Abstract
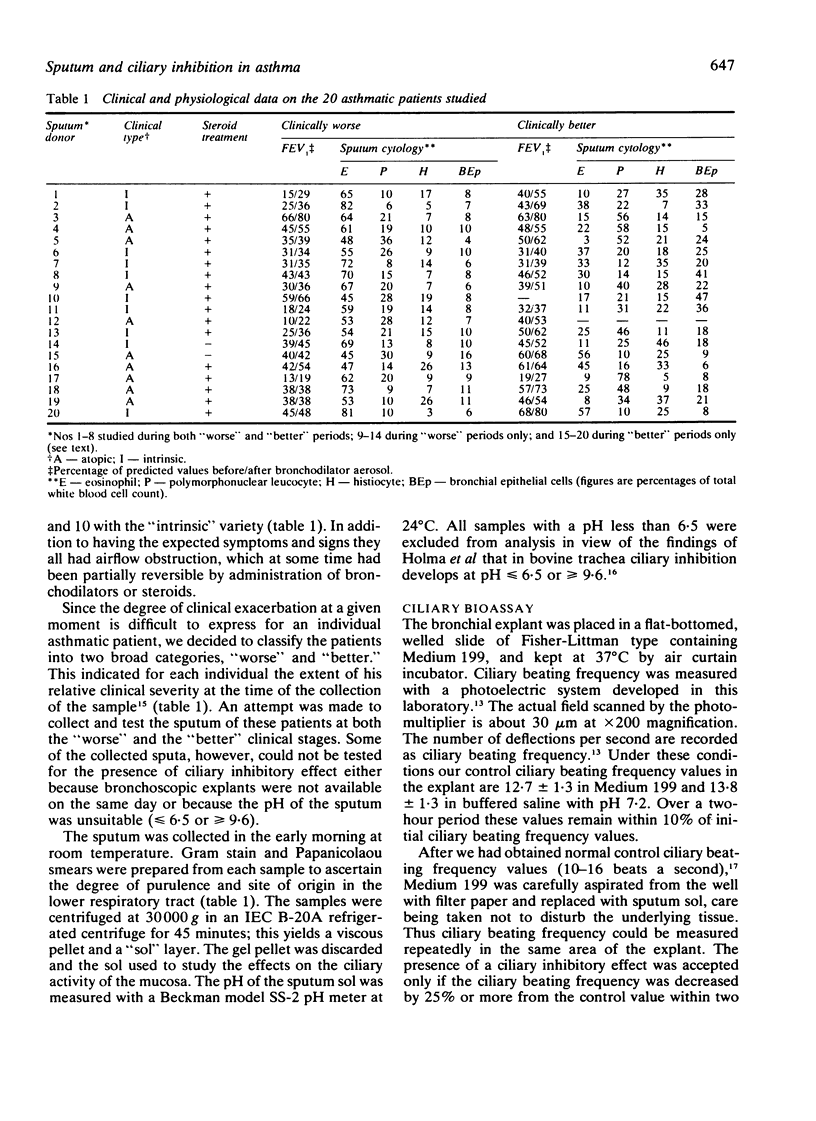
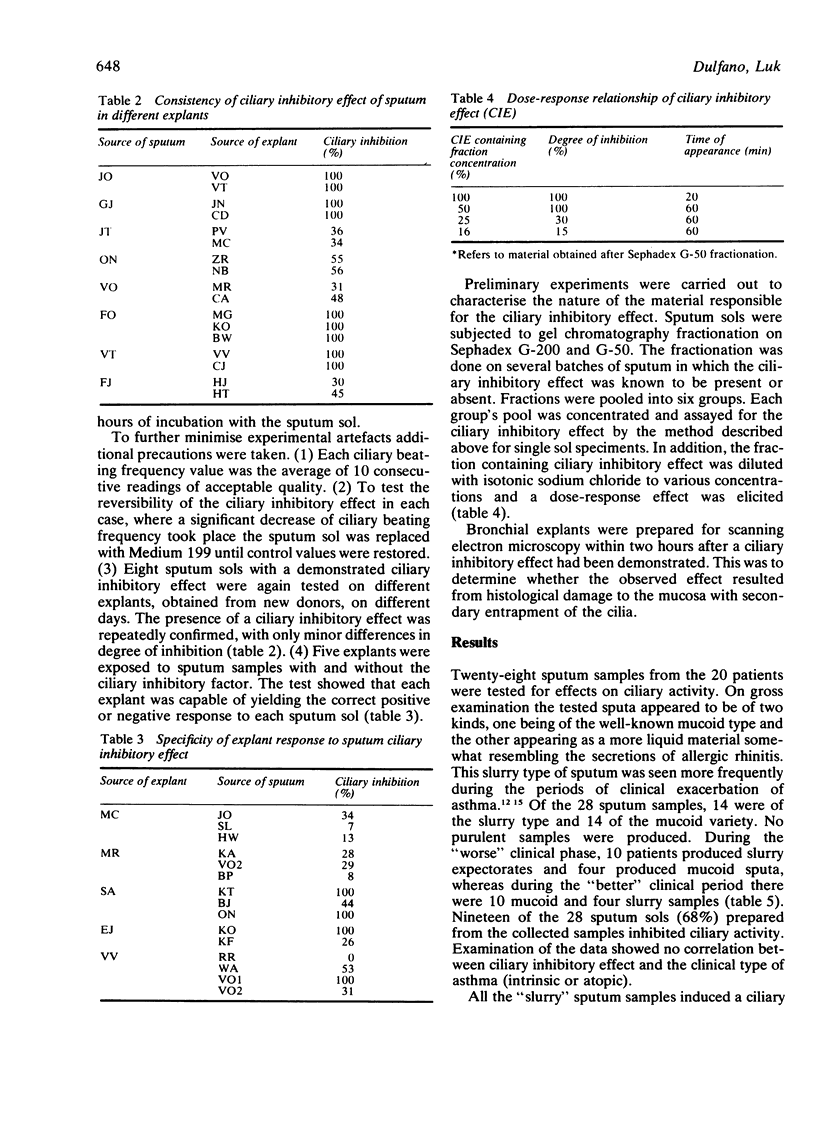
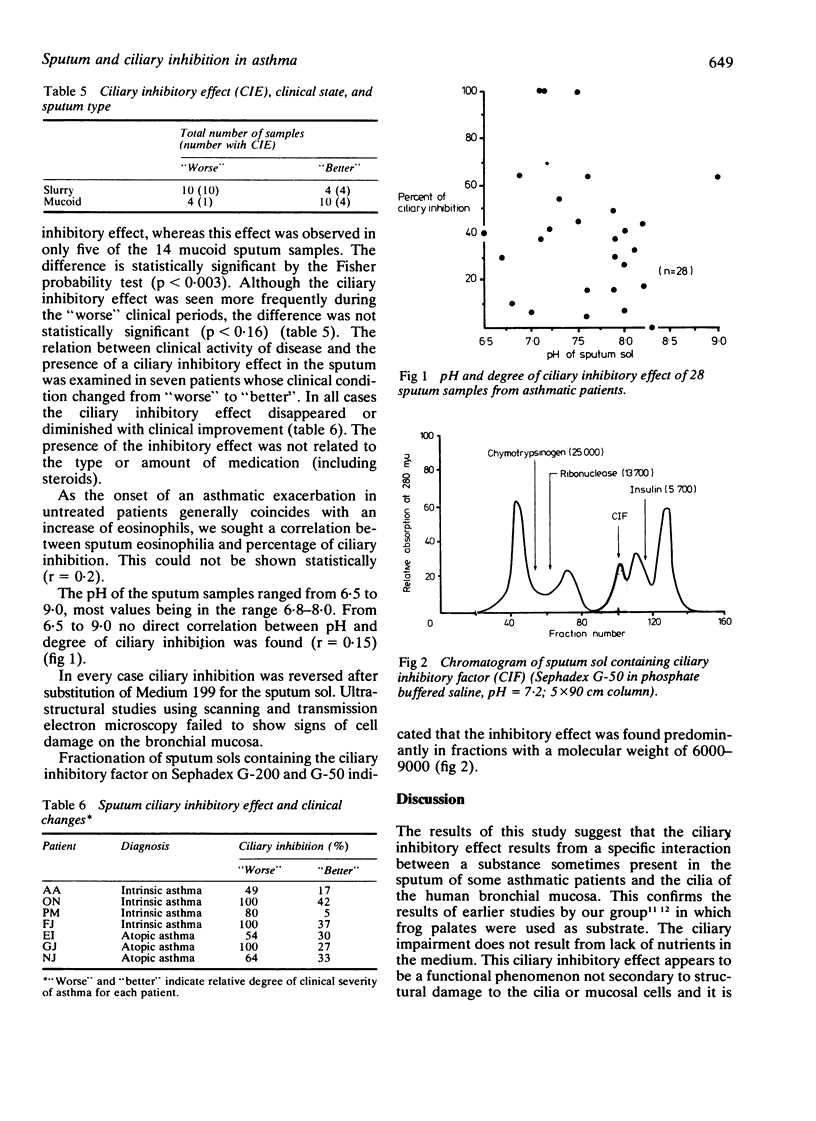
Twenty-eight sputum samples collected from 20 patients with chronic bronchial asthma of atopic and intrinsic clinical types were incubated with human bronchial explants to study their influence on ciliary motility. Of these, 19 (68%) of the sputa exerted a ciliary inhibitory effect of varying degree in a two-hour period. Analysis of the data indicates that (1) the ciliary inhibitory effect was invariably present when patients produced a distinctive slurry sputum; (2) this occurred more frequently during clinical exacerbations; (3) the induced ciliary inhibition was reversible on removal of the sputum; (4) the intensity of the ciliary inhibitory effect decreased with clinical improvement of the patient; (5) the inhibitory effect was unrelated to the medications used; (6) it was equally common in the atopic and the intrinsic types of asthmatic patients; (7) the effect was not pH dependent or related to the degree of eosinophilia. The ciliary inhibitory factor in sputum was identified as having a molecular weight of 6000-8000. It may play a part in the pathogenesis of asthma and recognition of sputum containing it carries implications for treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. H., Lankford B. J., McNeely M. C., Carson S. D., Barnett D. R., Berg K. Cystic fibrosis: studies with the oyster ciliary assay. Clin Genet. 1977 Dec;12(6):333–343. doi: 10.1111/j.1399-0004.1977.tb00952.x. [DOI] [PubMed] [Google Scholar]
- Bowman B. H., Lockhart L. H., McCombs M. L. Oyster ciliary inhibition by cystic fibrosis factor. Science. 1969 Apr 18;164(3877):325–326. doi: 10.1126/science.164.3877.325. [DOI] [PubMed] [Google Scholar]
- Chen T. M., Dulfano M. J. Mucus viscoelasticity and mucociliary transport rate. J Lab Clin Med. 1978 Mar;91(3):423–431. [PubMed] [Google Scholar]
- Cherry J. D., Roden V. J., Rejent A. J., Dorner R. W. The inhibition of ciliary activity in tracheal organ cultures by sera from children with cystic fibrosis and control subjects. J Pediatr. 1971 Dec;79(6):937–942. doi: 10.1016/s0022-3476(71)80187-7. [DOI] [PubMed] [Google Scholar]
- Conover J. H., Bonforte R. J., Hathaway P., Paciuc S., Conod E. J., Hirschhorn K., Kopel F. B. Studies on ciliary dyskinesia factor in cystic fibrosis. I. Bioassay and heterozygote detection in serum. Pediatr Res. 1973 Apr;7(4):220–223. doi: 10.1203/00006450-197304000-00027. [DOI] [PubMed] [Google Scholar]
- Dulfano M. J., Luk C. K., Beckage M., Wooten O. Ciliary beat frequency in human respiratory explants. Am Rev Respir Dis. 1981 Jan;123(1):139–140. doi: 10.1164/arrd.1981.123.1.139. [DOI] [PubMed] [Google Scholar]
- Dulfano M. J., Luk C. K., Beckage M., Wooten O. Ciliary inhibitory effects of asthma patients' sputum. Clin Sci (Lond) 1982 Oct;63(4):393–396. doi: 10.1042/cs0630393. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Solley G. O., Farrow G. M., Gleich G. J. Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc. 1981 Jun;56(6):345–353. [PubMed] [Google Scholar]
- Holma B., Lindegren M., Andersen J. M. pH effects on ciliomotility and morphology of respiratory mucosa. Arch Environ Health. 1977 Sep-Oct;32(5):216–226. doi: 10.1080/00039896.1977.10667285. [DOI] [PubMed] [Google Scholar]
- Mossberg B., Strandberg K., Philipson K., Camner P. Tracheobronchial clearance in bronchial asthma: response to beta-adrenoceptor stimulation. Scand J Respir Dis. 1976;57(3):119–128. [PubMed] [Google Scholar]
- Santa Cruz R., Landa J., Hirsch J., Sackner M. A. Tracheal mucous velocity in normal man and patients with obstructive lung disease: effects of terbutaline. Am Rev Respir Dis. 1974 Apr;109(4):458–463. doi: 10.1164/arrd.1974.109.4.458. [DOI] [PubMed] [Google Scholar]
- Spock A., Heick H. M., Cress H., Logan W. S. Abnormal serum factor in patients with cystic fibrosis of the pancreas. Pediatr Res. 1967 May;1(3):173–177. doi: 10.1203/00006450-196705000-00003. [DOI] [PubMed] [Google Scholar]
- Verdugo P., Hinds T. R., Vincenzi F. F. Laser light-scattering spectroscopy: preliminary results on bioassay of cystic fibrosis factor(s). Pediatr Res. 1979 Feb;13(2):131–135. doi: 10.1203/00006450-197902000-00009. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Fudenberg H. H. Ciliary dyskinesia factors in cystic fibrosis and asthma. Nature. 1977 Mar 31;266(5601):463–464. doi: 10.1038/266463a0. [DOI] [PubMed] [Google Scholar]
- Yager J., Chen T. M., Dulfano M. J. Measurement of frequency of ciliary beats of human respiratory epithelium. Chest. 1978 May;73(5):627–633. doi: 10.1378/chest.73.5.627. [DOI] [PubMed] [Google Scholar]


