Abstract
Sequence analysis has revealed the presence of 22 putative methyl-accepting chemotaxis protein (mcp) genes in the Ralstonia pseudosolanacearum GMI1000 genome. PCR analysis and DNA sequencing showed that the highly motile R. pseudosolanacearum strain Ps29 possesses homologs of all 22 R. pseudosolanacearum GMI1000 mcp genes. We constructed a complete collection of single mcp gene deletion mutants of R. pseudosolanacearum Ps29 by unmarked gene deletion. Screening of the mutant collection revealed that R. pseudosolanacearum Ps29 mutants of RSp0507 and RSc0606 homologs were defective in chemotaxis to l-malate and amino acids, respectively. RSp0507 and RSc0606 homologs were designated mcpM and mcpA. While wild-type R. pseudosolanacearum strain Ps29 displayed attraction to 16 amino acids, the mcpA mutant showed no response to 12 of these amino acids and decreased responses to 4 amino acids. We constructed mcpA and mcpM deletion mutants of highly virulent R. pseudosolanacearum strain MAFF106611 to investigate the contribution of chemotaxis to l-malate and amino acids to tomato plant infection. Neither single mutant exhibited altered virulence for tomato plants when tested by root dip inoculation assays. In contrast, the mcpM mutant (but not the mcpA mutant) was significantly less infectious than the wild type when tested by a sand soak inoculation assay, which requires bacteria to locate and invade host roots from sand. Thus, McpM-mediated chemotaxis, possibly reflecting chemotaxis to l-malate, facilitates R. pseudosolanacearum motility to tomato roots in sand.
INTRODUCTION
Chemotaxis, the movement of motile bacteria with reference to a chemical agent, is a widespread phenomenon (1). Bacteria sense and respond behaviorally to a wide variety of chemical stimuli, including amino acids, sugars, organic acids, aromatic compounds, and phosphate (2–5). Bacterial chemotaxis also can be viewed as an important prelude to ecological interactions such as symbiosis, infection, and root colonization (6). Indeed, chemotaxis has been shown to be involved in nodulation by Rhizobium leguminosarum (7) and root colonization by Pseudomonas fluorescens (8–10).
The molecular mechanisms that underlie bacterial chemotaxis have been studied intensively in Escherichia coli and Salmonella enterica serovar Typhimurium (11, 12). Chemotactic ligands are detected by cell surface chemoreceptors called methyl-accepting chemotaxis proteins (MCPs). Upon binding a chemotactic ligand, a MCP generates chemotaxis signals that are communicated to the flagellar motor via a series of chemotaxis (Che) proteins. E. coli possesses 5 MCPs and 6 Che proteins (CheA, CheB, CheR, CheW, CheY, and CheZ).
Ralstonia solanacearum is a Gram-negative plant-pathogenic bacterium that causes bacterial wilt in economically important crops, including tomato, potato, eggplant, tobacco, and banana (13, 14). This soilborne bacterium usually enters plant roots through wounds, root tips, and secondary root emerging points, from which the organism invades the xylem vessels and spreads to aerial parts (15). R. solanacearum is motile and shows chemotactic responses to plant-related compounds such as amino acids, carboxylic acids, and sugars (16). The chemotactic mechanism in R. solanacearum is similar to that in enteric bacteria (16). R. solanacearum is a heterogeneous species and termed the “R. solanacearum species complex” (17, 18). The R. solanacearum species complex can be subdivided into four phylotypes (19). Safni et al. proposed to emend the description of R. solanacearum and reclassify current R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis and current R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum (20). By this reclassification, R. solanacearum consists of strains of current R. solanacearum phylotype II only. In this study, we follow the new nomenclature for the R. solanacearum species complex.
Yao and Allen observed previously that cheA and cheW single mutants of R. solanacearum K60, which were nonchemotactic but motile, were less infectious than the wild-type strain in biologically realistic sand soak virulence assays (16). When tomato plants were coinoculated with a 1:1 mixture of each nonchemotactic mutant and its wild-type parent, the wild-type strain outcompeted these nonchemotactic mutants. From these results, these authors concluded that chemotaxis is required for full virulence in R. solanacearum K60 and that this bacterium depends on taxis to locate and colonize plant roots. Yao and Allen also demonstrated that aerotaxis (energy taxis) contributed to the ability of R. solanacearum K60 to locate and effectively interact with host plants (21). However, when tested by biologically realistic sand soak virulence assays, nonchemotactic cheA and cheW single mutants were more impaired in virulence than was the mutant defective in aerotaxis. These data suggested that taxis other than aerotaxis is involved in the migration of R. solanacearum K60 cells to plant roots.
Complete genomic sequences have been generated for several strains of the R. solanacearum species complex (22). Genomic analysis revealed that these strains each encode >20 MCPs. Among these MCPs, two have been identified as aerotaxis sensors (21), but other MCPs have not yet been functionally characterized, which hampers the identification of chemoattractants involved in plant infection by the R. solanacearum species complex. In the present study, we identified MCPs for amino acids and l-malate in R. pseudosolanacearum. We also investigated the involvement of these MCPs in plant colonization and infection using R. pseudosolanacearum mutant strains defective in chemotaxis to amino acids and l-malate.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1 (see also Table S1 in the supplemental material). R. pseudosolanacearum Ps29 (formerly R. solanacearum Ps29 [phylotype I, race 1, and biovar 3]) (isolated from tobacco) and R. pseudosolanacearum MAFF106611 (formerly R. solanacearum MAFF106611 [phylotype I, race 1, and biovar 4]) (isolated from eggplant) were obtained from the Leaf Tobacco Center (Japan Tobacco Inc.) and the National Institute of Agrobiological Sciences, Japan, respectively (23). Highly motile R. pseudosolanacearum strain Ps29 and its derivatives were used for chemotaxis research, and R. pseudosolanacearum MAFFF106611 and its derivatives were used for tomato plant virulence assays and competitive tomato plant colonization assays. E. coli strains JM109 (24) and S17-1 (25) were used for plasmid construction and transconjugation, respectively. R. pseudosolanacearum strains were cultivated at 28°C in CPG medium (29) or in R. solanacearum minimal (RSM) medium. RSM medium contained 1.75 g/liter K2HPO4, 0.75 g/liter KH2PO4, 0.15 g/liter trisodium citrate dihydrate, 1.25 g/liter (NH4)2SO4, 0.25 g/liter MgSO4·7H2O, and 5 g/liter glucose. E. coli strains were grown at 37°C in 2× YT medium supplemented with appropriate antibiotics when necessary (24).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference |
|---|---|---|
| Strains | ||
| Ralstonia pseudosolanacearum | ||
| Ps29 | Wild-type strain; race 1, biovar 3, phylotype I | 23 |
| DPS01 | Ps29 derivative; ΔmcpA | This study |
| DPS14 | Ps29 derivative; ΔmcpM | This study |
| MAFF106611 | Wild type; race 1, biovar 4, phylotype I | 23 |
| DMF01 | MAFF106611 derivative; ΔmcpA | This study |
| DMF14 | MAFF106611 derivative; ΔmcpM | This study |
| DMFcheA | MAFF106611 derivative; ΔcheA | This study |
| MFK | MAFF106611 derivative; Kmr | This study |
| Escherichia coli | ||
| JM109 | recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) e14 negative (mcrA negative) supE44 relA1 Δ(lac-proAB) F′ [traD36 proAB+ lacIq lacZΔM15] | 24 |
| S17-1 | MM294 derivative; RP4-2 Tc::Mu-Km::Tn7; chromosomally integrated | 25 |
| Plasmids | ||
| pK18mobsacB | Kmr pUC18 derivative; lacZα mobs site sacB | 26 |
| pNMPS01 | pK18mobsacB with a 1.2-kb PCR fragment upstream of mcpA and a 1.1-kb PCR fragment downstream of mcpA from the Ps29 genome; Kmr | |
| pNMPS14 | pK18mobsacB with a 0.9-kb PCR fragment upstream of mcpM and a 0.6-kb PCR fragment downstream of mcpM from the Ps29 genome; Kmr | This study |
| pNMMF01 | pK18mobsacB with a 1.2-kb PCR fragment upstream of mcpA and a 1.1-kb PCR fragment downstream of mcpA from the MAFF106611 genome; Kmr | This study |
| pNMMF14 | pK18mobsacB with a 0.9-kb PCR fragment upstream of mcpM and a 0.6-kb PCR fragment downstream of mcpM from the MAFF106611 genome; Kmr | This study |
| pNMMFcheA | pK18mobsacB with a 0.8-kb PCR fragment upstream of cheA and a 0.9-kb PCR fragment downstream of cheA from the MAFF106611 genome; Kmr | This study |
| pTBS | pK18mobsacB with 0.8-kb and 1.2-kb PCR fragments from the MAFF106611 genome; Kmr | This study |
| pINkanR | pTBS with a 1.0-kb fragment including the kanamycin resistance gene; Kmr | This study |
| pUCP18 | E. coli-Pseudomonas shuttle vector derived from pUC18; lac promoter lacZ Cbr | 27 |
| pKZ27 | Broad-host-range transcriptional fusion vector; IncQ lacZ Kmr | 28 |
| pRCII | E. coli-Ralstonia shuttle vector derived from pKZ27; IncQ lac promoter Kmr | This study |
| pPS01 | pRCII with a 2.1-kb PCR fragment including mcpA in Ps29 | This study |
| pPS14 | pRCII with a 2.0-kb PCR fragment including mcpM in Ps29 | This study |
Kmr, kanamycin resistance; Cbr, carbenicillin resistance.
Chemotaxis assay.
The computer-assisted capillary assay method was carried out as described previously (30). Cells in a 10-μl suspension were placed onto a coverslip, and the assay was started by placing the coverslip upside down on the U-shaped spacer to fill the chemotaxis chamber with the cell suspension. Cells were videotaped. Digital image processing was used to count the number of bacteria accumulating toward the mouth of a capillary containing a known concentration of an attractant plus 1% (wt/vol) agarose. The strength of the chemotactic response was determined by the number of bacterial cells per frame. Chemotaxis buffer consisted of 10 mM HEPES buffer (pH 7.0).
DNA manipulation.
Standard procedures were used for plasmid DNA preparations, restriction enzyme digestions, ligations, transformations, and agarose gel electrophoresis (24). PCR was carried out by using Kod FX Neo polymerase (Toyobo, Tokyo, Japan) according to the manufacturer's instructions. Oligonucleotides used for PCR are listed in Table 2. Plasmids were introduced into R. pseudosolanacearum strains by transconjugation using E. soli S17-1 or by electroporation using the CM 630 system (BTX Instrument Division of Genetronics Inc., MA, USA) with a capacitance of 25 μF and an electric field of 12.5 kV/cm.
TABLE 2.
Oligonucleotides used in this study

Construction of unmarked deletion mutants of R. pseudosolanacearum Ps29 and MAFF106611.
The putative mcp and cheA genes in R. pseudosolanacearum Ps29 and MAFF106611 were deleted by an unmarked-gene-deletion technique using suicide plasmid pK18mobsacB that harbors a kanamycin resistance (kan) gene as a selection marker and the sacB gene as a counterselection marker (26). The general procedure was as follows. All PCR primers (Table 2) were designed based on the genome sequence of R. pseudosolanacearum GMI1000 (formerly R. solanacearum GMI1000), and additional nucleotides with appropriate restriction sites were added at the 5′ ends of primers for convenience of plasmid construction. Four primers were used to amplify 0.6- to 1.2-kb regions upstream and downstream, respectively, of the target gene from R. pseudosolanacearum Ps29 and MAFF106611. The amplified DNA fragments were digested with appropriate restriction enzymes and ligated with the backbone of pK18mobsacB digested with appropriate restriction enzymes. The resulting plasmid was introduced into R. pseudosolanacearum Ps29 or MAFF106611. Single-crossover recombination between homologous regions of genomic DNA and the plasmid resulted in the integration of the plasmid into the genome. Cells containing the integrated plasmid were selected by kanamycin resistance (Kmr), and sucrose sensitivity was confirmed. Cells undergoing the second single-crossover recombination (plasmid excision) were then selected on plates containing 6% sucrose, yielding sucrose-resistant, kanamycin-sensitive cells. Depending on the excision crossover, the resulting strain harbored either the wild-type gene or an unmarked deletion of the target gene. The latter genotype was confirmed by visualizing the size of the amplicon generated by using PCR primers flanking the target gene.
Construction of plasmids for complementation analysis.
pRCII was constructed to provide a plasmid vector for complementation analysis of R. pseudosolanacearum mutants. The construction scheme and physical map of pRCII are shown in Fig. S1 in the supplemental material. To construct pRCII, regions corresponding to the origin of replication from pKZ27 (28), the kan gene from pUC4K (31), and the lac promoter and multiple-cloning sites from pUCP18 (27) were amplified by PCR using primer pairs RCIIoriVf/RCIIoriVr, CLkanRf/CLkanRr2, and RCIIMCSf/RCIIMCSr, respectively. The amplified kan gene and the origin of replication were digested with NdeI and SacII and ligated together to obtain pRC. The amplified region including the lac promoter and multiple-cloning sites was digested with NdeI and ligated with NdeI-digested pRC to obtain pRCII. To construct pPS01, a 2.1-kb region containing the RSc0606 homolog gene of R. pseudosolanacearum Ps29 was amplified by PCR using primer pair CLRS01f/CLRS01r. The amplified fragments were digested with EcoRI and BamHI and ligated with EcoRI- and BamHI-digested pRCII. To construct pPS14, a 2.0-kb region containing the RSp0507 homolog gene of R. pseudosolanacearum Ps29 was amplified by PCR using primer pair CLRS14f/CLRS14r. The amplified fragments were digested with EcoRI and BamHI and ligated with the backbone of EcoRI- and BamHI-digested pRCII.
Construction of the Kmr strain of R. pseudosolanacearum.
Monteiro et al. showed previously that one of the longest intergenic regions in R. pseudosolanacearum GMI1000 was a permissive site, that is, that integration of insertion elements into this interval did not affect viability or pathogenicity (32). The kan gene cassette was inserted into the corresponding intergenic region of R. pseudosolanacearum MAFF106611 to generate a Kmr strain for use in competitive tomato colonization assays. For this purpose, suicide plasmid pINkanR was constructed. PCR using primer pairs TBSUf/TBSUr and TBSDf/TBSDr was conducted to amplify 0.8-kb and 1.2-kb regions of the intergenic region, respectively. The amplified regions were digested with BamHI and EcoRV and with EcoRV and PstI, respectively, and ligated with the backbone of BamHI- and PstI-digested pK18mobsacB to obtain pTBS. The kan gene was amplified from pUC4K by PCR using primer pair CLkanRf/CLkanRr, and the PCR product was ligated with EcoRV-digested pTBS to generate pINkanR. The chromosomal insertion of the kan gene in R. pseudosolanacearum MAFF106611 was obtained by transforming the strain with pINkanR and selecting for Kmr, thereby selecting for a homologous double-crossover recombination event. One such transformant showing a growth rate comparable to that of the wild-type strain was selected and designated MFK.
Virulence assay.
Fifty grams of quartz sand (grain size, 0.1 to 0.3 mm) was placed into each glass tube (35-mm inner diameter, 40-mm outer diameter, and 120-mm length). The open end of the tube was plugged with a silicone resin stopper. The tube was then autoclaved for 15 min at 121°C. PNS (plant nutrient solution) (33) contained 0.295 g/liter Ca(NO3)2·4H2O, 0.126 g/liter KNO3, 0.123 g/liter MgSO4·7H2O, 0.136 g/liter KH2PO4, and trace elements (4.6 mg/liter Fe [as FeEDTA], 0.5 mg/liter B, 0.05 mg/liter Zn, 0.02 mg/liter Cu, 0.01 mg/liter Mo). Sterile PNS (12.5 ml) was added to each autoclaved sand column. Tomato (Solanum lycopersicum cv. Oogata-fukuju) seeds were sterilized by gentle shaking for 10 min in a solution containing 8.75% (vol/vol) sodium hypochlorite supplemented with 0.1% (vol/vol) Tween 20 and then washed six times for 15 min/cycle in sterile deionized water. To synchronize germination, sterile seeds were kept overnight at 4°C in the dark. Seeds then were placed onto petri dishes containing PNS solidified with 1.5% (wt/vol) agar and incubated in a climate-controlled growth chamber (Sanyo, Osaka, Japan) for 7 days at 28°C with a 16-h-light/8-h-dark cycle. Seven-day-old tomato roots were wounded by cutting 1 cm away from the base of the stem. Bacterial cells were grown for 20 h in RSM medium, centrifuged (3,300 × g for 2 min), washed twice with sterile deionized water, and adjusted to 106 CFU/ml in sterile deionized water. For the sand soak inoculation method, the wounded seedling was planted near one wall of the tube, and 50 μl of the cell suspension was inoculated near the opposite wall of the tube (the distance between the seedling and the inoculation spot was 30 mm). For the root dip inoculation method, the wounded seedling was dipped into the cell suspension for 10 s and planted in the center of the tube. For both methods, the plants were maintained in a climate-controlled growth chamber (28°C with a 16-h-light/8-h-dark cycle) for 7 to 10 days and observed daily.
Competitive plant colonization assay.
Twenty grams of quartz sand was put into each glass tubes (22-mm inner diameter, 25-mm outer diameter, and 120-mm length). The tube was sterilized as described above for virulence assays. A germinated tomato seed was aseptically placed at the center of each growth tube 5 mm below the surface of the quartz sand and then grown in a climate-controlled growth chamber (28°C with a 16-h-light/8-h-dark cycle) for another 3 days. Bacterial cells were grown for 20 h in RSM medium, centrifuged (3,300 × g for 2 min), washed twice with sterile deionized water, and adjusted to 107 CFU/ml in sterile deionized water. For the competitive colonization assay, 50 μl of a 1:1 (vol/vol) mixture of the tested strain and the competitor (the Kmr strain of R. pseudosolanacearum MAFF106611) was mixed and inoculated toward the edge of each plant growth tube. The plant growth tubes were incubated in a climate-controlled growth chamber (28°C with a 16-h-light/8-h-dark cycle). After 2, 4, and 6 days of incubation, each tomato seedling was homogenized and shaken vigorously in 0.5 ml of sterile deionized water to suspend the bacteria. The bacterial suspension was diluted, and 50 μl was plated onto CPG agar plates with and without kanamycin.
Collection of tomato root exudate.
Root exudate was prepared from tomato plant (S. lycopersicum cv. Oogata-fukuju). Three-day-old germinated seeds were prepared as described above for the competitive plant colonization assay. Root exudate was collected in a Magenta GA-7 vessel (Sigma-Aldrich, St. Louis, MO, USA) equipped with a perforated tray and filled with a volume of 80 ml of sterile Milli-Q water up to the tray. Forty germinated seeds were placed onto the tray, with their roots in the solution. After 10 days of growth in a climate-controlled growth chamber (28°C with a 16-h-light/8-h-dark cycle), an aliquot of the exudate was taken directly from the Magenta vessel and tested for sterility on CPG and 2× YT agar plates. The rest of the exudate was filtered to remove solid plant material, snap-frozen in liquid nitrogen, and lyophilized; the resting solid material was redissolved in 2.0 ml of Milli-Q water. This 40-fold-concentrated exudate was stored at −20°C until use. Only root exudate in which no microbial growth was detected was used.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 22 mcp genes in R. pseudosolanacearum Ps29 have been deposited in the DDBJ, EMBL-Bank, and GenBank nucleotide sequence databases under accession numbers LC005226 to LC005247 (see Table S1 in the supplemental material). The nucleotide sequences of the mcpA, mcpM, and cheA genes of R. pseudosolanacearum MAFF106611 have been deposited in the same databases under accession numbers LC005224, LC005225, and LC005222, respectively.
RESULTS
Chemotactic responses of R. pseudosolanacearum Ps29 to various compounds.
It is important to select a strain that shows superior motility under chemotaxis assay conditions to effectively carry out chemotaxis research. Microscopic observations and swimming plate assays found that the motility of R. pseudosolanacearum Ps29 was superior to that of R. pseudosolanacearum MAFF106611 (see Fig. S2A and S2B in the supplemental material). Although the genome sequence of R. pseudosolanacearum Ps29 has not been determined, we presumed that the R. pseudosolanacearum GMI1000 database (http://sequence.toulouse.inra.fr/R.solanacearum.html) could be used as a reference for the identification of methyl-accepting chemotaxis protein genes (mcp genes) and chemotaxis-related genes, given that both strains GMI1000 and Ps29 belong to former R. solanacearum phylotype I, race 1, and biovar 3. To confirm our prediction, we performed PCR analysis of the R. pseudosolanacearum Ps29 genome based on the GMI1000 sequence. R. pseudosolanacearum GMI1000 possesses 22 putative mcp genes. PCR using primers specific to each of the 22 putative mcp genes in R. pseudosolanacearum GMI1000 and genomic DNA of R. pseudosolanacearum Ps29 as a template yielded PCR products with sizes matching those predicted from the R. pseudosolanacearum GMI1000 genome (data not shown). Furthermore, DNA sequencing confirmed that the amplified DNA fragments from the R. pseudosolanacearum Ps29 genome contained open reading frames encoding proteins with >99% identity to the counterparts of R. pseudosolanacearum GMI1000 MCPs (see Table S1 in the supplemental material). These results demonstrated that R. pseudosolanacearum Ps29 possesses homologs of 22 R. pseudosolanacearum GMI1000 mcp genes. Thus, we selected R. pseudosolanacearum Ps29 as a model strain for further chemotaxis research.
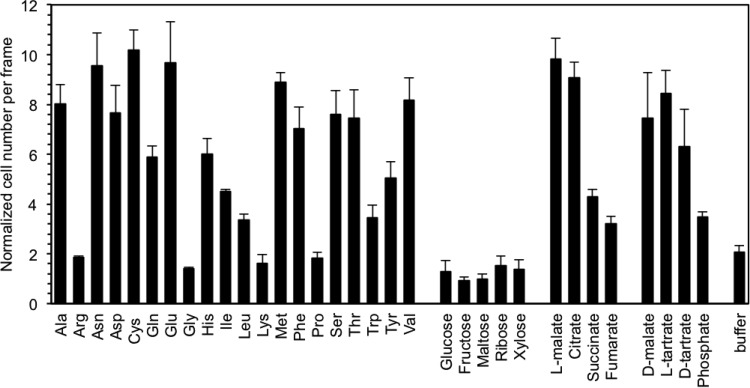
To identify chemoattractants, we measured chemotactic responses of wild-type R. pseudosolanacearum Ps29 to amino acids, organic acids, and sugars known to be major components of root exudate (34) by a computer-assisted capillary assay (30). In comparison with responses to a negative control (HEPES buffer), R. pseudosolanacearum Ps29 showed significant responses (P < 0.05 by Student's t test) to l-malate, citrate, fumarate, succinate, and 16 of the 20 standard amino acids (excepting arginine, glycine, lysine, and proline) (Fig. 1). R. pseudosolanacearum Ps29 was also attracted by growth substrates, including inorganic phosphate, l-tartrate, and d-tartrate. However, this strain did not respond to any of the tested utilizable (glucose and fructose) or nonutilizable (maltose, ribose, and xylose) sugars. Interestingly, R. pseudosolanacearum Ps29 showed chemotactic responses not only to naturally occurring l-malate but also to d-malate (Fig. 1), which is a nonphysiological isomer of malate and does not support growth of R. pseudosolanacearum Ps29 as a carbon and energy source (data not shown).
FIG 1.
Chemotactic responses to plant-related compounds by R. pseudosolanacearum wild-type strain Ps29. Bacterial cells were grown in RSM medium for 4 h after preculture in CPG medium. Digital image processing was used to count the number of bacteria around the mouth of a capillary containing the test compound and 1% (wt/vol) agarose. Compounds were used at a concentration of 5 mM. Buffer indicates 10 mM HEPES buffer as a negative control. Videotape frames were analyzed at the initiation of observation and 1 min after initiation. Normalized cell numbers were calculated by dividing the number of bacterial cells at 1 min by that at the initiation of observation. Vertical bars represent the standard errors for measurements done at least in triplicate.
Identification of MCPs for l-malate.
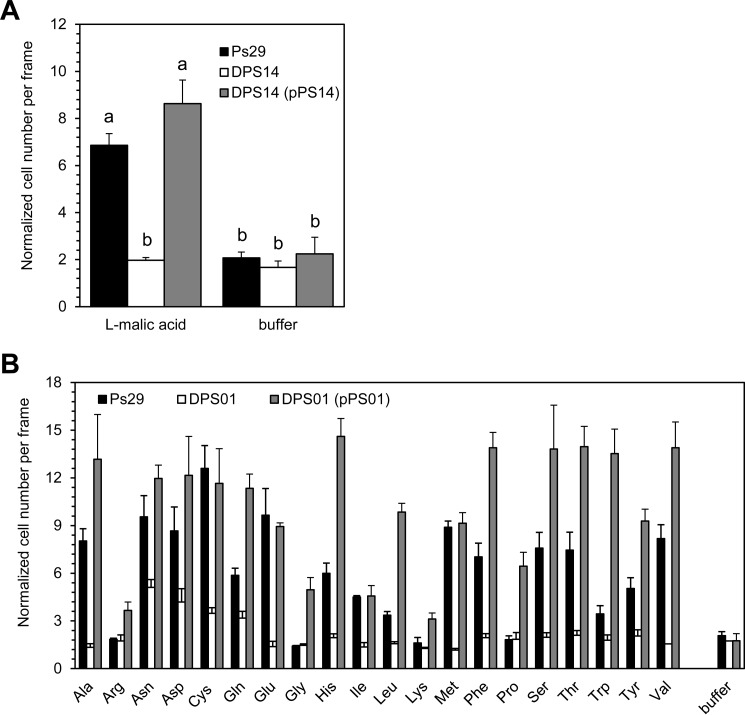
To identify genes encoding MCPs for specific chemoattractants, we constructed a library of R. pseudosolanacearum Ps29 single mutants harboring unmarked deletions in each of the 22 mcp genes. We then attempted to identify a MCP for l-malate by screening the library for a mutant deficient in the chemotactic response to l-malate. Among 22 mcp single-deletion mutants, strain DPS14, a mutant deleted for a homolog of R. pseudosolanacearum GMI1000 RSp0507, showed significantly lower-level responses to l-malate than did wild-type Ps29 (P < 0.05 by Student's t test) (Fig. 2A). The other 21 mcp single-deletion mutants showed responses to l-malate comparable to that of wild-type strain Ps29 (data not shown). The introduction of plasmid pPS14, which harbors the Ps29 RSp0507 homolog, restored the ability of strain DPS14 to respond to l-malate (Fig. 2A), demonstrating that the RSp0507 homolog encodes a MCP for l-malate. DNA sequencing revealed that the predicted RSp0507-homologous protein of Ps29 is 99% identical (596 amino acids [aa] with a 600-aa overlap) to the GMI1000 RSp0507 protein (see Table S1 in the supplemental material). We designated the RSp0507 homolog mcpM. The chemotactic responses of strain DPS14 to citrate, succinate, and fumarate did not differ significantly from those of wild-type strain Ps29 (data not shown), suggesting that McpM is not involved in chemotaxis toward organic acids other than l-malate. This result does not necessarily rule out the ability of McpM to sense these organic acids, but we infer that McpM is the major MCP for l-malate in R. pseudosolanacearum Ps29.
FIG 2.
Chemotactic responses to amino acids and l-malate by mutant and complemented R. pseudosolanacearum Ps29 strains. Bacterial cells were grown for 4 h in RSM medium after preculture in CPG medium with or without kanamycin. Buffer indicates 10 mM HEPES buffer as a negative control. (A) Chemotactic responses to 0.5 mM l-malate by R. pseudosolanacearum wild-type strain Ps29, the mcpM deletion mutant (DPS14), and DPS14 harboring the complementing plasmid [DPS14(pPS14)]. Videotape frames were analyzed at the initiation of observation and 1 min after initiation. Different letters indicate significant differences (P < 0.05 by Student's t test). (B) Chemotactic responses to 5 mM naturally occurring amino acids by R. pseudosolanacearum wild-type strain Ps29, the mcpA deletion mutant (DPS01), and DPS01 harboring the complementing plasmid [DPS01(pPS01)]. Videotape frames were analyzed at the initiation of observation and 1 min after initiation, except for Asp and Cys. Chemotactic responses to Asp and Cys were analyzed 1.5 min after initiation. There are significant differences in chemotaxis toward naturally occurring amino acids other than Arg, Gly, Lys, and Pro between the wild-type strain and DPS01 (P < 0.05 by Student's t test) and between DPS01 and DPS01(pPS01) (P < 0.05 by Student's t test). There were no significant differences in chemotaxis to Arg, Gly, Lys, and Pro between the wild-type strain and DPS01, although chemotactic responses of DPS01 and DPS01(pPS01) were significantly different (P < 0.05 by Student's t test). Vertical bars represent the standard errors for measurements done at least in triplicate.
McpM shows structural characteristics typical of MCPs: a positively charged N terminus followed by a hydrophobic membrane-spanning region, a hydrophilic periplasmic domain, a second hydrophobic membrane-spanning region, and a hydrophilic cytoplasmic domain (35). Chemotactic ligands are known to bind to the periplasmic domains (ligand binding domains [LBDs]) of MCPs, thereby initiating chemotactic signaling. The diverse ligand specificities among MCPs reflect the amino acid sequence diversity of the LBDs. BLASTP analysis using the putative LBD of McpM (155 amino acids; residues 33 to 187) as a query sequence revealed that other strains of the R. solanacearum species complex and Ralstonia pickettii strains possess MCPs with LBDs highly similar to that of McpM (78 to 100% identity), while the LBDs of MCPs of Burkholderia species such as Burkholderia ambifaria and Burkholderia cenocepacia shared up to 43% identity with the LBD of McpM. Pseudomonas putida F1 McfS (Pput_4520) (36), P. putida KT2440 McpS (PP4658) (37), Pseudomonas aeruginosa PAO1 McpS (PA2652) (38), and Pseudomonas fluorescens Pf0-1 McpS (Pfl01_0728) and McpT (Pfl01_3768) (10) have been identified as MCPs for malate. However, we did not detect an apparent similarity between the LBDs of these known MCPs for malate and that of R. pseudosolanacearum Ps29 McpM (data not shown).
Identification of MCPs for amino acids.
To identify a Ps29 MCP for an amino acid(s), we also screened the mutant library for the ability to respond to leucine. Strain DPS01, a deletion mutant with a homolog of R. pseudosolanacearum GMI1000 RSc0606, was defective in chemotaxis to leucine (Fig. 2B). Other mutants strains showed responses to leucine comparable to that of wild-type strain Ps29 (data not shown). Out of the 16 amino acid attractants, strain DPS01 failed to respond to 12 amino acids and showed significantly lower-level responses to 4 amino acids (asparagine, aspartate, cysteine, and glutamine) than did wild-type strain Ps29 (P < 0.05 by Student's t test) (Fig. 2B). The introduction of plasmid pPS01 (encoding the RSc0606 homolog of Ps29) restored the ability of strain DPS01 to respond to 16 amino acids (Fig. 2B), demonstrating that the RSc0606 homolog is a MCP for amino acids in R. pseudosolanacearum Ps29. We additionally noted that strain DPS01 harboring pPS01 (carrying the RSc0606 homolog) showed significant chemotactic responses to arginine, lysine, glycine, and proline (P < 0.05), amino acids to which wild-type R. pseudosolanacearum strain Ps29 did not respond, compared to the response to the negative control (HEPES buffer) (Fig. 2B). We postulate that this effect is due to an overexpression of the RSc0606 homolog in strain DPS01. This result suggests that the RSc0606 MCP has the potential to sense all 20 naturally occurring amino acids. DNA sequence data revealed that the RSc0606-homologous protein from Ps29 is completely identical to the GMI1000 RSc0606 protein (see Table S1 in the supplemental material). We designated the RSc0606 gene mcpA.
Protein BLAST analysis revealed that, like McpM, MCPs with LBDs similar to that of McpA (239 amino acids; residues 49 to 287) are distributed in R. solanacearum species complex and R. pickettii strains (80 to 100% identity) and Burkholderia species (up to 66% identity). The LBD of McpA showed 27% identity to that of P. aeruginosa PctA (PA4309), a protein that is the major MCP for amino acids in this pseudomonad (39).
Virulence assays of wild-type and mutant strains.
Although we used R. pseudosolanacearum Ps29 for our motility assays, we noted that Ps29 yielded weaker virulence on tomato plants (see Fig. S2C in the supplemental material). We therefore returned to R. pseudosolanacearum MAFF106611 for assessing the role of mcpA and mcpM in bacterial wilt virulence on tomato. PCR analysis and DNA sequencing revealed that R. pseudosolanacearum MAFF106611 also possesses mcpA and mcpM homologs, the respective products of which are >99% identical to the R. pseudosolanacearum Ps29 counterparts. The unmarked R. pseudosolanacearum MAFF106611 mcpA deletion mutant (DMF01) and the mcpM deletion mutant (DMF14) showed chemotactic phenotypes similar to those of R. pseudosolanacearum Ps29 mcpA and mcpM deletion mutants, respectively (see Fig. S3 in the supplemental material). We also constructed an unmarked R. pseudosolanacearum MAFF106611 cheA deletion mutant (DMFcheA); as expected, this cheA mutant was nonchemotactic but motile (data not shown). We confirmed that there were no significant differences in growth in PNS medium supplemented with glucose among these mutants and wild-type strain MAFF106611 (see Fig. S4 in the supplemental material), suggesting that these mutations did not affect the growth of R. pseudosolanacearum MAFF106611.
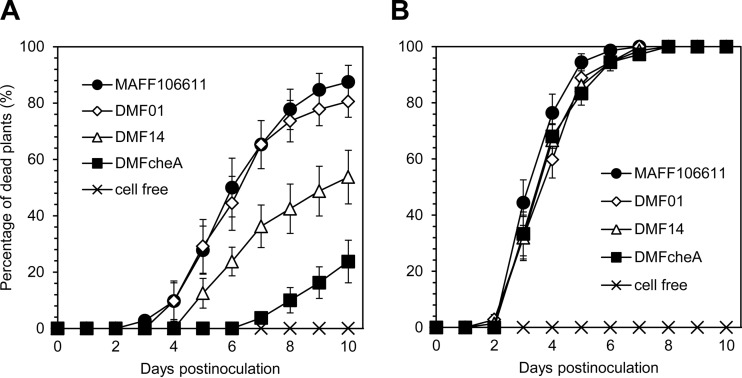
Virulence of the mutant strains was tested by the root dip inoculation method. For this method, the root tips of 7-day-old tomato plants were cut and then challenged by root dip inoculation with a cell suspension of R. pseudosolanacearum MAFF106611. Tomato plants inoculated with wild-type strain MAFF106611 started wilting at 3 days postinoculation (dpi) and died by 7 dpi. The time line of wilting in response to mutant strains DMF01, DMF14, and DMFcheA was similar to that seen with the wild-type parent, indicating that neither mcpA, mcpM, or cheA was required for virulence when bacterial cells were directly introduced into tomato plants (Fig. 3B).
FIG 3.
Virulence of R. pseudosolanacearum MAFF106611 mutants on tomato seedlings. Wild-type strain MAFF106611, the mcpA deletion mutant (DMF01), the mcpM deletion mutant (DMF14), and the cheA deletion mutant (DMFcheA) were tested. In each experiment, 8 tomato seedlings were examined and counted to calculate the percentage of dead plants. Means and standard errors were calculated from at least nine independent experiments. (A) Sand soak virulence assay. Bacterial cells were inoculated ∼30 mm away from 7-day-old wounded tomato seedlings. There were significant differences in the percentages of dead plants between the wild-type strain and DMF14 and between DMF14 and DMFcheA at 7, 8, 9, and 10 days postinoculation (P < 0.05 by Student's t test). (B) Root dip inoculation virulence assay. Bacterial cells were inoculated onto 7-day-old wounded tomato seedlings by dipping the root tip in a cell suspension. The strains were not significantly different (P < 0.05 by Student's t test).
We then tested plant infection by the mutants using the sand soak inoculation method. For this method, a bacterial cell suspension was inoculated into the sand at a spot ∼30 mm away from a tomato plant. Plant infection by this assay requires bacterial cells to locate and invade host plants from a distance. Wild-type strain MAFF106611 yielded wilting at 4 dpi, killing 90% of tomato plants at 10 dpi, while strain DMFcheA was significantly less infectious (Fig. 3A). This result suggested that the virulence assay using the sand soak inoculation method permits evaluation of chemotactic effects on plant infection. Testing of the mcp mutant strains revealed that strain DMF14 was significantly less infectious than wild-type strain MAFF106611 (P < 0.05), killing only 54% of tomato plants at 10 dpi in sand soak inoculation virulence assays (Fig. 3A). The infectivity of strain DMF01 did not differ significantly from that of wild-type strain MAFF106611 (Fig. 3A). These results suggest that McpM-mediated chemotaxis is required for full virulence of R. pseudosolanacearum MAFF106611; in contrast, McpA-mediated chemotaxis to amino acids does not play a crucial role in the initial location of plant roots by the bacterium in this sand soak inoculation virulence assay. Notably, the level of infectivity of strain DMF14, though attenuated compared to that of the wild-type strain, remained significantly higher than that of strain DMFcheA.
Chemotactic responses to root exudate and competitive plant colonization.
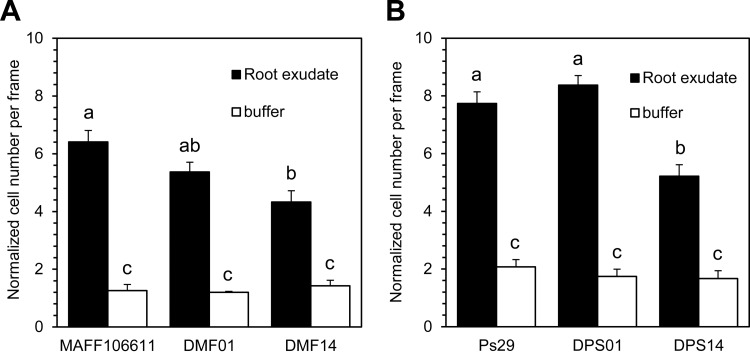
The attenuated infectivity of R. pseudosolanacearum MAFF106611 mutant strain DMF14 in the sand soak inoculation virulence assay presumably reflected a decreased ability of the mutant strain to locate tomato roots. To test this hypothesis, we evaluated the chemotactic responses of R. pseudosolanacearum MAFF106611 mcp mutants to tomato root exudate. Strain DMF14 showed a significantly lower-level chemotactic response to root exudate than did wild-type strain MAFF106611 (P < 0.05); responses did not differ significantly between DMF01 and the wild-type parent (Fig. 4A). Similar effects were observed in comparisons of wild-type and mutant strains of highly motile R. pseudosolanacearum Ps29 (Fig. 4B).
FIG 4.
Chemotactic responses to tomato root exudate by R. pseudosolanacearum MAFF106611 and Ps29 strains. The total organic carbon content of tomato root exudate used in this assay was 3.59 g C/liter total organic carbon. Buffer indicates 10 mM HEPES buffer as a negative control. (A) Chemotaxis of R. pseudosolanacearum wild-type strain MAF106611, the mcpA deletion mutant (DMF01), and the mcpM deletion mutant (DMF14). Videotape frames were analyzed at the initiation of observation and 2 min after initiation. (B) Chemotaxis of R. pseudosolanacearum wild-type strain Ps29, the mcpA deletion mutant (DPS01), and the mcpM deletion mutant (DPS14). Videotape frames were analyzed at the initiation of observation and 1 min after initiation. Vertical bars represent the standard errors for measurements done at least in triplicate. Different letters indicate significant differences (P < 0.05 by Student's t test).
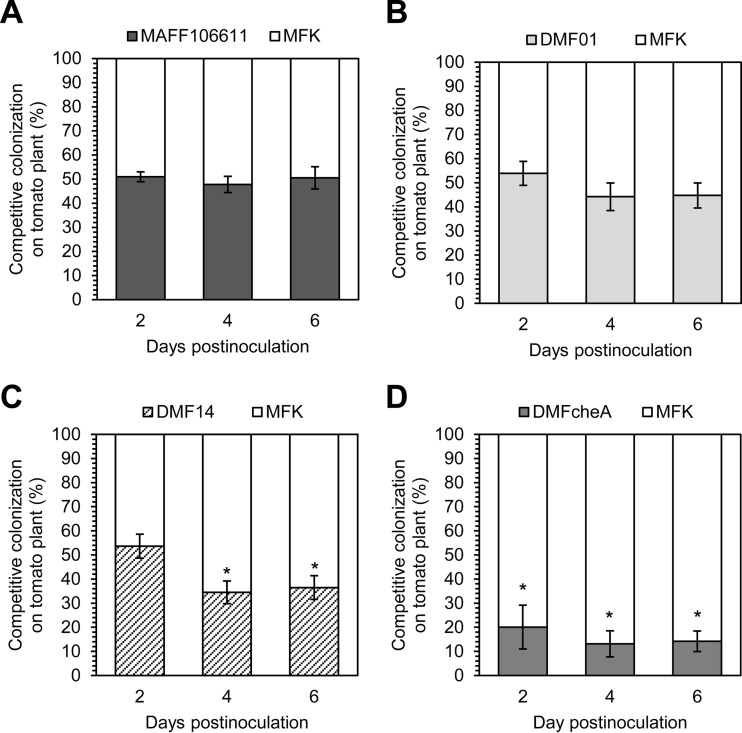
We further tested this hypothesis using competitive tomato colonization assays, specifically by inoculating tomato seedlings with a 1:1 mixture of test and competitor strains. Because the R. pseudosolanacearum MAFF106611 Kmr mutant (MFK) competed fully with wild-type strain MAFF106611 (Fig. 5A), we used MFK as the competitor strain in competitive plant colonization assays to distinguish the competitor strain from test strains; the Kmr phenotype facilitated the distinction between the test and competitor strains. The results of the competitive plant colonization assays were consistent with those of virulence assays: strain DMF14, like DMFcheA, showed an inferior plant colonization ability compared to that of MFK, while strain DMF01 fully competed with MFK (Fig. 5B to D).
FIG 5.
Plant colonization assay for competition between the R. pseudosolanacearum MAFF106611 kanamycin-resistant strain (MFK) and wild-type strain MAFF106611 (A), the mcpA deletion mutant (DMF01) (B), the mcpM deletion mutant (DMF14) (C), or the cheA deletion mutant (DMFcheA) (D). Tomato plants were sampled in at least six independent experiments conducted in triplicate per time point. Vertical bars represent the standard errors for measurements. Asterisks indicate statistically significant differences between MFK and mutants (P < 0.05 by the Wilcoxon-Mann-Whitney test).
DISCUSSION
Genomic analysis revealed that R. pseudosolanacearum GMI1000 possesses 22 putative mcp genes (see Table S2 in the supplemental material for GenBank accession numbers of genome sequences). In the present study, we demonstrated that R. pseudosolanacearum Ps29 possesses homologs of all 22 R. pseudosolanacearum GMI1000 mcp genes. Complete genome sequences of R. pseudosolanacearum FQY4 (formerly R. solanacearum FQY4 [phylotype I]), R. pseudosolanacearum CMR15 (formerly R. solanacearum CMR15 [phylotype III]), R. solanacearum CFBP2957 (phylotype IIA), R. solanacearum Po82 (phylotype IIB), and R. syzygii subsp. indonesiensis PSI07 (formerly R. solanacearum [phylotype IV]) have been determined. Although these strains belong to different phylotypes, all the sequenced strains possess 21 to 23 putative mcp genes, 19 to 21 of which are homologs of the R. pseudosolanacearum GMI1000 mcp genes. Notably, the LBDs of nominally homologous MCPs exhibit >71% respective identity. Thus, mcp genes are conserved among members of the R. solanacearum species complex.
R. pseudosolanacearum Ps29 showed chemotactic responses to amino acids, dicarboxylic acids (l-malate, d-malate, succinate, fumarate, and tartrates), tricarboxylic acid (citrate), and inorganic phosphate but not any of the tested sugars. Yao and Allen previously reported the chemotactic responses of R. solanacearum K60 (phylotype IIB) (isolated from tomato) to various plant-related organic compounds (16). The response pattern of Ps29 is similar to that of K60, although there are minor differences. Specifically, Ps29 did not respond to arginine, glycine, lysine, and proline, while K60 was attracted by proline, glycine, and lysine but failed to respond to arginine, cysteine, histidine, threonine, and tryptophan. Additionally, Ps29 was attracted by succinate, but K60 did not respond to succinate. A partial genome sequence of R. solanacearum K60 is available in the GenBank database. A BLAST search of this partial genome sequence showed the presence of a gene encoding a McpA homolog (GenBank accession number CCF97014). The LBD of the putative R. solanacearum K60 McpA protein exhibits 93% identity to the R. pseudosolanacearum Ps29 and MAFF106611 McpA proteins (data not shown). Differences in the patterns of chemotactic responses to 20 naturally occurring amino acids between Ps29/MAFF106611 and K60 may be attributed to differences in the amino acid sequences of the LBDs of their respective McpA proteins. Yao and Allen measured chemotactic responses to 8 compounds, including sugars and organic acids, by eight different strains of the R. solanacearum species complex and found that the strains varied significantly in their attraction to these compounds (16). Based on these results, these authors noted the possibility that chemotactic responses may be differentially selected traits that confer adaptation to various hosts or ecological conditions. Given that mcp genes are conserved among members of the R. solanacearum species complex, differences in expression patterns of a set of mcp genes may make a greater contribution to diverse chemotactic responses among the members of the R. solanacearum species complex than the diversity of MCPs. Therefore, comprehensive analysis of the expression of a set of mcp genes is important to understand the chemotactic response pattern in each strain of the R. solanacearum species complex.
LBDs of bacterial MCPs can be classified according to their sizes into cluster I (120 to 210 amino acids) and cluster II (220 to 299 amino acids) domains (40). The MCPs for amino acids in E. coli (Tar and Tsr) contain cluster II LBDs with 4-helix-bundle domains (41). The ligand specificity of Tar and Tsr is relatively narrow, and these MCPs sense limited numbers of amino acids (Tar, aspartate and glutamate; Tsr, serine, alanine, and glycine) (42). The PctA protein of P. aeruginosa PAO1, which senses as many as 18 naturally occurring amino acids (2), contains a cluster II LBD with a double-PDC (PhoQ/DcuS/CitA) domain (43, 44). R. pseudosolanacearum McpA, which (as we show here) is a MCP that is able to potentially sense 20 naturally occurring amino acids, also contains a cluster II LBD with a predicted LBD size of 243 amino acids. Structure prediction by the Phyre2 program (45) indicated that R. pseudosolanacearum Ps29 McpA contains a double-PDC domain in its LBD (see Fig. S5 in the supplemental material). In contrast, the LBD of R. pseudosolanacearum McpM is classified as a member of cluster I, with a predicted LBD size of 153 amino acids. Phyre2 structure analysis predicted the presence of a 4-helix-bundle domain in the LBD of McpM (see Fig. S5 in the supplemental material). Several MCPs have been reported to be chemoreceptors for malate. These MCPs include P. aeruginosa PAO1 McpS (PA2652) (38), P. putida KT2440 McpS (PP4658) (37), P. putida F1 McfS (Pput_4520) (36), and P. fluorescens Pf0-1 McpS (Pfl01_0728) and McpT (Pfl01_3768) (10). P. aeruginosa PAO1 McpS and P. fluorescens Pf0-1 McpT contain cluster I LBDs with CACHE (Ca2+ channels and chemotaxis receptors) domains (46), while the LBDs of P. putida KT2440 McpT, P. putida F1 McfS, and P. fluorescens Pf0-1 McpS belong to cluster II and contain helical bimodular (HBM) domains (47). Thus, the LBD of R. pseudosolanacearum McpM contains a different type of domain than the LBDs of Pseudomonas MCPs for malate, consistent with the lack of observed sequence similarity between the LBDs of R. pseudosolanacearum McpM and Pseudomonas MCPs for malate.
Our results showed that nonchemotactic but motile mutant strain DMFcheA (ΔcheA) displayed decreased infectivity to tomato plants in sand soak inoculation plant virulence assays and exhibited decreased tomato plant colonization in competitive plant colonization assays compared to the wild-type parent (MAFF106611). These data are consistent with those reported by Yao and Allen (16). These results confirmed that taxis is involved in migration to plants in soils and in plant infection by R. pseudosolanacearum. Our assays also demonstrated decreased plant infection, attenuated colonization, and weakened responses to tomato root exudate by a mcpM deletion mutant (strain DMF14) compared to the wild-type strain. These results indicate that in addition to Aer-mediated aerotaxis (energy taxis), McpM-mediated chemotaxis to certain components of root exudate is required for effective plant infection by R. pseudosolanacearum. Compared to the parent strain, the mcpM mutant showed decreased responses to malate but was not altered in responses to other organic acids (succinate, fumarate, and citrate). Notably, malate has been reported to constitute a major component of tomato root exudate (34). Therefore, it is highly likely that McpM-mediated chemotaxis to malate is involved in tomato plant infection by R. pseudosolanacearum. Although amino acids were also reported to be major components of tomato root exudate (48), the mcpA deletion mutant (strain DMF01) was as infectious as the wild-type strain in sand soak inoculation plant virulence assays and competed fully with the wild-type strain in competitive plant colonization assays. Since the response of the mcpA mutant to root exudate was as strong as that of the wild-type strain, it seems that the concentrations of amino acids in root exudate were too low to elicit strong chemotactic responses in R. pseudosolanacearum.
The cheA deletion mutant (DMFcheA) had decreased infectivity compared to that of the mcpM mutant. This distinction may reflect the fact that the cheA mutant is also deficient in Aer-mediated energy taxis as well as chemotaxis (21). Alternatively, a root exudate component(s) other than l-malate may be involved in plant colonization and plant infection by R. pseudosolanacearum. Citrate, which is abundant in tomato root exudate (34) and is a strong attractant of R. pseudosolanacearum, is a likely candidate for such a component. We are currently using our R. pseudosolanacearum mcp single mutant library to identify possible citrate-sensing MCPs.
Supplementary Material
ACKNOWLEDGMENTS
J.K. was supported by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-Oriented Industry of the Bio-Oriented Technology Research Advancement Institution (BRAIN), Japan; the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry of the Ministry of Agriculture, Forestry and Fisheries of Japan; and grant-in-aid for scientific research (B) 15H04478 from the Japan Society for Promotion of Science (JSPS). A.H. was supported by JSPS KAKENHI grant number 15J05572.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01870-15.
REFERENCES
- 1.Adler J. 1966. Chemotaxis in bacteria. Science 153:708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- 2.Taguchi K, Fukutomi H, Kuroda A, Kato J, Ohtake H. 1997. Genetic identification of chemotactic transducers for amino acids in Pseudomonas aeruginosa. Microbiology 143:3223–3229. doi: 10.1099/00221287-143-10-3223. [DOI] [PubMed] [Google Scholar]
- 3.Moulton RC, Montie TC. 1979. Chemotaxis by Pseudomonas aeruginosa. J Bacteriol 137:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moench TT, Konetzka WA. 1978. Chemotaxis in Pseudomonas aeruginosa. J Bacteriol 133:427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato J, Ito A, Nikata T, Ohtake H. 1992. Phosphate taxis in Pseudomonas aeruginosa. J Bacteriol 174:5149–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chet I, Mitchel R. 1976. Ecological aspects of microbial chemotactic behavior. Annu Rev Microbiol 30:221–239. [DOI] [PubMed] [Google Scholar]
- 7.Miller LD, Yost CK, Hynes MF, Alexandre G. 2007. The major chemotaxis gene cluster of Rhizobium leguminosarum bv viciae is essential for competitive nodulation. Mol Microbiol 63:348–362. doi: 10.1111/j.1365-2958.2006.05515.x. [DOI] [PubMed] [Google Scholar]
- 8.de Weert S, Vermeiren H, Mulders IHM, Kuiper I, Hendrickx N, Bloemberg GV, Vanderleyden J, De Mot R, Lugtenberg BJJ. 2002. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant Microbe Interact 15:1173–1180. doi: 10.1094/MPMI.2002.15.11.1173. [DOI] [PubMed] [Google Scholar]
- 9.Oku S, Komatsu A, Tajima T, Nakashimada Y, Kato J. 2012. Identification of chemotaxis sensory proteins for amino acids in Pseudomonas fluorescens Pf0-1 and their involvement in chemotaxis to tomato root exudate and root colonization. Microbes Environ 27:462–469. doi: 10.1264/jsme2.ME12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oku S, Komatsu A, Nakashimada Y, Tajima T, Kato J. 2014. Identification of Pseudomonas fluorescens chemotaxis sensory proteins for malate, succinate, and fumarate, and their involvement in root colonization. Microbes Environ 29:413–419. doi: 10.1264/jsme2.ME14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stock JB, Surette MG. 1996. Chemotaxis, p 1103–1129. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 12.Porter SL, Wandhams GH, Armitage JP. 2011. Signal processing in complex chemotaxis pathways. Nat Rev Microbiol 9:153–165. doi: 10.1038/nrmicro2505. [DOI] [PubMed] [Google Scholar]
- 13.Hayward AC. 1991. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87. doi: 10.1146/annurev.py.29.090191.000433. [DOI] [PubMed] [Google Scholar]
- 14.Hayward AC. 2000. Ralstonia solanacearum, p 32–42. In Lederberg J, Alexander M, Bloom BR, Hopwood D, Hull R, Iglewiski BH, Laskin AI, Oliver SG, Schaechter M, Summers WC (ed), Encyclopedia of microbiology, vol 4 Academic Press Inc, San Diego, CA. [Google Scholar]
- 15.Genin S. 2010. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol 187:920–928. doi: 10.1111/j.1469-8137.2010.03397.x. [DOI] [PubMed] [Google Scholar]
- 16.Yao J, Allen C. 2006. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J Bacteriol 188:3697–3708. doi: 10.1128/JB.188.10.3697-3708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillings MR, Fahy P. 1994. Genomic fingerprinting: towards a unified view of the Pseudomonas solanacearum species complex, p 95–112. In Hayward AC, Hartman GL (ed), Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. CAB International, Wallingford, United Kingdom. [Google Scholar]
- 18.Fegan M, Prior P. 2006. Diverse members of the Ralstonia solanacearum species complex cause bacterial wilt of banana. Australas Plant Pathol 35:93–101. doi: 10.1071/AP05105. [DOI] [Google Scholar]
- 19.Guidot J, Prior P, Schoenfeld J, Carrère S, Genin S, Boucher C. 2007. Genomic structure and phylogeny of the plant pathogen Ralstonia solanacearum inferred from gene distribution analysis. J Bacteriol 189:377–387. doi: 10.1128/JB.00999-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safni I, Cleenwerck I, De Vos P, Fegan M, Sly L, Kappler U. 2014. Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int J Syst Evol Microbiol 64:3087–3103. doi: 10.1099/ijs.0.066712-0. [DOI] [PubMed] [Google Scholar]
- 21.Yao J, Allen C. 2007. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J Bacteriol 189:6415–6424. doi: 10.1128/JB.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefeuvre P, Cellier G, Remenant B, Chiroleu F, Prior P. 2013. Constraints on genome dynamics revealed from gene distribution among the Ralstonia solanacearum species. PLoS One 8:e63155. doi: 10.1371/journal.pone.0063155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada T, Kawasaki T, Nagata S, Fujiwara A, Usami S, Fujie M. 2007. Isolation and characterization of bacteriophages that infect the phytopathogen Ralstonia solanacearum. Microbiology 153:2630–2639. doi: 10.1099/mic.0.2006/001453-0. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 25.Simon R, Prifer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 26.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer HP. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97:109–112. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Kato J, Kuroda A, Ikeda T, Takiguchi N, Ohtake H. 2000. Identification and characterization of two chemotactic transducers for inorganic phosphate in Pseudomonas aeruginosa. J Bacteriol 182:3400–3404. doi: 10.1128/JB.182.12.3400-3404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrick CA, Sequeira L. 1984. Lipopolysaccharide-defective mutants of the wilt pathogen Pseudomonas solanacearum. Appl Environ Microbiol 48:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikata T, Sumida K, Kato J, Ohtake H. 1992. Rapid method for analyzing bacterial behavioral responses to chemical stimuli. Appl Environ Microbiol 58:2250–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vieira J, Messing J. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 32.Monteiro F, Sole M, van Dijk I, Valls M. 2012. A chromosomal insertion toolbox for promoter probing, mutant complementation, and pathogenicity studies in Ralstonia solanacearum. Mol Plant Microbe Interact 25:557–568. doi: 10.1094/MPMI-07-11-0201. [DOI] [PubMed] [Google Scholar]
- 33.Hoffland E, Findenegg GR, Nelemans JA. 1989. Solubilization of rock phosphate by rape. Plant Soil 113:161–165. doi: 10.1007/BF02280176. [DOI] [Google Scholar]
- 34.Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B. 2006. Organic acids, sugars, and l-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact 19:250–256. doi: 10.1094/MPMI-19-0250. [DOI] [PubMed] [Google Scholar]
- 35.Falke JJ, Haselbauer GL. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem Sci 26:257–265. doi: 10.1016/S0968-0004(00)01770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parales RE, Luu RA, Chen GY, Lui X, Wu V, Lin P, Hughes JG, Nesteryuk V, Parales JV, Ditty JL. 2013. Pseudomonas putida F1 has multiple chemoreceptors with overlapping specificity for organic acids. Microbiology 159(Part 6):1086–1096. doi: 10.1099/mic.0.065698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacal J, Alfonso C, Liu X, Parales RE, Morel B, Conejero-Lara F, Rivas G, Duque E, Ramos J, Krell T. 2010. Identification of a chemoreceptor for tricarboxylic acid cycle intermediates: differential chemotactic response towards receptor ligands. J Biol Chem 285:23126–23136. doi: 10.1074/jbc.M110.110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Ortega C, Harwood CS. 2007. Identification of a malate chemoreceptor in Pseudomonas aeruginosa by screening for chemotaxis defects in an energy taxis-deficient mutant. Appl Environ Microbiol 73:7793–7795. doi: 10.1128/AEM.01898-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuroda A, Kumano T, Taguchi K, Nikata T, Kato J, Ohtake H. 1995. Molecular cloning and characterization of a chemotactic transducer gene in Pseudomonas aeruginosa. J Bacteriol 177:7019–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacal J, García-Fontana C, Muñoz-Martínez F, Ramos JL, Krell T. 2010. Sensing of environmental signals: classification of chemoreceptors according to the size of their ligand binding regions. Environ Microbiol 12:2873–2884. doi: 10.1111/j.1462-2920.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 41.Ulrich LE, Zhulin IB. 2005. Four-helix bundle: a ubiquitous sensory module in prokaryotic signal transduction. Bioinformatics 21(Suppl 3):iii45–iii48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart RC, Dahlquist FW. 1987. Molecular components of bacterial chemotaxis. Chem Rev 87:997–1025. doi: 10.1021/cr00081a007. [DOI] [Google Scholar]
- 43.Zhang Z, Hendrickson WA. 2010. Structural characterization of the predominant family of histidine kinase sensor domains. J Mol Biol 400:335–353. doi: 10.1016/j.jmb.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reyes-Darias JA, Yang Y, Sourjik V, Krell T. 2015. Correlation between signal input and output in PctA and PctB amino acid chemoreceptor of Pseudomonas aeruginosa. Mol Microbiol 96:513–525. doi: 10.1111/mmi.12953. [DOI] [PubMed] [Google Scholar]
- 45.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 Web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anantharaman V, Aravind L. 2000. Cache—a signaling domain common to animal Ca(2+)-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem 25:535–537. doi: 10.1016/S0968-0004(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 47.Ortega Á, Krell T. 2014. The HBM domain: introducing bimodularity to bacterial sensing. Protein Sci 23:332–336. doi: 10.1002/pro.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simons M, Permentier HP, de Weger LA, Wijffelman CA, Lugtenberg BJJ. 1997. Amino acid synthesis is necessary for tomato root colonization by Pseudomonas fluorescens strain WCS365. Mol Plant Microbe Interact 10:102–106. doi: 10.1094/MPMI.1997.10.1.102. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.