Abstract
Purpose
To determine whether the degree of change in Goldmann visual fields (GVFs) following oral administration of QLT091001 was related to baseline measures of retinal structure.
Methods
Oral QLT091001 was administered once daily for 7 days in all study patients. Comprehensive ophthalmic testing, including spectral-domain optical coherence tomography (SD-OCT), was conducted in 14 patients with Leber congenital amaurosis (LCA) and 18 patients with retinitis pigmentosa (RP) at seven international sites. Average thickness of the outer segment (OS) layer was calculated over central 20°. Both eyes of each subject were evaluated separately.
Results
Nineteen of 28 eyes (68%) with LCA and 13 of 36 eyes (36%) with RP responded to QLT091001. Among these responders, the average baseline thickness of the OS layer (central 20°) was 13.5 μm in the LCA cohort and 11.7 μm in the RP cohort. Nonresponders had average baseline OS thickness of less than 4.6 μm in both cohorts. The OS thickness in the central 20° was significantly shorter in nonresponders than responders in the LCA cohort (P = 0.01, t-test) and in the RP cohort (P = 0.02, Wilcoxon rank sum test). The OS thickness in the central 20° did not change significantly from baseline during the first 2 months (P = 0.09, t-test, paired).
Conclusions
The present findings suggest that there is a close parallel between the thickness of the photoreceptor layer and the potential for functional improvement in these patients.
Translational Relevance
SD-OCT thickness in the central retina may be useful for predicting the visual field response in the peripheral retina to QLT091001. (https://clinicaltrials.gov/ct2/show/NCT01014052 number, NCT01014052)
Keywords: retinitis pigmentosa, Leber congenital amaurosis, retinal degeneration, spectral-domain optical coherence tomography, segmentation, layer thickness, visual fields, clinical trial
Introduction
The retinal pigment epithelium (RPE) is a monolayer that provides metabolic support to the retinal photoreceptors. A series of biochemical reactions take place in the RPE, by which the chromophore of the rod photopigment, 11-cis-retinal, is renewed from all-trans-retinol. Retinal pigment epithelium-specific protein, 65-Kd (RPE65) (http://www.omim.org/entry/180069), and lecithin retinol acyltransferase (LRAT) (http://omim.org/entry/604863) are two of the three known enzymes that are involved in this rod photopigment renewal process.1,2 Leber congenital amaurosis (LCA) and retinitis pigmentosa (RP) have been found to be associated with mutations in RPE65 and LRAT.3–11 QLT, Inc. (Vancouver, BC, Canada) has been evaluating the safety and visual outcomes of QLT091001 for oral administration (ClinicalTrial gov identifier: NCT01014052) as therapy for these mutations. QLT091001 is a synthetic 9-cis-retinoid that has been shown to serve as a replacement for 11-cis-retinal in animal models of RPE65 deficiency.12–14 In the initial phase 1b trial, 19 of 28 eyes (68%) with LCA15 and 13 of 36 eyes (36%) with RP (Hendrik P.N. Scholl, Anthony T. Moore, Robert K. Koenekoop, et al., manuscript submitted) responded to QLT091001 (response is defined as expansion of Goldmann visual field retinal areas by ≥20% at two consecutive study visits for the primary isopter in first 2 months after treatment).
High-resolution spectral-domain optical coherence tomography (SD-OCT) allows visualization of the various layers of the human retina in vivo. SD-OCT is particularly important for quantifying decreases in photoreceptor outer segment (OS) thickness in RP and LCA. It is well established that OS thickness is related to traditional measures of visual function in RP, such as multifocal electroretinogram, full-field electroretinograms, and visual field sensitivity.16–20 However, it is unknown whether OS thickness of a study patient at baseline could be used as a predictor for the Goldmann visual field (GVF) response to QLT091001 or whether GVF treatment response is also related to increase in OS thickness. Therefore, we examined the relationship between macular photoreceptor OS thickness measured by SD-OCT and measurement of GVF improvement from baseline after treatment with QLT091001.
Methods
Regulatory Approval and Oversight
The phase 1b trial was an open-label, proof-of-concept study to evaluate the visual outcomes of 40 mg/m2 or 10 mg/m2 QLT091001 in LCA/RP subjects with mutations in RPE65 or LRAT. The clinical trial was conducted at seven international sites listed in the Appendix. The study was conducted in compliance with the approved protocol, which has been reviewed by the U.S. Food and Drug Administration (FDA), and with International Conference on Harmonization good clinical practice guidelines. Approvals were obtained from the institutional review boards of the participating institutions. The tenets of the Declaration of Helsinki were followed. Informed consent was obtained from all subjects in the study.
Study Eligibility and Protocol
Screening
Subjects underwent a screening period (day −21 to day −3), during which all visual function tests and safety assessments were conducted before starting study treatment. Cycloplegic refraction was performed at the first screening visit, followed by subjective refraction at a subsequent screening visit.
Baseline
On day −2/−1, subjects underwent repeat assessment of visual function tests, including GVF and other assessments, and a serum pregnancy test to confirm their eligibility to participate in the study. Subjective refraction and Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) were measured between day −2 and day 0.
Treatment
On day 0 through day 6, patients received a 40 mg/m2 or 10 mg/m2 oral dose of QLT091001 once daily for 7 consecutive days in the study clinic under medical supervision. ETDRS BCVA measurements were conducted on day 1, and BCVA and GVF were subsequently measured at approximately 7, 14, 30, and 60 days after the baseline visit. A baseline visit was available for each subject. While the visit on day 60 was the second most available, not all subjects had visits on day 60. When OS thickness was compared between baseline and day 60, comparison was made between the baseline visit and day 60 or a visit closest to day 60 chronologically.
Study Subjects and Genotype
The clinical trial was conducted in 14 patients with LCA and 18 patients with RP. In the LCA cohort, the average age was 17.9 years (±9.1 years, SD; range 6.6–38 years). There were 10 females and 4 males. There were seven Caucasians, three Asians, three Hispanics, and one of unknown race. Seven subjects in the LCA cohort harbored RPE65 mutations, and seven subjects harbored LRAT mutations. In the RP cohort, the average age was 28.6 years (±11.5 years, SD; range 6.4–55.9 years). There were 10 males and 8 females. There were 11 Caucasians, 6 Asians, and 1 Hispanic. Thirteen subjects in the RP cohort harbored RPE65 mutations, and five subjects harbored LRAT mutations.
Clinical Examinations and Visual Function
Protocol-defined assessments of visual function included BCVA testing using ETDRS protocol; visual field testing using Goldmann perimetry; full-field electroretinogram, SD-OCT, and fundus autofluorescence. Both eyes of each subject were evaluated separately.
As an evaluation of visual function response, responders were defined as eyes having improvements in GVF retinal area of ≥20% on two consecutive visits starting within 2 months of treatment. GVF evaluations were interpreted and quantified using a method described by Dagnelie21 by readers at the Wilmer Eye Institute (Johns Hopkins University, Baltimore, MD). Unless specified, the term responders refers to responding eyes. Nonresponders refers to nonresponding eyes.
SD-OCT Imaging and Segmentation
SD-OCT images were collected at the seven participating sites. Six of the sites used Spectralis (Heidelberg Engineering, Carlsbad, CA) and one site used Cirrus OCT (Carl Zeiss Meditec, Inc., Dublin, CA). High numbers of frames were averaged in order to improve image quality. In case of nystagmus, volume scans were used to ensure the successful capture of the foveal scan. The SD-OCT images were uploaded to QLT, Inc.'s, central server. At the Retina Foundation of the Southwest, these SD-OCT images were exported into tagged image file format (from Spectralis HRA+OCT), which also provided the scaling for the acquired OCT images. The standards for quality assessment are a discernable OS/RPE border and a discernable inner segment ellipsoid band. A total of 393 OCT assessments were documented in this phase 1b study (IRD01). Within these 393 visits, seven eyes in five visits were not analyzed (010103 OU, March 31, 2010, not centered; 010115 right eye, May 6, 2011, not centered; 010303 left eye, February 13, 2012, and June 13, 2012, not measurable; 010503 OU, August 6, 2012, not available). None of the aforementioned visits occurred in the pretreatment visit of the phase 1b trial (IRD01). A 30° (15° radius around the fovea) horizontal line scan was the default protocol for all patients. Thus, in case of parafoveal fixation, the retinal subfields of central 20° (10° radius around the fovea) were still available for analysis. Whenever the 30° horizontal line scans do not show discernable inner segment ellipsoid bands, line scans were derived from volume scans (30° or 20°) for analysis. When volume scans were achievable with discernable inner segment ellipsoid bands, the scans were typically centered on the fovea. Recentralization was done by defining the fovea as the reference point (0°). Thus, a typical analyzable scan covers −10° to 10° referenced around the fovea.
A custom-designed OCT segmentation program built in IGOR Pro (IGOR Pro 6.12; WaveMetrics, Inc., Lake Oswego, OR) was used to profile and measure the thickness of total retina and retinal layers represented in the OCT images. Average thickness of the OS layer (measured from the OS/RPE border to the inner segment ellipsoid band) in the central 20° (10° radius around the fovea) was calculated with the aforementioned OCT segmentation program. The segmentation software has been validated according to the principles in the applicable FDA guidance. The segmentation approach and strategy were comparable to the software (MATLAB based; MathWorks, Natick, MA) developed by Hood et al.22 For the site using Cirrus OCT (Carl Zeiss Meditec), only 6-mm scans were available in three subjects (010501, 010502, 010503). In these three subjects, OS thicknesses in the central 6 mm were used to represent the OS thickness in the central 20°.
Statistics
Because the OS thicknesses of the right eye (OD) and the left eye in the same subject were highly correlated (thus not independent measures), we used averaged thickness ([right eye + left eye]/2) in the same subject as the parameter to compare OS thickness in pre- and posttreatment visits. Because the datasets in pre- and posttreatment visits were not independent samples, paired tests were used. Averaged thickness ([right eye + left eye]/2) in the same subject was also used in the χ2 test for contingency tests shown in Table 1B.
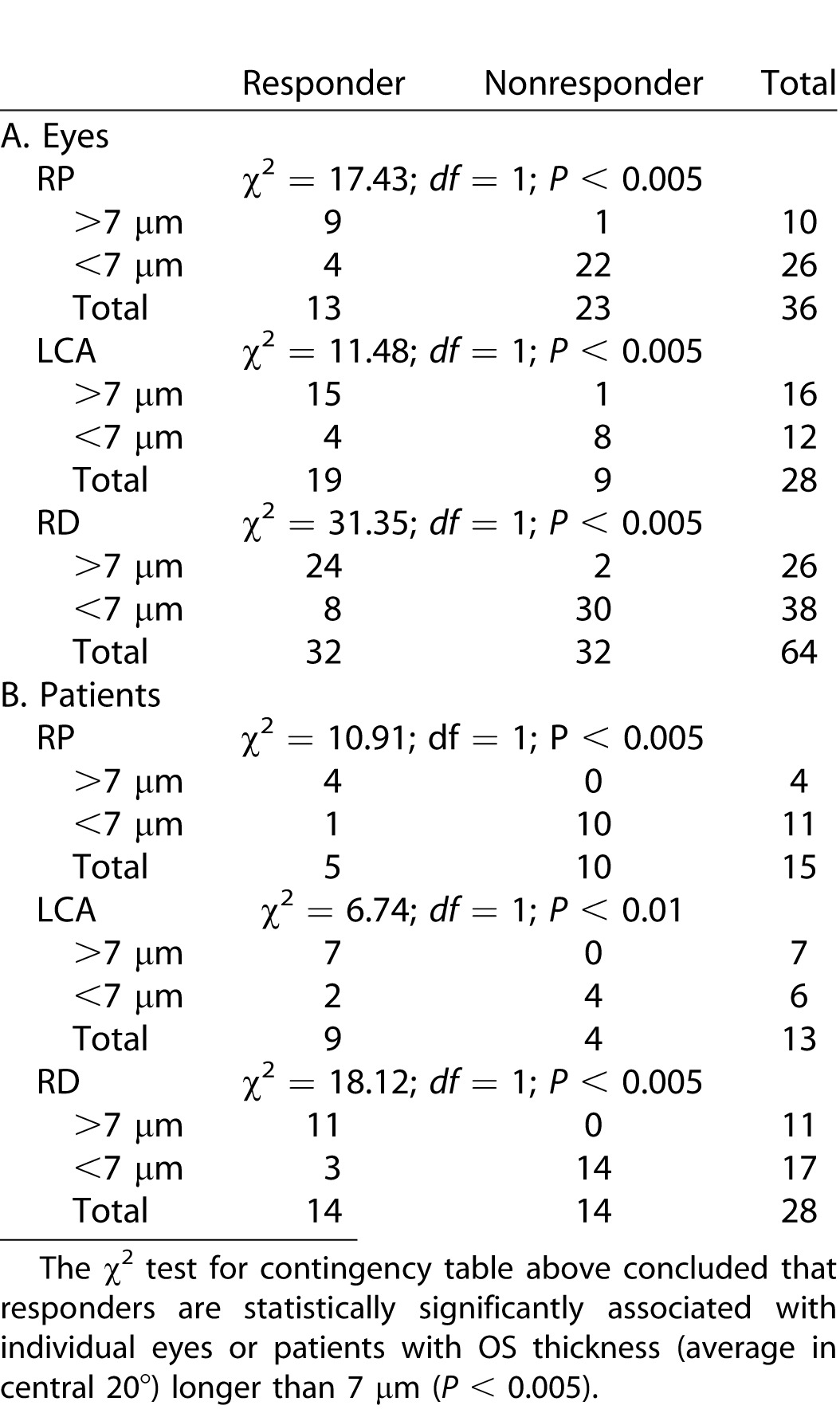
Table 1.
Distribution of eyes and patients as responders or nonresponders according to thickness in the central 20°.
Results
OS Thickness in GVF Responders and Nonresponders
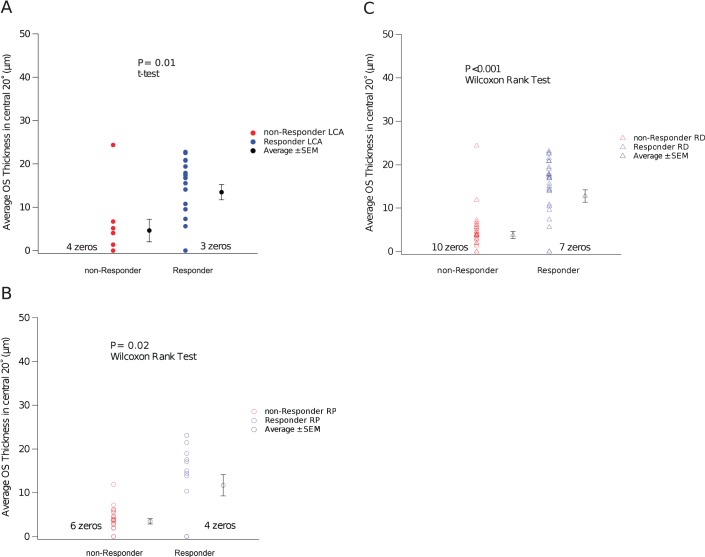
Nineteen of 28 eyes (68%) with LCA and 13 of 36 eyes (36%) with RP responded to QLT091001 (response is defined as expansion of GVF retinal area of the primary isopter by ≥20% at two consecutive study visits starting within 2 months of treatment; Table 1A). Combining all of the subjects into a single retinal degeneration (RD) cohort produced a population in which there were 32 of 64 eyes (50%) that responded. The OS thickness in the central 20° was compared between the responders and nonresponders for the various cohorts. The average OS thickness in the central 20° was significantly shorter in the nonresponders group than the responders group in both the LCA cohort (P = 0.01, t-test; equal variance, F test, P = 0.88) (Fig. 1A) and in the RP cohort (P = 0.02, Wilcoxon Rank test; unequal variance, F test, P < 0.001) (Fig. 1B). For the two groups combined (RD cohort), the average OS thickness in the central 20° was significantly shorter in nonresponders than responders (P < 0.001, Wilcoxon rank test; unequal variance, F test, P = 0.004) (Fig. 1C).
Figure 1.
(A) Leber congenital amaurosis cohort: The average OS thickness in the central 20° was significantly shorter in nonresponders than responders (P = 0.01, t-test; equal variance, F test, P = 0.88). (B) RP cohort: The average OS thickness in central 20° was significantly shorter in nonresponders than responders (P = 0.02, Wilcoxon rank test; unequal variance, F test, P < 0.001). (C) Retinal degeneration (RD; combined): The average OS thickness in the central 20° was significantly shorter in nonresponders than responders (P < 0.001, Wilcoxon rank test) in the combined group (unequal variance, F test, P = 0.004).
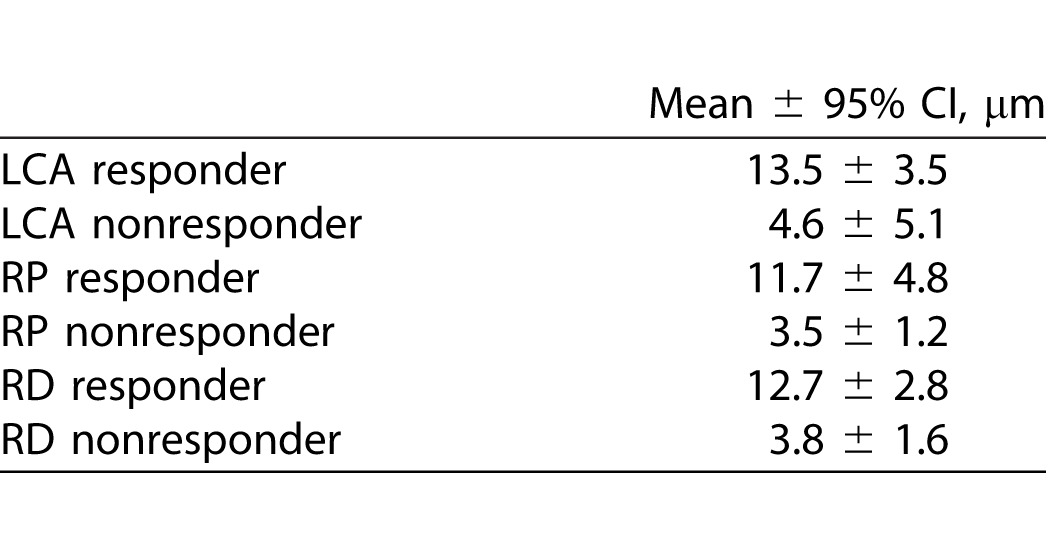
The average thickness over the central 20° of a healthy normal OS is approximately 32 μm.22 As shown in Table 2, the average baseline thickness of the OS layer (central 20°) for responders was 13.5 μm (58% reduced from the normal average) in the LCA cohort and 11.7 μm (64% reduction) in the RP cohort. Nonresponders had an average baseline OS thickness of less than 4.63 μm in both cohorts (86% reduction). In the RD group, the responders had average OS thickness of 12.7 μm at baseline (60% reduction), while the average OS thickness in the nonresponders (RD group) was 3.8 μm, which is reduced by 88% from the normal average.
Table 2.
95% CI for OS thickness (average in central 20°) at baseline visits in responders and nonresponders.
To investigate whether OS thickness changed during the first 60 days after treatment, we compared OS thickness in central 20° at baseline (8.4 ± 1.4 μm, mean ± SE) with the thickness in the visit on day 60 or in the visit closest to day 60 chronologically (7.5 ± 1.4 μm, mean ± SE). The average OS thickness in the central 20° did not change significantly from baseline during the first 2 months (P = 0.09, t-test, paired).
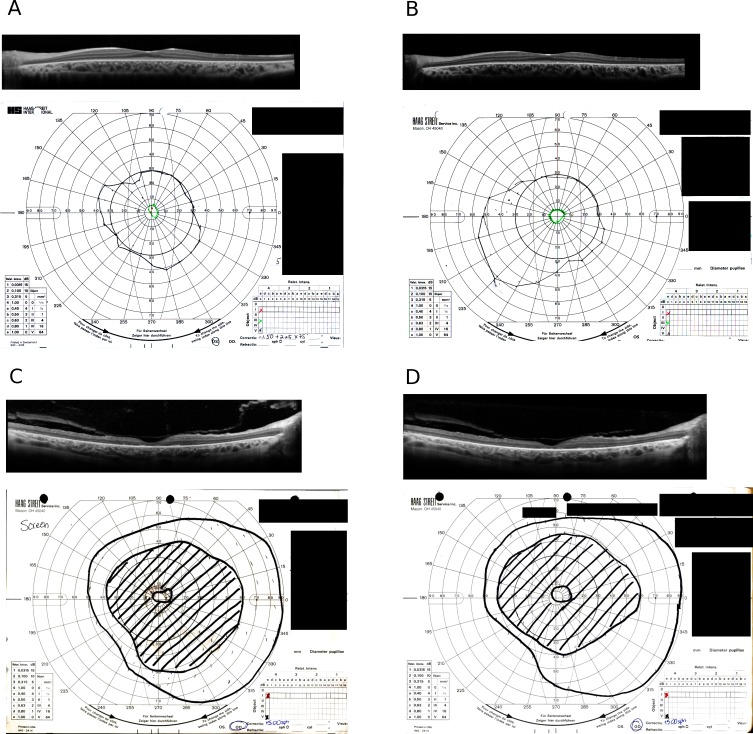
Figure 2 shows representative SD-OCT images and Goldmann fields in a GVF responder (Figs. 2A, 2B) and a GVF nonresponder (Figs. 2C, 2D) at two different time points, baseline (Figs. 2A, 2C) and at day 60 (±7 days) after the start of the treatment (Figs. 2B, 2D). The representative responder (Figs. 2A, 2B) was an 11-year-old male patient with RP associated with an RPE65 mutation. Fig. 2A shows obvious existence of the photoreceptor inner segment ellipsoid band in the foveal region. Measurement shows the average thickness of OS layer in the central 20° was 13.8 μm. The GVF of the V-4-e isopter spanned central 60°. At 2 months after treatment, the area of the primary GVF isopter (III-4-e) increased more than 20% in this patient (Fig. 2B vs. 2A), while no significant change was detected in OS thickness in the central 20°.
Figure 2.
(A) SD-OCT foveal scan (upper) and GVF (lower) in a responder at the screening visit. The average thickness of the OS layer in the central 20° was 13.8 μm. The subject is an 11-year-old male patient with RP associated with RPE65 mutation. (B) SD-OCT foveal scan (upper) and GVF (lower) in the responder (shown in [A]) at day 60 after the start of the treatment. The GVF shows more than 20% gain in area 2 months later (B vs. A). (C) SD-OCT foveal scan (upper) and GVF (lower) in a nonresponder at screening visit. The average thickness of the OS layer in the central 20° was 5.54 μm. The subject is a 42-year-old male patient with RP associated with LRAT mutation. (D) SD-OCT foveal scan (upper) and GVF (lower) in the nonresponder shown in (C) at 2 months after treatment. GVF shows less than 20% gain at 2 months (D vs. C).
A representative nonresponder (Figs. 2C, 2D) was a 42-year-old male patient with RP associated with an LRAT mutation. Figure 2C shows extensive loss of the photoreceptor inner segment ellipsoid band in the central 20°. Measurement shows the average thickness of OS layer in the central 20° was 5.54 μm. A large pericentral scotoma was present in the GVF (V-4-e isopter). At 2 months after treatment, the area of the primary GVF isopter (V-4-e) changed less than 20% (Figs. 2D vs. 2C) and there was no change in the average OS thickness measures.
Distribution of Eyes in Responders and Nonresponders According to Thickness Profile
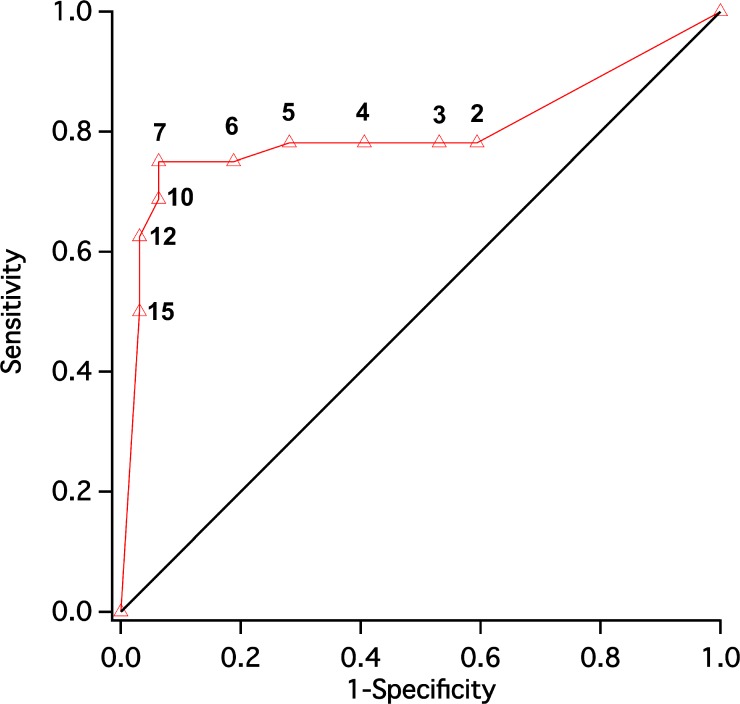
To determine the optimum criterion point in average OS thickness (in micrometers) that separates the responders and nonresponders with a combination of high sensitivity and high specificity, the receiver operating characteristic (ROC) curve was determined for the entire RD group (64 eyes; Fig. 3). It can be seen that 7 μm is the criterion cutoff point between GVF responders and nonresponders for the highest combination of sensitivity and specificity.
Figure 3.
Receiver operating characteristic (ROC) curve (whole RD group, 64 eyes) was plotted to find the best criterion to separate responder and nonresponder groups using thickness measurement (micrometers). It was found that 7 μm is the optimum criterion, with a combination of high sensitivity and high specificity.
Contingency tables for each of the average OS thickness measures were made between 2 μm and 15 μm. Table 1A shows the contingency tables when 7 μm was used as the criterion. The χ2 test for contingency tables showed that responders are statistically significantly associated with eyes with average OS thickness (average in central 20°) longer than 7 μm (P < 0.005; Table 1A). The χ2 tests were also statistically significant (P < 0.05) for all three groups (LCA, RP, RD) when the following criteria were used: 15 μm (P < 0.025), 12 μm (P < 0.01), 10 μm (P < 0.005), 6 μm (P < 0.005), 5 μm (P < 0.01). These results show that significant contingent association between average OS thickness and responsiveness to QLT091001 exists when various thickness criteria (5–15 μm) were used. In addition, 5 μm seems to be the lowest criterion for separating the responders group from nonresponders group effectively.
Not every patient in this study has comparable GVF response to QLT091001 in both eyes (right eye vs. left eye). Three patients with RP showed GVF response above the threshold (≥20% gain in GVF area in 2 months compared to pretreatment visit) in one eye, while not in the fellow eye. In addition, one patient with LCA showed GVF response above the threshold in one eye only. Thus, among these 32 patients, only 4 patients showed heterogeneous response to QLT091001. This suggests high concordance between fellow eyes in response to QLT091001 in 87.5% of the patients (28 of 32). Among these 28 patients, there were 14 bilateral responders and 14 bilateral nonresponders. To investigate whether these 14 patients with above-threshold response to QLT091001 are significantly associated with patients with OS thickness longer than 7 μm, we performed a χ2 test using individual patients as variable in Table 1B, unlike using individual eyes as variable in Table 1A. Results in Table 1B (χ2 test) showed that responding patients were statistically significantly associated with outer segment thickness (averaged in both eyes, [right eye + left eye]/2) that was longer than 7 μm (P < 0.01; Table 1B). In summary, Table 1A and Table 1B show that, regardless of using individual eyes or individual patients as variable, responders are consistently significantly associated with average OS thickness longer than 7 μm (P < 0.01; Table 1A and 1B).
Discussion
The results of the present study in patients with RPE65 or LRAT mutations indicate that a statistically significant association exists between average OS thickness and response to QLT091001 measured by GVF. The residual OS layer measured at baseline provides the structural basis for phototransduction, allowing QLT091001 to mediate the first step in the visual cascade. The improvement of GVF in responders suggests that, although significant photoreceptor degeneration occurs in LCA and RP, the bipolar cells and proximal visual system still effectively receive synaptic inputs from the surviving photoreceptors, despite neural remodeling found in degenerating retina.23–28 Thus, significant and sustained visual function improvements were shown with QLT091001.
This study also identified an optimal criterion cutoff value (micrometers) of 7 μm in OS thickness in the central 20° to effectively separate the GVF responders group from the nonresponders group (Fig. 3). Patients with OS thickness (average in central 20°) of longer than 7 μm measured at baseline of the trial are likely to be GVF responders. Indeed, only 2 eyes with OS > 7 μm at baseline among the 64 eyes (3%) turned out to be GVF nonresponders (false positives by criterion, Table 1A). Meanwhile, 8 eyes with OS < 7 μm at baseline among the 64 eyes (12.5%) turned out to be GVF responders (false negatives by criterion, Table 1A). Several factors could account for the existence of more false-negative GVF responders with shorter than 7 μm OS thickness (12.5%) than false-positive GVF nonresponders with longer than 7 μm OS thickness (3%). Current segmentation methodology identifies the viable inner segment ellipsoid band as a continuous line, rather than discrete pixels. In other words, if the inner segment ellipsoid band is broken into fine fragments that no longer register with the human eye as a continuous line, but rather as discrete pixels, the inner segment ellipsoid band cannot be segmented. As a result, OS thickness is not measurable in that region. Consequently, if a test subject/eye exhibited a pixelated, fragmented existence of inner segment ellipsoid band in certain regions, OS thickness could have been underestimated in these eyes by our manual segmentation method. For example, as shown in Figures 2C and 2D, high-density pixels exist above the RPE band in the fovea. We were not able to measure the thickness of foveal OS because these high-density pixels could be artifacts.29 Figures 2C and 2D also show that an isopter (V-4-e) exists in the foveal region before and after the treatment with QLT091001. This supports the existence of surviving photoreceptors in the fovea that are not measurable by our SD-OCT segmentation technique.
The optimum criterion value of 7 μm (responder/nonresponder group separation) is undoubtedly unique to this cohort. Studies of different cohorts could have different OS thickness criterion values to separate responders group from nonresponders group depending on study population, disease type, and study protocols. Nevertheless, these results support the concept that average OS thickness measured by SD-OCT at baseline could play an important role in future clinical trials in patients with LCA and RP due to RPE65 or LRAT mutations.
The axial resolution of the SD-OCT imager used in this trial (Spectralis HRA+OCT, Heidelberg Engineering) is 3.87 μm/pixel. Table 2 shows the 95% confidence interval (CI) of the 32 nonresponders range from 2.2 to 5.4 μm, which is close to the resolution of the SD-OCT imager. In fact, the mean OS thickness in all 32 nonresponders was 3.8 μm, very similar to the resolution of the SD-OCT imager (3.87 μm/pixel). Therefore, in this study, OS thicknesses that measured less than 5 μm (central 20°) had a much lower signal/noise ratio than OS thicknesses measuring more than 5 μm.
Due to field limitations of the SD-OCT imager and study protocol, SD-OCT images in the periphery were not available for analysis. Nevertheless, we found that average OS thickness measures in the central 20° were significantly associated with global visual field change measured by GVF, which includes not only central field, but also field in the periphery. This association is statistically significant when considering either individual eyes or individual patients (Table 1A and Table 1B).
This study has some inherent limitations. Average OS thickness provides only information of the first dimension (1D). Neither geographic area (2D) nor location (3D) of the structurally preserved retina was captured by this technique. In future studies based on volumetric scans, area (2D) and volume (3D) of the preserved retina could be measured aided by improved automated segmentation algorithms with options of manual correction.30–34 In this study, measurements of OS thickness were conducted manually using a computer program that is not commercially available. However, with improved automated segmentation algorithms with options of manual correction, OS thickness measure could be potentially incorporated into commercial software such as the Heidelberg Eye Explorer (Heidelberg Engineering).
In summary, although the primary defect in LCA and RP due to RPE65 or LRAT mutations lies in the visual (retinoid) cycle, photoreceptor degeneration frequently occurs at an early age, even in the first decade of life. The present findings suggest that there is a close parallel between photoreceptor structures and potential for function improvement in these eyes following medical therapy. Thus, SD-OCT thickness in the central retina may be useful as an indirect measure for predicting the response in the peripheral retina to QLT091001.
Acknowledgments
We thank Ronald Schuchard, PhD, for sharing his knowledge of study design and data interpretation. We thank Heather Kato for coordinating the study. We thank the study sites for providing us with high-quality SD-OCT and GVF data.
This study was supported by QLT, Inc., Vancouver, BC, Canada.
QLT091001 Retinoid Study Group: Gerald A. Fishman, MD (The Chicago Lighthouse, Pangere Center for Inherited Retinal Diseases, Chicago, IL, USA); Samuel G. Jacobson, MD, PhD (Scheie Eye Institute, University of Pennsylvania, Philadelphia, PA, USA); Robert Koenekoop, MD, PhD (McGill University Health Centre, Montreal, Quebec, Canada); Anthony T. Moore, MD, PhD (Moorfields Eye Hospital and Institute of Ophthalmology, University College London, London, United Kingdom); Hendrik Scholl, MD, MA, Gislin Dagnelie, PhD (Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, USA); L. Ingeborgh van den Born, MD, PhD (Rotterdam Eye Hospital and Ophthalmic Institute, Rotterdam, The Netherlands); Eberhart Zrenner, MD (Institute for Ophthalmic Research, Center for Ophthalmology, University of Tübingen, Tübingen, Germany).
PSI CRO Budapest (Budapest, Hungary) conducted medical monitoring (safety reporting), with Dr. Gyorgy Andor as the medical officer. PRA International conducted the sponsor's global medical monitoring, with Dr. L. Paul Starkey, MD, FAAFP, as the executive director.
Disclosure: Y. Wen, QLT, Inc. (C); D.G. Birch, QLT, Inc. (C)
References
- 1. Moiseyev G,, Chen Y,, Takahashi Y,, Wu BX,, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005. ; 102: 12413–12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xue L,, Gollapalli DR,, Maiti P,, Jahng WJ,, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 2004. ; 117: 761–771. [DOI] [PubMed] [Google Scholar]
- 3. Senechal A,, Humbert G,, Surget MO,, et al. Screening genes of the retinoid metabolism: novel LRAT mutation in Leber congenital amaurosis. Am J Ophthalmol. 2006. ; 142: 702–704. [DOI] [PubMed] [Google Scholar]
- 4. Thompson DA,, Li Y,, McHenry CL,, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet. 2001. ; 28: 123–124. [DOI] [PubMed] [Google Scholar]
- 5. den Hollander AI,, Lopez I,, Yzer S,, et al. Identification of novel mutations in patients with Leber congenital amaurosis and juvenile RP by genome-wide homozygosity mapping with SNP microarrays. Invest Ophthalmol Vis Sci 2007. ; 48: 5690–5698. [DOI] [PubMed] [Google Scholar]
- 6. Marlhens F,, Bareil C,, Griffoin JM,, et al. Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet. 1997. ; 17: 139–141. [DOI] [PubMed] [Google Scholar]
- 7. Gu SM,, Thompson DA,, Srikumari CR,, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997. ; 17: 194–197. [DOI] [PubMed] [Google Scholar]
- 8. Morimura H,, Fishman GA,, Grover SA,, Fulton AB,, Berson EL,, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998. ; 95: 3088–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson DA,, McHenry CL,, Li Y,, et al. Retinal dystrophy due to paternal isodisomy for chromosome 1 or chromosome 2, with homoallelism for mutations in RPE65 or MERTK, respectively. Am J Hum Genet. 2002. ; 70: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felius J,, Thompson DA,, Khan NW,, et al. Clinical course and visual function in a family with mutations in the RPE65 gene. Arch Ophthalmol. 2002. ; 120: 55–61. [DOI] [PubMed] [Google Scholar]
- 11. Yzer S,, van den Born LI,, Schuil J,, et al. A Tyr368His RPE65 founder mutation is associated with variable expression and progression of early onset retinal dystrophy in 10 families of a genetically isolated population. J Med Genet. 2003. ; 40: 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batten ML,, Imanishi Y,, Tu DC,, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005. ; 2: e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gearhart PM,, Gearhart C,, Thompson DA,, Petersen-Jones SM. Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs. Arch Ophthalmol. 2010. ; 128: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 14. Maeda T,, Dong Z,, Jin H,, et al. QLT091001, a 9-cis-retinal analog, is well-tolerated by retinas of mice with impaired visual cycles. Invest Ophthalmol Vis Sci. 2013. ; 54: 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koenekoop RK,, Sui R,, Sallum J,, et al. Oral 9-cis retinoid for childhood blindness due to Leber congenital amaurosis caused by RPE65 or LRAT mutations: an open-label phase 1b trial. Lancet. 2014. [DOI] [PubMed]
- 16. Acton JH,, Smith RT,, Hood DC,, Greenstein VC. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012. ; 53: 7618–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birch DG,, Locke KG,, Wen Y,, Locke KI,, Hoffman DR,, Hood DC. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol. 2013; 131: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hood DC,, Ramachandran R,, Holopigian K,, Lazow M,, Birch DG,, Greenstein VC. Method for deriving visual field boundaries from OCT scans of patients with retinitis pigmentosa. Biomed Opt Express. 2011. ; 2: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen Y,, Klein M,, Hood DC,, Birch DG. Relationships among multifocal electroretinogram amplitude, visual field sensitivity, and SD-OCT receptor layer thicknesses in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012. ; 53: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Birch DG,, Wen Y,, Locke K,, Hood DC. Rod sensitivity, cone sensitivity, and photoreceptor layer thickness in retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2011. ; 52: 7141–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dagnelie G. Conversion of planimetric visual field data into solid angles and retinal areas. Clin Vis Sci ; 1990: 95–100.
- 22. Hood DC,, Lin CE,, Lazow MA,, Locke KG,, Zhang X,, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009. ; 50: 2328–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bisti S. Degeneration/re-organization coupling in retinitis pigmentosa. Clin Neurophysiol. 2010. ; 121: 270–271. [DOI] [PubMed] [Google Scholar]
- 24. John SK,, Smith JE,, Aguirre GD,, Milam AH. Loss of cone molecular markers in rhodopsin-mutant human retinas with retinitis pigmentosa. Mol Vis. 2000. ; 6: 204–215. [PubMed] [Google Scholar]
- 25. Jones BW,, Kondo M,, Terasaki H,, Lin Y,, McCall M,, Marc RE. Retinal remodeling. Jpn J Ophthal. 2012. ; 56: 289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li ZY,, Kljavin IJ,, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci. 1995. ; 15: 5429–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marc RE,, Jones BW,, Watt CB,, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003. ; 22: 607–655. [DOI] [PubMed] [Google Scholar]
- 28. Wang J,, Zhang N,, Beuve A,, Townes-Anderson E. Mislocalized opsin and cAMP signaling: a mechanism for sprouting by rod cells in retinal degeneration. Invest Ophthalmol Vis Sci. 2012. ; 53: 6355–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rii T,, Itoh Y,, Inoue M,, Hirakata A. Foveal cone outer segment tips line and disruption artifacts in spectral-domain optical coherence tomographic images of normal eyes. Am J Ophthalmol. 2012. ; 153: 524–529. [DOI] [PubMed] [Google Scholar]
- 30. Garvin MK,, Abramoff MD,, Kardon R,, Russell SR,, Wu X,, Sonka M. Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search. IEEE Trans Med Imaging. 2008. ; 27: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garvin MK,, Abramoff MD,, Wu X,, Russell SR,, Burns TL,, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. IEEE Trans Med Imaging. 2009. ; 28: 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Q,, Reisman CA,, Chan K,, Ramachandran R,, Raza A,, Hood DC. Automated segmentation of outer retinal layers in macular OCT images of patients with retinitis pigmentosa. Biomed Opt Express. 2011. ; 2: 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Q,, Reisman CA,, Wang Z,, et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. Opt Express 2010. ; 18: 21293–21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L,, Buitendijk GH,, Lee K,, et al. Validity of Automated Choroidal Segmentation in SS-OCT and SD-OCT. Invest Ophthalmol Vis Sci. 2015. ; 56: 3202–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]