Abstract
Although fibrosis is becoming increasingly recognized as a major cause of morbidity and mortality in chronic inflammatory diseases, available treatment strategies are limited. Tenascins constitute a family of matricellular proteins, primarily modulating interactions of cells with other matrix components and growth factors. Data obtained from tenascin C deficient mice show important roles of this molecule in several models of fibrosis. Moreover there is growing evidence that tenascin C has a strong impact on chronic inflammation, myofibroblast differentiation and recruitment. Tenascin C as well as tenascin X has furthermore been shown to affect TGF-β activation and signaling. Taken together these data suggest that these proteins might be important factors in fibrosis development and make them attractive both as biological markers and as targets for therapeutical intervention. So far most clinical research in fibrosis has been focused on tenascin C. This review aims at summarizing our up-to-date knowledge on the involvement of tenascin C in the pathogenesis of fibrotic disorders.
Keywords: biomarker, extracellular matrix remodeling, fibrosis, inflammation, tenascin
Abbreviations
- MAP—kinases
Mitogen-activated protein kinases
- PI3K
Phosphatidylinositol-4, 5-bisphosphate 3-kinase
- PKB
protein kinase B
Introduction
There is growing recognition in medical and scientific communities that fibrosis, defined as the accumulation of excess extracellular matrix components, is one of major causes of morbidity and mortality in most chronic inflammatory diseases. In normal wound healing process, usually reversible, collagen deposition is an essential and beneficial part of wound healing process. When the wound healing process becomes dysregulated, uncontrolled accumulation of matrix components may lead to organ malfunction and death as seen in end-stage liver disease, kidney disease, idiopathic pulmonary fibrosis (IPF), and heart failure. Fibrosis is also observed in many chronic autoimmune diseases, including scleroderma, rheumatoid arthritis, Crohn's disease, ulcerative colitis, myelofibrosis and systemic lupus erythematosus. Despite progress in understanding the mechanisms of fibrosis, treatment strategies specifically targeting its pathogenesis are scarce.1
Tenascins—a family of large oligomeric extracellular matrix (ECM) glycoproteins consists of 4 members: tenascin C, R, X, and W2 sharing similar structure but having different time- and tissue-specific expression patterns.3,4 Although present in ECM, tenascins have rather a signaling than structural role and mostly affect the interactions of cells with other ECM components and growth factors in a cell-type- and context- dependent manner.2
This review focuses on 2 of them, tenascin C and X, as they had been described in the context of tissue remodeling. Tenascin C (TNC) is expressed during organ development but its expression in adult tissue is highly restricted to tissues exposed to high tensile stress or to high cell turnover. It has been demonstrated that de novo expression of tenascin C in the adult is usually associated with injury or cancer.2-5 In contrast to tenascin C, tenascin X expression remains high after birth6 and mutations in this protein cause Ehlers Danlos syndrome associated with mild myopathy.7
Lessons from Animal Models
Growing evidence from animal studies suggests that tenascins, particularly tenascin C, are crucial to the development of fibrosis (see Table 1). For example, the contribution of tenascin C to liver fibrogenesis was demonstrated by El-Karef and colleagues in the model of immune-mediated hepatitis, induced by intravenous injections of concanavalin A. Collagen deposition and procollagen I and III transcripts levels were significantly lower in tenasin C deficient (TNKO) mice than in wild type (WT) littermates. Inflammation, measured by the prominence of inflammatory infiltrates and levels of proinflammatory cytokines mRNA (interferon-γ, tumor necrosis factor-α, and interleukin-4), were higher in WT mice than in TNKO mice, as was the presence of activated hepatic stellate cells (HSCs) and myofibroblasts. Moreover, transforming growth factor (TGF)-β1 mRNA expression was significantly upregulated in WT mice, but not in TNKO mice. It was concluded that tenascin C can promote liver fibrogenesis through enhancement of the inflammatory response by cytokine upregulation, HSC recruitment, and TGF-β expression during progression of hepatitis to fibrosis.8
Table 1.
Selected animal studies examining the role of TNC in models relevant to fibrotic diseases
| Organ | Species | Strain / background | Model | Use of knockout animals | Findings |
|---|---|---|---|---|---|
| Cornea | Mouse | C57BL/6 | Incision injury | Yes | Delayed wound healing in TNKO mice with less myofibroblats, reduction in expression of collagen 1α1, fibronectin, TGFβ1 63 |
| Heart | Rat | Lewis | Immunization with cardiac C-protein fragments 2 and complete Freund's adjuvant, followed by intraperitoneal injection of pertussis toxin | No | Increase of TNC in experimental autoimmune myocarditis 64 |
| Heart | Mouse | BALB/c | Infusion of angiotensin II | No | Increased TNC expression upon myocardial fibrosis 65 |
| Heart | Mouse | BALB/c | Myocardial injury by an electric pulse | Yes | Delayed recruitment of myofibroblasts in TNKO mice 12 |
| Heart | Mouse | BALB/c | Ligation of coronary arteries | Yes | Less fibrosis in TNKO mice 66 |
| Joints | Mouse | 129/sv | Zymosan-induced inflammation | Yes | Rapid resolution of acute inflammation in TNKO mice 13 |
| Kidney | Rat | Wistar | Diabetic nephropathy induced by high-carbohydrate-fat food and injection of streptozotocin | No | TNC increased in diabetic nephropathy model. Deferiprone anti-fibrotic effect is accompanied by decrease of TNC expression 67 |
| Lens | Mouse | C57BL/6 | Injury by needle puncture | Yes | Attenuated EMT in TNKO mice 68 |
| Liver | Mouse | BALB/c | Immune-mediated chronic hepatitis induced by concanavalin A injections | Yes | Attenuated fibrosis in TNKO mice 8 |
| Liver | Rat | Wistar | Thioacetamide-induced liver cirrhosis Fibrosis after bile duct ligation | No | TNC expressed in most areas of the chronically injured livers up to 3 and 6 months in bile duct-ligated and chemically-injured livers, respectively 69 |
| Lung | Mouse | C57BL/6 | Bleomycin-induced fibrosis | No | TNC expression is increased in fibrotic tissue and significantly correlates with de novo synthesized collagen70,71 |
| Lung | Mouse | 129/sv | Bleomycin-induced fibrosis | Yes | Ameliorated fibrosis and reduced Smad-3 protein levels in TNKO mice 10 |
| Lung | Rat | Sprague-Dawley | Bleomycin-induced fibrosis | No | Induction of TNC upon fibrosis 9 |
| Skin | Rat | Sprague-Dawley | Healing skin wounds | No | TNC expressed during wound healing but not present in scars 72 |
| Skin | Pig | Exposure to radiation | No | TNC expressed in fibrotic tissue 73 | |
| Skin | Mouse | Swiss | Pressure ulcer formation caused by ischemia–reperfusion injury induced by external application of magnetic plates | No | Enhanced TNC and reduced collagen deposition following propranolol administration 74 |
| Skin | Mouse | MLR/MpJ | Full-thickness excisional skin wound | No | TNC expressed by blastemal cells 75 |
| Skin | Mouse | BALB/c | Dermatitis induced by application of hapten to the ear skin | Yes | More severe dermatitis in TNKO mice 76 |
In the model of acute lung injury (ALI) induced by intratracheal bleomycin instillation, tenascin C was greatly induced, primarily during the early inflammatory phase. A patchy distribution of tenascin C protein was found in alveolar septal walls and secondary septal tips in the areas of damaged tissues.9 Mice lacking tenascin C are protected from interstitial fibrosis in this model, because 3 weeks after exposure to bleomycin, TNKO mice had accumulated 85% less lung collagen than wild-type mice. The lung interstitium of TNKO mice also appeared to contain fewer myofibroblasts and fewer cells with intranuclear Smad-2/3 staining, suggesting impaired TGF-activation or signaling.10
Moreover, tenascin C was expressed during the acute stage in a rat model of myocardial infarction. Additionally, smooth muscle actin (SMA)-positive myofibroblasts appeared in tenascin C positive areas.11 Further studies using TNKO mice revealed that tenascin C controls the dynamics of myofibroblast recruitment after electrical injury to the myocardium. Although myocardial repair seemed to proceed normally in TNKO mice, the appearance of myofibroblasts was delayed.12
Although data obtained from knock-out animals should be treated with caution due to fact that some effects might be masked by adaptive response to transgene and even some phenotypes might be caused by adaptive mechanisms themselves, we demonstrate in this review that these observations are consistent with evidence obtained both from in vitro experiments and clinic.
From Bench: How can Tenascins Influence Fibrosis?
As presented above, studies in various animal models of fibrosis or tissue repair show an important role of tenascin in the outcome of these disorders. It is therefore valid to ask what is known about the cellular and molecular mechanisms of tenascins and their influence on fibrotic processes (see Fig. 1).
Figure 1.
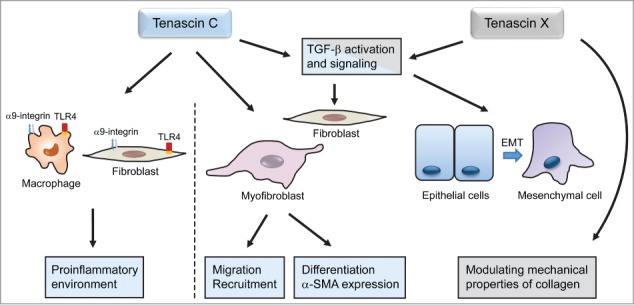
Cellular and molecular mechanisms by which Tenascin C and X influence fibrosis. Tenascin C participates in maintaining a proinflammatory environment and increases migration of fibroblasts and myofibroblasts. Due to their effects on TGF-β signaling pathway, tenascin C and X influence fibroblast and epithelial cells differentiation into myofibroblasts. Tenascin X modulates mechanical properties of collagen.
Tenascins in chronic inflammation
Fibrosis is often a consequence of chronic/unresolved inflammation. Midwood and coworkers reported that tenascin C acts as an endogenous activator of TLR4-mediated immunity mediating persistent synovial inflammation and tissue destruction in arthritic joint disease.13 In vitro, TLR4 ligation to the fibrinogen-like (FBG) domain of tenascin C at the C-terminus of the molecule stimulated synthesis of TNF-α, IL-6 and IL-8 in primary human macrophages and IL-6 in synovial fibroblasts. Interestingly, tenascin C does not influence the initiation of joint inflammation but is required for its maintenance, perhaps reflecting that it is absent from healthy tissue and needs induction by inflammatory mediators.14 In addition to acting as a TLR4 activator, tenascin C can also stimulate cytokine synthesis in murine synovial fibroblasts via activation of α9-integrins.15
Tenascin C expression in adult tissue is usually associated with ongoing inflammation for example it is rapidly and transiently induced in myeloid immune cells in response to tissue injury and infection. It therefore appears that induction of tenascin C in an inflammatory setting would drive TLR4 activation leading to synthesis of more tenascin C, perhaps resulting in a nonresolving loop of chronic inflammation.14
Moreover, tenascin C appears to be involved in regulation of lymphocyte migration as it supports adhesion and rolling of primary human peripheral blood and tonsillar lymphocytes.16 Furthermore, tenascin C may be involved in lymphocyte activation, although both stimulatory and inhibitory effects have been reported. Tenascin C significantly stimulates the secretion of IL-5, IL-13, IFN-γ and immunoglobulin-E from spleen lymphocytes.17 However, it has an inhibitory effect on the anti-CD3-induced activation of human peripheral blood T cells.18,19
Tenascins and myofibroblasts
Myofibroblasts are contractile cells expressing α-smooth muscle actin (α-SMA). They play a crucial role in physiologic wound healing as well as in profibrotic processes by synthesizing collagens and exerting strong contraction forces to minimize wound areas (for a comprehensive review of myofibroblast differentiation and functions see ref.20) Their recruitment is thought to be mediated by cellular damage and the release of inflammatory mediators including TLR agonists.21 Tamaoki and coworkers observed that although myocardial repair appeared to proceed normally in tenascin C-null mice, the appearance of myofibroblasts was delayed.12 Moreover, cardiac fibroblasts from TNKO mice showed lower cell migration and α-SMA expression than WT fibroblasts. Both cell migration and α-SMA expression could be recovered by exposing the fibroblasts to exogenous tenascin C. Interestingly, the different tenascin C domains were mapped as responsible for inducing myofibroblast differentiation and migration: alternatively spliced FNIII repeats and the FBG domain are responsible for myofibroblast differentiation while the molecular signal that promoted migration of cardiac fibroblasts was mapped to the domain of conserved FNIII repeats and the FBG domain.12 Full-length tenascin C was also shown to promote fibroblast migration within fibrin-fibronectin matrices. Noteworthy, in opposition to full length molecule, specific fragments of tenascin C have an inhibitory effect on the process.22
Tenascins and TGF-β activation and signaling
The differentiation of fibroblasts into collagen-secreting myofibroblasts can be directly induced by transforming growth factor (TGF-β)23—one of the key drivers of fibrosis. TGF-β1 production correlates with the progression of liver, lung, kidney, skin and cardiac fibrosis, and inhibition of its signaling pathway has been shown to reduce the development of fibrosis in many experimental models.1 As an example, overexpression of TGF-β1 by renal tubular epithelial cells results in tubulointerstitial fibrosis in the absence of any injury and, conversely, a blocking antibody to TGF-β ameliorates interstitial matrix accumulation in the fibrosis model of unilateral ureteral obstruction (UUO).24 Likewise, overexpression of Smad7, an inhibitory factor in the TGF-β signaling pathway, or genetic deletion of the agonistic signaling molecule Smad3, reduced renal fibrosis in UUO.25
TGF-β family members (TGF-β1, 2, and 3) are synthesized as pro-proteins that are proteolytically processed before secretion. Mature TGF-β remains inactive and noncovalently associated with latency-associated peptide (LAP) in a small latent complex (SLC). Further binding of LAPs to the latent TGF-β–binding proteins (LTBPs) form large latent complexes (LLCs) and allows incorporation of the different latent TGF-β isoforms into extracellular matrices via the LTBPs binding to ECM proteins including fibrillins and fibronectin.26 Activation of the latent TGF complex—a crucial step in the regulation of TGF-β function can be mediated by proteolytic cleavage of the LAP and release of TGF-β and/or by a conformational change in the LAP. Activation of TGF-β might involve either various cell surface receptors, such as RGD-dependent integrins, or the ECM protein thrombospondin 1.27
Alcaraz and colleagues have shown that tenascin X activates the latent TGF-β into an active molecule, most likely through a conformational change in the latent complex. Authors demonstrated that fibrinogen-like (FBG) domain of tenascin X physically interacts with the small latent TGF-β complex in vitro and in vivo and is crucial for the cytokine activation. Moreover, α11β1 integrin has been identify as a cell surface receptor for tenascin X.27
Active TGF-β transduces its signal from cell surface to nucleus via the canonical Smad-dependent pathway or the non-canonical pathways including the MAP kinase pathway or PI3K/Akt/PKB kinase pathway. Upon TGF-β binding active receptor I and II complex is formed on the cell surface and phosphorylates the serine residues at SSXS motif of cytoplasmic Smad2 and Smad3. The phosphorylated active Smad2/Smad3 heterodimerize with co-Smad Smad4 and translocate to the nucleus where Smads interact with Smad binding element (SBE) and also recruit p300 to the transcriptional complex of the target gene.28
Interestingly, Carey and colleagues observed that the lung interstitium of TNKO mice treated with bleomycin appeared to contain fewer myofibroblasts and fewer cells with intranuclear Smad-2/3 staining, suggesting impaired TGF-β activation or signaling. In vitro, TGF-β response in TNKO lung fibroblasts was significantly decreased. Impaired TGF-β responsiveness was correlated with dramatically reduced Smad-3 protein levels and diminished nuclear translocation of Smad-2 and Smad-3 in TGF-β-exposed TNKO cells. Reduced Smad-3 in TNKO cells was due to both decreased transcription and enhanced ubiquitin-proteasome mediated protein degradation.10
Tenascins and collagen synthesis
A crucial role for tenascin X in collagen biology is suggested by the fact that its deficiency is associated with Ehlers-Danlos syndrome in humans.29 Major clinical symptoms consist of skin hyperextensibility and joint laxity, while ultrastructural analyses reveal abnormalities in collagen fibril networks and elastic fiber morphology. Mice deficient in tenascin X partly reproduced this phenotype.30 In vitro studies revealed that tenascin X interacts with fibrillar collagen type I, III and V when they are in native conformation.31 Although the presence of tenascin X does not significantly influence the main parameters of fibrillogenesis and diameter of fibrils, mechanical analysis of collagen gels showed an increased compressive resistance of the gels containing tenascin X, indicating that this protein might be directly involved in determining the mechanical properties of collagen-rich tissues in vivo.32
To Bedside: Tenascin C in Human Fibrotic Disorders
There is growing interest in tenascins in the bio-medical field including studies on wound healing and fibrotic disorders. So far the best studied member of the family is tenascin C. Tenascin C expression is increased in inflammatory and fibrotic diseases in various organ systems including the lung/pleura, liver, cardiovascular system, intestine and skin (see Table 2).
Table 2.
Studies examining the expression of TNC in specimens taken from patients with a variety of fibrotic diseases
| Number of cases/controls | Organ | Material | Cases | Controls | Findings |
|---|---|---|---|---|---|
| 51 | Lung | Biopsy | Patients with UIP, DIP, sarcoidosis, BOOP or allergic alveolitis | Increased TNC in all types, especially in UIP. In patients with UIP, increased TNC was associated with shortened survival time. 34 | |
| 15/6 | Lung | Biopsy | Patients with interstitial lung disease (UIP, NSIP, COP) | Normal lung tissue from cancer resections | Increase of TNC in UIP, NSIP and COP. 36 |
| 71/5 | Lung/pleura | Biopsy | Patients with pleural inflammatory and fibrotic diseases | Pleural tissue from patients undergone surgery for lung carcinoma | Increase of TNC in areas with myofibroblasts.35 |
| 44/23 | Lung | Serum | Patients with COP, IPF, NSIP | Healthy volunteers | Serum TNC elevated in patients with COP. 39 |
| 31/15 | Lung | Serum/ BALF | Patients with sarcoidosis | Healthy volunteers | TNC levels in BALF bur not serum correlated with pulmonary infiltrates. 40 |
| 62/10 | Lung | Serum | Patients with systemic sclerosis | Healthy volunteers | Increased TNC in sera in SSc patients with pulmonary fibrosis. 37 |
| 22/6 | Lung | BALF | Patients with UIP, sarcoidosis, allergic alveolitis | Increase of TNC in patients with UIP, sarcoidosis and allergic alveolitis. 38 | |
| 192/328 | Colon | Biopsy | Collagenous colitis, pronounced and minimal | Normal mucosa, biopsies from IBD patients, infectious colitis, lymphocytic colitis, pseudomembranous colitis, ischemic colitis | Increased subepithelial expression of TNC is highly specific for collagenous colitis. 41 |
| 47 | Liver | Serum | Children with serologically and biopsy-verified chronic hepatitis B | Serum TNC decreased significantly during interferon treatment. 42 | |
| 247 | Liver | Serum | Histologically verified precirrhotic liver fibrosis and history of heavy alcohol consumption | TNC significantly correlated to the stage of fibrosis at baseline but not with change over time. 43 | |
| 54/176 | Kidney | Serum | Patients with different types of glomerulonephritis | Healthy blood bank donors | Circulating levels of TNC moderately higher in patients with chronic renal disease. 56 |
| 4/4 | Skin | Biopsy | Keloid | Normal | TNC expression was increased in keloids compared to normal skin in biopsy specimens and in keloidal fibroblasts compared with normal fibroblasts in vitro. 59 |
| 35/10 | Cornea | Biopsy | Patients undergone penetrating keratoplasty | Normeal corneas from globes enucleated for choroidal melanoma | TNC increased in inflammation. 60 |
| 40/18 | Cornea | Biopsy | Patients undergone penetrating keratoplasty | Autopsy corneas | TNC increased in corneas affected by bullous keratopathy. 61 |
| 80/15 | Systemic collagen disease | Serum | Patients with various systemic collagen diseases | Healthy volunteers | Serum TNC elevated in patients with SSc, SSD and LSc. 62 |
| Cardiovascular disease with fibrotic component | Pulmonary arterial hypertension, stenosis/restenosis, aortic aneurysm, ischemic or dilated cardiomyopathy, calcified heart valves, myocardial infarction. | Increased expression of TNC in areas with proliferating myofibroblasts reviewed by refs. 44,45 | |||
Abbreviations: UIP usual interstitial pneumonia, DIP, desquamative interstitial pneumonia, BOOP bronchiolitis obliterans organizing pneumonia, NSIP non-specific interstitial pneumonia, COP cryptogenic organizing pneumonia, IPF idiopathic pulmonary fibrosis, BALF bronchoalveolar lavage fluid, IBD inflammatory bowel disease, SSc systemic sclerosis, SSD scleroderma spectrum disorder, LSc localized scleroderma
In human lung and pleural disease, high tissue levels of tenascin C has been reported in diseases such as usual interstitial pneumonia (UIP)/idiopathic pulmonary fibrosis (IPF), non-specific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia (COP), asbestos-induced reactions, postcardiac injury syndrome, parapneumonic infection and/or empyema, tuberculosis, systemic sclerosis-associated pulmonary fibrosis and other rheumatoid diseases.33-37 Tenascin C concentration is furthermore increased in serum and/or epithelial lining fluid of patients with usual interstitial pneumonia, sarcoidosis, extrinsic allergic alveolitis, cryptogenic organizing pneumonia and systemic sclerosis-associated pulmonary fibrosis.37-40 Increased tissue expression, especially beneath metaplastic bronchiolar-type epithelium has been associated with a shortened survival time in patients with usual interstitial pneumonia.34
In the gastrointestinal tract, the utility of tenascin C staining in the diagnosis of minimal collagenous colitis has been suggested.41 Collagenous colitis is a subgroup of microscopic colitis that causes watery diarrhea. Biopsy specimens of collagenous colitis, other forms of colitis and normal mucosa were analyzed by tenascin C immunostaining and compared to conventional histological and histochemical detection. Selective subepithelial expression of tenascin-C was found to be highly specific and sensitive for collagenous colitis, especially in minimal collagenous colitis.
In the liver, the usefulness of tenascin C and 3 other matrix-derived proteins as serum markers of fibrosis in children with chronic hepatitis B was investigated.42 During interferon treatment, tenascin C was significantly decreased in the whole group and in nonresponders, but there were no significant differences in mean serum levels of tenascin between children with mild and advanced liver fibrosis or with mild and severe hepatic inflammation. Another study43 showed a significant correlation of serum levels of tenascin C with the stage of fibrosis in patients with precirrhotic alcoholic liver disease. However, baseline levels of tenascin C were not significantly correlated to change in histological stage of fibrosis over 24 months.
There are numerous studies investigating tenascin C in relation to cardiovascular disease reviewed in refs.44,45 To mention some, tenascin C levels are increased in ischemic or dilated cardiomyopathy46,47, calcified heart valves48, after myocardial infarction49, in vein grafts50, in coronary and carotid atheromas51-52, in pulmonary arteries with pulmonary arterial hypertension53 and in aortic aneurysms.54,55
In patients with chronic renal disease tenascin C was elevated in serum and urine, and increased with progressive reduction in renal function, but was unrelated to proteinuria.56
In skin, transient tenascin C expression is seen during normal wound healing after excisional wounds and punch biopsies.57,58 However in keloids, representing a pathological fibrotic response in skin, increased expression of tenascin C was sustained years after onset of disease. Tenascin C expression was also higher in cultured fibroblasts isolated from keloid lesions compared to fibroblasts from normal skin.59 In cornea, tenascin C is induced in regions of inflammation, fibrosis and neovascularisation but absent in mature, avascular scar tissue.60 Expression of tenascin C in corneas from patients with bullous keratopathy was higher than in normal corneas.61 Bullous keratopathy is a disorder characterized by endothelial dysfunction leading to bullae formation and subsequent stromal scar formation.
Lastly, tenascin C is elevated in collagen diseases which are disorders characterized by inflammation, autoimmune attack and vascular damage, often leading to fibrosis. Serum levels of tenascin C was elevated in patients with systemic sclerosis, scleroderma spectrum disorder and localized scleroderma compared to normal controls.62 The percentage of diffuse cutaneous systemic sclerosis, severity of skin thickness and the incidence of pulmonary fibrosis or pitting scar/ulcers were higher in patients with elevated tenascin C levels than in those without.
In summary, data from human studies has shown increased levels of tenascin C in tissue, serum and urine in several inflammatory and fibrotic diseases. However, studies on the therapeutic and diagnostic value of tenascin C are few. Perhaps the usefulness as a serological biomarker is somewhat limited by the fact that tenascin C more reflect activity of a disease but is unspecific in terms of etiology. Further studies involving also other members of family, for example tenascin X, are needed to evaluate possible potential of tenascins as diagnostic markers and therapeutic targets in fibrotic diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by grants from the South-Eastern Norway Regional Health Authority to CH and MK.
References
- 1. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012; 18:1028-40; PMID:22772564; http://dx.doi.org/ 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. Tenascins in stem cell niches. Matrix Biol 2014; 37:112-23; PMID:24472737. [DOI] [PubMed] [Google Scholar]
- 3. Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol 2003; 200:488-99; PMID:12845616; http://dx.doi.org/ 10.1002/path.1415 [DOI] [PubMed] [Google Scholar]
- 4. Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol 2011; 3; PMID:21441591; http://dx.doi.org/ 10.1101/cshperspect.a004960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sato I, Shimada K. Quantitative analysis of tenascin in chordae tendineae of human left ventricular papillary muscle with aging. Ann Anat 2001; 183:443-8; PMID:11677810; http://dx.doi.org/ 10.1016/S0940-9602(01)80202-8 [DOI] [PubMed] [Google Scholar]
- 6. Geffrotin C, Garrido JJ, Tremet L, Vaiman M. Distinct tissue distribution in pigs of tenascin-X and tenascin-C transcripts. Eur J Biochem 1995; 231:83-92; PMID:7543048; http://dx.doi.org/ 10.1111/j.1432-1033.1995.0083f.x [DOI] [PubMed] [Google Scholar]
- 7. Voermans NC, Verrijp K, Eshuis L, Balemans MC, Egging D, Sterrenburg E, van Rooij IA, van der Laak JA, Schalkwijk J, van der Maarel SM, et al. . Mild muscular features in tenascin-X knockout mice, a model of Ehlers-danlos syndrome. Connect Tissue Res 2011; 52:422-32; PMID:21405982; http://dx.doi.org/ 10.3109/03008207.2010.551616 [DOI] [PubMed] [Google Scholar]
- 8. El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol 2007; 211:86-94; PMID:17121418; http://dx.doi.org/ 10.1002/path.2099 [DOI] [PubMed] [Google Scholar]
- 9. Zhao Y, Young SL, McIntosh JC. Induction of tenascin in rat lungs undergoing bleomycin-induced pulmonary fibrosis. Am J Physiol 1998; 274:L1049-57; PMID:9609745 [DOI] [PubMed] [Google Scholar]
- 10. Carey WA, Taylor GD, Dean WB, Bristow JD. Tenascin-C deficiency attenuates TGF-ss-mediated fibrosis following murine lung injury. Am J Physiol Lung Cell Mol Physiol 2010; 299:L785-93; PMID:20833777; http://dx.doi.org/ 10.1152/ajplung.00385.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imanaka-Yoshida K, Hiroe M, Nishikawa T, Ishiyama S, Shimojo T, Ohta Y, Sakakura T, Yoshida T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab Invest 2001; 81:1015-24; PMID:11454990; http://dx.doi.org/ 10.1038/labinvest.3780313 [DOI] [PubMed] [Google Scholar]
- 12. Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol 2005; 167:71-80; PMID:15972953; http://dx.doi.org/ 10.1016/S0002-9440(10)62954-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, et al. . Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009; 15:774-80; PMID:19561617; http://dx.doi.org/ 10.1038/nm.1987 [DOI] [PubMed] [Google Scholar]
- 14. Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol 2010; 184:2655-62; PMID:20107185; http://dx.doi.org/ 10.4049/jimmunol.0903359 [DOI] [PubMed] [Google Scholar]
- 15. Kanayama M, Kurotaki D, Morimoto J, Asano T, Matsui Y, Nakayama Y, Saito Y, Ito K, Kimura C, Iwasaki N, et al. . Alpha9 integrin and its ligands constitute critical joint microenvironments for development of autoimmune arthritis. J Immunol 2009; 182:8015-25; PMID:19494327; http://dx.doi.org/ 10.4049/jimmunol.0900725 [DOI] [PubMed] [Google Scholar]
- 16. Clark RA, Erickson HP, Springer TA. Tenascin supports lymphocyte rolling. J Cell Biol 1997; 137:755-65; PMID:9151679; http://dx.doi.org/ 10.1083/jcb.137.3.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakahara H, Gabazza EC, Fujimoto H, Nishii Y, D'Alessandro-Gabazza CN, Bruno NE, Takagi T, Hayashi T, Maruyama J, Maruyama K, et al. . Deficiency of tenascin C attenuates allergen-induced bronchial asthma in the mouse. Eur J Immunol 2006; 36:3334-45; PMID:17125141; http://dx.doi.org/ 10.1002/eji.200636271 [DOI] [PubMed] [Google Scholar]
- 18. Hemesath TJ, Marton LS, Stefansson K. Inhibition of T cell activation by the extracellular matrix protein tenascin. J Immunol 1994; 152:5199-207; PMID:7514630 [PubMed] [Google Scholar]
- 19. Hibino S, Kato K, Kudoh S, Yagita H, Okumura K. Tenascin suppresses CD3-mediated T cell activation. Biochem Biophys Res Commun 1998; 250:119-24; PMID:9735343; http://dx.doi.org/ 10.1006/bbrc.1998.9258 [DOI] [PubMed] [Google Scholar]
- 20. Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech 2010; 43:146-55; PMID:19800625; http://dx.doi.org/ 10.1016/j.jbiomech.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 21. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214:199-210; PMID:18161745; http://dx.doi.org/ 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans 2007; 35:695-7; PMID:17635125; http://dx.doi.org/ 10.1042/BST0350695 [DOI] [PubMed] [Google Scholar]
- 23. Kaarteenaho-Wiik R, Paakko P, Sormunen R. Ultrastructural features of lung fibroblast differentiation into myofibroblasts. Ultrastruct Pathol 2009; 33:6-15; PMID:19191196; http://dx.doi.org/ 10.1080/01913120802608430 [DOI] [PubMed] [Google Scholar]
- 24. Gewin L, Zent R. How does TGF-beta mediate tubulointerstitial fibrosis? Semin Nephrol 2012; 32:228-35; PMID:22835453; http://dx.doi.org/ 10.1016/j.semnephrol.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boor P, Sebekova K, Ostendorf T, Floege J. Treatment targets in renal fibrosis. Nephrol Dial Transplant 2007; 22:3391-407; PMID:17890247; http://dx.doi.org/ 10.1093/ndt/gfm393 [DOI] [PubMed] [Google Scholar]
- 26. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009; 326:1216-9; PMID:19965464; http://dx.doi.org/ 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alcaraz LB, Exposito JY, Chuvin N, Pommier RM, Cluzel C, Martel S, Sentis S, Bartholin L, Lethias C, Valcourt U. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J Cell Biol 2014; 205:409-28; PMID:24821840; http://dx.doi.org/ 10.1083/jcb.201308031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003; 425:577-84; PMID:14534577; http://dx.doi.org/ 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- 29. Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet 1997; 17:104-8; PMID:9288108; http://dx.doi.org/ 10.1038/ng0997-104 [DOI] [PubMed] [Google Scholar]
- 30. Mao JR, Taylor G, Dean WB, Wagner DR, Afzal V, Lotz JC, Rubin EM, Bristow J. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat Genet 2002; 30:421-5; PMID:11925569; http://dx.doi.org/ 10.1038/ng850 [DOI] [PubMed] [Google Scholar]
- 31. Lethias C, Carisey A, Comte J, Cluzel C, Exposito JY. A model of tenascin-X integration within the collagenous network. FEBS Lett 2006; 580:6281-5; PMID:17078949; http://dx.doi.org/ 10.1016/j.febslet.2006.10.037 [DOI] [PubMed] [Google Scholar]
- 32. Margaron Y, Bostan L, Exposito JY, Malbouyres M, Trunfio-Sfarghiu AM, Berthier Y, Lethias C. Tenascin-X increases the stiffness of collagen gels without affecting fibrillogenesis. Biophys Chem 2010; 147:87-91; PMID:20089348; http://dx.doi.org/ 10.1016/j.bpc.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 33. Wallace WA, Howie SE, Lamb D, Salter DM. Tenascin immunoreactivity in cryptogenic fibrosing alveolitis. J Pathol 1995; 175:415-20; PMID:7540684; http://dx.doi.org/ 10.1002/path.1711750409 [DOI] [PubMed] [Google Scholar]
- 34. Kaarteenaho-Wiik R, Tani T, Sormunen R, Soini Y, Virtanen I, Paakko P. Tenascin immunoreactivity as a prognostic marker in usual interstitial pneumonia. Am J Respir Crit Care Med 1996; 154:511-8; PMID:8756830; http://dx.doi.org/ 10.1164/ajrccm.154.2.8756830 [DOI] [PubMed] [Google Scholar]
- 35. Kaarteenaho-Wiik R, Lakari E, Soini Y, Pollanen R, Kinnula VL, Paakko P. Tenascin expression and distribution in pleural inflammatory and fibrotic diseases. J Histochem Cytochem 2000; 48:1257-68; PMID:10950882; http://dx.doi.org/ 10.1177/002215540004800909 [DOI] [PubMed] [Google Scholar]
- 36. Fitch PM, Howie SE, Wallace WA. Oxidative damage and TGF-beta differentially induce lung epithelial cell sonic hedgehog and tenascin-C expression: implications for the regulation of lung remodelling in idiopathic interstitial lung disease. Int J Exp Pathol 2011; 92:8-17; PMID:21039988; http://dx.doi.org/ 10.1111/j.1365-2613.2010.00743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brissett M, Veraldi KL, Pilewski JM, Medsger TA, Jr., Feghali-Bostwick CA. Localized expression of tenascin in systemic sclerosis-associated pulmonary fibrosis and its regulation by insulin-like growth factor binding protein 3. Arthritis Rheum 2012; 64:272-80; PMID:21898349; http://dx.doi.org/ 10.1002/art.30647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaarteenaho-Wiik R, Mertaniemi P, Sajanti E, Soini Y, Paakko P. Tenascin is increased in epithelial lining fluid in fibrotic lung disorders. Lung 1998; 176:371-80; PMID:9780295; http://dx.doi.org/ 10.1007/PL00007619 [DOI] [PubMed] [Google Scholar]
- 39. Hisatomi K, Sakamoto N, Mukae H, Hayashi T, Amenomori M, Ishimoto H, Fujita H, Ishii H, Nakayama S, Ishimatsu Y, et al. . Elevated levels of tenascin-C in patients with cryptogenic organizing pneumonia. Intern Med 2009; 48:1501-7; PMID:19721293; http://dx.doi.org/ 10.2169/internalmedicine.48.2233 [DOI] [PubMed] [Google Scholar]
- 40. Fujita H, Sakamoto N, Ishimatsu Y, Kakugawa T, Nakashima S, Hara S, Hara A, Mukae H, Kohno S. Elevated tenascin-C levels in bronchoalveolar lavage fluid of patients with sarcoidosis. Lung 2012; 190:537-43; PMID:22760918; http://dx.doi.org/ 10.1007/s00408-012-9400-1 [DOI] [PubMed] [Google Scholar]
- 41. Muller S, Neureiter D, Stolte M, Verbeke C, Heuschmann P, Kirchner T, Aigner T. Tenascin: a sensitive and specific diagnostic marker of minimal collagenous colitis. Virchows Arch 2001; 438:435-41; PMID:11407470; http://dx.doi.org/ 10.1007/s004280000375 [DOI] [PubMed] [Google Scholar]
- 42. Lebensztejn DM, Sobaniec-Lotowska ME, Kaczmarski M, Voelker M, Schuppan D. Matrix-derived serum markers in monitoring liver fibrosis in children with chronic hepatitis B treated with interferon alpha. World J Gastroenterol 2006; 12:3338-43; PMID:16733849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lieber CS, Weiss DG, Paronetto F. Value of fibrosis markers for staging liver fibrosis in patients with precirrhotic alcoholic liver disease. Alcohol Clin Exp Res 2008; 32:1031-9; PMID:18422837; http://dx.doi.org/ 10.1111/j.1530-0277.2008.00664.x [DOI] [PubMed] [Google Scholar]
- 44. Golledge J, Clancy P, Maguire J, Lincz L, Koblar S. The role of tenascin C in cardiovascular disease. Cardiovasc Res 2011; 92:19-28; PMID:21712412; http://dx.doi.org/ 10.1093/cvr/cvr183 [DOI] [PubMed] [Google Scholar]
- 45. Imanaka-Yoshida K, Aoki H. Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front Physiol 2014; 5:283; PMID:25120494; http://dx.doi.org/ 10.3389/fphys.2014.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schenke-Layland K, Stock UA, Nsair A, Xie J, Angelis E, Fonseca CG, Larbig R, Mahajan A, Shivkumar K, Fishbein MC, et al. . Cardiomyopathy is associated with structural remodelling of heart valve extracellular matrix. Eur Heart J 2009; 30:2254-65; PMID:19561339; http://dx.doi.org/ 10.1093/eurheartj/ehp267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kotby AA, Abdel Aziz MM, El Guindy WM, Moneer AN. Can serum tenascin-C be used as a marker of inflammation in patients with dilated cardiomyopathy? Int J Pediatr 2013; 2013:608563; PMID:24106506; http://dx.doi.org/ 10.1155/2013/608563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jian B, Jones PL, Li Q, Mohler ER, 3rd, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-C in calcific aortic stenosis. Am J Pathol 2001; 159:321-7; PMID:11438479; http://dx.doi.org/ 10.1016/S0002-9440(10)61698-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol 1996; 179:321-5; PMID:8774490; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199607)179:3%3c321::AID-PATH555%3e3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- 50. Wallner K, Li C, Fishbein MC, Shah PK, Sharifi BG. Arterialization of human vein grafts is associated with tenascin-C expression. J Am Coll Cardiol 1999; 34:871-5; PMID:10483972; http://dx.doi.org/ 10.1016/S0735-1097(99)00272-7 [DOI] [PubMed] [Google Scholar]
- 51. Kenji K, Hironori U, Hideya Y, Michinori I, Yasuhiko H, Nobuoki K. Tenascin-C is associated with coronary plaque instability in patients with acute coronary syndromes. Circ J 2004; 68:198-203; PMID:14993772; http://dx.doi.org/ 10.1253/circj.68.198 [DOI] [PubMed] [Google Scholar]
- 52. Pedretti M, Rancic Z, Soltermann A, Herzog BA, Schliemann C, Lachat M, Neri D, Kaufmann PA. Comparative immunohistochemical staining of atherosclerotic plaques using F16, F8 and L19: Three clinical-grade fully human antibodies. Atherosclerosis 2010; 208:382-9; PMID:19699478; http://dx.doi.org/ 10.1016/j.atherosclerosis.2009.07.043 [DOI] [PubMed] [Google Scholar]
- 53. Ihida-Stansbury K, McKean DM, Lane KB, Loyd JE, Wheeler LA, Morrell NW, Jones PL. Tenascin-C is induced by mutated BMP type II receptors in familial forms of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2006; 291:L694-702; PMID:16782755; http://dx.doi.org/ 10.1152/ajplung.00119.2006 [DOI] [PubMed] [Google Scholar]
- 54. Satta J, Soini Y, Pollanen R, Paakko P, Juvonen T. Tenascin expression is associated with a chronic inflammatory process in abdominal aortic aneurysms. J Vasc Surg 1997; 26:670-5; PMID:9357470; http://dx.doi.org/ 10.1016/S0741-5214(97)70068-5 [DOI] [PubMed] [Google Scholar]
- 55. Paik DC, Fu C, Bhattacharya J, Tilson MD. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp Mol Med 2004; 36:524-33; PMID:15665585; http://dx.doi.org/ 10.1038/emm.2004.67 [DOI] [PubMed] [Google Scholar]
- 56. Horstrup JH, Gehrmann M, Schneider B, Ploger A, Froese P, Schirop T, Kampf D, Frei U, Neumann R, Eckardt KU. Elevation of serum and urine levels of TIMP-1 and tenascin in patients with renal disease. Nephrol Dial Transplant 2002; 17:1005-13; PMID:12032189; http://dx.doi.org/ 10.1093/ndt/17.6.1005 [DOI] [PubMed] [Google Scholar]
- 57. Betz P, Nerlich A, Tubel J, Penning R, Eisenmenger W. Localization of tenascin in human skin wounds–an immunohistochemical study. Int J Legal Med 1993; 105:325-8; PMID:7686039; http://dx.doi.org/ 10.1007/BF01222116 [DOI] [PubMed] [Google Scholar]
- 58. Latijnhouwers MA, Bergers M, Van Bergen BH, Spruijt KI, Andriessen MP, Schalkwijk J. Tenascin expression during wound healing in human skin. J Pathol 1996; 178:30-5; PMID:8778312; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199601)178:1%3c30::AID-PATH442%3e3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- 59. Dalkowski A, Schuppan D, Orfanos CE, Zouboulis CC. Increased expression of tenascin C by keloids in vivo and in vitro. Br J Dermatol 1999; 141:50-6; PMID:10417515; http://dx.doi.org/ 10.1046/j.1365-2133.1999.02920.x [DOI] [PubMed] [Google Scholar]
- 60. Maseruka H, Bonshek RE, Tullo AB. Tenascin-C expression in normal, inflamed, and scarred human corneas. Br J Ophthalmol 1997; 81:677-82; PMID:9349157; http://dx.doi.org/ 10.1136/bjo.81.8.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ljubimov AV, Saghizadeh M, Spirin KS, Khin HL, Lewin SL, Zardi L, Bourdon MA, Kenney MC. Expression of tenascin-C splice variants in normal and bullous keratopathy human corneas. Invest Ophthalmol Vis Sci 1998; 39:1135-42; PMID:9620072 [PubMed] [Google Scholar]
- 62. Inoue K, Jinnin M, Hara Y, Makino K, Kajihara I, Makino T, Sakai K, Fukushima S, Inoue Y, Ihn H. Serum levels of tenascin-C in collagen diseases. J Dermatol 2013; 40:715-9; PMID:23834524; http://dx.doi.org/ 10.1111/1346-8138.12218 [DOI] [PubMed] [Google Scholar]
- 63. Sumioka T, Kitano A, Flanders KC, Okada Y, Yamanaka O, Fujita N, Iwanishi H, Kao WW, Saika S. Impaired cornea wound healing in a tenascin C-deficient mouse model. Lab Invest 2013; 93:207-17; PMID:23207449; http://dx.doi.org/ 10.1038/labinvest.2012.157 [DOI] [PubMed] [Google Scholar]
- 64. Matsumoto Y, Niimi N, Kohyama K. Characterization of fibrosis-promoting factors and siRNA-mediated therapies in C-protein-induced experimental autoimmune myocarditis. Cell Immunol 2012; 279:70-7; PMID:23099153; http://dx.doi.org/ 10.1016/j.cellimm.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 65. Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, Hiroe M, Imanaka-Yoshida K. Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol 2007; 49:261-8; PMID:17513943; http://dx.doi.org/ 10.1097/FJC.0b013e318033dfd4 [DOI] [PubMed] [Google Scholar]
- 66. Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, et al. . Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 2010; 298:H1072-8; PMID:20081106; http://dx.doi.org/ 10.1152/ajpheart.00255.2009 [DOI] [PubMed] [Google Scholar]
- 67. Zou C, Xie R, Bao Y, Liu X, Sui M, M S , Li S, Yin H. Iron chelator alleviates tubulointerstitial fibrosis in diabetic nephropathy rats by inhibiting the expression of tenascinC and other correlation factors. Endocrine 2013; 44:666-74; PMID:23468095; http://dx.doi.org/ 10.1007/s12020-013-9907-0 [DOI] [PubMed] [Google Scholar]
- 68. Tanaka S, Sumioka T, Fujita N, Kitano A, Okada Y, Yamanaka O, Flanders KC, Miyajima M, Saika S. Suppression of injury-induced epithelial-mesenchymal transition in a mouse lens epithelium lacking tenascin-C. Mol Vis 2010; 16:1194-205; PMID:20664686 [PMC free article] [PubMed] [Google Scholar]
- 69. Richter HB, Franke H, Dargel R. Expression of tenascin, fibronectin, and laminin in rat liver fibrogenesis–a comparative immunohistochemical study with two models of liver injury. Exp Toxicol Pathol 1998; 50:315-22; PMID:9784003; http://dx.doi.org/ 10.1016/S0940-2993(98)80011-0 [DOI] [PubMed] [Google Scholar]
- 70. Blaauboer ME, Boeijen FR, Emson CL, Turner SM, Zandieh-Doulabi B, Hanemaaijer R, Smit TH, Stoop R, Everts V. Extracellular matrix proteins: a positive feedback loop in lung fibrosis? Matrix Biol 2014; 34:170-8; PMID:24291458; http://dx.doi.org/ 10.1016/j.matbio.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 71. Blaauboer ME, Emson CL, Verschuren L, van Erk M, Turner SM, Everts V, Hanemaaijer R, Stoop R. Novel combination of collagen dynamics analysis and transcriptional profiling reveals fibrosis-relevant genes and pathways. Matrix Biol 2013; 32:424-31; PMID:23648810; http://dx.doi.org/ 10.1016/j.matbio.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 72. Mackie EJ, Halfter W, Liverani D. Induction of tenascin in healing wounds. J Cell Biol 1988; 107:2757-67; PMID:2462568; http://dx.doi.org/ 10.1083/jcb.107.6.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Geffrotin C, Tricaud Y, Crechet F, Castelli M, Lefaix JL, Vaiman M. Unlike tenascin-X, tenascin-C is highly up-regulated in pig cutaneous and underlying muscle tissue developing fibrosis after necrosis induced by very high-dose gamma radiation. Radiat Res 1998; 149:472-81; PMID:9588358; http://dx.doi.org/ 10.2307/3579787 [DOI] [PubMed] [Google Scholar]
- 74. Assis de Brito TL, Monte-Alto-Costa A, Romana-Souza B. Propranolol impairs the closure of pressure ulcers in mice. Life Sci 2014; 100:138-46; PMID:24560961; http://dx.doi.org/ 10.1016/j.lfs.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 75. Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E, et al. . Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol 2010; 29:690-700; PMID:20797438; http://dx.doi.org/ 10.1016/j.matbio.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 76. Koyama Y, Kusubata M, Yoshiki A, Hiraiwa N, Ohashi T, Irie S, Kusakabe M. Effect of tenascin-C deficiency on chemically induced dermatitis in the mouse. J Invest Dermatol 1998; 111:930-5; PMID:9856798; http://dx.doi.org/ 10.1046/j.1523-1747.1998.00401.x [DOI] [PubMed] [Google Scholar]


