Abstract
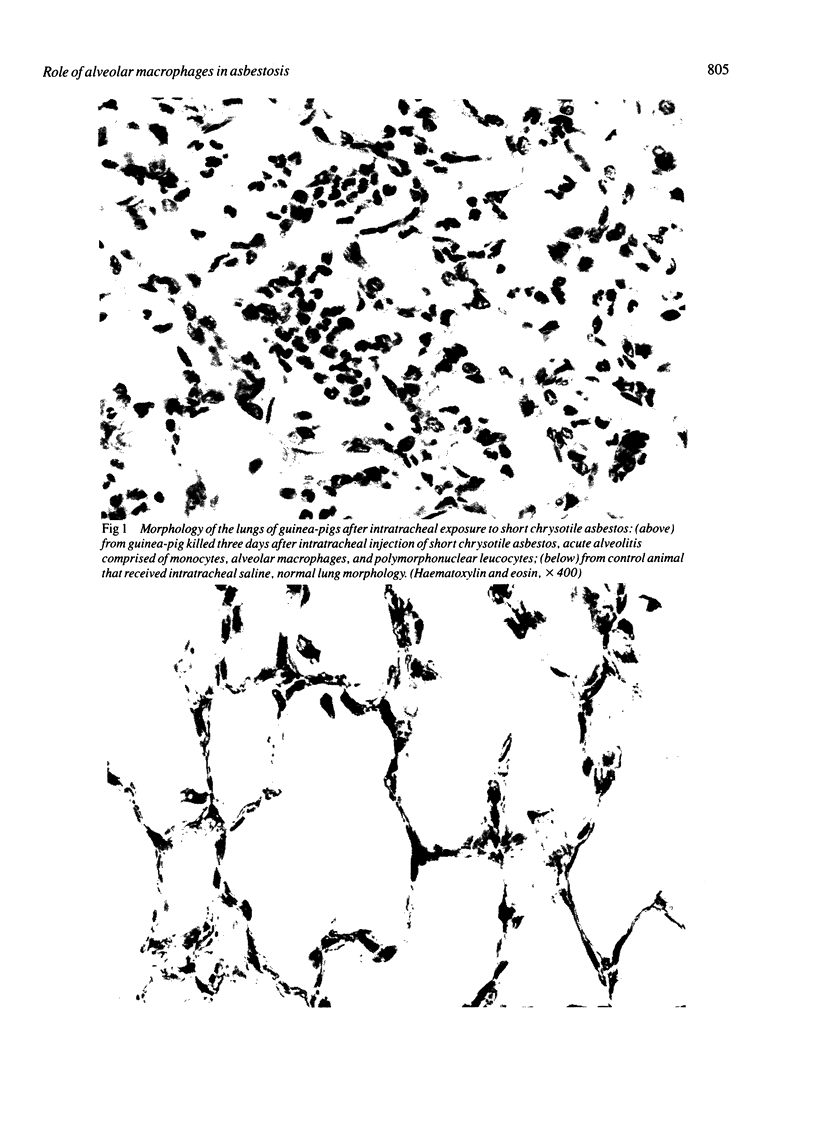
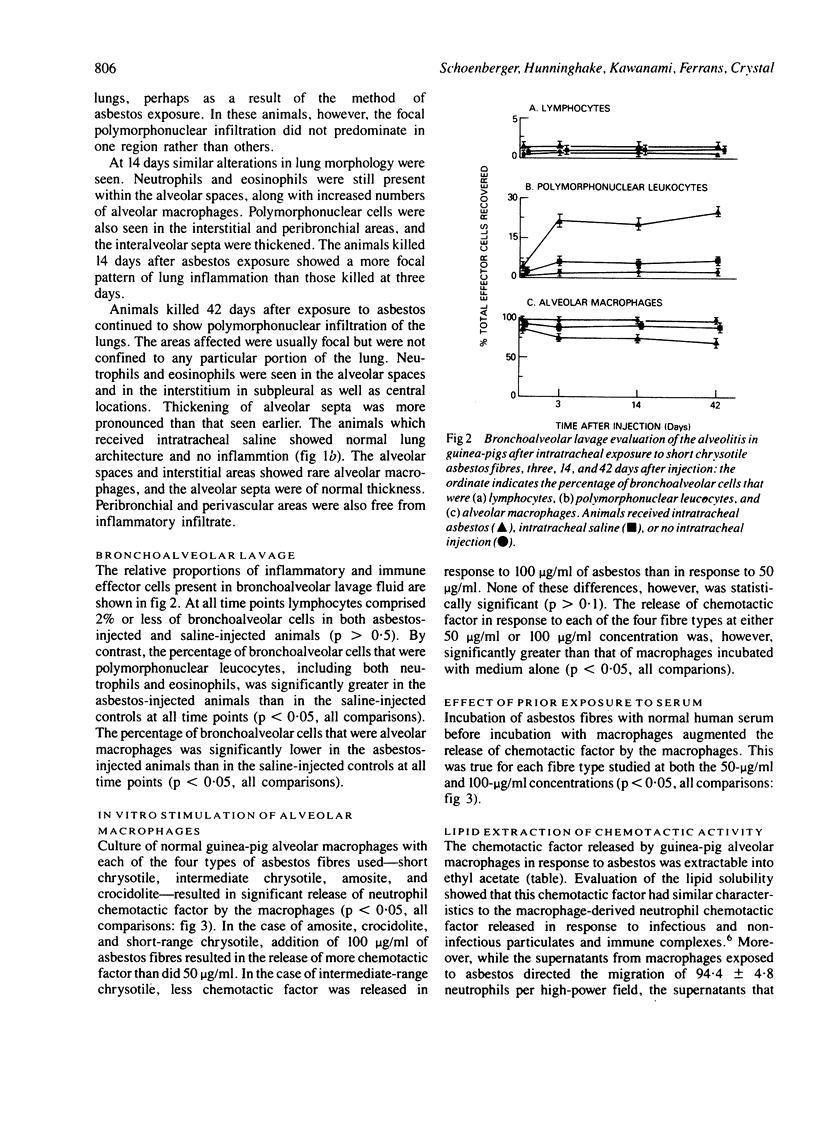
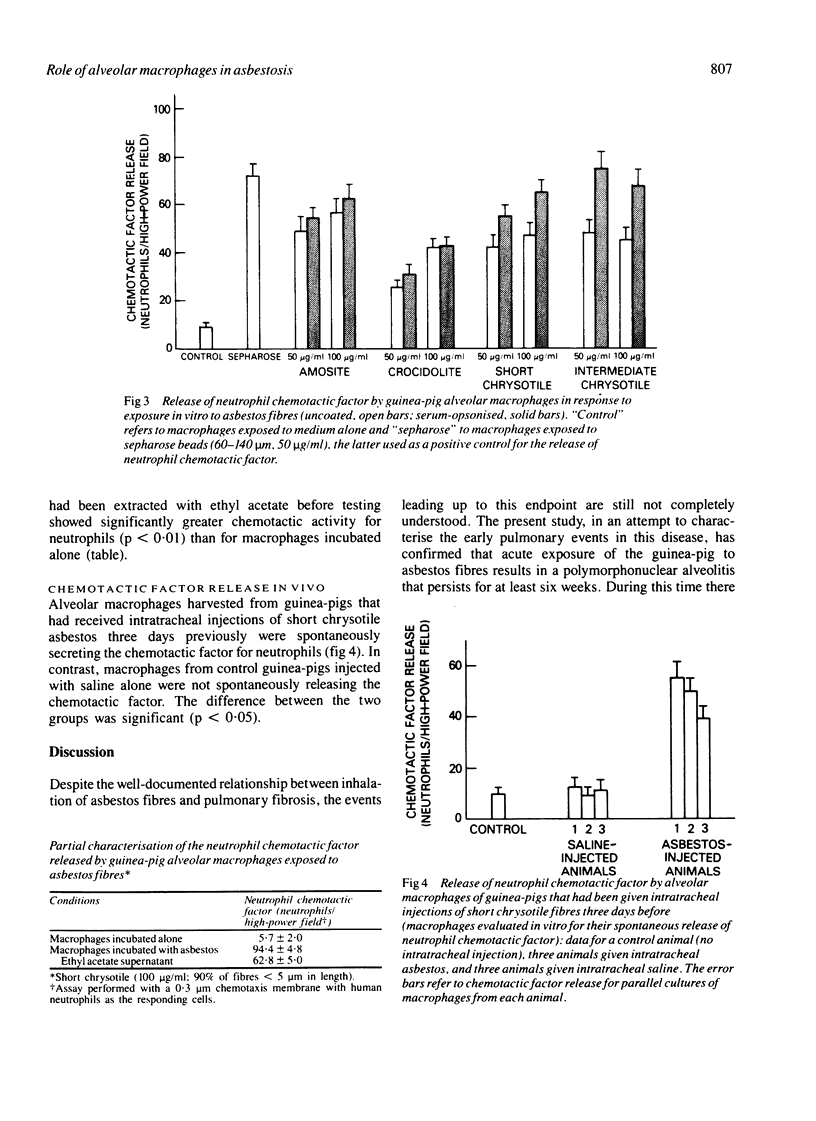
After intratracheal injection of short chrysotile asbestos fibres in guinea-pigs an intense neutrophil alveolitis was observed within three days. Evaluation by bronchoalveolar lavage of the inflammatory and immune effector cells producing the alveolitis by three days showed an increased proportion of polymorphonuclear leucocytes, which comprised 21% +/- 3% of the total leucocytes compared with 9% +/- 2% for the controls (p less than 0.05), persisting for at least six weeks (after which time the polymorphonuclear leucocytes comprised 28% +/- 2% compared with 7% +/- 1% for the controls: p less than 0.05). One mechanism by which asbestos fibres may cause polymorphonuclear leucocytes to be attracted to the alveolar structures is by induced release of neutrophil chemotactic factor by alveolar macrophages. When exposed in vitro to short or intermediate chrysotile fibres or amosite or crocidolite fibres guinea-pig alveolar macrophages released appreciable amounts of neutrophil chemotactic factor. The release of this chemotactic factor was augmented when the asbestos fibres had been previously exposed to normal serum. The chemotactic factor was lipid soluble, and was similar to the neutrophil chemotactic factor spontaneously released by alveolar macrophages recovered from guinea-pigs exposed in vivo to short chrysotile fibres. These observations suggest that alveolar macrophages may play an important part in the early stages of asbestosis by modulating the migration of neutrophils to the lung.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Hunninghake G. W., Zimmerman R. L., Crystal R. G. Regulation of the release of alveolar macrophage-derived neutrophil chemotactic factor. Am Rev Respir Dis. 1980 Apr;121(4):723–733. doi: 10.1164/arrd.1980.121.4.723. [DOI] [PubMed] [Google Scholar]
- Hasselbacher P. Binding of immunoglobulin and activation of complement by asbestos fibers. J Allergy Clin Immunol. 1979 Oct;64(4):294–298. doi: 10.1016/0091-6749(79)90147-7. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Lawley T. J., Crystal R. G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981 Jul;68(1):259–269. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Kazmierowski J. A., Gallin J. I., Reynolds H. Y. Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest. 1977 Feb;59(2):273–281. doi: 10.1172/JCI108638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. The effects of asbestos on macrophages. CRC Crit Rev Toxicol. 1978 Sep;5(4):319–354. doi: 10.3109/10408447809081010. [DOI] [PubMed] [Google Scholar]
- Wilson M. R., Gaumer H. R., Salvaggio J. E. Activation of the alternative complement pathway and generation of chemotactic factors by asbestos. J Allergy Clin Immunol. 1977 Oct;60(4):218–222. doi: 10.1016/0091-6749(77)90133-6. [DOI] [PubMed] [Google Scholar]