Abstract
Acid-sensing ion channels (ASICs) are proton-gated cation channels that are widely expressed in both the peripheral and central nervous systems. ASICs contribute to a variety of pathophysiological conditions that involve tissue acidosis, such as ischemic stroke, epileptic seizures and multiple sclerosis. Although much progress has been made in researching the structure-function relationship and pharmacology of ASICs, little is known about the trafficking of ASICs and its contribution to ASIC function. The recent identification of the mechanism of membrane insertion and endocytosis of ASIC1a highlights the emerging role of ASIC trafficking in regulating its pathophysiological functions. In this review, we summarize the recent advances and discuss future directions on this topic.
Keywords: accessory proteins, acid-sensing ion channels, motifs, signaling pathways, surface expression, trafficking
Introduction
Acid-sensing ion channels (ASICs) are a proton-gated subgroup of the degenerin/epithelial Na+ channel (DEG/ENaC) family of cation channels, which are trimeric protein complexes composed of different combinations of subunits.1 To date, 6 ASIC isoforms (ASIC1a, 1b, 2a, 2b, 3 and 4) arising from 4 genes (Accn1, 2, 3 and 4) have been identified. ASICs are expressed in both the peripheral and central nervous systems. ASIC1a, 2a and 2b are the major isoforms in the brain and spinal cord; whereas the expression of ASIC1b and ASIC3 is restricted to peripheral sensory neurons.2 As the key receptors for extracellular protons, ASICs have been implicated in many pathophysiological processes related to acidosis, such as pain, ischemic stroke, and fear-/anxiety-related psychiatric disorders. The function of ASICs in these processes depends on the number of channels on the cell surface. Thus, the dynamic control of surface ASICs under normal and pathological conditions is currently being researched.
The number and function of receptors on the plasma membrane is partially determined by the dynamic trafficking processes, which include sorting and forward trafficking of receptors from the endoplasmic reticulum (ER) through the Golgi apparatus to the plasma membrane; endocytosis of surface receptors; resorting of receptors following endocytosis; recycling of receptors back to the plasma membrane; and targeting receptors for degradation.3 Elucidating the detailed molecular mechanisms that govern ASIC trafficking will improve our understanding of their pathophysiological functions in the brain.
Recent Advances in ASIC Trafficking
Defects in trafficking cause the dysfunction of ion channels, and ultimately lead to a variety of disorders. ENaCs share substantial homology with ASICs.4 Disruption of ENaC endocytosis, which is primarily regulated by Nedd4–2, an E3 ubiquitin ligase, has been shown to be involved in Liddle syndrome.5–7 However, it is not yet known whether the same trafficking pathways regulate ASICs or if other mechanisms are involved. Cumulative evidence indicates that the function of these channels can be increased or decreased by modulating the level of trafficking-related proteins or mutating their trafficking motifs, both of which are discussed below.
PICK1 and ASIC trafficking
Protein-interacting with C kinase-1 (PICK1) regulates the trafficking of multiple membrane proteins,8 and is an established ASIC binding partner that binds to the C-terminus of ASICs via its PDZ domain.9-11 It has long been speculated that PICK1 plays a role in the trafficking of ASICs. Recent evidence has shown that the genetic disruption of PICK1 leads to a decreased plasma membrane level of ASIC1 and ASIC2a, which attenuates the function of ASICs in mouse cortical neurons.12 This work indicates a possible regulatory role of PICK1 in ASIC trafficking. Further supporting evidence shows that overexpression of PICK1 increases the surface level of ASIC1a and ASIC1a-mediated acidotoxicity via interactions with the PICK1 BAR and lipid binding domain.13 However, more details regarding the exact process of PICK1-mediated ASIC trafficking are yet to be elucidated. PICK1 can facilitate either endocytosis or exocytosis of its binding partners, depending on the distribution of these proteins in different pools and the nature of the stimulation. For instance, PICK1 increases the surface expression of dopamine transporters (DATs) and enhances DAT uptake activity,14 which is similar to the effects of PICK1 on ASICs. In contrast, overexpression of PICK1 decreases the surface expression of AMPA receptors through calcium-dependent endocytosis.15,16 A model that incorporates these differences is that PICK1 may maintain an intracellular reserve pool of membrane proteins, which engages in exchanging with cell surface proteins in a regulated manner. It would be interesting to determine whether PICK1-mediated trafficking of ASICs is regulated by a similar mechanism.
The role of the ASIC extracellular domain in channel maturation and trafficking
The large extracellular domain of ASICs (∼318 of the 528 residues in ASIC1a) has led to the speculation that it may have additional functions besides sensing protons.1 The trafficking of membrane proteins is tightly controlled by post-translational modification and protein maturation. As the most common type of modification, glycosylation through the secretory pathway, plays an important role in the maturation and trafficking of proteins within the cell.17 Jing et al.18 carried out studies to assess the role of N-glycosylation in the biogenesis and surface expression of ASICs. They found that the surface fraction of ASIC1a in the mouse brain contains a higher percentage of EndoH-resistant mature N-linked glycans than cytoplasmic ASIC1a, which indicates that mature ASIC1a is preferentially transported to the cell surface. Furthermore, they found that the extracellular asparagine site at 393 (Asn393; Fig. 1), but not those at other sites, was preferentially processed in middle-to-late Golgi; whereas mutation of Asn393 (N393Q) reduced ASIC1a maturation and surface expression. Importantly, inhibition of glycosylation with tunicamycin or by N393Q mutation reduced ASIC1a dendritic targeting; moreover, the N393Q mutation caused ASIC1a to be resistant to acidosis-induced spine loss. These findings suggest that glycosylation of ASICs has an important role in regulating synaptic morphogenesis and determining long-term consequences in tissue acidosis. Consistent with a previous study,19 the Asn393 site, located between the α6 and α7 helices in the crystal structure, is conserved among all ASICs. In addition to conventional trafficking signals that are composed typically of short linear peptide sequences, the tertiary structure, within the extracellular domain of ASICs, can form a signal patch for trafficking. In support of this hypothesis, Jing et al.20 showed that mutations of Tyr71 (Y71G) and Trp287 (W287G; Fig. 1), involved in the TM1-thumb interaction, decreased the surface expression and dendritic targeting of ASIC1a. In a separate study, a highly conserved salt bridge at the extracellular loop (D107-R153 of rat ASIC3, and D107-R160 of human ASIC1a; Fig. 1), which stabilizes a rigid signal patch, was found to be critical for surface expression of ASICs.21 These data indicate that both the post-translational modification sites and tertiary structure within the extracellular domains regulate ASIC trafficking.
Figure 1.
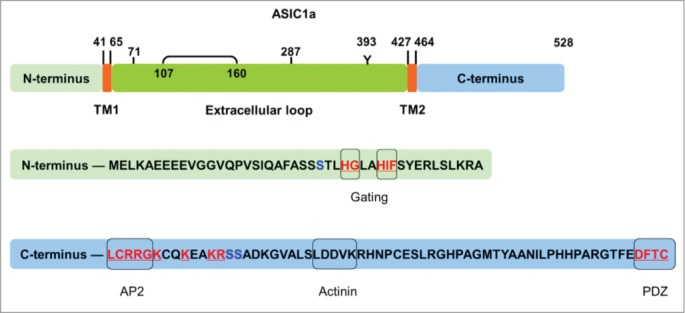
Diagram of motifs within ASIC1a. The trafficking motifs in the extracellular loop and cytoplasmic domains are indicated. Tyr71 and Trp287 are involved in the TM1-thumb interaction. Asp107–Arg160 (linked by the line) is a conserved salt bridge. Asn393 is the N-glycosylation site. The blue characters indicate the phosphorylation sites, and red characters indicate motifs mentioned in the manuscript. The red characters at the C-terminus indicate the motifs that are critical for ASIC1a surface expression.
The emerging role of ASIC1a dynamic trafficking
Our recent studies have demonstrated the molecular mechanism of ASIC1a dynamic trafficking and its pathophysiological role (Fig. 2).22,23 We found that application of brain-derived neurotropic factor (BDNF) upregulates ASIC1a channel activity in cultured mouse spinal dorsal horn (SDH) neurons and that ASIC1a is required for sustained BDNF-induced mechanical hyperalgesia. BDNF sensitizes ASIC1a function through enhancing its forward trafficking and surface expression via the downstream tropomyosin-related kinase B (TrkB)-phosphoinositide 3 kinase (PI3K)-protein kinase B (PKB/Akt) cascade and phosphorylation of the cytoplasmic residue Ser25 of ASIC1a. Moreover, this enhancement is required for BDNF-mediated hypersensitivity of SDH nociceptive neurons and central mechanical hyperalgesia in rat and mouse models. We further demonstrated that this process was abolished by intrathecal application of a peptide representing the N-terminal region of ASIC1a encompassing Ser25.22 These results reveal a novel mechanism underlying ASIC1a forward trafficking, and indicate that targeting specific trafficking process of pain-facilitating receptors may more efficiently treat chronic pain.24 In the future, it will be interesting to examine whether this regulatory signaling pathway of ASICs is involved in other physiological or pathological conditions.
Figure 2.
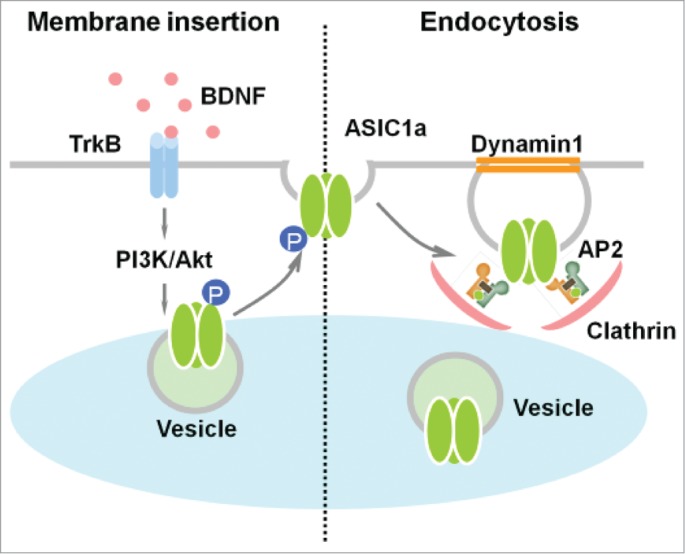
Dynamic trafficking of ASIC1a. Left: activation of TrkB receptor by BDNF Facilitates the PI3K/Akt pathway, and then induces ASIC1a phosphorylation and forward targeting to the cell surface. Right: the endocytosis of ASIC1a is mediated by clathrin and dynamin-dependent processes.
In contrast to the regulation of ASIC surface expression, the endocytosis of ASIC channels is not well understood. Given that epithelial sodium channels (ENaCs), which have substantial homology with the ASIC family, are regulated by clathrin-dependent endocytosis,25,26 and their dysfunction leads to Liddle syndrome5-7; we speculated that ASICs are also regulated by the same pathway. As expected, we found that ASIC1a is associated with several subunits of adaptor protein 2 (AP2) and undergoes constitutive endocytosis in a clathrin- and dynamin-dependent manner in both mouse cortical neurons and heterologous cell cultures. We have further shown that the membrane-proximal residues LCRRG, located at the cytoplasmic C-terminus of ASIC1a, are critical for interaction with the endogenous adaptor protein complex.23 Endocytic pathway dysfunctions have been found in various neurodegenerative disorders, such as Alzheimer disease, lateral sclerosis, and ischemia.27-29 In light of these studies, we hypothesized that dysfunction of ASIC1a endocytosis in neurodegeneration may exacerbate acidosis-induced neuronal injury. In our in vitro model of acidosis-induced neuronal death, inhibition of ASIC1a internalization by dynasore, a small inhibitor of dynamin, strongly exacerbated the acidosis-induced death of cortical neurons from wild-type, but not from ASIC1a knock-out mice.23 Our results indicate the importance of endocytic pathways in acidosis-induced neuronal death and suggest that this regulation is mediated mainly via membrane retention of ASIC1a proteins (Fig. 2).
Constitutive Trafficking of ASICs
As membrane proteins, ASICs undergo constitutive trafficking. To date, their trafficking motifs and pathway-specific accessory proteins remain largely unknown. Investigating the constitutive trafficking cascade of ASICs will help us to understand the molecular mechanisms that regulate these channels and provide potential targets for preventing acidosis-induced cell death.30 In this section, we summarize the current knowledge about trafficking motifs and accessory proteins of ASICs that have a profound influence on their constitutive trafficking processes.
Trafficking motifs within the cytoplasmic domain of ASICs
Motifs within the cytoplasmic domains of membrane proteins play important roles in regulating their biogenesis, surface expression and trafficking through interactions with different accessory proteins.31 The type of motif varies among different trafficking pathways. For instance, the di-acidic motifs [(D/E)X(D/E)], di-hydrophobic motifs (FF, YY, LL or FY), YXXXNPF and LXXLE are ER-exit signals that play a role in the transport of specific cargo from the ER exit sites31,32; whereas the KDEL, di-lysine (KK) and RXR motifs lead to ER retention32; and the di-leucine (LL) and tyrosine-based (YXXФ, where Ф is a hydrophobic amino acid) motifs are canonical signals for clathrin-mediated endocytosis.33,34 Investigations are currently underway to unravel the trafficking motifs within ASICs. Several motifs within the cytoplasmic domains of ASIC1a are critical for its channel gating and surface expression. The N-termini of ASICs contain 2 highly conserved channel gating motifs. Mutation of the HIF motif (Fig. 1) abolished the proton-gated current density,37 without affecting the surface expression of ASIC1a. In addition, the HG motif (Fig. 1) is completely conserved among ENaC family members and mutating it reduces the open probability of ENaC channels.35,36 Although it is yet unknown whether the HG motif is also involved in the functional properties of ASICs, it is possible that the N-terminus of ASIC1a contains multiple channel gating motifs. In contrast, cumulative evidence shows that the trafficking motifs are located within the C-terminus of ASIC1a. All ASICs contain a PDZ-binding motif at the end of their C-termini that regulates ASIC surface expression and channel activity through interacting with several PDZ-domain proteins.9–11 In accordance with a previous study in which coexpression of postsynaptic density protein 95 (PSD95) and ASIC3 reduced the amplitude of ASIC3 proton-gated currents,38 mutating the PDZ-binding motif of ASIC1a (Fig. 1) increased its surface expression and current density.39 These data suggest that the regulation of trafficking by PDZ-binding proteins is conserved among ASICs. We then wanted to find out whether ASICs contained isoform-specific trafficking motifs. By thoroughly scanning the C-terminus of ASIC1a, we found that the membrane-proximal residues LCRRG, at the cytoplasmic C-terminus, are critical for surface expression (unpublished data) and responsible for the interaction with AP2, which regulates the constitutive endocytosis of ASIC1a in both mouse cortical neurons and heterologous cell cultures.23 Consistent with our observation, Jing et al.39 showed that mutating the positively-charged amino acids that overlap the LCRRG motif (from RRGK to AAGA), or deleting these residues, significantly reduced ASIC1a surface expression and proton-gated current density. Among ASIC isoforms in higher vertebrates, the LCRRG motif is unique for ASIC1a/1b, indicating that these residues act as an ASIC1a/1b-specific regulatory motif through recruiting distinctive trafficking machineries. In addition, mutating the KEAKR motif (from KEAKR to AEAAG), located next to the LCRRG motif of ASIC1a (Fig. 1), has similar effects.39 These data suggest that the K/R motif on the C-terminus of ASIC1a plays a critical role in the regulation of channel trafficking. Because the total protein level of ASIC1a in wild-type and RRGK/KEAKR mutants remains unchanged,39 it appears that these mutations do not affect the stability of the protein or protein degradation pathways. The ASIC1a C-terminal juxtamembranous motif is localized for optimal interaction with the membranous protein dynamin and the AP2 complex. It will therefore be interesting to identify the binding partners and elucidate the exact functions of these cytosolic K/R motifs in future studies.
Accessory proteins of ASICs
Several lines of evidence have shown that accessory proteins can regulate the constitutive trafficking of ASICs (summarized in Table 1).30
Table 1.
Accessory proteins that regulate trafficking of ASICs
| Accessory proteins | ASIC isoforms | Interaction sites | Surface expression | Refs |
|---|---|---|---|---|
| Annexin II/p11 | 1a | N-terminus | ↑ by overexpression | 66 |
| AP2μ2 | 1a | C-terminal LCRRG motif | ↑ by knockdown | 23 |
| Dynamin1 | 1a | NR | ↑ by inhibition | 23 |
| Hsc70 | 2a | NR | ↑ by knockdown | 67,68 |
| Lin7b | 3 | C-terminal PDZ-binding motif | ↑ by overexpression | 38 |
| NHERF | 3 | C-terminal PDZ-binding motif | ↑ by overexpression | 69 |
| PICK1 | 1a, 2a | C-terminal PDZ-binding motif | ↑ by overexpression | 9,10,12,13 |
| PSD-95 | 2a, 3 | C-terminal PDZ-binding motif | ↓ by overexpression | 38,70 |
| Stomatin, STOML3 | 1a, 2a, 3 | NR | 1a: NR | |
| 2a, 3: no changes | 71-73 | |||
| SGK1.1 | 1 | NR | ↓ by activation | 52 |
Symbols: ↑, increases; ↓, decreases. Abbreviations: AP2μ2, adaptor protein 2 μ2 subunit; Hsc70, heat shock cognate protein 70; Lin7b, abnormal cell lineage 7b; NHERF, Na+/H+ exchanger regulatory factor 1; PICK1, protein interacting with C-kinase 1; PSD-95, postsynaptic density protein 95; STOML3, stomatin-like protein 3; SGK1.1, serum- and glucocorticoid-induced kinase isoform 1.1; NR, not reported.
Although we are continually adding to our knowledge of ASIC accessory proteins, some questions remain unclear. Further in vivo evidence of the binding and regulatory effects of these accessory proteins on ASICs under various pathophysiological conditions is needed. For example, understanding which interactions happen in vivo, which trafficking processes involved, and what's the pathophysiological consequence will undoubtedly advance our understanding of the pathophysiological role of ASICs.
Regulated Trafficking of ASICs
It is now well documented that membrane proteins, especially ion channels, receptors, and transporters, expressed at the surface, undergo both constitutive and regulated trafficking, which act cooperatively to achieve homeostasis and/or plasticity in response to different environmental changes.40–44 Indeed, dynamic regulation of the rate of either insertion or retrieval (or both) of integral membrane proteins in response to stimuli embodies the strategic regulation of their surface expression. Examples of receptors and transporters whose trafficking is modulated by stimuli or neuronal activity are the G protein-activated inwardly rectifying K+ channels,45,46 dopamine D2 receptors,47 ENaCs,48 cystic fibrosis transmembrane conductance regulator,48 as well as ionotropic ligand-gated receptors such as AMPA receptors, NMDA receptors, GABAA receptors, and the purinergic receptor P2X4.3,49 Interestingly, several studies have demonstrated that regulated trafficking is also commonly used by ASICs for modulation of their physiological function.22,50 In this section, we will discuss the regulatory pathways of ASIC trafficking and its pathophysiological roles, with particular emphasis on ASIC1a.
The insulin pathway
Insulin depletion has been shown to increase ASIC1a surface expression and proton-gated current density, without affecting ASIC2a, in cultured neurons and Chinese hamster ovary cells.50 Cerebral ischemia results in reduced blood flow and delivery of insulin to the damaged brain region, and therefore the effect of insulin on ASIC1a expression observed in vitro is relevant to the pathogenesis of the stroke. However, more evidence is needed to elucidate the underlying mechanism. Insulin activates the insulin receptor (IR) tyrosine kinase, which results in the activation of a variety of signaling pathways, including PI3K/Akt, mitogen-activated protein kinase, and Cbl/CAP pathway.51 Insulin signaling also activates serum- and glucocorticoid-induced kinase 1 (SGK1), and the brain-specific isoform SGK1.1 decreases ASIC1a surface expression and proton-gated current density;52 it would therefore be interesting to investigate whether activation of SGK1.1 facilitates the endocytosis or inhibits the membrane insertion of ASIC1a in the brain. Moreover, the expression level of SGK1.1 is highly associated with neuronal activity,52 and it could therefore provide insights into the neuronal activity-dependent trafficking of ASICs.
The PI3K/Akt pathway
It has long been speculated that the trafficking of ASICs can be regulated under pathological conditions that relate to tissue acidosis, such as ischemic stroke, epileptic seizures and chronic pain. However, evidence shows that acidosis itself has no influence on the trafficking processes of ASICs.50 A possible interplay between ASICs and neurotrophins, or other mediators of pathological development, is worthy of investigation. Our recent work examined the interaction between neurotrophin signaling and ASIC1a channel function, as well as its significance in chronic pain, using both in vitro and in vivo approaches.22 Because ASIC1a is the major component of ASICs and is required for central sensitization and pain hypersensitivity in SDH neurons, we screened several neurotrophins for ASIC1a function, and found that BDNF upregulates the activity of ASIC1a via the PI3K/Akt signaling pathway.22 BDNF and its receptor, TrkB, have been implicated in the development of spinal central sensitization that underlies persistent pain.53,54 We found that BDNF facilitates ASIC1a membrane insertion, a process for which the Ser25 site on the ASIC1a N-terminus is crucial. Blockade of ASIC1a trafficking by peptides that mimic the Ser25 phosphorylation site attenuates pain sensitization.22 Our results provide novel insights into the cellular processes of BDNF/TrkB signaling-mediated central sensitization. Because activity-dependent expression and release of BDNF is essential for synaptic plasticity and fear conditioning in the CNS,55,56 it is possible that BDNF-mediated ASIC1a trafficking also plays a role in fear memory57–59 at the central nucleus where ASIC1a is robustly expressed, for example, at the lateral and basolateral nuclei of the amygdala58,60 and striatum.60,61
Summary and Outlook
Our knowledge on the trafficking of ASICs has expanded in the past few years. However, more studies are needed to unravel the basic cell biological processes of ASICs to understand the pathophysiological role of ASICs. Future directions of ASIC trafficking studies are discussed below.
Imaging the trafficking and pathophysiology of ASICs in vivo. There remains a challenge to elucidate the mechanisms of ASIC trafficking between the plasma membrane and intracellular compartments with excellent spatial and temporal resolutions. To address this issue, more sensitive molecular probes and microscopy methods for ASICs should be developed. For example, pH-sensitive GFP, a superecliptic pHluorin, has been demonstrated to be a powerful probe used to monitor the dynamics of several ion channels, when it is fused to their extracellular domains.62 In combination with total internal reflection fluorescent microscopy, it is possible to visualize the rapid appearance of surface ion channel clusters within a specific region.47,63,64 However, the extracellular domain of ASICs is compact, and it is therefore important to test whether a pHluorin fusion protein would work. The use of an extracellular HA-tag within ASICs would provide a good start for such studies.22,23,65
Identifying novel physiological and pathophysiological mechanisms of ASIC trafficking. Investigations should be undertaken to address whether ASICs have isoform-specific trafficking motifs and related accessory proteins; the role of ASIC1a endocytosis in acidosis-induced neuronal death; and the contribution of ASIC trafficking to synapse development and synaptic transmission.
In summary, to understand the role of ASICs in disease more thoroughly and to explore new clinical treatments, the above issues should be addressed with a combination of traditional and the latest techniques.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by grants from the National Basic Research Program of China and the National Natural Science Foundation of China (Nos. 2014CB910300, 31230028, and 91213306).
References
- 1. Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci 2006; 29:578-86; PMID:16891000; http://dx.doi.org/ 10.1016/j.tins.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 2. Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007; 282:17325-9; PMID:17430882; http://dx.doi.org/ 10.1074/jbc.R700011200 [DOI] [PubMed] [Google Scholar]
- 3. Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 2007; 23:613-43; PMID:17506699; http://dx.doi.org/ 10.1146/annurev.cellbio.23.090506.123516 [DOI] [PubMed] [Google Scholar]
- 4. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 2002; 82:735-67; PMID:12087134 [DOI] [PubMed] [Google Scholar]
- 5. Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr., Ulick S, Milora RV, Findling JW, et al. Liddle's syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 1994; 79:407-14; PMID:7954808; http://dx.doi.org/ 10.1016/0092-8674(94)90250-X [DOI] [PubMed] [Google Scholar]
- 6. Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell 1995; 83:969-78; PMID:8521520; http://dx.doi.org/ 10.1016/0092-8674(95)90212-0 [DOI] [PubMed] [Google Scholar]
- 7. Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC. Identification of a PY motif in the epithelial na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J 1996; 15:2381-7; PMID:8665845 [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J, Xia J. Structure and function of PICK1. Neurosignals 2006; 15:190-201; PMID:17215589; http://dx.doi.org/ 10.1159/000098482 [DOI] [PubMed] [Google Scholar]
- 9. Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase c stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J Biol Chem 2002; 277:50463-8; PMID:12399460; http://dx.doi.org/ 10.1074/jbc.M208848200 [DOI] [PubMed] [Google Scholar]
- 10. Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with c kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem J 2002; 361:443-50; PMID:11802773; http://dx.doi.org/ 10.1042/0264-6021:3610443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duggan A, Garcia-Anoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNac1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem 2002; 277:5203-8; PMID:11739374; http://dx.doi.org/ 10.1074/jbc.M104748200 [DOI] [PubMed] [Google Scholar]
- 12. Hu ZL, Huang C, Fu H, Jin Y, Wu WN, Xiong QJ, Xie N, Long LH, Chen JG, Wang F. Disruption of PICK1 attenuates the function of ASICs and PKC regulation of ASICs. Am J Physiol Cell Physiol 2010; 299:C1355-62; PMID:20826761; http://dx.doi.org/ 10.1152/ajpcell.00569.2009 [DOI] [PubMed] [Google Scholar]
- 13. Jin W, Shen C, Jing L, Zha XM, Xia J. PICK1 regulates the trafficking of ASIC1a and acidotoxicity in a BAR domain lipid binding-dependent manner. Mol Brain 2010; 3:39; PMID:21176140; http://dx.doi.org/ 10.1186/1756-6606-3-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, Caron MG. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 2001; 30:121-34; PMID:11343649; http://dx.doi.org/ 10.1016/S0896-6273(01)00267-7 [DOI] [PubMed] [Google Scholar]
- 15. Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J 2005; 24:3266-78; PMID:16138078; http://dx.doi.org/ 10.1038/sj.emboj.7600801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci 2004; 24:5381-90; PMID:15190111; http://dx.doi.org/ 10.1523/JNEUROSCI.4378-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat Rev Mol Cell Biol 2012; 13:448-62; PMID:22722607; http://dx.doi.org/ 10.1038/nrm3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J Neurosci 2012; 32:4080-91; PMID:22442073; http://dx.doi.org/ 10.1523/JNEUROSCI.5021-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kadurin I, Golubovic A, Leisle L, Schindelin H, Grunder S. Differential effects of N-glycans on surface expression suggest structural differences between the acid-sensing ion channel (ASIC) 1a and ASIC1b. Biochem J 2008; 412:469-75; PMID:18307415; http://dx.doi.org/ 10.1042/BJ20071614 [DOI] [PubMed] [Google Scholar]
- 20. Jing L, Jiang YQ, Jiang Q, Wang B, Chu XP, Zha XM. The interaction between the first transmembrane domain and the thumb of ASIC1a is critical for its N-glycosylation and trafficking. PLoS One 2011; 6:e26909; PMID:22046405; http://dx.doi.org/ 10.1371/journal.pone.0026909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang Y, Yu Y, Cheng J, Liu Y, Liu DS, Wang J, Zhu MX, Wang R, Xu TL. Highly conserved salt bridge stabilizes rigid signal patch at extracellular loop critical for surface expression of acid-sensing ion channels. J Biol Chem 2012; 287:14443-55; PMID:22399291; http://dx.doi.org/ 10.1074/jbc.M111.334250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duan B, Liu DS, Huang Y, Zeng WZ, Wang X, Yu H, Zhu MX, Chen ZY, Xu TL. PI3-kinase/Akt pathway-regulated membrane insertion of acid-sensing ion channel 1a underlies BDNF-induced pain hypersensitivity. J Neurosci. 2012; 32:6351-63; PMID:22553040; http://dx.doi.org/ 10.1523/JNEUROSCI.4479-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng WZ, Liu DS, Duan B, Song XL, Wang X, Wei D, Jiang W, Zhu MX, Li Y, Xu TL. Molecular mechanism of constitutive endocytosis of acid-sensing ion channel 1a and its protective function in acidosis-induced neuronal death. J Neurosci 2013; 33:7066-78; PMID:23595764; http://dx.doi.org/ 10.1523/JNEUROSCI.5206-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma W, Quirion R. Targeting cell surface trafficking of pain-facilitating receptors to treat chronic pain conditions. Expert Opin Ther Targets 2014; 18:459-72; PMID:24512266; http://dx.doi.org/ 10.1517/14728222.2014.887683 [DOI] [PubMed] [Google Scholar]
- 25. Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, et al. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem 2006; 281:14129-35; PMID:16574660; http://dx.doi.org/ 10.1074/jbc.M512511200 [DOI] [PubMed] [Google Scholar]
- 26. Staruschenko A, Pochynyuk O, Stockand JD. Regulation of epithelial Na+ channel activity by conserved serine/threonine switches within sorting signals. J Biol Chem 2005; 280:39161-7; PMID:16203727; http://dx.doi.org/ 10.1074/jbc.M509608200 [DOI] [PubMed] [Google Scholar]
- 27. Kelly BL, Ferreira A. Beta-amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem 2006; 281:28079-89; PMID:16864575; http://dx.doi.org/ 10.1074/jbc.M605081200 [DOI] [PubMed] [Google Scholar]
- 28. Wu Y, Liang S, Oda Y, Ohmori I, Nishiki T, Takei K, Matsui H, Tomizawa K. Truncations of amphiphysin I by calpain inhibit vesicle endocytosis during neural hyperexcitation. EMBO J 2007; 26:2981-90; PMID:17541403; http://dx.doi.org/ 10.1038/sj.emboj.7601741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rudinskiy N, Grishchuk Y, Vaslin A, Puyal J, Delacourte A, Hirling H, Clarke PG, Luthi-Carter R. Calpain hydrolysis of alpha- and beta2-adaptins decreases clathrin-dependent endocytosis and may promote neurodegeneration. J Biol Chem 2009; 284:12447-58; PMID:19240038; http://dx.doi.org/ 10.1074/jbc.M804740200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zha XM. Acid-sensing ion channels: trafficking and synaptic function. Mol Brain 2013; 6:1; PMID:23281934; http://dx.doi.org/ 10.1186/1756-6606-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma D, Jan LY. ER transport signals and trafficking of potassium channels and receptors. Curr Opin Neurobiol 2002; 12:287-92; PMID:12049935; http://dx.doi.org/ 10.1016/S0959-4388(02)00319-7 [DOI] [PubMed] [Google Scholar]
- 32. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 2006; 7:548-62; PMID:16791144; http://dx.doi.org/ 10.1038/nrn1938 [DOI] [PubMed] [Google Scholar]
- 33. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem 2009; 78:857-902; PMID:19317650; http://dx.doi.org/ 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- 34. Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol 2009; 10:583-96; PMID:19696796; http://dx.doi.org/ 10.1038/nrm2751 [DOI] [PubMed] [Google Scholar]
- 35. Grunder S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J 1997; 16:899-907; PMID:9118951; http://dx.doi.org/ 10.1093/emboj/16.5.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grunder S, Jaeger NF, Gautschi I, Schild L, Rossier BC. Identification of a highly conserved sequence at the N-terminus of the epithelial Na+ channel alpha subunit involved in gating. Pflugers Arch 1999; 438:709-15; PMID:10555570; http://dx.doi.org/ 10.1007/s004240051097 [DOI] [PubMed] [Google Scholar]
- 37. Pfister Y, Gautschi I, Takeda AN, van Bemmelen M, Kellenberger S, Schild L. A gating mutation in the internal pore of ASIC1a. J Biol Chem 2006; 281:11787-91; PMID:16497675; http://dx.doi.org/ 10.1074/jbc.M513692200 [DOI] [PubMed] [Google Scholar]
- 38. Hruska-Hageman AM, Benson CJ, Leonard AS, Price MP, Welsh MJ. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+- gated current. J Biol Chem 2004; 279:46962-8; PMID:15317815; http://dx.doi.org/ 10.1074/jbc.M405874200 [DOI] [PubMed] [Google Scholar]
- 39. Jing L, Chu XP, Zha XM. Three distinct motifs within the C-terminus of ASIC1a regulate its surface trafficking. Neuroscience 2013; 247:321-7; PMID:23727453; http://dx.doi.org/ 10.1016/j.neuroscience.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 2000; 28:511-25; PMID:11144360; http://dx.doi.org/ 10.1016/S0896-6273(00)00129-X [DOI] [PubMed] [Google Scholar]
- 41. Martin S, Henley JM. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J 2004; 23:4749-59; PMID:15549132; http://dx.doi.org/ 10.1038/sj.emboj.7600483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang L, Garuti R, Kim BY, Wade JB, Welling PA. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest 2009; 119:3278-89; PMID:19841541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, Takemiya T, Mizoguchi A, Yagita Y, Sakurai T, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron 2007; 56:456-71; PMID:17988630; http://dx.doi.org/ 10.1016/j.neuron.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron 2007; 54:771-85; PMID:17553425; http://dx.doi.org/ 10.1016/j.neuron.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 45. Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci U S A 2009; 106:629-34; PMID:19118198; http://dx.doi.org/ 10.1073/pnas.0811615106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiser O, Qian X, Ehlers M, Ja WW, Roberts RW, Reuveny E, Jan YN, Jan LY. Modulation of basal and receptor-induced GIRK potassium channel activity and neuronal excitability by the mammalian PINS homolog LGN. Neuron 2006; 50:561-73; PMID:16701207; http://dx.doi.org/ 10.1016/j.neuron.2006.03.046 [DOI] [PubMed] [Google Scholar]
- 47. Duan L, Song J, Li X, Yuan H, Li N, Xu Y. Thallium concentrations and sources in the surface sediments of Bohai Bay. Mar Environ Res 2012; 73:25-31; PMID:22088832; http://dx.doi.org/ 10.1016/j.marenvres.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 48. Peters KW, Qi J, Johnson JP, Watkins SC, Frizzell RA. Role of SNARE proteins in CFTR and ENaC trafficking. Pflugers Arch 2001; 443(Suppl 1):S65-9; PMID:11845306; http://dx.doi.org/ 10.1007/s004240100647 [DOI] [PubMed] [Google Scholar]
- 49. Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 2002; 12:279-86; PMID:12049934; http://dx.doi.org/ 10.1016/S0959-4388(02)00329-X [DOI] [PubMed] [Google Scholar]
- 50. Chai S, Li M, Branigan D, Xiong ZG, Simon RP. Activation of acid-sensing ion channel 1a (ASIC1a) by surface trafficking. J Biol Chem 2010; 285:13002-11; PMID:20185828; http://dx.doi.org/ 10.1074/jbc.M109.086041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Virkamaki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest 1999; 103:931-43; PMID:10194465; http://dx.doi.org/ 10.1172/JCI6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arteaga MF, Coric T, Straub C, Canessa CM. A brain-specific SGK1 splice isoform regulates expression of ASIC1 in neurons. Proc Natl Acad Sci U S A 2008; 105:4459-64; PMID:18334630; http://dx.doi.org/ 10.1073/pnas.0800958105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci 2006; 29:507-38; PMID:16776595; http://dx.doi.org/ 10.1146/annurev.neuro.29.051605.112929 [DOI] [PubMed] [Google Scholar]
- 54. Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol 2008; 85:297-317; PMID:18514997; http://dx.doi.org/ 10.1016/j.pneurobio.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 55. Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J Neurosci 2009; 29:12764-7; PMID:19828787; http://dx.doi.org/ 10.1523/JNEUROSCI.3566-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci 2007; 10:1089-93; PMID:17726474; http://dx.doi.org/ 10.1038/nn1971 [DOI] [PubMed] [Google Scholar]
- 57. Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr., Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 2002; 34:463-77; PMID:11988176; http://dx.doi.org/ 10.1016/S0896-6273(02)00661-X [DOI] [PubMed] [Google Scholar]
- 58. Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 2009; 139:1012-21; PMID:19945383; http://dx.doi.org/ 10.1016/j.cell.2009.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A 2004; 101:3621-26; PMID:14988500; http://dx.doi.org/ 10.1073/pnas.0308753101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr., Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci 2003; 23:5496-502; PMID:12843249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience 2009; 162:55-66; PMID:19376200; http://dx.doi.org/ 10.1016/j.neuroscience.2009.04.029 [DOI] [PubMed] [Google Scholar]
- 62. Ashby MC, Ibaraki K, Henley JM. It's green outside: tracking cell surface proteins with pH-sensitive gfp. Trends Neurosci 2004; 27:257-61; PMID:15111007; http://dx.doi.org/ 10.1016/j.tins.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 63. Li Y, Roy BD, Wang W, Zhang L, Sampson SB, Lin DT. Imaging pHluorin-tagged receptor insertion to the plasma membrane in primary cultured mouse neurons. J Vis Exp 2012; pii: 4450; PMID:23208071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci 2009; 12:879-87; PMID:19503082; http://dx.doi.org/ 10.1038/nn.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen X, Grunder S. Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a. J Physiol 2007; 579:657-70; PMID:17204502; http://dx.doi.org/ 10.1113/jphysiol.2006.120733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Donier E, Rugiero F, Okuse K, Wood JN. Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASIC1a. J Biol Chem 2005; 280:38666-72; PMID:16169854; http://dx.doi.org/ 10.1074/jbc.M505981200 [DOI] [PubMed] [Google Scholar]
- 67. Grifoni SC, McKey SE, Drummond HA. Hsc70 regulates cell surface ASIC2 expression and vascular smooth muscle cell migration. Am J Physiol Heart Circ Physiol 2008; 294:H2022-30; PMID:18310515; http://dx.doi.org/ 10.1152/ajpheart.01271.2007 [DOI] [PubMed] [Google Scholar]
- 68. Vila-Carriles WH, Zhou ZH, Bubien JK, Fuller CM, Benos DJ. Participation of the chaperone Hsc70 in the trafficking and functional expression of ASIC2 in glioma cells. J Biol Chem 2007; 282:34381-91; PMID:17878160; http://dx.doi.org/ 10.1074/jbc.M705354200 [DOI] [PubMed] [Google Scholar]
- 69. Deval E, Friend V, Thirant C, Salinas M, Jodar M, Lazdunski M, Lingueglia E. Regulation of sensory neuron-specific acid-sensing ion channel 3 by the adaptor protein Na+/H+ exchanger regulatory factor-1. J Biol Chem 2006; 281:1796-807; PMID:16234233; http://dx.doi.org/ 10.1074/jbc.M509669200 [DOI] [PubMed] [Google Scholar]
- 70. Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci 2009; 29:8438-46; PMID:19571134; http://dx.doi.org/ 10.1523/JNEUROSCI.1284-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Price MP, Thompson RJ, Eshcol JO, Wemmie JA, Benson CJ. Stomatin modulates gating of acid-sensing ion channels. J Biol Chem 2004; 279:53886-91; PMID:15471860; http://dx.doi.org/ 10.1074/jbc.M407708200 [DOI] [PubMed] [Google Scholar]
- 72. Lapatsina L, Jira JA, Smith ES, Poole K, Kozlenkov A, Bilbao D, Lewin GR, Heppenstall PA. Regulation of ASIC channels by a stomatin/STOML3 complex located in a mobile vesicle pool in sensory neurons. Open Biol 2012; 2:120096; PMID:22773952; http://dx.doi.org/ 10.1098/rsob.120096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature 2007; 445:206-9; PMID:17167420; http://dx.doi.org/ 10.1038/nature05394 [DOI] [PubMed] [Google Scholar]


