Abstract
Current views suggest that apoptosis eliminates genetically damaged cells that may otherwise form tumors. Prior human studies link elevated insulin and reduced apoptosis to risk of colorectal adenomas. We hypothesized that hyperinsulinemia associated with obesity would lead to reduced colon epithelial cell (CEC) apoptosis after radiation and that this effect would be altered by deletion of the insulin-like growth factor (IGF) 1 receptor (IGF1R) or the insulin receptor (IR). Mice with villin-Cre-mediated IGF1R or IR deletion in CECs and floxed littermates were fed a high-fat diet to induce obesity and hyperinsulinemia or control low-fat chow. Mice were exposed to 5-Gy abdominal radiation to induce DNA damage and euthanized 4 h later for evaluation of apoptosis by localization of cleaved caspase-3. Obese mice exhibited decreased apoptosis of genetically damaged CECs. IGF1R deletion did not affect CEC apoptosis in lean or obese animals. In contrast, IR loss increased CEC apoptosis in both diet groups but did not prevent antiapoptotic effects of obesity. Levels of p53 protein were significantly reduced in CECs of obese mice with intact IR but increased in both lean and obese mice without IR. Levels of mRNAs encoding proapoptotic Perp and the cell cycle inhibitor Cdkn1b/p27 were reduced in CECs of obese mice and increased in lean mice lacking IR. Together, our studies provide novel evidence for antiapoptotic roles of obesity and IR, but not IGF1R, in colonic epithelium after DNA damage. However, neither IR nor IGF1R deletion prevented a reduction in radiation-induced CEC apoptosis during obesity and hyperinsulinemia.
Keywords: obesity, insulin receptor, insulin-like growth factor 1 receptor, apoptosis, colon
obesity and type 2 diabetes have been linked to increased risk of a number of cancers (2, 13, 26, 40), including colorectal cancer (CRC) (7, 35, 68, 86). Hyperinsulinemia occurs during obesity and type 2 diabetes and is a compensatory response to impaired insulin receptor (IR) signaling, which may contribute to increased CRC risk, decreased response to chemotherapy or radiotherapy, and increased CRC recurrence and mortality (19, 27, 31, 56, 70). However, the mechanisms underlying CRC risk and poor treatment outcome during obesity and hyperinsulinemia remain unclear.
Current views support the concept that tumors can arise due to survival and subsequent expansion of genetically damaged stem or progenitor cells (10, 73). Apoptosis is a programmed cell death that eliminates cells with DNA damage that may otherwise contribute to neoplasia. The frequency of basal, spontaneous apoptosis in the nonchallenged small intestinal epithelium is very low (61, 62, 82). Spontaneous apoptosis in the colonic epithelium is even rarer, consistent with relatively high levels of the antiapoptotic protein BCL-2 and low levels of the proapoptotic p53 (50, 51, 61, 79). Therefore, models of DNA damage induced by low-dose (1–6 Gy) ionizing radiation with evaluation of maximal apoptosis at 3–4.5 h after radiation have served as useful systems to study apoptosis in the intestinal epithelium (62, 64, 65, 82). Whereas in the small intestine both spontaneous and radiation-induced apoptosis occur primarily at the crypt base, where stem cells reside, apoptosis in the colon is scattered along the crypt and is not associated with the position of putative stem cells (61, 62).
Our group and others have reported that insulin-like growth factor (IGF) 1 (IGF1) inhibits radiation-induced apoptosis in the intestinal crypts (64, 65, 82). IGFs potently stimulate intestinal growth (15, 34, 53, 69) and are widely considered mediators of survival or proliferation of cancer cells (32, 59). Levels of bioavailable IGF1 can be increased by elevated plasma insulin, which inhibits hepatic production of IGF-binding protein (IGFBP) 1 (22, 55). Both insulin and IGF1 can exert mitogenic and antiapoptotic actions in normal and cancer cells via the IR or the IGF1 receptor (IGF1R) (11, 15, 30, 32), both of which belong to the receptor tyrosine kinase family and are highly homologous in structure (81). Because of the similarity between these receptors and their ability to mediate the actions of both IGF1 and insulin when these ligands are present at high circulating levels, studies of the specific roles of IGF1R vs. IR in proliferation and antiapoptosis during hyperinsulinemia have been challenging.
Traditionally, IGF1R has been viewed as the main mediator of the trophic, antiapoptotic, and protumorigenic actions of IGFs, and IGF1R overexpression has been reported in colorectal adenocarcinomas (3, 9, 33, 46, 80). In contrast, IR has been considered to play a larger role in mediating the metabolic actions of insulin (1, 17, 44). However, a concept that IR may promote tumor growth is based on evidence that a proliferative isoform of IR (IR-A) is highly expressed in cancer cells or tumors and studies showing that IR promoted tumor cell survival or growth when IGF1R was inhibited (5, 12, 18, 36, 39, 76). Opposing this evidence was a study showing that knockdown of IR-A in SW480 colon cancer cells promoted cell viability, which was associated with enhanced IGF1R phosphorylation (16). Therefore, the role of IR in colon epithelial cell (CEC) growth and survival is unclear.
Our prior human studies found that high plasma insulin and low apoptosis in normal rectal mucosa were consistently associated with colorectal adenomas in multiple patient cohorts (42, 43, 66, 77). Therefore, defining the roles of obesity-associated hyperinsulinemia and IGF1R vs. IR in promoting survival of genetically damaged CECs is relevant to mechanisms by which obesity may promote risk of early-stage colon tumors. In this study we used a model of high-fat diet (HFD)-induced obesity and hyperinsulinemia challenged with DNA damage caused by low-dose radiation to directly evaluate the impact of obesity on apoptosis of genetically damaged CECs. These studies were performed in mice with genetic disruption of IGF1R or IR in intestinal epithelial cells (IECs) and littermate controls to more directly test whether loss of IGF1R- or IR-linked signaling affected radiation-induced CEC apoptosis in lean mice or during obesity and hyperinsulinemia. We hypothesized that obesity/hyperinsulinemia would lead to decreased apoptosis of genetically damaged CECs and that these antiapoptotic effects would be prevented or attenuated by deletion of IGF1R or IR. Our results provide novel evidence that obesity and hyperinsulinemia promote reduced apoptosis of CECs following DNA damage. Furthermore, our data indicate that IR, but not IGF1R, deletion increases apoptosis in lean and obese mice but does not prevent a reduction in apoptosis during obesity.
MATERIALS AND METHODS
Animals.
Mice with loxP sites flanking exon 3 of the Igf1r gene (IGF1Rfl/fl) were provided by Dr. Argiri Efstratiadis (Columbia University, New York, NY) and have been described by Xuan et al. (85) and Zhang et al. (87). Mice with loxP sites flanking exon 4 of the Insr gene (IRfl/fl) were provided by Dr. Ronald Kahn and have been described by Brüning et al. (17). To generate mice with IEC-specific IGF1R or IR gene disruption, IGF1Rfl/fl and IRfl/fl mice were crossed with villin-Cre (VC) mice expressing a Cre recombinase transgene driven by the villin promoter (Jackson Laboratory, Bar Harbor, ME) (29). The VC transgene is expressed throughout small intestinal and colonic epithelium. All mice were on a C57BL/6 background. Study animals were derived by cross-breeding WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ mice or WT-IRfl/fl and VC-IRΔ/Δ mice. Genotyping on tail DNA was performed as previously described (14, 87). Animals were housed in a pathogen-free facility at the University of North Carolina (Chapel Hill, NC), and food and water were provided ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Diet-induced obesity.
Male WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ mice or WT-IRfl/fl and VC-IRΔ/Δ mice (10–12 wk old) were assigned to a standard rodent chow control diet (14% kcal from fat) or a HFD (product no. D12451, Research Diets, New Brunswick, NJ). The D12451 HFD contains 45% kcal from fat and comprises 31.4% saturated fat, 35.5% monosaturated fat, and 33.1% polyunsaturated fat. WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ or WT-IRfl/fl and VC-IRΔ/Δ animals on a given diet were co-housed throughout the study. In C57BL/6 mice, this HFD leads to obesity, hyperinsulinemia, and insulin resistance within 14–16 wk of onset of the diet (28, 84). Male mice were used, because our prior studies demonstrated more reproducible and consistent development of obesity and hyperinsulinemia in males than females on the C57BL/6 background (4). In the current study, after 16 wk on the control diet or HFD, blood was collected from the tail vein after an overnight fast and plasma was isolated by centrifugation at 600 relative centrifugal force for 6 min. Fasting blood glucose levels were measured using a glucometer (OneTouch Ultra 2, LifeScan, Milpitas, CA). Mice were fed the control diet or HFD for an additional 2 wk before radiation and euthanasia.
Radiation and tissue harvest.
After 18 wk on diets, mice were anesthetized with isoflurane (Baxter, Deerfield, IL) and exposed to a single 5-Gy dose of abdominal radiation using an XRad 320 (Precision X-Ray, North Branford, CT) at a rate of 1.07 Gy/min. Euthanasia was carried out with a lethal dose of pentobarbital sodium (Nembutal; 150 μg/g ip) 4 h after radiation, a time corresponding to maximal apoptosis of IECs after radiation-induced DNA damage (8, 21, 47, 60, 65). The colon was collected and flushed with cold 1× PBS (pH 7.4). All mesenteric fat was removed and discarded. Length and weight of the entire colon were recorded. For the first group of mice, the entire colon was splayed open and fixed in 4% paraformaldehyde overnight for histological scoring of apoptosis. For the second group, the most proximal and distal ∼1-cm segments of the colon were fixed in 10% zinc formalin overnight to confirm histological apoptosis data obtained from the first group. The remaining tissue was splayed open, and 3- to 4-cm sections of the distal colon were used for CEC isolation for gene and protein expression experiments. The distal colon was the focus of both histological and biochemical analyses, because, in mouse models of CRC, tumors develop mainly in the distal colon, and prior studies have documented reduced apoptosis in rectal biopsies from patients with elevated plasma insulin (43, 77).
Blood and plasma measurements.
Fasting plasma insulin was measured by ELISA (Mercodia, Uppsala, Sweden). Insulin sensitivity was estimated by calculation of homeostatic model assessment (HOMA) values using the following formula: fasting glucose (mmol/l) × fasting insulin (mU/l)/22.5. Higher HOMA values indicate decreased insulin sensitivity. Blood was collected by cardiac puncture at the time of euthanasia, and plasma was extracted using acid-ethanol to remove IGFBPs, as previously described (74). IGF1 levels in plasma were measured by ELISA (R & D Systems, Minneapolis, MN).
Immunohistochemistry and histological analyses.
Histological sections were visualized with a bright-field microscope (Axio Imager.A2, Zeiss, Thornwood, NY) and a ProgRes CF Scan camera (Jenoptik, Jena, Thuringia, Germany). After overnight fixation in 10% zinc formalin, closed cross sections were embedded in paraffin for hematoxylin-and-eosin staining. Crypt depth in 20 well-oriented crypts for each animal was measured using the software ProgRes Capture Pro 2.7. Splayed-open colons fixed in 4% paraformaldehyde were cryopreserved by two subsequent overnight incubations in 10% and 30% sucrose at 4°C. Tissues were embedded in optimal cutting temperature compound (OCT, Sakura, Torrance, CA) and allowed to freeze on dry ice. Sections (6 μm thick) were placed on microscope slides and baked at 37°C overnight and then at 60°C for 2 h. Heat-induced epitope retrieval (HIER) was performed using HIER Buffer L (pH 6.0; catalog no. TA-135-HBL, Thermo Scientific). Slides were washed in distilled water and incubated in 3% hydrogen peroxide for 10 min at room temperature and then blocked with 10% normal goat serum for 1 h. Slides were incubated with a cleaved caspase-3 antibody (Cell Signaling Technology, Danvers, MA) at 4°C overnight. Incubation in biotinylated goat anti-rabbit antibody (Jackson Immunoresearch, West Grove, PA) was performed for 1 h at room temperature. Apoptosis was assessed by quantification of cells positive for cleaved caspase-3 staining in 40 well-oriented crypts for each mouse. The total number of cells per crypt was quantified on the basis of hematoxylin-stained nuclei. The apoptotic indexes were determined for each crypt by calculation of the percentage of cleaved caspase-3-positive cells relative to the total number of cells.
CEC isolation.
Distal colon segments were placed in a solution containing 30 mM EDTA-1.5 mM DTT-1× PBS for 20 min on ice. The tissues were transferred to a 30 mM EDTA-1× PBS solution and incubated in a 37°C water bath for 10 min. Samples were shaken vigorously for 1 min to separate the epithelium from the submucosal and muscularis layers and centrifuged at 1,750 rpm for 5 min. After two washes with 1× PBS, samples were pelleted and resuspended in RLT buffer (Qiagen, Valencia, CA) containing 1:100 2-mercaptoethanol (Gibco, Grand Island, NY) for RNA extraction or flash-frozen for protein extraction.
Protein extraction and Western blotting.
Isolated CECs were lysed with hot Laemmli buffer, boiled, and sonicated. Protein samples were run on NuPAGE 4–12% Bis-Tris 1.0-mm gels (Life Technologies, Carlsbad, CA) and transferred to a 0.45-mm-pore polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were blocked with Blocker Casein in Tris-buffered saline (Thermo Fisher Scientific, Waltham, MA) for 1 h and incubated at 4°C overnight in primary antibodies: IR-β (Santa Cruz Biotechnology, Santa Cruz, CA); IGF1R-β, phosphorylated H2AX (pH2AX), p53, phosphorylated JNK (pJNK), and JNK (Cell Signaling Technologies, Danvers, MA); and β-actin (Sigma Aldrich, St. Louis, MO). Membranes were washed with 1× Tris-buffered saline-0.1% Tween buffer and incubated in DyLight 800 goat anti-rabbit or anti-mouse IgG secondary antibody (1:10,000 dilution; Pierce, ThermoScientific, Rockford, IL) at room temperature for 2 h. Protein was visualized with an infrared imager (Odyssey CLx, version 3, LI-COR, Lincoln, NE), and band intensity was measured with ImageJ software (National Institutes of Health).
RNA isolation and high-throughput real-time PCR.
RNA was isolated from CECs using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. High-quality RNA as determined by the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) was utilized for high-throughput gene expression analyses using the BioMark HD System (Fluidigm, San Francisco, CA), as previously described (48), based on the manufacturer's protocol. An RNA pool derived from pooling equal amounts of all samples studied was used as the reference sample. All test mRNAs were normalized to the mRNA encoding the housekeeping gene hydroxymethylbilane synthase (Hmbs). TaqMan primer/probes for data presented here include Mm01143545_m1 (Hmbs), Mm00432051_m1 (Bax), Mm00477631_m1 (Bcl2), Mm00480750_m1 (Perp), and Mm00438168_m1 (Cdkn1b) and were purchased from Applied Biosystems (Carlsbad, CA).
Statistical analyses.
Data were collected from multiple independent experiments, each consisting of four to eight mice. Subsets of mice were used for different assays. Sample size was selected on the basis of prior studies of apoptosis and potential downstream effectors in genetically manipulated mice (38, 64, 65, 82). Apoptosis, which was the primary end point, was studied in five to eight mice per group, but protein analyses involved fewer animals per group because of limiting amount of colon tissue, and data reflect animals from multiple litters. Values are means ± SE. Morphological measurements and quantification of apoptosis, protein, and mRNA levels were analyzed by two-way analysis of variance for main effects of diet or genotype and interactions between diet and genotype. Multiple comparisons were performed by Tukey's test. All analyses were performed using GraphPad Prism 6 software (La Jolla, CA), and P < 0.05 was considered statistically significant.
RESULTS
IEC-specific disruption of IGF1R or IR.
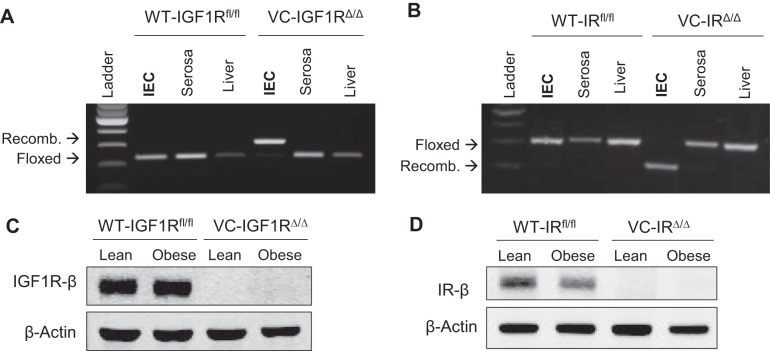
The PCR gel in Fig. 1 confirms that VC-IGF1RΔ/Δ and VC-IRΔ/Δ (Fig. 1, A and B) mice are homozygous for the recombined allele of IGF1R and IR, respectively, specifically in IECs. Only floxed but intact alleles are observed in serosa and liver of VC mutants and IECs of WT-IGF1Rfl/fl and WT-IRfl/fl controls. Western immunoblotting on isolated CECs confirmed complete absence of IGF1R protein in VC-IGF1RΔ/Δ animals and no change in IGF1R levels during obesity (Fig. 1C). Similarly, we confirmed loss of IR protein expression in CECs from VC-IRΔ/Δ mice and unchanged IR protein levels in obese vs. lean WT-IRfl/fl mice (Fig. 1D).
Fig. 1.
Intestinal epithelial cell (IEC)-specific villin-Cre (VC)-mediated deletion of insulin-like growth factor 1 receptor (IGF1R) and insulin receptor (IR). A and B: PCR gels for floxed regions in exon 3 of the Igf1r gene and exon 4 of the Insr gene in IECs, intestinal serosa, and liver DNA from WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ mice and WT-IRfl/fl and VC-IRΔ/Δ mice. Only mice carrying the villin-Cre recombinase are homozygous for the recombined (recomb) alleles specifically in IECs, and not in other tissues. C and D: isolated colon epithelial cells (CECs) from VC-IGF1RΔ/Δ and VC-IRΔ/Δ mice showing complete absence of IGF1R and IR protein.
IGF1R or IR loss in IECs does not impact weight gain, hyperinsulinemia, hyperglycemia, or insulin insensitivity resulting from long-term HFD feeding.
Table 1 describes weight gain, fasting glucose, insulin, HOMA, and plasma IGF1 in mice after 18 wk on HFD or control diet. All HFD-fed mice gained significantly more weight than control diet-fed mice. Body weight gain was significantly greater in HFD-fed WT-IGF1Rfl/fl than HFD-fed WT-IRfl/fl mice (P = 0.0007, by Student's t-test). The trend for body weight gain was greater in HFD-fed VC-IGF1RΔ/Δ than VC-IRΔ/Δ mice but did not reach statistical significance (P = 0.053, by Student's t-test). This illustrates that mice from different colonies can show quantitatively different body weight gain in response to the HFD. Importantly, however, HFD-induced weight gain in VC-IGF1RΔ/Δ mice did not differ from that in WT-IGF1Rfl/fl mice. Similarly, weight gain was virtually the same in VC-IRΔ/Δ and WT-IRfl/fl mice when fed the HFD. Thus, loss of IGF1R or IR has no discernible effect on HFD-induced weight gain. All HFD-fed animals exhibited increased fasting levels of blood glucose, plasma insulin, and HOMA, indicative of decreased insulin sensitivity. There were no significant differences across genotypes in any of these HFD-induced metabolic parameters. Plasma IGF1 levels did not differ between diet groups or genotypes. Our data, therefore, demonstrate that IEC-specific loss of IGF1R or IR does not affect the metabolic phenotype associated with HFD (45% kcal fat) feeding for 18 wk, at least in the parameters measured.
Table 1.
IEC-specific loss of IGF1R or IR does not affect obesity-associated changes in body weight, blood glucose, plasma insulin, and insulin sensitivity
| WT-IGF1Rfl/fl |
VC-IGF1RΔ/Δ |
WT-IRfl/fl |
VC-IRΔ/Δ |
|||||
|---|---|---|---|---|---|---|---|---|
| CTL | HFD | CTL | HFD | CTL | HFD | CTL | HFD | |
| Body weight gain, % vs. start of diet | 39.5 ± 10.0 (6) | 101.6 ± 4.2a (9) | 31.2 ± 8.1 (5) | 93.9 ± 10.5a (7) | 24.9 ± 1.7 (8) | 70.4 ± 6.2a,b (9) | 25.7 ± 3.2 (7) | 71.0 ± 5.8a (12) |
| Fasting blood glucose, mg/dl | 127.1 ± 11.3 (5) | 164.6 ± 16.4 (7) | 127.6 ± 17.3 (5) | 167.1 ± 13.8 (6) | 108.2 ± 2.3 (6) | 151.8 ± 11.3a (7) | 108.7 ± 8.8 (6) | 167.5 ± 10.4a (9) |
| Fasting plasma insulin, μg/l | 0.2 ± 0.03 (5) | 1.1 ± 0.2a (7) | 0.3 ± 0.1 (5) | 1.3 ± 0.3a (6) | 0.27 ± 0.03 (6) | 1.16 ± 0.23a (7) | 0.27 ± 0.07 (6) | 1.14 ± 0.19a (9) |
| HOMA | 2.3 ± 0.4 (5) | 13.9 ± 3.0a (7) | 2.5 ± 0.9 (5) | 15.0 ± 3.89a (6) | 2.0 ± 0.2 (6) | 12.6 ± 2.7a (7) | 2.2 ± 0.8 (6) | 15.4 ± 2.8a (9) |
| Plasma IGF1, ng/ml | 31.8 ± 11.2 (3) | 46.7 ± 3.8 (6) | 40.8 ± 4.0 (3) | 37.1 ± 6.6 (5) | 49.3 ± 2.6 (5) | 52.2 ± 4.5 (5) | 49.7 ± 3.3 (5) | 51.2 ± 2.2 (5) |
Values are means ± SE of number of mice in parentheses. IEC, intestinal epithelial cell; IGF1R, insulin-like growth factor 1 (IGF1) receptor; IR, insulin receptor; HOMA, homeostatic model assessment; CTL, control diet; HFD, high-fat diet.
P < 0.05 vs. CTL of the same genotype (by 2-way ANOVA and Tukey's multiple-comparisons test).
P < 0.05 vs. HFD-fed WT-IGF1Rfl/fl (by Student's t-test).
Loss of IGF1R in CECs does not impact measures of colon growth in obese or lean mice.
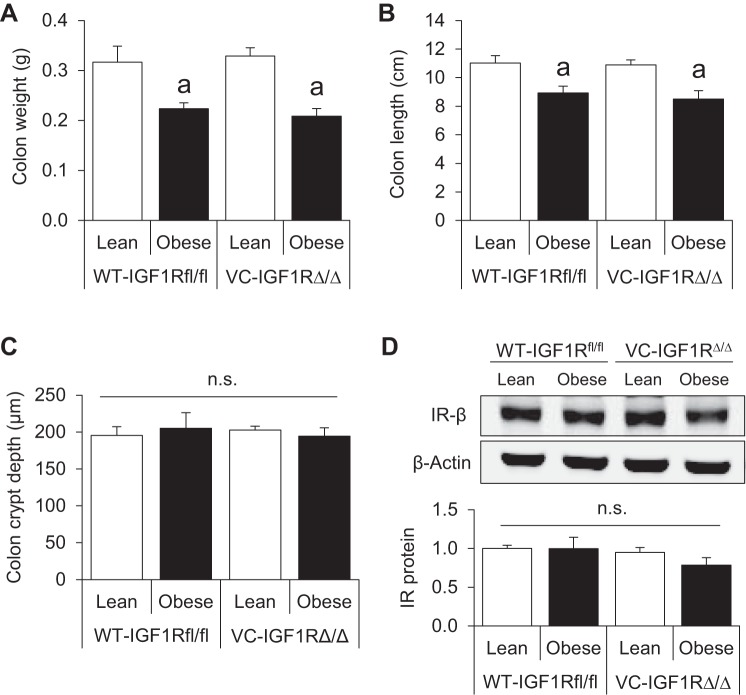
We next examined the effects of obesity and IGF1R deletion on colon weight, length, and morphometry. Obese mice had lighter and shorter colons, as previously observed in the small intestine in our recently published studies (4, 48), but disruption of IGF1R did not impact this phenotype (Fig. 2, A and B). Colon crypt depth was unaffected by obesity or loss of IGF1R (Fig. 2C). These data suggest that basal morphology and morphometry of the colon are not altered by epithelial loss of IGF1R in lean or obese mice.
Fig. 2.
IGF1R loss does not alter measures of colon growth in lean or obese mice, and neither obesity nor IGF1R deletion impacts IR protein levels in CECs. A–C: colon weight, length, and crypt depth. Values are means ± SE (n = 6 WT-Lean, 4 WT-Obese, 5 VC-Lean, and 4 VC-Obese for colon weight and length; n = 7 WT-Lean, 6 WT-Obese, 6 VC-Lean, and 5 VC-Obese for crypt depth). D: representative Western blot showing IR protein in isolated CECs of lean and obese WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ animals and quantification of IR protein levels relative to β-actin. Values are means ± SE (n = 3 WT-Lean, 4 WT-Obese, 3 VC-Lean, and 3 VC-Obese). aP < 0.05 vs. lean of the same genotype (by 2-way ANOVA with Tukey's multiple-comparisons test); ns, no significance.
IGF1R loss in CECs does not cause a compensatory increase in IR protein.
Since no differences were observed in gross or histological morphology of colons lacking epithelial IGF1R, we asked if IR protein levels in CECs were increased in response to IGF1R loss. Expression of IR was similar in obese and lean WT-IGF1Rfl/fl mice, and IGF1R loss did not significantly affect IR protein levels in CECs of mice from either diet group (Fig. 2D).
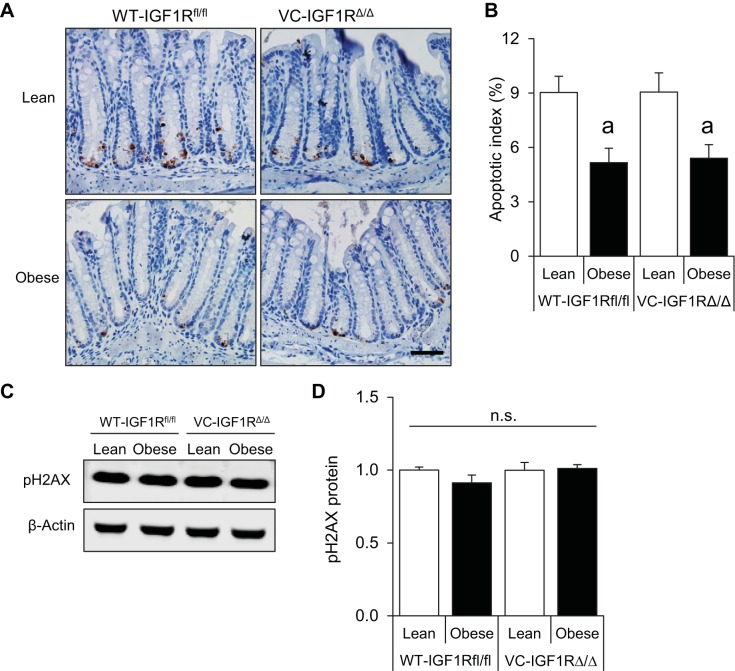
Obesity promotes decreases in radiation-induced apoptosis of CECs, and this is unaffected by IGF1R deletion.
We hypothesized that diet-induced obesity and hyperinsulinemia would decrease the ability of CECs to undergo apoptosis following DNA damage. Quantification of cleaved caspase-3-positive cells in colon crypts revealed a 42.8% lower level of apoptosis in obese WT-IGF1Rfl/fl animals than in their lean counterparts at 4 h after 5-Gy radiation (Fig. 3, A and B). We next asked if the antiapoptotic effects of obesity/hyperinsulinemia were affected by IGF1R deletion. Surprisingly, no differences in the apoptotic index were observed between WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ mice in lean or obese groups (Fig. 3, A and B). pH2AX, a well-established biomarker of DNA damage, was assessed by Western immunoblotting to verify that obesity or IGF1R deletion did not affect DNA damage. Levels of pH2AX were similar in all groups (Fig. 3, C and D). Together, these findings indicate that obesity and hyperinsulinemia promote resistance of CECs to apoptosis after radiation-induced DNA damage, and, surprisingly, IGF1R loss did not impact these effects.
Fig. 3.
Reduced apoptosis of genetically damaged CECs in obese mice with intact IGF1R or IGF1R deletion and no changes in levels of the DNA damage marker phosphorylated H2AX (pH2AX) across groups. A: representative images showing cleaved caspase-3 staining in distal colon of lean and obese WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ mice (×40 objective, scale bar = 50 μm). B: apoptotic index calculated using the following formula: number of cleaved caspase-3-positive cells/total number of cells × 100. Values are means ± SE (n = 7 WT-Lean, 6 WT-Obese, 6 VC-Lean, and 5 VC-Obese). C: representative Western blot showing pH2AX protein in isolated CECs of lean and obese WT-IGF1Rfl/fl and VC-IGF1RΔ/Δ animals. D: quantification of pH2AX levels relative to β-actin. Values are means ± SE (n = 3 WT-Lean, 4 WT-Obese, 3 VC-Lean, and 3 VC-Obese). aP < 0.05 vs. lean of the same genotype (by 2-way ANOVA with Tukey's multiple-comparisons test); ns, no significance.
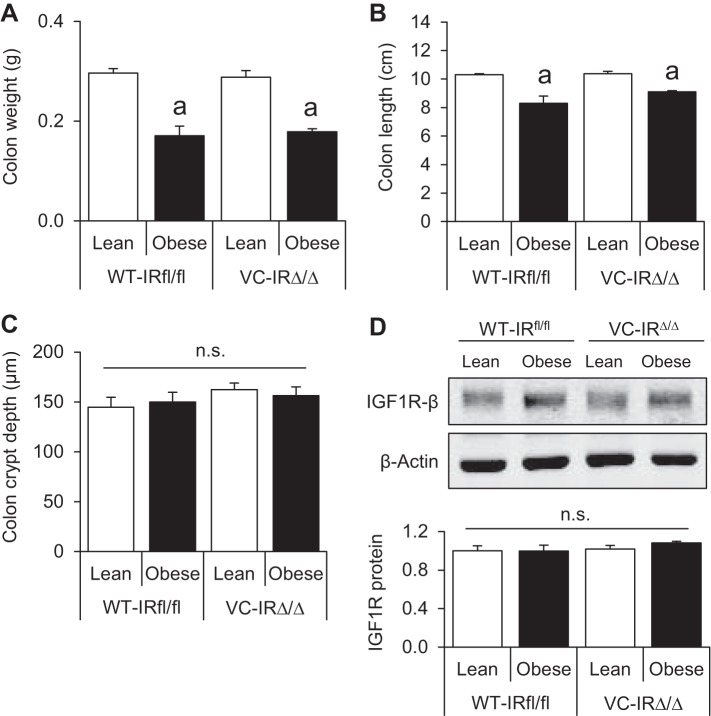
Loss of IR in CECs does not affect basal colon growth and does not alter IGF1R protein expression in obese or lean mice.
Consistent with findings in the IGF1R mouse colony, obesity was associated with lighter and shorter colons in WT-IRfl/fl and VC-IRΔ/Δ mice (Fig. 4, A and B). Colon crypt depth was similar across all groups (Fig. 4C), and neither obesity nor IR loss affected IGF1R protein levels (Fig. 4D). These data indicate that loss of IR in CECs has no detectable effects on colon weight, length, or morphometry and does not lead to a compensatory increase in IGF1R protein.
Fig. 4.
IR deletion does not affect measures of colon growth and does not impact IGF1R protein expression in CECs of lean or obese mice. A–C: colon weight, length, and crypt depth. Values are means ± SE (n = 7 WT-Lean, 5 WT-Obese, 5 VC-Lean, and 7 VC-Obese for colon weight and length; n = 5 WT-Lean, 4 WT-Obese, 4 VC-Lean, and 4 VC-Obese for crypt depth). D: representative Western blot and quantification of IGF1R protein levels in isolated CECs of lean and obese WT-IRfl/fl and VC-IRΔ/Δ animals. Values are means ± SE (n = 4 for all groups); ns, no significance. aP < 0.05 vs. lean of the same genotype (by 2-way ANOVA with Tukey’s multiple-comparisons test).
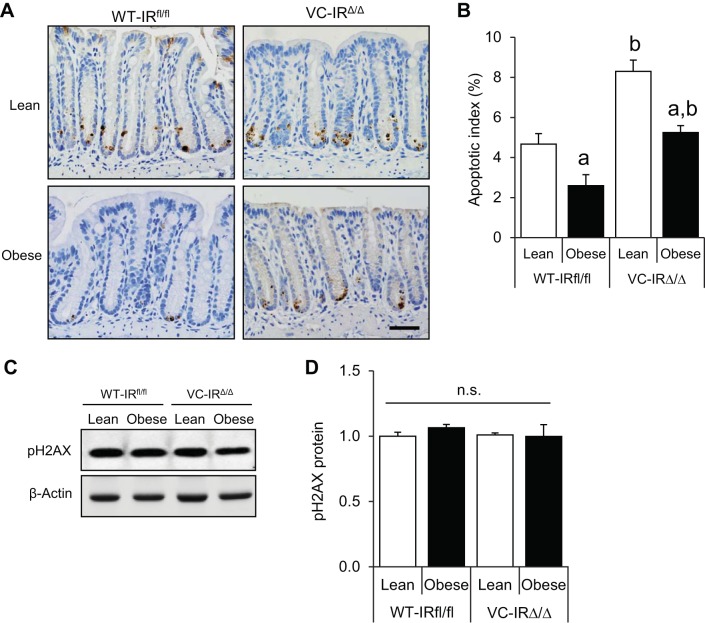
IR loss increases apoptosis of genetically damaged CECs in lean and obese animals.
We next examined if IR, rather than IGF1R, was required for the antiapoptotic effects of obesity and hyperinsulinemia on CECs with DNA damage. In obese WT-IRfl/fl mice, apoptosis levels were reduced by 44.5% relative to lean WT-IRfl/fl mice (Fig. 5, A and B), consistent with findings in obese WT-IGF1Rfl/fl mice. Interestingly, lean VC-IRΔ/Δ mice had significantly more cleaved caspase-3-positive cells than lean WT-IRfl/fl mice, providing evidence that IR loss increases early radiation-induced apoptosis of CECs. Obese VC-IRΔ/Δ mice also had a higher apoptotic index than obese WT-IRfl/fl animals, further indicating antiapoptotic roles of IR. However, apoptosis levels were significantly (36.7%) lower in obese VC-IRΔ/Δ than lean VC-IRΔ/Δ mice (Fig. 5, A and B). Levels of the DNA damage marker pH2AX were not affected by diet or genotype (Fig. 5, C and D). Overall, these results show that disruption of IR leads to increased apoptosis of genetically damaged CECs in lean and obese mice but does not prevent a reduction in radiation-induced apoptosis associated with obesity/hyperinsulinemia.
Fig. 5.
IR loss increases apoptosis of genetically damaged CECs in lean and obese mice, and levels of pH2AX do not change across groups. A: representative images showing cleaved caspase-3 staining in distal colon of lean and obese WT-IRfl/fl and VC-IRΔ/Δ mice (×40 objective, scale bar = 50 μm). B: apoptotic index calculated using the following formula: number of cleaved caspase-3-positive cells/total number of cells × 100. Values are means ± SE (n = 6 WT-Lean, 6 WT-Obese, 5 VC-Lean, and 8 VC-Obese). C: representative Western blot showing pH2AX protein in isolated CECs of lean and obese WT-IRfl/fl and VC-IRΔ/Δ animals. D: quantification of pH2AX expression relative to β-actin. Values are means ± SE (n = 4 for all groups). aP < 0.05 vs. lean of the same genotype; bP < 0.05 vs. WT of the same diet group (by 2-way ANOVA with Tukey's multiple-comparisons test); ns, no significance.
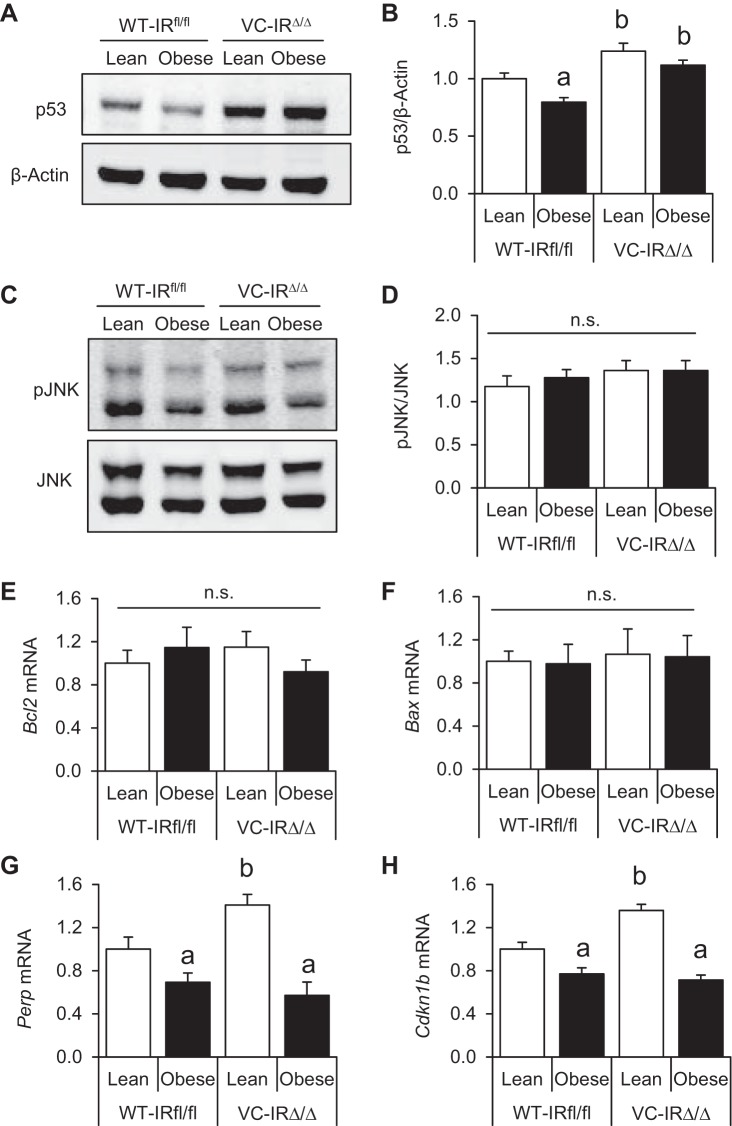
Obesity and IR loss impact protein expression of p53 and mRNA levels of Perp and Cdkn1b/p27 in genetically damaged CECs.
We next evaluated mediators known to contribute to radiation-induced apoptosis to gain insight into mechanisms by which obesity promotes reduced apoptosis and IR deletion enhances apoptosis. CECs were isolated from WT-IRfl/fl and VC-IRΔ/Δ mice, and levels of proapoptotic and antiapoptotic mediators were assessed. We examined p53, a key sensor of DNA damage that is a required mediator of apoptosis in the intestinal crypts within the initial hours following radiation (20, 50). At 4 h after 5-Gy radiation, there was a small, but significant, reduction in p53 protein levels in CECs of obese vs. lean WT-IRfl/fl animals (Fig. 6, A and B). Furthermore, lean and obese VC-IRΔ/Δ mice had significantly increased p53 protein levels compared with their WT-IRfl/fl counterparts (Fig. 6, A and B). We also evaluated c-Jun amino-terminal kinase (JNK) activation, which has been functionally linked to insulin resistance and apoptosis in other systems (23, 37, 58, 75). However, phosphorylated JNK was unaltered in CECs of obese vs. lean mice or mice with intact or deleted IR (Fig. 6, C and D). Next, we performed high-throughput quantitative RT-PCR on 96 mRNAs encoding regulators of apoptosis or cell cycle progression, but relatively few mRNAs showed significant changes. We found no differences in mRNA levels of proapoptotic Bax or antiapoptotic Bcl2 in obese vs. lean mice or across genotypes (Fig. 6, E and F). However, mRNA levels of p53 targets Perp (a p53 apoptosis effector related to PMP-22) and tumor suppressor Cdkn1b (which encodes for p27) were significantly downregulated in CECs of obese WT-IRfl/fl and VC-IRΔ/Δ mice vs. lean mice of the same genotype (Fig. 6, G and H). Perp and Cdkn1b/p27 mRNAs were also significantly higher in CECs from lean VC-IRΔ/Δ mice than lean WT-IRfl/fl controls. These data indicate that obesity and IR deletion promote changes in expression of p53 protein and Perp and p27 transcripts that may contribute to effects of obesity or IR deletion on apoptosis of CECs after DNA damage.
Fig. 6.
Obesity and IR deletion alter levels of p53 protein and p53-regulated mRNAs. A and B: Western blot showing p53 protein levels in isolated CECs of lean and obese WT-IRfl/fl and VC-IRΔ/Δ mice and quantification relative to β-actin by densitometry. C and D: Western blot for phosphorylated JNK (pJNK) and quantification relative to total JNK by densitometry. Protein data were normalized to Lean-WT on the same membrane. Values are means ± SE (n = 4 in duplicates for all groups). E–H: mRNA levels of antiapoptotic Bcl-2, proapoptotic Bax, proapoptotic Perp, and the tumor suppressor Cdkn1b. Data are expressed as fold change vs. lean WT-IRfl/fl control. Values are means ± SE (n = 6 WT-Lean, 7 WT-Obese, 5 VC-Lean, and 7 VC-Obese). aP < 0.05 vs. lean of the same genotype, bP < 0.05 vs. WT of the same diet group (by 2-way ANOVA with Tukey's multiple-comparisons test); ns, no significance.
DISCUSSION
This study investigated the effects of obesity and hyperinsulinemia on apoptosis of CECs after radiation-induced DNA damage. Using a mouse model of HFD-induced obesity/hyperinsulinemia, we found that obesity led to reduced CEC apoptosis 4 h after 5-Gy radiation. To assess the mechanisms linked to reduced apoptosis in obesity and hyperinsulinemia, we used mice with genetic deletion of IGF1R or IR specifically in IECs. We found that apoptosis levels in lean and obese mice were unaffected by IGF1R loss. However, IR loss significantly increased apoptosis of genetically damaged CECs in lean and obese mice but did not prevent the reduction in apoptosis in obese animals. Together, our findings in two independent mouse colonies show that obesity and hyperinsulinemia decrease apoptosis of CECs after radiation-induced DNA damage. We provide novel and unexpected evidence that loss of IR, but not IGF1R, increases apoptosis of genetically damaged CECs in lean or obese mice, indicating novel antiapoptotic roles of IR in the colon. However, IR does not appear to mediate the reduction in apoptosis associated with obesity and hyperinsulinemia.
A large body of clinical data has linked obesity, hyperinsulinemia, insulin therapies, and diabetes to CRC risk (7, 35, 43, 66, 83, 86), and considerable interest in the potential role of the insulin/IGF system in mediating this risk has emerged. Because of the structural similarities and overlapping functions of IGF1R and IR, as well as their ability to bind both insulin and IGF1 when present at high levels, defining the individual roles of these receptors in cell growth and death has been challenging. We therefore generated mice with disruption of IGF1R or IR specifically in intestinal epithelium to evaluate their roles in apoptosis of genetically damaged CECs, which may reflect an important mechanism to remove cells that could harbor potentially oncogenic mutations. The colons of lean mice with IEC-specific IR or IGF1R deletion showed no obvious basal growth defects, as demonstrated by the unaltered colon length, weight, and crypt depth with loss of either receptor. This demonstrates that colon growth is unaffected by loss of IR or IGF1R in healthy adult mice. Reduced colon weight and length in mice with HFD-induced obesity are consistent with findings in the small intestine (4, 48, 71). The cause and significance of this observation warrant further study and may reflect high fat content, reduced fiber, or altered microbiota that occur as a result of a HFD. Findings by Soares et al. (71) also suggest that altered myenteric innervation may play a role in responses of the small intestine to a HFD. Future studies can address whether altered myenteric innervation accompanies the shortened colon in obese mice.
Our initial pilot studies attempted to evaluate colon epithelial apoptosis in nonirradiated lean and obese animals. We observed 0.005 ± 0.003 apoptotic cells per crypt in lean animals but none in obese mice, and we believed that these numbers were too low for valid quantification. We therefore studied the impact of obesity on radiation-induced apoptosis. After radiation-induced DNA damage, apoptosis was decreased in CECs of obese and hyperinsulinemic mice. These findings in two independent colonies of mice provide, to our knowledge, new and direct evidence that obesity and hyperinsulinemia promote reduced apoptosis of genetically damaged CECs. This evidence in preclinical models supports our previous findings in humans linking increased plasma insulin to reduced apoptosis in normal rectal mucosa, which in fact predicted risk of colorectal polyps (42, 43).
The role of the IR in the gut epithelium has been understudied, while significantly more attention has been focused on IGF1R because of its well-known role in growth and cancer (3, 15, 59). Elevated plasma insulin promotes increases in circulating “free” IGF1 because of inhibition of hepatic production of IGFBPs (22). Elevated plasma IGF1 has been associated with normal and aberrant colon growth (54, 69, 72), and IGF1R has been traditionally considered a major mediator of the proliferative and antiapoptotic effects of IGF1 or insulin. Our findings that loss of IGF1R did not impact radiation-induced apoptosis of CECs in obese/hyperinsulinemic mice suggest that, in the colon, IGF1R is not required for antiapoptotic effects of obesity-associated elevations in plasma insulin. In vitro evidence for antiapoptotic roles of IR has been reported in primary hepatocytes of newborn mice and mouse embryo fibroblasts (52, 63). Our work showing dramatic increases in apoptosis of genetically damaged CECs lacking IR, therefore, provides novel evidence that IR exerts antiapoptotic actions in the adult colonic epithelium in vivo. However, our observations that IR deletion does not prevent the reduction in apoptosis in obese vs. lean mice indicate that other mechanisms, possibly independent of hyperinsulinemia, contribute to obesity-associated reductions in apoptosis and require further study. Future studies may focus on the role of the microbiota in CEC apoptosis, as it is well established that obesity results in changes in the gut microbial composition (25, 45).
Early radiation-induced apoptosis as studied here is known to require p53 (50). Insulin is known to activate Akt, a key signaling mediator downstream of IR, and Akt can promote phosphorylation of Mdm2, which decreases p53 levels (49). Our findings of reduced p53 levels in CECs from obese/hyperinsulinemic mice provide mechanistic evidence that reduced p53 may contribute to reduced CEC apoptosis in obesity and hyperinsulinemia. Increased p53 and apoptosis in CECs of mice with IR deletion also support roles of elevated p53 in mediating proapoptotic consequences of loss of IR signaling. However, the effects of obesity and IR deletion on levels of p53 protein were relatively small, and the increased p53 in IR-deficient CECs did not prevent antiapoptotic effects of obesity. Additional work is therefore needed to further explore the mechanistic and functional relationships between obesity, IR, and p53 that may impact apoptosis in the colonic epithelium.
Another approach we took to address mechanisms underlying the impact of obesity or IR deletion on apoptosis was high-throughput quantitative RT-PCR for mRNAs encoding 96 mediators linked to apoptosis or the cell cycle. Relatively few mediators changed. We included data showing lack of effects of obesity or IR loss on Bax or Bcl2 mRNA, which argues against a role of these key regulators of apoptosis. Perp is transcriptionally induced by p53 in normal and cancer cells specifically during apoptosis (6, 24, 41). Cdkn1b encodes the tumor suppressor and cell cycle inhibitor p27, which is downregulated in tumors from p53 null mice and can be upregulated by p53 to protect normal IECs from the cytotoxic effects of chemotherapy (57, 67, 78). Our findings of reduced levels of Perp and Cdkn1b mRNAs in CECs of obese mice are consistent with potential roles of these known p53 targets in the obesity-associated reductions in apoptosis. Increases in Perp and Cdkn1b mRNAs, as well as p53, in lean VC-IRΔ/Δ mice also support a mechanism whereby p53-mediated induction of these mediators may contribute to the elevated apoptosis due to IR loss. However, factors other than p53 likely contribute to reduced Perp and Cdkn1b mRNAs in obese VC-IRΔ/Δ mice, since p53 expression was not reduced in CECs from these mice. Therefore, although some evidence points to involvement of p53 and p53 targets in effects of obesity or IR on apoptosis, additional work is required to fully define the mechanisms driving altered apoptosis. It is noteworthy that mechanistic studies at the level of activated signaling proteins and mRNAs are a challenge, because even after radiation, relatively few of the total CECs undergo apoptosis. Therefore, approaches such as analyses of single cells may be necessary to better define mechanisms and mediators that regulate apoptosis.
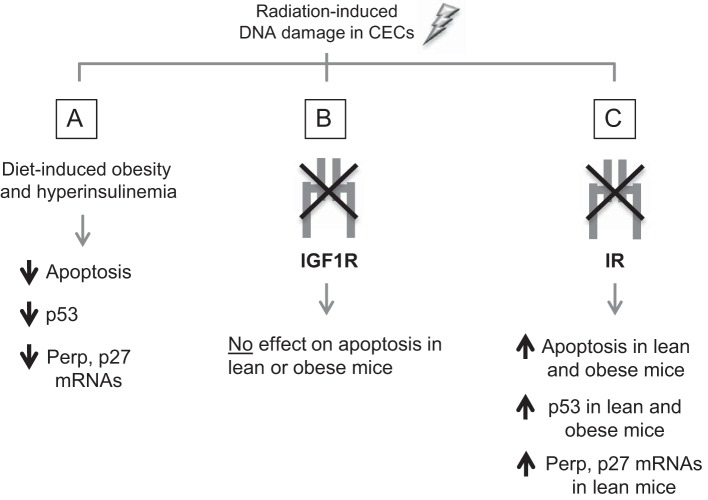
Figure 7 summarizes our current findings. Our study is, to our knowledge, the first to directly show that obesity and hyperinsulinemia promote resistance of CECs to apoptosis after DNA damage, and this effect is associated with reduced p53 and known targets. Furthermore, we provide novel evidence that IR, and not IGF1R, normally protects genetically damaged CECs from apoptosis in both lean and obese hyperinsulinemic mice. Overall, our work suggests that 1) more attention should be given to the physiological roles of IR in cell death and survival in the colon crypts to better understand the mechanisms underlying normal or aberrant colon growth and 2) neither colon epithelial IR nor IGF1R is required for the antiapoptotic effects of obesity in CECs after DNA damage, and additional mechanisms should be explored.
Fig. 7.
Summary of findings. After radiation-induced DNA damage in CECs, diet-induced obesity/hyperinsulinemia was associated with reduced apoptosis and reduced p53 and p53 targets (A), deletion of IGF1R did not impact apoptosis in lean or obese mice (B), and deletion of IR promoted increases in apoptosis in lean and obese animals, and this was associated with increased p53 and p53 targets (C).
GRANTS
This research was supported by National Institutes of Health Grants RO1 DK-040247 (P. K. Lund), AG-041198 (P. K. Lund), P30 DK-034987 (UNC Center for Gastrointestinal Biology and Disease), and F31 AG040943 (S. F. Andres), a grant from the National Cancer Center (L. Van Landeghem), and the UNC Lovick P. Corn Dissertation Fellowship (M. A. Santoro).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.S. designed and conducted the majority of the experiments, analyzed and interpreted the data, and wrote the manuscript; R.E.B. assisted with all radiation procedures and mouse dissections and conducted PCR on IGF1R recombination; S.F.A. generated the intestinal epithelial IR knockout model and conducted PCR on IR recombination; A.T.M. assisted with high-throughput qRT-PCR experiments; L.V. designed the study, interpreted the data, and generated the intestinal epithelial IGF1R knockout model; P.K.L. supervised the study, designed all experiments, interpreted the data, and wrote the manuscript. All authors reviewed and approved the manuscript.
ACKNOWLEDGMENTS
We thank the following core facilities whose services contributed to our studies: the University of North Carolina (UNC) Center for Gastrointestinal Biology and Disease Advance Analytics and Histology Core Facilities, the UNC Department of Cell Biology and Physiology Histology Research Core Facility, and the UNC Nutrition Obesity Research Core. We particularly thank Dr. Scott Magness, the UNC Intestinal Stem Cell Group, and Joe Galanko for useful discussions; Kirk McNaughton, Ashley Ezzell, Carlton Anderson, and Qing Shi for technical assistance; and Elaine Glenny and Christian Agosto Burgos for helping with histology pilot experiments.
REFERENCES
- 1.Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet 12: 106–109, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology 146: 357–373, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison AS, McIntyre MA, McArdle C, Habib FK. The insulin-like growth factor type 1 receptor and colorectal neoplasia: insights into invasion. Hum Pathol 38: 1590–1602, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Andres SF, Santoro MA, Mah AT, Keku JA, Bortvedt AE, Blue RE, Lund PK. Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs. Am J Physiol Gastrointest Liver Physiol 308: G100–G111, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres SF, Simmons JG, Mah AT, Santoro MA, Van Landeghem L, Lund PK. Insulin receptor isoform switching in intestinal stem cells, progenitors, differentiated lineages and tumors: evidence that IR-B limits proliferation. J Cell Sci 126: 5645–5656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, Jacks T. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev 14: 704–718, 2000. [PMC free article] [PubMed] [Google Scholar]
- 7.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 62: 933–947, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res 55: 249–252, 1995. [PubMed] [Google Scholar]
- 10.Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol 16: 201–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30: 586–623, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocrine-Related Cancer 18: R125–R147, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Berger NA. Obesity and cancer pathogenesis. Ann NY Acad Sci 1311: 57–76, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell 3: 25–38, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Bortvedt SF, Lund PK. Insulin-like growth factor 1: common mediator of multiple enterotrophic hormones and growth factors. Curr Opin Gastroenterol 28: 89–98, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brierley GV, Macaulay SL, Forbes BE, Wallace JC, Cosgrove LJ, Macaulay VM. Silencing of the insulin receptor isoform A favors formation of type 1 insulin-like growth factor receptor (IGF-IR) homodimers and enhances ligand-induced IGF-IR activation and viability of human colon carcinoma cells. Endocrinology 151: 1418–1427, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild R, Epstein D, Pachter JA, Miglarese MR. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther 9: 2652–2664, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Caudle AS, Kim HJ, Tepper JE, O'Neil BH, Lange LA, Goldberg RM, Bernard SA, Calvo BF, Meyers MO. Diabetes mellitus affects response to neoadjuvant chemoradiotherapy in the management of rectal cancer. Ann Surg Oncol 15: 1931–1936, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene 9: 1767–1773, 1994. [PubMed] [Google Scholar]
- 21.Clarke AR, Howard LA, Harrison DJ, Winton DJ. p53, mutation frequency and apoptosis in the murine small intestine. Oncogene 14: 2015–2018, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Conover CA, Lee PD, Kanaley JA, Clarkson JT, Jensen MD. Insulin regulation of insulin-like growth factor binding protein-1 in obese and nonobese humans. J Clin Endocrinol Metab 74: 1355–1360, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Cui J, Zhang M, Zhang YQ, Xu ZH. JNK pathway: diseases and therapeutic potential. Acta Pharmacol Sin 28: 601–608, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Davies L, Spiller D, White MR, Grierson I, Paraoan L. PERP expression stabilizes active p53 via modulation of p53-MDM2 interaction in uveal melanoma cells. Cell Death Dis 2: e136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013: 11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O'Connell MJ, Wolmark N. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 98: 1647–1654, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLos One 5: e12191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Marjou F, Janssen KP, Hung-Junn Chang B, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 114: 23–37, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care 36 Suppl 2: S233–S239, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab 21: 610–618, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallardo A, Lerma E, Escuin D, Tibau A, Munoz J, Ojeda B, Barnadas A, Adrover E, Sanchez-Tejada L, Giner D, Ortiz-Martinez F, Peiro G. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer 106: 1367–1373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrison AP, Dekaney CM, von Allmen DC, Lund PK, Henning SJ, Helmrath MA. Early but not late administration of glucagon-like peptide-2 following ileo-cecal resection augments putative intestinal stem cell expansion. Am J Physiol Gastrointest Liver Physiol 296: G643–G650, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86: s836–s842, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Heni M, Hennenlotter J, Scharpf M, Lutz SZ, Schwentner C, Todenhöfer T, Schilling D, Kühs U, Gerber V, Machicao F, Staiger H, Häring HU, Stenzl A. Insulin receptor isoforms A and B as well as insulin receptor substrates-1 and -2 are differentially expressed in prostate cancer. PLos One 7: e50953, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, Kolesnick R. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 143: 1266–1276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J, Morehouse C, Streicher K, Higgs BW, Gao J, Czapiga M, Boutrin A, Zhu W, Brohawn P, Chang Y, Viner J, LaVallee T, Richman L, Jallal B, Yao Y. Altered expression of insulin receptor isoforms in breast cancer. PLos One 6: e26177, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hursting SD. Obesity, energy balance, and cancer: a mechanistic perspective. Cancer Treat Res 159: 21–33, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, Attardi LD. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol 13: 1985–1990, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Keku T, Sandler R, Simmons J, Galanko J, Woosley J, Proffitt M, Omofoye O, McDoom M, Lund P. Local IGFBP-3 mRNA expression, apoptosis and risk of colorectal adenomas. BMC Cancer 8: 143, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 14: 2076–2081, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura T, Kahn CR, Accili D. Insulin receptor knockout mice. Annu Rev Physiol 65: 313–332, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Liu R, Hu LL, Sun A, Cao YJ, Tang T, Zhang XP, Zhang QH. mRNA expression of IGF-1 and IGF-1R in patients with colorectal adenocarcinoma and type 2 diabetes. Arch Med Res 45: 318–324, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Lund PK. Fixing the breaks in intestinal stem cells after radiation: a matter of DNA damage and death or DNA repair and regeneration. Gastroenterology 143: 1144–1147, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Mah AT, Van Landeghem L, Gavin HE, Magness ST, Lund PK. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 155: 3302–3314, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA 98: 11598–11603, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 54: 614–617, 1994. [PubMed] [Google Scholar]
- 51.Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Nakayama K, Hickman JA. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci 108: 2261–2271, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Nevado C, Benito M, Valverde AM. Role of insulin receptor and balance in insulin receptor isoforms A and B in regulation of apoptosis in simian virus 40-immortalized neonatal hepatocytes. Mol Biol Cell 19: 1185–1198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohneda K, Ulshen MH, Fuller CR, D'Ercole AJ, Lund PK. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology 112: 444–454, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Olivo-Marston SE, Hursting SD, Lavigne J, Perkins SN, Maarouf RS, Yakar S, Harris CC. Genetic reduction of circulating insulin-like growth factor-1 inhibits azoxymethane-induced colon tumorigenesis in mice. Mol Carcinogenesis 48: 1071–1076, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ooi GT, Tseng LY, Tran MQ, Rechler MM. Insulin rapidly decreases insulin-like growth factor-binding protein-1 gene transcription in streptozotocin-diabetic rats. Mol Endocrinol 6: 2219–2228, 1992. [DOI] [PubMed] [Google Scholar]
- 56.Pantano F, Guida FM, Vivaldi C, Vincenzi B, Grande E, Garrote MR, Caraglia M, Silvestris N, Vasile E, Masi G, Falcone A, Tonini G, Santini D. Body mass index and impaired fasting blood glucose as predictive factor of time to progression (TTP) in cetuximab-based colorectal cancer treatment. Cancer Biol Ther 14: 467–468, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Philipp-Staheli J, Kim KH, Liggitt D, Gurley KE, Longton G, Kemp CJ. Distinct roles for p53, p27Kip1, and p21Cip1 during tumor development. Oncogene 23: 905–913, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Picco V, Pages G. Linking JNK activity to the DNA damage response. Genes Cancer 4: 360–368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12: 159–169, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Potten C. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 11: 179–195, 1992. [DOI] [PubMed] [Google Scholar]
- 61.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Phil Trans R Soc Lond Ser B Biol Sci 353: 821–830, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells 15: 82–93, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Prisco M, Romano G, Peruzzi F, Valentinis B, Baserga R. Insulin and IGF-I receptors signaling in protection from apoptosis. Horm Metab Res 31: 80–89, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 29: 1622–1632, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramocki NM, Wilkins HR, Magness ST, Simmons JG, Scull BP, Lee GH, McNaughton KK, Lund PK. Insulin receptor substrate-1 deficiency promotes apoptosis in the putative intestinal crypt stem cell region, limits Apcmin/+ tumors, and regulates Sox9. Endocrinology 149: 261–267, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santoro MA, Andres SF, Galanko JA, Sandler RS, Keku TO, Lund PK. Reduced insulin-like growth factor 1 receptor and altered insulin receptor isoform mRNAs in normal mucosa predict colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 23: 2093–2100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sgambato A, Cittadini A, Faraglia B, Weinstein IB. Multiple functions of p27Kip1 and its alterations in tumor cells: a review. J Cell Physiol 183: 18–27, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Siddiqui AA. Metabolic syndrome and its association with colorectal cancer: a review. Am J Med Sci 341: 227–231, 2011. [DOI] [PubMed] [Google Scholar]
- 69.Simmons JG, Ling Y, Wilkins H, Fuller CR, D'Ercole AJ, Fagin J, Lund PK. Cell-specific effects of insulin receptor substrate-1 deficiency on normal and IGF-I-mediated colon growth. Am J Physiol Gastrointest Liver Physiol 293: G995–G1003, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sinicrope FA, Foster NR, Yothers G, Benson A, Seitz JF, Labianca R, Goldberg RM, Degramont A, O'Connell MJ, Sargent DJ, Adjuvant Colon Cancer Endpoints Group. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 119: 1528–1536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soares A, Beraldi EJ, Ferreira PE, Bazotte RB, Buttow NC. Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice. BMC Gastroenterol 15: 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soubry A, Il'yasova D, Sedjo R, Wang F, Byers T, Rosen C, Yashin A, Ukraintseva S, Haffner S, D'Agostino R Jr. Increase in circulating levels of IGF-1 and IGF-1/IGFBP-3 molar ratio over a decade is associated with colorectal adenomatous polyps. Int J Cancer 131: 512–517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol 13: 579–590, 2012. [DOI] [PubMed] [Google Scholar]
- 74.Srivenugopal K, Singh SP, Yuan XH, Ehmann S, Snyder AK. Differential removal of insulin-like growth factor binding proteins in rat serum by solvent extraction procedures. Experientia 50: 451–455, 1994. [DOI] [PubMed] [Google Scholar]
- 75.Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 344: 174–179, 2014. [DOI] [PubMed] [Google Scholar]
- 76.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci USA 107: 10791–10798, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vidal AC, Lund PK, Hoyo C, Galanko J, Burcal L, Holston R, Massa B, Omofoye O, Sandler RS, Keku TO. Elevated C-peptide and insulin predict increased risk of colorectal adenomas in normal mucosa. BMC Cancer 12: 389, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, Zhu S, Qian L, Gao J, Wu M, Gao J, Zhang Y, Chan GL, Yu Y, Han W. IL-1Ra selectively protects intestinal crypt epithelial cells, but not tumor cells, from chemotoxicity via p53-mediated upregulation of p21WAF1 and p27KIP1. Pharmacol Res 82: 21–33, 2014. [DOI] [PubMed] [Google Scholar]
- 79.Watson AJ, Pritchard DM. Lessons from genetically engineered animal models. VII. Apoptosis in intestinal epithelium: lessons from transgenic and knockout mice. Am J Physiol Gastrointest Liver Physiol 278: G1–G5, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Werner H, Le Roith D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncogenesis 8: 71–92, 1997. [DOI] [PubMed] [Google Scholar]
- 81.Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Arch Physiol Biochem 114: 17–22, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Wilkins HR, Ohneda K, Keku TO, D'Ercole AJ, Fuller CR, Williams KL, Lund PK. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol Gastrointest Liver Physiol 283: G457–G464, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Wong P, Weiner MG, Hwang WT, Yang YX. Insulin therapy and colorectal adenomas in patients with diabetes mellitus. Cancer Epidemiol Biomarkers Prev 21: 1833–1840, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, Accili D, Efstratiadis A. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest 110: 1011–1019, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol 3: 587–594, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277: 44005–44012, 2002. [DOI] [PubMed] [Google Scholar]