also suggests a rapid evolutionary pace for this species, and ensures high levels of adaptation for Bt strains to their target organisms [167].
Considering the vast amount of work done on the aspects related to Bt’s capability as a biological control agent (i.e., about its pathogenic behavior against mostly insect-pests), it is important to realize that such abilities may be intimately associated with human interference on Earth’s face. Human-related changes in the environment began more noticeably with the onset of civilization and agriculture at the Neolithic era, ~12,000 years ago [168], with practices related to clearing vegetation and opening areas for crop cultivation and cities construction, as well as related to destructions caused by war movements and battles. Since then, and as largely acknowledged today, crop cultivation in cleared areas narrows down the genetic background and trophic interactions, so that selective pressure favors few species that circumstantially become most adapted, such as insects, nematodes and others. Therefore, it appears conceivable that evolution of Bt as an insect pathogen (occupying agricultural and other man-affected niches) has long been favored by a direct human interference in the environment. Nevertheless, as an environmental bacterium occupying a variety of niches, with very many ways to disperse, Bt is capable of grow vegetatively (although slower and for few generations if not on other animals) and survive adverse conditions for long periods through sporulation. In fact, it can be logically posed that Bt populations dwelling in the environment had probably been the major evolutionary raw material (ancestors) for development of specifically adapted insect-pathogen strains found later on. Likely, this process is yet ongoing around the globe, generating a vast array of different strains, and so, justifying the ever continuing research for novel strains and toxins with biocontrol capabilities [28,62,88,89,90,169]. In this scenario, it is relevant to note that studies have shown no differences in competitive ability in plant colonization between strains with and without production of Cry proteins [34], suggesting that the metabolic burden of insecticidal crystal proteins (ICP) production apparently does not significantly reduce fitness. Taking all these ideas into account, the ecological role of Bt appears to be as multi-host-environmental-pathogen [11,170].
Several factors may be involved in the ability of infecting multiple hosts. As discussed above, mutations can alter existing genes and grant them new and/or improved functions [171], as well as the conjugation of cry genes that widens the range of possible hosts [167]. Another factor is host speciation, in which a microorganism affecting ancestor species can also infect their derived species [172]. Genetic similarity among different host species also influences the success of a multiple-infection pattern, since the related defenses, metabolism, niches and habits are similar, thereby favoring a faster adaptation by the pathogen [170]. Finally, the introduction of exotic hosts in areas inhabited by the pathogen, and vice versa, can promote successful infection if the host does not offer resistance by not having come into previous contact with the pathogen [173]. Being capable of infecting various hosts and inhabiting different environments multiplies the chances of Bt in succeeding at survival and proliferation.
4. Conclusions and Perspectives
After over 100 years of Bt discovery, new target species have constantly been found and its mechanism of action is yet under discussion. With the studies conducted to date, we realize that different strains of Bt appear to have co-evolved with different taxa, making it difficult to clarify the real ecology of such a highly versatile bacterium. Although it is currently well acknowledged by the scientific community that Bt is a pathogenic bacteria to insects, little has been studied and discussed about the interactive patterns of Bt with other taxa and the ecological relevance of them. Therefore, a discussion still remains on whether Bt has a role in nature as an opportunistic pathogen, and/or a saprophyte, and/or simply as a cosmopolitan microbe that occupies an array of different niches with various efficient forms of dispersal. The not so many studies available that directly addressed this issue, as well as inherent difficulties recognized in this type of research, have hindered a better clarification over what is the real ecology of this bacterium. Part of this scenario is also explained by the fact that the majority of studies to date have focused on (and so, limited to) pest-insects of agricultural or public health relevance, thereby leaving important gaps on the knowledge about Bt interactions with other organisms/hosts.
In this review, we have intended to collect and integrate available and relevant information in this context, perhaps a bit overlooked thus far. Based on the available studies, we have shown that Bt is capable of being present, both in vegetative and sporulated forms, in a variety of environments without the need of hosts; however, in each environment, it can reach hosts from a diverse set of taxa, and colonize their bodies in the pathogenic or paratenic manner, as well as their cadavers and excrements. Hence, Bt displays a high capacity of proliferation and dispersion, mostly due to its combined ability of grow and produce spores almost everywhere, and passively reach all sorts of environments. Coupled with a reasonable genomic plasticity, rapid co-evolutionary events have changed some of these isolates into host interactive ones that became pathogenic at various levels. The overall Bt classification remains open until more conclusive studies are reported, which should consider such a great genetic plasticity, spectrum of action, possibility of paratenic and intermediate hosts, and versatility to occupy different niches, all within more integrative approaches. The current available knowledge strongly leads us to accept a broader idea that Bt is a multi-host-enviromental-pathogen, or simply an enviromental-pathogen. Further ecologically-oriented research on Bt will benefit from basing the generation of working hypotheses on this point of view, rather than on the predominant view of it as a potential biocontrol agent.
It is also worth mentioning, as a next step for research, that some Bt strains produce crystalline parasporal proteins during sporulation that, until today, have shown to be non-toxic to insects and to differ from other Cry and Cyt proteins. Mizuki et al. [174] first described the cytotoxic action of these proteins on human cancer cells. A year later these proteins were collectively named as ‘parasporins’ [175]. Although invertebrate targets for them have not been identified so far, these proteins share the same nomenclature of Cry proteins. To date, there are six parasporin families described (PS1–PS6), containing 19 different proteins according to their genetic homology. Recent work have shown cytotoxic activity of parasporins against nine human cancer cell lines and, with less intensity, against five types of normal human cells [176,177,178,179,180]. Katayama et al. [177] have claimed that the PS1 is not a pore-forming toxin, at least in mammal cells. It appears to act by inducing apoptosis through decreasing the levels of DNA synthesis and cellular proteins, and by increasing the levels of intracellular Ca2+. The formation of pores in the plasmatic membrane of the cells under the action of PS2 or PS3 has been observed [178,179,180,181]. No information is available about the mechanism of action of other parasporins. Taking these data together, strains carrying parasporins may represent novel species within the B. cereus sensu lato group, requiring more in-depth taxonomic studies to sort this out. The true ecological function of parasporins is yet unknown. However, one might speculate that, due to their toxicity against cancerous white blood cells, parasporins can attack host-defense cells in a similar way to what occurs with hemolysins; or yet, its function might be related to β pore-forming toxins (β-PFT), due to its homology to those toxins from Aeromonas hydrophila and Biomphalaria glabrata [182,183].
Acknowledgments
The authors are deeply grateful to the anonymous reviewers of ‘Insects’, as their questions and comments on the first submitted version of this manuscript made us to ponder and rethink our points of view about Bt’s ecology, resulting in a much improved and refreshed paper. We also thank the State University of Santa Cruz (UESC) for the infra-structure and facilities provided for the work on this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Aronson A.I., Beckman W., Dunn P. Bacillus thuringiensis and related insect pathogens. Microbiol. Rev. 1986;50:1–24. doi: 10.1128/mr.50.1.1-24.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnepf E., Crickmore N., van Rie J., Lereclus D., Baum J., Feitelson J., Zeigler D.R., Dean D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. R. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bravo A., Sarabia S., Lopez L., Ontiveros H., Abarca C., Ortiz A., Ortiz M., Lina L., Villalobos F.J., Peña G., et al. Characterization of cry genes in a mexican Bacillus thuringiensis strain collection. Appl. Environ. Microb. 1998;64:4965–4972. doi: 10.1128/aem.64.12.4965-4972.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lecadet M.M., Frachon E., Dumanoir V.C., Ripouteau H., Hamon S., Laurent P., Thiéry I. Updating the H-antigen classification of Bacillus thuringiensis. J. Appl. Microbiol. 1999;86:660–672. doi: 10.1046/j.1365-2672.1999.00710.x. [DOI] [PubMed] [Google Scholar]
- 5.Xu D., Côté J.C. Sequence diversity of Bacillus thuringiensis flagellin (H antigen) protein at the intra-H serotype level. Appl. Environ. Microbiol. 2008;74:5524–5532. doi: 10.1128/AEM.00951-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raymond B., Johnston P.R., Nielsen-LeRoux C., Lereclus D., Crickmore N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010;18:189–194. doi: 10.1016/j.tim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Crickmore N. Beyond the spore: Past and future developments of Bacillus thuringiensis as a biopesticide. J. Appl. Microbiol. 2006;101:616–619. doi: 10.1111/j.1365-2672.2006.02936.x. [DOI] [PubMed] [Google Scholar]
- 8.Hendriksen N.B., Hansen B.M. Long-term survival and germination of Bacillus thuringiensis var. kurstaki in a field trial. Can. J. Microbiol. 2002;48:256–261. doi: 10.1139/w02-009. [DOI] [PubMed] [Google Scholar]
- 9.Marco G., Manuel P. Ecological mysteries: Is Bacillus thuringiensis a real insect pathogen? Bt Res. 2012;3:1–2. [Google Scholar]
- 10.Jensen G.B., Hansen B.M., Eilenberg J., Mahillon J. The hidden lifestyles of Bacillus cereus and relatives. Appl. Environ. Microbiol. 2003;5:631–640. doi: 10.1046/j.1462-2920.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 11.Cangelosi G.A., Freitag N.E., Buckley M.R. From Outside to Inside: Environmental Microorganisms as Human Pathogens. American Society for Microbiology; Washington, DC, USA: 2004. [Google Scholar]
- 12.Martin P.A.W., Travers R.S. Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl. Environ. Microbiol. 1989;55:2437–2442. doi: 10.1128/aem.55.10.2437-2442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiuza L., Nielsen-Leroux C., Goze E., Frutos R., Charles J. Binding of Bacillus thuringiensis Cry1 toxins to the midgut brush border membrane vesicles of Chilo. suppressalis (Lepidoptera: Pyralidae): Evidence of shared binding sites. Appl. Environ. Microbiol. 1996;62:1544–1549. doi: 10.1128/aem.62.5.1544-1549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappello M., Bungiro R.D., Harrison L.M., Bischof L.J., Griffitts J.S., Barrows B.D., Aroian R.V. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma. ceylanicum. Proc. Natl. Acad. Sci. USA. 2006;103:15154–15159. doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y., Li Z., Liu J., Shu C., Wang X., Zhang X., Yu X., Zhao D., Liu G., Hu S., et al. A pangenomic study of Bacillus thuringiensis. J. Genet. Genomics. 2011;38:567–576. doi: 10.1016/j.jgg.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Akiba Y. Microbial ecology of Bacillus thuringiensis VI. Germination of Bacillus thuringiensis spore in the soil. Appl. Entomol. Zool. 1986;21:76–80. [Google Scholar]
- 17.West A.W., Burges H.D., Dixon T.J., Wyborn C.H. Effect of incubation in non-sterilised and autoclaved arable soil on survival of Bacillus thuringiensis and Bacillus cereus spore inocula. N. Z. J. Agric. Res. 1985;28:559–566. doi: 10.1080/00288233.1985.10418003. [DOI] [Google Scholar]
- 18.West A.W., Burges H.D., Dixon T.J. Survival of Bacillus thuringiensis and Bacillus cereus spore inocula in soil: Effects of ph, moisture, nutrient availability and indigenous microorganisms. Soil Biol. Biochem. 1985;17:657–665. doi: 10.1016/0038-0717(85)90043-4. [DOI] [Google Scholar]
- 19.Saleh S.M., Harris R.F., Allen O.N. Fate of Bacillus thuringiensis in soil: Effect of soil pH and organic amendment. Can. J. Microbiol. 1970;16:677–680. doi: 10.1139/m70-116. [DOI] [PubMed] [Google Scholar]
- 20.Polanczyk R.A., Zanúncio J.C., Alves S.B. Relationship between chemical properties of the soil and the occurrence of Bacillus thuringiensis. Ciênc. Rural. 2009;39:1–5. doi: 10.1590/S0103-84782009000100001. [DOI] [Google Scholar]
- 21.Akiba Y., Sekijima Y., Aizawa K., Fujiyoshi N. Microbial ecological studies on Bacillus thuringiensis. II. Dynamics of Bacillus thuringiensis in sterilized soil. Jpn. J. Appl. Entomol. Zool. 1977;21:41–46. doi: 10.1303/jjaez.21.41. [DOI] [Google Scholar]
- 22.West A.W., Burges H.D., White R.J., Wyborn C.H. Persistence of Bacillus thuringiensis parasporal crystal insecticidal activity in soil. J. Invertebr. Pathol. 1984;44:128–133. doi: 10.1016/0022-2011(84)90002-8. [DOI] [Google Scholar]
- 23.Akiba Y. Microbial ecological studies on Bacillus thuringiensis. IV. The growth of Bacillus thuringiensis in soils of mulberry plantations. Jpn. J. Appl. Entomol. Zool. 1980;24:13–17. doi: 10.1303/jjaez.24.13. [DOI] [Google Scholar]
- 24.Crecchio C.S.G. Insecticidal activity and biodegradation of the toxin from Bacillus thuringiensis ssp. kurstaki bound to humic acids from soil. Soil Biol. Biochem. 1998;30:463–470. doi: 10.1016/S0038-0717(97)00147-8. [DOI] [Google Scholar]
- 25.Tapp L., Calamai L., Stotzky G. Adsorption and binding of the insecticidal proteins from Bacillus thuringiensis subsp. kurstaki and subsp. tenebrionis on clay minerals. Soil Biol. Biochem. 1994;26:663–679. doi: 10.1016/0038-0717(94)90258-5. [DOI] [Google Scholar]
- 26.Ohba M., Shisa N., Thaithanun S., Nakashima K., Lee D.-H., Ohgushi A., Wasano N. A unique feature of Bacillus thuringiensis H-serotype flora in soils of a volcanic island of Japan. J. Gen. Appl. Microbiol. 2002;48:233–235. doi: 10.2323/jgam.48.233. [DOI] [PubMed] [Google Scholar]
- 27.Konecka E., Baranek J., Hrycak A., Kaznowski A. Insecticidal activity of Bacillus thuringiensis strains isolated from soil and water. Sci. World J. 2012;2012:1–5. doi: 10.1100/2012/710501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monnerat R., Martins E., Queiroz P., Ordúz S., Jaramillo G., Benintende G., Cozzi J., Real M.D., Martinez-Ramirez A., Rausell C., et al. Genetic variability of Spodoptera. frugiperda Smith (Lepidoptera: Noctuidae) populations from Latin America is associated with variations in susceptibility to Bacillus thuringiensis cry toxins. Appl. Environ. Microbiol. 2006;72:7029–7035. doi: 10.1128/AEM.01454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidi V., Patocchi N., Lüthy P., Tonolla M. Distribution of Bacillus thuringiensis subsp. israelensis in soil of a Swiss Wetland reserve after 22 years of mosquito control. Appl. Environ. Microbiol. 2011;77:3663–3668. doi: 10.1128/AEM.00132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petras S.F., Casida L.E., Jr. Survival of Bacillus thuringiensis spores in soil. Appl. Environ. Microbiol. 1985;50:1496–1501. doi: 10.1128/aem.50.6.1496-1501.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen J.C., Damgaard P.H., Eilenberg J., Hansen B.M. Dispersal of Bacillus thuringiensis var. kurstaki in an experimental cabbage field. Can. J. Microbiol. 1995;41:118–125. doi: 10.1139/m95-016. [DOI] [Google Scholar]
- 32.Akiba Y. Assessment of rainwater-mediated dispersion of field-sprayed Bacillus thuringiensis in the soil. Appl. Entomol. Zool. 1991;26:477–483. [Google Scholar]
- 33.Wilcks A., Smidt L., Bahl M.I., Hansen B.M., Andrup L., Hendriksen N.B., Licht T.R. Germination and conjugation of Bacillus thuringiensis subsp. israelensis in the intestine of gnotobiotic rats. J. Appl. Microbiol. 2008;104:1252–1259. doi: 10.1111/j.1365-2672.2007.03657.x. [DOI] [PubMed] [Google Scholar]
- 34.Bizzarri M.F., Bishop A.H. The Ecology of Bacillus thuringiensis on the phylloplane: Colonization from soil, plasmid transfer, and interaction with larvae of Pieris brassica. Microb. Ecol. 2008;56:133–139. doi: 10.1007/s00248-007-9331-1. [DOI] [PubMed] [Google Scholar]
- 35.Ammons D.R., Reyna A., Granados J.C., Samlal M.S., Rampersad J.N. An investigation of Bacillus thuringiensis in rectal-collected fecal samples of cows. Curr. Microbiol. 2009;59:532–536. doi: 10.1007/s00284-009-9472-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Peng Y., Wu S., Sun L., Huang E., Huang T., Xu L., Wu C., Gelbic I., Guan X. Microbial ecology and association of Bacillus thuringiensis in chicken feces originating from feed. Curr. Microbiol. 2012;65:784–791. doi: 10.1007/s00284-012-0231-3. [DOI] [PubMed] [Google Scholar]
- 37.Naryanan M.S. MSc Thesis. University of Oxford; Oxford, UK: 2006. Competitive Ability and Host Exploitation in Bacillus thuringiensis. [Google Scholar]
- 38.Dubois T., Faegri K., Perchat S., Lemy C., Buisson C., Nielsen-LeRoux C., Gohar M., Jacques P., Ramarao N., Kolstø A.-B., et al. Necrotrophism is a quorum-sensing-regulated lifestyle in Bacillus thuringiensis. PLoS Pathog. 2012;8:e1002629. doi: 10.1371/journal.ppat.1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichimatsu T., Mizuki E., Nishimura K., Akao T., Saitoh H., Higuchi K., Ohba M. Occurrence of Bacillus thuringiensis in fresh waters of Japan. Curr. Microbiol. 2000;40:217–220. doi: 10.1007/s002849910044. [DOI] [PubMed] [Google Scholar]
- 40.Lachhab K., Tyagi R.D., Valéro J.R. Production of Bacillus thuringiensis biopesticides using wastewater sludge as a raw material: Effect of inoculum and sludge solids concentration. Process Biochem. 2001;37:197–208. doi: 10.1016/S0032-9592(01)00198-4. [DOI] [Google Scholar]
- 41.Maheswaran S., Sreeramanan S., Josephine C.M.R., Marimuthu K., Xavier R. Occurrence of Bacillus thuringiensis in faeces of herbivorous farm animals. Afr. J. Biotechnol. 2010;9:8013–8019. [Google Scholar]
- 42.Maduell P., Armengol G., Llagostera M., Orduz S., Lindow S. B. thuringiensis is a poor colonist of leaf surfaces. Microb. Ecol. 2008;55:212–219. doi: 10.1007/s00248-007-9268-4. [DOI] [PubMed] [Google Scholar]
- 43.Prabhakar A., Bishop A.H. Effect of Bacillus thuringiensis naturally colonising Brassica. campestris var. chinensis leaves on neonate larvae of Pieris. brassicae. J. Invertebr. Pathol. 2009;100:193–194. doi: 10.1016/j.jip.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez-Sánchez C., Sittenfeld A., Janzen D.H., Espinoza A.M. Bacillus thuringiensis in caterpillars and associated materials collected from protected tropical forests in northwestern Costa Rica. Rev. Biol. Trop. 2005;54:265–271. doi: 10.15517/rbt.v54i2.13867. [DOI] [PubMed] [Google Scholar]
- 45.Bora R.S., Murty M.G., Shenbagarathai R., Sekar V. Introduction of a Lepidopteran-specific insecticidal crystal protein gene of Bacillus thuringiensis subsp. kurstaki by conjugal transfer into a Bacillus megaterium strain that persists in the cotton phyllosphere. Appl. Environ. Microbiol. 1994;60:214–222. doi: 10.1128/aem.60.1.214-222.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devi V.S., Rao P.A., Sharma S.P., Sharma H.C. Interaction of acid exudates in chickpea with biological activity of Bacillus thuringiensis towards Helicoverpa. armigera. J. Appl. Entomol. 2013 doi: 10.1111/jen.12056. [DOI] [Google Scholar]
- 47.Rabinovitch L., Fátima C., Cavados G., Chaves J.Q., Silva K.R.A., Seldin L. A new strain of Bacillus thuringiensis serovar israelensis very active against Blackfly larvae. Mem. Inst. Oswaldo Cruz. 1999;94:683–685. doi: 10.1590/S0074-02761999000500024. [DOI] [PubMed] [Google Scholar]
- 48.Halverson L.J., Clayton M.K., Handelsman J. Population biology of Bacillus cereus UW85 in the rhizosphere of field-grown soybeans. Soil Biol. Biochem. 1993;25:485–493. doi: 10.1016/0038-0717(93)90074-L. [DOI] [Google Scholar]
- 49.Bisht S.C., Mishra P.K. Ascending migration of endophytic Bacillus thuringiensis and assessment of benefits to different legumes of N.W. Himalayas. Eur. J. Soil Biol. 2013;56:56–64. doi: 10.1016/j.ejsobi.2013.02.004. [DOI] [Google Scholar]
- 50.Subrahmanyan P., Reddy M.N., Rao A.S. Exudation of certain organic compounds from seeds of groundnut. Seed Sci. Technol. 1983;11:267–272. [Google Scholar]
- 51.Chanway C.P. Bacterial endophytes: Ecological and practical implications. Sydowia. 1998;50:149–170. [Google Scholar]
- 52.Coombs J.T., Franco C.M.M. Isolation and identification of Actinobacteria from surface-sterilized wheat roots. Appl. Environ. Microbiol. 2003;69:5603–5608. doi: 10.1128/AEM.69.9.5603-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallmann J., Quadt-Hallmann A., Mahaffee W.F., Kloepper J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997;43:895–914. doi: 10.1139/m97-131. [DOI] [Google Scholar]
- 54.Rosenblueth M., Martinez-Romero E. Rhizobium. etli maize populations and their competitiveness for root colonization. Arch. Microbiol. 2004;181:337–344. doi: 10.1007/s00203-004-0661-9. [DOI] [PubMed] [Google Scholar]
- 55.James E.K., Olivares F.L., de Oliveira A.L.M., dos Reis F.B., da Silva L.G., Reis V.M. Further observations on the interaction between sugar cane and Gluconacetobacter diazotrophicus under laboratory and greenhouse conditions. J. Exp. Bot. 2001;52:747–760. doi: 10.1093/jexbot/52.357.747. [DOI] [PubMed] [Google Scholar]
- 56.Bacon C. Endophytic and biological control potential of Bacillus mojavensis and related species. Biol. Contr. 2002;23:274–284. doi: 10.1006/bcon.2001.1016. [DOI] [Google Scholar]
- 57.Benhamou N., Kloepper J.W., Tuzun S. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: Ultrastructure and cytochemistry of the host response. Planta. 1998;204:153–168. doi: 10.1007/s004250050242. [DOI] [Google Scholar]
- 58.Bent E., Chanway C.P. The growth-promoting effects of a bacterial endophyte on lodgepole pine are partially inhibited by the presence of other rhizobacteria. Can. J. Microbiol. 1998;44:980–988. doi: 10.1139/w98-097. [DOI] [Google Scholar]
- 59.Chanway C.P. Inoculation of tree roots with plant growth promoting soil bacteria: An emerging technology for reforestation. For. Sci. 1997;43:99–112. [Google Scholar]
- 60.Praça L.B., Gomes A.C.M.M., Cabral G., Martins E.S., Sujii E.R., Monnerat R.G. Endophytic colonization by brazilian strains of Bacillus thuringiensis on cabbage seedlings grown in vitro. Bt Res. 2012;3:11–19. [Google Scholar]
- 61.McInroy J.A., Kloepper J.W. Population dynamics of endophytic bacteria in field-grown sweet corn and cotton. Can. J. Microbiol. 1995;41:895–901. doi: 10.1139/m95-123. [DOI] [Google Scholar]
- 62.Suzuki M.T., Hernández-Rodríguez C.S., Araújo W.L., Ferré J. Characterization of an endophytic Bacillus thuringiensis strain isolated from sugar cane; Proceedings of 41st Annual Meeting of the Society for Invertebrate Pathology and 9th International Conference on Bacillus thuringiensis; University of Warwick, Coventry, UK. 3–7 August 2008. [Google Scholar]
- 63.Mishra P.K., Mishra S., Selvakumar G., Bisht J.K., Kundu S., Gupta H.S. Coinoculation of Bacillus thuringiensis-KR1 with Rhizobium. leguminosarum enhances plant growth and nodulation of pea (Pisum. sativum L.) and lentil (Lens culinaris L.) World J. Microbiol. Biotechnol. 2009;25:753–761. doi: 10.1007/s11274-009-9963-z. [DOI] [Google Scholar]
- 64.Ornellas R.M.S. MSc Thesis. State University of Santa Cruz; Ilhéus-BA, Brazil: 2011. Bioprospection of Rizobacteria for Beneficial Effects on ‘Yellow’ Passion Fruit (Passiflora Edulis) Seedlings. [Google Scholar]
- 65.Da Silva C.B. MSc Thesis. State University of Santa Cruz; Ilhéus-BA, Brazil: 2013. Holobionte Cacaueiro: Diversidade Genética da Porção Microbiana Associada a Frutos de Diferentes Clones de Theobroma cacao L. (in portuguese) [Google Scholar]
- 66.Estruch J.J., Warren G.W., Mullins M.A., Nye G.J., Craig J.A., Koziel M.G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA. 1996;93:5389–5394. doi: 10.1073/pnas.93.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCully E.M. Niches for bacterial endophytes in crop plants: A plant biologist’s view. Funct. Plant Biol. 2001;28:983–990. doi: 10.1071/PP01101. [DOI] [Google Scholar]
- 68.Griego V.M., Spence K.D. Inactivation of Bacillus thuringiensis spores by ultraviolet and visible light. Appl. Environ. Microbiol. 1978;35:906–910. doi: 10.1128/aem.35.5.906-910.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Behle R., Mcguire M.R., Shasha B.S. Effects of sunlight and simulated rain on residual activity of Bacillus thuringiensis formulations. J. Econ. Entomol. 1997;90:1560–1566. [Google Scholar]
- 70.Ruan L., Yu Z., Fang B., He W., Wang Y., Shen P. Melanin pigment formation and increased UV resistance in Bacillus thuringiensis following high temperature induction. Syst. Appl. Microbiol. 2004;27:286–289. doi: 10.1078/0723-2020-00265. [DOI] [PubMed] [Google Scholar]
- 71.Stewart C.N., Adang M.J., All J.N., Boerma H.R., Cardineau C., Tucker D., Parrott W.A. Genetic transformation, recovery, and characterization of fertile soybean transgenic for a synthetic Bacillus thuringiensis cry1Ac gene. Plant Physiol. 1996;112:121–129. doi: 10.1104/pp.112.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischhoff D.A., Bowdish K.S., Perlak F.J., Marrone P.G., McCormick S.M., Niedermeyer J.G., Dean D.A., Kusano-Kretzmer K., Mayer E.J., Rochester D.E., et al. Insect tolerant transgenic tomato plants. Nat. Biotechnol. 1987;5:807–813. doi: 10.1038/nbt0887-807. [DOI] [Google Scholar]
- 73.Vaeck M., Reynaerts A., Hofte H., Jansens S., Debeuckeleer M., Dean C., Zabeau M., Vanmontagu M., Leemans J. Transgenic plants protected from insect attack. Nature. 1987;328:33–37. doi: 10.1038/328033a0. [DOI] [Google Scholar]
- 74.Lampel J.S., Canter G.L., Dimock M.B., Kelly J.L., Anderson J.J., Uratani B.B., Foulke J.S., Turner J.T. Integrative cloning, expression, and stability of the cryIA(c) gene from Bacillus thuringiensis subsp. kurstaki in a recombinant strain of Clavibacter xyli subsp. cynodontis. Appl. Environ. Microbiol. 1994;60:501–508. doi: 10.1128/aem.60.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skøt L., Timms E., Mytton L. The effect of toxin-producing Rhizobium strains, on larvae of Sitona flavescens feeding on legume roots and nodules. Plant Soil. 1994;163:141–150. [Google Scholar]
- 76.Menon A.S., Mestral J. Survival of Bacillus thuringiensis var. kurstaki in waters. Water Air Soil Pollut. 1985;25:265–274. [Google Scholar]
- 77.De Amorim G.V., Whittome B., Shore B., Levin D.B. Identification of Bacillus thuringiensis subsp. kurstaki strain HD1-Like bacteria from environmental and human samples after aerial spraying of Victoria, British Columbia, Canada, with foray 48B. Appl. Environ. Microbiol. 2001;67:1035–1043. doi: 10.1128/AEM.67.3.1035-1043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boisvert M., Boisvert J. Persistence of toxic activity and recycling of Bacillus thuringiensis var. israelensis in cold water: Field experiments using diffusion chambers in a pond. Biocontrol. Sci. Technol. 1999;9:507–522. doi: 10.1080/09583159929479. [DOI] [Google Scholar]
- 79.Nguyen T.T., Su T., Mulla M.S. Mosquito control and bacterial flora in water enriched with organic matter and treated with Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus formulations. J. Vector Ecol. 1999;24:138–153. [PubMed] [Google Scholar]
- 80.Manasherob R., Ben-Dov E., Zaritsky A., Barak Z. Germination, growth, and sporulation of Bacillus thuringiensis subsp. israelensis in excreted food vacuoles of the protozoan Tetrahymena pyriformis. Appl. Environ. Microbiol. 1998;64:1750–1758. doi: 10.1128/aem.64.5.1750-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kweon C., Choi S., Kwon H., Kim E., Kang H., Moon J., Jang G., Lee H., Kang S., Kim J., et al. Isolation, characterization, and evaluation of Bacillus thuringiensis isolated from cow milk. Korean J. Vet. Res. 2012;52:169–176. [Google Scholar]
- 82.Swiecicka I., Fiedoruk K., Bednarz G. The occurrence and properties of Bacillus thuringiensis isolated from free-living animals. Lett. Appl. Microbiol. 2002;34:194–198. doi: 10.1046/j.1472-765x.2002.01070.x. [DOI] [PubMed] [Google Scholar]
- 83.Peterson J.W. Bacterial pathogenesis. In: Baron S., editor. Medical Microbiology. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. [PubMed] [Google Scholar]
- 84.Rohmer L., Hocquet D., Miller M.I. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Ecol. Evol. 2012;19:341–348. doi: 10.1016/j.tim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanada Y., Kaya H.K. Insect Pathology. Academic Press; San Diego, CA, USA: 1993. [Google Scholar]
- 86.Johnston P.R., Crickmore N. Gut bacteria not required for Bacillus thuringiensis insecticidal activity towards the tobacco hornworm, Manduca sexta. Appl. Environ. Microbiol. 2009;75:5094–5099. doi: 10.1128/AEM.00966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cerstiaens A., Verleyen P., Rie J.V.A.N., Kerkhove E.V.A.N., Schwartz J., Laprade R., Loof A.D.E., Schoofs L. Effect of Bacillus thuringiensis Cry1 toxins in insect hemolymph and their neurotoxicity in brain cells of Lymantria dispar. Appl. Environ. Microbiol. 2001;67:3923–3927. doi: 10.1128/AEM.67.9.3923-3927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Argôlo Filho R.C., Gomes R.A., Barreto M.R., Lana U.G.P., Valicente F.H., Loguercio L.L. Growth variation among Bacillus thuringiensis strains can affect screening procedures for supernatant-secreted toxins against insect pests. Pest Manag. Sci. 2011;67:1184–1192. doi: 10.1002/ps.2206. [DOI] [PubMed] [Google Scholar]
- 89.Loguercio L.L., Santos C.G., Barreto M.R., Guimaraes C.T., Paiva E. Association of PCR and feeding bioassays as a large-scale method to screen tropical Bacillus thuringiensis isolates for a cry constitution with higher insecticidal effect against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Lett. Appl. Microbiol. 2001;32:362–367. doi: 10.1046/j.1472-765X.2001.00920.x. [DOI] [PubMed] [Google Scholar]
- 90.Loguercio L.L., Barreto M.L., Rocha T.L., Santos C.G., Teixeira F.F., Paiva E. Combined analysis of supernatant-based feeding bioassays and PCR as a first-tier screening strategy for Vip-derived activities in Bacillus thuringiensis strains effective against tropical fall armyworm. J. Appl. Microbiol. 2002;93:269–277. doi: 10.1046/j.1365-2672.2002.01694.x. [DOI] [PubMed] [Google Scholar]
- 91.Ohba M., Wasano N., Mizuki E. Bacillus thuringiensis soil populations naturally occurring in the Ryukyus, a subtropic region of Japan. Microbiol. Res. 2000;155:17–22. doi: 10.1016/S0944-5013(00)80017-8. [DOI] [PubMed] [Google Scholar]
- 92.Vallet-gely I., Lemaitre B., Boccard F. Bacterial strategies to overcome insect defences. Nat. Rev. Microbiol. 2008;6:302–313. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- 93.Fedhila S., Guillemet E., Nel P., Lereclus D. Characterization of two Bacillus thuringiensis genes identified by in vivo screening of virulence factors. J. Bacteriol. 2004;70:4784–4791. doi: 10.1128/AEM.70.8.4784-4791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dalhammar G., Steiner H. Characterization of inhibitor A, a protease from Bacillus thuringiensis which degrades attacins and cecropins, two classes of antibacterial proteins in insects. FEBS J. 1984;139:247–252. doi: 10.1111/j.1432-1033.1984.tb08000.x. [DOI] [PubMed] [Google Scholar]
- 95.Raymond B., Lijek R.S., Griffiths R.I., Bonsall M.B. Ecological consequences of ingestion of Bacillus cereus on Bacillus thuringiensis infections and on the gut flora of a lepidopteran host. J. Invertebr. Pathol. 2008;99:103–111. doi: 10.1016/j.jip.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 96.Cherif A., Rezgui W., Raddadi N., Daffonchio D., Boudabous A. Characterization and partial purification of entomocin 110, a newly identified bacteriocin from Bacillus thuringiensis subsp. entomocidus HD110. Microbiol. Res. 2008;163:684–692. doi: 10.1016/j.micres.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Wabiko H., Yasuda E. Bacillus thuringiensis protoxin: Location of toxic border and requirement of non-toxic domain for high-level in vivo production of active toxin. Microbiology. 1995;141:629–639. doi: 10.1099/13500872-141-3-629. [DOI] [PubMed] [Google Scholar]
- 98.Volwerk J.J., Koke J.A., Wetherwax P.B., Griffith O.H. Functional characteristics of phosphatidylinositol-specific phospholipases C from Bacillus cereus and Bacillus thuringiensis. FEMS Microbiol. Lett. 1989;52:237–241. doi: 10.1016/0378-1097(89)90203-6. [DOI] [PubMed] [Google Scholar]
- 99.Sampson M.N., Gooday G.W. Involvement of chitinases of Bacillus thuringiensis during pathogenesis in insects. Microbiology. 1998;144:2189–2194. doi: 10.1099/00221287-144-8-2189. [DOI] [PubMed] [Google Scholar]
- 100.Fedhila S., Nel P., Lereclus D. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 2002;184:3296–3304. doi: 10.1128/JB.184.12.3296-3304.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fortier M., Vachon V., Frutos R., Schwartz J.-L., Laprade R. Effect of insect larval midgut proteases on the activity of Bacillus thuringiensis Cry toxins. Appl. Environ. Microbiol. 2007;73:6208–6213. doi: 10.1128/AEM.01188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tran S.-L., Guillemet E., Lereclus D., Ramarao N. Iron regulates Bacillus thuringiensis haemolysin hlyII gene expression during insect infection. J. Invertebr. Pathol. 2013;113:205–208. doi: 10.1016/j.jip.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 103.Luo X., Chen L., Huang Q., Zheng J., Zhou W., Peng D., Ruan L., Sun M. Bacillus thuringiensis metalloproteinase Bmp1 functions as a nematicidal virulence factor. Appl. Environ. Microbiol. 2013;79:460–468. doi: 10.1128/AEM.02551-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramarao N., Lereclus D. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell. Microbiol. 2005;7:1357–1364. doi: 10.1111/j.1462-5822.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 105.Dillon R.J., Dillon V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 106.Wells E.V., Boulton M., Hall W., Bidol S.A. Reptile-associated salmonellosis in preschool-aged children in Michigan, January 2001–June 2003. Clin. Infect. Dis. 2004;39:687–691. doi: 10.1086/423002. [DOI] [PubMed] [Google Scholar]
- 107.Broderick N., Raffa K.F., Handelsman J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. USA. 2006;103:15196–15199. doi: 10.1073/pnas.0604865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Broderick N., Robinson C.J., McMahon M.D., Holt J., Handelsman J., Raffa K.F. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 2009;7:1–9. doi: 10.1186/1741-7007-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jarosz J. Gut flora of Galleria mellonella suppressing ingested bacteria. J. Invertebr. Pathol. 1979;34:192–198. doi: 10.1016/0022-2011(79)90101-0. [DOI] [PubMed] [Google Scholar]
- 110.Raymond B., Elliot S.L., Ellis R.J. Quantifying the reproduction of Bacillus thuringiensis HD1 in cadavers and live larvae of Plutella xylostella. J. Invertebr. Pathol. 2008;98:307–313. doi: 10.1016/j.jip.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 111.Guerchicoff A., Dele A. The Bacillus thuringiensis cyt genes for hemolytic endotoxins constitute a gene family. Appl. Environ. Microbiol. 2001;67:1090–1096. doi: 10.1128/AEM.67.3.1090-1096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bravo A., Gill S.S., Soberón M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kostichka K., Warren G.W., Mullins M., Mullins A.D., Palekar N.V., Craig J.A., Koziel M.G., Estruch J.J. Cloning of a cryV-type insecticidal protein gene from Bacillus thuringiensis: The CryV-encoded protein is expressed early in stationary phase. J. Bacteriol. 1996;178:2141–2144. doi: 10.1128/jb.178.7.2141-2144.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song F., Zhang J., Gu A., Wu Y., Han L., He K., Chen Z., Yao J., Hu Y., Li G., et al. Identification of cry1I-type genes from Bacillus thuringiensis strains and characterization of a novel cry1I-type gene. Appl. Environ. Microbiol. 2003;69:5207–5211. doi: 10.1128/AEM.69.9.5207-5211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li J.D., Carroll J., Ellar D.J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991;353:815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- 116.Vachon V., Laprade R., Schwartz J. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: A critical review. J. Invertebr. Pathol. 2012;111:1–12. doi: 10.1016/j.jip.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 117.Koller C.N., Bauer L.S., Hollingworth R.M. Characterization of the pH-mediated solubility of Bacillus thuringiensis var. san diego native delta-endotoxin crystals. Biochem. Biophys. Res. Commun. 1992;184:692–699. doi: 10.1016/0006-291X(92)90645-2. [DOI] [PubMed] [Google Scholar]
- 118.Soberón M., Gill S.S., Bravo A. Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cell. Mol. Life Sci. 2009;66:1337–1349. doi: 10.1007/s00018-008-8330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang X., Candas M., Griko N.B. Cytotoxicity of Bacillus thuringiensis Cry1Ab toxin depends on specific binding of the toxin to the cadherin receptor BT-R1 expressed in insect cells. Cell Death Differ. 2005;12:1407–1416. doi: 10.1038/sj.cdd.4401675. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X., Candas M., Griko N.B., Taussig R., Bulla L.A. A mechanism of cell death involving an adenylyl cyclase PKA signali ng pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA. 2006;103:9897–9902. doi: 10.1073/pnas.0604017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Glare T.R., O’Callaghan M. Bacillus thuringiensis: Biology, Ecology and Safety. John Wiley; Chichester, UK: 2000. [Google Scholar]
- 122.Butko P. Cytolytic toxin Cyt1A and its mechanism of membrane damage: Data and hypotheses. Appl. Environ. Microbiol. 2003;69:2415–2422. doi: 10.1128/AEM.69.5.2415-2422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parker M.W., Feil S.C. Pore-forming protein toxins: From structure to function. Prog. Biophys. Mol. Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 124.Sayyed A.L.I.H., Crickmore N., Wright D.J. Cyt1Aa from Bacillus thuringiensis subsp. israelensis is toxic to the diamondback moth, Plutella xylostella, and synergizes the activity of Cry1Ac towards a resistant strain. Appl. Environ. Microbiol. 2001;67:5859–5861. doi: 10.1128/AEM.67.12.5859-5861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oestergaard J., Ehlers R., Mart A.C., Real M.D. Binding of Cyt1Aa and Cry11Aa toxins of Bacillus thuringiensis serovar israelensis to brush border membrane vesicles of Tipula paludosa (Diptera: Nematocera) and subsequent pore formation. Appl. Environ. Microbiol. 2007;73:3623–3629. doi: 10.1128/AEM.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pérez C., Fernandez L.E., Sun J., Folch J.L., Gill S.S., Soberón M., Bravo A. Bacillus thuringiensis subsp. israelensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc. Natl. Acad. Sci. USA. 2005;102:18303–18308. doi: 10.1073/pnas.0505494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rincón-Castro M.C., Barajas-Huerta J., Ibarra J.E. Antagonism between Cry1Ac1 and Cyt1A1 toxins of Bacillus thuringiensis. Appl. Environ. Microbiol. 1999;65:2049–2053. doi: 10.1128/aem.65.5.2049-2053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu C.G., Mullins M.A., Warren G.W., Koziel M.G., Estruch J.J. The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Appl. Environ. Microbiol. 1997;63:532–536. doi: 10.1128/aem.63.2.532-536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rice W.C. Specific primers for the detection of vip3A insecticidal gene within a Bacillus thuringiensis collection. Lett. Appl. Microbiol. 1999;28:378–382. doi: 10.1046/j.1365-2672.1999.00536.x. [DOI] [Google Scholar]
- 130.Lee M.K., Walters F.S., Hart H., Palekar N., Chen J. The Mode of Action of the Bacillus thuringiensis Vegetative Insecticidal Protein Vip3A differs from that of Cry1Ab d-endotoxin. Appl. Environ. Microbiol. 2003;69:4648–4657. doi: 10.1128/AEM.69.8.4648-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hernández-Rodríguez C.S., Boets A., van Rie J., Ferré J. Screening and identification of vip genes in Bacillus thuringiensis strains. J. Appl. Microbiol. 2009;107:219–225. doi: 10.1111/j.1365-2672.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 132.Crickmore N., Baum J., Bravo A., Lereclus D., Narva K., Sampson K., Schnepf E., Sun M., Zeigler D.R. Bacillus thuringiensis toxin nomenclature. [(accessed on 20 October 2013)]. Available online: http://www.btnomenclature.info/
- 133.Warren W. Vegetative insecticidal proteins: Novel proteins for control of corn pests. In: Carozzi N.B., Koziel M., editors. Advances in Insect Control, the Role of Transgenic Plants. Taylors & Francis Ltd.; London, UK: 1997. [Google Scholar]
- 134.Barth H., Aktories K., Popoff M.R., Stiles B.G. Binary bacterial toxins: Biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol. Mol. Biol. Rev. 2004;68:373–402. doi: 10.1128/MMBR.68.3.373-402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peng D., Chen S., Ruan L., Li L., Yu Z., Sun M. Safety assessment of transgenic Bacillus thuringiensis with Vip insecticidal protein gene by feeding studies. Food Chem. Toxicol. 2007;45:1179–1185. doi: 10.1016/j.fct.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 136.Callegan M.C., Cochran D.C., Kane S.T., Gilmore M.S., Gominet M., Lereclus D. Contribution of membrane-damaging toxins to Bacillus endophthalmitis pathogenesis. Infect. Immun. 2002;70:5381–5389. doi: 10.1128/IAI.70.10.5381-5389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ikezawa H., Nakabayashi T., Suzuki K., Nakajima M., Taguchi T., Taguchi R. Complete purification of phosphatidylinositol-specific phospholipase C from a strain of Bacillus thuringiensis. J. Biochem. 1983;93:1717–1719. doi: 10.1093/oxfordjournals.jbchem.a134315. [DOI] [PubMed] [Google Scholar]
- 138.Hergenrother P.J., Martin S.F. Determination of the kinetic parameters for phospholipase C (Bacillus cereus) on different phospholipid substrates using a chromogenic assay based on the quantitation of inorganic phosphate. Anal. Biochem. 1997;251:45–49. doi: 10.1006/abio.1997.2251. [DOI] [PubMed] [Google Scholar]
- 139.Krieg A. Concerning alpha-exotoxin produced by vegetative cells of Bacillus thuringiensis and Bacillus cereus. J. Invertebr. Pathol. 1971;1:134–135. doi: 10.1016/0022-2011(71)90137-6. [DOI] [Google Scholar]
- 140.Faust R.M., Bulla A.L., Jr. Bacterial and their toxins as insecticides. In: Kurstaki E., editor. Microbial and Viral Pesticides. Marcel Dekker Inc.; Nova York, NY, USA: 1982. [Google Scholar]
- 141.Arora N., Ahmad T., Rajagopal R., Bhatnagar R.K. A constitutively expressed 36 kDa exochitinase from Bacillus thuringiensis HD-1. Biochem. Biophys. Res. Commun. 2003;307:620–625. doi: 10.1016/S0006-291X(03)01228-2. [DOI] [PubMed] [Google Scholar]
- 142.Regev A., Keller M., Strizhov N., Sneh B., Prudovsky E., Chet I., Ginzberg I., Koncz-Kalman Z., Koncz C., Schell J., et al. Synergistic activity of a Bacillus thuringiensis delta-endotoxin and a bacterial endochitinase against Spodoptera littoralis larvae. Appl. Environ. Microbiol. 1996;62:3581–3586. doi: 10.1128/aem.62.10.3581-3586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tantimavanich S., Pantuwatana S., Bhumiratana A., Panbangred W. Cloning of a chitinase gene into Bacillus thuringiensis subsp. aizawai for enhanced insecticidal activity. J. Gen. Appl. Microbiol. 1997;347:341–347. doi: 10.2323/jgam.43.341. [DOI] [PubMed] [Google Scholar]
- 144.Li E., Yousten A.A. Metalloprotease from Bacillus thuringiensis. Appl. Microbiol. 1975;30:354–361. doi: 10.1128/am.30.3.354-361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andrews R.E., Bibilos M.M., Bulla L.A. Protease activation of the entomocidal protoxin of Bacillus thuringiensis subsp. kurstaki. Appl. Environ. Microbiol. 1985;50:737–742. doi: 10.1128/aem.50.4.737-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guillemet E., Cadot C., Tran S.-L., Guinebretière M.-H., Lereclus D., Ramarao N. The InhA Metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 2010;192:286–294. doi: 10.1128/JB.00264-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donovan W.P., Engleman J.T., Donovan J.C., Baum J.A., Bunkers G.J., Chi D.J., Clinton W.P., English L., Heck G.R., Ilagan O.M., et al. Discovery and characterization of Sip1A: A novel secreted protein from Bacillus thuringiensis with activity against coleopteran larvae. Appl. Microbiol. Biot. 2006;72:713–719. doi: 10.1007/s00253-006-0332-7. [DOI] [PubMed] [Google Scholar]
- 148.Farkas J., Sebesta K., Horská K., Samek K., Dolejs L., Sorm F. The structure of exotoxin of Bacillus thuringiensis var. gelechiae. Collect. Czech. Chem. Commun. 1968;34:1118–1120. [Google Scholar]
- 149.Beebee T., Korner A., Bond R.P.M. Differential inhibition of mammalian ribonucleic acid polymerases by an exotoxin from Bacillus thuringiensis. The direct observation of nucleoplasmic ribonucleic acid polymerase activity in intact nuclei. Biochem. J. 1972;127:619–624. doi: 10.1042/bj1270619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.World Health Organization-WHO . Microbial Pest Control Agent: Bacillus thuringiensis. WHO; Geneva, Switzerland: 1999. p. 125. [Google Scholar]
- 151.Calberg G. Bacillus thuringiensis and microbial control of flies. MIRCEN J. Appl. Microb. Biotechnol. 1986;2:267–274. doi: 10.1007/BF00933492. [DOI] [Google Scholar]
- 152.Ohba M., Tantichodok A., Aizawa K. Production of heat-stable exotoxin by Bacillus thuringiensis and related bacteria. J. Invertebr. Pathol. 1981;38:26–32. doi: 10.1016/0022-2011(81)90030-6. [DOI] [Google Scholar]
- 153.Levinson B.L., Kasyan K.J., Chiu S.U.E.S., Currier T.C., González J.M., Jr. Identification of b-exotoxin production, plasmid encoding, and a new exotoxin in Bacillus thuringiensis by using high-performance liquid chromatograpy. J. Bacteriol. 1990;172:3172–3179. doi: 10.1128/jb.172.6.3172-3179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Domingos J.B., Longhinotti E., Gageiro V., Nome F. A química dos ésteres de fosfato. Quim. Nova. 2003;26:745–753. doi: 10.1590/S0100-40422003000500019. [DOI] [Google Scholar]
- 155.Espinasse S., Gohar M., Lereclus D., Sanchis V. An extracytoplasmic-function sigma factor is involved in a pathway controlling b-exotoxin I production in Bacillus thuringiensis subsp. thuringiensis strain 407–1. J. Bacteriol. 2004;186:3108–3116. doi: 10.1128/JB.186.10.3108-3116.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hassanain M.A., el Garhy M.F., Abdel-Ghaffar F.A., el-Sharaby A., Abdel Megeed K.N. Biological control studies of soft and hard ticks in Egypt: The effect of Bacillus thuringiensis varieties on soft and hard ticks (ixodidae) Parasitol. Res. 1997;83:209–213. doi: 10.1007/s004360050235. [DOI] [PubMed] [Google Scholar]
- 157.Zhioua E., Heyer K., Browning M., Ginsberg H.S., LeBrun R.A. Pathogenicity of Bacillus thuringiensis variety kurstaki to Ixodes scapularis (Acari: Ixodidae) J. Med. Entomol. 1999;36:900–902. doi: 10.1093/jmedent/36.6.900. [DOI] [PubMed] [Google Scholar]
- 158.El-Kelesh E.A.M., El-Refaii M.A.H. Insecticidal effect of Bacillus thuringiensis var. kurstaki against Hyalomma dromedarii on experimentally infested rabbits. Egypt. J. Agric. Res. 2006;83:993. [Google Scholar]
- 159.Fernández-Ruvalcaba M., Peña-Chora G., Romo-Martínez A., Hernández-Velázquez V., de La Parra A.B., de La Rosa D.P. Evaluation of Bacillus thuringiensis pathogenicity for a strain of the tick, Rhipicephalus microplus, resistant to chemical pesticides. J. Insect Sci. 2010;10:1–6. doi: 10.1673/031.010.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Habeeb S.M., El-hag H.A.A. Ultrastructural changes in hemocyte cells of hard tick (Hyalomma dromedarii: Ixodidae): A model of Bacillus thuringiensis var. thuringiensis H14 d-endotoxin mode of action. Am. Euras. J. Agric. Environ. Sci. 2008;3:829–836. [Google Scholar]
- 161.Erban T., Nesvorna M., Erbanova M., Hubert J. Bacillus thuringiensis var. tenebrionis control of synanthropic mites (Acari: Acaridida) under laboratory conditions. Exp. Appl. Acarol. 2009;49:339–346. doi: 10.1007/s10493-009-9265-z. [DOI] [PubMed] [Google Scholar]
- 162.Payne J., Cannon R.J.C., Ralph A.L. Bacillus thuringiensis Isolates for Controlling Acarides. 5350576A. US Patent. 1994 Sep 27;
- 163.Wei J.-Z., Hale K., Carta L., Platzer E., Wong C., Fang S.-C., Aroian R.V. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. USA. 2003;100:2760–2765. doi: 10.1073/pnas.0538072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mohammed S.H., El Saedy M.A.E., Enan M.R., Ibrahim N.E., Ghareeb A., Moustafa A. Biocontrol efficiency of Bacillus thuringiensis toxins against root-knot nematode, Meloidogyne incognita. J. Cell Mol. Biol. 2008;7:57–66. [Google Scholar]
- 165.Thompson M., Gaertner F.H. Bacillus thuringiensis Isolate having Anti-Protozoan Activity. 461799A2. European Patent. 1991 Dec 28;
- 166.Kondo S., Mizuki E., Akao T., Ohba M. Antitrichomonal strains of Bacillus thuringiensis. Parasitol. Res. 2002;88:1090–1092. doi: 10.1007/s00436-002-0692-6. [DOI] [PubMed] [Google Scholar]
- 167.Mahillon J., Rezsöhazy R., Hallet B., Delcour J. IS231 and other Bacillus thuringiensis transposable elements: A review. Genetica. 1994;93:13–26. doi: 10.1007/BF01435236. [DOI] [PubMed] [Google Scholar]
- 168.Weisdorf J.L. From foraging to farming: Explaining the neolithic revolution. J. Econ. Surv. 2005;19:561–586. doi: 10.1111/j.0950-0804.2005.00259.x. [DOI] [Google Scholar]
- 169.Loguercio L.L., Carneiro N.P., Carneiro A.A. Milho Bt: Alternativa biotecnológica para controle biológico de insetos-praga. Biotecnolog. Ciênc. Desenvolv. 2002;24:46–52. (in portuguese) [Google Scholar]
- 170.Bowden S.E., Drake J.M. Ecology of multi-host pathogens of animals. Nat. Educ. Knowl. 2013;4:5. [Google Scholar]
- 171.Pepin K.M., Lass S., Pulliam J.R.C., Read A.F., Lloyd-Smith J.O. Identifying genetic markers of adaptation for surveillance of viral host jumps. Nat. Rev. Microbiol. 2010;8:802–813. doi: 10.1038/nrmicro2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Garamszegi L.Z. Patterns of co-speciation and host switching in primate malaria parasites. Malar. J. 2009;8:1–15. doi: 10.1186/1475-2875-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peeler E.J., Oidtmann B.C., Midtlyng P.J., Miossec L., Gozlan R.E. Non-native aquatic animals introductions have driven disease emergence in Europe. Biol. Invasions. 2011;13:1291–1303. doi: 10.1007/s10530-010-9890-9. [DOI] [Google Scholar]
- 174.Mizuki E., Park Y.S., Saitoh H., Yamashita S., Akao T., Higuchi K., Ohba M. Parasporin, a human leukemic cell-recognizing parasporal protein of Bacillus thuringiensis. Clin. Diagn. Lab. Immun. 2000;7:625–634. doi: 10.1128/cdli.7.4.625-634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mizuki E., Ohba M., Akao T., Yamashita S., Saitoh H., Park Y.S. Unique activity associated with non-insecticidal Bacillus thuringiensis parasporal inclusions: In vitro cell-killing action on human cancer cells. J. Appl. Microbiol. 1999;86:477–486. doi: 10.1046/j.1365-2672.1999.00692.x. [DOI] [PubMed] [Google Scholar]
- 176.Ito A., Sasaguri Y., Kitada S., Kusaka Y., Kuwano K., Masutomi K., Mizuki E., Akao T., Ohba M. A Bacillus thuringiensis crystal protein with selective cytocidal action to human cells. J. Biol. Chem. 2004;279:21282–21286. doi: 10.1074/jbc.M401881200. [DOI] [PubMed] [Google Scholar]
- 177.Katayama H., Kusaka Y., Yokota H., Akao T., Kojima M., Nakamura O., Mekada E., Mizuki E. Parasporin-1, a novel cytotoxic protein from Bacillus thuringiensis, induces Ca2+ influx and a sustained elevation of the cytoplasmic Ca2+ concentration in toxin-sensitive cells. J. Biol. Chem. 2007;282:7742–7752. doi: 10.1074/jbc.M611382200. [DOI] [PubMed] [Google Scholar]
- 178.Yamashita S., Katayama H., Saitoh H., Akao T., Park Y.S., Mizuki E., Ohba M., Ito A. Typical three-domain cry proteins of Bacillus thuringiensis strain A1462 exhibit cytocidal activity on limited human cancer cells. J. Biochem. 2005;138:663–672. doi: 10.1093/jb/mvi177. [DOI] [PubMed] [Google Scholar]
- 179.Okumura S., Saitoh H., Ishikawa T., Wasano N., Yamashita S., Kusumoto K.-I., Akao T., Mizuki E., Ohba M., Inouye K. Identification of a novel cytotoxic protein, Cry45Aa, from Bacillus thuringiensis A1470 and its selective cytotoxic activity against various mammalian cell lines. J. Agric. Food Chem. 2005;53:6313–6318. doi: 10.1021/jf0506129. [DOI] [PubMed] [Google Scholar]
- 180.Kitada S., Abe Y., Shimada H., Kusaka Y., Matsuo Y., Katayama H., Okumura S., Akao T., Mizuki E., Kuge O., et al. Cytocidal actions of parasporin-2, an anti-tumor crystal toxin from Bacillus thuringiensis. J. Biol. Chem. 2006;281:26350–26360. doi: 10.1074/jbc.M602589200. [DOI] [PubMed] [Google Scholar]
- 181.Abe Y., Shimada H., Kitada S. Raft-targeting and oligomerization of Parasporin-2, a Bacillus thuringiensis crystal protein with anti-tumour activity. J. Biochem. 2008;143:269–275. doi: 10.1093/jb/mvm220. [DOI] [PubMed] [Google Scholar]
- 182.Fivaz M., Abrami L., Tsitrin Y., van der Goot F.G. Aerolysin from Aeromonas. hydrophila and related toxins. Curr. Top. Microbiol. Immunol. 2001;257:35–52. doi: 10.1007/978-3-642-56508-3_3. [DOI] [PubMed] [Google Scholar]
- 183.Galinier R., Portela J., Moné Y., Allienne J.F., Henri H., Delbecq S., Mitta G., Gourbal B., Duval D. Biomphalysin, a new β pore-forming toxin involved in Biomphalaria glabrata immune defense against Schistosoma mansoni. PLoS Pathog. 2013;9:e1003216. doi: 10.1371/journal.ppat.1003216. [DOI] [PMC free article] [PubMed] [Google Scholar]