Abstract
Exposure to polycyclic aromatic hydrocarbons (PAHs) has been associated with adverse health outcomes. Concentrations of urinary PAH metabolites (OH-PAHs) provide an integrated measure of human exposure to PAHs but measurement of urinary OH-PAHs has not been done in Australia and rarely in Vietnam, where air pollution is of concern. In this study, we assessed exposure to PAHs in 16 participants living in Brisbane, Australia and Hanoi, Vietnam, with 4 participants travelling between the two cities during the monitoring period. A total of 312 first morning urine samples were collected over 10 weeks and were analysed for nine OH-PAHs. Concentrations of the urinary OH-PAHs were 3-10 times higher in participants from Hanoi than those from Brisbane. For example, the median concentrations of 1-hydroxypyrene were 292 pg/mL in Hanoi, compared to 64 pg/mL in Brisbane. For participants travelling from Brisbane to Hanoi and back, differences in exposure to PAHs in these two cities resulted in corresponding changes of urinary OH-PAH concentrations, demonstrating that the more polluted environment in Hanoi was likely the source for higher PAH exposure there.
Keywords: OH-PAHs, PAH exposure, air pollution
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs), a class of hazardous air pollutants, are predominantly produced during the incomplete combustion of organic materials, e.g. fossil fuel, coal, and wood. PAHs are widely distributed in the atmosphere and they can be transported over long distances before depositing through atmospheric precipitation onto soils, vegetation or waters (Ravindra et al., 2008).
Exposure to PAHs is associated with a variety of health effects including lung, skin and bladder cancers in humans (Agudo 2006; IARC, 2010; Kim et al., 2013). Recent findings are suggestive of relationships between PAHs in placenta and the risk of neural tube defects and the alteration of the immune system (Langlois et al., 2012; Walker et al., 2013). Other study suggests that exposure to polycyclic aromatic hydrocarbons encountered in New York City air may play a role in childhood Attention Deficit Hyperactivity Disorder behaviour problems (Perera et al., 2014).
Due to the ubiquitous presence of PAHs in the atmosphere, exposure to atmospheric PAHs may likely impact large populations; as a consequence, it could be a major public health issue. This is especially important in developing countries, where severe air pollution from fossil fuel combustion, e.g. coal burning power plants and motor vehicles (Gurjar et al., 2010; Han and Naeher, 2006), usually exceeds air quality standards (Hopke et al., 2008). For example Vietnam ranked among the ten worst countries in the world in terms of air pollution (Emerson, 2012), with traffic emissions responsible for 70% of all urban air pollution (MoNRE, 2007). One study reported that atmospheric PAHs concentrations at 10 different roadside sites in Hanoi were significantly higher than those from other countries, and often exceeded the recommended maximum thresholds set by the World Health Organisation (Kishida et al. 2008). At the same time, developed countries like Australia are considered relatively clean in terms of air pollution with levels of atmospheric PAHs similar to other countries in Europe (Berko, 1999). Levels of atmospheric PAHs in Brisbane, a metropolitan city in Australia, have decreased throughout the last decade (Kennedy et al. 2010; Muller et al. 1998; Wang et al., 2013) probably due to strict emission regulations (Hopke et al., 2008).
To study the actual exposure to PAHs, urinary mono-hydroxylated PAHs (OH-PAHs), a group of PAH metabolites, have been used as biomarkers (Jacob and Seidel, 2002). Among the OH-PAHs, 1-hydroxypyrene (1-PYR) is the most commonly used PAH biomarker in both occupational as well as in the general population from various countries (Hansen et al. 2008). The use of PAH metabolites as biomarkers is more important when one wants to access the actual change in human exposure to different levels of PAHs (e.g. in different level of air pollution).
However, to our knowledge, there is no study to date using PAH metabolites to assess general human exposure to PAHs in Australia. There are only two known studies in Vietnam assessing PAH exposure by biomonitoring urinary OH-PAHs. One study systematically monitored 1-PYR urinary concentrations in 44 street workers in Hanoi over 4 weeks and consistently showed concentrations of 1-PYR up to 24 times higher than those in the US population. The study suggested substantially higher exposure to PAHs in Hanoi even when the workers wore activated carbon respirators to reduce exposure to PAHs and other air pollutants (Wertheim et al. 2012). The other study only analysed random spot urine samples of 23 middle-age people in Hanoi to compare the levels with those from other countries in Asia (Guo et al., 2013).
In this study, we report urinary concentrations of PAH metabolites in a small group of residents in Brisbane, Australia, and Hanoi, Vietnam, and those travelling between the two cities. Our goals were to assess a) the exposure to PAHs in the two cities through biomonitoring of urinary OH-PAH concentrations; b) the change of OH-PAH profile when people travelled between the two cities; and c) the influence of age on the concentrations of OH-PAHs.
2. Materials and Methods
2.1. Study participants
We recruited 16 healthy volunteers (9 adults and 7 children) representing 5 families (Table 1). Three families lived in metropolitan Brisbane and two families lived in metropolitan Hanoi. Their homes were not close to any heavy emission source or heavy traffic (at least 1 km away from heavy traffic). During the study, one family in Brisbane (two adults and two children) travelled to Hanoi, and then back to Brisbane. All participants were of Vietnamese origin, i.e. there was no race difference that could significantly affect the metabolism of PAHs. The adults were aged between 28 and 35 years and the children aged between 2 and 8 years. Participants gave written informed consent prior to inclusion; parents or guardians provided consent on behalf of their children. This study was approved by the Medical Research Ethics Committee of the University of Queensland (#2011000795), and the Internal Review Boards of the Centers for Disease Control and Prevention and the National Institute of Environmental and Occupational Health (Vietnam).
Table 1.
Participants’ information
| Participant code | Sex | Age (years) |
Number of urine sample | |
|---|---|---|---|---|
| Group 1 | Travel: Australia – Vietnam - Australia | |||
| 1 | TV1 | Male | 33 | 30 (10+9+11)a |
| 2 | TV2 | Female | 34 | 25 (9+10+6) |
| 3 | TV3 | Male | 7 | 22 (9+5+8) |
| 4 | TV4 | Female | 4 | 23 (9+9+5) |
| Group 2 | Australia | |||
| 5 | AU1 | Male | 31 | 20 |
| 6 | AU2 | Female | 28 | 17 |
| 7 | AU3 | Female | 3 | 19 |
| 8 | AU4 | Male | 31 | 16 |
| 9 | AU5 | Female | 31 | 12 |
| Group 3 | Vietnam | |||
| 10 | VN1 | Male | 34 | 18 |
| 11 | VN2 | Female | 34 | 18 |
| 12 | VN3 | Male | 8 | 17 |
| 13 | VN4 | Female | 5 | 18 |
| 14 | VN5 | Male | 37 | 19 |
| 15 | VN6 | Male | 2 | 18 |
| 16 | VN7 | Female | 6 | 19 |
the number of samples collected before, during and after travelling to Hanoi
All participants had no known occupational exposure to PAHs and all were non-smokers. The volunteers were instructed to avoid food with known high PAH-content (e.g. grilled or smoked food) during the study period. Participants from the travelling family (from Brisbane to Hanoi) took food from Brisbane to ensure that their diet in the first week in Hanoi was similar to their diet in Brisbane. Information about the participants is shown in Table 1.
A total of 312 urine samples were collected during the study period. In general, we asked the participants to collect first-morning urine voids twice a week (Tuesday and Friday) for 10 weeks in August and September 2011. The travelling family collected additional samples for one week in July 2011 and samples before and around 6 hours after their flights. There were occasions when the participants missed the sampling date and no sample was collected. After collection, the samples were frozen immediately in the freezer compartment of the participant’s refrigerator and then transported to the laboratory, and stored at −80 °C.
2.2. Urine analysis
After all samples were collected, urine samples were shipped on dry ice to the Centers for Disease Control and Prevention (Atlanta, GA, USA) and analysed for nine OH-PAHs using gas chromatography/high resolution mass spectrometry (GC-HRMS) according to a method described previously (Li et al., 2006). In brief, urine samples were spiked with 13C-labeled internal standards and sodium acetate buffer containing β-glucuronidase, urinary conjugates were hydrolysed overnight at 37 °C, and then the target analytes were extracted through semi-automated liquid–liquid extraction. The extracts were evaporated, and the target analytes were derivatised, and analysed on a 6890 gas chromatograph (Agilent Technology, Palo Alto, CA, USA) coupled with a MAT95XL high-resolution mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). All analyses were subjected to a series of quality control and quality assurance checks as described elsewhere (Li et al., 2006). All concentrations were subtracted with a method blank prepared and analyzed in the same sample run. The limits of detection (LODs) for the measured OH-PAHs ranged from 2.6-18 pg/mL. The overall coefficients of variation for 42 quality control samples prepared in 6 batches over 3 weeks were 2.8-3.4% for the 9 OH-PAHs. Urinary creatinine was measured on a Roche Hitachi 912 Chemistry Analyzer (Hitachi, Pleasanton, CA, USA) using the Creatinine Plus Assay, as described in Roche’s Creatinine Plus Product Application no. 03631761003.
Nine OH-PAHs, metabolites of naphthalene, 1-naphthol (1-NAP) and 2-naphthol (2-NAP), of fluorene, 9-hydroxyfluorene (9-FLU), 3-hydroxyfluorene (3-FLU), 2- hydroxyfluorene (2-FLU), of phenanthrene, 3-hydroxyphenanthrene (3-PHE), 1-hydroxyphenanthrene (1-PHE), 2-hydroxyphenanthrene (2-PHE), and of pyrene (1-PYR), were measured in urine.
2.3. Data and statistical analysis
We used both unadjusted and creatinine-adjusted concentrations for data analyses. All statistical analyses were performed through GraphPad Prism (GraphPad, La Jolla, CA, USA). Because of the small sample size, Mann–Whitney U test was used to examine the differences between groups. Results were considered statistically significant at p < 0.05.
3. Results
3.1. Concentrations of OH-PAHs in the participants’ urine
OH-PAHs concentrations were detectable in most urine samples, with a detection rate over 99% for all nine OH-PAHs. There were 11 samples in which one of the OH-PAHs concentrations was below the LOD. Values <LOD were replaced as LOD/squrt(2) in the statistical analysis (Hornung and Reed, 1990).
OH-PAHs urinary concentrations in samples collected in Hanoi and Brisbane are compared in Table 2 and stratified into two age groups (children and adults). Median unadjusted concentrations of the nine urinary OH-PAHs metabolites in samples collected in Hanoi were significantly higher (3 to 10 times, p < 0.01) than in samples collected in Brisbane for both age groups. No difference was observed between the unadjusted concentrations of the children and adults groups in the same cities. However, there were significant differences in concentrations of all urinary OH-PAHs between children and adults groups if the creatinine-adjusted concentrations were used, with higher concentrations observed in children’s samples (p < 0.05).
Table 2.
Urinary concentrationsof OH-PAHs in adults & children collected in Hanoi and Brisbane, expressed in pg/mL and in ng/g creatinine
| Adults (Age 28-37) |
Children (Age 2-8) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Analyte (Abbreviation) | Hanoi a |
Brisbane b |
Hanoi c |
Brisbane d |
||||
| Median (Range) | P95e | Median (Range) | P95 | Median (Range) | P95 | Median (Range) | P95 | |
| Unadjusted concentration (pg/mL urine) | ||||||||
| 1-Naphthol (1-NAP) | 2843 (377-45233) | 10722 | 998 (252-23899) | 5955 | 2583 (282-11855) | 8457 | 624 (163-3405) | 3103 |
| 2-Naphthol (2-NAP) | 2905 (469-12106) | 7386 | 1454 (329-44651) | 6227 | 3039 (535-59481) | 10272 | 1108 (364-7957) | 5349 |
| 2-Hydroxyfluorene (2-FLU) | 206(7-1057) | 773 | 103 (37-1146) | 582 | 222(7-770) | 719 | 92 (27-418) | 281 |
| 3-Hydroxyfluorene (3-FLU) | 130 (35-647) | 329 | 30 (10-602) | 159 | 122 (24-405) | 305 | 32 (10-152) | 131 |
| 9-Hydroxyfluorene (9-FLU) | 810 (127-3387) | 2395 | 113 (25-1015) | 720 | 496 (100-2266) | 1930 | 126 (38-386) | 377 |
| 1-Hydroxyphenanthrene (1-PHE) | 291 (67-1324) | 810 | 56 (12-445) | 197 | 220 (50-932) | 648 | 56 (16-335) | 281 |
| 2-Hydroxyphenanthrene (2-PHE) | 136 (29-731) | 437 | 33 (10-345) | 141 | 100 (27-274) | 197 | 20 (10-59) | 53 |
| 3-Hydroxyphenanthrene (3-PHE) | 163 (40-1352) | 589 | 52 (10-403) | 194 | 145 (37-524) | 400 | 57 (17-144) | 129 |
| 1-Hydroxypyrene (1-PYR) | 292 (54-1370) | 1155 | 56 (10-1011) | 200 | 292 (74-2447) | 1229 | 86 (24-399) | 329 |
| Creatinine-adjusted concentration (ng/gcreatinine) | ||||||||
| 1-Naphthol (1-NAP) | 2552 (969-25000) | 7827 | 732 (176-11042) | 6056 | 4260 (1122-20106) | 9589 | 955 (282-6824) | 3948 |
| 2-Naphthol (2-NAP) | 2331 (950-9677) | 7722 | 1096 (357-26683) | 4494 | 5203 (2089-252144) | 16663 | 1710 (790-9116) | 6889 |
| 2-Hydroxyfluorene (2-FLU) | 285 (3-565) | 499 | 83 (33-894) | 260 | 450 (12-939) | 846 | 144 (74-539) | 331 |
| 3-Hydroxyfluorene (3-FLU) | 123 (20-409) | 241 | 24 (9-469) | 105 | 220 (61-711) | 512 | 48 (24-373) | 164 |
| 9-Hydroxyfluorene (9-FLU) | 792 (129-2263) | 2044 | 89 (31-1363) | 359 | 1005 (114-6303) | 2669 | 173 (53-633) | 436 |
| 1-Hydroxyphenanthrene (1-PHE) | 301 (61-855) | 725 | 44 (10-279) | 112 | 486 (99-1673) | 1099 | 84 (44-378) | 360 |
| 2-Hydroxyphenanthrene (2-PHE) | 135 (32-346) | 314 | 25 (9-194) | 81 | 166 (45-517) | 437 | 30 (17-94) | 81 |
| 3-Hydroxyphenanthrene (3-PHE) | 165 (57-679) | 460 | 37 (9-314) | 101 | 279 (90-1052) | 599 | 89 (38-241) | 196 |
| 1-Hydroxypyrene (1-PYR) | 258 (46-1140) | 831 | 41 (5-417) | 164 | 534 (125-1905) | 1384 | 131 (45-573) | 354 |
5 subjects and 90 samples;
6 subjects and 101 samples;
6 subjects and 107 samples;
3 subjects and 50 samples;
95 percentile(Total 20 subjects because 4 subjects travelled from Brisbane to Hanoi and back)
We summed the total concentrations of metabolites from each parent PAH, e.g. 1-NAP and 2-NAP for naphthalene, to better reflect the exposure of the participants to the parent PAH. In this study, the total concentrations of metabolites of naphthalene (median values) were the highest followed, in decreasing order, by those of fluorene, phenanthrene and pyrene for both Hanoi and Brisbane samples.
3.2. Effect of travelling between cities
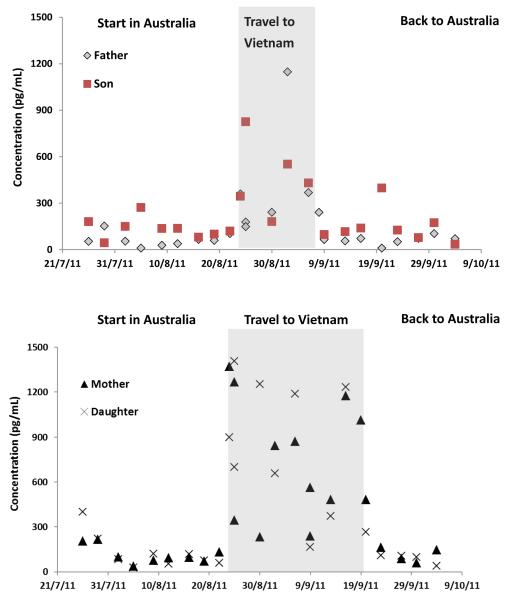
Figure 1 presents the urinary concentrations of 1-PYR (as a representative biomarker of OH-PAHs) in samples of the participants who travelled from Brisbane to Hanoi (on 23 August 2011) and then returned to Brisbane (07 September 2011 for father and son and 23 September for mother and daughter). Before going to Hanoi, the travelling participants had lived in Brisbane for at least 6 months and the median concentration of 1-PYR in their samples was 87 pg/mL. After arriving in Hanoi, the urinary median concentration of 1-PYR increased to 532 pg/mL. When the travelling participants returned to Brisbane from Hanoi, their 1-PYR median concentrations decreased to 105 pg/mL, a concentration comparable with the concentration among people who stayed in Brisbane during the entire study period. The level of urinary 1-PYR in the travelling participants did not change significantly before they travelled and after they returned home. The same effect was observed for all other PAH metabolites measured (Fig. S1-4)
Figure 1.
The concentrations of urinary 1-hydroxypyrene in a family who traveled between Brisbane, Australia and Hanoi, Vietnam
4. Discussion
This is the first study to monitor the biological response on urinary PAH biomarkers to the change in atmospheric PAH exposure for small groups of adults and children in a non-occupational setting while maintaining a similar diet. It is also the first study, to the best of our knowledge, which reports urinary OH-PAHs levels in a small group of residents living in Australia.
4.1. Profiles of urinary PAH metabolites
The OH-PAHs observed consistently at the highest concentrations were 1-NAP and 2-NAP which likely reflected the fact that naphthalene is one of the most abundant PAH in the atmosphere (Buckpitt et al., 2010). It is also because metabolites of smaller PAHs (i.e., two to three aromatic rings) have been reported to be excreted preferentially in the urine, but metabolites of larger PAHs are excreted primarily in the feces as the molecular structure and size of PAHs can affect absorption efficiency, and metabolic and excretion pathway (Ramesh et al., 2004). Metabolites of naphthalene, 1-NAP and 2-NAP, accounted for 73% and 83% total concentration of OH-PAHs in residents of Hanoi and Brisbane, respectively. Similar to other studies (Guo et al., 2013; Li et al., 2011), concentrations of metabolites of the higher molecular weight PAHs were lower. On average, the sum of metabolites of napthalene, fluorene and phenanthrene contributed >96% to the total urinary OH-PAH concentrations with 1-PYR accounts for the remaining part.
4.2. Difference in PAH exposure between two cities and its impact to the travellers
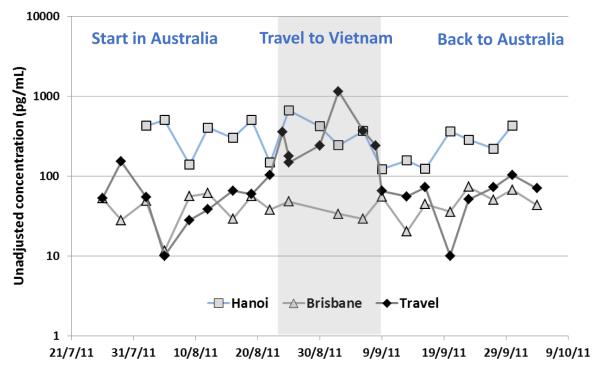
The differences in urinary OH-PAH concentrations were likely reflective of the differences in atmospheric concentrations of the parent PAHs between the two cities. The limited data available in the literature on atmospheric PAHs in the two cities, which were presented in Table 3, suggest that the ambient air concentrations of the parent PAHs (fluorene, phenanthrene, pyrene) in Hanoi were approximately 4.5 to 7.6 times higher than those measured in Brisbane around the period of this study (2011-2012). No data are available for naphthalene. The similar difference of urinary concentrations and the atmospheric concentrations of fluorene, phenanthrene and pyrene between the two cities indicated that OH-PAHs are good biomarkers for assessing population exposure to PAHs in the air. Albeit relying on a small sample size, this suggestive conclusion was specifically supported by the concentration profiles of travelling participants where food intake was controlled to remove its effect to the level of urinary OH-PAHs (see Figs. 1 and 2).
Table 3.
Atmospheric concentrations of parent PAHs (ng/m3) in Hanoi and Brisbane
Figure 2.
Urinary concentrations of 1-PYR of three husbands, one of whom travelled from Brisbane to Hanoi and back during the study period; the other two did not travel and remained at their regular place of residence for the whole study period
The urinary concentrations of OH-PAHs in Hanoi’s participants were lower compared with the data reported previously for this city. For example, Guo et al. (2013) recorded a median 1-PYR urinary concentration of 463 pg/mL for a middle-aged population in Hanoi (n=23), compared to the 292 pg/mL measured in this study. Meanwhile Wertheim et al. (2012) also reported a much higher concentrations of OH-PAHs in middle-aged street workers in Hanoi (n=44) with median 1-PYR concentrations of 1020 ng/g creatinine compared to 407 ng/g creatinine in this study. This is not surprising because street workers are occupationally exposed to traffic exhaust especially in Vietnam where the majority of the urban vehicle fleet consists of scooters with low emission standard. Similarly, it has been reported that children attending school in a heavy traffic area had much higher urinary OH-PAH levels than those attending a school far away from heavy traffic (Fan et al., 2012). Additionally, we minimised the PAH dietary input in this study by asking participants to eat low PAH-containing food, and the travel family further reduced dietary influence in Hanoi by bringing food to Hanoi during the sampling period. Therefore, the potentially lower dietary exposure could also contribute to the lower urinary OH-PAH level in this study compared to other reported levels for Hanoi.
Meanwhile, the urinary concentrations of OH-PAHs in participants from Brisbane, even with a small sample size, were comparable to those in more developed countries like Japan, Korea, Malaysia, Germany, and the United States as shown in Table 4. For example, median concentration of 1-PYR in Brisbane (101 urine samples from 6 participants) was 59 pg/mL while the corresponding values in Japan, Korea, Malaysia, were 75, 103, 65 pg/mL respectively (Guo et al., 2013). The urinary concentrations of 1-PYR in German and American populations were 140 and 113 pg/mL, respectively (CDC, 2015; Wilhelm et al., 2008).
Table 4.
Comparison of urinary concentrations of 1-PYR in Brisbane and Hanoi (this study) to other populations (pg/mL or creatinine-adjusted [ng/g creatinine])
| Population | N |
Median (pg/mL) |
Median (ng/g creatinine) |
Reference |
|---|---|---|---|---|
| Adults non-smokers - Hanoi (Vietnam) | 5(74)a | 292 | 258 | this study |
| Children non-smokers - Hanoi (Vietnam) | 6(101)a | 292 | 534 | this study |
| Adults non-smokers - Brisbane (Australia) | 6(87)a | 56 | 41 | this study |
| Children non-smokers - Brisbane (Australia) | 3(50)a | 86 | 131 | this study |
| Hanoi – street worker | 44 | NAb | 1020 | Wertheim et al. (2012) |
| Hanoi (Vietnam) | 23 | 463 | NA | Guo et al. (2013) |
| China | 84 | 378 | NA | Guo et al. (2013) |
| Japan | 34 | 75 | NA | Guo et al. (2013) |
| India | 38 | 424 | NA | Guo et al. (2013) |
| Malaysia | 29 | 65 | NA | Guo et al. (2013) |
| Korea | 60 | 103 | NA | Guo et al. (2013) |
| Kuwait | 38 | 220 | NA | Guo et al. (2013) |
| Children in parquet floor houses in Germany | 347 | NA | 148 | Heudorf and Angerer (2001a) |
| Adults in parquet floor houses in Germany | 495 | NA | 88 | Heudorf and Angerer (2001a) |
| Children and adults in Afghanistan | 55 | 1646 (1550; 3167)c | NA | Hemat et al. (2012) |
| US population (2011-2012) | 2487 | 113 (104; 127) c | 119 (112; 181) c | CDC (2015) |
| Children non smokers - German population | 351 | 140 | NA | Wilhelm et al. (2008) |
| Adults non smokers – German population | 389 | 100 | NA | Wilhelm et al. (2008) |
| Korean population | 4702 | 150 d | NA | Sul et al. (2012) |
Number of participants with number of urine samples in parenthesis
NA: not applicable
Adults median and children median are in parenthesis
Geometric mean value
We examined the impact of travel from Brisbane to Hanoi on urinary OH-PAH concentrations. As shown in Fig. 1 and 2, the urinary levels of 1-PYR among the travellers were similar to those of local participants. While in Hanoi, the median 1-PYR concentration increased by 5 folds within the same participants who consumed the same type of food during the sampling period. The same effect was observed for all other PAH metabolites measured in this study (Fig. S5). This suggests that urinary OH-PAH concentrations reflected the exposure to PAHs accurately, and that these PAH metabolites are effective biomarkers for monitoring PAH exposure. Furthermore, the more polluted ambient air in Hanoi contributed to the higher PAH exposure in the travellers while they were in Hanoi.
Vehicle exhaust was likely the main cause of higher exposure to PAHs in Hanoi compared to Brisbane. An earlier analysis of PAH and nitro-PAHs in atmospheric particulate matter showed that several million motorbikes with no-catalytic converter, and a poorly maintained car fleet were major pollution sources in Vietnam (Pham et al. 2013; Thuy et al. 2012). Raising Vietnam’s emission standard for motor vehicles is essential to improve air quality and reduce the human exposure to PAHs in Hanoi and in Vietnam in general. The current standard in Vietnam is Euro 2 which allows the emission of pollutants such as hydrocarbon several times higher than the Euro 5/6 standard applied in Australia (Delphi, 2015). Additionally, many low income people in Vietnam cook with poor quality fuels (e.g. beehive coal or coal briquette), which could emit high level of PAHs and contribute to indoor and outdoor air pollution (Kim Oanh and Dung, 1999).
4.3. Comparison between adults and children
This is the first study, to the best of our knowledge, followed entire families–both children and adults–over several months. The children and adults from the same families had similar environment and dietary intake. Both groups spent approximately the same amount of time indoor and outdoor although at different places during weekday (office/workplace for adults and school for children in daytime and at home in nighttime). This condition allowed a close examination of the effect of age on PAH biomarker levels. Our study found no significant differences between the unadjusted urinary concentrations (in pg/mL) of OH-PAHs in children compared with adults in both Brisbane and Hanoi (Table 3). For example, median 1-PYR concentrations in Hanoi was 292 pg/mL for both adult (74 samples from 5 adults) and children (87 samples from 6 children). It is not surprising because all participants were non-smokers and their diets and environments were similar (no special diet for children was recorded in any family participating in this study).
However, there were significant differences between adults and children if the OH-PAHs urinary concentrations were creatinine-adjusted. This could be caused by the lower level of urinary creatinine in children than in adults as reported previously (Barr et al., 2005). Several other studies have recognized that creatinine adjustment increases the calculated adjusted concentrations of chemicals in children compared with adults (Barr et al., 2005, Heudorf and Angerer, 2001b). This finding raises doubts about comparing creatinine-adjusted urine contaminants between different populations with biologically different creatinine levels – such as between children and adults. In such case, alternative adjustment methods should be considered to correct for urine dilution, e.g., specific gravity adjustment (Sauve et al., 2015, Suwazono et al., 2005).
4.4. Limitations
We acknowledge that there were some limitations to the study. First, the data were derived from a small number of participants, especially when stratified into children and adult groups. The participants were all non-smokers and students/office workers. Therefore the results are not likely to be representative for the whole population but rather provide an indication for similar sub-populations (e.g. students and office workers) in Brisbane and Hanoi. Second, there was no personal air monitoring data for direct comparison between ambient exposure and internal exposure although the ambient PAHs concentrations in the two cities around the time of this study were documented. Third, we did not provide food nor measure the dietary intake of PAHs although the diet was kept similar among participants during the study period. The similarity of OH-PAH concentrations between the travelling group and the local resident groups suggested that dietary intake does not likely to have considerable impact on the urinary concentration of OH-PAHs in this study.
5. Conclusion
This is the first study, to the best of our knowledge, reporting levels of urinary OH-PAHs in Brisbane, Australia. Even with a small sample size, we found that the urinary OH-PAH levels in Brisbane were consistent with those in developed countries. We found 3-10 times higher levels in residents in Hanoi, Vietnam than those in Brisbane suggesting that PAH exposure in Hanoi was substantially higher than in Brisbane, most likely because of the higher ambient air PAH concentrations. Travelling from Brisbane to Hanoi and back while keeping similar diets resulted in corresponding changes in the concentration of urinary OH-PAHs in the travellers; this demonstrated the effectiveness of the PAH metabolites as PAH exposure biomarkers and further confirmed that the more polluted environment in Hanoi was likely the source for the elevated PAH exposure there. This is also the first study that followed entire families–both children and adults–in various environments while kept similar diets, which allowed a close examination of age effects on exposure biomarkers. Our findings indicated no significant difference on the unadjusted urinary concentrations between children and adults, but the creatinine adjustment process could introduce bias as children and adults are biologically at different creatinine levels.
Supplementary Material
Figure S1. Concentrations of 8 urinary OH-PAHs in the father who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S2. Concentrations of 8 urinary OH-PAHs in the son who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S3. Concentrations of 8 urinary OH-PAHs in the mother who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S4. Concentrations of 8 urinary OH-PAHs in the daughter who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S5. Effect of traveling from Brisbane to Hanoi and back to Brisbane on the concentrations of urinary PAH metabolites (Travel), and comparison to two control groups, residents of Brisbane and residents of Hanoi during the entire study period (a and b are significantly different, p<0.01).
Acknowledgements and Disclaimer
We would like to thank the participants for their time and devotion. PT is partly funded by a UQ Postdoctoral Fellowship. JFM is funded by an ARC Future Fellowship (FT120100546). Entox is a joint venture of the University of Queensland and the Queensland Department of Health.
Footnotes
The co-authors of this manuscript do not have any financial conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Agudo A. Epidemiology of dietary exposure to polycyclic aromatic hydrocarbons (PAH) and cancer risk. Epidemiology. 2006;17:S77–S78. [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ Health Persp. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berko DHN. Technical Report No. 2: Polycyclic aromatic hydrocarbons (PAHs) in Australia. Department of Environmental Protection; Perth, Western Australia: 1999. pp. 1–55. [Google Scholar]
- Buckpitt A, Kephalopoulos S, Koistinen K, Kotzias D, Morawska L, Sagunski H. WHO Guidelines for Indoor Air Quality: Selected Pollutants. World Health Organization; Geneva: 2010. Naphthalene. [PubMed] [Google Scholar]
- CDC [accessed 4 March 2015];Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. 2015 Feb; 2015. http://www.cdc.gov/exposurereport/
- Delphi [accessed June 2015];Worldwide emissions standards. 2015 http://delphi.com/docs/default-source/catalogs/delphi-worldwide-emissions-standards-pc-ldv-15-16.pdf?sfvrsn=2.
- Eder E. Intraindividual variations of DNA adduct levels in humans. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1999;424:249–261. doi: 10.1016/s0027-5107(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Emerson JW, Hsu A, Levy MA, de Sherbinin A, Mara V, Esty DC, Jaiteh M. 2012 Environmental Performance Index and Pilot Trend Environmental Performance Index. Yale Center for Environmental Law and Policy; New Haven: 2012. [Google Scholar]
- Fan R, Wang D, Mao C, Ou S, Lian Z, Huang S, Lin Q, Ding R, She J. Preliminary study of children’s exposure to PAHs and its association with 8-hydroxy-2-deoxyguanosine in Guangzhou, China. Environ Int. 2012;42:53–58. doi: 10.1016/j.envint.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Guo Y, Senthilkumar K, Alomirah H, Moon HB, Minh TB, Mohd MA, Nakata H, Kannan K. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Tech. 2013;47:2932–2938. doi: 10.1021/es3052262. [DOI] [PubMed] [Google Scholar]
- Gurjar BR, Jain A, Sharma A, Agarwal A, Gupta P, Nagpure AS, Lelieveld J. Human health risks in megacities due to air pollution. Atmos Environ. 2010;44:4606–4613. [Google Scholar]
- Han X, Naeher LP. A review of traffic-related air pollution exposure assessment studies in the developing world. Environ Int. 2006;32:106–120. doi: 10.1016/j.envint.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Hansen ÅM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies-A review. Int J Hyg Envir Heal. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Hemat H, Wittsiepe J, Wilhelm M, Muller J, Goen T. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from afghanistan. J Expos Sci Environ Epidemiol. 2012;22:46–51. doi: 10.1038/jes.2011.33. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Angerer J. Internal exposure to PAHs of children and adults living in homes with parquet flooring containing high levels of PAHs in the parquet glue. Int Arch Occ Env Hea. 2001a;74:91–101. doi: 10.1007/s004200000214. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Angerer J. Metabolites of Organophosphorous Insecticides in Urine Specimens from Inhabitants of a Residential Area. Environ Res. 2001b;86:80–87. doi: 10.1006/enrs.2001.4237. [DOI] [PubMed] [Google Scholar]
- Hopke PK, Cohen DD, Begum BA, Biswas SK, Ni B, Pandit GG, Santoso M, Chung Y-S, Davy P, Markwitz A, Waheed S, Siddique N, Santos FL, Pabroa PCB, Seneviratne MCS, Wimolwattanapun W, Bunprapob S, Vuong TB, Duy Hien P, Markowicz A. Urban air quality in the Asian region. Sci Total Environ. 2008;404:103–112. doi: 10.1016/j.scitotenv.2008.05.039. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- IARC . IARC monographs on the evaluation of carcinogenic risks to humans. Volume 92. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. International Agency for Research on Cancer; Lyon: 2010. [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Seidel A. Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J Chrom B. 2002;778:31–47. doi: 10.1016/s0378-4347(01)00467-4. [DOI] [PubMed] [Google Scholar]
- Kennedy K, Macova M, Bartkow ME, Hawker DW, Zhao B, Denison MS, et al. Effect based monitoring of seasonal ambient air exposures in australia sampled by puf passive air samplers. Atmospheric Pollution Research. 2010;1:50–58. doi: 10.5094/apr.2010.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Jahan SA, Kabir E, Brown RJC. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Kim Oanh N.T., Dung NT. Emission of polycyclic aromatic hydrocarbons and particulate matter from domestic combustion of selected fuels. Environ Sci Technol. 1999;33:2703–2709. [Google Scholar]
- Kishida M, Imamura K, Takenaka N, Maeda Y, Viet PH, Bandow H. Concentrations of atmospheric polycyclic aromatic hydrocarbons in particulate matter and the gaseous phase at roadside sites in Hanoi, Vietnam. Bull Environ Contam Toxicol. 2008;81:174–179. doi: 10.1007/s00128-008-9450-5. [DOI] [PubMed] [Google Scholar]
- Langlois PH, Hoyt AT, Lupo PJ, Lawson CC, Waters MA, Desrosiers TA, et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons and risk of neural tube defect-affected pregnancies. Birth Defects Research Part a-Clinical and Molecular Teratology. 2012;94:693–700. doi: 10.1002/bdra.23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006;78:5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the us population. Environmental Research. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Li Z, Sjödin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, et al. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environment International. 2011;37:1157–1163. doi: 10.1016/j.envint.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Muller JF, Hawker DW, Connell DW. Polycyclic aromatic hydrocarbons in the atmospheric environment of brisbane, australia. Chemosphere. 1998;37:1369–1383. [Google Scholar]
- Ministry of Natural Resources and Environment (MoNRE) Vietnam urban air environment. Report from the Ministry of Natural Resources and Environment. 2007 [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, Huang TJ, Miller RL, Wang S, Rauh V. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. Plos One. 2014;9(11) doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CT, Kameda T, Toriba A, Hayakawa K. Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in particulates emitted by motorcycles. Environ Pollut. 2013;183:175–183. doi: 10.1016/j.envpol.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Phong TK, Wang X, Phuc DH, Mueller J. Temporal Trend of Polycyclic Aromatic Hydrocarbons (PAHs) Concentrations in Hanoi Air. 4th International Scientific Conference on Occupational and Environmental Health; Hanoi, Vietnam. November 2012. [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider K, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos Environ. 2008;42:2895–2921. [Google Scholar]
- Suwazono Y, Åkesson A, Alfvén T, Järup L, Vahter M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10:117–126. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- Sauvé J-F, Lévesque M, Huard M, Drolet D, Lavoué J, Tardif R, Truchon G. Creatinine and specific gravity normalization in biological monitoring of occupational exposures. J Occup Environ Hyg. 2015;12:123–9. doi: 10.1080/15459624.2014.955179. 2015. [DOI] [PubMed] [Google Scholar]
- Thuy PC, Kameda T, Toriba A, Tang N, Hayakawa K. Characteristics of atmospheric polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in hanoi-vietnam, as a typical motorbike city. Polycycl Aromat Comp. 2012;32:296–312. [Google Scholar]
- Walker AI, Kohli A, Syed A, Eisen EA, Noth EM, Pratt B, et al. Exposure to polycyclic aromatic hydrocarbons is associated with higher levels of total ige, decreased function of t regulatory cells and an increase of asthma occurrence in children. Journal of Allergy and Clinical Immunology. 2013;131:Ab54–Ab54. [Google Scholar]
- Wang X, Thai P, Li Y, Hawker D, Gallen M, Mueller J. Changes in concentrations of PAHs and PCBs in Brisbane atmosphere between summer 1994/95 and 2012/13. Organohalogen Compounds Electronic. http://www.dioxin20xx.org/pdfs/2013/4311.pdf.
- Wertheim HF, Ngoc DM, Wolbers M, Binh TT, Hi NTT, Loan NQ, et al. Studying the effectiveness of activated carbon r95 respirators in reducing the inhalation of combustion by-products in hanoi, vietnam: A demonstration study. Environmental Health: A Global Access Science Source. 2012;11 doi: 10.1186/1476-069X-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Hardt J, Schulz C, Angerer J. New reference value and the background exposure for the PAH metabolites 1-hydroxypyrene and 1- and 2-naphthol in urine of the general population in Germany: Basis for validation of human biomonitoring data in environmental medicine. Int J Hyg Envir Heal. 2008;211:447–453. doi: 10.1016/j.ijheh.2007.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Concentrations of 8 urinary OH-PAHs in the father who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S2. Concentrations of 8 urinary OH-PAHs in the son who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S3. Concentrations of 8 urinary OH-PAHs in the mother who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S4. Concentrations of 8 urinary OH-PAHs in the daughter who traveled between Brisbane, Australia and Hanoi, Vietnam
Figure S5. Effect of traveling from Brisbane to Hanoi and back to Brisbane on the concentrations of urinary PAH metabolites (Travel), and comparison to two control groups, residents of Brisbane and residents of Hanoi during the entire study period (a and b are significantly different, p<0.01).