Abstract
Alcohol abuse is comorbid with abuse of many other drugs, some with similar pharmacology and others quite different. This leads to the hypothesis of an underlying, unitary dysfunctional neurobiological basis for substance abuse risk and consequences. In this review, we discuss commonalities and distinctions of addiction to alcohol and other drugs. We focus on recent advances in pre-clinical studies using rodent models of drug self-administration. While there are specific behavioral and molecular manifestations common to alcohol, psychostimulant, opioid, and nicotine dependence, attempts to propose a unifying theory of the addictions inevitably face details where distinctions are found among classes of drugs. For alcohol, versus other drugs of abuse, we discuss and compare advances in: 1) neurocircuitry important for the different stages of drug dependence; 2) transcriptomics and genetical genomics; and 3) enduring effects. We note in particular the contributions of behavioral genetics and animal models: discussions of progress specifically relevant to treatment development can be found in the accompanying review (Karoly et al, this issue).
Keywords: alcohol, cocaine, drugs of abuse, animal models, addiction
Introduction
Commonalities in the interoceptive states, behaviors and pathophysiologies associated with dependence on alcohol, psychostimulant, and opioids (among other drugs) have been the impetus for proposing unifying theories of addiction that have led to important discoveries and ultimately shaped the trajectory of our field. Although unifying theories of addiction have aided clinical diagnosis, any unifying theory (e.g. psychomotor-stimulant, incentive-sensitization, hedonic allostasis, aberrant learning, and fronto-striatal dysfunction) inevitably cannot account for some behavioral and molecular features distinct to each class of abused drugs. Ignoring such distinctions may limit scientific and therapeutic advances. We adopt the framework of the hedonic allostasis model for convenience (Koob and Le Moal, 2008) and because it emphasizes developmental changes during the course of addiction, but make no attempt to compare the various unifying theories critically.
Some major commonalities are found in the existence of specific brain circuitry dedicated to hedonic processes, which is based on early studies showing that electrical stimulation of specific brain regions is rewarding in rats (Olds and Milner, 1954). Subsequent studies showed that natural and drug rewards recruit similar brain circuitry, namely the mesotelencephalic dopamine system (Koob and Volkow, 2010, Stuber et al., 2012). Additionally, different pharmacological classes of abused drugs increase expression of immediate early genes (i.e. c-Fos, Arc) and alter expression of proteins involved in long-term adaptive changes (i.e. ΔFosB) in the same brain regions (Lobo et al., 2013, McClung et al., 2004). Further, many studies implicate the same neurotransmitter systems (e.g. glutamate, dopamine, GABA, CRF and other peptides, as well as immune function genes) as mediators of behavioral responses to several classes of abused drugs (Koob and Volkow, 2010). Irrespective of the type of drug abused, there are similar hedonic states, feelings and behaviors associated with each stage of drug dependence. For example, “liking” is associated with the early “binge/intoxication” stage, where self-administration is postulated to be maintained by rewarding consequences. Negative affect is associated with cycles of acute and chronic drug abstinence leading to withdrawal, and feelings of “preoccupation/anticipation” are associated with drug seeking behavior during the later stage of allostatic dysregulation in the drug dependent state (Koob and Volkow, 2010).
We compare recent findings on the role of brain circuitry, gene expression, plasticity, and genetic risk factors for different classes of drugs in the context of rodent models. We highlight places where alcohol differs from other abused drugs. To keep the scope manageable, we concentrate on comparisons of recently published studies using voluntary drug self-administration. Consideration of similarities and differences specifically relevant to development of novel pharmacotherapies is a focus of the accompanying review (Karoly et al, this issue).
Brain Circuitry
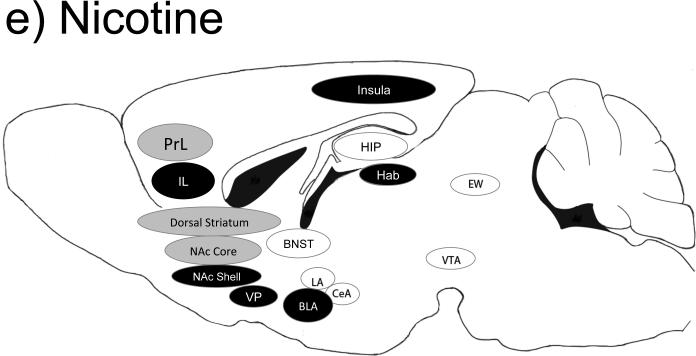
The mesotelencephalic dopamine system has long been a focus for addiction researchers. This system is comprised of the nigrostriatal, mesolimbic, and mesocortical pathways which consists of DA cell bodies in the substantia nigra and ventral tegmental area (VTA) that project to a number of regions, including the ventral striatum (nucleus accumbens (NAc) core and shell subdivisions), dorsal striatum, amygdala (5 distinct sub-regions), and medial prefrontal cortical regions (mPFC). There are a number of additional brain regions shown to be important for alcohol and other drug intake, such as the ventral pallidum (VP), bed nucleus of the stria terminalis (BNST), habenula (Hb), lateral septum, hippocampus (HIP), and insular cortex (Koob and Volkow, 2010, Marchant et al., 2014, Volkow et al., 2004). Figure 1 illustrates brain regions important for cocaine (a), methamphetamine (b), alcohol (c), heroin (d) and Nicotine (e) self-administration and drug-seeking behaviors. Table 1 provides additional information on the effects of region specific lesions, administration of intracranial injections of pharmacological agents, and optogenetic- or Designer Receptors Exclusively Activated by Designer Drugs (DREADD)- based activity manipulations on drug taking and seeking behaviors. Notably, Figure 1 and table 1 are not comprehensive, as the scope of this review is limited to recent studies that have further elucidated the importance of specific striatal cell types, neuronal firing patterns, and neuronal projections in reinforcing and drug-related behaviors.
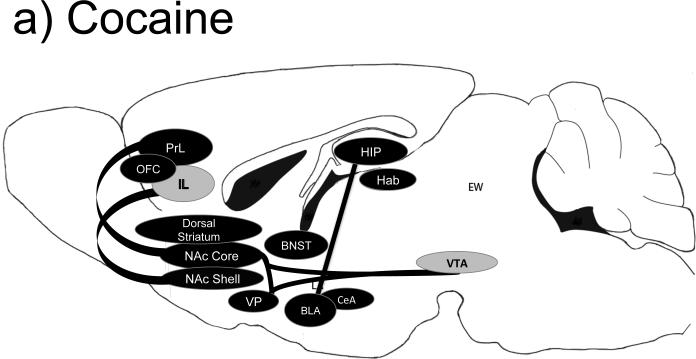
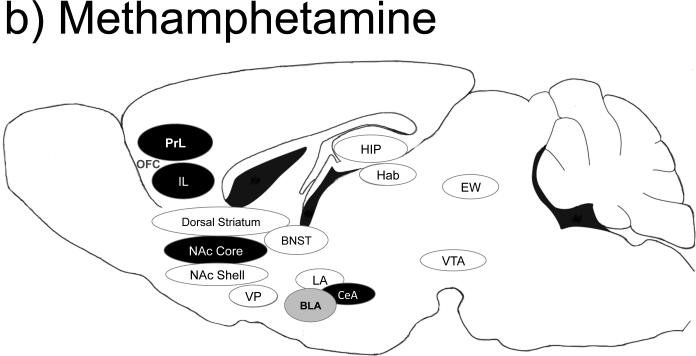
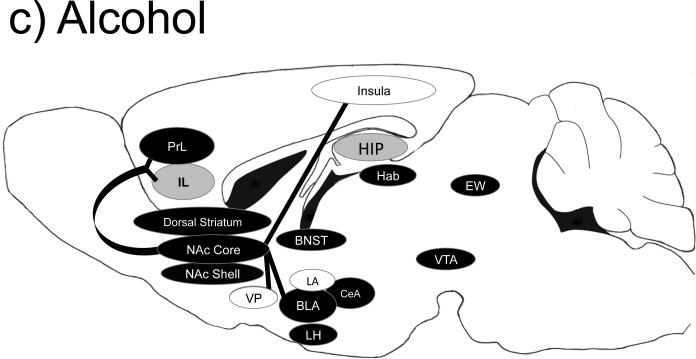
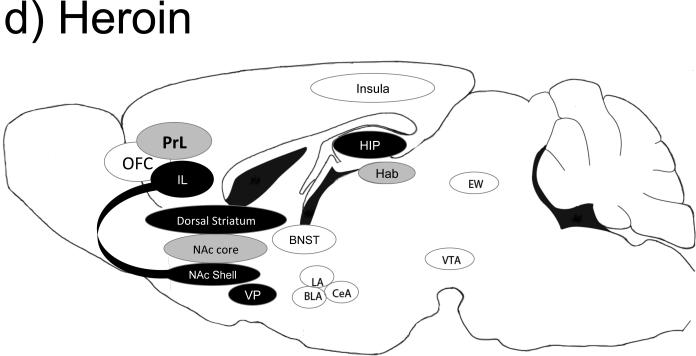
Figure 1.
Brain regions important for cocaine (a), methamphetamine (b), alcohol (c), heroin (d) and nicotine (e) self-administration behaviors. Black-filled oval= manipulation of this brain region is important for self-administration behaviors; Gray-filled oval= manipulation of this brain region has no known effects on self-administration behaviors; Open oval= manipulation of this region has not been tested in a similar manner; A line from one region to another indicates a specific projection shown to be important for self-administration-related behaviors. Abbreviations: BLa= basolateral amygdala; CeA= central amygdala; EW= Edinger-Westphal nucleus; Hab= habenula; HIP= hippocampus; IL= infralimbic cortex; LA= lateral amygdala; NAc= nucleus accumbens; PrL= prelimbic cortex; OFC= orbital frontal cortex; VP= ventral pallidum; VTA= ventral tegmental area.
Table 1.
Effects of region specific lesions, administration of intracranial injections of pharmacological agents, and optogenetic- or DREADD-based activity manipulations on drug taking and seeking behaviors.
| Brain Region(s) | Cocaine | Methamphetamine | Alcohol | Heroin | Nicotine | Reference(s) |
|---|---|---|---|---|---|---|
| dMPFC, PrL (dorsomedial prefrontal cortex; Prelimbic cortex) | TTX inactivation ↓seeking; optogenetic excitation ↓seeking and optogenetic inhibition ↓seeking | B+M inactivation had no effect on context seeking but ↓ drug+cue seeking | B+M inactivation ↓seeking | B+M inactivation has no effect on seeking | Bossert et al., 2011; Chen et al., 2013; Fuchs et al., 2005; Li et al., 2015; Rocha and Kalivas, 2010; Stefanik et al., 2012; Willcocks and McNally, 2013 | |
| vPFC, IL (dorsolateral prefrontal cortex; Inrfalimbic cortex) | TTX had no effect on seeking | B+M inactivation↓ or had no effect on seeking | B+M inactivation had no effect on seeking | B+M inactivation and Daun02 inactivation ↓seeking | Bossert et al., 2011; Fuchs et al., 2005; Li et al., 2015; Rocha and Kalivas, 2010; Willcocks and McNally, 2013 | |
| OFC (orbitofrontal cortex) | LOFC B+M inactivation ↓seeking | B+M inactivation had no effect on seeking | Lasseter et al., 2009, Li et al., 2015 | |||
| Ins (Insular cortex) | B+M inactivation ↓SA and seeking | Pushparaj et al., 2015 | ||||
| DStr (Dorsal striatum) | DLS M+B inactivation or α-flupenthixol ↓ seeking; CNQX or JNJ16259685 had no effect on seeking | DMS B+M inactivation prevents devalutation early in SA/habit learning; DLS B+M inactivation renew sensitivity to devaluation later in SA/habit learning | SCH23390 ↓seeking; LY379268 had no effect on seeking | Bossert et al., 2006, 2009; Corbit et al., 2012; Fuchs et al., 2006; Murray et al., 2014; Xie et al., 2012 | ||
| NAc core (Nucleus accumbens, core subregion) | optogenetic excitation of D2 MSNs ↓ SA; DREADD inhibition of D2 MSNs ↑SA; optogenetic inhibition, B+M inactivation, JNJ16259685 or CNQX, ↓seeking; DAUN02 inactivation had no effect on seeking | B+M inactivation ↓seeking | B+M inactivation or SCH23390 ↓seeking; high frequency DBS or lesion ↓intake | LY379268 or SCH23390 had no effect on seeking | Bock et al., 2013; Bossert et al., 2007; Cassataro et al., 2014; Chaudhri et al., 2009, 2010; Cruz et al., 2014; Fuchs et al., 2004, 2008; Rocha and Kalivas, 2010; Stefanik et al., 2012 Wilden et al., 2014; Xie et al., 2012 | |
| NAc shell (Nucleus accumbens, shell subregion) | B+M inactivation ↓context-induced seeking; B+M inactivation has no effect on cue-induced seeking | B+M inactivation, CART, CTAP or SCH23390 ↓seeking; ↓seeking | LY379268 or SCH23390 ↓seeking | Bossert et al., 2006, 2007; Chaudhri et al., 2009; Fuchs et al., 2004; Fuchs et al., 2008; Millan and McNally, 2012; Perry and McNally, 2013 | ||
| VP (Ventral pallidum) | lesion ↓seeking | lesion ↓seeking | Hubner and Koob, 1990 | |||
| BLA (Basolateral amygdala) | TTX inactivation ↓seeking | B+M inactivation had no effect on seeking | naloxone methiodide ↓seeking | SB-277011-A ↓seeking; B+M inactivation had no effect on SA | Fuchs et al., 2005; Li et al., 2015; Khaled et al., 2014; Marinelli et al., 2010; Yu and Sharp, 2015 | |
| CeA (Central nucleus of the amygdala) | SCH23390 ↓SA; LY37928 ↓seeking | B+M inactivation ↓seeking | UCN3 and D-Phe-CRF(12-41) ↓SA | Caine et al., 1995; Funk et al., 2006, 2007; Li et al., 2015; Lu et al.., 2007 | ||
| BNST (Bed nucleus of the stria terminalis) | B+M inactivation ↓seeking | LeuPro NPY ↓binge drinking; DREADD-mediated inhibition of CRF + neurons ↓binge drinking | Buffalari and See, 2011; Pleil et al., 2015 | |||
| HIP (Hippocampus) | TTX, AP5, PP2, Ro25-6981, JNJ16259685, and CNQX in dHip ↓seeking; M+B in CA3 or VHip ↓seeking; M+B in pDHip had no effect on seeking | blockade of opioid receptors in dHip had no effect on seeking | B+M inactivation of vSub ↓seeking | Bossert and Stern, 2014; Fuchs et al., 2005; Lasseter et al., 2010; Luo et al., 2011; Marinelli et al., 2010; Xie et al., 2010, 2013, 2014 | ||
| Hab (Habenula) | Lhab high+low combination DBS ↓seeking; Lhab lesion ↑seeking | Lhab lesions ↑SA | Hab lesion has no effect on SA | Lidocaine-induced inactivation mHab ↑SA; SB-277011-A ↓seeking | Fowler et al., 2011; Friedman et al., 2010; Haack et al., 2014; Khaled et al., 2014; Wang et al., 2009 | |
| VTA (Ventral tegmental area) | optogenetic excitation (tonic firing) ↓intake | Bass et al., 2013 | ||||
| LH (Lateral hypothalamus) | B+M inactivation ↓seeking | Marchant et al., 2014 | ||||
| EW (Edinger-westphal nucleus) | lesion ↓drinking | Bachtell et al., 2004 | ||||
| BLA - NAc | asymmetrical disconnection (M+B in BLA; NBQX in NAc shell) ↑seeking | Millan and McNally, 2011; | ||||
| VTA - NAc Core | optogenetic inhibition ↓seeking | Stefanik et al., 2013 | ||||
| mPFC - NAc Core | optogenetic inhibition ↓SA paired with aversive stimulus | Seif et al., 2013 | ||||
| Ins - NAc Core | optogenetic inhibition ↓SA paired with aversive stimulus | Seif et al., 2013 | ||||
| PrL - NAc Core | optogenetic inhibition ↓seeking | Stefanik et al., 2012 | ||||
| IL - NAc Shell | pharmacological inactivation or optogenetic induction of LTD ↑seeking | asymmetrical disconnection ↓seeking | Bossert et al., 2012; LaLumiere et al., 2012; Peters et al., 2009; Ma et al., 2014 |
Black box = manipulation changes drug self-administration or seeking behaviors (where arrow denotes increase or decrease in behavior, and underline denotes additional study showing no effect); Gray box = manipulation has no effect on drug self-administration or seeking; White box = area untested in a similar manner. Abbreviations: B+M, baclofen + muscimol; DREADD, designer receptor exclusively activated by designer drug; MSN, medium spiny neuron; SA, self-administration; TTX, tetrodotoxin.
Although there are similarities in the brain regions implicated in each stage of the addiction cycle, the role of these regions can differ depending on the class of drug and the type of paradigm employed. Together, the studies discussed in this section suggest that an understanding of only dopamine reward cannot account for the reinforcing properties of all abused drugs. Rather, studies point to the existence of multiple important inputs to the ventral and dorsal striatum that play an important role in the development of dependence for each class of drugs. Given some of the symptom commonalities during each stage of the addiction cycle, it is not surprising that similar brain regions are involved in these particular behaviors, regardless of the class of abused drug. The NAc plays a major role in pleasurable feelings during binge-like use, while the amygdala is implicated in increased anxiety and anhedonia during withdrawal. Cortical projections to the NAc are important for drug-seeking behavior. It is possible that the differences in cortical brain regions engaged by each class of drug during drug-seeking may reflect the different interoceptive effects of the drug, as well as the cues associated with that psychological state.
The Role of Dopamine in Alcohol and Drug Self-Administration
Different aspects of this circuitry are engaged during each of the three stages of addiction: binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation. The binge/intoxication stage is associated with the acute reinforcing properties drugs of abuse, including ethanol, cannabinoids, opiates, nicotine, amphetamine, and cocaine. Each these abused drugs activates the mesolimbic dopamine system and increases levels of dopamine in the striatum; however, different drugs effect this through different mechanisms (Di Chiara and Imperato, 1988, Kornetsky and Esposito, 1979, Oleson and Cheer, 2012, Koob and Volkow, 2010, Wang et al., 2014). Despite this commonality, activation of the midbrain dopamine system is not critical for the reinforcing effects of all drugs. For example, dopamine (DA) receptor blockade, as well as selective lesions of loci within the mesolimbic DA system, results in decreased cocaine and d-amphetamine self-administration, but not that of ethanol, heroin, or morphine (Koistinen et al., 2001, Koob and Le Moal, 2005, McGregor and Roberts, 1993, Pettit et al., 1984). Further, blockade of dopamine receptors in the NAc increases the reinforcing efficacy of nicotine (Laviolette and van der Kooy, 2003, Sun and Laviolette, 2015).
The NAc is composed mainly of GABAergic medium spiny neurons (MSN) that are differentiated by their enrichment in expression of dopamine receptor 1 (Drd1) or dopamine receptor 2 (Drd2) as well as several other genes, and by their distinct projections through the cortico-basal ganglia pathway (the direct and indirect pathways, respectively). Studies have uncovered opposing roles for these cell types and pathways in reinforcing behaviors. Optogenetic stimulation of the striatal direct (D1 MSNs) pathway mimicked reward (resulted in reinforcing behaviors), whereas stimulation of the indirect (D2 MSNs) pathway mimicked punishment (reduced the probability of performing future actions) (Kravitz et al., 2012). Additionally, both types of behavioral effects remained intact in the presence of dopamine receptor antagonists. Sources of glutamate input to the striatum and their role in alcohol and drug self-administration are discussed further below. Notably, similar cell-type and pathway specific effects have been found for cocaine self-administration; alcohol and other drugs have not been tested in a comparable way. Inhibition of D2-MSNs in the NAc core using a pharmacogenetic approach (DREADDs) enhanced the motivation to obtain cocaine, whereas optogenetic activation of D2-MSNs suppressed cocaine self-administration (Bock et al., 2013).
Circuitry Controlling Binge Use and the Transition to Habitual Behaviors
The transition to from binge use to addiction involves changes in mesolimbic regions that may begin with a cascade of neuroadaptations from the ventral striatum to dorsal striatum and orbitofrontal cortex and eventually dysregulation of the extended amygdala and cortical regions (prefrontal and cingulate) (Koob and Volkow, 2010). Other recent studies have also employed optogenetics and/or brain region specific delivery of various pharmacological agents to better understand this circuitry in models of drug self-administration. Optogenetic stimulation patterns can be used to mimic tonic and phasic patterns of DA firing with significant behavioral relevance. For instance, tonic, but not phasic, stimulation of VTA dopamine cells attenuated ethanol drinking in rats (Bass et al., 2013). It will be of interest to determine whether these effects are specific to a particular projection region, such as the ventral striatum (NAc) or mPFC. A study by Stefanik et al. (2013) suggests there may exist a distinction here between alcohol and cocaine. Stefanik et al. (2013) found that optogenetic inhibition of VTA neurons projecting to the NAc core abolished reinstated cocaine seeking. Together, these studies further support that reducing DA mediated signaling reduces intake of stimulants and introduce new evidence that increasing tonic firing of DA neurons decreases ethanol intake. It has been recently noted that dopaminergic projections to the NAc, but not the dorsal striatum, are capable of co-releasing glutamate under specific conditions (Stuber et al., 2010). However, the specific role of glutamate release from DA neurons in drug-related behaviors is currently understudied.
Frontal cortical and dorsal striatal regions have been implicated in the transition to dependence. Recent studies with natural (sucrose pellet) and drug (alcohol and cocaine) rewards have elegantly illustrated an important role for the shift in orbital frontal cortex and dorsal medial striatum control of goal-directed behaviors to dorsal lateral striatum control of habitual behaviors, which is of critical interest for research on the mechanisms mediating the transition from abuse to dependence (Corbit et al., 2012, Gremel and Costa, 2013, Belin-Rauscent et al., 2012, Murray et al., 2014). It has recently been discovered that dopaminergic terminals in the dorsal striatum are capable of releasing GABA (Tritsch et al., 2012). More studies are needed to understand how regional specific co-release of DA and GABA influences specific circuits and their role in drug-related behaviors. Moving forward, it will be important to determine whether there are distinct responses to different drugs for these regions. It would also be advantageous to identify pharmacotherapies that can effectively enable normal goal-directed behavior without loss of control. Such a drug might target drug-related maladaptive habits or the compulsive-like nature of drug self-administration by increasing neuroplasticity, or by rendering the OFC/dorsal medial striatum labile again.
Circuitry Underlying Negative Affect and the Aversive Effects Associated with Alcohol and Drug Self-Administration
Regulation of alcohol and other drug intake is thought to reflect a balance of the positive and negative affective properties of alcohol or drugs of abuse, where increases in self-administration occur when rewarding effects of the drug are of greater magnitude than the aversive effects (Davis and Riley, 2010). The aversive and negative emotional states associated with alcohol and drug withdrawal are thought to promote continued alcohol and drug abuse and engage the extended amygdala, including the CeA and BNST. Withdrawal and negative affect are associated with reductions in levels of synaptic mesolimbic DA, accumbal serotonin and dynorphin, and increased amygdala CRF (Cui et al., 2013, Koob and Volkow, 2010). An important, but understudied, feature in the development of dependence is an individual's loss of self-regulation by the negative consequences of chronic alcohol and/or drug abuse. This is manifested by coming to ignore the negative medical and social consequences of their abuse, as well as by a shift toward increasing strength of motivation to reduce unpleasant consequences of withdrawal during abstinence (i.e., self-medication). Recent research has attempted to model negative consequences by seeking evidence of persistent self-administration in dependent animals when punishment is explicitly associated with self-administration. The insular cortex and the lateral habenula are implicated in mediating interoceptive states and processing aversive information (learning to avoid negative outcomes), respectively. Seif et al. (2013) showed that optogenetic inhibition of glutamatergic projections from either the medial prefrontal (mPFC) or insular cortex to the NAc core specifically reduced alcohol intake that was paired with aversive stimuli (foot shock or quinine adulterated alcohol solution) in both an operant self-administration paradigm and two-bottle choice ethanol drinking. A major strength of this study is that the authors use a paradigm of self-administration that models a major symptom of dependence (continued use despite negative consequences). It will be important to further study whether the same circuitry is engaged for intake of other drugs accompanied by adverse consequences.
Matsumoto and Hikosaka (2007) identified the lateral Hb as a mediator of aversive-related stimuli (Matsumoto and Hikosaka, 2007). This study generated great interest for addiction researchers, since there was very little known about the brain processes important for negative or aversive events related to drugs of abuse. Interestingly, chronic intermittent alcohol (a procedure that produces escalated alcohol intake) results in reduced sensitivity to the aversive effects of alcohol (Lopez et al., 2012). Both the medial and lateral Hb have been implicated in mediating the aversive effects of alcohol, nicotine, cocaine, and morphine (Friedman et al., 2010, Haack et al., 2014, Velasquez et al., 2014). Although Hb lesions do not alter heroin self-administration, lesions or lidocaine-induced inactivation of the Hb can increase ethanol intake and attenuate the aversive effects of ethanol (LHb), increase nicotine self-administration (MHb), as well as prevent extinction of cocaine seeking (LHb) (Friedman et al., 2010, Fowler et al., 2011, Haack et al., 2014, Wang et al., 2009). Notably, different LHb stimulation patterns can result in increased (low freguency stimulation), unaltered (high frequency stimulation), or reduced (combined pattern of high and low stimulations) cocaine seeking (Friedman et al., 2010). The ability to bi-directionally modulate cocaine-seeking behaviors with different LHb stimulation patterns provides a unique opportunity for testing novel therapeutics using ex vivo and in vivo electrophysiology in animals with drug self-administration experience. More studies are needed to better understand the role of this brain region in mediating the aversive effects of alcohol and other drugs. Interventions at this stage would be extremely beneficial in harm reduction, a major goal for alcohol research.
Circuitry Controlling Alcohol- and Drug-Seeking Behaviors
In animals that have learned to acquire drug administration associated with a cue and have then extinguished responding, reinstatement of drug self-administration behaviors (i.e., alcohol- or drug-seeking) can be induced using priming (i.e., administering the drug to re-establish its interoceptive effects), presentation of drug-associated cues (including context – see Figure 1), and/or inducing a presumed negative emotional state by a stressor. While there are a number of studies examining the involvement of specific brain regions in drug-seeking, few direct comparisons across drug classes can be made due to procedural differences in methods used to induce reinstatement. The NAc shell is most consistently implicated in this behavior across different classes of drugs (Marchant et al., 2014). Antagonism of dopamine or glutamate receptors in the NAc shell reduces reinstatement responding for alcohol, cocaine, and heroin (Bossert et al., 2007, Chaudhri et al., 2009, Fuchs et al., 2008, Xie et al., 2012).
However, notable distinctions among drugs of abuse exist for the role of different brain regions that project to the NAc in context-induced drug seeking across drug classes. There are several studies of cocaine self-administration and seeking behavior, and only a few for alcohol, that identify pathway specific behavioral effects for the role of mPFC sub-regions in context-induced drug seeking. Dorsal (including prelimbic (PrL)) and ventral [including infralimbic (IL)] mPFC regions provide glutamatergic input to the NAc core, whereas the ventral mPFC provides glutamatergic input to the NAc shell (Brog et al., 1993, Sesack et al., 1989). Chen et al. (2013) found marked reductions in PrL excitability in a rat model of compulsive cocaine seeking, where optogenetic PrL stimulation decreased compulsive drug-seeking behaviors. This finding was specific to a subgroup of rats that exhibited continued cocaine seeking despite delivery of noxious foot shocks. In contrast, paradigms that do not model drug seeking despite negative consequence indicate an opposite role for the PrL in drug-seeking behaviors. Specifically, inactivation of the dorsal mPFC (including PrL) resulted in reduced context-induced alcohol or cocaine seeking (Fuchs et al., 2005, Willcocks and McNally, 2013). Additionally, inhibition of glutamatergic projections from the PrL to the NAc core increased cocaine-seeking behavior (Stefanik et al., 2012). Interestingly, pharmacological inactivation or optogenetic induction of long term depression (LTD) in glutamatergic projections from the IL-to-NAc shell can reinstate cocaine seeking, suggests opposite modulation of cocaine seeking by PrL-to-NAc core and IL-to-NAc shell projections (LaLumiere et al., 2012, Peters et al., 2009, Ma et al., 2014). In contrast to findings for cocaine, inactivation of the ventral mPFC (including IL) reduced context-induced reinstatement of heroin seeking (Bossert et al., 2007, 2011).
Another source of glutamatergic input to the NAc is the ventral HIP. Inactivation of the ventral HIP reduces context-induced cocaine and heroin seeking (Bossert and Stern, 2014, Lasseter et al., 2010). Additionally, theta burst stimulation of the ventral HIP reinstates responding for cocaine (Vorel et al., 2001). Further, inactivation of the dorsal HIP and the dorsal HIP-to-BLA projection reduces context-dependent reinstatement of responding for cocaine (Fuchs et al., 2005, Fuchs et al., 2007). The role of ventral and dorsal HIP have not been tested in comparable manner for alcohol. However, Marinelli et al. (2010) found that blockade of opioid receptors in the BLA, but not dorsal HIP, reduced context-induced reinstatement of alcohol seeking (Marinelli et al., 2010).
In summary, glutamatergic projections from the ventral and dorsal mPFC differentially contribute to cocaine seeking; the HIP mediates cocaine and heroin seeking; and opioid transmission in the BLA is an important mediator of context-induced alcohol seeking. These recently published drug self-administration studies have been highlighted because drug-seeking behavior could be compared across drug classes without the confounding influence of drug-priming (for different classes of drugs) or stressors (which may differentially affect the pathophysiological state associated with each type of drug abuse).
We described above the importance of specific dopaminergic and glutamatergic inputs to the NAc in alcohol and drug-seeking behavior. The NAc projects to the VP, a region implicated in alcohol and drug-taking and -seeking behaviors. For instance, lesions of the VP reduced heroin and cocaine self-administration (Hubner and Koob, 1990) and optogenetic inhibition of the NAc core projections specifically to the VP reduced the reacquisition of alcohol relapse-like behavior (Khoo et al., 2015). Further, optogenetic inhibition of NAc projections to the VP reduced cocaine relapse-like behaviors (Stefanik et al., 2013). Stefanik et al. (2013) further interrogated this pathway and found that optogenetic inhibition of VTA inputs to the NAcore reduced cocaine-plus-cue-induced reinstatement of cocaine seeking. Furthermore, Mahler et al. (2014) found that DREADD-mediated inhibition of VP neurons projecting to the VTA reduced cocaine-seeking behaviors. This work also revealed an important role for the rostral VP in cue-induced cocaine seeking and the caudal VP in cocaine-induced cocaine seeking behaviors, a sub-regional distinction previously undescribed (Mahler et al., 2014). An obvious gap exists in the literature regarding the importance of these circuits for other drugs of abuse (alcohol, methamphetamine, nicotine): thus, much insight can be gained in future studies with other drugs of abuse by employing context-induced drug-seeking paradigms.
Transcriptomics
Over the past 15 or so years, every aspect of high throughput measurement of gene expression has rapidly evolved, including types of tissues collected (whole brain, brain regions, cell types), types of RNA isolated, detection methods (differential display, serial analysis of gene expression, microarray, whole transcriptome sequencing), and of course, various statistical approaches for the analysis of large data sets. Furthermore, we continue to identify new genes and characterize their functions at an astounding rate. This means that genes identified in a study today may not have even been represented on an array or in a functional category in a study published 5 years ago.
Another complication in the comparison of gene expression findings across classes of drugs are the use of different species, strains, sexes, paradigms employed for drug experience, brain region of interest, time of tissue collection after last drug experience, platform for detection, and type of analysis employed. Much more work has been published using alcohol than other drugs of abuse. Therefore, this section of the review will focus on a few take-home messages from specific studies with alcohol thus far.
The first message is that different alcohol-drinking paradigms do not result in similar patterns of gene expression, even when the different paradigms result in comparable intake. Osterndorff-Kahanek et al. (2013) compared gene expression in the PFC of female C57BL/6J mice using three different paradigms for voluntary consumption of a 20% ethanol solution (for approximately one month): chronic continuous two bottle choice, two bottle choice available every other day (chronic intermittent paradigm) and limited access to one bottle of ethanol (Drinking In the Dark, or DID, paradigm). An unexpected finding from this work is the distinct effects of the three alcohol paradigms on the PFC transcriptome. Total ethanol consumption generally paralleled the relative amount of gene regulation; mice from the chronic continuous access and chronic intermittent access groups had greater ethanol consumption and a larger number of alcohol-regulated genes than the lower total intake DID group. There were 531 differentially expressed genes identified in the PFC of mice from the chronic continuous access group, 587 in the chronic intermittent group, and 445 in DID group, with significant overlap of specific genes observed between chronic continuous and chronic intermittent access groups. Studies by Rodd et al. (2008) and Bell et al. (2009) also illustrate distinct changes in rat brain gene expression depending on the alcohol paradigm employed (operant self-administration, multiple periods of scheduled (limited) access, and continuous access) (Bell et al., 2009, Rodd et al., 2008). That apparently limited consilience exists within a drug class suggests that it may be optimistic to expect firm conclusions from comparisons across multiple classes of drugs. Interestingly, there are a few published studies comparing the effects of different classes of abused drugs on gene expression from human post-mortem tissue (Enoch et al., 2014; Li et al., 2012; Zhou et al., 2011). However, using mouse models, Le Merrer et al (2012) examined expression levels of a set of approximately 100 mu-opioid receptor-dependent genes in the extended amygdala 4 weeks after experimenter administered chronic morphine, nicotine, THC or alcohol. This study identified 9 genes whose expression was changed in a similar pattern across all drugs of abuse (including Pde10a, discussed below). A meta-analysis or critical review of the existing data collected from animals that self-administered different drugs would be useful for identifying drug-specific, rather than drug-responsive, cellular adaptations. Such an analysis could synthesize results from several studies (Jacobs et al., 2005, Cadet et al., 2014, Fernandez-Castillo et al., 2012, Krasnova et al., 2013, Rodd et al., 2008, Yuferov et al., 2005).
The second message is more positive. Collaborative efforts of researchers have led to the successful identification of candidate genes for high alcohol intake, among other alcohol-related traits. Using pharmacological or RNAi targeting strategies, genes identified by their altered expression in multiple studies with alcohol have been shown to affect alcohol self-administration. Those genes include peroxisome proliferator-activated receptors (PPARs) and other neuroimmune modulators, circadian genes, phosphodiesterase 4 and phosphodiesterase 10a (Blednov et al., 2015, Blednov et al., 2014, Franklin et al., 2015, Ferguson et al., 2014, Perreau-Lenz et al., 2012, Ozburn et al., 2013, Robinson et al., 2014, Melendez et al., 2012, Logrip et al., 2014, Logrip and Zorrilla, 2014). Interestingly, PPARα agonists also decrease nicotine self-administration and nicotine-induced reinstatement in rats and monkeys, but did not alter food- or cocaine-reinforced operant behavior (Mascia et al., 2011). Administration of the PPARγ agonist, pioglitazone, decreased heroin self-administration and reduced opioid-induced excitation of VTA DA neurons (via reduction of presynaptic GABA release from the rostromedial tegmental nucleus) (de Guglielmo et al., 2015). Many of the target genes identified and validated, such as PPARs, are regulated in a circadian manner. Interestingly, PPARs are important for the integration of metabolic processes with the molecular clock (Chen and Yang, 2014). Reduced expression and function of circadian genes lead to increased alcohol (Clock and Per2) and cocaine (Clock), but not nicotine (Clock) self-administration (Abarca et al., 2002, Bernardi and Spanagel, 2013, Ozburn et al., 2012, Ozburn et al., 2013, Parekh et al., 2015).
The third message is that transcriptomics is a powerful tool for advancing addiction research, as well as for identifying basic biological processes. Transcriptomics has generally been characterized as hypothesis generating. However, one often-overlooked point is that these studies are critically important for building our foundational knowledge of cellular processes. With advances in whole genome and transcriptome sequencing, new transcriptional regulatory elements have been identified (e.g. eRNAs, piRNAs, snoRNAs) and transcriptional architecture and chromatin landscapes have been determined (Kim et al., 2010, Koike et al., 2012, Zhang et al., 2014). Further, advances in whole transcriptome sequencing allow for the identification of drug effects on the expression of not only protein-coding genes but also particular splice variants, miRNAs, or lncRNAs (long noncoding RNAs). Interestingly, recent studies have identified a role for piRNAs in memory, epigenetics, and transgenerational inheritance (Lutejin et al., 2013; Landry et al., 2013). The contributions of small RNA guided gene regulation to drug-related behaviors are not yet known, but represent an attractive mechanism for the long-term behavioral changes associated with alcohol and other drugs. In the future, to interpret a transcriptional experiment it will be necessary to better understand the role of RNAs that are considered to be the most important differentially expressed genes by differentiating them from simply one differentially expressed gene in a long list.
The alcohol field has embraced a variety of genetic animal models (discussed further below), and this genetic diversity allows for integration of genetics and gene expression, also known as genetical genomics. The integration of phenotypic, genetic, and transcriptome data sets has identified a number of behaviorally relevant genes (e.g. the role of Mpdz in alcohol withdrawal as described in Kruse et al., 2014). There is much left to learn about the effects of alcohol and other drug self-administration by interrogating the transcriptional architecture and chromatin landscapes via the integration of whole genome sequencing, RNA Seq, ChIP Seq, and DNA methylation data.
Differences in lasting effects of alcohol and other drugs
Pharmacology and the inflammatory response
As described above (in other paragraphs focused on anatomy, circuitry, behavior and transcriptomics) and in the accompanying review by Karoly et al. (submitted), there are similarities in the pharmacological and neurochemical long-term effects of abused substances, as well as significant differences. Unfortunately, there are missing data that prevents direct comparisons across abused substances and subsequent unifying conclusions on their mechanism(s) of action. One recently described potential mechanism that appears to be involved in multiple long-term drug effects is activation of the trace amine associated receptor 1 (TAAR1). As compared to its lack of direct effects at other biogenic amine receptors, methamphetamine and many of its analogues are agonists at TAAR1, as is lysergic acid diethylamide (LSD). However, cocaine is not an agonist at the TAAR1, and based on structure activity relationship studies, it is unlikely that heroin or ethanol directly stimulate the receptor (Bunzow et al., 2001; Borowsky et al., 2001; Wainscott et al., 2007). Consistent with this finding, there are behavioral effects of methamphetamine that are consistent with chronic activation of the TAAR1. Those effects are corroborated in knockout mice, but effects on conditioned place preference in the knockout animals, for instance, do not generalize to morphine (Achat-Mendes et al., 2012). Studies using selectively bred methamphetamine high- and low-drinking mice (described below) indicate that TAAR1 activation confers sensitivity to the aversive effects of the drug, and Taar1 knockout mice or mice with a non-synonymous SNP that results in a non-functional receptor freely ingest the drug as compared with animals with a functional receptor (Harkness et al., 2015). Interestingly, in a similar 2-bottle choice paradigm involving ethanol instead of methamphetamine, Taar1 knockout mice drank more ethanol than wild-type mice, and were more sensitive to its sedative-like effects (Lynch et al., 2013). TAAR1 receptors regulate dopamine release in an autoreceptor-like manner (Lindemann et al., 2008; Revel et al., 2012; Leo et al., 2014), and in vitro and ex vivo experiments suggest that TAAR1 regulates dopamine transporter function (Xie et al., 2008). Thus, it is quite possible that TAAR1 represents a step upstream from common pharmacological mechanisms of drug abuse involving dopamine. Long term consequences of TAAR1 stimulation are difficult to assess because drugs that stimulate the receptor, such as the amphetamines, or the trace amines, also alter the function of biogenic amine transporters. However, treatment of primary human astrocytes in culture with methamphetamine increases TAAR1 expression and function, and the effect on expression is increased by HIV-1 (Cisneros and Ghorpade 2014).
This latter finding involving astrocytes is important because of the potential role of the inflammatory/immune response in the behavioral consequences of long-term drug abuse and dependence. Long-term exposure to methamphetamine alters cytokine responses in animals and human subjects (Loftis et al., 2011). In addition, the methamphetamine high- and low-drinking mice differ in a number of inflammatory pathway markers (Wheeler et al., 2009). Likewise, Toll like receptor (TLR) 2 knockout animals have an attenuated response to morphine as measured by microglia activation and proinflammatory cytokine release (Zhang et al., 2011). Evidence that cocaine is involved in a cytokine signaling response, however, is either contradictory or suggests that the drug does not exert its effects directly through alteration of cytokine activity (Chang et al., 1995; Fernandez-Espejo 2009). Nicotine, an immunosuppressant, may exert it effects via alteration of IL-1β expression, and long-term exposure down regulates an initial IL-1β increase. Further, changes in the cytokine parallel changes in resistance to proinflammatroy stimulation (Razani-Boroujerdi et al., 2011). Ethanol's effects on cytokine release and on cytokine signaling responses are robust, and speculation on mechanisms for the effects include possible involvement of TLR4 and high-mobility group box 1 (HMGB-1) (reviewed in Crews and Vetreno, 2015).
Thus, long-term effects of a number of drugs of abuse could involve alterations in various intermediates in the immune/inflammatory response, in the brain regions described in various sections of this review. Interestingly, TAAR1 is expressed on lymphocytes (Nelson et al., 2007), and the evidence involving astrocytes described above is complemented by studies on TAAR1's role in cytokine signaling via alteration of T cell gene expression and function (Wasik et al., 2012; Panas et al., 2012; Babusyte et al., 2013). Because the immune system responds to environmental stimuli, epigenetics may well play a central role in how abused drugs affect this system.
Epigenetics
Alcohol and drug dependence are accompanied by long-term effects on behavior. The stability of behavioral alterations associated with chronic abuse suggests maladaptive neuroplasticity via epigenetic, transcriptional, electrophysiological, and morphological mechanisms. Epigenetic modifications of chromatin structure are carried by enzymes. One such class of enzymes is histone deacetylases (HDACs; a transcriptionally silencing enzyme). Treatment with HDAC inhibitors results in significantly reduced excessive alcohol intake in three different alcohol-drinking paradigms and reduced anxiety in dependent animals (Sakharkar et al., 2012, Simon-O'Brien et al., 2014). HDAC inhibitors have also been shown to reduce the reinforcing properties of and motivation for cocaine, but not sucrose, as well as reinstatement of cocaine-primed drug-seeking (Romieu et al., 2011, Romieu et al., 2008). While additional studies of the effects of HDAC inhibition on other classes of drug self-administration may uncover class specific drug effects, HDAC inhibitors have also been shown to enhance mood and cognition (Penney and Tsai, 2014). Comprehensive reviews highlight the important role of epigenetics in abuse of alcohol (Krishnan et al., 2014) and other drugs (Robison and Nestler, 2011; Walker et al., 2015).
Plasticity
During reinforcement learning, such as operant drug self-administration, phasic dopamine bursts may induce long-lasting changes in striatal plasticity, including long-term potentiation via D1 receptor activation and long-term depression via D2 receptor activation. Under conditions of repeated alcohol or drug use, co-occurrence of striatal inputs might change efficacy at corticostriatal and thalamostriatal synapses through glutamate receptor–driven plasticity. For instance, Jeanes et al. (2014) observed low frequency-induced long-term depression (LTD) of AMPA-mediated excitatory postsynaptic currents in D1-MSNs that was absent after chronic intermittent ethanol (CIE) vapor exposure. Interestingly, after CIE, LTD was observed only in D2-MSNs. These alterations in plasticity recovered after 2 weeks of withdrawal from CIE. Kasenetz et al. (2010) found that cocaine self-administration also suppresses LTD in the NAc. Some of the cocaine self-administering rats progressively developed an addiction-like phenotype and these animals exhibited permanently impaired LTD, whereas animals maintaining cocaine intake exhibit recovery of LTD (Kasenetz et al., 2010). Moreover, low perseverance and motivation for cocaine correlated with greater synaptic potentiation of glutamatergic inputs onto indirect pathway D2-MSNs of the NAc (Bock et al., 2013). Together, these results suggest that alcohol dependence and perseverant cocaine taking differentially alter long-lasting depression of excitatory synaptic transmission in the NAc. However, it appears that this plasticity is eventually restored during cocaine taking that is not perseverant in nature.
Behavioral Genetics and Animal Models
Alcohol research has long employed behavioral genetics tools in the form of rodent models. For example, an early comprehensive review of genetic contributions to drugs of abuse required 200 pages devoted to alcohol studies vs less than 100 pages to psychostimulants, opioids, nicotine, and barbiturates/benzodiazepines combined (Crabbe and Harris, 1991). The field of alcohol research remains distinguished from other abused substances by the large number of rodent lines selectively bred to respond differentially to alcohol. A recent compilation identified 20 pairs of rat or mouse lines selected mostly for high vs low drinking traits, but also for sensitivity, tolerance, and withdrawal severity (Crabbe, 2014). The early establishment of long-term, genetically stable animal models has allowed accumulation of much useful information over the years. For example, knowledge of the consequences of chronic drug use on gene expression is derived heavily from alcohol-selected rats and mice (see above).
The use of genetic selection is not confined to alcohol, as lines of mice have been bred for sensitivity to levorphanol and diazepam, but neither of these lines currently exist. Recently, lines of rats have been directionally selected for high vs low nicotine intake, but these animals have not yet been studied for other traits to date (Nesil et al, 2013). Tamara Phillips’ laboratory has been pursuing genetic contributions to methamphetamine self-administration in mice derived from C57BL/6J × DBA/2J crosses through repeated selections for high versus low voluntary drinking. Three sets of lines have been sequentially bred for 4-5 generations and several genetic correlates have been found in multiple pairs of these lines. The genetic structure underlying methamphetamine risk have been explored with gene mapping and network analyses (Eastwood et al, 2014: Eastwood and Phillips, 2014; Harkness et al, 2015; Belknap et al, 2013). Interestingly, substance abuse researchers have instead elected to select rats for extremes in traits thought to be comorbid with or predisposing to substance abuse, such as or intake of the sweet tastant, saccharin (Carroll et al., 2008). Rats selected for high saccharin intake (HiS), when compared with those selected for LoS, have been examined for many cocaine-related behaviors consistent with cocaine addiction liability (e.g., acquisition, maintenance, extinction, reinstatement) and generally exhibit greater responding. Limited studies extend this difference to heroin and alcohol (Gosnell et al., 2010;Carroll et al., 2008). Rats bred for differential response to novelty also differ for other related traits and respond differentially to cocaine (Flagel et al., 2014) but do not appear to have been tested for other drugs. Rats shown to have greater impulsive-like behavior also acquire cocaine self-administration more readily; however, because these studies are based on comparing high vs low scoring animals from a single generation, it cannot be determined whether such differences are heritable (Perry et al., 2008;Broos et al., 2012;Jupp et al., 2013).
In contrast with alcohol studies, genetic studies of abuse of other substances have concentrated heavily on the use of gene-targeted animals, usually knockout preparations. Systematic studies with null mutants targeting multiple opioid and cannabinoid receptors and peptides have shown effects on multiple drugs of abuse, including alcohol (Charbogne et al., 2014). Similar catalogs of single-gene studies specifically targeting alcohol responses have been published elsewhere (Crabbe et al., 2011; Bilbao, 2013). Such comparisons of gene-derived effects on similar behaviors and physiological systems across multiple drugs of abuse have proven valuable (Le Merrer et al., 2012). An older study systematically compared the responses of reference populations (standard inbred mouse strains), to multiple drugs of abuse in search of signs of genetic associations that could underly polydrug sensitivity (Belknap et al, 2008). Data continue to accumulate in these reference populations (for a recent excellent example, see Wiltshire et al, 2015), and much of it has been contributed to the Mouse Phenome Database (www.phenome.jax.org), but to our knowledge, there have been no recent large-scale systematic syntheses of data seeking genetic commonalities of influence across drugs. Notably, a number of QTL studies for alcohol drinking have revealed different QTLs for different measures of drinking (acceptance, drinking, preference, DID, etc.). Additionally, there are relatively few similar studies for other drugs of abuse. For more information related to this topic, refer to Crabbe et al. (1999) and Crabbe et al. (1994). The continued development of new genetic approaches, discussed above, should facilitate the extension of the early gene-by-gene and drug-by-drug studies. We currently must rely on to systematic comparisons of genetic influences across multiple drugs of abuse, further differentiating drug-specific characteristics from more general responses.
Conclusions
Alcohol abuse is comorbid with abuse of many other drugs, some with similar pharmacology and others quite different. For alcohol, versus other drugs of abuse, we discussed advances in: 1) neurocircuitry important for the different stages of drug dependence; 2) transcriptomics and genetical genomics; and 3) enduring effects. We note in particular the contributions of behavioral genetics and animal models. Similarities generally include the involvement of the mesotelencephalic system in self-administration behaviors, lasting effects on neuronal plasticity, and some overlap in affected signaling pathways. The distinctions are numerous, and often found in the details; even within a drug class (i.e. different alcohol drinking assays result in distinct transcriptional profiles). The distinctions highlight how each drug class differentially engages and alters neuronal activity (and regulatory mechanisms) in specific cell types and projections during each stage of addiction. In the future, acknowledging these differences may lead to more effective therapies.
Acknowledgments
Sources of support: ARO: Veterans Affairs Career Development Award 2 IK2 BX002488, NARSAD Young Investigator Award, NIH P60 AA010760. AJJ: NIH P50 DA018165. JCC: NIH AA13519, NIH AA10760, NIH AA020245 and the Department of Veterans Affairs.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Lynch LJ, Sullivan KA, Vallender EJ, Miller GM. Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol Biochem Behav. 2012;101(2):201–7. doi: 10.1016/j.pbb.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babusyte A, Kotthoff M, Fiedler J, Krautwurst D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J Leukoc Biol. 2013;93(3):387–94. doi: 10.1189/jlb.0912433. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Frontiers in behavioral neuroscience. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin-Rauscent A, Everitt BJ, Belin D. Intrastriatal shifts mediate the transition from drug-seeking actions to habits. Biological Psychiatry. 2012;72:343–345. doi: 10.1016/j.biopsych.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Metten P, Beckley EH, Crabbe JC. Multivariate analyses reveal common and drug-specific genetic influences on responses to four drugs of abuse. Trends Pharmacol Sci. 2008;29:537–543. doi: 10.1016/j.tips.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, McClintick JN, Strother WN, Carr LG, Liang T, Rodd ZA, Mayfield RD, Edenberg HJ, McBride WJ. Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacology, biochemistry, and behavior. 2009;94:131–147. doi: 10.1016/j.pbb.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Metten P, Beckley EH, Crabbe JC. Multivariate analyses reveal common and drug-specific genetic influences on responses to four drugs of abuse. Trends Pharmacol Sci. 2008;29:537–543. doi: 10.1016/j.tips.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Spanagel R. The ClockDelta19 mutation in mice fails to alter the primary and secondary reinforcing properties of nicotine. Drug and alcohol dependence. 2013;133:733–739. doi: 10.1016/j.drugalcdep.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Bilbao A. Advanced transgenic approaches to understand alcohol-related phenotypes in animals. Curr Top Behav Neurosci. 2013;13:271–311. doi: 10.1007/7854_2012_204. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Ferguson LB, Schoenhard GL, Goate AM, Edenberg HJ, Wetherill L, Hesselbrock V, Foroud T, Adron Harris R. Peroxisome Proliferator-Activated Receptors alpha and gamma are Linked with Alcohol Consumption in Mice and Withdrawal and Dependence in Humans. Alcoholism, clinical and experimental research. 2015;39:136–145. doi: 10.1111/acer.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Harris RA. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Frontiers in neuroscience. 2014;8:129. doi: 10.3389/fnins.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature neuroscience. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966–71. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL. Role of ventral subiculum in context-induced reinstatement of heroin seeking in rats. Addiction biology. 2014;19:338–342. doi: 10.1111/adb.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2102;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. The Journal of comparative neurology. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, de Vries TJ. Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: a bidirectional investigation. 2012;37:1377–1386. doi: 10.1038/npp.2011.323. npp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology. 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60(6):1181–8. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Jayanthi S, Krasnova IN. Transcriptional and Epigenetic Substrates of Methamphetamine Addiction and Withdrawal: Evidence from a Long-Access Self-Administration Model in the Rat. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Cassataro D, Bergfeldt D, Malekian C, Van Snellenberg JX, Thanos PK, Fishell G, Sjulson L. Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology. 2014;39:283–90. doi: 10.1038/npp.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SL, Kenigs V, Moldow RL, Zadina JE. Chronic treatment with morphine and ethanol, but not cocaine, attenuates IL-1 beta activation of FOS expression in the rat hypothalamic paraventricular nucleus. Adv Exp Med Biol. 373:201–8. doi: 10.1007/978-1-4615-1951-5_28. [DOI] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76(Pt B):204–217. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology. 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology. 2014;85:499–507. doi: 10.1016/j.neuropharm.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Use of animal models of alcohol-related behavior. In: Sullivan EV, Pfefferbaum A, editors. Alcohol and the Nervous sSstem. Vol. 125. Elsevier BV; San Diego, CA: 2014. pp. 71–86. (3rd Series). [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Buck KJ. Genetic animal models of alcohol and drug abuse. Science. 1994;264:1715–1723. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA. The Genetic Basis of Alcohol and Drug Actions. Plenum Press; New York: 1991. [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3906-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, Hope BT. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2104;34:7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, Kash TL, Roberto M, Wilcox MV. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2013;67:223–232. doi: 10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, Giordano A, Senzacqua M, Somaini L, Cippitelli A, Gaitanaris G, Demopulos G, Damadzic R, Tapocik J, Heilig M, Ciccocioppo R. PPARgamma Activation Attenuates Opioid Consumption and Modulates Mesolimbic Dopamine Transmission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:927–937. doi: 10.1038/npp.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood EC, Barkley-Levenson AM, Phillips TJ. Methamphetamine drinking microstructure in mice bred to drink high or low amounts of methamphetamine. Behav Brain Res. 2014;272:111–120. doi: 10.1016/j.bbr.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood EC, Phillips TJ. Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict Biol. 2014;19:370–379. doi: 10.1111/adb.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson LB, Most D, Blednov YA, Harris RA. PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption. Neuropharmacology. 2014;86:397–407. doi: 10.1016/j.neuropharm.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Castillo N, Orejarena MJ, Ribases M, Blanco E, Casas M, Robledo P, Maldonado R, Cormand B. Active and passive MDMA (‘ecstasy’) intake induces differential transcriptional changes in the mouse brain. Genes, brain, and behavior. 2012;11:38–51. doi: 10.1111/j.1601-183X.2011.00735.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo E, Ramiro-Fuentes S, Rodriguez de Fonseca F. The absence of a functional peroxisome proliferator-activated receptor-alpha gene in mice enhances motor sensitizing effects of morphine, but not cocaine. Neuroscience. 2009;164(2):667–75. doi: 10.1016/j.neuroscience.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76(Pt B):425–436. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, McClintick J, Ding ZM, McBride WJ, Bell RL. Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4). Psychopharmacology. 2015 doi: 10.1007/s00213-014-3852-3. [Epub ahead of print] PMID: 25585681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Ami-Ad L, Yaka R, Yadid G. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59:452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. The European journal of neuroscience. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell BA, Mitra A, Avant RA, Anker JJ, Carroll ME, Levine AS. Operant responding for sucrose by rats bred for high or low saccharin consumption. Physiol Behav. 2010;99:529–533. doi: 10.1016/j.physbeh.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nature communications. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PloS one. 2014;9:e92701. doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness JH, Shi X, Janowsky A, Phillips TJ. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.61. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain research. 1990;508:20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Long-term gene expression in the nucleus accumbens following heroin administration is subregion-specific and depends on the nature of drug administration. Addiction biology. 2005;10:91–100. doi: 10.1080/13556210412331284748. [DOI] [PubMed] [Google Scholar]
- Khaled MA, Pushparaj A, Di Ciano P, Diaz J, Le Foll B. Dopamine D3 receptors in the basolateral amygdala and the lateral habenula modulate cue-induced reinstatement of nicotine seeking. Neuropsychopharmacology. 2014;39:3049–3058. doi: 10.1038/npp.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo AT, Gibson GD, Prasad AA, McNally GP. Role of the striatopallidal pathway in renewal and reacquisition of alcohol seeking. Behavioral neuroscience. 2015;129:2–7. doi: 10.1037/bne0000036. [DOI] [PubMed] [Google Scholar]
- Jupp B, Caprioli D, Dalley JW. Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction. Dis Model Mech. 2013;6:302–311. doi: 10.1242/dmm.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, Markenscoff-Papadimitriou E, Kuhl D, Bito H, Worley PF, Kreiman G, Greenberg ME. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen M, Tuomainen P, Hyytia P, Kiianmaa K. Naltrexone suppresses ethanol intake in 6-hydroxydopamine-treated rats. Alcoholism, clinical and experimental research. 2001;25:1605–1612. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual review of psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Federation proceedings. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E, Kobeissy FH, Gold MS, Becker KG, Goldberg SR, Cadet JL. CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiology of disease. 2013;58:132–143. doi: 10.1016/j.nbd.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC. The epigenetic landscape of alcoholism. Int Rev Neurobiol. 2014;115:75–116. doi: 10.1016/B978-0-12-801311-3.00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse LC, Walter NA, Buck KJ. Mpdz expression in the caudolateral substantia nigra pars reticulate is crucially involved in alcohol withdrawal. Genes Brain Behavior. 2014;13:769–776. doi: 10.1111/gbb.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. The European journal of neuroscience. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry CD, Kandel ER, Rajasethupathy P. New mechanisms in memory storage: piRNAs and epigenetics. Trends Neurosci. 2013;36:535–542. doi: 10.1016/j.tins.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2010;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Molecular psychiatry. 2003;8:50–59. doi: 10.1038/sj.mp.4001197. 59. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, Becker JA, Kieffer BL. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR. Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors. Neuropharmacology. 2014;81:283–91. doi: 10.1016/j.neuropharm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2004;40:1297–1306. doi: 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324(3):948–56. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20(1):59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF, Zorrilla EP. Phosphodiesterase 10A regulates alcohol and saccharin self-administration in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1722–1731. doi: 10.1038/npp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Differential changes in amygdala and frontal cortex Pde10a expression during acute and protracted withdrawal. Frontiers in integrative neuroscience. 2014;8:30. doi: 10.3389/fnint.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcoholism, clinical and experimental research. 2012;36:1180–1187. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luteijn MJ, Ketting RF. PIWI-interacting RNAs: from generation to transgenerational epigenetics. Nat Rev Genet. 2013;14:523–534. doi: 10.1038/nrg3495. [DOI] [PubMed] [Google Scholar]
- Lynch LJ, Sullivan KA, Vallender EJ, Rowlett JK, Platt DM, Miller GM. Trace amine associated receptor 1 modulates behavioral effects of ethanol. Subst Abuse. 2013;7:117–26. doi: 10.4137/SART.S12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schluter OM, Huang YH, Dong Y. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature neuroscience. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain research. 2014 doi: 10.1016/j.brainres.2014.09.004. pii: S0006-8993(14)01191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Le AD. Opioid receptors in the basolateral amygdala but not dorsal hippocampus mediate context-induced alcohol seeking. Behavioural brain research. 2010;211:58–63. doi: 10.1016/j.bbr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biological psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain research. Molecular brain research. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain research. 1993;624:245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addiction biology. 2012;17:351–364. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, McNally GP. Cocaine- and amphetamine-regulated transcript in the nucleus accumbens shell attenuates context-induced reinstatement of alcohol seeking. Behav Neurosci. 2012;126:690–698. doi: 10.1037/a0029953. [DOI] [PubMed] [Google Scholar]
- Murray JE, Dilleen R, Pelloux Y, Economidou D, Dalley JW, Belin D, Everitt BJ. Increased impulsivity retards the transition to dorsolateral striatal dopamine control of cocaine seeking. Biological psychiatry. 2014;76:15–22. doi: 10.1016/j.biopsych.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Tolbert MD, Singh SJ, Bost KL. Expression of neuronal trace amine-associated receptor (Taar) mRNAs in leukocytes. J Neuroimmunol. 2007;192(1-2):21–30. doi: 10.1016/j.jneuroim.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Li MD, Pogun S. Nine generations of selection for high and low nicotine intake in outbred Sprague-Dawley rats. Behav Genet. 2013;43:436–444. doi: 10.1007/s10519-013-9605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of comparative and physiological psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harbor perspectives in medicine. 2012:2. doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, McClung CA. The role of clock in ethanol-related behaviors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:2393–2400. doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Larson EB, Self DW, McClung CA. Cocaine self-administration behaviors in ClockDelta19 mice. Psychopharmacology. 2012;223:169–177. doi: 10.1007/s00213-012-2704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas MW, Xie Z, Panas HN, Hoener MC, Vallender EJ, Miller GM. Trace amine associated receptor 1 signaling in activated lymphocytes. J Neuroimmune Pharmacol. 2012;7(4):866–76. doi: 10.1007/s11481-011-9321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh PK, Ozburn AR, McClung CA. Circadian clock genes: Effects on dopamine, reward and addiction. Alcohol. 2015 doi: 10.1016/j.alcohol.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney J, Tsai LH. Histone deacetylases in memory and cognition. Science signaling. 2014;7:re12. doi: 10.1126/scisignal.aaa0069. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Vengeliene V, Noori HR, Merlo-Pich EV, Corsi MA, Corti C, Spanagel R. Inhibition of the casein-kinase-1-epsilon/delta/ prevents relapse-like alcohol drinking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2121–2131. doi: 10.1038/npp.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, McNally GP. μ-Opioid receptors in the nucleus accumbens shell mediate context-induced reinstatement (renewal) but not primed reinstatement of extinguished alcohol seeking. Behav Neurosci. 2013 Aug;127(4):535–43. doi: 10.1037/a0032981. 2013. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, Olson DP, Lowell BB, Grant KA, Thiele TE, Kash TL. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushparaj A, Kim AS, Musiol M, Trigo JM, Le Foll B. Involvement of the rostral agranular insular cortex in nicotine self-administration in rats. Behav Brain Res. 2015;290:77–83. doi: 10.1016/j.bbr.2015.04.039. [DOI] [PubMed] [Google Scholar]
- Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, André CB, Haenggi M, Miss MT, Galley G, Norcross RD, Invernizzi RW, Wettstein JG, Moreau JL, Hoener MC. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology. 2012;37(12):2580–2592. doi: 10.1038/npp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, Mayfield J, Harris RA, Blednov YA. Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans. International review of neurobiology. 2014;118:13–39. doi: 10.1016/B978-0-12-801284-0.00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Kimpel MW, Edenberg HJ, Bell RL, Strother WN, McClintick JN, Carr LG, Liang T, McBride WJ. Differential gene expression in the nucleus accumbens with ethanol self-administration in inbred alcohol-preferring rats. Pharmacology, biochemistry, and behavior. 2008;89:481–498. doi: 10.1016/j.pbb.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Deschatrettes E, Host L, Gobaille S, Sandner G, Zwiller J. The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Current neuropharmacology. 2011;9:21–25. doi: 10.2174/157015911795017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcoholism, clinical and experimental research. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of comparative neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Simon-O'Brien E, Alaux-Cantin S, Warnault V, Buttolo R, Naassila M, Vilpoux C. The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals. Addiction biology. 2014 doi: 10.1111/adb.12161. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-0997-8. [Epub ahead of print]. PMID: 25663648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biological psychiatry. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Laviolette SR. Dopamine Receptor Blockade Modulates the Rewarding and Aversive Properties of Nicotine via Dissociable Neuronal Activity Patterns in the Nucleus Accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:791. doi: 10.1038/npp.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez KM, Molfese DL, Salas R. The role of the habenula in drug addiction. Frontiers in human neuroscience. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL. Pharmacologic characterization of the cloned human trace amine-associated receptor1 (TAAR1) and evidence for species differences with the rat TAAR1. J Pharmacol Exp Ther. 2007;320(1):475–85. doi: 10.1124/jpet.106.112532. [DOI] [PubMed] [Google Scholar]
- Walker DM, Cates HM, Heller EA, Nestler EJ. Regulation of chromatin states by drugs of abuse. Curr Opin Neurobiol. 2015;30:112–121. doi: 10.1016/j.conb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shang S, Kang X, Teng S, Zhu F, Liu B, Wu Q, Li M, Liu W, Xu H, Zhou L, Jiao R, Dou H, Zuo P, Zhang X, Zheng L, Wang S, Wang C, Zhou Z. Modulation of dopamine release in the striatum by physiologically relevant levels of nicotine. Nature communications. 2014;5:3925. doi: 10.1038/ncomms4925. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang F, Tang S, Lai M, Hao W, Zhang Y, Yang J, Zhou W. Lack of effect of habenula lesion on heroin self-administration in rats. Neurosci Lett. 2009;461:167–71. doi: 10.1016/j.neulet.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Wasik AM, Millan MJ, Scanlan T, Barnes NM, Gordon J. Evidence for functional trace amine associated receptor-1 in normal and malignant B cells. Leuk Res. 2012;36(2):245–9. doi: 10.1016/j.leukres.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8(8):758–71. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden JA, Qing KY, Hauser SR, McBride WJ, Irazoqui PP, Rodd ZA. Reduced ethanol consumption by alcohol-preferring (P) rats following pharmacological silencing and deep brain stimulation of the nucleus accumbens shell. J Neurosurg. 2014;120:997–1005. doi: 10.3171/2013.12.JNS13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. The European journal of neuroscience. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Wiltshire T, Ervin RB, Duan H, Bogue MA, Zamboni WC, Cook S, Chung W, Zou F, Tarantino LM. Initial locomotor sensitivity to cocaine varies widely among inbred mouse strains. Genes Brain Behav. 2015 doi: 10.1111/gbb.12209. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addiction biology. 2012;17:287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wells AM, Fuchs RA. Cocaine seeking and taking: role of hippocampal dopamine D1-like receptors. Int J Neuropsychopharmacol. 2014;17:1533–1538. doi: 10.1017/S1461145714000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Sharp BM. Basolateral amygdala and ventral hippocampus in stress-induced amplification of nicotine self-administration during reacquisition in rat. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3911-4. [Epub ahead of print]. PMID: 25772339. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Nielsen D, Butelman E, Kreek MJ. Microarray studies of psychostimulant-induced changes in gene expression. Addiction biology. 2005;10:101–118. doi: 10.1080/13556210412331308976. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li H, Li Y, Sun X, Zhu M, Hanley G, Lesage G, Yin D. Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice. Neurosci Lett. 2011;489(1):43–7. doi: 10.1016/j.neulet.2010.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]