Abstract
Objective
To examine the accuracies of body mass index (BMI) and skinfold thicknesses in classifying the body fatness of 7365 8- to 19-year-old subjects in a national sample.
Study design
We used percent body fat determined by dual-energy x-ray absorptiometry (PBFDXA) between 1999 and 2004. Categories of PBFDXA and the skinfold sum (triceps plus subscapular) were constructed so that that numbers of children in each category were similar to the number in each of 5 BMI categories based on the Centers for Disease Control and Prevention growth charts.
Results
Approximately 75% of the children and adolescents who had a BMI-for-age ≥ 95th percentile (considered obese) had elevated body fatness, but PBFDXA levels were more variable at lower BMIs. For example, only 41% of the boys who had a BMI < 25th percentile, had a similarly low PBFDXA. The use of the skinfold sum, rather than BMI, slightly improved the identification of elevated levels of body fatness among boys (P = .03), but not among girls (P > .10). A low sum of the triceps and subscapular skinfold thicknesses was a better indicator of low PBFDXA than was a low BMI, but differences were smaller among children with greater levels of body fatness. Among girls who had a PBFDXA above the median, for example, BMI and the skinfold sum were correlated similarly (r = 0.77-0.79) with body fatness.
Conclusions
Both BMI and skinfold thicknesses are fairly accurate in identifying children who have excess body fatness. In contrast, if the goal is to identify children who have low body fatness, skinfold thicknesses would be preferred.
Body mass index (BMI, kg/m2) is used as a screening tool for overweight and obesity in various settings, and a high BMI among children is associated with adverse levels of various cardiovascular risk factors, the initial stages of atherosclerosis, and adult obesity.1-3 However, because BMI is based on weight and height, both of which change greatly during growth, a high BMI can reflect a high level of either fat mass or fat-free mass.4 A child with a high BMI is likely to have elevated body fatness,5 but lower levels of BMI are a poor indicator of body fatness among children.6 In addition, several investigators7,8 have reported that the correlation between BMI and more accurate measures of body fatness among children and adolescents is only moderate (r < 0.70).
The thickness of various skinfolds is thought to give a more direct indication of body fatness than does BMI, and despite their large measurement errors,9 skinfold thicknesses are widely used.10-12 Most, but not all,13 studies have found that skinfold thicknesses are more strongly associated with the body fatness of children than is BMI.6,14-17 A stronger correlation, however, does not necessarily mean that skinfold thicknesses can more accurately identify children who have high levels of body fatness than can BMI. The greater correlation may reflect the more accurate prediction of low levels of body fatness by skinfold thicknesses.
Our objective was to determine whether the sum of 2 (subscapular and triceps) skinfold thicknesses is more strongly related to dual-energy X-ray absorptiometry (DXA)-calculated body fatness than is BMI in a nationally, representative sample of 8- to 19-year-old subjects (n = 7365). We also assessed whether differences are caused by the identification of children who have relatively low or relatively high levels of body fatness.
Methods
The 1999-2004 National Health and Nutrition Examination Survey (NHANES) is a representative, cross-sectional sample of the US civilian, noninstitutionalized population.18 NHANES 1999-2004 underwent institutional review board approval, and parental permission was obtained for minors younger than the age of 18 years. Children 7-17 years of age also were asked to provide documented assent. Consent was obtained for all subjects 18 years and older. Age was calculated as age in months at the time of examination. Race and ethnicity were self-reported, and in the current study, we classify subjects as non-Hispanic white, non-Hispanic black, Mexican American, and other. The overall examination rate for 6- to 19-year-old subjects in NHANES 1999-2004 was 85%.
DXA scans were acquired in NHANES 1999-2004 for boys and nonpregnant girls who were at least 8 years of age with the use of a Hologic QDR 4500A fan-beam densitometer (Hologic Inc, Bedford, Massachusetts).19,20 The scan for each survey participant was analyzed using Hologic Discovery software (version 12.1) by the Department of Radiology in the University of California, San Francisco. PBFDXA (ie, percent body fat determined by DXA) was calculated as 100 × (DXA – estimated total fat mass/DXA – estimated total mass). To protect patient confidentiality, the 1999-2000 DXA data for 8- to 17-year-old girls are available only in the Research Data Center. The current analyses do not include girls who were examined in the 1999-2000 cycle.
Our analyses used the NHANES DXA Multiple Imputation Data Files.20 Approximately 9.5% of the children and adolescents in the current study were missing at least one DXA measurement, and because missingness was associated with BMI, body fatness, and other characteristics, an analysis of only nonmissing data could be biased.21 Multiple imputation, performed by National Center for Health Statistics (NCHS) using sequential regression20 with 5 imputations, was used to estimate missing DXA values from nonmissing DXA measurements and other characteristics such as raceethnicity, age, BMI, and skinfold thicknesses. Although it has been suggested that a larger number of imputations may be necessary to arrive at accurate estimates of CIs and P values,22,23 these imputations were performed by NCHS before these reports were available.
Weight and height were measured using standardized techniques and equipment. BMI was calculated as weight (kg)/height (m)2, and BMI-for-age z-scores (that account for sex and age) were calculated relative to children who participated in national studies between 1963-1965 and 1988-1994.24 Overweight is defined as a BMI, relative to the 2000 Centers for Disease Control and Prevention (CDC) growth charts,24 between the 85th and 94th percentiles for a child’s sex and age; obesity is defined as a BMI ≥ 95th percentile of this reference population. On the basis of these cut points, 16% of children in the current study were considered to be overweight, and 17% were obese. Because we were interested in comparing the accuracies of BMI and skinfold thicknesses over the entire distribution of body fatness, we also used the 25th and 50th percentiles of BMI in the CDC reference population as cut-points in the analyses. Approximately 16% of subjects in the current study had a BMI < 25th percentile, 18% had a BMI that was between the 25th and 49th percentiles, and 33% had a BMI between the 50th and 84th percentiles.
The thickness of the triceps and subscapular skinfolds were measured to the nearest 0.1 mm by the use of Holtain skinfold calipers. These values were missing for about 7% (subscapular) and 4% (triceps) either because of measurement difficulties or because the skinfold exceeded the capacity of the caliper (45.0 mm).25 Because the probability of a missing data for the skinfold thicknesses was not random, with more than 70% of these children having a BMI ≥ 95th percentile, we used the “Amelia” package in R (http://cran.r-project.org/web/packages/Amelia/index.html)26,27 to impute values for the missing skinfolds based on levels of sex, race, age, BMI, PBFDXA, and other characteristics. The skinfold thicknesses were log-transformed to avoid imputing improbably large values and to linearize the associations with PBFDXA. We imputed one skinfold thickness value for each of the 5 sets of imputed DXA values; each set contained (if originally missing) an imputed value for the PBFDXA and the 2 skinfold thicknesses.
We focus on the ability of 5 categories (<25th percentile, 25th-49th percentile, 50th-84th percentiles, 85th-94th percentiles, and ≥95th percentile) of BMI-for-age and the sum of the triceps and subscapular skinfold thicknesses (SF sum) to correctly classify the body fatness of children. Because there is little agreement on the classification of “excess body fatness” among children or adults,28 we constructed 5 categories (low, low-normal, moderate, slightly elevated, and elevated) for both SF sum and PBFDXA, with each category having a similar number of children as the corresponding category of BMI-for-age.
This type of classification, with similar numbers of children in each BMI, SF sum, and percent body fat category, eliminates a possible bias in comparing screening performance. For example, if one compared extremely high values of the SF sum with less extreme values of BMI in identifying high levels of body fatness, the positive predictive value of the SF sum would likely be greater. This could be true even if BMI was a more accurate indicator of body fatness, and results from the differing prevalences of high levels of BMI and the SF sum.
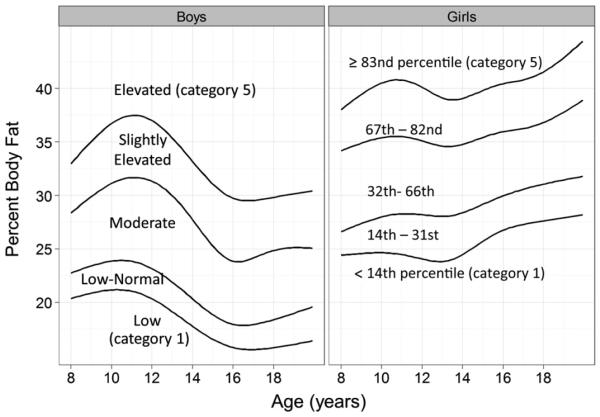
We used quantile regression29 to calculate sex- and age-specific cut-points for 5 categories of the SF sum and percent body fat. For example, because 18% of the boys in the current study had a BMI-for-age ≥ 95th percentile, we considered boys with a PBFDXA at or above the 82nd percentile to have an elevated level of PBFDXA, and boys with a SF sum at or above the 82nd percentile to have an elevated SF sum. Age was modeled by the use of restricted cubic splines to account for nonlinearity, and a similar process was used to determine cut-points for other categories of the SF sum and body fatness. For example, because 14% of the girls had a BMI-for-age below the CDC 25th percentile, low levels of the SF sum and PBFDXA were defined as values <14th (age-specific) percentile. Levels of PBFDXA corresponding to each of the 5 categories of BMI-for-age, along with the terminology used throughout the article (Figure; available at www.jpeds.com).
Figure.
Cut-points for the 5 categories of PBFDXA by sex (boys, left panel; girls, right panel) and age. Within each sex, the 4 curves were estimated with the use of quantile regression (see Methods)29 and represent the boundaries of the 5 body fatness categories. These cut-points were chosen so that the number of children in each PBFDXA category would be similar to the number of children in the corresponding BMI-for-age category. The names of these categories are shown for boys, and the percentile cut points are shown for girls. The cut-points for the 5 categories of body fatness among boys were the 17th, 35th, 66th, and 82nd percentiles.
Statistical Analyses
All analyses were performed with the survey and mitools packages in R,27,30 and accounted for the sample weights, sample design, and multiple imputations. We focused on the relation of the skinfold thickness and BMI to PBFDXA, but additional analyses were performed that examined associations with total fat mass (kg).
We incorporated the uncertainty of the multiple imputations (for PBFDXA and log skinfold thicknesses) into all standard errors31 by analyzing each of the 5 imputation sets separately. Estimates were then averaged over the 5 sets, and the total variance was calculated as the within-imputation variance plus (1 + 1/5) times the between-imputation variance.20,32 The accuracy of the imputations was assessed by overimputing; a process in which each observed value was treated as if it had been missing. The correlation between the over-imputed and actual values was strong (r ~ 0.93), and almost all of the observed values were within the 90% CIs of the overimputed values.
Although the skinfold thicknesses were log transformed before the imputation process, BMI levels, to a lesser extent, were positively skewed. However, as we did not impute any BMI values, this variable was not transformed. It has been shown33 that even if the data are highly skewed, the results of regression models are accurate if the sample is relatively large (eg, >500).
The 15th, 50th, and 85th percentiles or proportions and SEs were used to summarize various characteristics of the sample. Sex-specific regression models were used to predict levels of PBFDXA from race, age, BMI, and the skinfold thicknesses; age and BMI were modeled with restricted cubic splines. We focused on the sex-specific (weighted) correlations of PBFDXA with BMI and SF sum. To control for the influence of age, these correlations were based on the residuals of regression models in which each body size measure was regressed on age. To determine the statistical significance of the observed differences (eg, the correlation between PBFDXA and BMI minus the correlation between PBFDXA and SF sum), we calculated standard errors with the “withReplicates” function,30 which uses jackknife replicate weights. The estimated variances were then combined across the 5 imputations, and P values were calculated using t tests with 44 degrees of freedom.
The abilities of elevated levels of BMI and the SF sum to identify children with elevated levels of PBFDXA were examined using the: (1) positive predictive value (eg, the proportion of children with an elevated BMI who had an elevated PBFDXA); (2) the positive likelihood ratio (prevalence of an elevated BMI among children who had an elevated PBFDXA divided by its prevalence among children without an elevated PBFDXA); and (3) the kappa statistic,34 a measure of chance-corrected agreement. The variance of the differences between kappa statistics also were calculated with the “withReplicates” function.30 A similar process was used to compare the accuracies of BMI and the SF sum in the identification of children with low PBFDXA levels.
Results
Various characteristics of the sample are shown among boys and girls in Table I (available at www.jpeds.com). The distributions of several variables were positively skewed, and we focus on the 15th, 50th, and 85th percentiles; in a normal distribution, the 15th and 85th percentiles would be about 1 SD from the mean. Overall, 14% of the children were non-Hispanic black and 11% were Mexican-American. The median BMI-for-age level of these children was 0.5 standard deviations greater than the median in the 1963-1994 CDC reference population, but BMI levels did not differ between boys and girls. In contrast, median levels of the SF sum and PBFDXA were about 40% to 50% greater among girls than boys.
Table I.
Descriptive characteristics of 8- to 19-year-old subject by sex: NHANES 1999-2004
| Characteristic | Boys | Girls |
|---|---|---|
| n (unweighted) | 4493 | 2872* |
| Age, years | 13.9 (9.8, 18.2)† | 13.8 (9.9, 17.9) |
| BMI, kg/m2 | 20.8 (16.8, 26.9) | 21.1 (17.0, 27.3) |
| BMI-for-age‡ | 0.5 (−0.8, 1.8) | 0.5 (−0.6, 1.7) |
| % obese§ | 18 ± 1% | 17 ± 1% |
| % overweight or obese§ | 34 ± 1% | 34 ± 1% |
| % non-Hispanic white | 61% | 62% |
| % non-Hispanic black | 15% | 14% |
| % Mexican-American | 11% | 11% |
| Skinfold thicknesses, mm | ||
| Triceps | 11.2 (7.0, 22.1) | 17.2 (10.4, 27.3) |
| Subscapular | 9.1 (5.7, 19.4) | 12.4 (7.2, 23.7) |
| SF sum | 20.2 (13.4, 41.5) | 30.2 (18, 50.1) |
| DXA-calculated percent body fat, PBFDXA |
23.6 (17.2, 34.6) | 32.3 (25.6, 41.1) |
Data for girls are from 2001-2004 rather than 1999-2004.
With the exception of the sample size, values are medians for continuous variables or percents for categorical variables. The distribution of each characteristic is indicated by the 15th and 85th percentile (for continuous variables) or by the SE (for categorical variables). The 15th and 85th percentiles are about 1 SD from the mean for a normally distributed variable.
Z-score (SD score) of subjects in the current study relative to those who were included in the 2000 CDC growth charts.24
Obesity is defined as a BMI-for-age ≥ CDC 95th percentile, and overweight is a BMI-for-age between the 85th and 94th percentiles.
As assessed by the multiple R2 values of various sex-specific models predicting PBFDXA from race, age, and either BMI or the SF sum, the SF sum accounted for more of the variability in PBFDXA than did BMI (Table II). Multiple R2 values for models that included BMI-for-age, in addition to race and age, resulted in R2 values of approximately 0.75 (second row), but the use of skinfold thicknesses yielded R2 values of 0.86 (boys) and 0.81 (girls). As shown in Table II, even after we accounted for the information conveyed by BMI-for-age, the SF sum provided additional information on PBFDXA among both boys (R2 increases from 0.75 to 0.87), and girls (0.76 to 0.84).
Table II.
Multiple R2 values for various regression models predicting PBFDXA
| Predictors* | Boys | Girls |
|---|---|---|
| Race, age (baseline) | 0.11 | 0.04 |
| + BMI | 0.75 | 0.76 |
| + triceps skinfold thickness | 0.85 | 0.77 |
| + subscapular skinfold thickness | 0.78 | 0.73 |
| + SF sum | 0.86 | 0.81 |
| Race, age, and BMI (baseline) | 0.75 | 0.76 |
| + triceps skinfold thickness | 0.86 | 0.83 |
| + subscapular skinfold thickness | 0.81 | 0.80 |
| + SF sum | 0.87 | 0.84 |
All models include race and age (top rows) or race, age, and BMI (bottom rows). Age, BMI, and the skinfold thicknesses were modeled with the use of restricted cubic splines.
Table III shows sex-specific correlations between BMI and the SF sum with PBFDXA. (We accounted for age in these analyses by using the residuals of regression models that predicted levels of PBFDXA, BMI and the SF sum from sex and age.) BMI was less strongly correlated with PBFDXA than was the SF sum among both boys (r = 0.83 vs 0.91, P < .001 for difference) and girls (r = 0.84 vs 0.89, P < .001). These differences varied somewhat by age, with a smaller difference seen among 18- to 19-year-olds (boys: r = 0.87 vs 0.91; girls: r = 0.89 vs 0.88). However, the magnitudes of the associations with PBFDXA, particularly for BMI, were substantially weaker among children whose body fatness was below the median for their sex and age. Among these thinner children, correlations ranged from r = 0.49-0.59 for BMI and from r = 0.68-0.75 for the SF sum. In contrast, among children with greater levels of body fatness, correlations ranged from 0.76 to 0.84. Furthermore, there was little difference in the magnitudes of the associations with BMI and SF sum among girls who had levels of PBFDXA above the median (r = 0.77 and r = 0.79).
Table III.
Stratified correlations between PBFDXA, BMI, and SF sum
| Boys |
Girls |
|||
|---|---|---|---|---|
| BMI | SF sum | BMI | SF sum | |
| Overall | 0.83* | 0.91* | 0.84* | 0.89* |
| Age group, years | ||||
| 8-11.9 | 0.86* | 0.92* | 0.84* | 0.90* |
| 12-14.9 | 0.78* | 0.91* | 0.86† | 0.90† |
| 15-17.9 | 0.84* | 0.90* | 0.83† | 0.87† |
| 18-19.9 | 0.87† | 0.91† | 0.89 | 0.88 |
| Race | ||||
| Non-Hispanic whites | 0.83* | 0.91* | 0.85* | 0.89* |
| Non-Hispanic blacks | 0.84* | 0.92* | 0.85* | 0.91* |
| Mexican-Americans | 0.86* | 0.93* | 0.82* | 0.90* |
| Body fatness‡ | ||||
| <Median | 0.49* | 0.68* | 0.59* | 0.75* |
| ≥Median | 0.76* | 0.84* | 0.77 | 0.79 |
P values assess whether the correlation between PBFDXA and BMI is equal to the correlation between PBFDXA and the SF sum.
P < .001;
P < .05. In a random sample of 3500, a correlation of 0.055 would be statistically significant (H0: r = 0) at the 0.001 level.
Body fatness was categorized into 2 groups on the basis of the median PBFDXA for a child’s sex, age (year), and imputation number.
Additional analyses of total fat mass (data not shown), rather than percent body fat, indicated that fat mass was more strongly correlated with BMI (r = 0.94-0.96) than with the SF sum (r = 0.85-0.87) among boys and girls. Furthermore, the SF sum did not convey additional information on fat mass if the BMI level was already known.
Table IV shows a cross-classification of the 5 PBFDXA categories with those of BMI (top) and SF sum (bottom). As assessed by the proportion of children along the diagonal, the SF sum was a more accurate indicator of PBFDXA than was BMI among both boys (60% vs 48% along the diagonal) and girls (59% vs 51%). (Similar differences were seen for the intraclass correlations across the 5 categories, with correlations of 0.65-0.66 for BMI among boys and girls vs 0.76-0.79 for the SF sum.) These differences, however, were largely due to the more accurate classification of thinner children by the SF sum. For example, whereas only 41% of boys in the lowest BMI category were also in the lowest PBFDXA category, the comparable proportion (row percent) for the SF sum was 61%. In contrast, differences in the abilities of BMI and the SF sum to correctly identify children with elevated levels of PBFDXA were much smaller among both boys (75%, BMI vs 79%, SF sum) and girls (76% for both BMI and SF sum). The classification of slightly elevated levels of PBFDXA (category #4) was relatively poor for both BMI and SF sum.
Table IV.
Cross-classification of categories* of PBFDXA with either BMI-for-age or SF sum
| Boys: PBFDXA category |
Girls: PBFDXA category |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI-for-age category | 1 (low) | 2 | 3 | 4 | 5 (elevated) | 1 (low) | 2 | 3 | 4 | 5 (elevated) |
| <25th percentile | 41%† | 35% | 23% | 2% | 0 | 48% | 32% | 19% | 1% | 0 |
| 25th-49th percentile | 32% | 28% | 37% | 3% | 0 | 29% | 30% | 39% | 1% | 0 |
| 50th-84th percentile | 14% | 20% | 50% | 14% | 2% | 7% | 21% | 54% | 16% | 2% |
| 85th-94th percentile | 0 | 4% | 28% | 46% | 22% | 1% | 1% | 35% | 43% | 20% |
| ≥95th percentile (obese) | 0 | 0 | 4% | 21% | 75% | 0 | 0 | 4% | 20% | 76% |
| SF sum category | ||||||||||
| 1 (low) | 61% | 28% | 1% | 0 | 0 | 62% | 32% | 6% | 0 | 0 |
| 2 (low-normal) | 28% | 42% | 30% | 0 | 0 | 27% | 40% | 33% | 0 | 0 |
| 3 (moderate) | 5% | 17% | 63% | 13% | 1% | 2% | 16% | 65% | 16% | 2% |
| 4 (slightly elevated) | 0 | 1% | 25% | 53% | 21% | 0 | 1% | 31% | 46% | 22% |
| 5 (elevated) | 0 | 0 | 1% | 20% | 79% | 0 | 0 | 4% | 20% | 76% |
Categories of PBFDXA and the SF sum were constructed so the that number of children in each category would be the same as the number of children in each BMI-for-age category.
Values are row percents, representing the proportion of children in the specified BMI or SF sum category who are in specified category of body fatness.
Table V focuses on the screening performance of BMI and the SF sum in the identification of elevated (left columns) or low (right) levels of PBFDXA based on the results of several 2 × 2 tables. In general, elevated levels of both BMI and SF sum were good indicators of elevated PBFDXA levels (Table V) with positive likelihood ratios ranging from 14 to 18 among both boys and girls. (For example, boys with an elevated PBFDXA were 14.3 times more likely to have an elevated BMI than were those with a lower PBFDXA level.) As assessed by kappa statistics (H0: no difference in the abilities of BMI and SF sum to classify body fatness), an elevated SF sum was a better indicator of an elevated PBFDXA level among boys (kappas of 0.70 vs 0.75, P = .03) but not girls (kappas of 0.71 for both measures). Kappa statistics varied somewhat across race-ethnicity groups, but the only statistically significant difference between BMI and the SF sum was among non-Hispanic black boys (0.68, BMI vs 0.77, SF sum). The positive predictive values, however, were lower among non-Hispanic black girls, with values of 0.61 (BMI) and 1.64 (SF sum) versus values of 0.79 or more among other girls.
Table V.
Screening characteristics of BMI and SF sum for elevated and low levels of PBFDXA
| Children with elevated PBFDXA |
Thin children (Low PBFDXA) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Race-ethnicity | Measure | Prevalence | Positive predictive value |
Positive likelihood ratio |
Kappa statistic |
Prevalence | Positive predictive value |
Positive likelihood ratio |
Kappa statistic |
| Boys | Overall | BMI | 0.18† | 0.75 | 14.3 | 0.70† | 0.17 | 0.41 | 3.3 | 0.28§ |
| SF sum | 0.18 | 0.79 | 18.0 | 0.75† | 0.17 | 0.61 | 7.7 | 0.53§ | ||
| Girls | Overall | BMI | 0.17 | 0.76 | 15.3 | 0.71 | 0.14 | 0.48 | 5.7 | 0.40§ |
| SF sum | 0.17 | 0.77 | 15.7 | 0.71 | 0.14 | 0.62 | 10.0 | 0.55§ | ||
| Boys | Non-Hispanic Whites | BMI | 0.16 | 0.77 | 16.8 | 0.71 | 0.16 | 0.37 | 3.6 | 0.29§ |
| SF sum | 0.16 | 0.80 | 12.0 | 0.76 | 0.17 | 0.54 | 7.1 | 0.51§ | ||
| Non-Hispanic Blacks | BMI | 0.19 | 0.65 | 11.0 | 0.68‡ | 0.17 | 0.71 | 4.4 | 0.29§ | |
| SF sum | 0.17 | 0.75 | 17.4 | 0.77‡ | 0.24 | 0.86 | 11.2 | 0.56§ | ||
| Mexican-Americans | BMI | 0.24 | 0.82 | 12.7 | 0.72 | 0.16 | 0.28 | 3.7 | 0.26§ | |
| SF sum | 0.23 | 0.85 | 16.5 | 0.73 | 0.13 | 0.50 | 9.5 | 0.52§§ | ||
| Girls | Non-Hispanic Whites | BMI | 0.16 | 0.80 | 19.9 | 0.73 | 0.15 | 0.47 | 5.6 | 0.41‡ |
| SF sum | 0.16 | 0.79 | 18.2 | 0.73 | 0.15 | 0.59 | 9.0 | 0.55‡ | ||
| Non-Hispanic Blacks | BMI | 0.26 | 0.61 | 7.5 | 0.66 | 0.10 | 0.67 | 7.6 | 0.34§ | |
| SF sum | 0.24 | 0.64 | 8.9 | 0.69 | 0.13 | 0.81 | 16.0 | 0.56§ | ||
| Mexican Americans | BMI | 0.17 | 0.81 | 17.8 | 0.70 | 0.13 | 0.28 | 4.8 | 0.29§ | |
| SF sum | 0.16 | 0.87 | 27.3 | 0.73 | 0.12 | 0.45 | 9.8 | 0.51§ | ||
*Values represent various screening characteristics of BMI or the SF sum in the identification of children with elevated levels of PBFDXA (left side) or low levels of PBFDXA (right side). For example, a cross-classification of elevated levels of BMI and PBFDXA among boys (first row, top left) indicated that the positive predictive value of an elevated BMI was 0.75 with a kappa statistic of 0.70.
P-values are based on the differences between kappa statistics for BMI and the SF sum, and were calculated for each imputation using jackknife replicate weights:
P < .05;
P < .01;
P < .001.
In general, a low level of the SF sum was a much better indicator of a low PBFDXA level than was a low BMI (Table V) among both boys (kappas of 0.28 vs 0.53) and girls (0.40 vs 0.55); P < .001 for both differences. Furthermore, BMI appeared to perform worse among non-Hispanic black and Mexican-American girls (kappas of 0.34 and 0.29) than among non-Hispanic white girls (kappa = 0.41). Only 28% of Mexican-American boys and girls with a low BMI had a low PBFDXA.
Discussion
It is frequently assumed that thickness of skinfolds at various sites, typically expressed as a sum or as percent body fat based on published equations,6 is a better indicator of body fatness than is BMI.10,35 Our results indicate that although the SF sum (subscapular plus triceps) is more strongly associated with PBFDXA than is BMI, with correlations of about 0.90 (SF sum) versus 0.84 (BMI), the importance of this difference may depend upon the objective of the study. We found that the largest difference between BMI and SF sum was in their abilities to correctly identify children with a low level of PBFDXA: a low BMI-for-age could identify only 40% (boys) to 50% (girls) of these thin children, and the SF sum could identify 75%. In contrast, differences between BMI and SF sum were greatly reduced among children with elevated body fatness. Among boys, an elevated SF sum was slightly more accurate than was an elevated BMI (kappas of 0.75 and 0.70) in identifying those with a high PBFDXA level. Among girls, however, BMI and the SF sum performed equally well (kappas of 0.71 for both measures).
It has previously been emphasized that although BMI is a useful surrogate for body adiposity among fatter children, it is “almost useless” in assessing the body fatness of normal-weight children.6,36 However, even when the goal is to identify children with low levels of body fatness, BMI may be of some use. We found, for example, that boys with a low (<16th percentile) PBFDXA were 3.3 times more likely to have a low BMI than were other boys. This, however, should be contrasted with our findings that boys with an elevated PBFDXA were 14 times more likely to have an elevated BMI than were other boys. It is likely that these differing associations between BMI and body fatness may account for the inter-study differences that have been observed between BMI and PBFDXA.7,8,36 For example, Kerruish et al8 reported a correlation of only r = 0.46 between BMI and PBFDXA, but they focused on girls with anorexia nervosa (mean PBFDXA, 14%). In contrast, we and others37 have found multiple R2 values of 0.75 or greater for the prediction of PBFDXA from BMI (or 1/BMI), race, sex, and age in more representative samples.
It should also be realized that although approximately 25% of the children with an elevated BMI did not have an elevated PBFDXA level, most (~80%) of these misclassified children had a PBFDXA level considered to be slightly elevated, corresponding to BMIs in the overweight category (between the CDC 85th and 94th percentiles). Of the children with a BMI-for-age ≥ 95th percentile, only 5% had a PBFDXA corresponding to levels in the moderate or normal range. However, there was a wide range of levels of body fatness among children who had slightly lower, but still high, BMIs (85th-94th percentiles). Among these overweight children, approximately 40%-50% had a body fatness that was in the expected range, but about 20% had a greater-than-expected PBFDXA level and another 30% had a PBFDXA corresponding to levels between the CDC 50th and 84th percentiles.
We also found that the screening ability of BMI for elevated body fatness varied across race-ethnic groups, with BMI having a lower positive predictive value among non-Hispanic black children. Whereas about 80% of non-Hispanic white and Mexican-American children with a BMI ≥ CDC 95th percentile had an elevated PBFDXA, these predictive values were 61% (girls) and 65% (boys) among non-Hispanic black children. This difference is comparable with that observed by Flegal et al38 despite differences in the cut-points used for body fatness. The authors of previous studies37,39 have reported that at similar levels of age and BMI, the mean PBFDXA of non-Hispanic black children is approximately 2%-3% lower than that of non-Hispanic white children. If the 95th percentile of BMI is used to identify children who have excess body fatness, these differences could result in a large number of false positives among non-Hispanic black children.
Although we found that the use of skinfold thickness significantly improved the identification of boys (but not girls) who had a high PBFDXA, it is not certain if the higher positive predictive value (75%, BMI vs 79%, SF sum) would have a substantial impact on screening and interventions. The errors associated with skinfold thicknesses measurements can be large,9,40 particularly among inexperienced observers, and these measurements, some of which require disrobing, are generally more intrusive than are those for weight and height. It is also known that the accuracy of skinfolds in predicting body fatness varies according to the selected sites and the equation used. For example, Bray et al6 found that most skinfold thickness equations were better predictors of body fatness (determined from a 4-compartment model) than was BMI, but that one resulted in very poor prediction (multiple R2 of 0.51). Furthermore, 29% of obese children in the current study had at least one skinfold that could not be measured. We imputed these missing values, but in practice, it may be difficult to use skinfold thicknesses to track the progress of extremely obese persons over time.
There are several limitations of the current analyses that should be considered. DXA estimates of body fatness are known to vary across machines, manufacturers, and software versions.6,41-46 Because the Hologic QDR-4500-A (Hologic Inc, Bedford, Massachusetts) in the current study has been found47-49 to overestimate lean mass among adults, the NCHS decreased the recorded DXA lean mass values by 5% and added an equivalent weight to each subject’s fat mass.50 This adjustment may be the reason for the greater PBFDXA values in this sample than in other studies of children who have similar BMI levels. However, if this proportional adjustment was valid across the range of body fatness in the current study, it is unlikely to have substantially influenced our findings concerning the screening performances of BMI and skinfold thicknesses. Relying on a fixed cutpoint (eg, 30%) for high body fatness would be influenced by a bias, but we used cut-points that resulted in equivalent numbers of children with elevated levels BMI, SF sum, and PBFDXA. A somewhat-similar approach has been taken by other investigators51,52 in the construction of body fat reference curves. It should also be realized that we were primarily interested in PBFDXA, rather than fat mass, as a measure of body fatness. Additional analyses of total fat mass, however, indicated that this characteristic was: (1) more strongly correlated with BMI than with skinfold thicknesses; and (2) that skinfolds provided no additional information beyond that conveyed by BMI.
The results of the current study indicate that sum of the thicknesses of triceps and subscapular skinfolds is more strongly associated with DXA-calculated body fatness of children than is BMI. This stronger association, however, does not necessarily indicate that skinfolds rather than BMI should always be used in the classification of body fatness of children. If the objective is to identify children with low body fatness, which may be associated with slower bone development, 53 skinfold thicknesses are superior. However, if the goal is to identify girls with elevated levels of PBFDXA, who are at increased risk for obesity-related complications, skinfold thicknesses and BMI perform equally well. If the goal is to identify boys with elevated levels of percent body fat, skinfold thickness provides some additional information concerning health risks and correctly identifies an additional 4 of 100 boys who have an elevated PBFDXA, but the importance of this additional information is uncertain. The relatively small improvement obtained with the measurement of skinfold thicknesses should be balanced with the additional training needed to standardize these measurements and the difficulties in obtaining these measurements among obese children.
Glossary
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- DXA
Dual-energy X-ray absorptiometry
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- PBFDXA
Percent body fat determined by dual-energy x-ray absorptiometry
- SF sum
Sum of the triceps and subscapular skinfold thicknesses
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors declare no conflicts of interest.
References
- 1.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 2.Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Arch Dis Child. 2003;88:748–52. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, Wathern AK, et al. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes. 2009;33:866–77. doi: 10.1038/ijo.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes. 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 5.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, DeLany JP, Volaufova J, Harsha DW, Champagne C. Prediction of body fat in 12-y-old African American and white children: evaluation of methods. Am J Clin Nutr. 2002;76:980–90. doi: 10.1093/ajcn/76.5.980. [DOI] [PubMed] [Google Scholar]
- 7.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics. 1997;99:804–7. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- 8.Kerruish KP, O’Connor J, Humphries IR, Kohn MR, Clarke SD, Briody JN, et al. Body composition in adolescents with anorexia nervosa. Am J Clin Nutr. 2002;75:31–7. doi: 10.1093/ajcn/75.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–77. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 10.Laurson KR, Eisenmann JC, Welk GJ. Body fat percentile curves for U.S. children and adolescents. Am J Prev Med. 2011;41:S87–92. doi: 10.1016/j.amepre.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Addo OY, Himes JH. Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am J Clin Nutr. 2010;91:635–42. doi: 10.3945/ajcn.2009.28385. [DOI] [PubMed] [Google Scholar]
- 12.Leitao R, Rodrigues LP, Neves L, Carvalho GS. Changes in adiposity status from childhood to adolescence: a 6-year longitudinal study in Portuguese boys and girls. Ann Hum Biol. 2011;38:520–8. doi: 10.3109/03014460.2011.571220. [DOI] [PubMed] [Google Scholar]
- 13.Gaskin PS, Walker SP. Obesity in a cohort of black Jamaican children as estimated by BMI and other indices of adiposity. Eur J Clin Nutr. 2003;57:420–6. doi: 10.1038/sj.ejcn.1601564. [DOI] [PubMed] [Google Scholar]
- 14.Sarria A, Garcia-Llop LA, Moreno LA, Fleta J, Morellon MP, Bueno M. Skinfold thickness measurements are better predictors of body fat percentage than body mass index in male Spanish children and adolescents. Eur J Clin Nutr. 1998;52:573–6. doi: 10.1038/sj.ejcn.1600606. [DOI] [PubMed] [Google Scholar]
- 15.Sardinha LB, Going SB, Teixeira PJ, Lohman TG. Receiver operating characteristic analysis of body mass index, triceps skinfold thickness, and arm girth for obesity screening in children and adolescents. Am J Clin Nutr. 1999;70:1090–5. doi: 10.1093/ajcn/70.6.1090. [DOI] [PubMed] [Google Scholar]
- 16.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong CP, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes. 2005;29:1346–52. doi: 10.1038/sj.ijo.0803026. [DOI] [PubMed] [Google Scholar]
- 17.Freedman DS, Wang J, Ogden CL, Thornton JC, Mei Z, Pierson RN, et al. The prediction of body fatness by BMI and skinfold thicknesses among children and adolescents. Ann Hum Biol. 2007;34:183–94. doi: 10.1080/03014460601116860. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention National Center for Health Statistics: National Health and Nutrition Examination Survey: Questionnaires, Datasets, and Related Documentation. 2012 Available from http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 19.National Center for Health Statistics The 1999-2004 Dual Energy X-ray Absorptiometry (DXA) Multiple Imputation Data Files and Technical Documentation. 2010 Available from http://www.cdc.gov/nchs/nhanes/dxx/dxa.htm.
- 20.National Center for Health Statistics National Health and Nutrition Examination Survey: Technical Documentation for the 1999-2004 Dual Energy X-Ray Absorptiometry (DXA) Multiple Imputation Data files. 2008 Available from: http://www.cdc.gov/nchs/data/nhanes/dxa/dxa_techdoc.pdf.
- 21.Schenker N, Borrud LG, Burt VL, Curtin LR, Flegal KM, Hughes J, et al. Multiple imputation of missing dual-energy X-ray absorptiometry data in the National Health and Nutrition Examination Survey. Stat Med. 2011;30:260–76. doi: 10.1002/sim.4080. [DOI] [PubMed] [Google Scholar]
- 22.Bodner TE. What improves with increased missing data imputations? Structural Equation Modeling. 2008;15:651–75. [Google Scholar]
- 23.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–13. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 25.National Center for Health Statistics Anthropometry Procedures Manual. 2004 Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/BM.pdf.
- 26.Honaker J, King G, Blackwell M. Amelia: Amelia II: A Program for Missing Data. 2012 Available from http://CRAN.R-project.org/package=Amelia.
- 27.R Development Core Team R: A language and environment for statistical computing (version 2.15.0) 2012 R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available from: http://www.r-project.org/
- 28.World Health Organization . Physical status: the use and interpretation of anthropometry. WHO; Geneva: 1995. p. 420. Report of a WHO Expert Committeee. Technical report series No. 854. Available from http:// www.who.int/childgrowth/publications/physical_status/en/index.html. [PubMed] [Google Scholar]
- 29.Koenker R. Quantreg: Quantile Regression. 2008 R package version 4.24. Available from http://cran.r-project.org/web/packages/quantreg/index.html.
- 30.Lumley T. Complex surveys: A guide to analysis using R. Wiley; Hoboken, NJ: 2010. [Google Scholar]
- 31.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley-Interscience; Hoboken, NJ: 2004. [Google Scholar]
- 32.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd John Wiley & Sons; Hoboken, NJ: 2002. [Google Scholar]
- 33.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–69. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 34.Kraemer HC, Bloch DA. Kappa coefficients in epidemiology: an appraisal of a reappraisal. J Clin Epidemiol. 1988;41:959–68. doi: 10.1016/0895-4356(88)90032-7. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez G, Moreno LA, Blay MG, Blay VA, Fleta J, Sarria A, et al. Body fat measurement in adolescents: comparison of skinfold thickness equations with dual-energy X-ray absorptiometry. Eur J Clin Nutr. 2005;59:1158–66. doi: 10.1038/sj.ejcn.1602226. [DOI] [PubMed] [Google Scholar]
- 36.Bray GA, DeLany JP, Harsha DW, Volaufova J, Champagne CC. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton Rouge Children’s Study. Am J Clin Nutr. 2001;73:687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- 37.Dugas LR, Cao G, Luke AH, Durazo-Arvizu RA. Adiposity is not equal in a multi-race/ethnic adolescent population: NHANES 1999-2004. Obesity. 2011;19:2099–101. doi: 10.1038/oby.2011.52. [DOI] [PubMed] [Google Scholar]
- 38.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, et al. High adiposity and high BMI-for-age in US children and adolescents by race-ethnic group. Am J Clin Nutr. 2010;91:1020–6. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman DS, Wang J, Thornton JC, Mei Z, Pierson RN, Jr, Dietz WH, et al. Racial/ethnic differences in body fatness among children and adolescents. Obesity (Silver Spring) 2008;16:1105–11. doi: 10.1038/oby.2008.30. [DOI] [PubMed] [Google Scholar]
- 40.WHO Multicentre Growth Reference Study Group Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl. 2006;450:38–46. doi: 10.1111/j.1651-2227.2006.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 41.Fields DA, Goran MI. Body composition techniques and the four-compartment model in children. J Appl Physiol. 2000;89:613–20. doi: 10.1152/jappl.2000.89.2.613. [DOI] [PubMed] [Google Scholar]
- 42.Wong WW, Hergenroeder AC, Stuff JE, Butte NF, Smith EO, Ellis KJ. Evaluating body fat in girls and female adolescents: advantages and disadvantages of dual-energy X-ray absorptiometry. Am J Clin Nutr. 2002;76:384–9. doi: 10.1093/ajcn/76.2.384. [DOI] [PubMed] [Google Scholar]
- 43.Sopher AB, Thornton JC, Wang J, Pierson RN, Jr, Heymsfield SB, Horlick M. Measurement of percentage of body fat in 411 children and adolescents: a comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics. 2004;113:1285–90. doi: 10.1542/peds.113.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plank LD. Dual-energy X-ray absorptiometry and body composition. Curr Opin Clin Nutr Metab Care. 2005;8:305–9. doi: 10.1097/01.mco.0000165010.31826.3d. [DOI] [PubMed] [Google Scholar]
- 45.Williams JE, Wells JC, Wilson CM, Haroun D, Lucas A, Fewtrell MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr. 2006;83:1047–54. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- 46.Wells JC, Haroun D, Williams JE, Wilson C, Darch T, Viner RM, et al. Evaluation of DXA against the four-component model of body composition in obese children and adolescents aged 5-21 years. Int J Obes. 2010;34:649–55. doi: 10.1038/ijo.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis KJ, Shypailo RJ. Bone mineral and body composition measurements: cross-calibration of pencil-beam and fan-beam dual-energy X-ray absorptiometers. J Bone Miner Res. 1998;13:1613–8. doi: 10.1359/jbmr.1998.13.10.1613. [DOI] [PubMed] [Google Scholar]
- 48.Tylavsky F, Lohman T, Blunt BA, Schoeller DA, Fuerst T, Cauley JA, et al. QDR 4500A DXA overestimates fat-free mass compared with criterion methods. J Appl Physiol. 2003;94:959–65. doi: 10.1152/japplphysiol.00732.2002. [DOI] [PubMed] [Google Scholar]
- 49.Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, et al. QDR 4500A dual-energy X-ray absorptiometer underes-timates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–25. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 50.National Center for Health Statistics National Health and Nutrition Examination Survey 2003-2004. 2008 Documentation, Codebook, and Frequencies: Dual-Energy X-ray Absorptiometry. Available from http:// www.cdc.gov/nchs/data/nhanes/dxa/dxx_c.pdf.
- 51.Taylor RW, Jones IE, Williams SM, Goulding A. Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3-18 y. Am J Clin Nutr. 2002;76:1416–21. doi: 10.1093/ajcn/76.6.1416. [DOI] [PubMed] [Google Scholar]
- 52.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes. 2006;30:598–602. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- 53.Wells JC, Fewtrell MS. Is body composition important for paediatricians? Arch Dis Child. 2008;93:168–72. doi: 10.1136/adc.2007.115741. [DOI] [PubMed] [Google Scholar]