Abstract
Objective
To investigate the effect of demographics including age and sex on excretion of four key urinary factors (calcium (Ca), magnesium (Mg), oxalate (Ox) and uric acid (UA)) related to kidney stone risk.
Methods
Twenty-four hour urine samples were collected from non-Hispanic white sibships in Rochester, MN. Height, weight, blood pressure, serum creatinine and cystatin C (CC) were measured. Diet was assessed using the Viocare food frequency questionnaire. Effects of demographics and dietary elements on urinary excretions were evaluated in univariate, multivariate, and interaction models that included age, sex, and body mass index (BMI).
Results
Samples were available from 709 individuals. In multivariate models, sex was a significant predictor of all four urinary factors, age was significant for all but UA excretion, and serum creatinine was significant only for Ca and Mg excretion (p<0.05). BMI or weight positively correlated with Mg, Ox and UA excretion (p<0.05). Use of a thiazide diuretic (lower) and dietary protein (higher) were associated with Ca excretion, while dietary Ca was associated with higher Mg excretion. Urinary UA excretion increased with animal protein intake and CC estimated glomerular filtration rate (eGFR), and was lower with concurrent loop diuretic use. Significant interaction effects on urinary UA excretion were observed for loop diuretic use and sex, eGFR and sex, age and animal protein intake, and BMI and eGFR (p<0.05).
Conclusions
Age and sex influence excretion of key urinary factors related to kidney stone risk, and should be taken into account when evaluating kidney stone patients.
Keywords: Calcium, Diet, Nephrolithiasis, Oxalate, Uric acid
INTRODUCTION
Kidney stones are common, affecting up to 10% of individuals over their lifetime (1). Certain urinary traits are thought to influence stone formation (2). For example, high urinary excretion of calcium, oxalate, and uric acid may increase stone risk (2, 3), while magnesium may be protective (4). Thus these urinary factors are often measured in 24- hour urine samples as part of metabolic evaluation to guide treatment. Although some evidence suggests that age, sex and weight, as well as dietary intake, can influence excretion of these substances (3, 5), such modifying factors are rarely taken into account during the metabolic evaluation of stone formers. Thus a single adult reference range is often used during interpretation of results.
Therefore in the current study we took advantage of urinary and food frequency questionnaire data obtained from a well-studied cohort of largely non-stone forming individuals to assess the effects of demographic features and diet on the excretion of key urinary factors. Our findings highlight the substantial influence of age and sex on the excretion of urinary factors related to kidney stone risk, and suggests these should be taken into account when evaluating an individual patient.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board.
GENOA cohort
The Genetic Epidemiology Network of Arteriopathy (GENOA), a member of the Family Blood Pressure Program (FBPP), recruited non-Hispanic white hypertensive sibships from Rochester, Minnesota for linkage and association studies to investigate the genetic underpinnings of hypertension in Phase I (1996–2001)(6). The Genetic Determinants of Urinary Lithogenicity (GDUL) study (2006–2012) is an ancillary study of the Phase III GENOA Genetics of Chronic Kidney Disease (CKD) Study conducted in GENOA participants. Participants in the Rochester, MN GENOA cohort were invited to collect 24 hour urine samples and complete a food frequency questionaire (FFQ, Viocare Technologies, Princeton, NJ, USA). Participants were excluded from this study if they were in endstage renal failure (stage 5 CKD).
Study visit
After informed consent, participants completed at least one 24 hour urine collection and the FFQ at a CKD and/or GDUL study visit. A total of 333, 295, and 183 participants had a total of one, two, or three urine collections, respectively. For individuals with two or three urine collections, values were averaged for analysis. The mean time between the earliest (CKD) and latest (GDUL) urine collections was 1.73 years (range = 0.9 to 3.6 years). The average time between the two GDUL collections was 22 days. Intraclass correlation coefficients (ICCs) for urine factors across collections revealed that the majority of urine measures were relatively stable across time. Of the urine factors, oxalate had the lowest ICC (0.46) and calcium had the highest (0.73). Participants also completed a detailed Kidney Stone Questionnaire, a Viocare Food Frequency Questionnaire (7), and data from a GENOA CKD Study Questionnaire was available as needed. Subjects completed the questionnaires at the time of a study visit, which was in general within one to two days of the urine collection.
Urine collection
Urine was collected with toluene as a preservative. Twenty-four hour urinary concentrations of oxalate, calcium and other determinants of supersaturation were measured in the Mayo Clinic Renal Testing Laboratory. Serum creatinine was assessed using a standardized enzymatic assay on a Roche Cobas chemistry analyzer (c311) (Roche Diagnostics; Indianapolis, USA) while cystatin C was measured using an immunoturbidometric assay (Gentian; Moss, Norway) that was traceable to an international reference material. Glomerular filtration rate (GFR) was independently estimated using creatinine (eGFRCr)(8) or cystatin C (eGFRCys) (9).
Descriptive statistics
Data management and statistical analyses were conducted in SAS version 9.3 (SAS Institute Inc., Cary, NC). Urine measures appeared to have relatively normal distributions; thus, no variable transformations were applied. Values that were ≥ 4 standard deviations from the mean of any urine or diet measure were removed. Linear mixed effects models (LMM) that included sibship as a random intercept (to properly account for family structure) were used to test whether there were significant differences by sex for the urinary and diet measures.
Association testing
A randomly selected, independent subset of the GENOA cohort (one individual per sibship; n=414) was used for stepwise linear regression to determine the variables that were associated with each urinary measures. Variables available for selection included: body mass index (BMI), smoking status (current- or ever-smoker/ never smoker), diabetes status (yes/no), fasting blood glucose level, systolic blood pressure (SBP), diastolic blood pressure (DBP), eGFRCr, eGFRCys, diuretic loop use (yes/no), diuretic thiazide use (yes/no), and dietary animal protein, calcium, fructose, oxalate, total protein, and sucrose intakes. The entry criterion was p<0.05, and the exit criterion was p<0.10. Age, sex, and serum creatinine were forced into each model.
After model selection, LMM was performed on the full GENOA sample to assess significant predictors of the urinary measures, accounting for the sibship structure in GENOA. Interaction models were also conducted to assess age, sex, and BMI (if BMI was included in the model selection as a predictor) interactions with the variables included in the models. Interactions were considered significant at an alpha level of 0.05.
RESULTS
A total of 709 individuals from 414 sibships participated in this study (Table 1). The sibship structure of the sample was as follows: 211 singletons, 148 sibpairs, 35 sibships with three siblings and 20 sibships with four or more siblings. The mean age was 66±9 years and 59% of the participants were female. A minority were diabetic (14%) or on loop diuretics (5%), while thiazides were commonly used (36.5%). Out of 709 participating individuals, 537 provided information on kidney stone history, of whom 63 (11.73 %) had had a previous stone, reflecting urinary stone disease prevelence in the general population (10). Three individuals were on medications for stone prevention (potassium citrate). As expected, the mean daily urinary excretions of calcium (157 mg), magnesium (109 mg), oxalate (0.29 mmol) and uric acid (451 mg) were well within the expected reference ranges.
Table 1.
Descriptive statistics
| Combined | Female | Male | |||
|---|---|---|---|---|---|
| n=416 | n=293 | ||||
| n | Mean (SD) or n (%) |
Mean (SD) or n (%) |
Mean (SD) or n (%) |
p- value |
|
| Age, yr | 709 | 65.4 (9.0) | 64.6 (8.9) | 66.5 (9.0) | 0.05 |
| Weight, kg | 709 | 87.7 (19.1) | 81.3 (17.5) | 96.8 (17.5) | <0.001 |
| BMI, kg/m2 | 709 | 31.0 (5.9) | 30.9 (6.5) | 31.0 (5.0) | 1.00 |
| Serum creatinine, mg/dL | 612 | 0.9 (0.2) | 0.8 (0.2) | 1.0 (0.2) | <0.001 |
| SBP, mmHg | 705 | 149 (25) | 150 (25) | 147 (25) | 0.09 |
| DBP, mmHg | 705 | 84 (11) | 82 (11) | 86 (11) | 0.95 |
| eGFRCys, ml/min/1.73m2 | 601 | 85.6 (24.7) | 87.9 (25.8) | 82.5 (22.8) | 0.02 |
| eGFRCr, ml/min/1.73m2 | 612 | 81.0 (18.7) | 81.0 (18.8) | 81.1 (18.5) | 0.58 |
| Diabetes status | 625 | 0.21 | |||
| Yes | 87 (14) | 45 (12) | 42 (16) | ||
| No | 538 (86) | 319 (88) | 219 (84) | ||
| Blood Glucose, mg/dL | 612 | 96.1 (22.9) | 94.8 (23.0) | 98.0 (22.7) | 0.24 |
| Dietary measures | |||||
| Oxalate, mg/d | 511 | 216 (126) | 218 (121) | 213 (133) | 0.64 |
| Animal protein, g/d | 521 | 53 (25) | 49 (21) | 59 (29) | <0.001 |
| Total protein, g/d | 521 | 80 (34) | 76 (30) | 87 (38) | <0.001 |
| Sucrose, g/d | 519 | 37 (21) | 38 (20) | 36 (21) | 0.34 |
| Calcium, mg/d | 521 | 1059 (542) | 1060 (517) | 1057 (578) | <0.001 |
| Diuretic use | |||||
| Loop | 709 | 0.61 | |||
| Yes | 35 (5) | 22 (5) | 13 (4) | ||
| No | 674 (95) | 394 (95) | 280 (96) | ||
| Thiazide | 709 | 0.99 | |||
| Yes | 259 (37) | 152 (37) | 107 (37) | ||
| No | 450 (64) | 264 (64) | 186 (64) | ||
| Urinary traits | |||||
| Uric acid, mg/d | 707 | 451 (172) | 393 (135) | 534 (185) | <0.001 |
| Calcium, mg/d | 706 | 157 (90) | 153 (85) | 162 (96) | 0.09 |
| Magnesium, mg/d | 709 | 109 (41) | 100 (35) | 120 (45) | <0.001 |
| Oxalate, mmol/d | 703 | 0.29 (0.097) | 0.27 (0.085) | 0.33 (0.099) | <0.001 |
SD: standard deviation, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, eGFR: estimated Glomerular Filtration Rate (cystatin calculation or creatinine calculation)
P-values are from linear mixed models used to assess sex differences, accounting for sibships.
Univariate analysis revealed that many demographic factors, comorbidities and dietary measures influenced urinary calcium, magnesium, oxalate and uric acid levels (Table 2). There was an age-related decline in calcium, magnesium, and uric acid excretion (Figure 1), while men excreted, on average, more magnesium, oxalate, and uric acid. Weight was positively associated with excretion of each of the four urinary factors, while BMI was positively associated with all but calcium.
Table 2.
Bivariate and multivariate associations with excretions of urinary factors related to kidney stone risk
| Urinary trait (outcome measure) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium, mg/d | Magnesium, mg/d | Oxalate, mmol/d | Uric acid, mg/d | |||||
| Bi (β) | MV (β) | Bi (β) | MV (β) | Bi (β1) | MV (β1) | Bi (β) | MV (β) | |
| Intercept | 477.21*** | 178.58*** | 0.25*** | 121.70 | ||||
| Age, yr | −3.75*** | −2.72*** | −0.94*** | −0.74** | 0.017 | 0.0865* | −2.31** | 1.54 |
| Sex (male) | 11.47 | 31.52*** | 20.50*** | 21.26*** | 0.3373*** | 0.321*** | 144.3*** | 132.23*** |
| Weight, kg | 0.71*** | 0.55*** | 0.32** | 0.35*** | 0.22*** | 3.38*** | ||
| BMI, kg/m2 | 1.13 | 0.79** | 0.18*** | 5.72*** | 6.74*** | |||
| DBP, mmHg | 0.68* | 0.26 | 0.063 | 2.36*** | 1.17 | |||
| Serum creatinine, mg/dL | −105.8*** | −133.08*** | −10.1 | −29.26** | 0.121** | −0.081 | 10.56 | −34.44 |
| eGFRCys, ml/min/1.73m2 | 1.38*** | 0.28*** | −0.020 | 1.31*** | 1.51*** | |||
| eGFRCr, ml/min/1.73m2 | 1.68*** | 0.49*** | 0.052 | 1.96*** | −0.0023 | |||
| Diabetes status (yes) | −21.99* | −5.57 | 0.080* | 15.22 | ||||
| Blood glucose, mg/dL | 0.18 | 0.030 | 0.086* | 0.74* | −0.30 | |||
| Dietary measures | ||||||||
| Oxalate, mg/d | 0.037 | 0.040** | 0.023 | 0.11* | 0.12** | 0.14* | 0.12* | |
| Animal protein, g/d | 0.67*** | 0.26*** | −0.16 | 0.083* | 1.40*** | 0.83** | ||
| Total protein, g/d | 0.51*** | 0.40** | 0.24*** | 0.12** | 1.04*** | |||
| Sucrose, g/d | 0.45* | 0.18* | −0.0021 | −0.051 | −0.66 | |||
| Calcium, mg/d | 0.025*** | 0.011** | 0.012* | −0.0054 | 0.013 | |||
| Diuretic use | ||||||||
| Loop (yes) | −6.28 | −9.06 | 0.04* | −56.52 | −93.65** | |||
| Thiazide (yes) | −28.80*** | −20.73** | 1.85 | 0.019* | 27.06* | |||
Bi: Bivariate associations, MV: Multivariate associations, β: beta estimate, BMI: body mass index, DBP: diastolic blood pressure, eGFR: estimated Glomerular Filtration Rate (cystatin calculation or creatinine calculation);
β values are standardized.
p-value<0.05;
p-value:<0.01;
p-value:<0.001
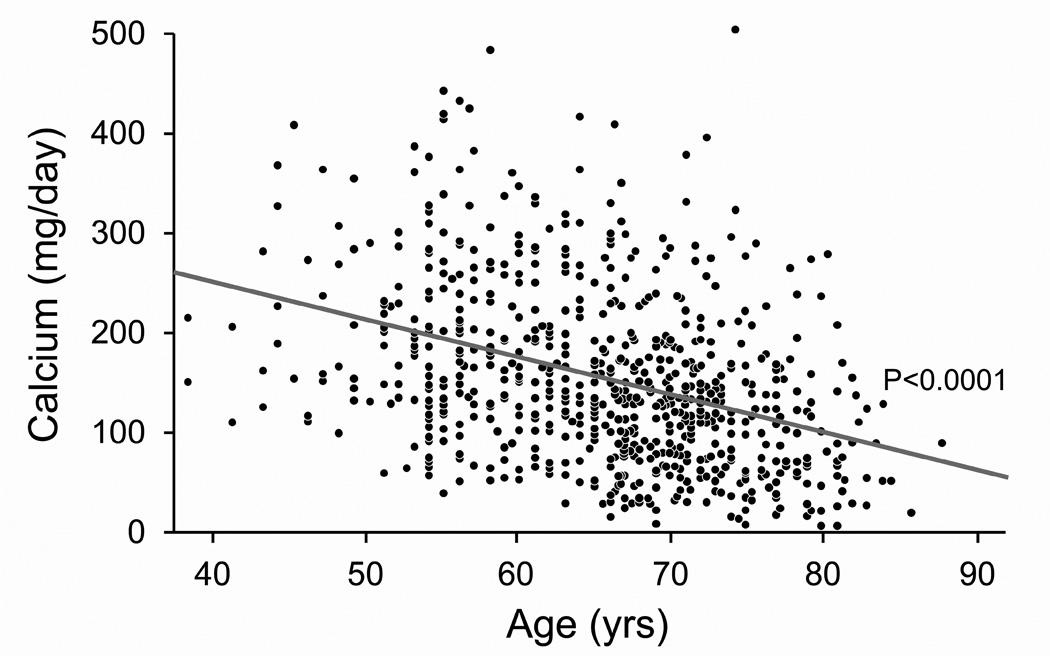
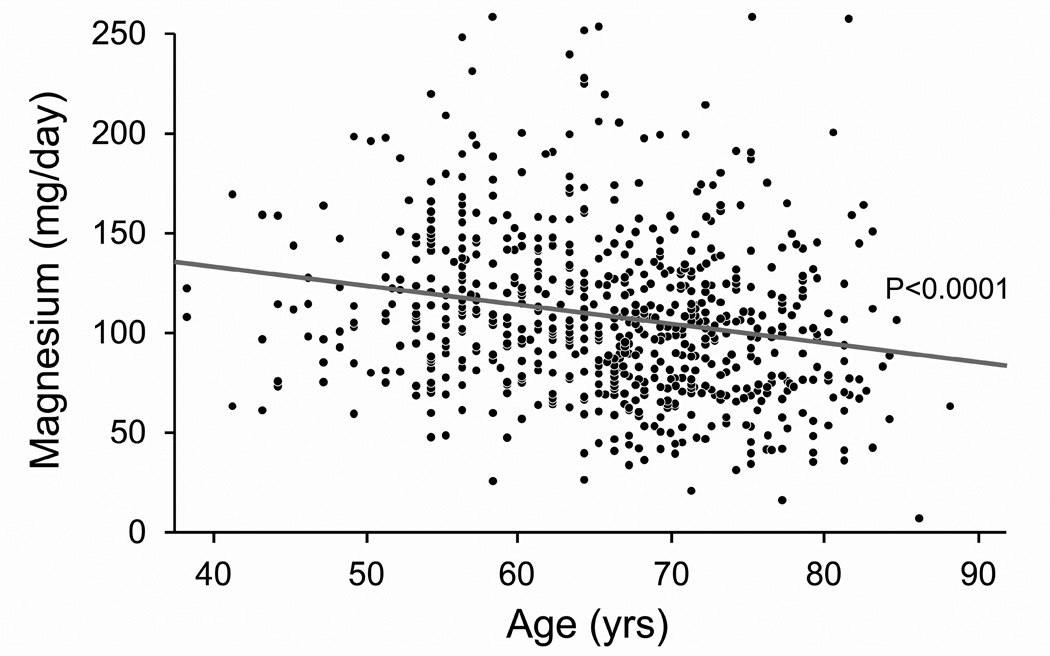
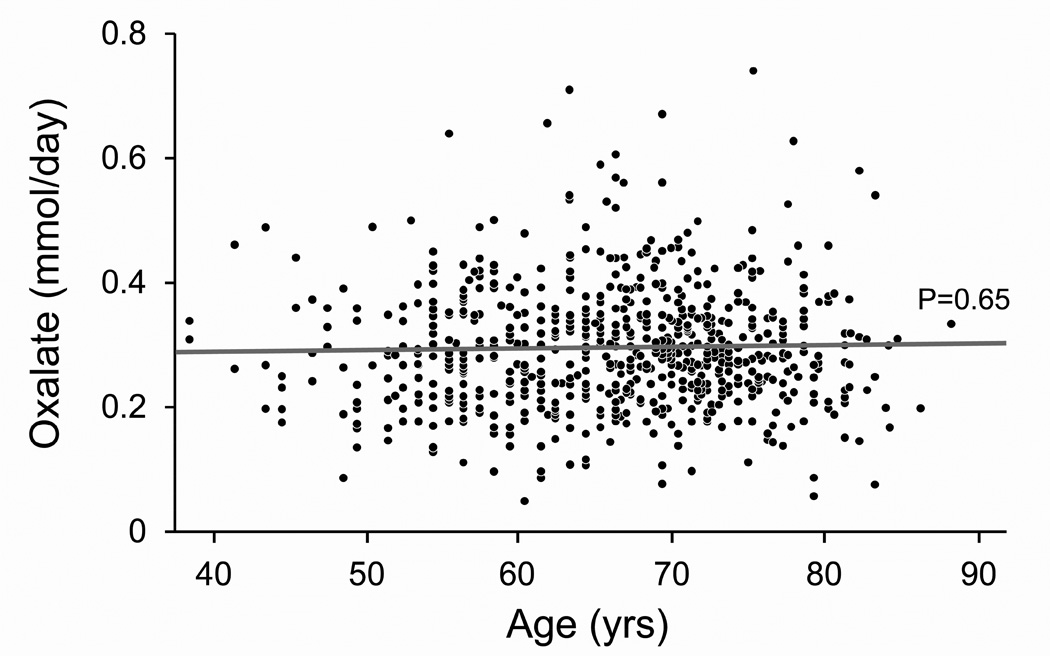
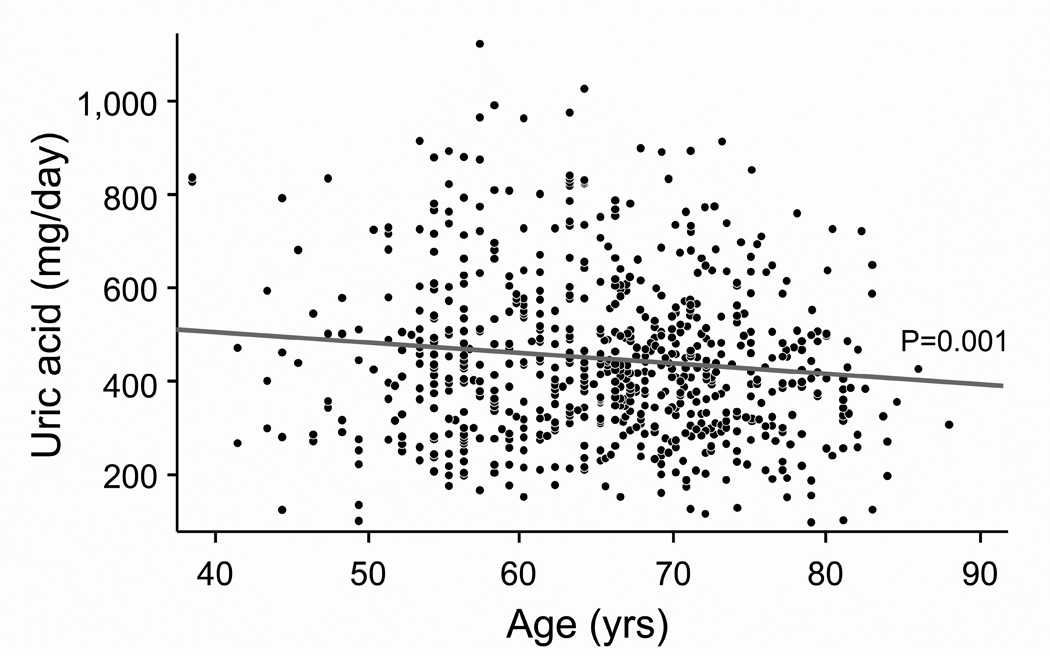
Figure 1. Effect of age on 24-hour urinary excretions of calcium, magnesium, oxalate and uric acid after accounting for sibships.
Panel A: Calcium excretion fell linearly with age (Calcium (mg/day) = 401.65 −3.7482 *Age (yrs)). Panel B: Magnesium excretion fell linearly with age (Magnesium (mg/day) = 169.67 – 0.9370*Age(yrs)). Panel C: Oxalate excretion did not vary with age. Panel D: Uric acid excretion fell linearly with age (Uric acid (mg/day) = 601.67 – 2.3058*Age (yrs)).
Urinary calcium excretion fell with declining GFR as assessed by serum creatinine concentration alone, eGFRCr or eGFRCys. Use of loop diuretics was associated with higher oxalate excretion, while thiazides were associated with higher uric acid and oxalate but lower calcium excretion. Several dietary measures also associated with urinary values. In particular, total protein and animal protein were associated with greater urine calcium and uric acid excretions. Correlations between urinary sulfate excretion and dietary animal protein (r=0.42; p<0.0001) and total dietary protein intake (r = 0.39239; p-value <0.0001) were both very significant. These data serve to validate accuracy of the food frequency tool for protein intake.
Multivariable models were constructed for each of the four urinary factors, using variables selected from step-wise regression, and forcing age, sex and serum creatinine into each model (Table 2). Demographics and patient characteristics influenced many urinary traits. Sex was a significant predictor of all four factors, while age was significant for all but uric acid. Serum creatinine concentration, an indication of GFR, was a significant predictor of calcium and magnesium excretion. Weight was positively associated with magnesium and oxalate excretion, while BMI was positively associated with uric acid. Dietary factors and medications were also important. Calcium excretion was associated with use of a thiazide diuretic (lower) and dietary protein intake (higher). Intake of dietary calcium was associated with higher urinary magnesium excretion. Given the large effect of age and sex on urinary calcium excretion, and the key role this value plays in stone risk, an equation was formulated to account for these factors and estimate the linear fall in mean urinary calcium excretion with age (Supplementary Table 1). Dietary oxalate was an independent predictor of higher urine oxalate excretion. Urinary uric acid excretion was positively associated with animal protein intake and eGFRCys and negatively associated with use of loop diuretic.
Significant interactions between age, sex, and BMI with dietary factors, loop diuretic use, serum creatinine and eGFRCys were observed in the context of urinary uric acid excretion (Figure 2), but none on the other urinary excretions we studied. Specifically, we found significant interactions between sex and loop diuretic use (β=−151.18, p=0.05), eGFRCys (β=2.22, p=0.0002), dietary oxalate intake (β=0.21, p=0.04), and serum creatinine (β=−149.09, p=0.04); age and dietary animal protein intake (β=−0.06, p=0.02), and serum creatinine (β=−8.78, p=0.03); and BMI and eGFRCys (β=−0.09, p=0.04).
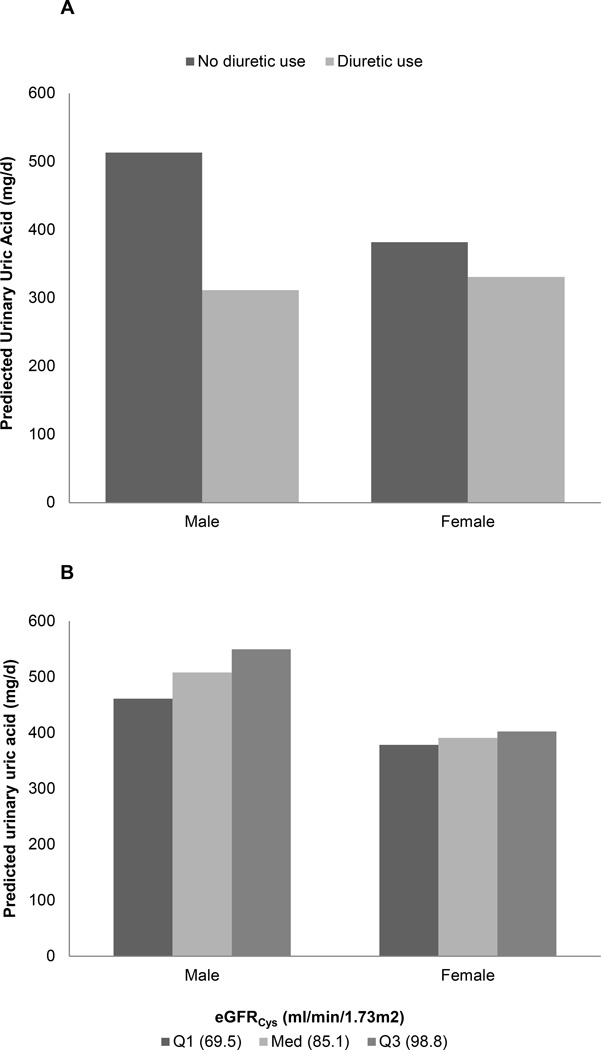
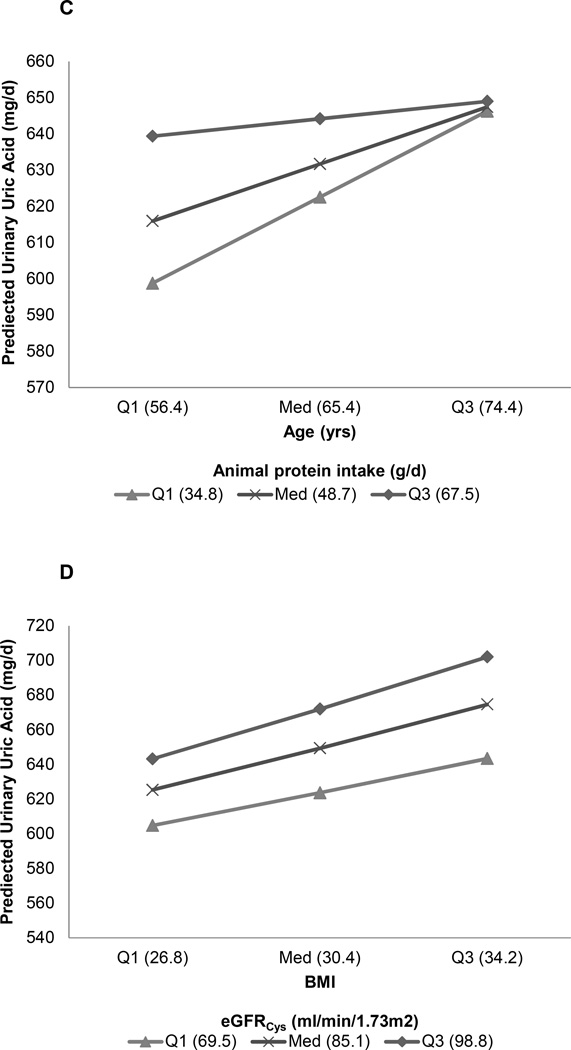
Figure 2. Interaction models for urinary uric acid excretion.
Panel A: Loop diuretics decreased urinary uric acid proportionately more in men than women (p=0.05). Panel B: Uric acid excretion fell proportionately more with declining GFR in men than women (p<0.001). Panel C: Urinary uric acid excretion varied more with age in individuals with a lower animal protein intake as opposed to a higher intake (p=0.02). Panel D: Urinary uric acid excretion increased more with BMI in persons with higher eGFRCys (p=0.04).
DISCUSSION
The current study assessed the effects of demographics and diet on excretion of 4 key urinary factors related to kidney stone risk: calcium, magnesium, oxalate, and uric acid. Results demonstrate that age, sex and weight influence each, even after controlling for diet. Thus physicians should consider these factors when assessing urinary values obtained for the purpose of kidney stone risk. For example, a urinary calcium level that might be average in a younger individual might be relatively high in an older one.
Urinary calcium excretion strongly decreased with age (Figure 1A). Many studies that examined the effect of age on urinary parameters studied cohorts solely comprised of stone forming individuals. Goldfarb and colleagues (5) found that urine calcium excretion was significantly lower in older stone formers of both sexes. This observation was confirmed in a recent study (11) that documented lower urine calcium excretion in both men and women stone formers that were older than 60 years of age. A study of 2800 stone formers in Southampton Stone Clinic, Gallway, Ireland reported decreases in urinary calcium with age in males but not females greater than 60 years old (12). Conversely, we did not observe any sex difference in the age-related decline in urinary calcium excretion. A study of 246 non-stone formers found urine calcium excretion remained relatively constant until the eighth decade and then decreased (13). Our data, however, suggests that the decline in calcium excretion is progressive across the adult age spectrum (Figure 1A). Supplementary table 1 provides the mean urine calcium excretion in 5 year intervals for both sexes, and could be used as a guide when evaluating individual patients. This decrease in urine calcium with age might contribute to the reported decline in stone incidence after the 5th decade (14).
Physiologic changes in calcium metabolism with aging could contribute to these trends. Intestinal calcium absorption decreases significantly with normal aging(15). The levels of serum 25-OH-D (25-hydroxy cholecalciferol) remained constant while 1.25(OH)2D significantly decreased after age 65, thus implicating reduced renal 1-alpha hydroxylation activity(15). The normal gentle decline in kidney function with age could contribute to this decline in calcium absorption and renal excretion. Age related differences in calcium intake and exposure to sunlight could also be important (16).
Females excreted less calcium than males in the main effects model, as was observed by others (12). Curhan and colleagues (3) reported a slightly higher urinary calcium excretion in male versus female stone formers, but no sex differences in the non-stone formers. Bulusu and colleagues (13) reported that the daily excretion of calcium was higher in men regardless of stone forming status, but was entirely related to body weight. In the current study weight and BMI were not independent predictors of calcium excretion, while diet protein was, suggesting diet rather than bone mass might be a key variable. Previous studies (17) have found an association between protein intake and urine calcium, sometimes attributed to the associated acid load. Taylor and colleagues (18) also found that an association between BMI and urinary calcium excretion was eliminated by adjusting for urinary sodium and phosphate excretion.
In the current study determinants of urinary magnesium excretion were very similar to those for urinary calcium, perhaps because both divalent cations are largely handled in a parallel fashion by the intestine and kidney. Unlike calcium, however, urinary magnesium levels increased with weight in multivariate models. Parallel increases in urinary magnesium and calcium excretion might serve to protect against stone formation, since magnesium is thought to be a crystallization inhibitor (4).
Females excreted slightly less oxalate than males. Other studies have also found that men excreted slightly more oxalate (12). Studies suggest that between 10 and 50% of urinary oxalate is derived from the diet (19), and in our study dietary oxalate was an independent predictor of urinary oxalate (and calcium) excretion (Table 2). However, we did not find a significant sex difference in oxalate intake. Thus, our study is consistent with the hypotheses that there are sex differences in either the intake of other foods that increase urinary oxalate excretion (e.g. vitamin C, xylitol (sweetener), or hydroxyproline (20)), mechanisms of oxalate transport in the kidney or intestine, and/or endogenous oxalate production. Studies in rats suggest that testosterone increases and estrogen decreases urinary oxalate excretion(21). A recent study in mice that lack the hepatic androgen receptor (AR) documented reduced oxalate biosynthesis (22), and that mice lacking kidney proximal or distal epithelial AR also had lower CaOx crystal formation. Other in vivo studies found that collagen synthesis was inhibited by estrogen but not affected by testosterone (23). Notably hydroxyproline, a component of collagen, accounts for 5–20% of the urinary oxalate derived from endogenous synthesis (24). Thus higher collagen turnover in males might contribute to the sex difference in urinary oxalate excretion.
Several interesting interactions were noted among factors associated with urinary uric acid excretion. Loop diuretic use strongly reduced uric acid excretion, as has been previously noted (25). However, in our study the effect was more dramatic in males than females (Figure 2A). The underlying reasons are not readily apparent. BMI was positively associated with urinary uric acid excretion, as previously noted by others (18). However, urinary uric acid excretion was also lower in females than males, independent of BMI, perhaps lowering their relative risk of both calcium oxalate and uric acid stones (2). Participants with lower animal protein intake had a larger increase in urinary uric acid excretion with age (Figure 2C). The reasons behind this observation are not clear, but intake of other purine rich foods (e.g., legumes) is one possibility. Ingestion of fructose or sucrose is a known cause of hyperuricemia (26), but in our study the effect of sucrose on urinary uric acid excretion was, if anything, modest (p=0.09)(Table 2). It is interesting that eGFRCys was an independent positive predictor of urinary uric acid excretion. Although this serum biomarker has been proposed as a better indicator of GFR than serum creatinine (27), cystatin C has also been linked to mortality, inflammation, cardiovascular- and chronic kidney disease (28). Thus it might not be surprising if hyperuricemia, inflammation and cystatin C are interrelated. We found eGFRCys had a stronger effect on uric acid excretion in males than females (Figure 2B). Male participants had higher animal protein intake and lower eGFRCys than females. In addition, BMI had a larger effect on urinary uric acid excretion in individuals with a high eGFRCys (Figure 2D). Differences in body habitus, muscle mass, protein intake, and true GFR might underlie some of these interactions. However, further studies are needed to elucidate underlying mechanisms.
Our study has certain limitations. Participants were white Americans of European descent, of relatively older age, and limited socioeconomic diversity. Taylor and colleagues (29) found that 24-hour urinary calcium excretion was less in non-stone forming African American as opposed to white women. We also examined a largely non stone forming population, and certain individuals with consistently high urinary calcium may not necessarily form stones (30). However, studying non-stone formers allowed more precise assessment of age and sex influences without being confounded by dietary changes initiated after a stone event. Also, the observed changes in urine chemistry are consistent with most published studies performed in stone forming cohorts (3, 5, 12, 18). Furthermore, ours in one of the largest studies to date that systematically examined the effects of demographics and diet on excretion of key urinary factors related to kidney stone risk. Thus results should aid clinicians in assessing the effects of age and gender when evaluating kidney stone patients and interpreting lab values.
In conclusion, data in this large cohort suggests an age-related decline in urinary calcium, and that urinary calcium excretion is on average lower in women of all ages. This is consistent with other studies and the observed incidence of calcium oxalate stone events. Uric acid excretion was also higher in males, and influenced by BMI. Urinary oxalate excretion was less in females than males with no difference in oxalate intake. Thus sex differences in oxalate transport and/or endogenous oxalate production may exist. Regardless, demographic factors should be taken into account when evaluating urinary risk factors for kidney stone formation.
Supplementary Material
Acknowledgments
GRANT FUNDING
This work was supported by R01 DK077950, R01 DK073537, U01 HL054457, R01 HL087660, R01 HL119443, the Mayo Clinic O’Brien Urology Research Center P50 DK083007, and Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), all funded by the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med. 2009;76:583–591. doi: 10.3949/ccjm.76a.09043. [DOI] [PubMed] [Google Scholar]
- 3.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59:2290–2298. doi: 10.1046/j.1523-1755.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 4.Johansson G, Backman U, Danielson BG, Fellstrom B, Ljunghall S, Wikstrom B. Effects of magnesium hydroxide in renal stone disease. J Am Coll Nutr. 1982;1:179–185. doi: 10.1080/07315724.1982.10718985. [DOI] [PubMed] [Google Scholar]
- 5.Goldfarb DS, Parks JH, Coe FL. Renal stone disease in older adults. Clin Geriatr Med. 1998;14:367–381. [PubMed] [Google Scholar]
- 6.Daniels PR, Kardia SL, Hanis CL, Brown CA, Hutchinson R, Boerwinkle E, Turner ST. Familial aggregation of hypertension treatment and control in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. The American journal of medicine. 2004;116:676–681. doi: 10.1016/j.amjmed.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Kristal AR, Kolar AS, Fisher JL, Plascak JJ, Stumbo PJ, Weiss R, Paskett ED. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J Acad Nutr Diet. 2014;114:613–621. doi: 10.1016/j.jand.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR. Medical management of kidney stones: AUA guideline. J Urol. 2014;192:316–324. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander JI, Moreira DM, Hartman C, Elsamra SE, Smith AD, Okeke Z. Age-related changes in 24- hour urine composition must be considered in the medical management of nephrolithiasis. J Endourol. 2014;28:871–876. doi: 10.1089/end.2014.0002. [DOI] [PubMed] [Google Scholar]
- 12.Walker V, Stansbridge EM, Griffin DG. Demography and biochemistry of 2800 patients from a renal stones clinic. Ann Clin Biochem. 2013;50:127–139. doi: 10.1258/acb.2012.012122. [DOI] [PubMed] [Google Scholar]
- 13.Bulusu L, Hodgkinson A, Nordin BE, Peacock M. Urinary excretion of calcium and creatinine in relation to age and body weight in normal subjects and patients with renal calculus. Clin Sci. 1970;38:601–612. doi: 10.1042/cs0380601. [DOI] [PubMed] [Google Scholar]
- 14.Lieske JC, Pena de la Vega LS, Slezak JM, Bergstralh EJ, Leibson CL, Ho KL, Gettman MT. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int. 2006;69:760–764. doi: 10.1038/sj.ki.5000150. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979;64:729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaqueiro M, Bare M, Anton E, Andreu E, Moya A, Sampere R, Villar E, Gimeno C. [Hypovitaminosis D associated to low sun exposure in the population over 64 years old] Med Clin (Barc) 2007;129:287–291. doi: 10.1157/13109116. [DOI] [PubMed] [Google Scholar]
- 17.Itoh R, Nishiyama N, Suyama Y. Dietary protein intake and urinary excretion of calcium: a cross-sectional study in a healthy Japanese population. Am J Clin Nutr. 1998;67:438–444. doi: 10.1093/ajcn/67.3.438. [DOI] [PubMed] [Google Scholar]
- 18.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001;59:270–276. doi: 10.1046/j.1523-1755.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 20.Holmes RP, Knight J, Assimos DG. Origin of Urinary Oxalate. AIP Conference Proceedings. 2007;900:176–182. [Google Scholar]
- 21.Yagisawa T, Ito F, Osaka Y, Amano H, Kobayashi C, Toma H. The influence of sex hormones on renal osteopontin expression and urinary constituents in experimental urolithiasis. J Urol. 2001;166:1078–1082. [PubMed] [Google Scholar]
- 22.Liang L, Li L, Tian J, Lee SO, Dang Q, Huang CK, Yeh S, Erturk E, Bushinsky D, Chang LS, He D, Chang C. Androgen receptor enhances kidney stone-CaOx crystal formation via modulation of oxalate biosynthesis & oxidative stress. Mol Endocrinol. 2014;28:1291–1303. doi: 10.1210/me.2014-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, Silbiger S. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50:1173–1179. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- 24.Knight J, Jiang J, Assimos DG, Holmes RP. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int. 2006;70:1929–1934. doi: 10.1038/sj.ki.5001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascual E, Perdiguero M. Gout, diuretics and the kidney. Ann Rheum Dis. 2006;65:981–982. doi: 10.1136/ard.2005.049023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinig M, Johnson RJ. Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med. 2006;73:1059–1064. doi: 10.3949/ccjm.73.12.1059. [DOI] [PubMed] [Google Scholar]
- 27.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Taylor EN, Curhan GC. Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol. 2007;18:654–659. doi: 10.1681/ASN.2006080854. [DOI] [PubMed] [Google Scholar]
- 30.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.