Abstract
Prisoners have among the highest incidence of tuberculosis (TB) globally. However, the contribution of the prison environment on transmission is not well understood and structural characteristics have received little attention as effective epidemiological interventions in TB control. We evaluated architectural characteristics and estimated ventilation rates in 141 cells in three prisons in central west Brazil using steady-state exhaled carbon dioxide (CO2) levels. We used a modified Wells–Riley equation to estimate the probability of infection for inmates sharing a cell with an infectious case and projected the impact of interventions, including early diagnosis and improved ventilation. Overall, prison cells were densely populated (mean 2.1 m2 per occupant) and poorly ventilated, with only three cells meeting World Health Organization (WHO) standards for per-person ventilation (60 L/s) applied in infection control settings. In the absence of interventions, projected mean risk of infection was 78.0% during a 6-month period. Decreasing time-to-diagnosis by 25% reduced transmission risk by 8.3%. Improving ventilation to WHO standards decreased transmission by 38.2%, whereas optimizing cross-ventilation reduced transmission by 64.4%. Prison environments promote high infection risk over short-time intervals. In this context, enhanced diagnostics have a limited impact on reducing transmission. Improving natural ventilation may be required to effectively control TB in prisons.
Introduction
Despite global progress in the control of tuberculosis (TB),1 the burden of disease, particularly in medium- and low-burden countries, may be increasingly concentrated in high-risk subpopulations. Prisoners consistently have among the highest rates of TB in the world, in many settings exceeding 20 times the incidence of the general population.2 Penal institutions may represent “institutional amplifiers” of TB epidemics,3 given the dynamic, transient nature of prisoner populations, which in turn may fuel broader transmission in community settings.
Although the burden of TB in prisons has been well characterized, there have been few data on successful interventions for reducing the incidence of disease in correctional facilities. Nearly all studies have focused on early diagnosis of active TB through radiography or rapid diagnostics.4–7 Such interventions do not address the fundamental conditions favoring the transmission of TB, which may be critical for making a durable impact on epidemics in prisons. To do so, a better understanding of the role of underlying environmental epidemiology of prisons on TB transmission is needed.
Since the pioneering studies of Wells, Riley and others in the 1950s, the importance of ventilation on transmission of TB has been evident.8 Subsequent work has clarified the role of specific “real-world” conditions and TB risks.9–13 However, most of this work has focused on hospital settings, which has resulted in guidelines for infection control in health-care environments.14,15 There has been a dearth of primary data on ventilation in other high-risk environments, including prisons, which has hampered our ability to identify effective strategies for addressing transmission in these settings.
Brazil has the fourth largest prison population in the world, with over 545,000 inmates residing in 1,478 prisons.16 Multiple studies in prisons throughout Brazil have documented active TB prevalence in excess of 2,000 cases per 100,000 inmates.14,17–20 The central west state of Mato Grosso do Sul has the highest incarceration rate in the country. In this setting, we obtained empirical data on architecture, ventilation, and occupancy and projected the impact of diagnostic and environmental interventions on TB transmission.
Methods
Setting.
The state of Mato Grosso do Sul in Brazil has a population of 2.5 million. In 2012, the incarceration rate was 497 per 100,000, the highest in the country.21 We selected three medium-security prisons located near international or domestic borders, where drug-trafficking crimes are prevalent, as study sites. Estabelecimento Penal de Corumbá (EPC), near the Brazil–Bolivia border, has an occupant capacity of 208 inmates in 35 cells. Penitenciária de Três Lagoas (PTL), near the state border with São Paulo state, has a capacity of 255 inmates in 97 cells. Unidade Penal Ricardo Brandão (UPRB), near the Brazil–Paraguay border, has a capacity of 155 inmates in 26 cells. Overcrowding in all three prisons is persistently high. The reported state average occupancy rate was 175.4% in 2012.21
Architectural and environmental measurements.
The study team entered all 141 cells in the three prisons to survey architectural and environmental characteristics: occupancy, floor area, ceiling height, number of beds, bed layout, building materials, wall thickness, area of open windows and doors, window and door type, presence of cross-ventilation design, temperature, relative humidity, presence of openings facing prevailing winds, indoor ventilation obstructions, and portable fans. In addition, the study team surveyed area of courtyard, outdoor temperature, and wind speed.
Ventilation measurements.
To assess room ventilation, diffusion of carbon dioxide (CO2) is frequently measured because of its safety and ease of measurement.22,23 Typically, the tracer gas concentration decay technique is used to estimate ventilation, wherein a room is cleared of occupants and a cartridge containing a high concentration of CO2 is released. However, when CO2 cartridges cannot be released (or a room cannot be cleared of its occupants for a substantial period of time), exhaled CO2 can be used as a natural tracer gas to assess ventilation.23 For security reasons, the latter approach was used. Steady-state CO2 concentration after at least 14 hours of closed cell occupancy was measured using a portable continuous CO2 sampling device (Extech Instruments, Waltham, MA).
Analytic approach.
The absolute ventilation rate (Q; in L/s) for each cell was calculated based on the equilibrium between ventilation and CO2 production through respiration23 according to the following equation:
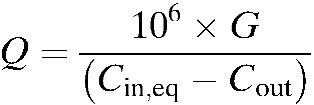 |
The CO2 generation rate in the space (G) is a function of the average CO2 generation rate per person at rest, 0.0052 L/s, and the number of occupants.23 Here, equilibrium CO2 concentration in the cell (Cin,eq) and outdoor CO2 concentration (Cout) are expressed in parts per million (ppm).
Statistical analysis.
One-way random effects analysis of variance was used to determine the extent to which cells within the same prison were similar to each other in terms of absolute ventilation. We used univariable regression to assess the association between environmental characteristics of cells and absolute ventilation estimates. Significant variables at P < 0.2 were then included in a full multivariable hierarchical linear model. Backward elimination was used to remove variables not significant at P < 0.05 until a parsimonious model was achieved. For all hierarchical models, absolute ventilation was normalized by log10-transformation. Data were analyzed using SAS 9.4 (Cary, NC).
Spatial distribution of ventilation.
Although air changes per hour (ACH) has classically been used as a metric for assessing infection control risks, per person ventilation rates are a better measure of risk of airborne infection.15 World Health Organization (WHO) guidelines recommend a minimum of 60 L/s/person for the prevention of airborne infections in naturally ventilated health-care settings.15 To identify macro- and microenvironments of ventilation within the prisons, quintile values of ventilation rates were calculated and overlaid on architectural floor plans.
Transmission modeling.
We estimated transmission probabilities by applying a non-steady-state version of the Wells–Riley equation, which describes airborne transmission probabilities within a space with defined ventilation characteristics.12,24–26 The probability of infection (P) is given by the following equation:
Rudnick and Milton revised the classic Wells–Riley equation to allow the estimation of the probability of infection in a confined space when total exposure occurs in a single time period:
 |
Here, the probability of infection is a function of the number of infectious individuals in a room (I), respiration rate (p), quantum generation rate by an infected person (q), total exposure time (t), volume of the confined space (V), time elapsed from when the room becomes occupied (θ), and germ-free ventilation rate (Q). We estimated the probability of TB infection in the presence of one infectious individual in a cell.
Model parameters.
Germ-free ventilation rate was estimated for each prison cell as described above (Supplemental Table 1). The existing literature provides variable estimates for the infectious quanta rate (q). Wells and others estimated q to be 1.25 quanta per hour for smear-positive individuals, whereas Escombe and others estimated q to be 8.2 quanta per hour.8,27 We used the more conservative estimate of 1.25 for our model and adjusted for the proportion of smear-positive individuals reported among these prisons (34.6%). We assumed the relative infectiousness of smear-negative individuals at 0.2.28 The mean ventilatory rate of adults at rest was assumed to be 360 L/hour. The period of infectiousness is variable among different study populations; by comparing cross-sectional prevalence with TB incidence (notifications) among inmates of Mato Grosso do Sul,29 the average duration of infectiousness prior to diagnosis was estimated to be 180 days.
Interventions.
We then examined the effects of improved ventilation and case finding. To project the effects of improvements to case finding, enhanced diagnosis was modeled as a 25% (45 days) reduction in time-to-diagnosis, as may be achieved by enhanced diagnostic interventions. We examined three improved ventilation scenarios: 1) increasing ventilation in cells with less than 12 ACH to achieve 12 ACH14; 2) increasing per-person ventilation to the WHO recommendation for naturally ventilated health care settings (60 L/s/person)15; and 3) optimizing cross-ventilation design conditions, within the existing opening sizes. Wind-driven natural ventilation rate through a cross-ventilated room can be estimated by the following equation15:
 |
For each prison cell, we estimated cross-ventilated ACH assuming existing architectural (e.g., room volume and area of smallest opening) and environmental conditions (e.g., wind speed). Smallest opening area was calculated as the mean window size in each corresponding prison.
Results
Architecture, occupancy, and ventilation.
In total, the three prisons housed 1,217 inmates in 141 cells (mean: 8.6 inmates per cell), who were locked in cells for a mean of 18.4 hours per day, with an average of 2.1 m2 per prisoner (Table 1). PTL, the most recently built prison, had several differences in the design in comparison with EPC and UPRB: lower mean cell occupancy (5.8 versus 13.2 and 11.7 inmates per cell); larger mean floor area per inmate (2.5 m2 versus 1.0 and 1.6 m2); and more cells per courtyard (19.4 versus 3.8 and 4.6). Mean area of open windows and doors in EPC and UPRB was 4.3 and 5.1 m2, respectively, with all cells designed for single-sided ventilation. By contrast, PTL had a smaller mean opening area (2.8 m2), and 94.1% of cells had a stack ventilation design, wherein an upper room window is used to ventilate air that is driven upward by thermal buoyancy. PTL also had a longer average lockdown time (20.0 hours versus 16.8 and 14.7 hours).
Table 1.
Description of the sample, by prison (N = 141 cells)*
| Cell characteristic† | EPC (N = 32) | PTL (N = 85) | UPRB (N = 24) | P value‡ |
|---|---|---|---|---|
| Individuals per cell | 13.2 ± 5.9 | 5.8 ± 2.7 | 11.7 ± 6.0 | < 0.001 |
| Beds per cell | 6.4 ± 4.1 | 2.9 ± 1.5 | 5.7 ± 1.7 | < 0.001 |
| Absolute ventilation (L/s) | 119.1 ± 54.0 | 128.7 ± 74.4 | 202.3 ± 217.5 | 0.008 |
| ACH | 12.6 ± 4.6 | 15.0 ± 12.5 | 21.8 ± 26.7 | 0.066 |
| Steady-state CO2 (ppm) | 989 ± 204 | 652 ± 159 | 775 ± 158 | < 0.001 |
| Floor area (m2) | 13.1 ± 6.9 | 13.3 ± 4.7 | 13.0 ± 3.6 | 0.959 |
| Ceiling height (m) | 2.9 ± 0.2 | 3.2 ± 0.2 | 2.9 ± 0.2 | < 0.001 |
| Opening area (m2)§ | 4.3 ± 0.8 | 2.8 ± 0.3 | 5.1 ± 3.9 | < 0.001 |
| Openings facing prevailing winds, n (%)∥ | 14 (43.8) | 40 (50.0) | 8 (33.3) | 0.346 |
| Interior concrete walls | 2.5 ± 1.1 | 2.3 ± 1.0 | 2.0 ± 0.5 | 0.144 |
| Portable fans | 7.5 ± 8.0 | 2.6 ± 1.5 | 5.7 ± 2.8 | < 0.001 |
| Cells per courtyard | 3.8 ± 1.4 | 19.4 ± 6.1 | 4.6 ± 1.7 | < 0.001 |
| Courtyard area (m2) | 55.0 ± 17.1 | 316.3 ± 94.7 | 54.0 ± 15.9 | < 0.001 |
| Recreational time (hours) | 7.2 ± 4.5 | 4.0 ± 2.4 | 9.3 ± 1.0 | < 0.001 |
ACH = air changes per hour; EPC = Estabelecimento Penal de Corumbá; PTL = Penitenciária de Três Lagoas; SD = standard devaition; UPRB = Unidade Penal Ricardo Brandão.
A total of 1,047 inmates in 141 cells.
Table values are mean ± SD for continuous variables and n (column %) for categorical variables.
P value is for t test (continuous variables) or χ2 test (categorical variables).
Area of open windows and/or doors.
Windows and/or doors facing prevailing winds during winter.
With existing architectural features, mean absolute ventilation ranged from 119.1 to 202.3 L/s (P = 0.008). EPC, PTL, and UPRB had mean per person ventilation rates of 9.5 L/s/person (95% confidence interval [CI]: 8.3–10.8), 23.9 L/s/person (95% CI: 21.1–26.7), and 16.4 L/s/person (95% CI: 11.9–20.8), respectively (P < 0.001), for a combined mean of 19.4L/s/person (95% CI: 17.3–21.5) (Figure 1A ). The overlay of per person ventilation on UPRB's architectural floor plan illustrates the heterogeneity in ventilation (Figure 1B). Half (49.6%) of cells had less than 12 ACH and 97.9% had less than 60 L/s/person.
Figure 1.
Ventilation (L/s/person) (colored dots) and mean value (dark solid line) in general population cells of three prisons, Mato Grosso do Sul, Brazil, 2013. The horizontal dashed line indicates the World Health Organization (WHO)-recommended 60 L/s/person for naturally ventilated, general wards (A). Overall floor plan of UPRB illustrating the distribution of ventilation (colored dots) in proportion to the number of inmates (B). Photograph of typical courtyard in Unidade Penal Ricardo Brandão (UPRB) (C).
In multivariable analysis, absolute ventilation was positively associated with floor area, ceiling height, opening area, opening to floor area ratio, opening to volume ratio, and area of courtyard space (Table 2). Number of cells per courtyard was negatively associated with absolute ventilation.
Table 2.
Hierarchical linear model of factors associated with absolute ventilation (log10 L/s)
| Characteristic | Coefficient (95% CI) | P value |
|---|---|---|
| Floor area (m2) | 0.045 (0.01, 0.08) | 0.005 |
| Ceiling height (m) | 0.700 (0.08, 1.32) | 0.028 |
| Opening area (m2) | 0.082 (0.01, 0.15) | 0.025 |
| Opening area to floor area ratio | 11.955 (5.80, 18.11) | < 0.001 |
| Opening to volume ratio | 30.491 (13.65, 47.33) | < 0.001 |
| Cells per courtyard | −0.016 (−0.03, 0.01) | 0.023 |
| Courtyard area (m2) | 0.002 (0.001, 0.003) | < 0.001 |
CI = confidence interval.
Estimated risk of TB transmission.
The mean estimated risk of TB transmission after exposure for 180 days to an infectious cellmate under the existing conditions was 78.9% (95% CI: 69.7–88.1) for EPC, 81.1% (95% CI: 75.2–87.1) for PTL, and 65.6% (95% CI: 54.6–76.5) for UPRB, (P < 0.001) (Figure 2 ). There was marked variability of risk between cells, from 14.2% to 99.9%, reflecting variability in ventilation.
Figure 2.
Projected probability of tuberculosis (TB) infection over time for each inhabitant of a prison cell in which one infectious case is present, in Estabelecimento Penal de Corumbá (EPC) (A), Penitenciária de Três Lagoas (PTL) (B), and Unidade Penal Ricardo Brandão (UPRB) (C), Mato Grosso do Sul, Brazil, 2013. The grey lines indicate TB transmission probability for individual prison cells. The dark red lines indicate the mean TB transmission probability for each prison.
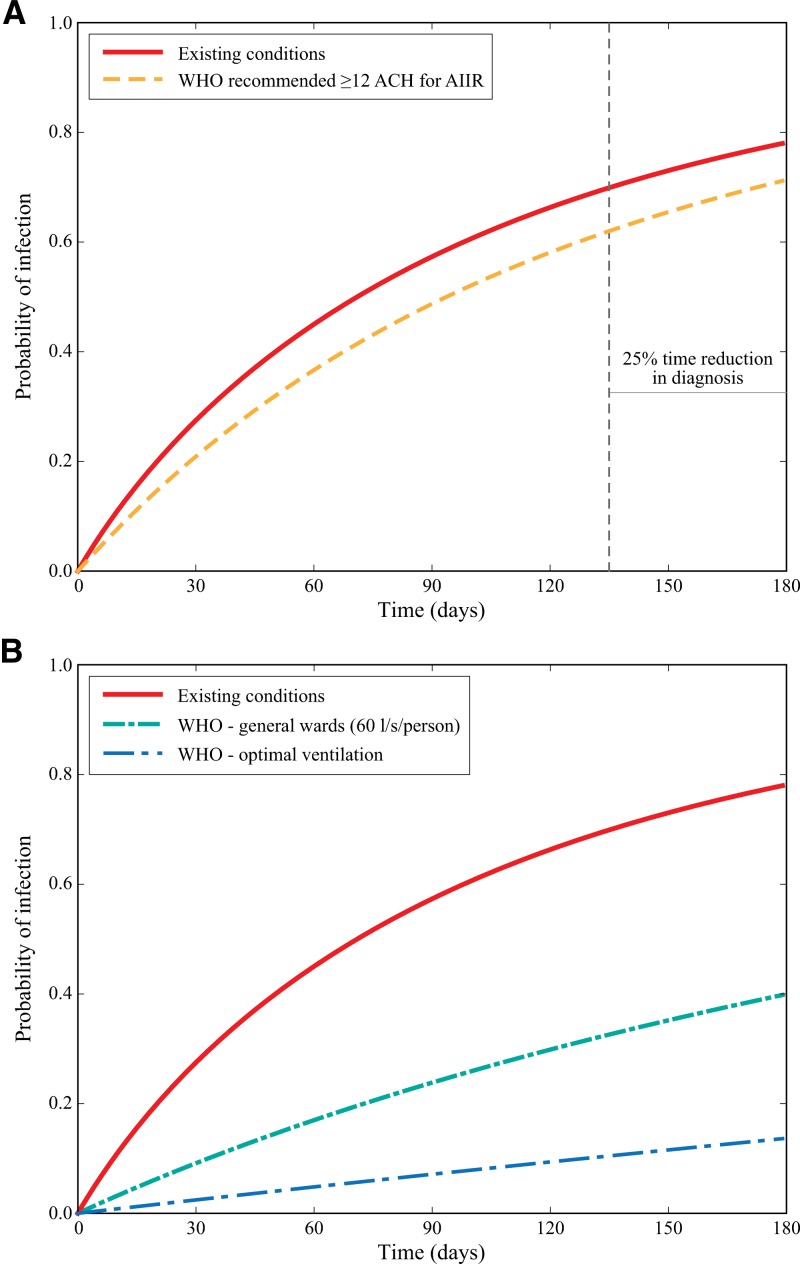
Improved case finding resulted in an 8.3% reduction to transmission probabilities for existing scenarios (Figure 3A ). When cells were modeled as having minimum ventilation rates of 12 ACH, mean transmission probability slightly improved (6.8% reduction). By combining intervention strategies, decreasing time-to-diagnosis and improving minimum ventilation to 12 ACH, existing transmission probabilities were reduced by 16.2%.
Figure 3.
The effects of improving cell ventilation and time-to-diagnosis on tuberculosis (TB) transmission probabilities by the time of infectiousness up to 180 days. Two values of ventilation scenarios are shown (A): the current estimated mean absolute ventilation (m3/hour) and the mean of improving ventilation in all cells to ≥12 air changes per hour (ACH) as recommended for airborne infection isolation rooms (AIIR). The vertical dashed line represents a 25% reduction in time to diagnosis. Three values of ventilation scenarios are shown (B): the current estimated mean absolute ventilation (m3/hour); improved ventilation rate in all cells to 60 L/s/person; and improved ventilation rate in all cells to reflect ACH under a cross-ventilation design for naturally ventilated settings.
Compared with the limited reductions achieved with improved case finding and meeting minimum ACH, large reductions in TB transmission were observed when ventilation was improved to meet WHO recommendations for health-care settings (Figure 3B). Improving minimal ventilation to 60 L/s/person reduced TB transmission risk by 38.2%, whereas implementing an optimal cross-ventilation design reduced TB transmission risk by 64.4%.
Discussion
The state of TB transmission in prisons remains a public health crisis throughout much of the world. The majority of the programmatic work and research aimed at addressing this burden have focused on early diagnosis, but the fundamental structural drivers of transmission have received less attention. We performed detailed architectural evaluation and estimation of ventilation in three prisons in Brazil, and used this data to project transmission risks over time, and to model the impact of diagnostics and improved ventilation as epidemiological interventions. We found that transmission in crowded cells with limited ventilation occurs quickly. As a result, enhanced diagnostics used among symptomatic patients (e.g., passive diagnosis) may have a limited impact on transmission. However, based on the findings of our models, improving ventilation substantially reduces the daily transmission risk, providing a longer window for diagnosis to avert infections. These findings highlight the need to address the underlying environment to control TB in prisons.
This work builds on the classical studies of Wells and Riley8,30 and more recent studies evaluating the relationship between ventilation, infectiousness, and transmission,12,24,25 and applies these findings to a nonhospital setting. A previous model in a South African prison projected even higher rates of TB transmission, but did not use empirical data on ventilation.25 We performed detailed measurements of architectural layout, cell inhabitance, wind speed, and CO2 to characterize ventilation in 141 cells. We found substantial heterogeneity in ventilation between cells, identified architectural characteristics that facilitated ventilation, and then used these data to project the impact of optimizing ventilation on reducing TB transmission.
In high-transmission environments, the window for averting secondary infections through early diagnosis may be small. We projected that over three quarters of cellmates of an infectious case would be infected within 6 months, which is the average duration of infectiousness estimated based on comparison of prevalence and notification data from these prisons. This finding is consistent with, if not higher than estimates of secondary infections from household contact investigation studies.31 However, because the majority of transmission occurs outside households in high-burden settings,32,33 “saturation” of local susceptible contacts does not limit diagnostic impact as greatly.
The situation in prisons differs considerably from that observed in household settings in that inmates are typically in their cells in close contact for 16–20 hours per day. Previous models evaluating the impact of early diagnostics in prisons have assumed homogenous mixing and neglected saturation dynamics, which overestimates the impact of diagnostics.6 We found that enhanced diagnostics targeted at symptomatic patients have a limited impact on TB transmission; however, improving ventilation would slow the secondary infection rate, providing a greater window for diagnosing a case before the majority of cellmates are infected.
In the absence of standards pertaining to prison design and minimum ventilation, we demonstrated the potential impact of applying WHO standards for naturally ventilated health-care settings (which only 3 of 141 cells met). Given that the prevalence of TB in many prisons approaches that of hospitals, we believe that it would be reasonable to use ventilation guidelines for health-care settings as a starting point in developing guidelines for prisons. In this context, improving ventilation to the WHO-recommended threshold of 12 ACH (for mechanically ventilated isolation rooms) does not confer a large benefit.14 Despite its lack of applicability, the literature has interpreted this guideline as the target metric for TB control in congregate settings.25,34
In contrast, standards for natural, per-person ventilation rates are far more appropriate for quantifying transmission reduction in prison settings. The WHO recommends a minimum ventilation rate of 60 L/s/person for general wards and outpatient departments and even higher ventilation rates (160 L/s/person) for airborne precaution rooms.15 We found that transmission risk greatly improved when ventilation was increased to 60 L/s/person. Improvements to natural ventilation systems are often low cost, compared with mechanical ventilation, and may thereby be more feasible in resource-limited settings.15,35 However, natural ventilation is highly variable12,36 and consequently requires specific planning to achieve desired airflow patterns.
Present multivariable analyses demonstrate that spacious floor areas, high ceilings, and generous opening area were associated with higher absolute ventilation, albeit insufficient to achieve WHO-recommended values. Though these findings are intuitive, the models also suggest the important association of decreased number of cells per courtyard and larger floor area of courtyards with absolute ventilation, which are unique to prison settings; factors that should be considered for new constructions or renovations.
One of the main obstacles identified was the absence of cross-ventilation. Cross-ventilation, two adjacent openings through which ventilation can pass through unobstructed, is considered to be more effective than single-sided ventilation for airborne infection control.15 Here, security and public health concerns may be at odds with one another. These issues will need to be weighed in the design of new prisons in low- and middle-income countries, where mechanical ventilation is not commonly available.
Crowding remains a critical problem in prisons throughout the world, as was the case in the prisons surveyed in this study. The mean of 2.1 m2 per inmate was less than half of the minimum standard recommended by the American Public Health Association (60 ft2, or 5.6 m2). Other prisons in Mato Grosso do Sul have up to 35–40 inmates per cell and are operating at 500% capacity. Addressing overcrowding is an urgent problem on both human rights and public health grounds. However, de-crowding prisons alone is not enough to lower transmission risks. The most recently constructed prison, PTL, was significantly less crowded than EPC and UPRB, yet was no better ventilated and thus TB transmission risk remains great.
The conclusions of this study should be interpreted within the context of the quality of the data used. We collected observations of steady-state exhaled CO2 to estimate ventilation, rather than the more commonly used tracer gas approach, owing to security concerns in the prisons. Although this approach is well described in the literature, it may be less precise owing to interindividual variability in CO2 production. The measurement of CO2 after at least 14 hours of overnight confinement ensured that ventilation estimates reflected well-mixed, equilibrium conditions. Only one measure of CO2, under varying climatological conditions, was recorded per cell; therefore, we cannot assess variability that would be expected in naturally ventilated settings, nor accurately compare cells within or across prisons. Nevertheless, ventilation rates were low across all three prisons and across multiple days of measurements.
The use of model-based projections for TB transmission risk has several limitations. We used the conservative literature estimate for the quantum generation rate and based our projections on an “average” individual with this level of infectiousness. It is likely that there is considerable interindividual and intraindividual variability in infectiousness,37 which are not captured in these projections. We assumed that transmission risk was restricted to events occurring within cells based on the observation that nearly all congregation outside of cells occurs in outdoor courtyards, where transmission is unlikely to occur. We calculated transmission risk for prisoners who shared a cell with an infectious case throughout the period that the case was infectious, prior to diagnosis. In reality, prisoners may be relocated between cells, creating further opportunities for transmission by renewing the susceptible pool of contacts.
Another limitation of the study is that it was conducted in three prisons in central west Brazil, which may or may not be generalizable to prisons in other settings. However, the architectural styles of these prisons reflect different eras of construction and are common to other states. Furthermore, crowding and poor ventilation are thought to be typical in prisons worldwide.25
The present state of TB in the world's prisons represents a social justice crisis and a threat to broader disease control, yet to date we have had few successful examples of programs that mitigate this epidemic. Given the poor structural environment of prisons, our findings indicate that interventions targeted after individuals develop symptoms may be too late to control spread. Reducing the burden of TB from the present incidence of over 1,500 per 100,000 will require 1) decreasing the contact rate in prison, by addressing overcrowding; 2) reducing the rate of infection, by improving cell ventilation; and 3) early diagnosis and treatment of infectious cases, either through active detection (e.g., annual mass screening) or enhanced passive surveillance (e.g., rapid diagnostics, symptom screening). These epidemiological interventions may be synergistic, as improved ventilation widens the window for diagnosis. Further empirical studies are urgently needed to validate these findings and provide an additional evidence base for development of policies and interventions to address the burden of TB in the world's prisons.
Supplementary Material
Footnotes
Financial support: This work was supported by Foundation for the Development of Teaching, Science, and Technology of the State of Mato Grosso do Sul (0067/12 and 0059/13); Secretariat of Health Surveillance, Brazilian Ministry of Health (20/2013); Ciências sem Fronteiras Program of the Brazilian National Research Council; Fogarty Global Health Equity Scholars Program (NIH 1 R25 TW009338); Jan A. J. Stolwijk Fellowship at the Yale School of Public Health (to J.U.); and National Institute of Allergy and Infectious Disease (K01 AI104411 to J.R.A.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' addresses: Juliana Urrego and Catherine W. Yeckel, Yale School of Public Health, New Haven, CT, E-mails: urrego.juliana@gmail.com and catherine.yeckel@yale.edu. Albert I. Ko, Yale School of Public Health, New Haven, CT, and Fundação Oswaldo Cruz, Salvador, Brazil, E-mail: albert.ko@yale.edu. Andrea da Silva Santos Carbone, Dayse Sanchez Guimarães Paião, and Renata Viebrantz Enne Sgarbi, Hospital Universitário de Dourados, Dourados, Brazil, E-mails: andrea.santos.enf@gmail.com, daysesanchez@hotmail.com, and renata_enne@hotmail.com. Jason R. Andrews, Stanford University School of Medicine, Stanford, CA, E-mail: jandr@stanford.edu. Julio Croda, Faculdade de Ciências da Saúde, Universidade Federal da Grande Dourados, Dourados, Brazil, E-mail: juliocroda@gmail.com.
References
- 1.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, Wang H, Duber HC, Naghavi M, Dicker D, Dandona L, Salomon JA, Heuton KR, Foreman K, Phillips DE, Fleming TD, Flazman AD, Phillips BK, Johnson EK, Coggeshall MS, Abd-Allah F, Abera SF, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Adeyemo AO, Adou AK, Adsuar JC, Agardh EE, Akena D, Al Kahbouri MJ, Alasfoor D, Albittar MI, Alcalá-Cerra G, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allen PJ, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Amini H, Ammar W, Anderson BO, Antonio CA, Anwari P, Arnlöv J, Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Banerjee A, Basu S, Beardsley J, Bekele T, Bell ML, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Abdulhak AB, Binagwaho A, Blore JD, Basara BB, Bose D, Brainin M, Breitborde N, Castañeda-Orjuela CA, Catalá-López F, Chadha VK, Chang JC, Chiang PP, Chuang TW, Colomar M, Cooper LT, Cooper C, Courville KJ, Cowie BC, Criqui MH, Dandona R, Dayama A, De Leo D, Degenhardt L, Del Pozo-Cruz B, Deribe K, Des Jarlais DC, Dessalegn M, Dharmaratne SD, Dilmen U, Ding EL, Driscoll TR, Durrani AM, Ellenbogen RG, Ermakov SP, Esteghamati A, Faraon EJ, Farzadfar F, Fereshtehnejad SM, Fijabi DO, Forouzanfar MH, Fra Paleo U, Gaffikin L, Gamkrelidze A, Gankpé FG, Geleijnse JM, Gessner BD, Gibney KB, Ginawi IA, Glaser EL, Gona P, Goto A, Gouda HN, Gugnani HC, Gupta R, Gupta R, Hafezi-Nejad N, Hamadeh RR, Hammami M, Hankey GJ, Harb HL, Haro JM, Havmoeller R, Hay SI, Hedayati MT, Pi IB, Hoek HW, Hornberger JC, Hosgood HD, Hotez PJ, Hoy DG, Huang JJ, Iburg KM, Idrisov BT, Innos K, Jacobsen KH, Jeemon P, Jensen PN, Jha V, Jiang G, Jonas JB, Juel K, Kan H, Kankindi I, Karam NE, Karch A, Karema CK, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Keren A, Kereselidze M, Khader YS, Khalifa SE, Khan EA, Khang YH, Khonelidze I, Kinfu Y, Kinge JM, Knibbs L, Kokubo Y, Kosen S, Defo BK, Kulkarni VS, Kulkarni C, Kumar K, Kumar RB, Kumar GA, Kwan GF, Lai T, Balaji AL, Lam H, Lan Q, Lansingh VC, Larson HJ, Larsson A, Lee JT, Leigh J, Leinsalu M, Leung R, Li Y, Li Y, De Lima GM, Lin HH, Lipshultz SE, Liu S, Liu Y, Lloyd BK, Lotufo PA, Machado VM, Maclachlan JH, Magis-Rodriguez C, Majdan M, Mapoma CC, Marcenes W, Marzan MB, Masci JR, Mashal MT, Mason-Jones AJ, Mayosi BM, Mazorodze TT, Mckay AC, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Melaku YA, Memish ZA, Mendoza W, Miller TR, Mills EJ, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Moore AR, Mori R, Moturi WN, Mukaigawara M, Murthy KS, Naheed A, Naidoo KS, Naldi L, Nangia V, Narayan KM, Nash D, Nejjari C, Nelson RG, Neupane SP, Newton CR, Ng M, Nisar MI, Nolte S, Norheim OF, Nowaseb V, Nyakarahuka L, Oh IH, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orisakwe OE, Pandian JD, Papachristou C, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pereira DM, Pervaiz A, Pesudovs K, Petzold M, Pourmalek F, Qato D, Quezada AD, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Ur Rahman S, Raju M, Rana SM, Razavi H, Reilly RQ, Remuzzi G, Richardus JH, Ronfani L, Roy N, Sabin N, Saeedi MY, Sahraian MA, Samonte GM, Sawhney M, Schneider IJ, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Sheikhbahaei S, Shibuya K, Shin HH, Shiue I, Shivakoti R, Sigfusdottir ID, Silberberg DH, Silva AP, Simard EP, Singh JA, Skirbekk V, Sliwa K, Soneji S, Soshnikov SS, Sreeramareddy CT, Stathopoulou VK, Stroumpoulis K, Swaminathan S, Sykes BL, Tabb KM, Talongwa RT, Tenkorang EY, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Towbin JA, Traebert J, Tran BX, Dimbuene ZT, Tsilimbaris M, Uchendu US, Ukwaja KN, Uzun SB, Vallely AJ, Vasankari TJ, Venketasubramanian N, Violante FS, Vlassov VV, Vollset SE, Waller S, Wallin MT, Wang L, Wang X, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Westerman R, White RA, Wilkinson JD, Williams TN, Woldeyohannes SM, Wong JQ, Xu G, Yang YC, Yano Y, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis M, Yu C, Jin KY, El Sayed Zaki M, Zhao Y, Zheng Y, Zhou M, Zhu J, Zou XN, Lopez AD, Vos T. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baussano I, William BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS Med. 2010;7:e1000381. doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu S, Stuckler D, McKee M. Addressing institutional amplifiers in the dynamics and control of tuberculosis epidemics. Am J Trop Med Hyg. 2011;84:30–37. doi: 10.4269/ajtmh.2011.10-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez A, Massari V, Gerhardt G, Espinola AB, Siriwardana M, Camacho LA, Larouze B. X ray screening at entry and systematic screening for the control of tuberculosis in a highly endemic prison. BMC Public Health. 2013;13:983. doi: 10.1186/1471-2458-13-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez A, Larouze B, Espinola AB, Pires J, Capone D, Gerhardt G, Cesconi V, Procopio MJ, Hijjar M, Massari V. Screening for tuberculosis on admission to highly endemic prisons? The case of Rio de Janeiro State prisons. Int J Tuberc Lung Dis. 2009;13:1247–1252. [PubMed] [Google Scholar]
- 6.Winetsky DE, Negoescu DM, DeMarchis EH, Almukhamedova O, Dooronbekova A, Pulatov D, Vezhnina N, Owens DK, Goldhaber-Fiebert JD. Screening and rapid molecular diagnosis of tuberculosis in prisons in Russia and eastern Europe: a cost-effectiveness analysis. PLoS Med. 2012;9:e1001348. doi: 10.1371/journal.pmed.1001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira SM, Ruffino-Netto A, Paniago AM, Oliveira OA, Marques M, Cunha RV, Andreotti R. Tuberculin skin test: operational research in the state of Mato Grosso do Sul, Brazil. J Bras Pneumol. 2011;37:646–654. doi: 10.1590/s1806-37132011000500012. [DOI] [PubMed] [Google Scholar]
- 8.Riley RL, Mills CC, O'Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 9.Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144:302–306. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 10.Ko G, Burge HA, Nardell EA, Thompson KM. Estimation of tuberculosis risk and incidence under upper room ultraviolet germicidal irradiation in a waiting room in a hypothetical scenario. Risk Anal. 2001;21:657–673. doi: 10.1111/0272-4332.214142. [DOI] [PubMed] [Google Scholar]
- 11.Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, Jensen PA, First MW, Parsons S, McMurray DN, Orme IM, Nardell EA. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 2011;91:329–338. doi: 10.1016/j.tube.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escombe AR, Oeser CC, Gilman R, Navincopa M, Ticona E, Pan W, Martínez C, Chacaltana J, Rodríguez R, Moore DA, Friedland JS, Evans CA. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escombe AR, Moore DA, Gilman RH, Navincopa M, Ticona E, Mitchell B, Noakes C, Martínez C, Sheen P, Ramirez R, Quino W, Gonzalez A, Friedland JS, Evans CA. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e43. doi: 10.1371/journal.pmed.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . WHO Policy on TB Infection Control in Health-Care Facilities, Congregate Settings and Households. Geneva, Switzerland: World Health Organization; 2009. http://www.ncbi.nlm.nih.gov/books/NBK179249/ Available at. Accessed November 14, 2013. [PubMed] [Google Scholar]
- 15.World Health Organization . In: Natural Ventilation for Infection Control in Health-Care Settings. Atkinson J, Chartier Y, Pessoa-Silva CL, Jensen P, Li Y, Seto WH, editors. Geneva, Switzerland: World Health Organization; 2009. http://www.ncbi.nlm.nih.gov/books/NBK1432284 Available at. Accessed January 8, 2014. [PubMed] [Google Scholar]
- 16.International Centre for Prison Studies . World Prison Brief: Brazil. London, United Kingdom: International Centre for Prison Studies; 2013. http://www.prisonstudies.org/country/brazil Available at. Accessed November 14, 2013. [Google Scholar]
- 17.Estevan AO, Oliveira SM, Croda J. Active and latent tuberculosis in prisoners in the central west region of Brazil. Rev Soc Bras Med Trop. 2013;46:515–518. doi: 10.1590/0037-8682-1441-2013. [DOI] [PubMed] [Google Scholar]
- 18.Kuhleis D, Ribeiro AW, Costa ER, Cafrune PI, Schmid KB, Costa LL, Ribeiro MO, Zaha A, Rossetti ML. Tuberculosis in a southern Brazilian prison. Mem Inst Oswaldo Cruz. 2012;107:909–915. doi: 10.1590/s0074-02762012000700012. [DOI] [PubMed] [Google Scholar]
- 19.Nogueira PA, Abrahao RM, Galesi VM. Tuberculosis and latent tuberculosis in prison inmates. Rev Saude Publica. 2012;46:119–127. doi: 10.1590/s0034-89102011005000080. [DOI] [PubMed] [Google Scholar]
- 20.Fournet N, Sanchez A, Massari V, Penna L, Natal S, Biondi E, Larouzé B. Development and evaluation of tuberculosis screening scores in Brazilian prisons. Public Health. 2006;120:976–983. doi: 10.1016/j.puhe.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Brazilian Ministry of Justice . Statistical Report: Analysis of the Prison System of Mato Grosso do Sul, Brazil. Brasilia, Brazil: National Penitenciary Department of the Ministry of Justice; 2012. http://www.justica.gov.br/seus-direitos/politica-penal/acesso-a-informacao/estatisticas-prisional/anexos-sistema-prisional/ Available at. Accessed February 10, 2014. [Google Scholar]
- 22.Turiel I, Rudy JV. Occupant-generated CO2 as an indicator of ventilation rate. ASHRAE Trans. 1982;88:12. [Google Scholar]
- 23.Persily A. Evaluating building IAQ and ventilation with indoor carbon dioxide. ASHRAE Trans. 1997;103:1–12. [Google Scholar]
- 24.Andrews JR, Morrow C, Wood R. Modeling the role of public transportation in sustaining tuberculosis transmission in South Africa. Am J Epidemiol. 2013;177:556–561. doi: 10.1093/aje/kws331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnstone-Robertson S, Lawn SD, Welte A, Bekker LG, Wood R. Tuberculosis in a South African prison: a transmission modelling analysis. S Afr Med J. 2011;101:809–813. [PMC free article] [PubMed] [Google Scholar]
- 26.Rudnick SN, Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13:237–245. doi: 10.1034/j.1600-0668.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 27.Escombe AR, Moore DA, Gilman RH, Pan W, Navincopa M, Ticona E, Martínez C, Caviedes L, Sheen P, Gonzalez A, Noakes CJ, Friedland JS, Evans CA. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 29.Carbone A da SS, Paiao DSG, Sgarbi RVE, Lemos EF, Cazanti RF, Ota MM, Junior AL, Bampi JVB, Elias VPF, Simionatto S, Motta-Castro ARC, Pompilio MA, Oliveria SM do V, Ko AI, Andrews JR, Croda J. Active and latent tuberculosis in Brazilian correctional facilities: a cross-sectional study. BMC Infect Dis. 2015;15:24. doi: 10.1186/s12879-015-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sultan L, Nyka W, Mills C, O'Grady F, Wells W, Riley RL. Tuberculosis disseminators. A study of the variability of aerial infectivity of tuberculous patients. Am Rev Respir Dis. 1960;82:358–569. doi: 10.1164/arrd.1960.82.3.358. [DOI] [PubMed] [Google Scholar]
- 31.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 32.Verver S, Warren RM, Munch Z, Richardson M, van der Spuy GD, Borgdorff MW, Behr MA, Beyers N, van Helden PD. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363:212–214. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 33.Middelkoop K, Bekker LG, Morrow C, Lee N, Wood R. Decreasing household contribution to TB transmission with age: a retrospective geographic analysis of young people in a South African township. BMC Infect Dis. 2014;14:221. doi: 10.1186/1471-2334-14-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lygizos M, Shenoi SV, Brooks RP, Bhushan A, Brust JC, Zelterman D, Deng Y, Northrup V, Moll AP, Friedland GH. Natural ventilation reduces high TB transmission risk in traditional homes in rural KwaZulu-Natal, South Africa. BMC Infect Dis. 2013;13:300. doi: 10.1186/1471-2334-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spengler JD, Chen Q. Indoor air quality factors in designing a healthy building. Annu Rev Energy Environ. 2000;25:567–600. [Google Scholar]
- 36.Qian H, Li Y, Seto WH, Ching P, Ching WH, Sun HQ. Natural ventilation for reducing airborne infection in hospitals. Build Environ. 2010;45:559–565. doi: 10.1016/j.buildenv.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein RA. Super-spreaders in infectious diseases. Int J Infect Dis. 2011;15:e510–e513. doi: 10.1016/j.ijid.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinna GD, Maestri R, La Rovere MT, Gobbi E, Fanfulla F. Effect of paced breathing on ventilatory and cardiovascular variability parameters during short-term investigations of autonomic function. Am J Physiol Heart Circ Physiol. 2006;290:H424–H433. doi: 10.1152/ajpheart.00438.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.