Abstract
Despite a wealth of fossils of Mesozoic birds revealing evidence of plumage and other soft-tissue structures, the epidermal and dermal anatomy of their wing’s patagia remain largely unknown. We describe a distal forelimb of an enantiornithine bird from the Lower Cretaceous limestones of Las Hoyas, Spain, which reveals the overall morphology of the integument of the wing and other connective structures associated with the insertion of flight feathers. The integumentary anatomy, and myological and arthrological organization of the new fossil is remarkably similar to that of modern birds, in which a system of small muscles, tendons and ligaments attaches to the follicles of the remigial feathers and maintains the functional integrity of the wing during flight. The new fossil documents the oldest known occurrence of connective tissues in association with the flight feathers of birds. Furthermore, the presence of an essentially modern connective arrangement in the wing of enantiornithines supports the interpretation of these primitive birds as competent fliers.
Exceptional fossil preservation allows access to crucial information regarding the integumentary structures1, internal organs2, and other soft-tissues3 of early birds. These rare occurrences have proven crucial for a better understanding of the origin and early evolution of avian physiology2,4,5, behavior4,5, and particularly, the characteristics of their flight4,5,6. The anatomy of the connective tissue of the forelimb of basal birds, however, is largely unknown beyond general observations (i.e., evidence of propatagium7,8). Nonetheless, the characteristics of the wing’s connective tissue are central to flight mechanics given that muscles, tendons, ligaments, and conjunctive tissues connect anatomically the skeletal forelimb with an airfoil composed almost entirely of feathers9,10. Here we describe a new fossil (MCCMLH31444; Museo de las Ciencias de Castilla-La Mancha, Cuenca, Spain) (Fig. 1) of an upper Barremian (~125 MYa) bird from the Las Hoyas Konservat-Lagerstätten11,12. Consisting of the distal portion of a left wing, MCCMLH31444 displays a metacarpal III projecting distally further than metacarpal II (following the digit identity formula of the avian hand as I–II–III13), a synapomorphy of Enantiornithes, and an overall morphology consistent with the anatomy of this clade14. The fossil preserves the anatomy of the wing’s patagia10 and different connective structures associated with the attachment of the remigial feathers9 in exceptional detail.
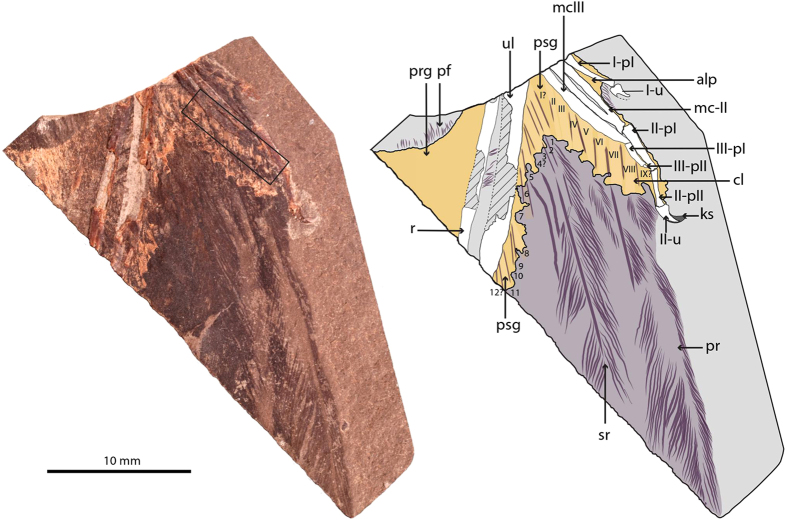
Figure 1. MCCMLH31444, enantiornithine bird (Lower Cretaceous, Las Hoyas, Spain).
Photograph and interpretive drawing of slab. Black-lined inset in the photograph delineates the area of the transition between bone and soft tissue magnified in Fig. 2A. Abbreviations: alp, alular patagium; cl, calamus; ks, keratinous sheath; mc-II; metacarpal II; mc-III, metacarpal III; pf, plumulaceous feathers; pr, primary remex; prg, propatagium; psg, postpatagium; r, radius; sr, secondary remex; ul, ulna; I-pI, first phalanx of digit I; I-u, ungual phalanx of digit I; II-pI, first phalanx of digit II; II-pII, second phalanx of digit II; II-u, ungual phalanx of digit II; III-pI, first phalanx of digit III; III-pII, second phalanx of digit III.
Results
The distal wing of MCCMLH31444 is likely exposed in ventral view, as evidenced by the presence of ventral flexor depressions in the exposed side of the pre-ungual phalanges of digit I and II and the metacarpal II15,16. The preserved portions of the ulna, radius, and metacarpals, and the complete manual digits, are all surrounded by abundant remains of epidermal and dermal connective tissues in close association with plumage. Eight to nine large and strongly asymmetrical primaries, 10–12 secondaries, and the remains of plumulaceous coverts are preserved as carbonized keratinous structures. A halo of brownish-to-yellowish epidermic and dermic tissue outlines the skeletal elements of the wing except for the ungual phalanges of digits I and II and their keratinous sheaths. The connective tissue associated with the plumage shows the same post-mortem folding of the skeletal elements, indicating that these soft tissues are preserved in anatomical position.
Three epidermal structures (i.e., patagia10) are noticeable in MCCMLH31444: the propatagium, the alular patagium, and the postpatagium (Fig. 1). The morphology of the propatagium is remarkably similar to that of modern birds10,17, other enantiornithines7, and the Chinese Early Cretaceous Confuciusornis sanctus8, in which this large epidermal fold connects the wrist to the shoulder (Fig. 1). The propatagium shows a scattered pattern of feather follicles distributed across its entire surface (Suplementary Fig. S1, online). The alular patagium extends from the cranial surface of the midshaft of the metacarpal II to almost the caudodistal end of the first phalanx of digit I (Fig. 1), thus showing a similar distribution to that of most modern birds10,17. Particularly remarkable is the presence of an almost completely preserved postpatagium, extending from the caudoproximal base of the ungual phalanx of digit II to the preserved proximal-most caudal portion of the ulna and in clear association with the wing’s plumage (Fig. 1). The preserved calami of flight feathers (i.e., primaries and secondaries) are embedded in the postpatagium, forming the same sinuous caudal outline visible in the naked (i.e., unfeathered) wing of modern birds10,17 (Fig. 1). Also, the reduced digit III appears to be completely lodged in the postpatagium, as in modern birds10,17 (Fig. 1), thus confirming previous claims that in Enantiornithes this reduced digit was attached to the digit II by soft-tissue structures18.
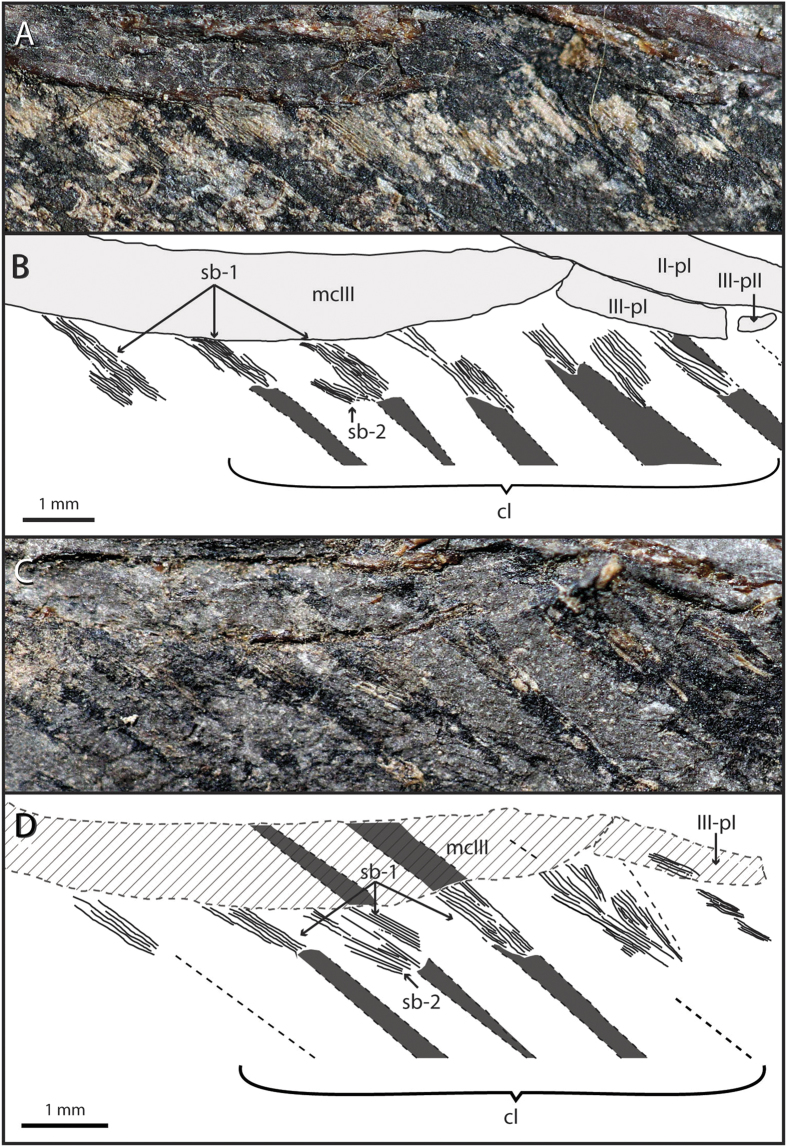
The connective tissue surrounding the proximal-most portion of the calami of the remiges is characterized by a serial striation pattern (Fig. 2). The striations are arranged in discrete and truncated cone-shaped to ribbon-shaped bundles that attach diagonally to the medial portion of the calamus of each feather (Fig. 2). In some of the best-preserved regions, it is possible to identify several striated bundles of fibers that attach to the calamus at different angles and one above the other (Figs 2 and 3A). In the proximal-most region of each bundle—opposite to their feather attachment—the striation lines are more tightly joined together, slightly curving with respect to the main orientation of the bundle (Fig. 2).
Figure 2. Arrangement of striated bundles in the manual region of postpatagium.
(A,B) Photograph and interpretive drawing of tissue and details (Type 1 and 2 bundles of stripes) below skin level of the postpatagium (inset shown in Fig. 1). (C,D) Photograph and interpretive drawing of the same region in the counterslab. Abbreviations: cl, calami; mcIII, metacarpal III; sb-1, Type 1 striated bundle; sb-2, Type 2 striated bundle; II-pI, first phalanx of digit II; III-pI, first phalanx of digit III; III-pII, second phalanx of digit III. (Photograph by José Antonio P. Gracia).
Figure 3. Microstructure of striated bundles.
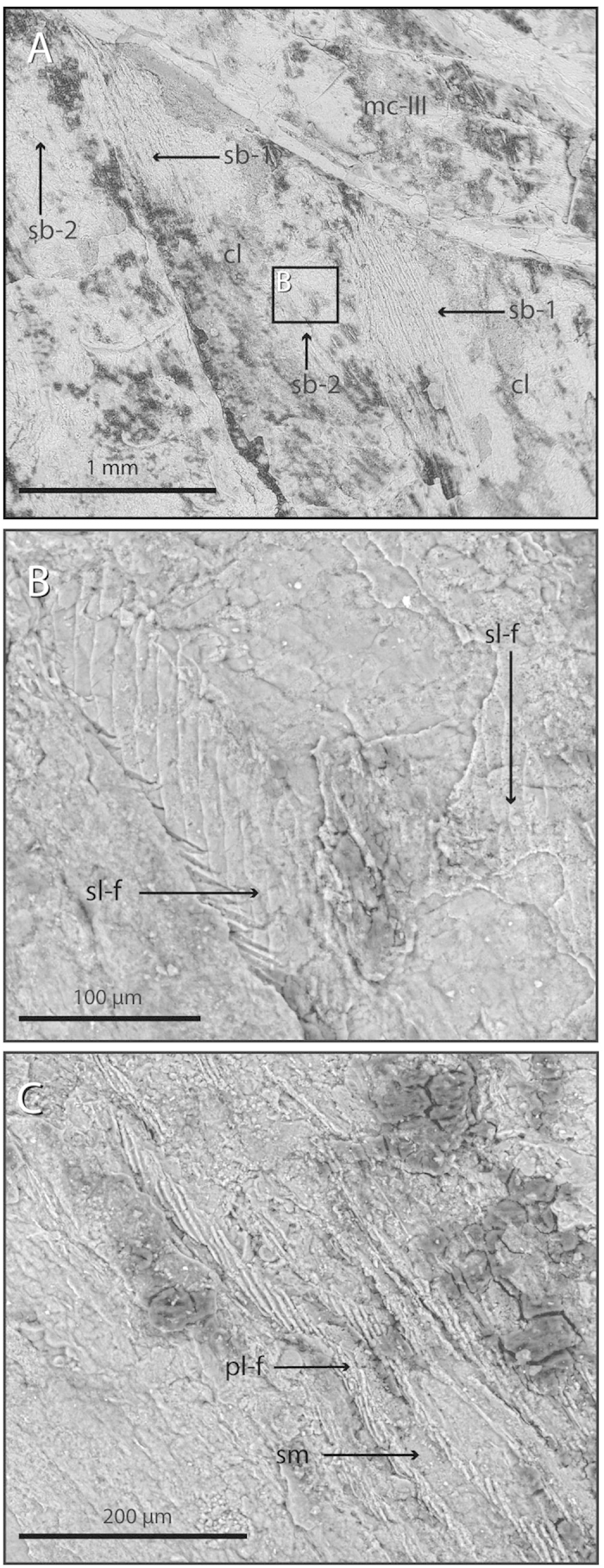
(A) SEM image of two consecutive primary feather calami and their respective Type 1 and Type 2 striated bundles. (B) SEM close-up image of the area delineated by an inset in (A), of a Type 2 striated bundle containing strap-like fibers. (C) SEM close-up of a Type 1 striated bundle showing its alternating pattern of parallel plait-like fibers and smooth matrix. Abbreviations: cl, calami; mcIII, metacarpal III; sb-1, type 1 striated bundle; sb-2, type 2 striated bundle; pl-f, plait-like fibers; sm, smooth matrix; sl-f, strap-like fibers.
SEM inspection reveals that the majority of these striated bundles are composed of plait-like fibers arranged in parallel and alternating with smooth regions of subequal width (here label as Type 1 striated bundles, Fig. 3A,C). The plait-like fibers are made of smaller fibers rolled in a helical pattern (Fig. 3C); the size range and arrangement of these smaller structures is congruent with their interpretation as fibrous collagenous fibers19. Additionally, we label as Type 2 some of the striated bundles in the postpatagium containing strap-like fibers. These are flat and wider than the plait-like ones, and are patterned with diagonal striations with respect to the main axis of the fibers (Fig. 3B). These strap-like fibers are only visible in two of the best-preserved striated bundles and they are associated diagonally with the calami at a higher angle than the plait-like fibers that compose the Type 1 striated bundles (Figs 2 and 3).
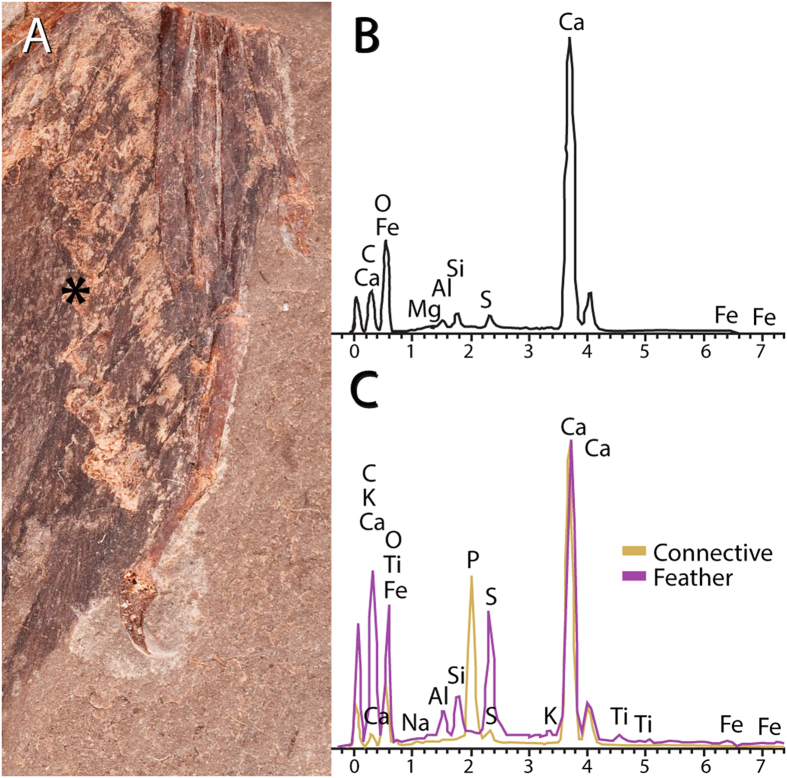
Element analyses (EDAX) indicate that the connective tissues of MCCMLH31444 are mainly preserved as calcium phosphate while the plumage has likely undergone a carbonization process (Fig. 4). Phosphatization of soft tissues is generally associated with changes in pH levels and conditions that allow steady mineral precipitation20. These decay conditions fit the combination of taphonomic factors—obruption, stagnation, and bacterial sealing—proposed as responsible for the exceptional preservation of the Konservat Lagerstätte of Las Hoyas11.
Figure 4. EDAX data from different regions of the slab of MCCMLH31444.
(A) Photograph of part of MCCMLH31444 indicating the region (asterisk) where the EDAX analysis was performed (over the remains of connective tissue). (B) EDAX profile of matrix, primarily showing a high abundance of Ca as expected for a limestone locality. (C) EDAX profile of feathers (purple profile) and connective tissues (brownish). Feathers are composed by higher concentrations of K, O, S, C and Fe as well as traces of Ti; connective tissues show a remarkably different composition from both the matrix and the fossil feathers, with a much more flat profile and a higher peak of P and Ca, evidencing the underlying calcium phosphatization process. One sample was collected from each region.
Discussion
The parallel pattern of plait-like fibers alternating with an unorganized matrix in MCCMLH31444 is characteristic of modern tendons and ligaments that are subject to multidirectional mechanical stresses19,21. Even though the structural composition of modern tendons and ligaments is highly variable depending on their size and mechanical requirements19,21, the strap-like fibers also preserved in the new fossil also show an overall morphology similar to that of structures interpreted as fossilized muscle tissues in theropods22,23. The general morphology and arrangement of the system of ligaments, elastic tendons, and smooth muscles associated with the follicles of flight feathers in the postpatagium of MCCMLH31444 is strikingly similar to that present in living birds9,10, strongly suggesting that a comparably elaborate dermal system is preserved in the new fossil.
In modern birds, networks of smooth muscles attached by elastic tendons to the outer walls of feather follicles control the coordinated movement of groups of feathers9,10,24,25. In the wing, a well-developed array of such tendons and feather muscles—erectors, depressors, retractors and rotators9—together with other ligamental structures (i.e., Ligg. phalangoremigalia distalia, digitationes remigiales10) bestows structural strength and allows the range of movement crucial for controlling lift and maneuverability during flight9,25. For instance, the contraction of rotators results in a counter-clockwise movement of the flight feathers9,25, which counteracts the passive rotation of the feathers caused by aerodynamic stresses and impacting the wing’s ability to generate lift9,25. The comparable arrangement and microstructure of the plait-like muscle fibers observed in MCCMLH31444 suggest that they were capable of providing structural support and buffer against multidirectional forces, such as the ones exerted on the remigial feathers during flight in modern birds. This overall similarity suggests that the wing of MCCMLH31444, and most likely that of other flighted enantiornithines, formed an airfoil capable of enduring important aerodynamic stresses.
The evolution of the modern network of smooth muscles, elastic tendons and ligaments involved in the function of the wing’s flight feathers was a paramount event in the fine-tuning of aerodynamic capabilities in birds. By allowing these feathers to be repositioned in tandem, this sophisticated dermal system helped the wing to maneuver as a single functional unit and to cope with the strenuous aerodynamic stresses of flapping flight. The 125-million-year-old MCCMLH31444 provides the oldest reported evidence of this intricate connective network and its earliest phylogenetic occurrence. The remarkably modern anatomy and arrangement of the connective tissues preserved in the wing of MCCMLH31444 implies that Early Cretaceous enantiornithines had already developed forelimbs morphologically well adapted for flight, losing most of the primitive grasping functions attributed to the dinosaurian forerunners of birds5.
Although still showing a suite of primitive skeletal traits4,14, even the earliest enantiornithines (i.e., Protopteryx fengningensis26) had already developed forelimb elements of modern proportions, a carinate sternum, and an advanced pectoral girdle including a triosseal canal for the passage of flight muscles4,14, all of which suggest active flapping flight and a wing stroke similar to that of present-day birds4,14. Integumentary similarities with modern birds, such as wings with identical feather arrangement and a well-developed alula (i.e., bastard wing)18,27, also point to the same functional conclusions4,18,27. The preservation of three important patagia—propatagium, alular patagium, and postpatagium—together with the above-mentioned dermal system in the wing of MCCMLH31444, an Early Cretaceous enantiornithine, lends strong support to the notion that these primitive avians had achieved aerodynamic competence comparable to those of many modern birds.
Additional Information
How to cite this article: Navalón, G. et al. Soft-tissue and dermal arrangement in the wing of an Early Cretaceous bird: Implications for the evolution of avian flight. Sci. Rep. 5, 14864; doi: 10.1038/srep14864 (2015).
Supplementary Material
Acknowledgments
We thank Marta Furió for SEM assistance at MNCN (Museo de Ciencias Naturales de Madrid) and José Antonio P. Gracia for photographs. We are also grateful to Tyler Hayden (NHM, Natural History Museum of Los Angeles County) for editorial assistance and to Stephanie Abramowicz (NHM) for helping us producing the figures. Support and funds were provided by the projects CGL2009-1183 BTE and CGL-2013-42643-P, Juntas de Comunidades de Castilla-La Mancha and by donations from Mrs. Gretchen Augustyn to the Dinosaur Institute of the Natural History Museum of Los Angeles County.
Footnotes
Author Contributions G.N., J.M., L.C. and A.B. designed the project and conducted the analyses. All authors co-wrote the manuscript.
References
- Fucheng Z., Zhonghe Z. & Dyke G. Feathers and ‘feather‐like’ integumentary structures in Liaoning birds and dinosaurs. Geological Journal. 41, 395–404 (2006). [Google Scholar]
- O’Connor J. K., Zheng X., Wang X., Wang Y. & Zhou Z. Ovarian follicles shed new light on dinosaur reproduction during the transition towards birds. National Science Review. 0, 1–3 (2013). [Google Scholar]
- Zheng X. et al. On the absence of sternal elements in Anchiornis (Paraves) and Sapeornis (Aves) and the complex early evolution of the avian sternum. Proceedings of the National Academy of Sciences. 111, 13900–13905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor J. K., Chiappe L. M. & Bell A. In Living dinosaurs: the evolutionary history of modern birds (eds Dyke G. & Kaiser G. ) Ch. 3, 39–114 (Wiley-Blackwell, 2011). [Google Scholar]
- Chiappe L. M. In Glorified dinosaurs: the origin and early evolution of birds. (John Wiley, 2007). [Google Scholar]
- Wang X. et al. Insights into the evolution of rachis dominated tail feathers from a new basal enantiornithine (Aves: Ornithothoraces). Biological Journal of the Linnean Society. 113, 805–819 (2014). [Google Scholar]
- Chiappe L. & Lacasa-Ruiz A. Noguerornis gonzalezi (Aves: Ornithothoraces) from the Early Cretaceous of Spain in Mesozoic birds: above the heads of dinosaurs. 230–239 (University of California Press, 2002). [Google Scholar]
- Chiappe L. M., Ji S.-A., Ji Q. & Norell M. A. Anatomy and systematics of the Confuciusornithidae (Theropoda, Aves) from the late Mesozoic of northeastern China. Bulletin of the AMNH. 242 (1999). [Google Scholar]
- Lucas A. M. & Stettenheim P. R. Avian anatomy: integument in Agricultural Handbook. Vol. 362 (US Government Printing Office Washington, 1972). [Google Scholar]
- Baumel J. & Witmer L. In Handbook of avian anatomy: Nomina anatomica avium (eds Baumel J. J., King A. S., Breazile J. E., Evans H. E. & Vanden Berge J. C. ). 45–132 (Publications of the Nuttall Ornithological Club, 1993). [Google Scholar]
- Buscalioni A. D. & Fregenal-Martínez M. A. A holistic approach to the palaeoecology of Las Hoyas Konservat-Lagerstätte (La Huérguina Formation, Lower Cretaceous, Iberian Ranges, Spain). Journal of Iberian Geology. 36, 297–326 (2010). [Google Scholar]
- Sanz J. L. et al. An Early Cretaceous faunal and floral continental assemblage: Las Hoyas fossil site (Cuenca, Spain). Geobios. 21, 611–635 (1988). [Google Scholar]
- Towers M., Signolet J., Sherman A., Sang H. & Tickle C. Insights into bird wing evolution and digit specification from polarizing region fate maps. Nature communications 2, 426 (2011). [DOI] [PubMed] [Google Scholar]
- Chiappe L. M., Walker C. A., Chiappe L. & Witmer L. Skeletal morphology and systematics of the Cretaceous Euenantiornithes (Ornithothoraces: Enantiornithes) in Mesozoic birds: above the heads of dinosaurs. 240–267 (University of California Press, 2002). [Google Scholar]
- Sereno P., Rao C. & Li J. Sinornis santensis (Aves: Enantiornithes) from the Early Cretaceous of Northeastern China. Mesozoic birds: above the heads of dinosaurs. 184–208 (University of California Press, 2002). [Google Scholar]
- Sanz J., Pérez-Moreno B., Chiappe L. & Buscalioni A. The Birds from the Lower Cretaceous of Las Hoyas (Province of Cuenca, Spain). Mesozoic birds: above the heads of dinosaurs. 209–229 (University of California Press, 2002). [Google Scholar]
- Ghetie V. Chitescu., Cotofan, V. & Hillebrand A. In Anatomical atlas of domestic birds. 195–208 (1981) [Google Scholar]
- Zhou Z., Chiappe L. M. & Zhang F. Anatomy of the Early Cretaceous bird Eoenantiornis buhleri (Aves: Enantiornithes) from China. Canadian Journal of Earth Sciences. 42, 1331–1338 (2005). [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scandinavian Journal of Medicine & Science in Sports. 10, 312–320 (2000). [DOI] [PubMed] [Google Scholar]
- Briggs D. E. & Wilby P. R. The role of the calcium carbonate-calcium phosphate switch in the mineralization of soft-bodied fossils. Journal of the Geological Society. 153, 665–668 (1996). [Google Scholar]
- Fratzl P. In Collagen: structure and mechanics. (Springer Science & Business Media, 2008). [Google Scholar]
- Kellner A. W. Fossilized theropod soft tissue. Nature. 379, 32–33 (1996). [Google Scholar]
- Dal Sasso C. & Maganuco S. Scipionyx samniticus (Theropoda: Compsognathidae) from the lower Cretaceous of Italy. Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 37, 283 (2011). [Google Scholar]
- Homberger D. G. & de Silva K. N. Functional microanatomy of the feather-bearing integument: Implications for the evolution of birds and avian flight. American Zoologist. 40, 553–574 (2000). [Google Scholar]
- Videler J. J. In Avian flight. (Oxford University Press, 2006). [Google Scholar]
- Zhang F. & Zhou Z. A primitive enantiornithine bird and the origin of feathers. Science. 290, 1955–1959 (2000). [DOI] [PubMed] [Google Scholar]
- Sanz J. L. et al. An Early Cretaceous bird from Spain and its implications for the evolution of avian flight. Nature. 382, 442–444 (1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.