Abstract
Human embryonic stem cells (hESCs) are pluripotent and capable of generating new β-cells, but current in vitro differentiation protocols generally fail to produce mature, glucose-responsive, unihormonal β-cells. Instead, these methods tend to produce immature polyhormonal endocrine cells which mature in vivo into glucagon-positive α-cells. PAX4 is an established transcription factor in β-cell development and function, and is capable of converting glucagon-positive cells to insulin-positive cells in mice. Work in human and mouse ESCs has shown that constitutive PAX4 expression promotes the development of insulin-positive cells, but whether acute PAX4 expression is sufficient to guide specific endocrine cell fates has not been addressed in hESCs. In this study, we applied recombinant adenovirus to ectopically express human PAX4 in hESC-derived pancreatic progenitors, with the aim of influencing the endocrine developmental cascade away from polyhormonal cells toward unihormonal insulin-positive cells. Gene delivery to pancreatic progenitors was efficient and dose-dependent. By the end of in vitro differentiation, PAX4 reduced ARX expression, but only the high dose tested significantly reduced glucagon release. Single cell analysis revealed that while PAX4 did not alter the proportion of endocrine cells, it did reduce the number of glucagon-positive cells and increased the number of unihormonal insulin-positive cells. These data suggest that acute PAX4 overexpression can reduce expression of ARX and glucagon resulting in improved numbers of unihormonal insulin-positive cells.
Keywords: ARX, PAX4, adenovirus, glucagon, human embryonic stem cells, insulin, β-cell
Introduction
Transplantation of healthy human islets from recent cadavers remains the most effective cellular therapy for type 1 diabetes, yet the demand for donor islets exceeds their availability. Accordingly, many researchers are engaged in the search for novel and unrestricted sources of β-cells. In this search, many have turned to pluripotent stem cell populations and their differentiated derivatives as a potential new source of functional β-cells.1 While pluripotent human embryonic stem cells (hESCs) have the ability to generate any cell type by definition, the protocols and methods required to generate fully functional pancreatic endocrine cells have been relatively unsuccessful using in vitro culture approaches alone.1,2 However, these fully in vitro protocols have been relatively efficient at achieving targeted differentiation of pancreatic progenitor populations from undifferentiated cells, and to a lesser extent the generation of pancreatic endocrine cells that express a variety of islet hormones.2 The majority of in vitro hESC-derived pancreatic endocrine cells express multiple hormones within the same cell.3-8 While these polyhormonal cells are a natural component of human development,9-11 the eventual bias of these cells, which most notably co-express glucagon and insulin, is to an α-cell fate.3,12-14 Therefore, new methods that shift the polyhormonal nature of cells away from a glucagon-positive lineage could significantly improve the generation of functional β-cells in vitro.
One of the influences in pancreatic development that determines pancreatic endocrine cell specificity is the expression of transcription factors. Many factors are known to play a key role in the generation of the pancreas as an organ (eg PDX1, PTF1A, MNX1, HNF1B, GATA6), or the endocrine sub-compartment (eg NEUROG3).15,16 Although the specification of endocrine cells to specific mature fates (eg α-, β-, δ-, ε-, and PP-cells) is incompletely understood, ARX (aristaless related homeobox) and PAX4 (paired box 4) are known to mutually repress each other's transcription within pancreatic endocrine precursors, and ultimately only one or the other is predominantly expressed in mature endocrine cells. When ARX expression predominates, PAX4 is repressed and the genesis of α-cells is favored; conversely, when PAX4 expression is high, ARX levels are reduced and the specification of β- and δ-cells is enhanced.17-19 Furthermore, the PAX4 knockout mouse displays an increased number of α-cells and an absence of β- and δ-cells, which suggests that PAX4 regulates both the β- and δ-cell lineages.20 This role in pancreatic development may also be true in humans as both PAX4 and ARX are simultaneously expressed in the developing human fetal pancreas beginning at week 8–9 of gestation.9,21,22 Furthermore, PAX4 dysfunction has been implicated in human MODY9 (maturity onset diabetes of the young), further supporting the hypothesis that this factor plays a role in the maintenance of the adult β-cell phenotype.23
Based on the established role of PAX4 in pancreatic endocrine development as a positive regulator of β-cell specification, PAX4 is an attractive potential tool for increasing the proportion of β-cells derived from stem cell populations, including embryonic stem cells. Indeed, it has been shown that in both mESCs and hESCs, constitutive overexpression of PAX4 has broadly beneficial effects in terms of improved pancreatic endocrine differentiation of insulin-positive cells.24,25 Using relatively non-specific differentiation conditions these studies found that PAX4 increased the expression of insulin, a number of β-cell-associated transcription factors, and insulin processing pathway components.24,25 These improvements over control cultures were achieved despite the lack of temporal control on PAX4 expression or the more recently developed stage-specific differentiation methods such as those used in this study.
In order to influence the developmental fate specification of hESC derived pancreatic endocrine cells in vitro, we generated an adenoviral vector to allow for acute expression of human PAX4 in pancreatic progenitors during differentiation. Viral delivery of PAX4 to developing monolayers of cells resulted in dose-dependent and robust PAX4 overexpression. Ultimately, increased levels of PAX4 resulted in reduced ARX and glucagon expression and led to decreased numbers of polyhormonal cells and maintenance of insulin-positive cells which lost expression of glucagon.
Results
Adenoviral gene delivery of PAX4 to hESCs
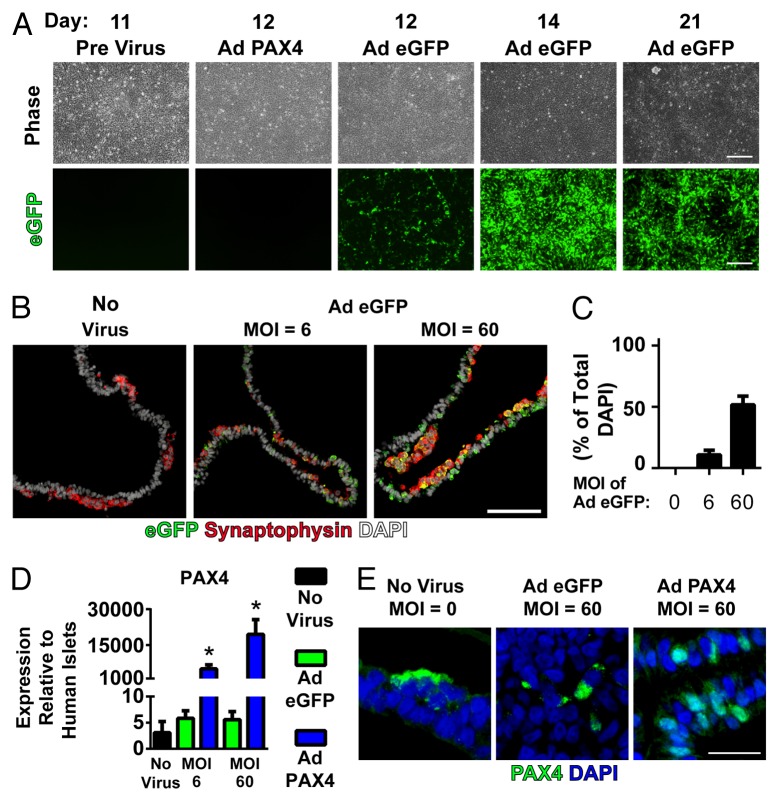
Based on the potential to improve endocrine fate specification of hESCs toward the β-cell lineage, we examined the effect of increased PAX4 expression during staged in vitro pancreatic differentiation. To do this, we used a 21-d protocol designed to mimic the changing embryonic environment that induces the development of hESCs into pancreatic endocrine cells (Fig. 1A). We have previously shown that the expression of PAX4 and its antagonistic partner ARX begins between day 11 and 14 of this protocol.4 Based on this timeline, and one of the presumed PAX4 targets being ARX, we performed infections of developing hESC cultures at day 11 with either control virus expressing enhanced green fluorescent protein (Ad eGFP) or PAX4 virus (Ad PAX4) at a multiplicity of infection (MOI) of 6 or 60 based on a day 11 cell density of 4.3 x 105 cells/cm.2,4 By day 12, eGFP-positive cells were observed in the control group, and eGFP expression was maintained until day 21 (Fig. 1B). At day 21, cultures treated with a MOI of 6 and 60 were 10.7% and 51.6% positive respectively for eGFP expression (Fig. 1C and D). These cells were predominantly non-endocrine cells, based on their lack of synaptophysin immunoreactivity, although some cells did co-express eGFP and synaptophysin. This suggests that transgene expression was more efficient at targeting pancreatic progenitor populations with a lower efficiency of maintained expression in differentiated progeny. Similar to the transduction efficiency results, overexpression of PAX4 in day 21 cultures was found to be dose-dependent with the MOI of 6 and 60 for Ad PAX4 resulting in ~5,000-, and ~19,000-fold overexpression compared with adult human islet levels, respectively. PAX4 overexpression levels were also significantly elevated compared with the 3–6-fold human islet levels seen in non-virally treated and Ad eGFP treated cultures (Fig. 1E). Delivery of Ad PAX4 was also associated with nuclear PAX4 immunoreactivity, which was in contrast to the cytoplasmic immunoreactivity seen in rare cells of control cultures (Fig. 1F).
Figure 1.
Pancreatic differentiation and PAX4 overexpression in hESCs. (A) Pancreatic endocrine differentiation according to Gage et al.4 modified from Rezania et al.3 Stage-specific sequential development of hESCs from undifferentiated cells through foregut-derived endodermal progenitors to hormone-positive endocrine cells. (B) Adenoviral infection of 11 d differentiated hESCs yields eGFP expression (green) by 24 h post-viral delivery which persists through the culture period. (C) 21-d differentiated pancreatic endocrine cultures were immunostained for eGFP (green) to mark expression of the adenoviral vector and synaptophysin (red) to mark the endocrine cell population as a portion of the total cells (DAPI, white). (D) Quantification of the total number of eGFP-positive cells as a percentage of the total number of nuclei. (E) Infection of day 11 cells with an adenoviral human PAX4 expression construct resulted in a dose-dependent increase in PAX4 transcript levels as measured by RT-qPCR of day 21 samples relative to expression in adult human islets. (F) PAX4 delivery was also associated with nuclear immunoreactivity (PAX4, green), in contrast to rare cytoplasmic PAX4 immunoreactivity seen in uninfected and control infected day 21 cultures (nuclei, blue). * Indicates significant overexpression of human PAX4 compared with control virus (Ad eGFP) at the same dose (P < 0.05 by 1-way ANOVA with Bonferroni post-hoc test). Scale bar is 200 μm in (B), 100 μm in (C) and 25 μm in (F).
PAX4 blocks glucagon expression
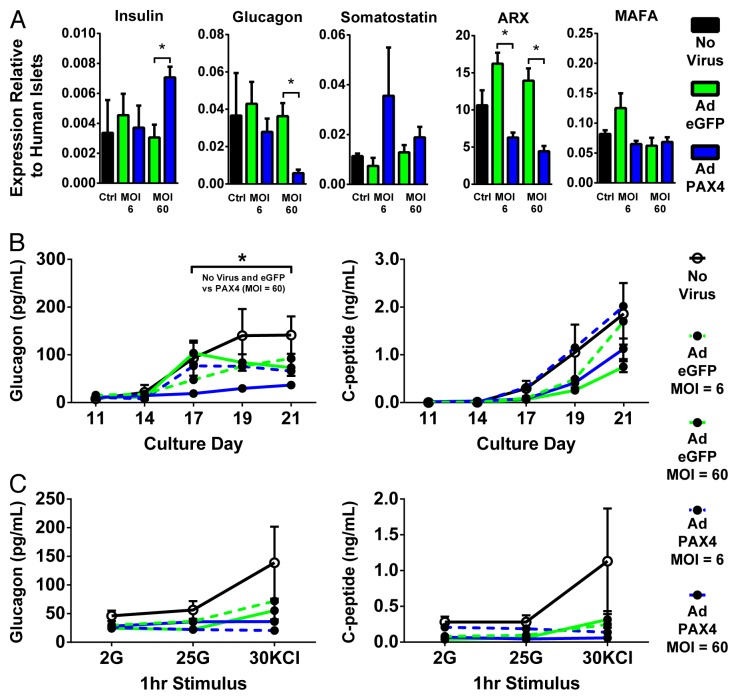
To examine the effect of PAX4 for the last 10 d of hESC in vitro differentiation, we tested the bulk cell population for expression of islet hormones and key transcription factors. Adenoviral delivery of Ad PAX4 at both low dose (MOI = 6) and high dose (MOI = 60) significantly reduced ARX transcript levels compared with control virus (Ad eGFP) treated cultures (Fig. 2A). This approximately 50% reduction of ARX correlates well with the ~50% transgene expression efficiencies observed in the high viral dose (Figs. 2A and 1D). Furthermore, high dose Ad PAX4, but not low dose, reduced glucagon levels and modestly increased insulin levels with no significant change in somatostatin or MAFA levels (Fig. 2A). Since the low viral dose infected relatively few cells compared with the high viral dose, and these were predominantly non-endocrine (Fig. 1C and D), we speculate that this biased gene delivery could explain the reduction of ARX at both doses yet reduction of glucagon only at the high dose.
Figure 2.
PAX4 overexpression blocks glucagon expression. (A) 21 d differentiated hESC cultures uninfected or infected with either Ad eGFP or Ad PAX4 at an MOI of 6 or 60 were assessed for expression of a number of targets by RT-qPCR relative to adult human islets. * Indicates significant overexpression of human PAX4 compared with control virus (Ad eGFP) at the same dose (P < 0.05 by 1-way ANOVA with Bonferroni post-hoc test). (B) Twenty-four hour static media samples were taken between days 11 and 21 were assayed by radioimmunoassay for glucagon and C-peptide content. (C) Nineteen day differentiated hESCs were tested for glucose regulated glucagon and C-peptide release in response to 1 h incubations in low glucose (2 mM), high glucose (25 mM), and potassium chloride (30 mM).
To assess the hormone release capacity of PAX4 cultures, we next examined media samples taken between days 11 and 21 for glucagon and C-peptide levels. High dose, but not low dose, PAX4 overexpression significantly reduced glucagon release with a non-significant trend to decreased C-peptide levels (Fig. 2B). To examine the potential for improved stimulus-coupled hormone secretion in hESCs, day 19 differentiated cells were tested by a static sequential secretion assay including low and high glucose levels and potassium chloride. Under these conditions, no significant effect was observed in terms of C-peptide or glucagon release in either glucose or potassium chloride stimulated conditions (Fig. 2C). Notably, a trend is evident that Ad eGFP may have had a negative effect on the stimulated release of both glucagon and C-peptide although this did not reach statistical significance. Taken together, these data suggest that overexpression of PAX4 has dose-dependent effects on the expression of ARX and glucagon which, in high dose PAX4 conditions, results in a reduction of glucagon release under static conditions.
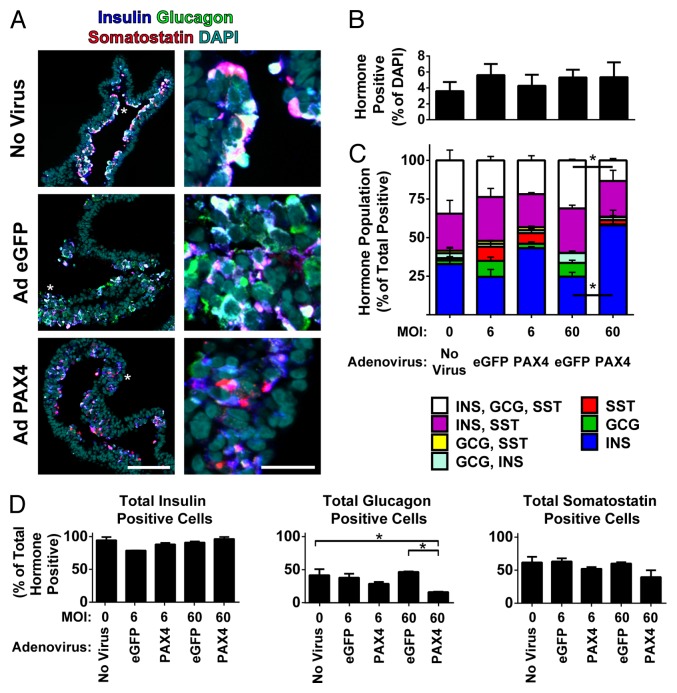
While the bulk population data suggested a loss of glucagon expression upon PAX4 overexpression we next tested if this effect was based on changes in the number of endocrine cells and, more specifically, in a change within subpopulations of hormone positive cells. To do this, we examined the single-cell hormone expression of 21 d differentiated hESCs treated with low or high doses of Ad eGFP or Ad PAX4 as well as untreated controls. Staining for insulin, glucagon, and somatostatin in paraffin sections of agarose-embedded cell sheets revealed that, in all conditions tested, approximately the same number of cells expressing any combination of hormones were present (Fig. 3A and B). Quantification of the individual hormone expression status of each cell using unbiased image analysis software revealed that the generally polyhormonal nature of untreated and Ad eGFP treated cultures significantly shifted upon treatment with a high dose of Ad PAX4. This shift included a significant decrease in the triple positive fraction (insulin-positive, glucagon-positive, and somatostatin-positive; white bar) fraction and a significant increase in the unihormonal insulin-positive (insulin-only; blue bar) fraction comparing Ad PAX4 and Ad eGFP at an MOI of 60 (Fig. 3C). The reason for this shift toward insulin-only cells was not due to a change in the total number of insulin- or somatostatin-positive cells but was a specific decrease in the number of glucagon-positive cells upon treatment with a high dose of PAX4 (Fig. 3D). This maintenance of insulin and loss glucagon correlates well with the observation of maintenance of static C-peptide release and loss of static glucagon release in PAX4 treated cultures (Fig. 2B).
Figure 3.
PAX4 overexpression reduces the number of glucagon-positive cells. (A) Agarose-embedded sections of 21 d differentiated hESC cultures uninfected or infected with either Ad eGFP or Ad PAX4 (MOI = 60) were immunostained for insulin (blue), glucagon (green), and somatostatin (red) with a nuclear counterstain (DAPI, cyan). Right image is an enlargement of the region indicated by the white *. Right scale bar is 25 μm, left scale bar is 100 μm. (B) Single cell quantification of the number of total nuclei that are positive for any combination of insulin, glucagon, or somatostatin. (C) Single cell population profile of hormone-positive cells as a percentage of the total number of hormone-positive cells. * Indicates significant change in cell population between PAX4 and eGFP treated cultures given an MOI of 60 (P < 0.05). (D) The total number cells positive for insulin, glucagon, and somatostatin were examined regardless of polyhormonal nature. * Indicates significant change in the glucagon-positive cell population between untreated and PAX4 or eGFP treated cultures at an MOI of 60 (P < 0.05 by 1-way ANOVA with Bonferroni post-hoc test).
Discussion
The goal of this study was to develop and apply a model of acute PAX4 expression in hESCs under defined in vitro pancreatic endocrine differentiation conditions. To do this, we built upon our previous work differentiating CA1S hESCs to pancreatic endocrine cells4,26 and applied a newly generated adenoviral human PAX4 expression vector that allowed temporally controlled gene delivery and maintained gene expression. This model system generated the expected nuclear localizing PAX4 immunoreactivity, and PAX4 transcript levels were found to be overly high (~5,000–19,000-fold greater than in adult human islets) in both the low and high viral doses. While a decreased viral dose was able to reduce the average PAX4 expression, there was a significant reduction of infection efficiency, to an extent that the low viral dose delivered PAX4 to relatively few cells. From these data we can extrapolate that in the gene delivery method applied in this study, a relatively high PAX4 expression level was generated even on a cell-by-cell basis. Thus, changing the viral dose altered the number of cells which obtained PAX4 overexpression at a high level. The high expression results from our use of the CMV promoter element, similar to that of other PAX4 overexpression studies.19,24,25 In order to achieve more physiologically relevant levels of overexpression even when high MOI's are employed to maintain infection efficiency, alternate promoters, such as EF1α or an inducible expression system such as that used by Brun et al. (2004 and 2008), could be employed.
In our study, PAX4 had no effect on the number of endocrine cells. Since the number of endocrine cells is generally controlled by expression of NEUROG3,27,28 the absence of an effect of PAX4 on endocrine cell number is consistent with reports that PAX4 expression lies directly downstream of NEUROG3.29 However, PAX4 overexpression significantly influenced which endocrine cell types were formed by repressing glucagon production, leaving unihormonal insulin-positive cells to predominate the endocrine fraction of the cultures. The modest effect of human PAX4 that we observed follows work done on human and rat islets, which found that adenoviral overexpression of human PAX4 was relatively ineffective compared with murine PAX4 at inducing proliferation of human and rat islet cells.30 While this work was focused on the pro-proliferative effect of PAX4 on islet cells, it highlights the importance of examining the effects of human transcription factors in human cell types as the murine homologs do not necessarily show the same effects. Moreover, building upon studies by Blyszczuk et al. who overexpressed murine PAX4 in mouse ESCs, Liew et al. examined the effects of constitutive overexpression of human PAX4 in hESCs. Using the same human PAX4 sequence as used in our study, the authors found that PAX4 expression generally accelerated the endocrine differentiation time-line including the formation of a small proportion of cells that were zinc positive (based on Newport green dye uptake) and found to be enriched for C-peptide. While the polyhormonal nature of these Newport green positive cells or the total cell population was not reported in the PAX4 overexpressing hESCs, improved responsiveness to potassium chloride depolarization was noted specifically in the PAX4 expressing cells. While our data supports a role for PAX4 in the repression of glucagon-positive cells, we observed a trending reduction in potassium chloride stimulated hormone secretion (both glucagon and C-peptide) in PAX4 treated cultures, similar to control virus treated cells. Ultimately this negative effect of the viral gene delivery vector on hormone secretion precludes functional analysis of PAX4 treated cultures.
PAX4 may have further roles in the maintenance of β-cell function once a mature cell type is formed. In mice constitutively overexpressing PAX4 in PDX1-, PAX6-, and glucagon- positive lineages, young mice have neonatal hypoglycemia due to reduced numbers of glucagon producing α-cells and improved glucose tolerance during a glucose challenge due to increased β-cell mass.19 Over time, despite increased numbers of β-cells overexpressing PAX4, these mice developed hyperglycemia, decreased insulin secretion and elevated blood glucose during a glucose challenge which suggests a failure of these older β-cells to functionally respond to elevated glucose levels.19 Therefore, while PAX4 is beneficial and critical to the genesis and specification of β-cells, sustained high expression of PAX4 is detrimental to maintenance of functional aspects of the β-cell phenotype. This notion of transcription factor expression peaking during human pancreas development and decreasing in more mature cell types has also been reported for PDX1 and contrasts the expression profile of MAFA, which has peak expression in mature β-cells.22 Additionally PAX4 has an established role of repressing the α-cell phenotype,18,31,32 and acts as a transcriptional repressor of both the human glucagon and insulin genes.23 This repressive activity of PAX4 suggests that in the mature β-cell, low PAX4 expression is required to permit efficient insulin production while also helping to maintain β-cell identity. A strong negative autorepression via PAX4 binding to and repressing the human PAX4 promoter may help to limit PAX4 expression levels.33 Given that we used a PAX4 expression system which results in artificially sustained overexpression, it is possible that fully functional maturation of β-cells including high insulin expression could have been actively repressed by high levels of PAX4 or the adenoviral vector itself. While our data show a small increase in insulin expression, which was unexpected given the repressive nature of PAX4, the predominant role of PAX4 is associated with pancreatic endocrine fate specification of hESCs. However, it remains to be seen if more physiological expression levels of PAX4 through a gene delivery method which has no deleterious effects on cellular secretory capacity can modulate hESC differentiation to a fully functional cell type.
In addition to effects on the β-cell lineage, PAX4 has also been shown to influence the formation of somatostatin-positive δ-cells as PAX4 null mice have decreased numbers of both β-and δ-cells.20 Remarkably, in mice that lack both PAX4 and ARX, δ-cell numbers are dramatically elevated, suggesting that neither factor is required for the genesis of δ-cells.34 Based on this developmental data we examined our model of PAX4 overexpression for effects on δ-cell formation. We found no changes in somatostatin transcript levels or the numbers of cells immunoreactive for somatostatin protein. These data correlate well with conditional PAX4 overexpression mice where PAX4 is constitutively expressed in cells from PDX1-, PAX6- or glucagon-positive embryonic lineages.19 Islets from these mice display biased formation of insulin-positive cells with no increase in somatostatin-positive cells.19 Together this suggests that while PAX4 may be a positive regulator of the development of insulin- and somatostatin-positive lineages, PAX4 is not required for somatostatin cell formation, and high levels of PAX4 acts as a selective driver the insulin-positive lineage.
Taken within the context of other PAX4 overexpression studies, most evidence supports the roles of PAX4 as a cell fate specification and endocrine induction transcription factor with a key attribute of repressing the α-cell biasing factor ARX during both murine and human development. In mature β-cells, PAX4 may be associated more with activating proliferation in response to unknown extracellular cues, seemingly at the expense of cellular function if left unchecked. While this study has focused on a defined hESC differentiation system and the role of human PAX4 in endocrine cell fate specification, the work builds upon the growing knowledge of the multitude of roles of PAX4 during pancreatic development and maturity.
Materials and Methods
Generation of human PAX4 adenovirus
A human PAX4 cDNA was generated by RT-PCR using high-fidelity Accuprime Taq polymerase (Invitrogen) and cloning primers (fwd, 5′ CCACCATCTAGAGGGATCAGCAGCATGAACCAGCTTG 3′; rev, 5′ CCACCAGCGGCCGCTCATTCCAAGCCATACAGTAGTGGGCAGC 3′). The primers contain heterologous XbaI and NotI sites to facilitate cloning. A 1.05 kb amplicon was produced from adult human islet cDNA, digested with XbaI and NotI, and cloned into a shuttle vector, pScore3. pScore3 is a derivative of pShuttle (Clontech), containing a rabbit β-globin intron (GenBank #V00878.1, nucleotides 557–1187), followed by a custom polylinker, between the CMV promoter and BGH polyadenylation sequences of pShuttle. The cloned PAX4 ORF was validated by sequencing to be the same as that used by Liew et al. (2008), and is identical to the ORF in GenBank #NM_006193, bases 207–1238. The recombinant CMV-RBGI-PAX4 transgene cassette was excised from pScore3, and subcloned into pAdeno-X (Clontech), using the homing endonucleases I-CeuI and PI-SceI (New England Biolabs). Complete virions were generated by transient transfection of HEK293 cells with CMV-RBGI-PAX4 loaded pAdeno-X plasmid followed by amplification and purification to high titer (3 x 1010 PFU/ml) by ViraQuest Inc. Control adenoviral virions expressing eGFP from the CMV promoter were similarly generated (4 x 109 PFU/ml).
Culture of hESCs
Undifferentiated CA1S hESCs were maintained on 1:30 diluted growth factor reduced Matrigel (BD Biosciences) in mTeSR1 media (STEMCELL Technologies) as previously described.26 hESCs were passaged using Accutase (STEMCELL Technologies) to maintain subconfluent cultures which were amenable to stimulated differentiation conditions.
Pancreatic differentiation and adenoviral delivery of hESCs
Subconfluent CA1S hESCs were seeded in 1:30 diluted Matrigel coated, 12-well culture plates at a previously established optimal density of 5.3 x 104 cells/cm2 in 1.5ml of mTeSR1 in the absence of Rho Kinase inhibition.4 Approximately 16 h after seeding, undifferentiated cultures were 95–100% confluent and were subjected to a pancreatic endocrine differentiation cascade as previously described for CA1S hESCs4 originally developed for H1 hESCs.3 On day 11 of culture, randomly assigned 12-wells were transduced over 24 h with either an Ad eGFP, Ad PAX4 or no virus in the standard day 11 culture media. Viral delivery was at a multiplicity of infection (MOI) of 6 for the low dose or 60 for the high dose based on a cell count of 4.3 x 105 cells/cm2. During the protocol, 24-h static media samples were collected, centrifuged to remove cell debris, individually aliquoted and stored at -20 °C on days 11, 14, 17, 19, and 21 of culture. On day 19 of culture, a sequential static glucose and potassium chloride stimulated hormone secretion assay was performed on differentiated cells as follows. Remaining daily culture medium was aspirated and the cells were washed with PBS without calcium and magnesium chloride (PBS-) twice before the addition of 1.5 ml/well RPMI media (Sigma, Cat# 11879) supplemented with 2 mM D-glucose (Sigma). This last wash was incubated for 2 h at 37 °C, 5% CO2 before aspiration, followed by sequential 1 h incubations in RPMI + 2 mM glucose, RPMI + 25 mM glucose, and RPMI + 30 mM potassium chloride (Sigma). After collection the secretion assay samples were prepared and stored similarly to daily media samples for later analysis by radioimmunoassay.
Quantitative reverse transcriptase PCR (RT-qPCR)
Quantitative reverse transcriptase PCR (RT-qPCR) was performed on day 21 cell samples using previously frozen cell pellets according to manufacture recommended protocols. RNA was extracted using a RNeasy MiniKit (Quiagen) with DNase digestion performed on the column. Two-hundred fifty nanograms of total RNA was used to prepare cDNA using iSCRIPT (BioRad). qPCR was performed using SsoFast EvaGreen Supermix (BioRad) in 96-well plates on a StepOnePlus instrument (Applied Biosystems). Primers were optimized for identical fast 2-step cycling conditions with a Tm of 62 °C. All reactions were performed in technical duplicate and biological triplicate with gene expression normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) then to a pooled sample of adult human islet cDNA. Primer sequences can be found in Table 1. Human islets were kindly provided by Drs. Ao and Warnock from the Irving K. Barber Human Islet Isolation Laboratory.
Table 1. RT-qPCR primers. Primers, product sizes and applicable references for transcripts tested in this study.
| Gene Name | Gene Accession | Product Size (bp) |
Primer Sequence Forward / Reverse 5′→3′ |
Reference |
| Insulin | NM_000207.2 | 245 | AGCCTTTGTGAACCAACACC GCTGGTAGAGGGAGCAGATG |
36 |
| Glucagon | NM_002054.4 | 275 | CATTCACAGGGCACATTCAC CGGCCAAGTTCTTCAACAAT |
36 |
| Somatostatin | NM_001048.3 | 126 | AGCTGCTGTCTGAACCCAAC CCATAGCCGGGTTTGAGTTA |
36 |
| PAX4 | NM_006193 | 169 | AGCAGAGGCACTGGAGAAAGAGTT CAGCTGCATTTCCCACTTGAGCTT |
36 |
| ARX | NM_139058.2 | 141 | CTGCTGAAACGCAAACAGAGGC CTCGGTCAAGTCCAGCCTCATG |
4 |
| MAFA | NM_201589 | 195 | CTTCAGCAAGGAGGAGGTCA TTGTACAGGTCCCGCTCTTT |
4 |
| HPRT | NM_000194.2 | 148 | TGTTGTAGGATATGCCCTTGACTAT GCGATGTCAATAGGACTCCAGA |
4 |
Immunocytochemistry
hESCs differentiated for 21 d were detached from the 12-well culture plates and fixed overnight at 4 °C in 4% paraformaldehyde in PBS-. After 2 washes in PBS-, fixed cell sheets were embedded in 2% w/v agarose (Sigma), chilled on ice, and post fixed for 1 h in 4% PFA before storage at 4 °C in 70% ethanol for less than 1 week. Agarose embedded cell sheets were prepared and sectioned as 5 μm paraffin sections by Wax-it Histology Services. Immunostaining using primary and secondary antibodies described in Table 2, was performed as previously described.4,35 Image acquisition and subsequent semi-automated nucleocentric quantification of immunostained slides was performed using an ImageXpress MicroTM automated microscope and associated MetaXpress NX software (Molecular Devices Corp.). Image acquisition of differentiating hESC cultures expressing eGFP was acquired using an Axiovert 200 microscope (Carl Zeiss Canada), Retiga 2000R CCD camera (Qimaging) controlled using Openlab 5.0 imaging software (Improvision).
Table 2. Antibody Sources and Conditions for Immunocytochemistry Antibody concentrations, sources, and relevant staining condition as applicable for this study.
| Gene Name | Host Species |
Supplier / Catalogue number |
Staining Method | Dilution | Antigen Retrieval |
| PAX4 | Goat | R&D Systems AF2614 |
PFA fixed, paraffin section of agarose embedded cell pellet | 1:250 | HIER |
| eGFP | Mouse | Clontech 632375 |
PFA fixed, paraffin section of agarose embedded cell pellet | 1:500 | HIER |
| Synaptophysin | Rabbit | Novus NB120 - 16659 |
PFA fixed, paraffin section of agarose embedded cell pellet | 1:50 | HIER |
| Insulin | Guinea Pig | Sigma I8510 |
PFA fixed, paraffin section of agarose embedded cell pellet | 1:1000 | HIER |
| Glucagon | Rabbit | Cell Signaling 8233P |
PFA fixed, paraffin section of agarose embedded cell pellet | 1:500 | HIER |
| Somatostatin | Mouse | BCBC AB1985 |
PFA fixed, paraffin section of agarose embedded cell pellet | 1:1000 | HIER |
HIER (heat induced epitope retrieval): 15 min at 95 °C in 10mM Citrate buffer with 0.05% Tween-20 pH 6.0. BCBC (Beta Cell Biology Consortium)
Radioimmunoassay
Static and stimulated media samples were assessed for hormone content by radioimmunoassay following manufacture recommended protocols with volumes halved for all reagents. Analysis for human C-peptide (Millipore, HCP-20K) and glucagon (Millipore, GL-32K) was performed in technical duplicate and biological triplicates.
Statistical analysis
Unless otherwise stated data are reported as mean ± SEM. All numerical data sets were tested for binomial distribution using a Shapiro-Wilk normality test as calculated by Statistical Package for Social Sciences (SPSS Inc., version 15.0.0 for Windows). On normally distributed data, significance was set at P ≤ 0.05 based on the results of 1-way ANOVA and Bonferonni post-hoc tests using Prism (GraphPad Software Inc. version 6.01 for Windows).
Ethics
This work was approved by the Canadian Stem Cell Oversight Committee and the UBC Clinical Research Ethics Board.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by the Canadian Institutes of Health Research (CIHR) Regenerative Medicine and Nanomedicine Initiative, Stem Cell Network (SCN), Juvenile Diabetes Research Foundation (JDRF), and STEMCELL Technologies Inc. BKG received PhD funding from the Natural Sciences and Engineering Research Council, Michael Smith Foundation for Health Research and the University of British Columbia. We thank Ali Asadi and Travis Webber for their technical assistance, and Drs Garth Warnock and Ziliang Ao of the Irving K. Barber Human Islet Transplantation Laboratory for providing human islets.
Glossary
Abbreviations:
- hESCs
human embryonic stem cells
- PAX4
paired box gene 4
- ARX
aristaless related homeobox
- PDX1
pancreas/duodenum homeobox protein 1
- PTF1A
pancreas transcription factor 1 alpha subunit
- MNX1
motor neuron and pancreas homeobox 1
- HNF1B
HNF1 homeobox B
- GATA6
gata-binding protein 6
- NEUROG3
neurogenin 3
- MAFA
v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog A
- PAX6
paired box gene 6
- MODY
maturity onset diabetes of the young
- MOI
multiplicity of infection
- eGFP
enhanced green fluorescent protein
References
- 1.Gage BK, Wideman RD, Kieffer TJ. Generating Pancreatic Endocrine Cells From Pluripotent Stem Cells. In: Islam MS, ed. Islets of Langerhans: Springer, 2014. [Google Scholar]
- 2.Bruin J, Kieffer T. Differentiation of Human Embryonic Stem Cells into Pancreatic Endocrine Cells. In: Hayat MA, ed. Stem Cells and Cancer Stem Cells, Volume 8: Springer Netherlands, 2012:191-206. [Google Scholar]
- 3.Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL, Kieffer TJ. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239–47. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gage BK, Webber TD, Kieffer TJ. Initial cell seeding density influences pancreatic endocrine development during in vitro differentiation of human embryonic stem cells. PLoS One. 2013;8:e82076. doi: 10.1371/journal.pone.0082076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–71. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 7.Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265–74. doi: 10.1634/stemcells.22-3-265. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–53. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 9.Riedel MJ, Asadi A, Wang R, Ao Z, Warnock GL, Kieffer TJ. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia. 2012;55:372–81. doi: 10.1007/s00125-011-2344-9. [DOI] [PubMed] [Google Scholar]
- 10.Polak M, Bouchareb-Banaei L, Scharfmann R, Czernichow P. Early pattern of differentiation in the human pancreas. Diabetes. 2000;49:225–32. doi: 10.2337/diabetes.49.2.225. [DOI] [PubMed] [Google Scholar]
- 11.Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57:811–24. doi: 10.1369/jhc.2009.953307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, Li X, Guo Q, Elefanty AG, Stanley EG, Keller G, et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358–71. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- 13.Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D’Amour KA, Kroon E, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–6. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 14.Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O’Neil JJ, Kieffer TJ. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–42. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 15.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKnight KD, Wang P, Kim SK. Deconstructing pancreas development to reconstruct human islets from pluripotent stem cells. Cell Stem Cell. 2010;6:300–8. doi: 10.1016/j.stem.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collombat P, Hecksher-Sørensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–70. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–62. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 21.Lyttle BM, Li J, Krishnamurthy M, Fellows F, Wheeler MB, Goodyer CG, Wang R. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia. 2008;51:1169–80. doi: 10.1007/s00125-008-1006-z. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar SA, Kobberup S, Wong R, Lopez AD, Quayum N, Still T, Kutchma A, Jensen JN, Gianani R, Beattie GM, et al. Global gene expression profiling and histochemical analysis of the developing human fetal pancreas. Diabetologia. 2008;51:285–97. doi: 10.1007/s00125-007-0880-0. [DOI] [PubMed] [Google Scholar]
- 23.Plengvidhya N, Kooptiwut S, Songtawee N, Doi A, Furuta H, Nishi M, Nanjo K, Tantibhedhyangkul W, Boonyasrisawat W, Yenchitsomanus PT, et al. PAX4 mutations in Thais with maturity onset diabetes of the young. J Clin Endocrinol Metab. 2007;92:2821–6. doi: 10.1210/jc.2006-1927. [DOI] [PubMed] [Google Scholar]
- 24.Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liew CG, Shah NN, Briston SJ, Shepherd RM, Khoo CP, Dunne MJ, Moore HD, Cosgrove KE, Andrews PW. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS One. 2008;3:e1783. doi: 10.1371/journal.pone.0001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caron NJ, Gage BK, O’Connor MD, Eaves CJ, Kieffer TJ, Piret JM. A human embryonic stem cell line adapted for high throughput screening. Biotechnol Bioeng. 2013;110:2706–16. doi: 10.1002/bit.24936. [DOI] [PubMed] [Google Scholar]
- 27.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–42. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 28.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 29.Smith SB, Gasa R, Watada H, Wang J, Griffen SC, German MS. Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem. 2003;278:38254–9. doi: 10.1074/jbc.M302229200. [DOI] [PubMed] [Google Scholar]
- 30.Brun T, Hu He KH, Lupi R, Boehm B, Wojtusciszyn A, Sauter N, Donath M, Marchetti P, Maedler K, Gauthier BR. The diabetes-linked transcription factor Pax4 is expressed in human pancreatic islets and is activated by mitogens and GLP-1. Hum Mol Genet. 2008;17:478–89. doi: 10.1093/hmg/ddm325. [DOI] [PubMed] [Google Scholar]
- 31.Sosa-Pineda B. The gene Pax4 is an essential regulator of pancreatic beta-cell development. Mol Cells. 2004;18:289–94. [PubMed] [Google Scholar]
- 32.Smith SB, Ee HC, Conners JR, German MS. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol Cell Biol. 1999;19:8272–80. doi: 10.1128/mcb.19.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SB, Watada H, Scheel DW, Mrejen C, German MS. Autoregulation and maturity onset diabetes of the young transcription factors control the human PAX4 promoter. J Biol Chem. 2000;275:36910–9. doi: 10.1074/jbc.M005202200. [DOI] [PubMed] [Google Scholar]
- 34.Collombat P, Hecksher-Sørensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–80. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- 35.Fujita Y, Asadi A, Yang GK, Kwok YN, Kieffer TJ. Differential processing of pro-glucose-dependent insulinotropic polypeptide in gut. Am J Physiol Gastrointest Liver Physiol. 2010;298:G608–14. doi: 10.1152/ajpgi.00024.2010. [DOI] [PubMed] [Google Scholar]
- 36.Gage BK, Riedel MJ, Karanu, F, Rezania A, Fujita Y, Webber TD, Baker RK, Kieffer TJ. Cellular reprogramming of human amniotic fluid cells to express insulin. Differentiation. 2010;80:130-9. doi: 10.1016/j.diff.2010.05.007. [DOI] [PubMed] [Google Scholar]