Abstract
Treponema pallidum ssp. pallidum (TPA) causes over 10 million new cases of syphilis worldwide whereas T. pallidum ssp. pertenue (TPE), the causative agent of yaws, affects about 2.5 million people. Although penicillin remains the drug of choice in the treatment of syphilis, in penicillin-allergic patients, macrolides have been used in this indication since the 1950s. Failures of macrolides in syphilis treatment have been well documented in the literature and since 2000, there has been a dramatic increase in a number of clinical samples with macrolide-resistant TPA. Scarce data regarding the genetics of macrolide-resistant mutations in TPA suggest that although macrolide-resistance mutations have emerged independently several times, the increase in the proportion of TPA strains resistant to macrolides is mainly due to the spread of resistant strains, especially in developed countries. The emergence of macrolide resistance in TPA appears to require a two-step process including either A2058G or A2059G mutation in one copy of the 23S rRNA gene and a subsequent gene conversion unification of both rRNA genes. Given the enormous genetic similarity that was recently revealed between TPA and TPE strains, there is a low but reasonable risk of emergence and spread of macrolide-resistant yaws strains following azithromycin treatment.
Introduction
Syphilis is a sexually transmitted disease caused by Treponema pallidum ssp. pallidum (TPA), a spirochete that is genetically closely related to the causative agent of yaws, T. pallidum ssp. pertenue (TPE). Both TPA and TPE strains are highly related, whole genome analyses of TPA and TPE treponemes revealed a sequence identity of 99.8%.1
Although penicillin remains the drug of choice in the treatment of syphilis, macrolide antibiotics were historically recommended in penicillin-allergic patients for the treatment of primary, secondary, and latent syphilis.2
In 2007, World Health Organization (WHO) launched a program to eradicate yaws by the year 2020. The considered treatment regimens include a single dose of azithromycin or a single dose of benzathine penicillin G.3,4
The antibiotic resistance of TPA was comprehensively reviewed by L. Stamm.5,6 This article focuses on the occurrence of macrolide resistance in TPA strains with respect to the de novo emergence of macrolide resistance and spread of existing macrolide-resistant TPA strains.
Macrolide Failures in Syphilis Treatment and Frequency of Mutations in the 23S rRNA Gene
The commonly used macrolide antibiotics include erythromycin, clarithromycin, roxithromycin, azithromycin, and spiramycin. Different macrolide antibiotics interact with different regions of the 23S rRNA gene.7
Failures of macrolide treatment of syphilis have been well documented in the literature8–18 and included cases of erythromycin–clarithromycin–azithromycin–spiramycin treatment failures. Until now, no macrolide treatment failures in the treatment of yaws have been reported.19–21
A wide spectrum of macrolide-resistance mutations in 23S rRNA gene have been reported in other bacteria. However, only the A2058G and A2059G mutations (the position numbers correspond to positions on the Escherichia coli 23S rRNA gene) have been identified in TPA from clinical samples.16,22
In several bacteria including Mycoplasma smegmatis, Mycobacterium avium, and Treponema denticola, the frequency of macrolide-resistant mutants was 0.7 × 10−8,23 10−8 to 10−9,24 and 5 × 10−9,25 respectively. For T. pallidum, no data on mutation rates leading to macrolide-resistant mutations are available.
In general, the predicted mutation rate per genome varies around 3.3 × 10−3 per genome and replication for a variety of organisms including phages, bacteria, and yeasts.26 For E. coli it is estimated to be 5.4 × 10−10 mutations per base, per DNA replication.27 Assuming that the nucleotide TPA mutation rate is similar to that frequently observed in other bacteria, the TPA mutation rate can be estimated to be 0.22 − 0.38 × 10−8 per base and DNA replication.
In other spirochetes, the number of infecting bacteria in whole blood samples were found within the range of 104 to 105 bacteria/mL.28,29 In human TPA infections, a reported number of bacteria/mL of whole blood reached 1 × 103 and 3 × 104.30,31 The total blood volume (~5 L) therefore likely contains in one point of TPA infection at least 108 treponemes. Assuming a relatively low number of infecting spirochetes, the number of DNA replications approaches a number of microorganisms in human body. The number of DNA replications (getting closer to at least 108) in the course of human infection is within the same range of estimated reciprocal number of mutation rate (0.22 − 0.38 × 10−8 per base and DNA replication).
Presence of A2058G or A2059G Mutation in both TPA rRNA Operons
In all completely sequenced treponemal strains and in several additionally analyzed strains,1,32–42 comprising altogether 21 strains, sequences of 5S, 16S, and 23S rRNA genes in both rrn operons were identical in particular strains including syphilis-, yaws- and endemic syphilis-causing strains. The sequences of 5S, 16S, and 23S rRNA genes of the two rrn operons appear to undergo gene conversion unification leading to sequence identity of rRNA paralogs.43 Sequence analysis of amplified 23S rDNA from 144 patients revealed no sequence differences between both loci in the individual samples, although only a regions spanning several hundred base pairs were evaluated.44,45 Moreover, both copies of the 23S rDNA were amplified during the detection of macrolide resistance in many studies (listed in Table 1), and in all analyzed TPA DNA samples (N = 396) from these studies, the same macrolide resistance mutations (A2058G or A2059G) were found in both copies of the 23S rRNA gene. In addition, both mutations were never found combined.44–47 Altogether, no evidence of sequence heterogeneity of 23S rRNA gene copies exists in TPA to date. However, in T. denticola, the presence of an A2058G mutation in one 23S rRNA gene has been reported.25 Recent analysis of 23S rRNA genes in 184 prokaryotic species with multiple rrn operons revealed that 38.6% of the analyzed genomes contained identical 23S rRNA sequences within the individual genome.66 Although the reasons for identical 23S rDNA loci are unknown, the presence of either A2058G or A2059G mutation in TPA in both copies of the 23S rRNA gene suggests not only an efficient mechanism for copying between the two operons but also a general need for this genomic constitution. At the same time, the probability of emergence of macrolide-resistant mutation in TPA is lowered by several orders of magnitude. In Escherichia coli, recombination between rrn operons occurs at frequencies of 10−4 − 10−5.67
Table 1.
An overview of studies mapping the prevalence of A2058G and A2059G mutations in clinical samples containing TPA
| Country | Year | City, geographic area | No. of analyzed samples | No. of samples containing macrolide-resistant TPA (%) | No. of samples containing TPA with A2058G (%) | No. of samples containing TPA with A2059G (%) | References |
|---|---|---|---|---|---|---|---|
| Australia | 2004–2011 | Sydney | 409 | 345 (84.4) | 345 (84.4) | –* | 46 |
| Canada | 2000–2003 | British Columbia | 56 | 5 (8.9) | 5 (8.9) | – | 47 |
| 2007–2008 | Alberta | 14 | 4 (28.6) | 4 (28.6) | – | 48 | |
| 2007–2009 | Alberta and Northwest Territories | 43 | 7 (16.3) | 7 (16.3) | – | 49† | |
| China | 2007–2008 | Shanghai | 38 | 38 (100) | 38 (100) | – | 50 |
| 2008–2011 | – | 211 | 194 (91.9)‡ | 194 (91.9) | – | 51 | |
| 2010–2012 | Shandong | 66 | 66 (100) | 61 (92.4) | 5 (7.6) | 52 | |
| Czech Republic | 2004–2010 | – | 75 | 28 (37.3)‡ | 17 (22.7) | 11 (14.7) | 44 |
| 2011–2013 | – | 69 | 46 (66.7)‡ | 39 (56.5) | 7 (10.1) | 45 | |
| Great Britain | 2006–2008 | London | 18 | 12 (66.7)‡ | 11 (61.1) | 1 (5.6) | 53 |
| Ireland | 2002 | Dublin | 17 | 15 (88.2)‡ | 15 (88.2) | – | 54 |
| 2009–2010 | Dublin | 29 | 27 (93.1) | 27 (93.1) | – | 5 | |
| Madagascar | 2000–2007 | – | 141 | 1 (0.7) | 0 (0.0) | 1 (0.7) | 56, 57, 58 |
| South Africa | 2005–2010 | – | 100 | 1 (1.0)‡ | 1 (1.0) | 0 (0.0) | 59 |
| Taiwan | 2009–2011 | – | 102 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 60 |
| 2009–2013 | – | 268 | 2 (0.7) | 2 (0.7) | 0 (0.0) | 61§ | |
| United States | 1999–2003 | San Francisco | 55 | 12 (21.8)‡ | 12 (21.8) | – | 54 |
| 2000–2004 | San Francisco | 124 | 46 (37.1) | 46 (37.1) | – | 15 | |
| 2004–2007 | San Francisco | 62 | 42 (67.7) | 42 (67.7) | – | 62 | |
| 2001–2003 | Seattle | 23 | 3 (13.0)‡ | 3 (13.0) | – | 54 | |
| 2001–2010 | Seattle | 128 | 92 (71.9) | 79 (61.7) | 13 (10.2) | 56‖ | |
| 1998–2000 | Baltimore | 19 | 2 (10.5)‡ | 2 (10.5) | – | 54 | |
| 2001–2005 | – | 58 | 20 (34.5) | 20 (34.5) | – | 63 | |
| 2007–2009 | – | 141 | 75 (53.2) | 75 (53.2) | – | 64 | |
| 2007–2009 | – | 129 | 83 (64.3)‡ | 66 (51.2) | 17 (13.2) | 65# |
TPA = Treponema pallidum ssp. pallidum.
Mutation A2059G was not tested in this study. The occurrence of A2059G mutation16 was published 9 years after publication of the A2058G mutation.22
This study also analyses samples investigated in a previous study by Martin and others.48
These studies clearly stated that both copies of 23S rRNA genes were amplified. In all cases, macrolide resistance was found in both copies of 23S rDNA.
This study also analyses samples investigated in a previous study by Wu and others.60
This study also analyses samples investigated in a previous study by Lukehart and others54 and Marra and others.63
This study analyses portion of samples investigated in a previous study by Su and others.64
Prevalence of Macrolide-Resistant Samples of TPA, Patient*s Characteristics, and TPA Genotypes
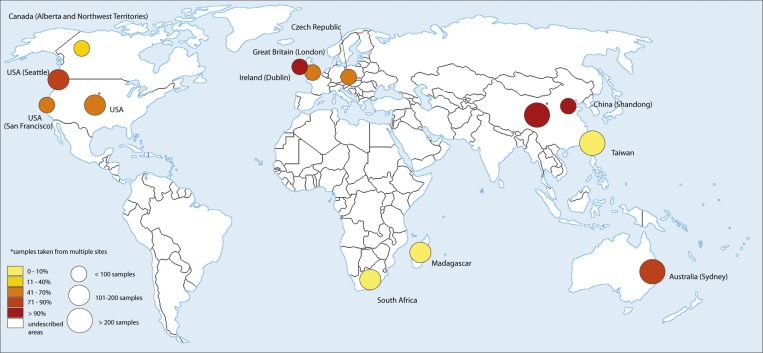
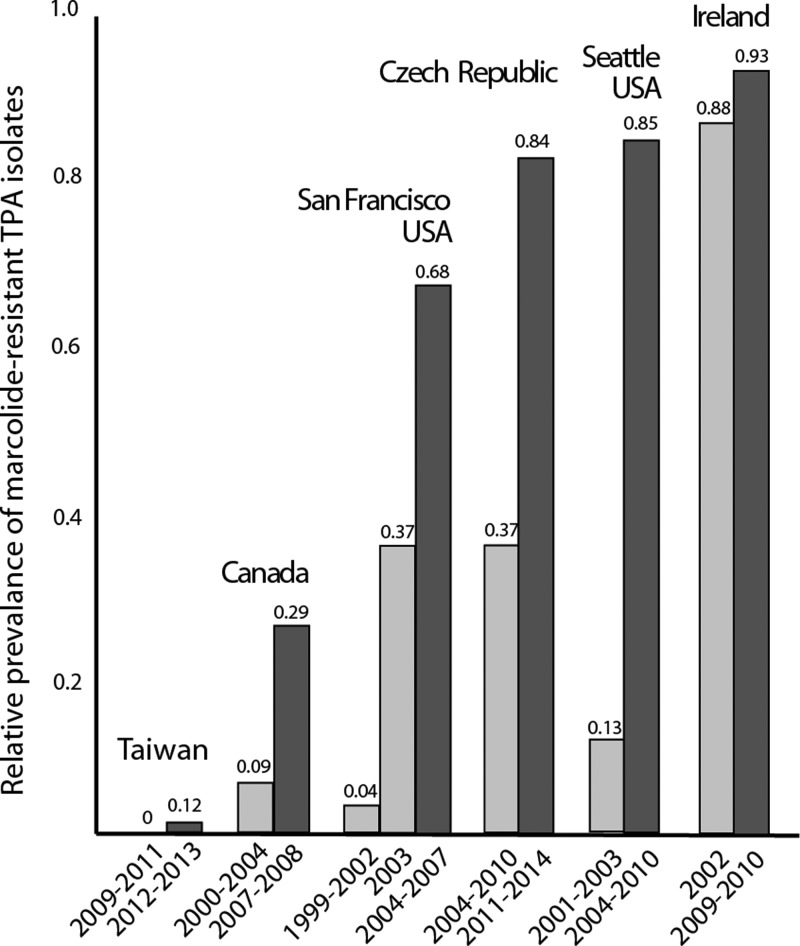
The presence of A2058G and A2059G mutations causing macrolide resistance was screened in TPA clinical samples in a number of studies from different countries including Australia,46 Canada,47–49 China,50–52 Czech Republic,44,45 United Kingdom,53 Ireland,54,55 Madagascar,56,57 South Africa,59 Taiwan,60,61 and United States15,54,56,62–65 (Table 1). A geographical distribution of clinical samples containing macrolide-resistant TPA in studies with samples isolated after 2004 is shown in Figure 1 . In all published studies from the same area, it was clear that the number of macrolide-resistant TPA in clinical samples increased over time (Figure 2 ). The prevalence of macrolide-resistant TPA samples appears to show geographical differences with lower prevalence on islands (Madagascar, Taiwan) and relatively remote areas (Northwest Territories, Canada; Lesotho, South Africa), whereas the highest prevalence was in large cities with a high level of tourism and business travel (Sydney, Shanghai, London, Dublin, San Francisco, Seattle; Table 1).
Figure 1.
A geographical distribution of clinical samples containing macrolide-resistant TPA in different countries. Only studies with samples collected after 2004 are shown (other studies are shown in Table 1) and only most recent study from a particular area is shown. The exception is the study by Grimes and others56 where analyzed samples were collected between 2001 and 2010 and the study of Van Damme and others57 where the time of sample collection was not indicated. However, according to the work of Tipple and Taylor,58 these samples were taken between 2000 and 2007. The frequencies of macrolide resistant strains are shown based on a color code (lower left). The size of circle indicates the number of tested samples. The circle position indicates prevalent origin of samples within the country. Note that Treponema pallidum ssp. pallidum (TPA) has been analyzed from only a limited number of countries. The map template was downloaded from the web (http://imgkid.com/world-map-blank-color.shtml).
Figure 2.
The relative prevalence of A2058G and A2059G mutations in clinical samples containing Treponema pallidum ssp. pallidum (TPA) in different countries. The number of strains harboring macrolide-resistance causing mutations has increased over time in all countries with available results from multiple studies/years.
Several studies have shown that treponemes harboring the A2058G mutation or one of the two A2058G or A2059G mutation are statistically more often found among men who have sex with men (MSM) patients compared with non-MSM patients.45,49,64 Moreover, Su and others64 found that treponemes harboring the A2058G mutation were more often associated with HIV-positive patients. These associations could reflect differences in the sexual networks and/or the differences in the use of macrolide antibiotics between different groups of patients.
Some studies44,45,53,56analyzed resistance genotypes together with sequence data from TP0548 gene locus that is used as a chromosomal target in sequencing-based typing44,45 and enhanced Centers for Disease Control and Prevention typing schemes.68,69 Some of them described predominance56 or an association between individual TPA genotypes and the A2058G mutation.53 In addition, several unique alleles (U2, U5, and U6) of TP0548 identified by sequencing-based typing were found to be associated with macrolide resistance mutations while the S allele from TP0548 was associated with macrolide susceptibility.44,45
On the other hand, TPA samples with identical genotypes based on the typing of at least two independent genomic loci but with different mutations within the 23S rRNA genes have been reported44,45,53,57 (Table 2) indicating that macrolide resistance emerged several times, independently of strain background.
Table 2.
Clinical samples containing TPA with identical genotypes based on the typing of at least two independent chromosomal loci but with different versions of the 23S rRNA gene
| 23S rRNA gene | ||||
|---|---|---|---|---|
| Genotype* | Wildtype | A2058G | A2059G | References |
| SS† | + | +‡ | + | 44, 45 |
| SU2 | + | + | −§ | 44 |
| 14d/f | + | + | −§ | 46, 53 |
| 14d/i | + | + | −§ | 56 |
TPA = Treponema pallidum ssp. pallidum.
Genotype was determined using sequencing-based typing or enhanced Centers for Disease Control and Prevention typing.44,68,69
Besides wild-type 23S rDNA, the SS genotype can have either the A2058G mutation or the A2059G mutation.
In all tested loci, the TPA in this sample is sequentially identical to the laboratory reference strain TPA SS14.
Not found yet.
Efficacy of Azithromycin in the Treatment of Syphilis caused by Macrolide-Susceptible TPA
The work of Riedner and others70 revealed that treatment of 155 persons with syphilis in Tanzania with 2 g of single oral dose of azithromycin was as successful as treatment with penicillin G. Kiddugavu and others71 showed that 1 g of oral azithromycin achieved higher cure rates compared with penicillin in 165 patients from Uganda. As shown by Hook and others,72 none of the 40 participants after 1 g of oral azithromycin, who in the preceding 30 days had been exposed to partners with infectious syphilis, developed signs of syphilis. Moreover, patients from Louisiana with early syphilis treated with azithromycin (N = 46) had similar cumulative response rates as patients treated with penicillin (N = 14).73 Similarly to TPA, treatment of 110 TPE-infected children with single oral dose of azithromycin in Papua New Guinea revealed slightly better results than treatment with benzathine benzylpenicillin.74 In Ghana, the analysis of 90 children with ulcers after azithromycin mass treatment to control trachoma revealed no evidence of yaws based on serology and polymerase chain reaction (PCR).75 In addition, no evidence of azithromycin treatment failure due to macrolide resistance has been detected during mass treatment of yaws patients.20 The above presented data indicate that de novo emergence of macrolide-resistance per treated patient is very rare, in order of 10−2 or lower.
Risk of Macrolide Treatment Failure in Yaws
The first effort to eradicate yaws by the WHO and the United Nations Children*s Fund (UNICEF) during 1952–1964, using injectable penicillin, led to a decrease in the global yaws prevalence from 50 to 2.5 million cases. Although the number of cases was reduced by 95%, the disease was not eradicated mainly due to the failure to identify and treat contacts and because of the existence of latent cases.4 In 2007, WHO launched a new program to eradicate yaws by the year 2020. The preferred treatment includes a single, peroral dose of azithromycin (30 mg/kg). Benzathine penicillin G (1.2 million units for adults and 0.6 million units for children) serves as a back-up treatment scheme since its administration is more complicated (involving painful intramuscular injections, requiring trained medical staff, etc.).4,74 Although the development of penicillin resistance appears to be extremely unlikely,76,77 azithromycin use could be complicated by treatment failures linked to the emergence of macrolide resistance mutations in the 23S rRNA genes.
Genotype analyses of macrolide-resistant TPA from clinical samples and estimates of TPA mutation rate and bacterial load during infection suggest that macrolide-resistance mutations emerged, independently, several times, during syphilis treatment, or during macrolide use for other indications of TPA infected people. The presence of mutations in both 23S rRNA genes suggests that emergence of macrolide resistance in TPA appears to require a two-step process including either A2058G or A2059G mutation in one copy of the 23S rRNA gene and a subsequent gene conversion unification of rRNA gene copies. The accelerated increase in the proportion of TPA strains resistant to macrolides since 2000 is likely due to the spread of already-existing resistant strains. Although this increase coincides with the introduction and the increased use of azithromycin in the United States78 and other countries, the exact role of azithromycin relative to the increase is not known. The spread of preexisting macrolide-resistant TPA strains is supported by 1) efficacy of azithromycin treatment in several trials, 2) increased prevalence in large cities with a high level of travel, and 3) increased prevalence among several groups of patients. Given the fact that the whole genome nucleotide divergence between TPA and TPE subspecies is only few times higher than the divergence within TPA and TPE subspecies,1 respectively, the WHO campaign to eradicate yaws with a perorally administered single dose of azithromycin possesses a low but reasonable risk of emergence and spread of macrolide-resistant TPE strains.
ACKNOWLEDGMENTS
We thank M. Strouhal for his careful reading of the manuscript and suggestions. This work was supported by the Grant Agency of the Czech Republic (P302/12/0574) to DS.
Footnotes
Authors* addresses: David Šmajs, Lenka Paštěková, and Linda Grillová, Masaryk University, Department of Biology, Faculty of Medicine, Masaryk University, Brno, Czech Republic, E-mails: dsmajs@med.muni.cz, lmikal@med.muni.cz, and lindagrillova@gmail.com.
References
- 1.Čejková D, Zobaníková M, Chen L, Pospíšilová P, Strouhal M, Qin X, Mikalová L, Norris SJ, Muzny DM, Gibbs RA, Fulton LL, Sodergren E, Weinstock GM, Šmajs D. Whole genome sequences of three Treponema pallidum ssp. pertenue strains: yaws and syphilis treponemes differ in less than 0.2% of the genome sequence. PLoS Negl Trop Dis. 2012;6:e1471. doi: 10.1371/journal.pntd.0001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines 1982. MMWR Morb Mortal Wkly Rep. 1982;31((Suppl 2)):33S–60S. [PubMed] [Google Scholar]
- 3.Mitjà O, Hays R, Rinaldi AC, McDermott R, Bassat Q. New treatment schemes for yaws: the path toward eradication. Clin Infect Dis. 2012;55:406–412. doi: 10.1093/cid/cis444. [DOI] [PubMed] [Google Scholar]
- 4.WHO Summary Report of the Consultative Meeting on Eradication of Yaws, 5–7 March 2012, Morges, Switzerland. 2012. http://apps.who.int/iris/bitstream/10665/75528/1/WHO_HTM_NTD_IDM_2012.2_eng.pdf Available at. Accessed April 21, 2015.
- 5.Stamm LV. Global challenge of antibiotic-resistant Treponema pallidum. Antimicrob Agents Chemother. 2010;54:583–589. doi: 10.1128/AAC.01095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamm LV. Syphilis: antibiotic treatment and resistance. Epidemiol Infect. 2015;143:1567–1574. doi: 10.1017/S0950268814002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfister P, Jenni S, Poehlsgaard J, Thomas A, Douthwaite S, Ban N, Böttger EC. The structural basis of macrolide-ribosome binding assessed using mutagenesis of 23S rRNA positions 2058 and 2059. J Mol Biol. 2004;342:1569–1581. doi: 10.1016/j.jmb.2004.07.095. [DOI] [PubMed] [Google Scholar]
- 8.Hashisaki P, Wertzberger GG, Conrad GL, Nichols CR. Erythromycin failure in the treatment of syphilis in a pregnant woman. Sex Transm Dis. 1983;10:36–38. doi: 10.1097/00007435-198301000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Stapleton JT, Stamm LV, Bassford PJ., Jr Potential for development of antibiotic resistance in pathogenic treponemes. Rev Infect Dis. 1985;7((Suppl 2)):S314–S317. doi: 10.1093/clinids/7-supplement_2.s314. [DOI] [PubMed] [Google Scholar]
- 10.Stamm LV, Stapleton JT, Bassford PJ., Jr In vitro assay to demonstrate high-level erythromycin resistance of a clinical isolate of Treponema pallidum. Antimicrob Agents Chemother. 1988;32:164–169. doi: 10.1128/aac.32.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan WC. Failure of erythromycin to cure secondary syphilis in a patient infected with the human immunodeficiency virus. Arch Dermatol. 1988;125:82–84. [PubMed] [Google Scholar]
- 12.Sands M, Markus A. Lues maligna, or ulceronodular syphilis, in man infected with human immunodeficiency virus: case report and review. Clin Infect Dis. 1995;20:387–390. doi: 10.1093/clinids/20.2.387. [DOI] [PubMed] [Google Scholar]
- 13.Klausner JD, Kohn RP, Kent CK. Azithromycin versus penicillin for early syphilis. N Engl J Med. 2006;354:203–205. [PubMed] [Google Scholar]
- 14.Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, Engelman J, Mitchell SJ, Rompalo AM, Marra CM, Klausner JD. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell SJ, Engelman J, Kent CK, Lukehart SA, Godornes C, Klausner JD. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004. Clin Infect Dis. 2006;42:337–345. doi: 10.1086/498899. [DOI] [PubMed] [Google Scholar]
- 16.Matějková P, Flasarová M, Zákoucká H, Bořek M, Křemenová S, Arenberger P, Woznicová V, Weinstock GM, Šmajs D. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J Med Microbiol. 2009;58:832–836. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhou P, Li K, Lu H, Qian Y, Gu X, Gong W, Tucker JD, Cohen MS. Azithromycin treatment failure among primary and secondary syphilis patients in Shanghai. Sex Transm Dis. 2010;37:726–729. doi: 10.1097/OLQ.0b013e3181e2c753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woznicová V, Matějková P, Flasarová M, Zákoucká H, Vališová Z, Šmajs D, Dastychová E. Clarithromycin treatment failure due to macrolide resistance in Treponema pallidum in a patient with primary syphilis. Acta Derm Venereol. 2010;90:206–207. doi: 10.2340/00015555-0774. [DOI] [PubMed] [Google Scholar]
- 19.Ayove T, Houniei W, Wangnapi R, Bieb SV, Kazadi W, Luke LN, Manineng C, Moses P, Paru R, Esfandiari J, Alonso PL, de Lazzari E, Bassat Q, Mabey D, Mitjà O. Sensitivity and specificity of a rapid point-of-care test for active yaws: a comparative study. Lancet Glob Health. 2014;2:e415–e421. doi: 10.1016/S2214-109X(14)70231-1. [DOI] [PubMed] [Google Scholar]
- 20.Mitjà O, Houinei W, Moses P, Kapa A, Paru R, Hays R, Lukehart S, Godornes C, Bieb SV, Grice T, Siba P, Mabey D, Sanz S, Alonso PL, Asiedu K, Bassat Q. Mass treatment with single-dose azithromycin for yaws. N Engl J Med. 2015;372:703–710. doi: 10.1056/NEJMoa1408586. [DOI] [PubMed] [Google Scholar]
- 21.Chi KH, Danavall D, Taleo F, Pillay A, Ye T, Nachamkin E, Kool JL, Fegan D, Asiedu K, Vestergaard LS, Ballard RC, Chen CY. Molecular differentiation of Treponema pallidum subspecies in skin ulceration clinically suspected as yaws in Vanuatu using real-time multiplex PCR and serological methods. Am J Trop Med Hyg. 2015;92:134–138. doi: 10.4269/ajtmh.14-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamm LV, Bergen HL. A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob Agents Chemother. 2000;44:806–807. doi: 10.1128/aac.44.3.806-807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sander P, Prammananan T, Meier A, Frischkorn K, Böttger EC. The role of ribosomal RNAs in macrolide resistance. Mol Microbiol. 1997;26:469–480. doi: 10.1046/j.1365-2958.1997.5811946.x. [DOI] [PubMed] [Google Scholar]
- 24.Heifets L, Mor N, Vanderkolk J. Mycobacterium avium strains resistant to clarithromycin and azithromycin. Antimicrob Agents Chemother. 1993;37:2364–2370. doi: 10.1128/aac.37.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SY, Ning Y, Fenno JC. 23S rRNA point mutation associated with erythromycin resistance in Treponema denticola. FEMS Microbiol Lett. 2002;207:39–42. doi: 10.1111/j.1574-6968.2002.tb11025.x. [DOI] [PubMed] [Google Scholar]
- 26.Drake JW. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci USA. 1991;88:7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agampodi SB, Moreno AC, Vinetz JM, Matthias MA. Utility and limitations of direct multi-locus sequence typing on qPCR-positive blood to determine infecting Leptospira strain. Am J Trop Med Hyg. 2013;88:184–185. doi: 10.4269/ajtmh.2012.12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liveris D, Schwartz I, McKenna D, Nowakowski J, Nadelman RB, DeMarco J, Iyer R, Cox ME, Holmgren D, Wormser GP. Quantitation of cell-associated borrelial DNA in the blood of Lyme disease patients with erythema migrans. Eur J Clin Microbiol Infect Dis. 2012;31:791–795. doi: 10.1007/s10096-011-1376-x. [DOI] [PubMed] [Google Scholar]
- 30.Tipple C, Hanna MO, Hill S, Daniel J, Goldmeier D, McClure MO, Taylor GP. Getting the measure of syphilis: qPCR to better understand early infection. Sex Transm Infect. 2011;87:479–485. doi: 10.1136/sti.2011.049494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gayet-Ageron A, Ninet B, Toutous-Trellu L, Lautenschlager S, Furrer H, Piguet V, Schrenzel J, Hirschel B. Assessment of a real-time PCR test to diagnose syphilis from diverse biological samples. Sex Transm Infect. 2009;85:264–269. doi: 10.1136/sti.2008.034314. [DOI] [PubMed] [Google Scholar]
- 32.Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, Sodergren E, Hardham JM, McLeod MP, Salzberg S, Peterson J, Khalak H, Richardson D, Howell JK, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton MD, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith HO, Venter JC. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 33.Matějková P, Strouhal M, Šmajs D, Norris SJ, Palzkill T, Petrosino JF, Sodergren E, Norton JE, Singh J, Richmond TA, Molla MN, Albert TJ, Weinstock GM. Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol. 2008;8:76. doi: 10.1186/1471-2180-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacani L, Jeffrey BM, Molini BJ, Le HT, Lukehart SA, Centurion-Lara A, Rockey DD. Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J Bacteriol. 2010;192:2645–2646. doi: 10.1128/JB.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Šmajs D, Zobaníková M, Strouhal M, Čejková D, Dugan-Rocha S, Pospíšilová P, Norris SJ, Albert T, Qin X, Hallsworth-Pepin K, Buhay C, Muzny DM, Chen L, Gibbs RA, Weinstock GM. Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS One. 2011;6:e20415. doi: 10.1371/journal.pone.0020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pětrošová H, Zobaníková M, Čejková D, Mikalová L, Pospíšilová P, Strouhal M, Chen L, Qin X, Muzny DM, Weinstock GM, Šmajs D. Whole genome sequence of Treponema pallidum ssp. pallidum, strain Mexico A, suggests recombination between yaws and syphilis strains. PLoS Negl Trop Dis. 2012;6:e1832. doi: 10.1371/journal.pntd.0001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zobaníková M, Mikolka P, Čejková D, Pospíšilová P, Chen L, Strouhal M, Qin X, Weinstock GM, Šmajs D. Complete genome sequence of Treponema pallidum strain DAL-1. Stand Genomic Sci. 2012;7:2615838. doi: 10.4056/sigs.2615838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pětrošová H, Pospíšilová P, Strouhal M, Čejková D, Zobaníková M, Mikalová L, Sodergren E, Weinstock GM, Šmajs D. Resequencing of Treponema pallidum ssp. pallidum strains Nichols and SS14: correction of sequencing errors resulted in increased separation of syphilis Treponema subclusters. PLoS One. 2013;8:e74319. doi: 10.1371/journal.pone.0074319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zobaníková M, Strouhal M, Mikalová L, Čejková D, Ambrožová L, Pospíšilová P, Fulton LL, Chen L, Sodergren E, Weinstock GM, Šmajs D. Whole genome sequence of the Treponema Fribourg-blanc: unspecified simian isolate is highly similar to the yaws subspecies. PLoS Negl Trop Dis. 2013;7:e2172. doi: 10.1371/journal.pntd.0002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giacani L, Iverson-Cabral SL, King JC, Molini BJ, Lukehart SA, Centurion-Lara A. Complete genome sequence of the Treponema pallidum subsp. pallidum Sea81-4 strain. Genome Announc. 2014;2:e00333–14. doi: 10.1128/genomeA.00333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Štaudová B, Strouhal M, Zobaníková M, Čejková D, Fulton LL, Chen L, Giacani L, Centurion-Lara A, Bruisten SM, Sodergren E, Weinstock GM, Šmajs D. Whole genome sequence of the Treponema pallidum subsp. endemicum strain Bosnia A: the genome is related to yaws treponemes but contains few loci similar to syphilis treponemes. PLoS Negl Trop Dis. 2014;8:e3261. doi: 10.1371/journal.pntd.0003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Čejková D, Zobaníková M, Pospíšilová P, Strouhal M, Mikalová L, Weinstock GM, Šmajs D. Structure of rrn operons in pathogenic non-cultivable treponemes: sequence but not genomic position of intergenic spacers correlates with classification of Treponema pallidum and Treponema paraluiscuniculi strains. J Med Microbiol. 2013;62:196–207. doi: 10.1099/jmm.0.050658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao D. Gene conversion drives within genic sequences: concerted evolution of ribosomal RNA genes in bacteria and archaea. J Mol Evol. 2000;51:305–317. doi: 10.1007/s002390010093. [DOI] [PubMed] [Google Scholar]
- 44.Flasarová M, Pospíšilová P, Mikalová L, Vališová Z, Dastychová E, Strnadel R, Kuklová I, Woznicová V, Zákoucká H, Šmajs D. Sequencing-based molecular typing of Treponema pallidum strains in the Czech Republic: all identified genotypes are related to the sequence of the SS14 strain. Acta Derm Venereol. 2012;92:669–674. doi: 10.2340/00015555-1335. [DOI] [PubMed] [Google Scholar]
- 45.Grillová L, Ptrošová H, Mikalová L, Strnadel R, Dastychová E, Kuklová I, Kojanová M, Kreidlová M, Vaňousová D, Hercogová J, Procházka P, Zákoucká H, Krchňáková A, Vašků V, Šmajs D. Molecular typing of Treponema pallidum in the Czech Republic during 2011 to 2013: increased prevalence of identified genotypes and of isolates with macrolide resistance. J Clin Microbiol. 2014;52:3693–3700. doi: 10.1128/JCM.01292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Read P, Jeoffreys N, Tagg K, Guy RJ, Gilbert GL, Donovan B. Azithromycin resistant syphilis-causing strains in Sydney: prevalence and risk factors. J Clin Microbiol. 2014;52:2776–2781. doi: 10.1128/JCM.00301-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morshed MG, Jones HD. Treponema pallidum macrolide resistance in BC. CMAJ. 2006;174:349. doi: 10.1503/cmaj.1050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin IE, Tsang RSW, Sutherland K, Tilley P, Read R, Anderson B, Roy C, Singh AE. Molecular characterization of syphilis in patients in Canada: azitromycin resistance and detection of Treponema pallidum DNA in whole-blood samples versus ulcerative swabs. J Clin Microbiol. 2009;47:1668–1673. doi: 10.1128/JCM.02392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin IE, Tsang RSW, Sutherland K, Anderson B, Read R, Roy C, Yanow S, Fonseca K, White W, Kandola K, Kouadjo E, Singh AE. Molecular typing of Treponema pallidum strains in western Canada: predominance of 14d subtypes. Sex Transm Dis. 2010;37:544–548. doi: 10.1097/OLQ.0b013e3181d73ce1. [DOI] [PubMed] [Google Scholar]
- 50.Martin IE, Gu W, Yang Y, Tsang RS. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin Infect Dis. 2009;49:515–521. doi: 10.1086/600878. [DOI] [PubMed] [Google Scholar]
- 51.Chen XS, Yin YP, Wei WH, Wang HC, Peng RR, Zheng HP, Zhang JP, Zhu BY, Liu QZ, Huang SJ. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin Microbiol Infect. 2012;19:975–979. doi: 10.1111/1469-0691.12098. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, Hou J, Zheng R, Li Z, Wen J, Liu D, Liu R, Chu T, Liu B, Yu G, Tian H, Zhang F. Two mutations associated with macrolide resistance in Treponema pallidum in Shandong, China. J Clin Microbiol. 2013;51:4270–4271. doi: 10.1128/JCM.01261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tipple C, McClure MO, Taylor GP. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex Transm Infect. 2011;87:486–488. doi: 10.1136/sextrans-2011-050082. [DOI] [PubMed] [Google Scholar]
- 54.Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, Engelman J, Mitchell SJ, Rompalo AM, Marra CM, Klausner JD. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 55.Muldoon EG, Walsh A, Crowley B, Mulcahy F. Treponema pallidum azithromycin resistance in Dublin, Ireland. Sex Transm Dis. 2012;39:784–786. doi: 10.1097/OLQ.0b013e318269995f. [DOI] [PubMed] [Google Scholar]
- 56.Grimes M, Sahi SK, Godornes BC, Tantalo LC, Roberts N, Bostick D, Marra CM, Lukehart SA. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex Transm Dis. 2012;39:954–958. doi: 10.1097/OLQ.0b013e31826ae7a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Damme K, Behets F, Ravelomanana N, Godornes C, Khan M, Randrianasolo B, Rabenja NL, Lukehart S, Cohen M, Hook E. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar. Sex Transm Dis. 2009;36:775–776. doi: 10.1097/OLQ.0b013e3181bd11dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tipple C, Taylor GP. Syphilis testing, typing, and treatment follow-up: a new era for an old disease. Curr Opin Infect Dis. 2015;28:53–60. doi: 10.1097/QCO.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 59.Müller EE, Paz-Bailey G, Lewis DA. Macrolide resistance testing and molecular subtyping of Treponema pallidum strains from southern Africa. Sex Transm Infect. 2012;88:470–474. doi: 10.1136/sextrans-2011-050322. [DOI] [PubMed] [Google Scholar]
- 60.Wu H, Chang SY, Lee NY, Huang WC, Wu BR, Yang CJ, Liang SH, Lee CH, Ko WC, Lin HH, Chen YH, Liu WC, Su YC, Hsieh CY, Wu PY, Hung CC. Evaluation of macrolide resistance and enhanced molecular typing of Treponema pallidum in patients with syphilis in Taiwan: a prospective multicenter study. J Clin Microbiol. 2012;50:2299–2304. doi: 10.1128/JCM.00341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu BR, Yang CJ, Tsai MS, Lee KY, Lee NY, Huang WC, Wu H, Lee CH, Chen TC, Ko WC, Lin HH, Lu PL, Chen YH, Liu WC, Yang SP, Wu PY, Su YC, Hung CC, Chang SY. Multicentre surveillance of prevalence of the 23S rRNA A2058G and A2059G point mutations and molecular subtypes of Treponema pallidum in Taiwan, 2009–2013. Clin Microbiol Infect. 2014;20:802–807. doi: 10.1111/1469-0691.12529. [DOI] [PubMed] [Google Scholar]
- 62.Katz KA, Pillay A, Ahrens K, Kohn RP, Hermanstyne K, Bernstein KT, Ballard RC, Klausner JD. Sex Transm Dis. 2010;37:660–663. doi: 10.1097/OLQ.0b013e3181e1a77a. [DOI] [PubMed] [Google Scholar]
- 63.Marra CM, Colina AP, Godornes C, Tantalo LC, Puray M, Centurion-Lara A, Lukehart SA. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J Infect Dis. 2006;194:1771–1773. doi: 10.1086/509512. [DOI] [PubMed] [Google Scholar]
- 64.Su JR, Pillay A, Hook EW, Ghanem KG, Wong W, Jackson D, Smith LD, Pierce E, Philip SS, Wilson S, Golden MR, Workowski KA, Chi KH, Parrish DD, Chen CY, Weinstock HS. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex Transm Dis. 2012;39:794–798. doi: 10.1097/OLQ.0b013e31826f36de. [DOI] [PubMed] [Google Scholar]
- 65.Chen CY, Chi KH, Pillay A, Nachamkin E, Su JR, Ballard RC. Detection of the A2058G and A2059G 23S rRNA gene point mutations associated with azithromycin resistance in Treponema pallidum by use of a TaqMan real-time multiplex PCR assay. J Clin Microbiol. 2013;51:908–913. doi: 10.1128/JCM.02770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pei A, Nossa CW, Chokshi P, Blaser MJ, Yang L, Rosmarin DM, Pei Z. Diversity of 23S rRNA genes within individual prokaryotic genomes. PLoS One. 2009;4:e5437. doi: 10.1371/journal.pone.0005437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harvey S, Hill CW. Exchange of spacer regions between rRNA operons in Escherichia coli. Genetics. 1990;125:683–690. doi: 10.1093/genetics/125.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marra C, Sahi S, Tantalo L, Godomes C, Reid T, Behets F, Rompalo A, Klausner JD, Yin Y, Mulcahy F, Golden MR, Centurion-Lara A, Lukehart SA. Enhanced molecular typing of Treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J Infect Dis. 2010;202:1380–1388. doi: 10.1086/656533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pillay A, Liu H, Chen CY, Holloway B, Sturm AW, Steiner B, Morse SA. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis. 1998;25:408–414. doi: 10.1097/00007435-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Riedner G, Rusizoka M, Todd J, Maboko L, Hoelscher M, Mmbando D, Samky E, Lyamuya E, Mabey D, Grosskurth H, Hayes R. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–1244. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 71.Kiddugavu MG, Kiwanuka N, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, Makumbi F, Li X, Reynolds SJ, Quinn TC, Gray RH, Rakai Study Group Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda. Sex Transm Dis. 2005;32:1–6. doi: 10.1097/01.olq.0000148297.48590.d8. [DOI] [PubMed] [Google Scholar]
- 72.Hook EW, 3rd, Stephens J, Ennis DM. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann Intern Med. 1999;131:434–437. doi: 10.7326/0003-4819-131-6-199909210-00007. [DOI] [PubMed] [Google Scholar]
- 73.Hook EW, 3rd, Martin DH, Stephens J, Smith BS, Smith K. A randomized, comparative pilot study of azithromycin versus benzathine penicillin G for treatment of early syphilis. Sex Transm Dis. 2002;29:486–490. doi: 10.1097/00007435-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Mitjà O, Hays R, Ipai A, Penias M, Paru R, Fagaho D, de Lazzari E, Bassat Q. Single-dose azithromycin versus benzathine benzylpenicillin for treatment of yaws in children in Papua New Guinea: an open-label, non-inferiority, randomised trial. Lancet. 2012;379:342–347. doi: 10.1016/S0140-6736(11)61624-3. [DOI] [PubMed] [Google Scholar]
- 75.Ghinai R, El-Duah P, Chi KH, Pillay A, Solomon AW, Bailey RL, Agana N, Mabey DC, Chen CY, Adu-Sarkodie Y, Marks M. A cross-sectional study of ‘yaws* in districts of Ghana which have previously undertaken azithromycin mass drug administration for trachoma control. PLoS Negl Trop Dis. 2015;9:e0003496. doi: 10.1371/journal.pntd.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weigel LM, Radolf JD, Norgard MV. The 47-kDa major lipoprotein immunogen of Treponema pallidum is a penicillin-binding protein with carboxypeptidase activity. Proc Natl Acad Sci USA. 1994;91:11611–11615. doi: 10.1073/pnas.91.24.11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cha JY, Ishiwata A, Mobashery S. A novel beta-lactamase activity from a penicillin-binding protein of Treponema pallidum and why syphilis is still treatable with penicillin. J Biol Chem. 2004;279:14917–14921. doi: 10.1074/jbc.M400666200. [DOI] [PubMed] [Google Scholar]
- 78.Hicks LA, Taylor TH, Jr, Hunkler RJ. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;369:1175–1176. doi: 10.1056/NEJMc1306863. [DOI] [PubMed] [Google Scholar]