Abstract
In CD8+ T cells, engagement of the TCR with agonist peptide:MHC molecules causes dynamic redistribution of surface molecules including the CD8 co-receptor to the immunological synapse. CD8 associates with the Src-family kinase Lck, which in turn initiates the rapid tyrosine phosphorylation events that drive cellular activation. Compared to naïve T cells, Ag-experienced CD8+ T cells make shorter contacts with APC, are less dependent on costimulation, and are triggered by lower concentrations of Ag, yet the molecular basis of this more efficient response of memory T cells is not fully understood. Here we show differences between naïve and Ag-experienced CD8+ T cells in colocalisation of the Src-family kinases and their negative regulator, Csk. In naïve CD8+ T cells there was pronounced colocalisation of Src-family kinases and Csk at the site of TCR triggering, while in Ag-experienced cells, Csk displayed a bipolar distribution with a proportion of the molecules sequestered within a cytosolic area in the distal pole of the cell. The data show that there is differential redistribution of a key negative regulator away from the site of TCR engagement in Ag-experienced CD8+ T cells, which might be associated with the more efficient responses of these cells upon re-exposure to antigen.
Keywords: T cell signaling, Immunological Synapse, Csk, Fyn, Lck, PAG, CD8 T cells
Introduction
A hallmark of immunological memory is that upon Ag re-encounter previously activated T cells have a faster response than naive T cells. Memory CD8+ T cells may be activated by peptide concentrations up to 50 times lower than those required to activate naive CD8+ T cells (1) and show a shorter lag time for entry into cell cycle and production of cytokines (2). However the molecular basis of the increased sensitivity of memory T cells remains poorly understood. Activation of αβ CD8+ T cells requires binding of the TCR together with the co-receptor, CD8, to MHC class I molecules containing antigenic peptides (pMHCI) resulting in the redistribution of these molecules to a discrete area called the immunological synapse (IS). The CD8 co-receptor is directly associated with a member of the Src-family kinases (SFK), p56lck (Lck) (3) which is important for the co-receptor function of CD8 (4). The immediate targets of Lck are the ITAMs of the TCR-associated CD3 and ζ chains and ZAP70. In resting T cells the activity of the SFKs is regulated by the balancing interactions of the phosphatase, CD45 (5, 6) and the kinase, Csk (7, 8). Precisely how signal transduction is initiated is unclear, but it involves a combination of colocalisation of the TCR and its co-receptor, and possible further oligomerisation of these complexes, which increases the local concentrations of kinases and their substrates (9). In addition, the active exclusion of negative regulators of signaling, such as phosphatases, particularly CD45 (10) and carboxy-terminal Src kinase (Csk) from the IS is thought to play an important role in signal initiation (11). Indeed, resting T cells are maintained in a quiescent state by the local action of phosphatases, as shown by the ability of phosphatase inhibitors to activate T cells in the absence of receptor engagement (12).
One possible explanation for the increased sensitivity of effector/memory CD8+ T cells to antigenic stimulation is that the balance or distribution of protein tyrosine kinases (PTK) and protein tyrosine phosphatases (PTP) is regulated differently than in naïve cells, resulting in their characteristic lower activation threshold. The availability of Lck is a key determinate of the activation threshold of primary T cells (13, 14), therefore how Csk regulates the activity of Lck has an important influence on T cell activation (15, 16). Csk is a cytoplasmic PTK that down-regulates SFK activity by phosphorylating the carboxy-terminal inhibitory tyrosine and promoting a closed, inactive conformation of the kinase (17). The activity of Csk is regulated by its cellular localisation (15, 16, 18) and by interactions via its SH2 domain which increases the kinase activity of Csk (15, 19). Csk is recruited to the plasma membrane (PM) and thus the vicinity of its SFK target proteins via binding of its SH2 domain to phosphorylated transmembrane adapter proteins such as Csk-Binding Protein/Phosphoprotein Associated with Glycosphingolipid-enriched microdomains (Cbp/PAG) (hereafter named PAG) (20, 21). The second major SFK expressed in T cells, p59fyn (Fyn), plays a role in the recruitment of Csk to the PM by phosphorylating PAG following T cell activation. Fyn selectively phosphorylates PAG on Tyr314 which is the primary residue that interacts with the Csk SH2 domain (20). Together these enzymes modulate signal duration downstream of the TCR, forming an integral part of a negative feedback system that regulates T cell activation. Overexpression of Csk resulted in inhibition of TCR-induced Tyr phosphorylation and IL-2 production (8). Similarly, constitutively targeting Csk to the PM inhibited T cell activation (15, 16) emphasising the importance of cellular localisation for Csk function. Furthermore, loss of PAGpY314 in Fyn knock-out (FynKO) CD8+ T cells decreased the duration of pMHC stimulation required to stimulate IL-2 production, suggesting that Fyn is involved in a negative feedback loop involving Csk (13).
Early localisation of TCR signaling mediators following TCR engagement has been well characterised in CD8+ cytotoxic effector cells (CTL) (22). Positive regulators of TCR signaling, such as Lck and Zap-70, were shown to concentrate at the site of contact within 5-10 minutes of interaction of the TCR with pMHCI on the APC. However, little is known about the localisation of negative regulators in CD8+ T cells. Furthermore, relatively few studies using naïve or memory CD8+ T cells have addressed the localisation of TCR signaling mediators at a single cell level. Biochemical analysis showed that early after T cell activation, PAGpY314 and Csk colocalised at the PM, which correlated with increased phosphorylation of Lck on its inhibitory residue Tyr505 (23, 24). However it was unclear where these interactions occurred relative to the site of T cell:APC contact, or how these proteins were distributed. In this study we asked whether there was differential localisation of key signaling mediators between naive and Ag-experienced CD8+ T cells that could account for the differences in their kinetics of response.
Using four colour confocal microscopy and an imaging flow cytometer we followed the redistribution of the TCR and CD8 co-receptor together with critical PTKs during early TCR engagement. We found that Lck was more efficiently recruited to the site of TCR engagement in Ag-experienced rather than naïve CD8+ T cells. Moreover, in Ag-experienced CD8+ T cells, Csk and Fyn shared a bipolar distribution which was distinct from that found in naive cells. In both cell types Csk and Fyn colocalised with LckpY505 at the site of TCR engagement, but in Ag-experienced CD8+ T cells a distinct pool of Csk and Fyn concentrated within the cytosol at the distal pole of the cell. Our results suggest that altered and more efficient signal transduction intrinsic to Ag-experienced CD8+ T cells may be due in part to the spatial reorganisation of critical T cell signaling mediators.
Materials and Methods
Mice
F5 Rag-1−/− mice, transgenic for a class 1MHC-restricted TCR with a cognate peptide Ag derived from an influenza virus nucleoprotein, NP68 have been described previously (25). The generation of F5 FynKO mice have previously been described (13). Mice were bred in the specific pathogen free facility at the University of Edinburgh (UK). All experiments were approved under a Project License granted by the Home Office (UK) and conducted in accordance with the institutional and ethical guidelines of the University of Edinburgh.
Mouse T cell primary cultures and T cell stimulation
Cell preparation and in vitro generation of Ag-experienced CD8+ T cells
Single cell suspensions from total lymph node (LN) and spleen (SPL) of mice were cultured in IMDM medium supplemented with 5% FCS, L-glutamine, 100 U/mL penicillin and streptomycin (GIBCO) and 50 μM β-mercaptoethanol, additionally supplemented with 10nM NP68 for 3 days. The NP68 nonamer corresponds to residues 366-374 of nucleoprotein from strain A/NT/60/68 and is the naturally occurring epitope presented to T cells by H-2Db (26). Activated CD8+ T cells were then rested for 4 days in 5 ng/mL IL-2 and 10 ng/mL IL-15 (all from PeproTech, UK).
Cell stimulation
For confocal and Imagestream analysis CD8+ T cells were pre-incubated with a cocktail of 10 μg/mL biotinylated anti-TCRβ (H57-597, eBiosciences) and anti-CD8α (KT15, Abcam) Abs on ice for 30 min. Post-washing in 1×PBS, crosslinking was achieved by addition of 5 μg/mL of streptavidin-543 on ice for 10 min. Cells were then incubated at 37°C until termination of cell activation by addition of 4% PFA.
Naïve F5 LN cells or Ag-experienced CD8 T cells were incubated in 96-well tissue culture plates (Nunc, Denmark) at a density of 2.5×105 cells /well in complete media containing soluble peptide at concentrations of 10−7–10−11M NP68 or 10−7M control peptide from the GAG protein of the SF2 strain of HIV (a.ac. 390-398). Alternately, cells were incubated for 30 min on ice with biotin-labeled TCR (H57-597, eBiosciences) and biotin-labeled CD8 (KT15, Abcam) at 10ug/mL, washed once and then seeded into 96-well tissue culture plates containing complete media supplemented with streptavidin AF-405 at 1/1000 (Molecular Probes, UK).
To inhibit phosphatases, 100 μM pervanadate was added for 20 min at 37°C prior to downstream applications.
T cell:APC conjugation assay
RMA-S cells were incubated overnight at 1×107/mL in RPMI/0.5% FCS in a 25°C water bath to enable stable expression of the MHC I molecules at the cell surface. Cells were pulsed for 30 min with 1 μM NP68 or GAG at 25°C, then returned to 37°C/5% CO2 for a minimum of 3 hr to facilitate the decay of empty MHC I molecules from the surface. CD8+ T cells were preincubated on coverslips for 15 min at 37°C/5% CO2. Pulsed RMA-S cells were labelled with 1 μM CellTracker® CMTMR (Molecular Probes, UK), at 5×106/mL, for a minimum of 30 min at 37°C/5% CO2. Labelled cells were washed twice in complete media and incubated for 3 hr at 37°C/5% CO2 to eliminate excess dye. RMA-S cells were mixed with T cells and centrifuged for 1 min at 800 rpm to commence conjugate formation. Cells were then placed at 37°C/5% CO2 for the indicated time points, until fixed by addition of 4% PFA.
Flow Cytometry
Cells were washed twice with 1×PBS then incubated in LIVE/DEAD® (Invitrogen) for 10 min at RT, followed by incubation with anti-FcR blocking mAb (2.4G2, Biolegend) for 10 min at RT and then stained 30 min at 4°C withPE-labelled anti-Vb11 (eBiosciences). For detection of intracellular Tyr phosphorylated proteins, cells were fixed in 2% final concentration PFA for 30 minutes on ice, washed and permeabilised in 90% ice-cold methanol for 30 minutes on ice, then stained with anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (clone 197G2, Cell Signaling) for 1 hr at RT Cells were washed twice in FACS buffer washed and incubated with a second layer streptavidin conjugated to an AF (Molecular Probes, UK) for 30 min at RT, washed and resuspended in FACS buffer for acquisition on FACSCalibur™ or LSR II cytometers (BD). Data were analyzed in FlowJo software (TreeStar, Ashland, USA).
Confocal microscopy
Cells were stimulated as described above and fixed in 4% paraformaldehyde, with residual PFA quenched in 50 mM glycine for 10 min at RT. Cells were permeabilised in 0.1% TritonX-100 for 6 min at RT, washed 4 times in 1× PBS, before staining with anti-Lck (Cell Signaling), anti-phospho-Lck Tyr505 (Cell Signaling), anti-Csk (Abcam) and anti-Fyn (Millipore), anti-γTubulin (Sigma) and BODIPY®FL phallacidin (Invitrogen) at 4°C overnight in PBS/2% BSA. Cells were washed 4 times in PBS/2% BSA and further incubated with species specific anti-F(ab′)2-AF488 or 647 conjugates (Molecular Probes) for 15 min at 4°C. The secondary Ab was removed by washing 4 times in PBS/2% BSA, followed by a 1× PBS wash. Nuclei were stained with DAPI supplemented at 1μg/mL in ProLong Fade Gold (Invitrogen) mounting media. Samples were examined on the Leica SP5 II (Leica Microsystems) with lasers exciting at 405, 488, 543 and 647 nm with the 63× objective, using LAS AP software (Leica, USA). Representative images of the localisation of each molecule are shown. All confocal analysis were multiple repeats and at least 50 images were analysed for each molecule. Data was rendered and analysed using Volocity software (Improvision) and Image J (NIH, USA). Pearson’s correlation coefficient was calculated using Volocity software to determine the pair-wise co-localisation of the signals with a student’s paired two-tailed t-test used to determine statistical significance between two protein data sets.
For quantitation of protein distribution within a single cell image, a line parallel to the plane of the TCR cap was used to identify a 50% region of the cell, termed as proximal, with the distal half of the cell posterior to the site of TCR capping termed distal. A background threshold was set for the channel to be measured, applied to each image and the sum of fluorescence in each region was determined using Volocity software. The sum of fluorescence above the threshold minimum generated by the imaging software was recorded and entered into the following formulae: (P−D)/(P+D) , where: P = the fluorescence in the proximal half of the cell, D = the fluorescence in the distal half of the cell. A student’s paired two-tailed t-test was used to determine statistical significance between two protein data sets.
Imagestream Analysis
Samples were acquired on a 5-laser, 6 channel ImageStream X (ISx, Amnis Corp, Seattle USA) Imaging Flow Cytometer (IFC) with violet (120mW), blue (100mW) and red (120mW) laser excitation turned on. The system was ASSIST calibrated immediately prior to acquisition (27). Single stained controls were collected with bright field illumination turned off in order to generate a compensation matrix post acquisition using the wizard imbedded within the IDEAS analysis software package (Amnis corp, an example matrix are included in Supplementary Figure 2). TCR/CD8 AF450 emission light was collected in camera channel 1, SFK AF488 in channel 2 and CSK AF647 in channel 5. Bright field illumination was set in channel 4 and a cell classifier of 20 was set on the area of the bright filed image to exclude debris from the data file. A minimum of 10,000 single, live cells was collected per sample at 40× magnification. Raw image files (rif) were compensated using the defined matrix values and the gating analysis strategy set out in Supplementary Figure 2 was implemented across all samples to identify live, single in focus AF450, AF488 and AF647 triple positive cells. A Pearson’s-correlation coefficient-based feature within the IDEAS analysis software called “Bright Detail Similarity R3” (BDS-R3) was used to determine the pair-wise co-localisation of the signals on a per cell basis (28).
Results
TCR plus CD8 engagement optimally redistributes Lck to the site of activation in naïve and Ag-experienced CD8+ T cells
In order to compare naive and in vitro generated Ag-experienced CD8+ T cells we used Rag−/− F5 TCR transgenic mice, in which all CD8+ T cells recognise NP68 peptide presented by H-2Db (25), providing a homogenous population of CD8+ T cells. Naive CD8+ T cells were obtained from peripheral LN while Ag-experienced cells were generated in vitro by stimulation with peptide for 3 days followed by 4 days incubation in IL-2 and IL-15 supplemented medium. We confirmed that Ag-experienced F5 T cells were more sensitive to stimulation than naïve F5 T cells by measuring TCR down-regulation and Erk phosphorylation after stimulation with either peptide or Ab-mediated cross-linking (Supplementary Fig. 1). Lower doses of peptide were required to down-regulate TCR (Supplementary Fig. 1A), while phospho-Erk was observed with faster kinetics and in more cells in the Ag-experienced population (Suppl Fig. 1B), confirming that they were more sensitive to stimulation than naïve T cells, as described previously (1).
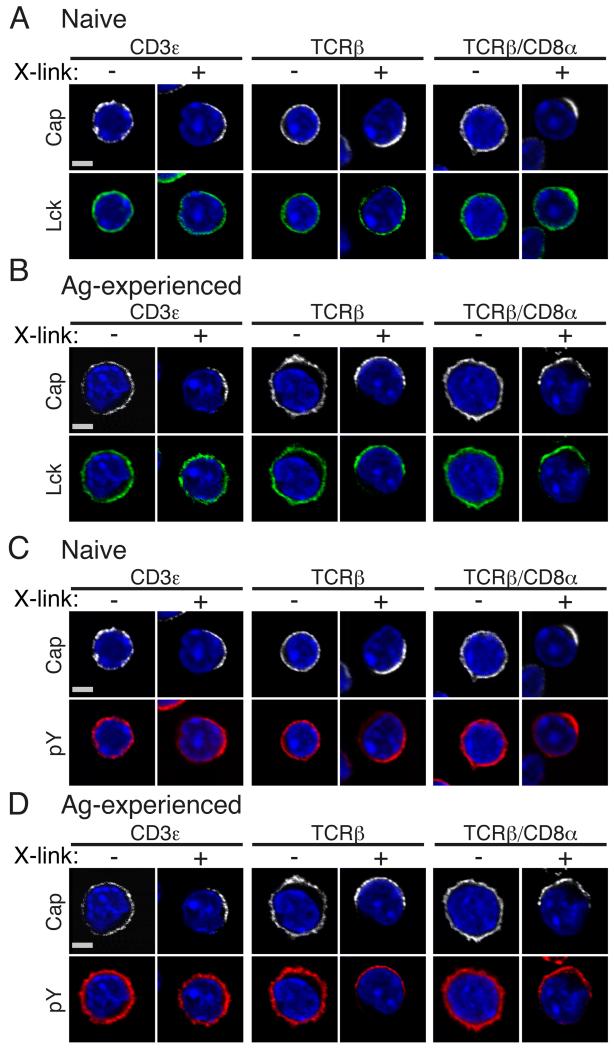
To investigate whether the heightened responses of Ag-experienced CD8+ T cells to TCR stimulation could be due to differences in the distribution of key signaling mediators between naïve and Ag-experienced cells, we asked how the distribution and activation of Lck was influenced by engagement of the TCR and/or co-receptor. Cross-linking Abs were used to stimulate T cells in order to follow redistribution of molecules to defined stimuli in the absence of APC and additional costimulatory or accessory molecules. We addressed the efficiency of mAb cross-linking to CD3ε or TCRβ alone or the combination of TCRβ + CD8α and measured Lck and phosphorylated Tyr (pTyr) residues by confocal microscopy. Cross-linking for 5 minutes with CD3ε alone, TCRβ alone or TCRβ plus CD8α drove discrete capping in both naïve and Ag-experienced CD8+ T cells as expected (Fig 1). In naïve CD8+ T cells, crosslinking CD3ε alone caused only a small proportion of cells (6%) to redistribute Lck to the CD3ε cap (Table 1). In contrast, crosslinking with TCRβ Ab alone caused more cells (20%) to redistribute Lck (Fig 1A, Table 1). The strongest colocalisation of Lck with capped TCR occurred following TCRβ coligation with CD8α, whereupon 28% of cells showed redistribution of Lck to the cap (Fig 1A, Table 1). Similarly, pTyr recruitment to the cap site occurred in more cells following TCRβ and TCRβ/CD8α crosslinking and considerably fewer following crosslinking of CD3ε alone (Fig 1C and Table 1), despite the latter generally being considered to be a better stimulus for T cell activation. Ag-experienced CD8+ T cells behaved similarly to naïve T cells, although cells showed tighter colocalisation of Lck and pTyr residues to the cap site for all the stimuli (Fig 1B, D and Table 1). In regard to crosslinking of TCRβ and TCRβ/CD8α coligation there was a two-fold increase in the number of cells that co-capped Lck in Ag-experienced compared to naïve CD8+ T cells, a trend seen also in pTyr localisation (Table 1). Clearly for both naïve and Ag-experienced CD8+ T cells direct engagement of the co-receptor with TCR optimised recruitment of Lck to the site of capping, although this was improved in Ag-experienced cells.
Figure 1. TCRβ/CD8α ligation is required for optimal redistribution of Lck and Tyr-phosphorylated proteins.
CD8 T cells from naïve F5 mice (A and C) or following in vitro differentiation into Ag-experienced CD8+ T cells (B and D) were stimulated by crosslinking of biotinylated CD3ε, TCRβ and TCRβ/CD8α mAb, as indicated, with streptavidin conjugated to Alexa Fluor (AF) 543 for 5 min. Following fixation and permeabilisation, cells were stained for Lck (A-B) and pY (C-D) and nuclei stained with DAPI. Scale bar represents 3 μM (Naïve) and 3.5 μM (Ag-experienced). A single 2D optical section (along x-y axis) is shown in each panel, with data representative of at least 50 cells for each crosslinking mAb from 2 independent experiments.
I.
Efficiency of Ab crosslinking in redistributing Lck and pY.
| Capping Ab | Co-capping Ab | T cell subset | No. of cells counted | % of cells capped | % of cells co-capped |
|---|---|---|---|---|---|
| Anti-CD3ε | Lck | Naïve | 1260 | 27 | 6.1 |
| Ag.experienced | 1385 | 16 | 13 | ||
| pY | Naïve | 1260 | 27 | 12 | |
| Ag.experienced | 1385 | 16 | 16 | ||
| Anti-TCRβ | Lck | Naïve | 1637 | 32 | 20 |
| Ag.experienced | 760 | 34 | 40 | ||
| pY | Naïve | 1637 | 32 | 28 | |
| Ag.experienced | 760 | 34 | 56 | ||
| Anti-TCRβ/CD8α | Lck | Naïve | 1325 | 20 | 28 |
| Ag.experienced | 826 | 23 | 66 | ||
| pY | Naïve | 1325 | 20 | 45 | |
| Ag.experienced | 826 | 23 | 62 |
Volocity software was used to manually count individual Ab crosslinked naive and Ag-experienced CD8+ T cells and count the number of cells co-capping Lck and pY.
Differential distribution of Csk and Fyn between naïve and Ag-experienced CD8+ T cells
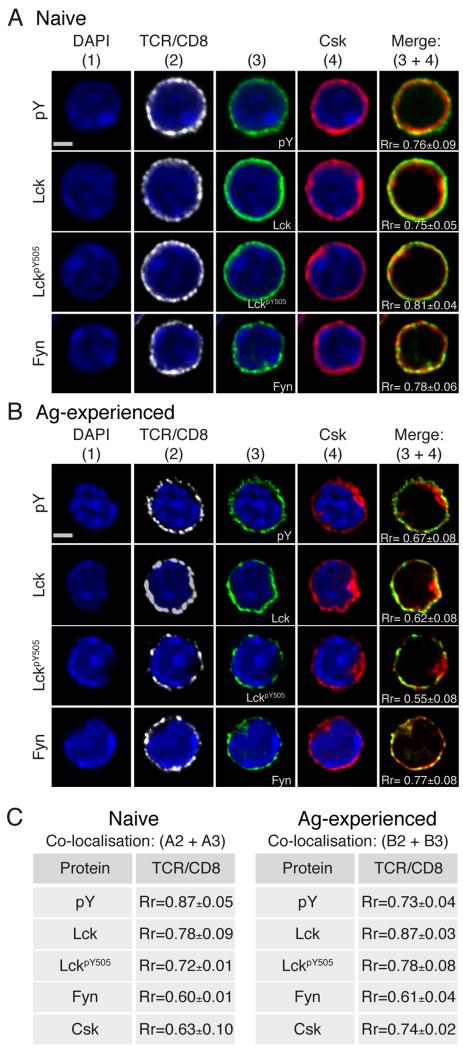
We asked whether other upstream positive and negative regulators of TCR signals were differentially distributed upon TCR-triggering in naïve versus Ag-experienced CD8+ T cells. Lck is the most proximal kinase to be activated upon TCR activation, with recent literature suggesting that there is dynamic regulation of Lck activity already at the basal state, partly due to the localisation of Csk, which determines TCR signaling initiation and sensitivity (16, 29). Csk is critical for down-regulating Lck signals as it is the sole kinase able to phosphorylate the SFK negative regulatory Tyr, LckY505. In the absence of Csk (30) or upon mutation of LckY505 to alanine (Ala) (31) TCR signaling is dysregulated and following activation T cell signal termination is impaired. Fyn has also been implicated in negative regulation of T cell signaling as it phosphorylates Tyr314 on the transmembrane adapter PAG facilitating recruitment of cytosolic Csk to the PM. In the absence of Fyn T cell responses are slower to turn off and can predispose to autoimmunity (32). Naive CD8+ T cells, in a resting state, display a homogeneous distribution of pTyr, Lck, LckpY505 and Fyn at the cell periphery identified by TCRβ and CD8α staining (Fig 2A). Despite lacking the amino-terminal modifications of palmitylation, myristoylation and S-acetylation that direct Lck and Fyn to the PM (33-35), Csk was also observed to localise with these two SFK members. The average Pearson’s correlation coefficient indicated strong colocalisation of Csk with both Lck and Fyn (Rr= 0.75 and 0.78 respectively) (Fig 2A). As Csk is the only kinase to phosphorylate LckY505 (7, 23), the relative distribution of Csk and LckpY505 was investigated. In naïve CD8+ T cells LckpY505 was homogenously distributed at the cell periphery, highly colocalised with Csk (Rr= 0.81) (Fig 2A).
Figure 2. Naive and Ag-experienced CD8+ T cells differentially localise Csk and Fyn.
Confocal immunofluorescence of resting naïve (A) and Ag-experienced (B) CD8+ T cells stained with anti-TCRβ plus CD8α, DAPI, Csk, pY, Lck, LckpY505, and Fyn, as indicated. A single 2D optical section (along x-y axis) is shown in each panel with colocalisation shown as a merge image of green and red pixels (column 5). Scale bar represents 3 μM (Naïve) and 3.5 μM (Ag-experienced). (C)Tables show values for colocalisation of white (column 2) and green (column 3) pixels. Pearson’s correlation coefficient (Rr) was calculated using Volocity software from 2 independent experiments.
In Ag-experienced CD8+ T cells, basal levels of pTyr were again detected at the cell periphery together with Lck (Fig 2B). Strikingly, in contrast to naïve cells, Csk distributed not only with SFKs at the cell periphery of Ag-experienced CD8+ T cells but was also observed within a discrete cytoplasmic area in the majority of cells analysed (Fig 2B). As a consequence the average localisation score between Csk with Lck and Csk with LckpY505 decreased significantly in Ag-experienced compared to naïve CD8+ T cells (p≤0.05 and p≤0.01 respectively) . Interestingly, the correlation between Csk and Fyn remained similar between naïve and Ag-experienced CD8+ T cells (Rr= 0.78 and 0.77 respectively, p>0.05) (Fig 2A, B). In Ag-experienced CD8+ T cells Fyn distributed similarly to Csk, both at the cell periphery and within the cytosolic area in which Csk was present (Fig 2B). Similarly to naïve cells, Csk demonstrated a significant loss of association with TCRβ/CD8α (Rr= 0.74, p≤0.0001) (Fig 2C) and maintained stronger colocalisation with Fyn compared to PM associated Lck (Rr= 0.77 and 0.62 respectively), reflecting the increased accumulation of Csk with Fyn within the defined cytosolic area. We asked whether the differential localisation of Csk in Ag-experienced versus naïve CD8+ T cells resulted in altered Lck activity, by examining phosphorylation of Shc which is a target of Lck (36). Ag-experienced CD8+ T cells exhibited faster kinetics and greater phospho-Shc following stimulation, consistent with stronger activation of Lck in this population compared to naïve CD8 T cells (Suppl. Fig. 1).
Bipolar distribution of Csk and Fyn upon TCR engagement of Ag-experienced, but not naïve CD8+ T cells
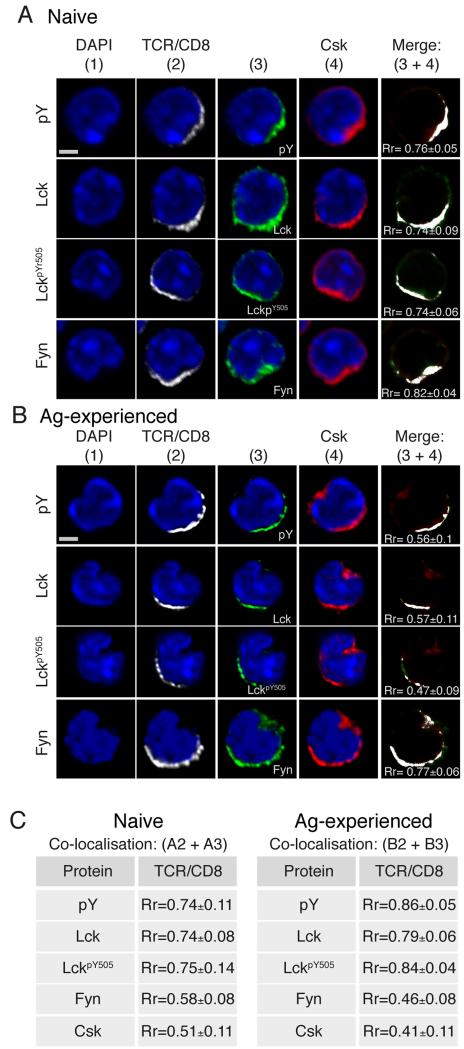
As Csk and Fyn are regulators of TCR signaling and having identified a second intracellular pool of colocalised Csk and Fyn in Ag-experienced cells that was not present in naïve CD8+ T cells, we asked how Csk and Fyn behaved upon TCR stimulation in both cell types. After TCRβ/CD8α cross-linking, naïve T cells showed strong association of Csk with pTyr, Lck, LckpY505 and Fyn (Rr = 0.76, 0.74, 0.74 and 0.82 respectively, Fig. 3A). These associations occurred at the site of capping only. In contrast, Ag-experienced CD8+ T cells showed much lower association of Csk with pTyr, Lck, and LckpY505 (Rr = 0.56, 0.57 and 0.47, respectively), while retaining a strong association between Csk and Fyn (Rr = 0.77, Fig 3B).
Figure 3. Csk and Fyn display bipolar distribution in Ag-experienced CD8+ T cells following TCRβ/CD8α mediated activation.
Confocal immunofluorescence of (A) naïve and (B) Ag-experienced CD8+ T cells stimulated with TCRβ/CD8α for 5 min. Cells were fixed, permeabilised stained for Csk, column 4, and in column 3 for pTyr (top row), Lck (second row), LckpY505 (third row) and Fyn (bottom row). A single 2D optical section (along x-y axis) is shown in each panel, and all red and green colocalised pixels represented as white pixels on a merge image of 3+4. Scale bar represents 3 μM for naïve cells and 3.5 μM for Ag-experienced cells. (C) Tables show values for colocalisation of white (column 2) and green (column 3) pixels. Pearson’s correlation coefficient (Rr) was calculated using Volocity software from at least 2 independent experiments (Rr 3+4 naïve, n=40; Rr 2+3 naive, n=50; Rr 3+4 n=50; Rr 2+3 n=50).
The reason that Csk was less well associated with Lck and pTyr in Ag-experienced CD8+ T cells after stimulation was because the cytoplasmic pool of Csk, which we identified in resting Ag-experienced cells (Fig 2B), remained in the area distal to the TCRβ/CD8α cap even after TCR cross-linking. As a consequence, although the capped TCR remained as, or even more, tightly associated with pTyr, Lck and LckpY505, the association between TCRβ/CD8α and Csk reduced from Rr = 0.51 in naïve T cells to Rr = 0.41 in Ag-experienced cells (Fig 3C). As before, Fyn retained stronger co-localisation with Csk than with capped TCR in both naïve and Ag-experienced CD8 T cells, and in the latter was seen in the distal cytoplasmic area.
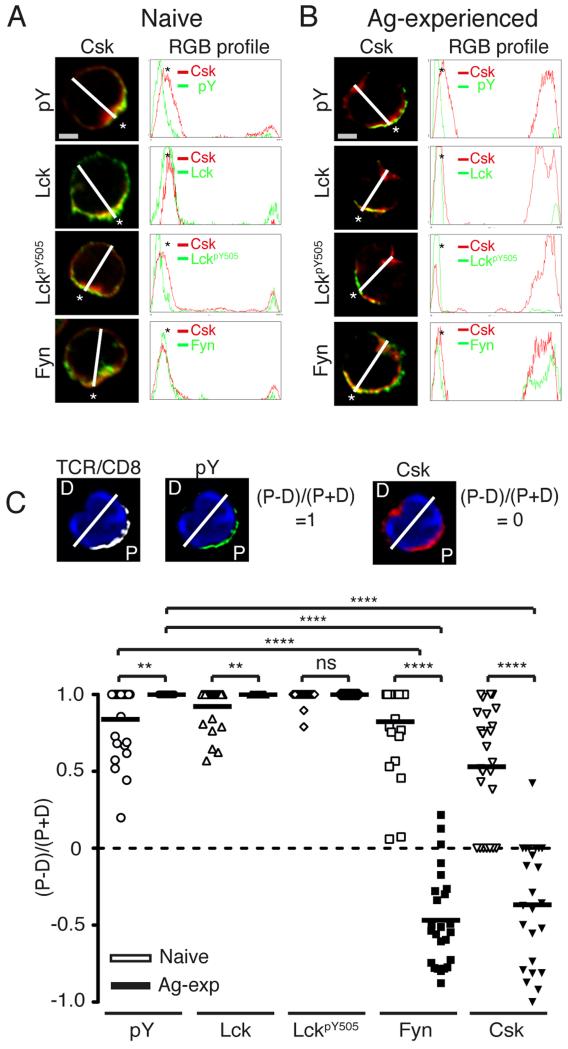
To visualize better the proportion of molecules that redistributed towards the proximal and distal poles of stimulated naïve and Ag-experienced CD8+ T cells, we used spectral overlaps and RGB histogram analysis. A merge image sectioned from the proximal (designated by *) to the opposing, distal end (trajectory represented by a white line) showed that in naive CD8+ T cells the majority of Lck, LckpY505, Fyn and Csk molecules polarised to the site of the TCRβ/CD8α cap (Fig 4A). Similar analysis of Ag-experienced CD8+ T cells identified a proportion Csk at the proximal pole of the cell, colocalising with pTyr, Lck and LckpY505 and Fyn (Fig 4B). In addition a large peak of both Csk and Fyn molecules was detected in close association with each other at the distal end of Ag-experienced (Fig 4B) but not naïve (Fig 4A) CD8+ T cells. Moreover, in Ag-experienced CD8+ T cells, the peak representing Csk at the distal pole was much broader than that at the proximal pole. The latter was more similar to the peak width of known membrane-associated molecules, such as Lck, while the former was in keeping with the notion that the distal pool of Csk is largely cytoplasmic.
Figure 4. Csk and Fyn are significantly less polarised to the site of activation in Ag-experienced CD8+ T cells than naïve T cells.
(A) Naive and (B) Ag-experienced CD8+ T cell merge images (first column) generated from the images in Figure 3 were sectioned (white line) from the proximal site of cell polarisation, indicated by the astrix (*), to the distal end, generating a RGB histogram (second column), using ImageJ software. The histogram lines indicate the distribution and MFI of protein throughout the cross-section of the cell. (C) Protein distribution was calculated within the merge images from experiments presented in Figure 3. Using Volocity software, the sum of fluorescence above background of each condition was calculated in both the proximal half of the cell, identified by the presence of the TCRβ/CD8α and denoted P as shown in the representative image. The amount of fluorescent units were entered into the formulae (P−D)/(P+D), where D, represents distal fluorescence. Thus a protein fully polarised at the TCRβ/CD8α cap will score 1 (pY, second panel), a protein with bipolar distribution will score closer to 0 (Csk, third panel) and a protein fully localised in the distal pole will score −1 (image not shown). The data set of 1 experiment, comprising 25 images for each condition was used to generate the protein distribution graph and calculate p-values as determined by the Student’s two-tailed t-test; where p≤0.05 = *, p≤0. 01 = **, p≤0.001 = ***, p≤0.001 = ****. The data are representative of at least 2 independent experiments.
In addition we calculated the proportion of each molecule found in the proximal and distal poles of a cell by sectioning the merge image into the proximal end (P), identified by the TCRβ/CD8α cap, and the opposing distal end of the cell (D) (Fig 4C). The results in Fig. 4C show clearly that Csk was significantly less polarised to the site of the TCRβ/CD8α cap in Ag-experienced CD8+ T cells than naïve T cells (p≤0.0001). Similarly, the majority of Fyn molecules were significantly more concentrated in the distal pole of stimulated Ag-experienced CD8+ T cells (Fig 4C) compared to naïve T cells (p≤0.0001), further confirming the close association between Fyn and Csk. Additionally, significantly more pTyr and Lck molecules were recruited to the TCRβ/CD8α cap in Ag-experienced rather than naïve CD8+ T cells (p≤0.01). These data show that distribution of signaling molecules changes between naïve and Ag-experienced CD8 T cells such that the latter have tighter association of Lck, a positive regulator of T cell signaling, and exclusion of Csk, a negative regulator of signaling from the IS.
Csk is sequestered from Lck and the TCR following activation
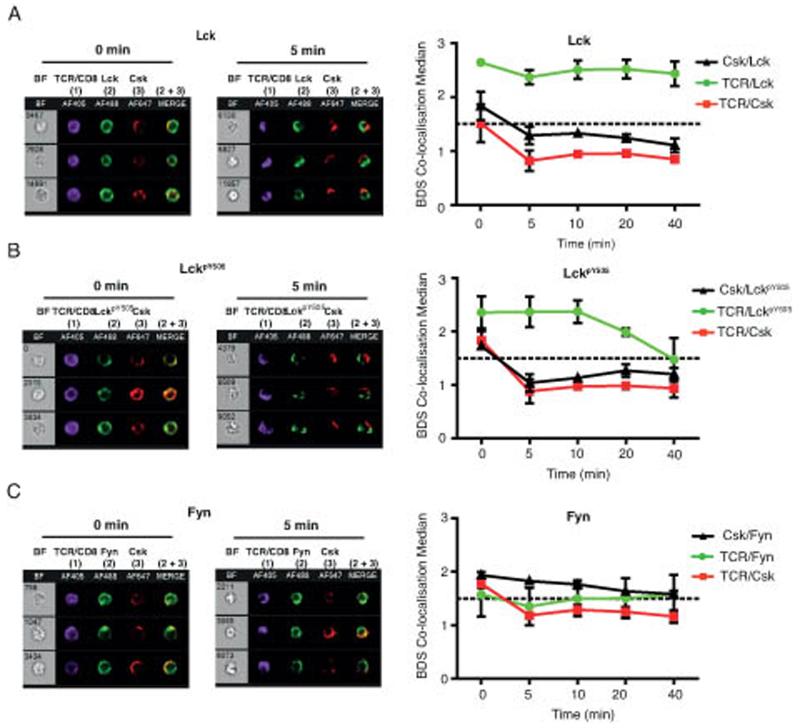
In order to follow the kinetics of redistribution of proximal PTKs upon T cell stimulation of Ag-experienced CD8+ T cells we utilised a high throughput imaging flow cytometer, Imagestream, which although lacking the high resolution afforded by the confocal studies, enabled us to obtain a measure of the spatial distribution of molecules from large numbers of cells over multiple time points. The Imagestream allows the separation of cells based on multiple parameters including removal of apoptotic cells based on Live/Dead staining so that only viable activated Ag-experienced CD8+ T cells were analysed (gating strategy is detailed in Supplementary Fig 1). A log-transformed Pearson’s correlation coefficient was used to measure colocalisation (Bright Detail Similarity score, BDS), and the morphometrically relevant biological (MRB) control (37) of Lck and TCRβ/CD8α signals was used to set a threshold on two signals known to heavily co-localise. On this basis we determined that median BDS scores of <1.5 (indicated by the broken line in the histograms, Fig 5B) corresponded to dissimilar image pairs, and conversely scores >1.5 were similar image pairs.
Figure 5. Csk is maintained away from the site of TCR activation in Ag-experienced CD8+ T cells.
Ag-experienced CD8+ T cells were stimulated with TCRβ/CD8α and Streptavidin AF405 (column 1) for the indicated times (0-40 min), fixed, permeabilised and intracellular proteins were stained for Csk, column 3, and in column 1 for Lck (A), or LckpY505 (B) or Fyn (C). Each panel shows representative cells from the median BDS-R3 value at 0 and 5 min, with colocalisation shown as a merge image of green and red pixels (2 + 3). Graphs of the median BDS-R3 score for the indicated image pairs on a per cell basis, are the mean of 3 independent experiments ± SEM (for some points the error bars are too small to be visible outwith the symbols). The dotted line denotes the MRB-determined threshold below which two signals are considered to be dissimilar by the BDS-R3 score (<1.5).
Analysis of Ag-experienced CD8+ T cells showed that at Time 0 the localisation of Csk significantly correlated with TCRβ/CD8α, Lck and LckpY505 (BDS ≥1.5) (Fig 5A-B), suggesting all proteins shared similar spatial location, in line with the confocal data (Fig 2). Following TCRβ/CD8α crosslinking, Lck retained strong colocalisation with TCRβ/CD8α (BDS >2), while 5 mins after stimulation the correlation between Csk with TCRβ/CD8α, Lck and LckpY505 was lost (BDS <1.5). The only molecule with which Csk remained colocalised was Fyn (BDS >1.5) up to 40 mins after activation. The loss of correlation between Csk and TCRβ/CD8α or Lck 5 minutes after TCR crosslinking (Fig 5A) was due to the bipolar distribution of Csk in Ag-experienced CD8+ T cells as seen in confocal analysis (Fig 3B). Interestingly, although LckpY505 maintained a strong colocalisation with TCRβ/CD8α early after crosslinking beyond 20 minutes this was reduced, so that by 40 minutes after TCR stimulation the BDS was ≤1.5, suggesting the negatively regulated form of Lck disappears from the TCRβ/CD8α cap over time. Overall these data indicate that exclusion of Csk and Fyn from the site of TCR engagement is a dynamic process which is maintained for at least 40 minutes following activation.
Csk localisation is not dependent on Fyn
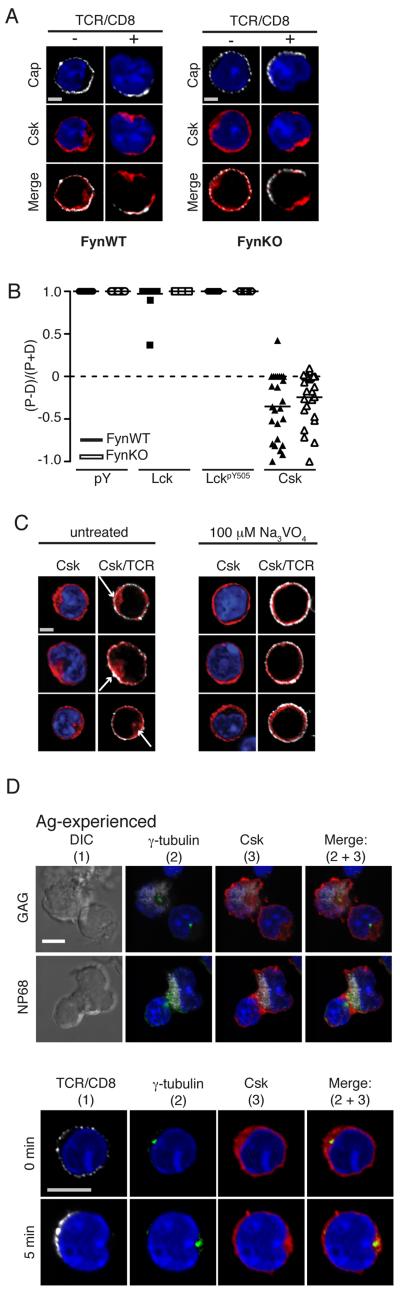
Given the close association between Csk and Fyn in these studies we asked whether the observed distribution of Csk required the presence of Fyn. Csk is recruited to the PM by interaction with membrane associated adaptor molecules including the transmembrane adaptor PAG (20, 21). In resting T cells PAG is phosphorylated on Y314 by Fyn, generating an SH2 binding site for Csk (20). Previously we showed that Fyn knockout (FynKO) CD8+ T cells were more sensitive than WT cells to Ag stimulation, which correlated with abrogation of phosphorylation of PAGpY314 and was consistent with a loss of negative feedback (13). Therefore we asked whether the spatiotemporal localisation of Csk would be altered in Ag-experienced FynKO F5 CD8+ T cells. Somewhat surprisingly, there appeared to be no difference to WT cells in the localisation of Csk in either unstimulated Ag-experienced FynKO CD8+ T cells or following TCRβ/CD8α mediated activation (Fig 6). In both WT and FynKO Ag-experienced CD8+ T cells, Csk was identified at the periphery of the cell as well as localising within a distinct cytosolic pool (Fig 6A). Following TCRβ/CD8α crosslinking, there was no difference in the localisation of Csk between WT and FynKO T cells, with Csk displaying a bipolar distribution (Fig 6A). Quantification of the distribution of Csk molecules in polarised WT and FynKO Ag-experienced CD8+ T cells showed no significant difference between the two genotypes (Fig 6B). These data suggests that the bipolar distribution of Csk observed in Ag-experienced CD8+ T cells is not dependent on PAGpY314.
Figure 6. Csk localisation does not require PAGpY314.
(A-B) Ag-experienced F5 (FynWT) and Ag-experienced F5 FynKO CD8+ T cells were crosslinked with TCRβ/CD8α and Streptavidin AF543 for 5 min fixed, permeabilised and intracellular proteins were double stained for Csk with Lck and LckpY505. (B) The sum of fluorescence above background of each condition was calculated using Volocity software in both the proximal and the distal half of the cell. The data set of 1 experiment, comprising 25 images for each condition was used to generate the protein distribution graph and calculate p-values as determined by the Student’s two-tailed t-test; where p≤0.05 = *, p≤0.01 = **, p≤0.001 = ***, p≤0.001 = ****. The data is representative of 2 independent experiments. (C) Ag-experienced CD8+ T cells were treated for 20 min with Na3VO4, stained with TCRβ/CD8α, fixed, permeabilised and stained for Csk, with nuclei stained with DAPI. A single 2D optical section (along x-y axis) is shown in each panel and an overlay of red and white pixels is represented as a merge image. Scale bar represents 1.3μM. Arrows indicate distribution of Csk in cytoplasm. Data is representative of at least 50 cells of each condition from 3 independent experiments. (D) Ag.experienced CD8+ T cells were conjugated to NP68- or GAG-pulsed RMA-S cells for 5-min (top 2 panels) or activated by TCRβ/CD8α cross-linking (bottom 2 panels) and fluorescently labeled with Abs to Csk and γ-tubulin to label the centrosome. Images were rendered with Volocity software. Representative conjugates containing GAG-pulsed RMA-S (first panel) or NP68-pulsed RMA-S (second panel) each intracellularly labelled with Mitotraker (white) and a single Ag.experienced CD8+ T cell. Scale bar represents 6 μM. Data is representative of at least 50 cells of each condition from 2 independent experiments.
In order to investigate the localisation of Csk at the distal structure, we first asked whether its presence was influenced by the action of PTPs. Many intracellular PTPs act as negative regulators of TCR signaling, so we inhibited PTP activity with pervanadate (Na3VO4), which gives increased pTyr and generation of SH2-binding domains, altered Csk localisation in Ag-experienced CD8+ T cells. Inhibition of cellular PTP activity by treatment of cells with Na3VO4 resulted in redistribution of Csk from the cytosol (indicated by arrows in unstimulated, Fig 6C) to the cell periphery, with almost complete loss of the cytosolic structure. These data argue that compartmentalisation and sequestration of Csk from the PM is maintained by PTP activity in Ag-experienced CD8+ T cells. Staining with γ-tubulin showed that the centrosome was present in the distal area of the Ag-experienced cell in which Csk was found to accumulate (Fig 6D). Somewhat surprisingly the centrosome did not re-localise to the cap following TCR pus CD8 cross-linking (Fig 6D). It has been shown that the centrosome localises to the IS following Ag stimulation of CTL and that docking of the centrosome at the IS requires signals from Lck (38). We confirmed that the formation of Ag-specific conjugates between Ag-experienced CD8 T cells and peptide-pulsed RMA-S cells relocated the centrosome to the IS (Fig 6D). In addition we followed the location of Csk in Ag-specific conjugates and found that Csk was distributed at both the IS and to a lesser extent at the distal pole of the cell at the 5min time point (Fig 6D). By 10 mins of conjugation most Csk molecules were associated with the IS and this association became more localised over time (Suppl Fig 3B). Therefore the re-distribution of Csk and the centrosome is influenced by the nature of the stimulus, and surprisingly, despite the observation that cross-linking with TCR and anti-CD8 Abs causes robust Lck phosphorylation, this is insufficient to re-localise the centrosome to the site of capping.
These results identify differences in the distribution of signaling molecules in naïve and Ag-experienced CD8+ T cells both in the resting state and following TCR activation with antibody stimulation. Taken together they indicate that loss of the critical negative regulator Csk from the PM reduces the threshold of TCR triggering in effector/memory CD8+ T cells. Furthermore, upon TCR triggering with antibodies, Csk and the centrosome remain sequestered at the distal pole of the cell.
Discussion
The molecular basis for the increased sensitivity of memory T cells to TCR triggering by Ag is currently unknown. Using confocal imaging of endogenous molecules, we addressed this issue by asking whether we could see differences in the distribution of key proximal signaling molecules between naïve and Ag-experienced CD8+ T cells, in both the resting state and following TCR engagement. We show that Csk, the primary negative regulator of the SFKs, Lck and Fyn, has a unique cytoplasmic location in Ag-experienced cells, which was not seen in naïve CD8+ T cells. In addition there was a pool of Csk associated with the cell periphery, which was common to both cell types. Upon TCR engagement the discrete cytoplasmic pool of Csk was maintained in the distal pole of the cell away from the TCR. The data suggested that this pool of Csk was located in a cytoplasmic structure, as it was disrupted upon removal of cholesterol. Fyn, but not Lck, was associated with the cytoplasmic pool of Csk, whereas both Fyn and Lck were associated with peripherally distributed Csk. However, there was no absolute requirement for Fyn to maintain the cytoplasmic pool, as it was undiminished in FynKO Ag-experienced CD8+ T cells. Our data support the hypothesis that the reduce threshold of activation seen in Ag-experienced CD8+ T cells could be facilitated by the spatial redistribution of proximal signalling mediators, such as sequestration of Csk into the cytosol away from its substrate Lck at the PM.
We showed previously that the availability of Lck was an important element in determining the activation threshold of primary T cells (36). More recently it was suggested that the activity of Lck was crucial for initiating T cell activation (29). Up to 40% of Lck in resting T cells was found to be constitutively active and a proportion of this activated pool was phosphorylated on the regulatory Tyr505 residue, previously thought to mark only inactive pools of Lck (29). Csk is the PTK responsible for phosphorylating the SFK members on their regulatory Tyr but, unlike the SFKs, Csk is not constitutively targeted to the PM. Instead Csk is recruited by interaction of its SH2 domain with a variety of Tyr phosphorylated proteins (15, 39-43). Constitutive targeting of Csk to the PM reduced the basal state of activity of primary T cells (16) indicating that the localisation of Csk is important in regulating the signaling threshold even in the absence of Ag.
The presence of a distal pole complex (DPC) that forms early after TCR activation has been previously identified in CD4+ T cells (44-46) and was shown to concentrate negative regulators of T cell signaling including, SHP-1 and PAG. Localisation of PAG to the DPC was potentially mediated by Ezrin, as EBP-50 which is the intermediary protein linking PAG to the actin cytoskeleton through Ezrin, was also localised to the DPC (47). However, the arrangement of molecules in the DPC was different from that observed here, as the DPC was distributed as if attached to the PM, whereas we found Csk to have a distinct cytoplasmic location. In addition molecules were reported to localise to the DPC only after pMHC Ag presentation (44, 45, 47), whereas we found Csk colocalising in the cytoplasmic pool in Ag-experienced CD8+ T cells to be constitutive and unchanged by Ab-mediated TCR crosslinking. Upon interaction with APC and formation of conjugates, T cells round up as they receive stop signals (48, 49). The assembly of the IS occurs in various stages which depend on distinct cytoskeletal rearrangements that facilitate the integration of signals, but the requirement of the IS may vary between CD4+ and CD8+ T cells, and whether the cells are naïve, have effector functions or are multifunctional memory cells. In naïve CD4+ T cells, microclusters containing CD3, TCR and PLCγ aggregate within the SMAC, the site of T cell signalling. In CTL, a correlation between the formation of the dSMAC and the docking of the centrosome at the PM has been demonstrated, the latter required for granule delivery and lytic function (50). The signals delivered through TCRβ/CD8α crosslinking were not sufficient to polarise nonmigratory Ag-experienced CD8+ T cells, perhaps reducing their full effector function or limiting their multifunctional potential. Regardless, early signalling events immediately downstream of the TCR were enhanced in Ag-experienced CD8+ T cells compared to naïve T cells, which may be attributed to the removal of Csk from the PM and its active sequestration within a cytoplasmic structure both in a basal state and following TCR triggering acting to dynamically regulate Csk activity, possibly by inhibiting activation by PKA at the PM (51).
It was striking that Fyn showed a very tight co-localisation with Csk both at the cell periphery and in the cytoplasmic pool, unlike Lck which was only co-localised with Csk at the PM. Fyn is responsible for phosphorylating PAGpY314, which is the major pTyr residue that recruits Csk (20, 52). We were unable to determine whether PAG co-localised with Csk in the cytoplasmic pool, as available anti-PAG antibodies gave excessive background staining in confocal microscopy. Certainly Fyn-mediated PAG phosphorylation was not essential for this localisation of Csk, as FynKO Ag-experienced cells showed similar distribution of Csk to WT CD8+ T cells. However, the absence of an overtly deleterious phenotype in PAGKO mice (53, 54) and in FynKO mice (55, 56) argues that molecules other than Fyn and PAG are also able to influence Csk localisation.
In this study we addressed the requirement of key signaling mediators associated with TCR-dependent activation and therefore triggered the TCR through Ab-crosslinking of CD3, or TCR plus CD8 in the absence of costimulation, which has been reported to affect IS formation between naive and memory T cells (57). It has been shown previously that Lck associates with the co-receptor (3, 58), whereas Fyn interacts with TCR/CD3 (59). Indeed, FynKO T cells became hyporesponsive upon anti-CD3 stimulation (55, 56), most likely because Lck was not activated to the same extent as when CD3 was engaged in combination with anti-CD4/CD8 (60). In support, our data identified that anti-CD3 alone was largely inadequate at redistributing Lck from around the cell periphery to the CD3 cap, compared to TCR, which generated a 3-fold increase in Lck coincident with the TCRβ cap. It was not until additional CD8 coligation that maximal colocalisation of Lck was observed in both naive and Ag-experienced CD8+ T cells. Of interest, Ag-experienced CD8+ T cells were twice as efficient in redistributing Lck as naïve CD8+ T cells, which is consistent with previous findings that a greater proportion of Lck molecules are found at the PM associated to the coreceptor in memory CD8+ T cells (61). This suggests that there is a rapid reorganisation of positive mediators of TCR signaling in Ag-experienced compared to naïve CD8+ T cells, which may contribute to the enhanced memory cell response.
Our data are consistent with the requirement for specific compartmentalisation of signaling mediators for the regulation of T cell homeostasis and the altered sensitivity to Ag stimulation that occurs between naïve and Ag-experienced CD8+ T cells. We propose that early TCR triggering events drive divergent signaling events between naive and memory CD8+ T cells, and it is the rapid reorganisation of signaling molecules that contribute to enhanced memory cell responses.
Supplementary Material
Acknowledgments
We would like to thank Derek Davies for discussion of Imagestream flow cytometry analysis and the Zamoyska laboratory for general discussions.
Abbreviations used in this paper
- Csk
C-terminal Src kinase
- LN
lymph nodes
- IS
immunological synapse
- PAG
phosphoprotein associated with glycoshingolipid-enriched microdomains
- PM
plasma membrane
- pMHCI
peptide MHC Class 1
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
- pTyr
phosphorylated tyrosine
- SFK
Src family kinase
- SPL
spleen
- WT
wild-type
Footnotes
Work was supported by Wellcome Trust Grant # WT086054MA and MRC Grant # G1100116; JGB was the recipient of an MRC UK PhD studentship. This work was also supported by a Strategic award from the Wellcome Trust for the Centre for Immunity, Infection and Evolution (Grant reference 095831)
References
- 1.Pihlgren M, Dubois PM, Tomkowiak M, Sjogren T, Marvel J. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J Exp Med. 1996;184:2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sprent J, Judge AD, Zhang X. Cytokines and memory-phenotype CD8+ cells. Adv Exp Med Biol. 2002;512:147–153. doi: 10.1007/978-1-4615-0757-4_20. [DOI] [PubMed] [Google Scholar]
- 3.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 4.Zamoyska R, Derham P, Gorman SD, von Hoegen P, Bolen JB, Veillette A, Parnes JR. Inability of CD8 alpha’ polypeptides to associate with p56lck correlates with impaired function in vitro and lack of expression in vivo. Nature. 1989;342:278–281. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]
- 5.Ashwell JD, D’Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–416. doi: 10.1016/s0167-5699(99)01505-4. [DOI] [PubMed] [Google Scholar]
- 6.Thomas ML, Brown EJ. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today. 1999;20:406–411. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 7.Bergman M, Mustelin T, Oetken C, Partanen J, Flint NA, Amrein KE, Autero M, Burn P, Alitalo K. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. Embo J. 1992;11:2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow LM, Fournel M, Davidson D, Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993;365:156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- 9.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 10.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 11.Acuto O, Di Bartolo V, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 12.Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 13.Filby A, Seddon B, Kleczkowska J, Salmond R, Tomlinson P, Smida M, Lindquist JA, Schraven B, Zamoyska R. Fyn regulates the duration of TCR engagement needed for commitment to effector function. J Immunol. 2007;179:4635–4644. doi: 10.4049/jimmunol.179.7.4635. [DOI] [PubMed] [Google Scholar]
- 14.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 15.Cloutier JF, Chow LM, Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Mol Cell Biol. 1995;15:5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci Signal. 2011;4:ra59. doi: 10.1126/scisignal.2001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 18.Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Horejsi V, Schraven B, Rolstad B, Mustelin T, Tasken K. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi S, Takayama Y, Ogawa A, Tamura K, Okada M. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J Biol Chem. 2000;275:29183–29186. doi: 10.1074/jbc.C000326200. [DOI] [PubMed] [Google Scholar]
- 20.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 22.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 23.Davidson D, Schraven B, Veillette A. PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. Mol Cell Biol. 2007;27:1960–1973. doi: 10.1128/MCB.01983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koneru M, Schaer D, Monu N, Ayala A, Frey AB. Defective proximal TCR signaling inhibits CD8+ tumor-infiltrating lymphocyte lytic function. J Immunol. 2005;174:1830–1840. doi: 10.4049/jimmunol.174.4.1830. [DOI] [PubMed] [Google Scholar]
- 25.Mamalaki C, Norton T, Tanaka Y, Townsend AR, Chandler P, Simpson E, Kioussis D. Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T-cell receptor transgenic mice. Proc Natl Acad Sci U S A. 1992;89:11342–11346. doi: 10.1073/pnas.89.23.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood P, Elliott T. Glycan-regulated antigen processing of a protein in the endoplasmic reticulum can uncover cryptic cytotoxic T cell epitopes. J Exp Med. 1998;188:773–778. doi: 10.1084/jem.188.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filby A, Perucha E, Summers H, Rees P, Chana P, Heck S, Lord GM, Davies D. An imaging flow cytometric method for measuring cell division history and molecular symmetry during mitosis. Cytometry A. 2011;79:496–506. doi: 10.1002/cyto.a.21091. [DOI] [PubMed] [Google Scholar]
- 28.Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 1998;394:901–904. doi: 10.1038/29802. [DOI] [PubMed] [Google Scholar]
- 31.Abraham KM, Levin SD, Marth JD, Forbush KA, Perlmutter RM. Thymic tumorigenesis induced by overexpression of p56lck. Proc Natl Acad Sci U S A. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 33.Shenoy-Scaria AM, Gauen LK, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koegl M, Zlatkine P, Ley SC, Courtneidge SA, Magee AI. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem J. 1994;303(Pt 3):749–753. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. Embo J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, Zamoyska R. Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol Cell Biol. 2006;26:8655–8665. doi: 10.1128/MCB.00168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filby A, Davies D. Reporting imaging flow cytometry data for publication: Why mask the detail? Cytometry A. 2012 doi: 10.1002/cyto.a.22091. [DOI] [PubMed] [Google Scholar]
- 38.Tsun A, Qureshi I, Stinchcombe JC, Jenkins MR, de la Roche M, Kleczkowska J, Zamoyska R, Griffiths GM. Centrosome docking at the immunological synapse is controlled by Lck signaling. J Cell Biol. 2011;192:663–674. doi: 10.1083/jcb.201008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabe H, Hata A, Okada M, Nakagawa H, Hanafusa H. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc Natl Acad Sci U S A. 1994;91:3984–3988. doi: 10.1073/pnas.91.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hur EM, Son M, Lee OH, Choi YB, Park C, Lee H, Yun Y. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J Exp Med. 2003;198:1463–1473. doi: 10.1084/jem.20030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simeoni L, Posevitz V, Kolsch U, Meinert I, Bruyns E, Pfeffer K, Reinhold D, Schraven B. The transmembrane adapter protein SIT regulates thymic development and peripheral T-cell functions. Mol Cell Biol. 2005;25:7557–7568. doi: 10.1128/MCB.25.17.7557-7568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao M, Janas JA, Niki M, Pandolfi PP, Van Aelst L. Dok-1 independently attenuates Ras/mitogen-activated protein kinase and Src/c-myc pathways to inhibit platelet-derived growth factor-induced mitogenesis. Mol Cell Biol. 2006;26:2479–2489. doi: 10.1128/MCB.26.7.2479-2489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277:8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 44.Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–750. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 45.Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 46.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, Murphy A, Ellis S, Smyth MJ, Kershaw MH, Darcy PK, Humbert PO, Russell SM. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, Freedman BD, Burkhardt JK. Ezrin and moesin function together to promote T cell activation. J Immunol. 2009;182:1021–1032. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 50.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 51.Vang T, Torgersen KM, Sundvold V, Saxena M, Levy FO, Skalhegg BS, Hansson V, Mustelin T, Tasken K. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J Exp Med. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol. 2003;23:2017–2028. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobenecker MW, Schmedt C, Okada M, Tarakhovsky A. The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol Cell Biol. 2005;25:10533–10542. doi: 10.1128/MCB.25.23.10533-10542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu S, Huo J, Tan JE, Lam KP. Cbp deficiency alters Csk localization in lipid rafts but does not affect T-cell development. Mol Cell Biol. 2005;25:8486–8495. doi: 10.1128/MCB.25.19.8486-8495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 56.Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 57.Watson AR, Lee WT. Differences in signaling molecule organization between naive and memory CD4+ T lymphocytes. J Immunol. 2004;173:33–41. doi: 10.4049/jimmunol.173.1.33. [DOI] [PubMed] [Google Scholar]
- 58.Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988;85:5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van’t Hof W, Resh MD. Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J Cell Biol. 1999;145:377–389. doi: 10.1083/jcb.145.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo KX, Sefton BM. Cross-linking of T-cell surface molecules CD4 and CD8 stimulates phosphorylation of the lck tyrosine protein kinase at the autophosphorylation site. Mol Cell Biol. 1990;10:5305–5313. doi: 10.1128/mcb.10.10.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bachmann MF, Gallimore A, Linkert S, Cerundolo V, Lanzavecchia A, Kopf M, Viola A. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. J Exp Med. 1999;189:1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.