Abstract
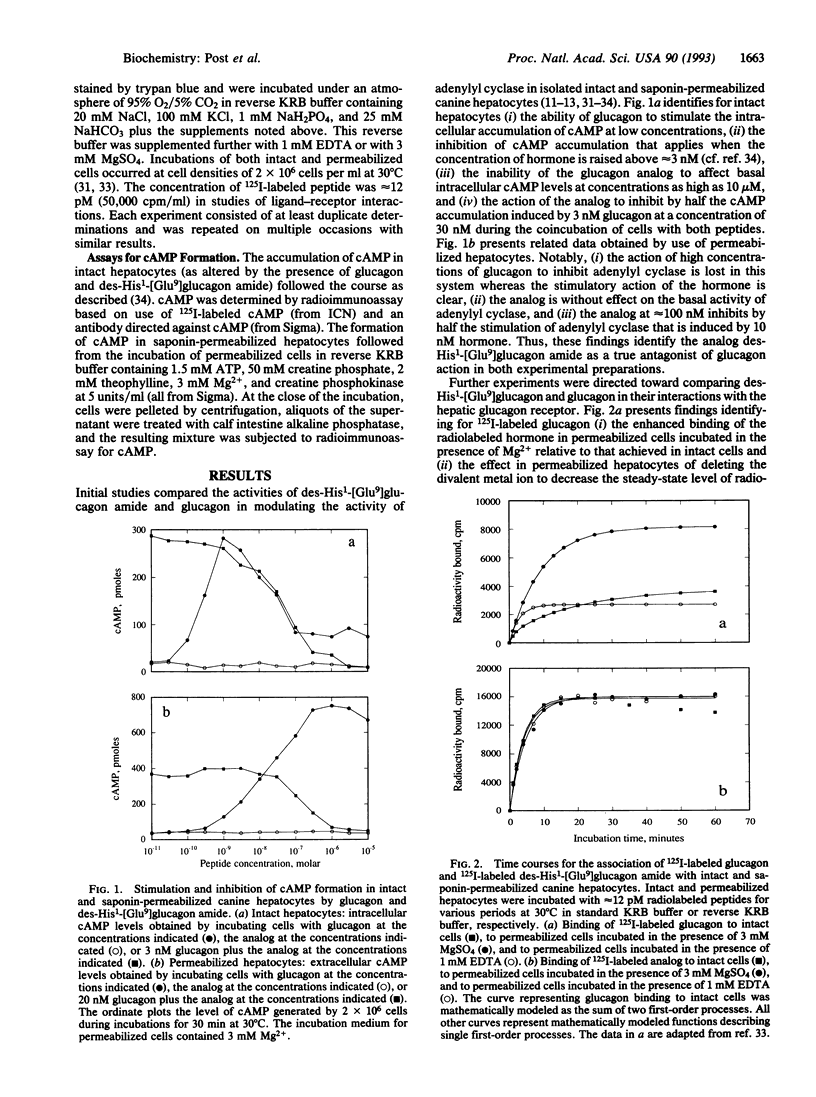
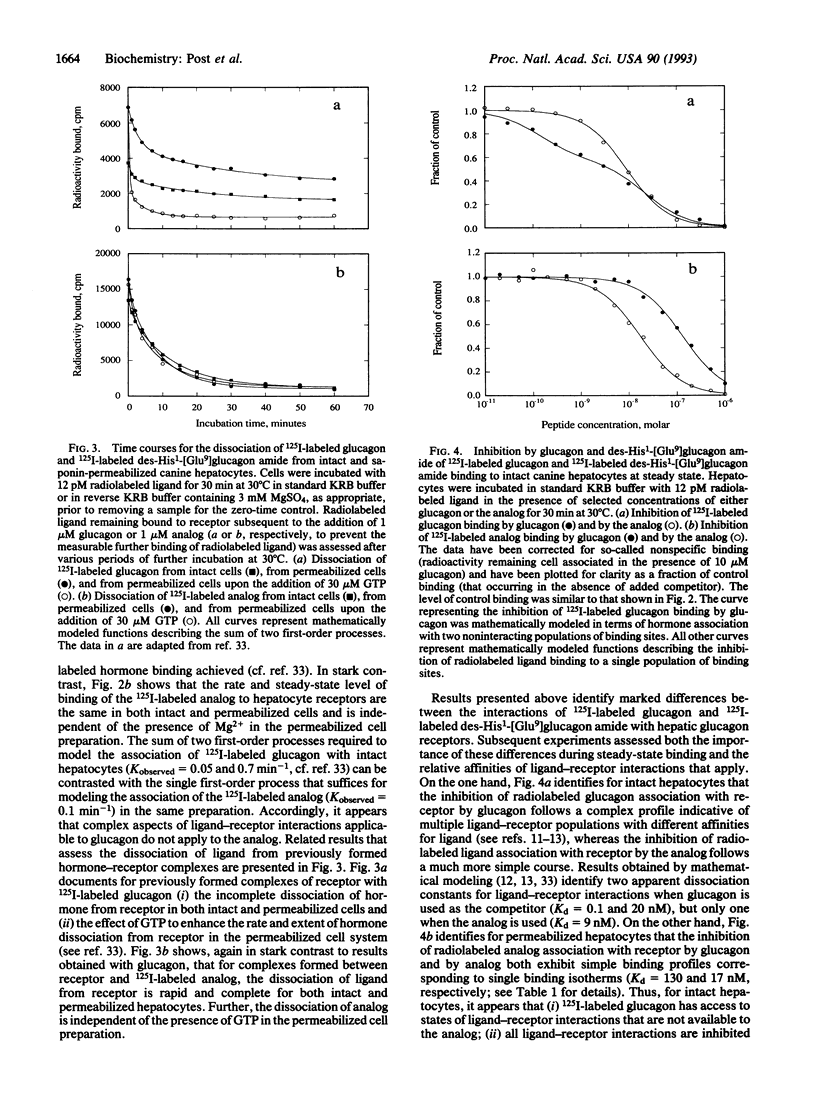
We have investigated the mechanisms through which des-His1-[Glu9]glucagon amide functions as a peptide antagonist of the glucagon receptor/adenylyl cyclase system. Studies with radiolabeled peptides identified that (i) the antagonist bound to intact hepatocytes according to a single first-order process, whereas the rate of association of glucagon with the same preparation could be described only by the sum of two first-order processes; (ii) the interaction of the antagonist with saponin-permeabilized hepatocytes was not affected by the addition of GTP to the incubation medium or by the elimination of Mg2+, whereas the interaction of glucagon with the same cell preparation was modified significantly by the presence of the nucleotide or by the absence of the divalent metal ion; (iii) the dissociation of antagonist from intact hepatocytes incubated in buffer was complete, whereas that of agonist was not; and (iv) the antagonist bound to intact hepatocytes at steady state according to a single binding isotherm (as did both agonist and antagonist in permeabilized hepatocytes), whereas glucagon bound to the intact cell system with two clearly defined apparent dissociation constants. A model is presented for the mechanism of action of the glucagon antagonist in which the analog binds to glucagon receptors in a Mg(2+)- and GTP-independent fashion and in which resulting ligand-receptor complexes fail to undergo sequential adjustments necessary for the stimulation of adenylyl cyclase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bharucha D. B., Tager H. S. Analysis of glucagon-receptor interactions on isolated canine hepatocytes. Formation of reversibly and irreversibly cell-associated hormone. J Biol Chem. 1990 Feb 25;265(6):3070–3079. [PubMed] [Google Scholar]
- Bird S. J., Maguire M. E. The agonist-specific effect of magnesium ion on binding by beta-adrenergic receptors in S49 lymphoma cells. Interaction of GTP and magnesium in adenylate cyclase activation. J Biol Chem. 1978 Dec 25;253(24):8826–8834. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. II. Comparison between glucagon- and fluoride-stimulated activities. J Biol Chem. 1971 Mar 25;246(6):1857–1860. [PubMed] [Google Scholar]
- Bonnevie-Nielsen V., Polonsky K. S., Jaspan J. J., Rubenstein A. H., Schwartz T. W., Tager H. S. Surface receptors for pancreatic hormones in dog and rat hepatocytes: qualitative and quantitative differences in hormone-target cell interactions. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2167–2171. doi: 10.1073/pnas.79.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie-Nielsen V., Tager H. S. Glucagon receptors on isolated hepatocytes and hepatocyte membrane vesicles. Discrete populations with ligand- and environment-dependent affinities. J Biol Chem. 1983 Sep 25;258(18):11313–11320. [PubMed] [Google Scholar]
- Bratusch-Marrain P. R. Insulin-counteracting hormones: their impact on glucose metabolism. Diabetologia. 1983 Feb;24(2):74–79. doi: 10.1007/BF00297384. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Chung F. Z., Wang C. D., Potter P. C., Venter J. C., Fraser C. M. Site-directed mutagenesis and continuous expression of human beta-adrenergic receptors. Identification of a conserved aspartate residue involved in agonist binding and receptor activation. J Biol Chem. 1988 Mar 25;263(9):4052–4055. [PubMed] [Google Scholar]
- Contreras M. L., Wolfe B. B., Molinoff P. B. Kinetic analysis of the interactions of agonists and antagonists with beta adrenergic receptors. J Pharmacol Exp Ther. 1986 Oct;239(1):136–143. [PubMed] [Google Scholar]
- Epand R. M., Rosselin G., Hoa D. H., Cote T. E., Laburthe M. Structural requirements for glucagon receptor binding and activation of adenylate cyclase in liver. Study of chemically modified forms of the hormone, including N alpha-trinitrophenyl glucagon, an antagonist. J Biol Chem. 1981 Feb 10;256(3):1128–1132. [PubMed] [Google Scholar]
- Flanders K. C., Horwitz E. M., Gurd R. S. Semisynthetic derivatives of glucagon. The contribution of histidine-1 to hormone conformation and activity. J Biol Chem. 1984 Jun 10;259(11):7031–7037. [PubMed] [Google Scholar]
- Fraser C. M., Chung F. Z., Wang C. D., Venter J. C. Site-directed mutagenesis of human beta-adrenergic receptors: substitution of aspartic acid-130 by asparagine produces a receptor with high-affinity agonist binding that is uncoupled from adenylate cyclase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5478–5482. doi: 10.1073/pnas.85.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V., McNeill J. R., Sulakhe P. V., Triggle C. R. Hepatic vasopressin receptor: differential effects of divalent cations, guanine nucleotides, and N-ethylmaleimide on agonist and antagonist interactions with the V1 subtype receptor. Endocrinology. 1988 Aug;123(2):922–931. doi: 10.1210/endo-123-2-922. [DOI] [PubMed] [Google Scholar]
- Grady T., Fickova M., Tager H. S., Trivedi D., Hruby V. J. Stimulation and inhibition of cAMP accumulation by glucagon in canine hepatocytes. J Biol Chem. 1987 Nov 15;262(32):15514–15520. [PubMed] [Google Scholar]
- Hagopian W. A., Tager H. S., Gysin B., Trivedi D., Hruby V. J. Interactions of glucagon and glucagon analogs with isolated canine hepatocytes. J Biol Chem. 1987 Nov 15;262(32):15506–15513. [PubMed] [Google Scholar]
- Hagopian W. A., Tager H. S. Receptor binding and cell-mediated metabolism of [125I]monoiodoglucagon by isolated canine hepatocytes. J Biol Chem. 1984 Jul 25;259(14):8986–8993. [PubMed] [Google Scholar]
- Heidenreich K. A., Weiland G. A., Molinoff P. B. Effects of magnesium and N-ethylmaleimide on the binding of 3H-hydroxybenzylisoproterenol to beta-adrenergic receptors. J Biol Chem. 1982 Jan 25;257(2):804–810. [PubMed] [Google Scholar]
- Horwitz E. M., Jenkins W. T., Hoosein N. M., Gurd R. S. Kinetic identification of a two-state glucagon receptor system in isolated hepatocytes. Interconversion of homogeneous receptors. J Biol Chem. 1985 Aug 5;260(16):9307–9315. [PubMed] [Google Scholar]
- Hruby V. J. Structure-conformation-activity studies of glucagon and semi-synthetic glucagon analogs. Mol Cell Biochem. 1982 Apr 16;44(1):49–64. doi: 10.1007/BF00573846. [DOI] [PubMed] [Google Scholar]
- Johnson D. G., Goebel C. U., Hruby V. J., Bregman M. D., Trivedi D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science. 1982 Feb 26;215(4536):1115–1116. doi: 10.1126/science.6278587. [DOI] [PubMed] [Google Scholar]
- Kofod H., Unson C. G., Merrifield R. B. Potentiation of glucose-induced insulin release in islets by desHis1[Glu9]glucagon amide. Int J Pept Protein Res. 1988 Dec;32(6):436–440. doi: 10.1111/j.1399-3011.1988.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Caron M. G. Regulation of beta-adrenergic receptors by guanyl-5'-yl imidodiphosphate and other purine nucleotides. J Biol Chem. 1976 Aug 10;251(15):4686–4692. [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May D. C., Ross E. M., Gilman A. G., Smigel M. D. Reconstitution of catecholamine-stimulated adenylate cyclase activity using three purified proteins. J Biol Chem. 1985 Dec 15;260(29):15829–15833. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Coverstone M., Lefkowitz R. J. Identification of adenylate cyclase-coupled beta-adrenergic receptors in frog erythrocytes with (minus)-[3-H] alprenolol. J Biol Chem. 1975 Jul 10;250(13):4869–4876. [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Post S. R., Miyazaki H., Tager H. S. Identification of a Mg(2+)- and guanyl nucleotide-dependent glucagon receptor cycle by use of permeabilized canine hepatocytes. J Biol Chem. 1992 Dec 25;267(36):25776–25785. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Register R. B., Candelore M. R., Rands E., Dixon R. A. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes. 1971 Dec;20(12):834–838. doi: 10.2337/diab.20.12.834. [DOI] [PubMed] [Google Scholar]
- Unson C. G., Andreu D., Gurzenda E. M., Merrifield R. B. Synthetic peptide antagonists of glucagon. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4083–4087. doi: 10.1073/pnas.84.12.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unson C. G., Gurzenda E. M., Iwasa K., Merrifield R. B. Glucagon antagonists: contribution to binding and activity of the amino-terminal sequence 1-5, position 12, and the putative alpha-helical segment 19-27. J Biol Chem. 1989 Jan 15;264(2):789–794. [PubMed] [Google Scholar]
- Unson C. G., Macdonald D., Ray K., Durrah T. L., Merrifield R. B. Position 9 replacement analogs of glucagon uncouple biological activity and receptor binding. J Biol Chem. 1991 Feb 15;266(5):2763–2766. [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]
- Williams L. T., Mullikin D., Lefkowitz R. J. Magnesium dependence of agonist binding to adenylate cyclase-coupled hormone receptors. J Biol Chem. 1978 May 10;253(9):2984–2989. [PubMed] [Google Scholar]
- Wyborski R. J., Horwitz E. M., Jenkins W. T., Mormol J. S., Gurd R. S. Guanine nucleotide regulation of the interconversion of the two-state hepatic glucagon receptor system of rat. Arch Biochem Biophys. 1988 May 1;262(2):532–542. doi: 10.1016/0003-9861(88)90405-5. [DOI] [PubMed] [Google Scholar]


