Abstract
Purpose of review
Idiopathic pulmonary fibrosis (IPF) is a fatal disease with limited treatment options and extensive gene expression changes identified in the lung parenchyma. Multiple lines of evidence suggest that epigenetic factors contribute to dysregulation of gene expression in IPF lung. Most importantly, risk factors that predispose to IPF – age, gender, cigarette smoke, and genetic variants – all influence epigenetic marks. This review summarizes recent findings of association of DNA methylation and histone modifications with the presence of disease and fibroproliferation.
Recent findings
In addition to targeted studies focused on specific gene loci, genome-wide profiles of DNA methylation demonstrate widespread DNA methylation changes in IPF lung tissue and a substantial effect of these methylation changes on gene expression. Genetic loci that have been recently associated with IPF also contain differentially methylated regions, suggesting that genetic and epigenetic factors act in concert to dysregulate gene expression in IPF lung.
Summary
While we are in very early stages of understanding the role of epigenetics in IPF, the potential for the use of epigenetic marks as biomarkers and therapeutic targets is high and discoveries made in this field will likely bring us closer to better prognosticating and treating this fatal disease.
Keywords: DNA methylation, histone modifications, pulmonary fibrosis, gene regulation
Introduction
Idiopathic pulmonary fibrosis (IPF) is a late-age-of-onset lung disease with a median survival of only 3 years characterized by progressive scarring of the pulmonary parenchyma that leads to progressive loss of lung function with dyspnea and hypoxemia, ultimately resulting in respiratory failure and death. The prevalence of IPF is estimated at 63 individuals in 100,000 in the United States (1), with the prevalence and mortality in pulmonary fibrosis increasing as our population ages (2). Treatment options for IPF are limited to two recently approved drugs that slow down disease progression (3, 4). We are therefore in need of prevention and additional treatment strategies for this fatal lung disease.
The paradigm about disease pathogenesis has shifted from beliefs that IPF is a result of chronic inflammation (5) to the idea that it results from excessive, sequential injury and/or aberrant wound healing of the alveolar epithelium (6) and to more recent suggestions that the distal airway epithelium may also be important in disease development (7–9). It is likely that the disease process underlying the IPF phenotype is heterogeneous and many different cell types (10–12) and molecular processes may be involved (7, 8, 13–27). Regardless of the specific pathways that lead to fibrosis, the end stage result is usual interstitial pneumonia (UIP) histological pattern of heterogeneous, subpleural regions of fibrotic and remodeled lung (28).
Gene expression profiling studies have demonstrated that extensive transcriptional changes are present in the lung parenchyma of individuals with IPF (29–35). Gene expression changes are dramatic and consistently have identified genes associated with extracellular matrix (ECM) formation, degradation, and signaling; smooth muscle markers; growth factors; developmental pathways; and genes encoding immunoglobulins, complement, chemokines, and other host defense/innate immune genes. We also recently identified two molecular subtypes of IPF based on a strong gene expression signature of cilium-associated genes (11). Expression of MUC5B, the strongest and most replicated genetic risk factor for IPF (8, 36–41), is highly correlated to expression of cilium genes. This is also the case for MMP7, an extracellular matrix gene that has emerged as the main expression biomarker for IPF (30, 33, 42) and was recently shown to play a role in attenuating ciliated cell differentiation during wound repair (43). While gene expression studies in aggregate have been successful in identifying molecular processes that are dysregulated in IPF lung, we know much less about how expression of these genes is regulated at the transcriptional, post-transcriptional, and post-translational levels.
Introduction to Epigenetic Mechanisms
Epigenetic processes translate environmental exposures into regulation of chromatin, which shapes the identity, gene expression profile, and activity of specific cell types that participate in disease pathophysiology (44). They are emerging as key mechanisms that mediate the effects of both genetics and the environment on gene expression and disease (45, 46). Traditionally epigenetic processes refer to DNA methylation and histone modifications. Although noncoding RNAs are often considered a part of the epigenome, this review will focus only on DNA methylation and histone modifications.
Methylation of cytosine residues in CpG dinucleotides within the context of CpG islands is the simplest form of epigenetic regulation. DNA methyltransferases (de novo DNMT3A/B and maintenance DNMT1) are enzymes responsible for DNA methylation while the TET family of enzymes actively demethylate DNA though the 5-hydoxymethylcytosine intermediate (47). Traditional view of DNA methylation is that hypermethylation of CpG islands in gene promoters leads to gene silencing while hypomethylation leads to active transcription (48, 49) but we now know that methylation of less CpG dense regions near islands (‘CpG island shores’) (50, 51) and within gene bodies (52, 53) is also important in regulation of gene transcription and alternative splicing. While the canonical inverse relationships of methylation and expression are predominant in the genome, direct relationships also exist especially for methylation marks in gene bodies and for those associated with alternative splicing (53, 54).
Acetylation and methylation are the most common modifications of histone tails that occur at specific sites and residues, and control gene expression by regulating DNA accessibility to RNA polymerase II and transcription factors. Histone acetyltransferases (HATs) acetylate histone tails, histone deacetylases (HDACs) remove acetyl groups from histone tails, and bromodomain (Brd) proteins are chromatin readers that recognize and bind acetylated histones and play a key role in transmission of epigenetic memory across cell divisions and transcription regulation (55, 56). Similarly, histone methyltransferases (HMTs) add the methyl groups to histone tails while histone demethylases (HDMs) remove them (55, 56). A number of modifications at specific sites residues regulate chromatin accessibility (57, 58).
Epigenetics and IPF – the Potential Links
Given what we know about IPF and epigenetic marks, several lines of evidence support a critical role for control of gene expression in IPF lung by DNA methylation and histone modifications (Figure 1). Firstly, IPF is a disease of the elderly and changes in DNA methylation, histone modifications, and gene expression occur as we age (59–61). Genome-wide studies in aging cells and tissues have revealed the occurrence of stochastic changes in DNA methylation, also referred to as drift (62). Stochastic profibrotic DNA methylation drift could predispose to the development of the disease in susceptible individuals (63). Similarly, gender is known to play a role in tissue specific DNA methylation patterns (64–68) and being of male gender is a risk factor for development of IPF (1).
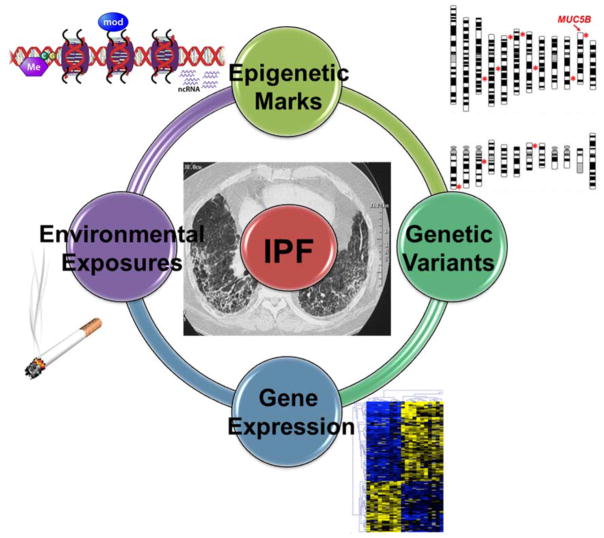
Figure 1.
An overview of epigenetic regulation of gene expression in IPF lung. Environmental exposures such as cigarette smoke and genetic variants are associated with changes in epigenetic marks which in turn are associated with altered gene expression. Because the causative nature of these associations has not been established, these molecular processes are drawn as a circle with no arrows. * symbols on the chromosome view plot indicate approximate location of IPF-associated genetic loci from the two published GWAS (Fingerlin. Nat Genet. 2013;45:613–20 and Noth. Lancet Resp Med. 2013;1:309–17).
Secondly, IPF is an environmental lung disease (69, 70). It is well established that environmental exposures strongly influence epigenetic marks (71). Cigarette smoke, the main environmental risk factor for IPF, has an influence on the methylome (72–74) and on methylation of specific promoters in genes involved in pathogenesis of IPF such as WNT7A(75). Recent work identified extensive genomic changes in DNA methylation in small airway epithelium (SAE) of smokers compared to smokers with corresponding modulation of gene expression (76). Other recent studies have shown how cigarette smoke influences histone modifications and chromatin accessibility (77–79).
Thirdly, IPF is also a genetic disease (38, 39, 80), and genetic factors also influence epigenetic marks. An individual’s genetic background influences epigenetic marks in two ways – by direct inheritance (imprinted loci) (81) and by genetic variants that segregate with disease exerting their effects through epigenetic modifications, such as the case of haplotype-specific methylation. In addition to investigation at specific loci, genomewide studies demonstrate a strong genetic component to inter-individual variation in methylation (82–84) and histone modification profiles (85–87).
Finally, epigenetic marks are crucial in lung development and aberrant recapitulation of the developmental program following injury is a hallmark feature of IPF (27). DNA methylation and histone modifications determine cell fate during organ development by controlling tissue-specific expression (88). Epigenetic control of gene expression is also involved in lung epithelial cell differentiation (89) and developmental pathway signaling (90, 91).
Targeted Studies of DNA Methylation and Histone Modifications in Lung Fibrosis
Several targeted studies have shown that epigenetic modulation (both DNA methylation and histone marks) regulates expression of genes and miRNAs involved in pathogenesis of IPF (Table 1), namely, cyclooxygenase-2 (COX2) (92, 93), chemokine IP-10 (94), Thy-1 (CD90) (95, 96), p14(ARF) (97), α-smooth muscle actin (α-SMA) (98, 99), and miR-17~92 cluster (100). Similarly, molecular processes of high relevance to pulmonary fibrosis are also epigenetically regulated; this has been demonstrated specifically for fibroblast apoptosis (101, 102), cell senescence (103), and innate immunity (104) in IPF. These studies of DNA methylation and histone modifications in specific genes, miRNAs, and molecular processes have also shown a direct link to fibroproliferative phenotypes. For example, Dakhlallah et al. identified a DNMT1-controlled feedback loop that contributes to the IPF fibroblast phenotype and ECM deposition (100). Another study has linked TGF-β1 signaling, lung development and histone modifications (105). Taken together, these targeted studies have provided crucial information on the role of DNA methylation and histone modifications in regulation of gene expression in some of the key genes, miRNAs, pathways, and molecular processes that are hallmarks of IPF.
Table 1.
Summary of studies of DNA methylation and histone modifications performed to date in pulmonary fibrosis.
| Genes/Genomic Loci | Epigenetic Mechanism | Tissue or Cell | Phenotypes and Outcomes |
|---|---|---|---|
| cyclooxygenase-2 (COX2) (92, 93) | DNA methylation; histone H3 and H4 acetylation; H3K9me3 and H3K27me3; DNMTs, HATs, and HMTs, | Human lung fibroblasts | IPF |
| chemokine IP-10 (94) | |||
| Thy-1 (CD90) (95, 96) | DNA methylation; H3K4me3 and H4 acetylation | Human and rat lung fibroblasts | Myofibroblast differentiation |
| p14(ARF) (97) | DNA methylation | Human lung fibroblasts | IPF; apopotosis |
| α-smooth muscle actin (α-SMA) (98, 99) | DNA metylation; methyl CpG binding protein 2 (MeCP2) | Myofibroblast differentiation; collagen deposition | |
| miR-17~92 cluster (100) | DNA methylation; DNMT1 | Human lung tissue and human lung fibroblasts | IPF; collagen deposition |
| BAK, BCL-XL, FAS (101, 102) | DNA methylation (no difference); histone H3 acetylation; H3K9Me3 | Human and mouse lung fibroblasts | Fibroblast apoptosis |
| NOX4 (103) | H4K16Ac and H4K20Me3; | IMR-90 human fetal lung fibroblast cell line | Cell senescence |
| TLR9 (104) | hypomethylated CpG DNA (ligand) | Human lung fibroblasts, mouse lung tissue, and A549 lung epithelial cell line | Innate immunity; EMT |
| Mmp9 (105) | histone acetylation; SIRT1 | Mouse lung tissue | TGF-β1 signaling and lung development |
| CpG islands (106, 107) | DNA methylation | Human lung tissue | IPF |
| CpG islands (108) | DNA methylation | Human lung fibroblasts | IPF |
| 2.1M CpG motifs in the genome (109) | DNA methylation | Human lung tissue | IPF |
Genomic Profiles of DNA Methylation in IPF
Genome wide assessments of epigenetic marks in IPF are limited to DNA methylation profiles at the present time (Table 1). The first two studies of genomic methylation profiles of IPF lung tissue used arrays with probes covering CpG islands and promoters. Despite the limited coverage of early array platforms the first two studies of genomic methylation profiles identified extensive DNA methylation changes in IPF lung tissue (106, 107). A more recent study used the same platform as in Sanders et al. (107) to also show substantial changes in DNA methylation in fibroblasts, the key effector cell in fibrosis, of patients with IPF compared to controls (108).
The most comprehensive study of IPF lung tissue to date was led by our group and interrogated 4.6 million CpG sites distributed across the human genome in lung tissue of 94 subjects with IPF and 67 controls (109). This analysis identified 2130 significant differentially methylated regions (DMRs), of which 60% are in CpG island shores, similar to published findings in cancer (110). 738 DMRs are associated with significant changes in gene expression and enriched for canonical inverse relationship between methylation and expression. An additional analysis of the relationship of methylation marks to expression changes identified methylation marks that control both cis and trans regulation of gene expression, with an enrichment for cis relationships. This analysis also identified five trans relationships where a methylation change at a single DMR is associated with transcriptional changes in a substantial number of genes; four of these DMRs are near transcription factors. Taken together, these findings suggest not only widespread DNA methylation changes in IPF lung tissue but also a substantial effect of these methylation changes on gene expression. While it is unknown whether the methylation changes we have identified are are the result of the disease or are causative, given that several risk factors for IPF are independently associated with changes in DNA methylation, it is likely that the latter at least contributes to the methylation pattern.
In addition to replication of the published findings, one of the most important directions for the field of IPF epigenomics is to begin to understand cell specific patterns of DNA methylation and gene expression in the lung. For example, our study showed hypermethylation and reduced expression of the CASZ1 transcription factor in whole lung tissue but the same DMRs were hypomethylated in alveolar type II cells isolated from IPF lungs (109). In accordance with these findings, immunohistochemical staining showed loss of expression in airway epithelium and concomitant increase in expression of CASZ1 in the alveolar epithelium in IPF lung tissue sections compared to histologically normal lung (109). Isolation of specific cell types would also allow for profiling of histone modifications to paint a more complete picture of the role of epigenetic regulation of gene expression in IPF lung.
Methylation Changes within the IPF Genetic Loci
Recent work in the field of genetics of IPF has made it clear that there is a strong genetic component to this disease. Common genetic variants in the 10 loci identified by Fingerlin et al using a genome-wide association study (GWAS) explain ~30% of the disease risk (38). We intersected the 2130 IPF-associated DMRs with recently loci identified by the two published GWAS in IPF (38, 39) and identified methylation changes in genes within 5 of these loci (109). Of special interest are genes that are differentially expressed in IPF lung and whose expression may be regulated by both genetic variants and DNA methylation. As more genetic discoveries are made and the loci that have been already been associated with fine mapped, it is highly likely that additional candidate genes will emerge.
MUC5B
The strongest genetic candidate gene is MUC5B whose expression appears to be regulated at least in part by the IPF-associated promoter polymorphism rs35705950 (8, 111). This MUC5B promoter variant is associated with a 34.1-fold increase in MUC5B expression in lung tissue among unaffected subjects and a 5.3-fold increase among IPF patients, with IPF patients expressing 14.1-fold more MUC5B than unaffected controls. While our genomic methylation study did not identify DMRs near the MUC5B gene, there is reasonable evidence for the potential role of DNA methylation in regulation of MUC5B expression. The variant is approximately 3kb upstream of the MUC5B transcriptional start site, in an area of open chromatin, a dense region of ChIP-seq hits, in a highly conserved genomic region and within a CpG island, strongly suggesting that this region is important for gene regulation. DNase hypersensitivity assays indicate areas of open transcriptionally active chromatin (112). In the ENCODE project, 19 of the 125 analyzed cell lines, including the lung epithelial carcinoma cell line A549, have open chromatin in the chromosomal region overlapping the MUC5B promoter polymorphism (chr11:1241201–1241350 for A549) (112). Areas of open chromatin are often associated with binding of enhancer, silencer and other regulator elements (112, 113). Large scale ChIP-seq analysis, also part of the ENCODE project, has demonstrate the binding of at least 20 transcription factors to the A549 specific DNase hypersensitivity region described above, with 18 transcription factors predicted to bind in the region overlapping the common polymorphism (114–116).
Moreover, the promoter polymorphism is located within a ~200bp CpG island motif (chr11:1241162–1241364), which is of particular interest given that the variant (G to T transversion) allele at rs35705950 disrupts a CpG motif and therefore directly affects methylability of the adjacent cytosine. Although rs35705950 provides a very pointed example of a potential epigenetic regulator, more global changes in DNA methylation have also been associated with MUC5B expression. Vincent et al. previously showed that in vitro exposure to 5-azacytidine, a global DNA demethylating agent, can alter MUC5B expression (117, 118). We also know that the region of chromosome 11 becomes differentially methylated in some forms of cancer (119). Understanding regulation of MUC5B expression by a combination of DNA methylation in IPF lung and the rs35705950 polymorphism is an important future direction.
TOLLIP
Toll interacting protein (TOLLIP), a gene involved in innate immunity and inflammation, has emerged as a potential genetic candidate in addition to MUC5B on chromosome 11 (39), is 1.6 fold downregulated, and we identified two intronic DMRs in TOLLIP that are ~11% hypermethylated in IPF compared to controls. Hypermethylation of TOLLIP has recently been observed in synovial fibroblasts of patients with rheumatoid arthritis (120).
DSP
Desmoplakin (DSP), a key component of tight junctions, is located in one of the most precisely mapped IPF-associated GWAS loci (38), is 1.8 fold upregulated and has two hypomethylated intronic DMRs with one of the DMRs in the first intron. DSP expression is regulated by DNA methylation in non-small cell lung cancer (121).
Promise for Treatment Options
Identification of key epigenetic marks that are shaped by the genetics and environment and influence transcription of specific genes will not only help us have a better understanding of etiology and heterogeneity of IPF but will also empower us to develop biologically driven therapeutics and biomarkers for secondary prevention of this disease. DNA methylation changes have been shown to drive tumor formation and malignant progression (122), and as such have established basic mechanisms for disease pathogenesis, as well as targets for intervention in cancer. DNMT inhibitors have been approved for the treatment of myelodysplastic syndrome (123, 124) and are in clinical trials for treatment of solid tumors (125, 126). While currently available DNMT inhibitors lack specificity for gene(s) of interest, locus-specific therapies are currently being developed using genome editing technologies (122, 127) or taking advantage of recently discovered DNMT1-interacting noncoding RNAs (128). Additionally, current FDA-approved and in development histone mark modifying drugs are effective in targeting specific gene loci and pathways (55, 129) and treating diseases such as lung cancer (130). As a proof of principle for IPF, profibrotic phenotypes have been reversed in primary fibroblasts and the bleomycin mouse model by the Brd4 inhibitor JQ1 (131) as well as HDAC inhibitors Spiruchostatin A (SpA) (132) and suberoylanilide hydroxamic acid (SAHA) (102).
Conclusions
While we are in very early stages of using epigenetic marks as biomarkers and therapeutic targets in IPF, the potential is high and the next several years are likely to bring many exciting discoveries in this field that will hopefully bring us closer to better prognosticating and treating this fatal disease.
Key Points.
Epigenetic marks, just like pulmonary fibrosis, are affected by ageing, environmental exposures, genetic variants, and are important in developmental pathways whose aberrantly recapitulated in IPF lung.
Expression of genes and pathways that are key in the fibroproliferative response is regulated by DNA methylation and histone modifications.
Genomic studies of DNA methylation and histone modifications in IPF lung tissue and specific cells from the IPF lung are just emerging.
Epigenetic marks that are shaped by the genetics and environment and influence transcription of specific genes will empower us to develop biologically driven therapeutics and biomarkers for secondary prevention of this disease.
Acknowledgments
Financial support and sponsorship
National Heart Lung and Blood Institute R21-HL121572
Footnotes
Conflicts of Interest
I.V.Y has received speaker fee, manuscript preparation fee, and travel support from Boehringer Ingelheim and is partially supported by grant R21-HL121572 received by the University of Colorado. B.A.H. has no conflicts of interest.
References
- 1.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clinical epidemiology. 2013;5:483–92. doi: 10.2147/CLEP.S54815. Epub 2013/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. American journal of respiratory and critical care medicine. 2007;176(3):277–84. doi: 10.1164/rccm.200701-044OC. Epub 2007/05/05. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345(7):517–25. doi: 10.1056/NEJMra003200. Epub 2001/08/25. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–61. doi: 10.1016/S0140-6736(11)60052-4. Epub 2011/07/02. [DOI] [PubMed] [Google Scholar]
- 7.Boucher RC. Idiopathic pulmonary fibrosis--a sticky business. N Engl J Med. 2011;364(16):1560–1. doi: 10.1056/NEJMe1014191. Epub 2011/04/22. [DOI] [PubMed] [Google Scholar]
- 8.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–12. doi: 10.1056/NEJMoa1013660. Epub 2011/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang IV, Fingerlin TE, Evans CM, Schwarz MI, DAS MUC5B and Idiopathic Pulmonary Fibrosis. Annals of the American Thoracic Society. 2015 doi: 10.1513/AnnalsATS.201503-110AW. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–83. doi: 10.1073/pnas.1117988108. Epub 2011/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68(12):1114–21. doi: 10.1136/thoraxjnl-2012-202943. Epub 2013/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–5. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. The American journal of pathology. 2005;166(5):1321–32. doi: 10.1016/s0002-9440(10)62351-6. Epub 2005/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–5. doi: 10.1073/pnas.0605669103. Epub 2006/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. American journal of respiratory and critical care medicine. 2009;180(7):657–65. doi: 10.1164/rccm.200903-0322OC. Epub 2009/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. American journal of respiratory cell and molecular biology. 2011;45(1):1–15. doi: 10.1165/rcmb.2010-0365TR. Epub 2010/11/09. [DOI] [PubMed] [Google Scholar]
- 17.Fattman CL. Apoptosis in pulmonary fibrosis: too much or not enough? Antioxid Redox Signal. 2008;10(2):379–85. doi: 10.1089/ars.2007.1907. Epub 2007/11/23. [DOI] [PubMed] [Google Scholar]
- 18.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proceedings of the American Thoracic Society. 2006;3(4):350–6. doi: 10.1513/pats.200601-001TK. Epub 2006/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nature medicine. 2009;15(9):1077–81. doi: 10.1038/nm.2005. Epub 2009/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, et al. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. The Journal of biological chemistry. 2011;286(35):30972–80. doi: 10.1074/jbc.M110.181164. Epub 2011/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108(26):10562–7. doi: 10.1073/pnas.1107559108. Epub 2011/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552–7. doi: 10.1073/pnas.0701009104. Epub 2007/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13051–6. doi: 10.1073/pnas.0804280105. Epub 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2008;178(7):729–37. doi: 10.1164/rccm.200804-550OC. Epub 2008/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chilosi M, Doglioni C, Murer B, Poletti V. Epithelial stem cell exhaustion in the pathogenesis of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(1):7–18. Epub 2010/11/23. [PubMed] [Google Scholar]
- 26.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. The Journal of clinical investigation. 2009;119(9):2550–63. doi: 10.1172/JCI33288. Epub 2009/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS medicine. 2008;5(3):e62. doi: 10.1371/journal.pmed.0050062. Epub 2008/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King T, Costabel U, Cordier J-F, Dopico GA, Du Bois RM, Lynch DA, et al. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) American journal of respiratory and critical care medicine. 2000;161(2 Pt 1):646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 29.Kaminski N. Microarray analysis of idiopathic pulmonary fibrosis. American journal of respiratory cell and molecular biology. 2003;29(3 Suppl):S32–6. [PubMed] [Google Scholar]
- 30.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2009;180(2):167–75. doi: 10.1164/rccm.200810-1596OC. Epub 2009/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PloS one. 2007;2(5):e482. doi: 10.1371/journal.pone.0000482. Epub 2007/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. American journal of respiratory and critical care medicine. 2006;173(2):188–98. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99(9):6292–7. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boon K, Bailey NW, Yang J, Steel MP, Groshong S, Kervitsky D, et al. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF) PloS one. 2009;4(4):e5134. doi: 10.1371/journal.pone.0005134. Epub 2009/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang IV, Burch LH, Steele MP, Savov JD, Hollingsworth JW, McElvania-Tekippe E, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. American journal of respiratory and critical care medicine. 2007;175(1):45–54. doi: 10.1164/rccm.200601-062OC. Epub 2006/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364(16):1576–7. doi: 10.1056/NEJMc1013504. Epub 2011/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stock CJ, Sato H, Fonseca C, Banya WA, Molyneaux PL, Adamali H, et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax. 2013 doi: 10.1136/thoraxjnl-2012-201786. Epub 2013/01/17. [DOI] [PubMed] [Google Scholar]
- 38.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nature genetics. 2013;45(6):613–20. doi: 10.1038/ng.2609. Epub 2013/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noth I, Zhang Y, Ma S-F, Flores C, Barber M, Huang Y, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Resp Med. 2013;1(4):309–17. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borie R, Crestani B, Dieude P, Nunes H, Allanore Y, Kannengiesser C, et al. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PloS one. 2013;8(8):e70621. doi: 10.1371/journal.pone.0070621. Epub 2013/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei R, Li C, Zhang M, Jones-Hall YL, Myers JL, Noth I, et al. Association between MUC5B and TERT polymorphisms and different interstitial lung disease phenotypes. Translational research : the journal of laboratory and clinical medicine. 2014;163(5):494–502. doi: 10.1016/j.trsl.2013.12.006. Epub 2014/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral Blood Proteins Predict Mortality in Idiopathic Pulmonary Fibrosis. American journal of respiratory and critical care medicine. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. Epub 2011/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gharib SA, Altemeier WA, Van Winkle LS, Plopper CG, Schlesinger SY, Buell CA, et al. Matrix metalloproteinase-7 coordinates airway epithelial injury response and differentiation of ciliated cells. American journal of respiratory cell and molecular biology. 2013;48(3):390–6. doi: 10.1165/rcmb.2012-0083OC. Epub 2012/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allis CD, Jenuwein T, Reinberg D, editors. Epigenetics. Cold Spring Harbor Laboratory Press; 2009. [Google Scholar]
- 45.Yang IV, Schwartz DA. Epigenetic control of gene expression in the lung. American journal of respiratory and critical care medicine. 2011;183(10):1295–301. doi: 10.1164/rccm.201010-1579PP. Epub 2011/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Portela A, Esteller M. Epigenetic modifications and human disease. Nature biotechnology. 2010;28(10):1057–68. doi: 10.1038/nbt.1685. Epub 2010/10/15. [DOI] [PubMed] [Google Scholar]
- 47.Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature reviews Genetics. 2012;13(1):7–13. doi: 10.1038/nrg3080. Epub 2011/11/16. [DOI] [PubMed] [Google Scholar]
- 48.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143):433–40. doi: 10.1038/nature05919. Epub 2007/05/25. [DOI] [PubMed] [Google Scholar]
- 49.Feinberg AP, Tycko B. The history of cancer epigenetics. Nature reviews Cancer. 2004;4(2):143–53. doi: 10.1038/nrc1279. Epub 2004/01/21. [DOI] [PubMed] [Google Scholar]
- 50.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature genetics. 2009;41(12):1350–3. doi: 10.1038/ng.471. Epub 2009/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji H, Ehrlich LI, Seita J, Murakami P, Doi A, Lindau P, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467(7313):338–42. doi: 10.1038/nature09367. Epub 2010/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews Genetics. 2012;13(7):484–92. doi: 10.1038/nrg3230. Epub 2012/05/30. [DOI] [PubMed] [Google Scholar]
- 53.Kulis M, Heath S, Bibikova M, Queiros AC, Navarro A, Clot G, et al. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nature genetics. 2012;44(11):1236–42. doi: 10.1038/ng.2443. Epub 2012/10/16. [DOI] [PubMed] [Google Scholar]
- 54.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer cell. 2014;26(4):577–90. doi: 10.1016/j.ccr.2014.07.028. Epub 2014/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nature reviews Drug discovery. 2012;11(5):384–400. doi: 10.1038/nrd3674. Epub 2012/04/14. [DOI] [PubMed] [Google Scholar]
- 56.Tarakhovsky A. Tools and landscapes of epigenetics. Nature immunology. 2010;11(7):565–8. doi: 10.1038/ni0710-565. Epub 2010/06/22. [DOI] [PubMed] [Google Scholar]
- 57.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nature reviews Genetics. 2012;13(5):343–57. doi: 10.1038/nrg3173. Epub 2012/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera CM, Ren B. Mapping human epigenomes. Cell. 2013;155(1):39–55. doi: 10.1016/j.cell.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–9. doi: 10.1073/pnas.0500398102. Epub 2005/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heyn H, Li N, Ferreira HJ, Moran S, Pisano DG, Gomez A, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci U S A. 2012;109(26):10522–7. doi: 10.1073/pnas.1120658109. Epub 2012/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ong ML, Holbrook JD. Novel region discovery method for Infinium 450K DNA methylation data reveals changes associated with aging in muscle and neuronal pathways. Aging cell. 2013 doi: 10.1111/acel.12159. Epub 2013/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Issa JP. Aging and epigenetic drift: a vicious cycle. The Journal of clinical investigation. 2014;124(1):24–9. doi: 10.1172/JCI69735. Epub 2014/01/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selman M, Pardo A. Stochastic age-related epigenetic drift in the pathogenesis of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2014;190(12):1328–30. doi: 10.1164/rccm.201411-1953ED. Epub 2014/12/17. [DOI] [PubMed] [Google Scholar]
- 64.Sarter B, Long TI, Tsong WH, Koh WP, Yu MC, Laird PW. Sex differential in methylation patterns of selected genes in Singapore Chinese. Human genetics. 2005;117(4):402–3. doi: 10.1007/s00439-005-1317-9. Epub 2005/06/02. [DOI] [PubMed] [Google Scholar]
- 65.Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M, et al. DNA methylation profiling of human chromosomes 6, 20 and 22. Nature genetics. 2006;38(12):1378–85. doi: 10.1038/ng1909. Epub 2006/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Maarri O, Becker T, Junen J, Manzoor SS, Diaz-Lacava A, Schwaab R, et al. Gender specific differences in levels of DNA methylation at selected loci from human total blood: a tendency toward higher methylation levels in males. Human genetics. 2007;122(5):505–14. doi: 10.1007/s00439-007-0430-3. Epub 2007/09/14. [DOI] [PubMed] [Google Scholar]
- 67.Boks MP, Derks EM, Weisenberger DJ, Strengman E, Janson E, Sommer IE, et al. The relationship of DNA methylation with age, gender and genotype in twins and healthy controls. PloS one. 2009;4(8):e6767. doi: 10.1371/journal.pone.0006767. Epub 2009/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cotton AM, Lam L, Affleck JG, Wilson IM, Penaherrera MS, McFadden DE, et al. Chromosome-wide DNA methylation analysis predicts human tissue-specific X inactivation. Human genetics. 2011;130(2):187–201. doi: 10.1007/s00439-011-1007-8. Epub 2011/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proceedings of the American Thoracic Society. 2006;3(4):293–8. doi: 10.1513/pats.200512-131TK. Epub 2006/06/02. [DOI] [PubMed] [Google Scholar]
- 70.Seibold MA, Schwartz DA. The lung: the natural boundary between nature and nurture. Annu Rev Physiol. 2011;73:457–78. doi: 10.1146/annurev-physiol-012110-142212. Epub 2010/11/09. [DOI] [PubMed] [Google Scholar]
- 71.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature reviews Genetics. 2007;8(4):253–62. doi: 10.1038/nrg2045. Epub 2007/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wan ES, Qiu W, Baccarelli A, Carey VJ, Bacherman H, Rennard SI, et al. Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome. Human molecular genetics. 2012 doi: 10.1093/hmg/dds135. Epub 2012/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Philibert RA, Sears RA, Powers LS, Nash E, Bair T, Gerke AK, et al. Coordinated DNA methylation and gene expression changes in smoker alveolar macrophages: specific effects on VEGF receptor 1 expression. Journal of leukocyte biology. 2012;92(3):621–31. doi: 10.1189/jlb.1211632. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. American journal of human genetics. 2011;88(4):450–7. doi: 10.1016/j.ajhg.2011.03.003. Epub 2011/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tennis MA, Vanscoyk MM, Wilson LA, Kelley N, Winn RA. Methylation of Wnt7a is modulated by DNMT1 and cigarette smoke condensate in non-small cell lung cancer. PloS one. 2012;7(3):e32921. doi: 10.1371/journal.pone.0032921. Epub 2012/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buro-Auriemma LJ, Salit J, Hackett NR, Walters MS, Strulovici-Barel Y, Staudt MR, et al. Cigarette smoking induces small airway epithelial epigenetic changes with corresponding modulation of gene expression. Human molecular genetics. 2013;22(23):4726–38. doi: 10.1093/hmg/ddt326. Epub 2013/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen D, Fang L, Li H, Tang MS, Jin C. Cigarette smoke component acrolein modulates chromatin assembly by inhibiting histone acetylation. The Journal of biological chemistry. 2013;288(30):21678–87. doi: 10.1074/jbc.M113.476630. Epub 2013/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29(25):3650–64. doi: 10.1038/onc.2010.129. Epub 2010 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagathihalli NS, Massion PP, Gonzalez AL, Lu P, Datta PK. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Molecular cancer therapeutics. 2012;11(11):2362–72. doi: 10.1158/1535-7163.MCT-12-0107. Epub 2012/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathai SK, Schwartz DA, Warg LA. Genetic susceptibility and pulmonary fibrosis. Current opinion in pulmonary medicine. 2014;20(5):429–35. doi: 10.1097/MCP.0000000000000074. Epub 2014/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abramowitz LK, Bartolomei MS. Genomic imprinting: recognition and marking of imprinted loci. Current opinion in genetics & development. 2012;22(2):72–8. doi: 10.1016/j.gde.2011.12.001. Epub 2011/12/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bell JT, Pai AA, Pickrell JK, Gaffney DJ, Pique-Regi R, Degner JF, et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome biology. 2011;12(1):R10. doi: 10.1186/gb-2011-12-1-r10. Epub 2011/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang D, Cheng L, Badner JA, Chen C, Chen Q, Luo W, et al. Genetic control of individual differences in gene-specific methylation in human brain. American journal of human genetics. 2010;86(3):411–9. doi: 10.1016/j.ajhg.2010.02.005. Epub 2010/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gibbs JR, van der Brug MP, Hernandez DG, Traynor BJ, Nalls MA, Lai SL, et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS genetics. 2010;6(5):e1000952. doi: 10.1371/journal.pgen.1000952. Epub 2010/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasowski M, Kyriazopoulou-Panagiotopoulou S, Grubert F, Zaugg JB, Kundaje A, Liu Y, et al. Extensive variation in chromatin states across humans. Science. 2013;342(6159):750–2. doi: 10.1126/science.1242510. Epub 2013/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kilpinen H, Waszak SM, Gschwind AR, Raghav SK, Witwicki RM, Orioli A, et al. Coordinated effects of sequence variation on DNA binding, chromatin structure, and transcription. Science. 2013;342(6159):744–7. doi: 10.1126/science.1242463. Epub 2013/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McVicker G, van de Geijn B, Degner JF, Cain CE, Banovich NE, Raj A, et al. Identification of genetic variants that affect histone modifications in human cells. Science. 2013;342(6159):747–9. doi: 10.1126/science.1242429. Epub 2013/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turner BM. Defining an epigenetic code. Nature cell biology. 2007;9(1):2–6. doi: 10.1038/ncb0107-2. Epub 2007/01/03. [DOI] [PubMed] [Google Scholar]
- 89.Marconett CN, Zhou B, Rieger ME, Selamat SA, Dubourd M, Fang X, et al. Integrated transcriptomic and epigenomic analysis of primary human lung epithelial cell differentiation. PLoS genetics. 2013;9(6):e1003513. doi: 10.1371/journal.pgen.1003513. Epub 2013/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hagood JS, Ambalavanan N. Systems biology of lung development and regeneration: current knowledge and recommendations for future research. Wiley interdisciplinary reviews Systems biology and medicine. 2013;5(2):125–33. doi: 10.1002/wsbm.1205. Epub 2013/01/08. [DOI] [PubMed] [Google Scholar]
- 91.Morrisey EE, Cardoso WV, Lane RH, Rabinovitch M, Abman SH, Ai X, et al. Molecular determinants of lung development. Annals of the American Thoracic Society. 2013;10(2):S12–6. doi: 10.1513/AnnalsATS.201207-036OT. Epub 2013/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Molecular and cellular biology. 2009;29(15):4325–39. doi: 10.1128/MCB.01776-08. Epub 2009/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coward WR, Feghali-Bostwick CA, Jenkins G, Knox AJ, Pang L. A central role for G9a and EZH2 in the epigenetic silencing of cyclooxygenase-2 in idiopathic pulmonary fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28(7):3183–96. doi: 10.1096/fj.13-241760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coward WR, Watts K, Feghali-Bostwick CA, Jenkins G, Pang L. Repression of IP-10 by interactions between histone deacetylation and hypermethylation in idiopathic pulmonary fibrosis. Molecular and cellular biology. 2010;30(12):2874–86. doi: 10.1128/MCB.01527-09. Epub 2010/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanders YY, Tollefsbol TO, Varisco BM, Hagood JS. Epigenetic Regulation of Thy-1 by Histone Deacetylase Inhibitor in Rat Lung Fibroblasts. American journal of respiratory cell and molecular biology. 2010 doi: 10.1165/rcmb.2010-0154OC. Epub 2010/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. American journal of respiratory cell and molecular biology. 2008;39(5):610–8. doi: 10.1165/rcmb.2007-0322OC. Epub 2008/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cisneros J, Hagood J, Checa M, Ortiz-Quintero B, Negreros M, Herrera I, et al. Hypermethylation-mediated silencing of p14(ARF) in fibroblasts from idiopathic pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2012;303(4):L295–303. doi: 10.1152/ajplung.00332.2011. Epub 2012/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu B, Gharaee-Kermani M, Wu Z, Phan SH. Epigenetic regulation of myofibroblast differentiation by DNA methylation. The American journal of pathology. 2010;177(1):21–8. doi: 10.2353/ajpath.2010.090999. Epub 2010/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu B, Gharaee-Kermani M, Wu Z, Phan SH. Essential role of MeCP2 in the regulation of myofibroblast differentiation during pulmonary fibrosis. The American journal of pathology. 2011;178(4):1500–8. doi: 10.1016/j.ajpath.2011.01.002. Epub 2011/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dakhlallah D, Batte K, Wang Y, Cantemir-Stone CZ, Yan P, Nuovo G, et al. Epigenetic Regulation of miR-17~92 Contributes to the Pathogenesis of Pulmonary Fibrosis. American journal of respiratory and critical care medicine. 2013 doi: 10.1164/rccm.201205-0888OC. Epub 2013/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang SK, Scruggs AM, Donaghy J, Horowitz JC, Zaslona Z, Przybranowski S, et al. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell death & disease. 2013;4:e621. doi: 10.1038/cddis.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanders YY, Hagood JS, Liu H, Zhang W, Ambalavanan N, Thannickal VJ. Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2014;43(5):1448–58. doi: 10.1183/09031936.00095113. [DOI] [PubMed] [Google Scholar]
- 103.Sanders YY, Liu H, Liu G, Thannickal VJ. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free radical biology & medicine. 2015;79:197–205. doi: 10.1016/j.freeradbiomed.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 104.Hogaboam CM, Murray L, Martinez FJ. Epigenetic mechanisms through which Toll-like receptor-9 drives idiopathic pulmonary fibrosis progression. Proceedings of the American Thoracic Society. 2012;9(3):172–6. doi: 10.1513/pats.201201-002AW. [DOI] [PubMed] [Google Scholar]
- 105.Warburton D, Shi W, Xu B. TGF-beta-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? American journal of physiology Lung cellular and molecular physiology. 2013;304(2):L83–5. doi: 10.1152/ajplung.00258.2012. Epub 2012/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rabinovich EI, Kapetanaki MG, Steinfeld I, Gibson KF, Pandit KV, Yu G, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PloS one. 2012;7(4):e33770. doi: 10.1371/journal.pone.0033770. Epub 2012/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanders YY, Ambalavanan N, Halloran B, Zhang X, Liu H, Crossman DK, et al. Altered DNA methylation profile in idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2012;186(6):525–35. doi: 10.1164/rccm.201201-0077OC. Epub 2012/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang SK, Scruggs AM, McEachin RC, White ES, Peters-Golden M. Lung fibroblasts from patients with idiopathic pulmonary fibrosis exhibit genome-wide differences in DNA methylation compared to fibroblasts from nonfibrotic lung. PloS one. 2014;9(9):e107055. doi: 10.1371/journal.pone.0107055. Epub 2014/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang IV, Pedersen BS, Rabinovich E, Hennessy CE, Davidson EJ, Murphy E, et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2014;190(11):1263–72. doi: 10.1164/rccm.201408-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nature genetics. 2009;41(2):178–86. doi: 10.1038/ng.298. Epub 2009/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Helling BA, Steele MP, Brown KK, Loyd JE, Cosgrove GP, Fingerlin TE, et al. A Common MUC5B Promoter Polymorphism (rs35705950) And Risk Of Interstitial Lung Disease. American journal of respiratory and critical care medicine. 2013;187:A3815. [Google Scholar]
- 112.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489(7414):75–82. doi: 10.1038/nature11232. Epub 2012/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roadmap Epigenomics C. Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–30. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang J, Zhuang J, Iyer S, Lin XY, Greven MC, Kim BH, et al. Factorbook.org: a Wiki-based database for transcription factor-binding data generated by the ENCODE consortium. Nucleic acids research. 2013;41(Database issue):D171–6. doi: 10.1093/nar/gks1221. Epub 2012/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gerstein MB, Kundaje A, Hariharan M, Landt SG, Yan KK, Cheng C, et al. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489(7414):91–100. doi: 10.1038/nature11245. Epub 2012/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J, Zhuang J, Iyer S, Lin X, Whitfield TW, Greven MC, et al. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome research. 2012;22(9):1798–812. doi: 10.1101/gr.139105.112. Epub 2012/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vincent A, Perrais M, Desseyn JL, Aubert JP, Pigny P, Van Seuningen I. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene. 2007;26(45):6566–76. doi: 10.1038/sj.onc.1210479. Epub 2007/05/02. [DOI] [PubMed] [Google Scholar]
- 118.Perrais M, Pigny P, Buisine MP, Porchet N, Aubert JP, Van Seuningen-Lempire I. Aberrant expression of human mucin gene MUC5B in gastric carcinoma and cancer cells. Identification and regulation of a distal promoter. The Journal of biological chemistry. 2001;276(18):15386–96. doi: 10.1074/jbc.M010534200. Epub 2001/03/30. [DOI] [PubMed] [Google Scholar]
- 119.de Bustros A, Nelkin BD, Silverman A, Ehrlich G, Poiesz B, Baylin SB. The short arm of chromosome 11 is a “hot spot” for hypermethylation in human neoplasia. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(15):5693–7. doi: 10.1073/pnas.85.15.5693. Epub 1988/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park SH, Kim SK, Choe JY, Moon Y, An S, Park MJ, et al. Hypermethylation of EBF3 and IRX1 genes in synovial fibroblasts of patients with rheumatoid arthritis. Molecules and cells. 2013;35(4):298–304. doi: 10.1007/s10059-013-2302-0. Epub 2013/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang L, Chen Y, Cui T, Knosel T, Zhang Q, Albring KF, et al. Desmoplakin acts as a tumor suppressor by inhibition of the Wnt/beta-catenin signaling pathway in human lung cancer. Carcinogenesis. 2012;33(10):1863–70. doi: 10.1093/carcin/bgs226. Epub 2012/07/14. [DOI] [PubMed] [Google Scholar]
- 122.Yu DH, Waterland RA, Zhang P, Schady D, Chen MH, Guan Y, et al. Targeted p16Ink4a epimutation causes tumorigenesis and reduces survival in mice. The Journal of clinical investigation. 2014;124(9):3708–12. doi: 10.1172/JCI76507. Epub 2014/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. The oncologist. 2005;10(3):176–82. doi: 10.1634/theoncologist.10-3-176. Epub 2005/03/29. [DOI] [PubMed] [Google Scholar]
- 124.Saba HI. Decitabine in the treatment of myelodysplastic syndromes. Therapeutics and clinical risk management. 2007;3(5):807–17. Epub 2008/05/14. [PMC free article] [PubMed] [Google Scholar]
- 125.Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358(11):1118–28. doi: 10.1056/NEJMoa0706550. Epub 2008/03/14. [DOI] [PubMed] [Google Scholar]
- 126.Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer discovery. 2011;1(7):598–607. doi: 10.1158/2159-8290.CD-11-0214. Epub 2012/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500(7463):472–6. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Di Ruscio A, Ebralidze AK, Benoukraf T, Amabile G, Goff LA, Terragni J, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503(7476):371–6. doi: 10.1038/nature12598. Epub 2013/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heller EA, Cates HM, Pena CJ, Sun H, Shao N, Feng J, et al. Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nature neuroscience. 2014;17(12):1720–7. doi: 10.1038/nn.3871. Epub 2014/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huffman K, Martinez ED. Pre-Clinical Studies of Epigenetic Therapies Targeting Histone Modifiers in Lung Cancer. Frontiers in oncology. 2013;3:235. doi: 10.3389/fonc.2013.00235. Epub 2013/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang X, Peng R, Phillips JE, Deguzman J, Ren Y, Apparsundaram S, et al. Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. The American journal of pathology. 2013;183(2):470–9. doi: 10.1016/j.ajpath.2013.04.020. Epub 2013/06/14. [DOI] [PubMed] [Google Scholar]
- 132.Davies ER, Haitchi HM, Thatcher TH, Sime PJ, Kottmann RM, Ganesan A, et al. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. American journal of respiratory cell and molecular biology. 2012;46(5):687–94. doi: 10.1165/rcmb.2011-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]