Abstract
FoxO transcription factors promote longevity across taxa. How they do so is poorly understood. In the nematode Caenorhabditis elegans, the A- and F-isoforms of the FoxO transcription factor DAF-16 extend life span in the context of reduced DAF-2 insulin-like growth factor receptor (IGFR) signaling. To elucidate the mechanistic basis for DAF-16/FoxO-dependent life span extension, we performed an integrative analysis of isoform-specific daf-16/FoxO mutants. In contrast to previous studies suggesting that DAF-16F plays a more prominent role in life span control than DAF-16A, isoform-specific daf-16/FoxO mutant phenotypes and whole transcriptome profiling revealed a predominant role for DAF-16A over DAF-16F in life span control, stress resistance, and target gene regulation. Integration of these datasets enabled the prioritization of a subset of 92 DAF-16/FoxO target genes for functional interrogation. Among 29 genes tested, two DAF-16A-specific target genes significantly influenced longevity. A loss-of-function mutation in the conserved gene gst-20, which is induced by DAF-16A, reduced life span extension in the context of daf-2/IGFR RNAi without influencing longevity in animals subjected to control RNAi. Therefore, gst-20 promotes DAF-16/FoxO-dependent longevity. Conversely, a loss-of-function mutation in srr-4, a gene encoding a seven-transmembrane-domain receptor family member that is repressed by DAF-16A, extended life span in control animals, indicating that DAF-16/FoxO may extend life span at least in part by reducing srr-4 expression. Our discovery of new longevity genes underscores the efficacy of our integrative strategy while providing a general framework for identifying specific downstream gene regulatory events that contribute substantially to transcription factor functions. As FoxO transcription factors have conserved functions in promoting longevity and may be dysregulated in aging-related diseases, these findings promise to illuminate fundamental principles underlying aging in animals.
Keywords: C. elegans, aging, longevity, insulin-like growth factor signaling, FoxO transcription factors
FOXO transcription factors control development, metabolism, stress responses, and aging in diverse animal species (Accili and Arden 2004; Barthel et al. 2005; van der Horst and Burgering 2007; Kenyon 2010). In invertebrates, FoxO promotes longevity in the context of reduced insulin-like signaling (Kenyon et al. 1993; Lin et al. 1997; Ogg et al. 1997; Slack et al. 2011; Yamamoto and Tatar 2011). Phenotypic analysis of knockout mice implicates FoxO transcription factors in the pathogenesis of aging-related diseases such as cancer (Paik et al. 2007; Gan et al. 2010; Sykes et al. 2011), type 2 diabetes (Kitamura et al. 2002; Nakae et al. 2002; Dong et al. 2008; Cheng et al. 2009), osteoporosis (Ambrogini et al. 2010; Rached et al. 2010), and atherosclerosis (Tsuchiya et al. 2012, 2013). Furthermore, FoxO3 is required for life span extension in mice subjected to dietary restriction (Shimokawa et al. 2015). In humans, FoxO1 and FoxO3 polymorphisms are associated with extreme longevity in multiple independent cohorts of centenarians (Lunetta et al. 2007; Willcox et al. 2008; Anselmi et al. 2009; Flachsbart et al. 2009; Li et al. 2009; Pawlikowska et al. 2009). The evolutionary conservation of FoxO functions in promoting longevity suggests that understanding the molecular basis for FoxO transcription factor action will illuminate fundamental mechanisms that govern aging.
The well-established role of FoxO transcription factors as targets of insulin-like signaling first came to light from studies in Caenorhabditis elegans, where the insulin/insulin-like growth factor receptor (IGFR) ortholog DAF-2 promotes reproductive development and limits adult life span by inhibiting the FoxO transcription factor DAF-16 via a conserved phosphoinositide 3-kinase (PI3K)/Akt pathway-dependent mechanism. Engagement of DAF-2/IGFR by agonist insulin-like ligands activates the PI3K/Akt pathway, resulting in Akt-dependent phosphorylation of three DAF-16/FoxO amino acid residues that lie within conserved RxRxxS/T motifs. Phosphorylated DAF-16/FoxO is subsequently exported from the nucleus and sequestered in the cytoplasm. When DAF-2/IGFR pathway activity is reduced, unphosphorylated DAF-16/FoxO translocates to the nucleus, where it regulates the expression of numerous genes, including those that control metabolism, immunity, and detoxification (Murphy and Hu 2013). The inhibition of FoxO by insulin-like signaling is evolutionarily conserved, as reduction of FoxO activity ameliorates biological phenotypes associated with reduced insulin-like signaling in flies (Junger et al. 2003; Slack et al. 2011; Yamamoto and Tatar 2011) and mice (Kitamura et al. 2002; Nakae et al. 2002; Dong et al. 2008; Cheng et al. 2009).
DAF-16/FoxO also promotes life span extension in animals lacking a germline (Hsin and Kenyon 1999). Although DAF-2/IGFR and the germline both control DAF-16/FoxO activity by regulating its subcellular localization (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001), they may do so through distinct mechanisms, as the molecular requirements for DAF-16/FoxO regulation by DAF-2/IGFR signaling and the germline differ (Berman and Kenyon 2006; Ghazi et al. 2009).
Work from multiple groups has identified hundreds of genes that are regulated by DAF-16/FoxO in the context of reduced DAF-2/IGFR signaling (Lee et al. 2003; McElwee et al. 2003; Murphy et al. 2003). Our understanding of the functional significance of DAF-16/FoxO target genes in mediating DAF-16/FoxO-dependent longevity is based primarily on a study in which nearly 60 DAF-16/FoxO target genes were tested for roles in DAF-16/FoxO-dependent life span extension using RNAi-based assays. This revealed that most single gene RNAi knockdowns have relatively small effects on longevity, leading to the conclusion that DAF-16/FoxO promotes longevity through the cumulative regulation of hundreds of genes (Murphy et al. 2003).
The roles of most individual DAF-16/FoxO-dependent gene regulatory events in life span control have not been assessed experimentally. This may be due in large part to the abundance of genes regulated by DAF-16/FoxO. Efforts to prioritize DAF-16/FoxO target genes based on direct binding of DAF-16/FoxO to promoters succeeded in identifying aakg-4 as a gene that is directly induced by DAF-16/FoxO and required for DAF-16/FoxO-dependent life span extension (Schuster et al. 2010; Tullet et al. 2014). Therefore, strategies to prioritize subsets of DAF-16/FoxO target genes can lead to the discovery of new longevity genes by permitting detailed functional analysis of a relatively small number of genes.
The daf-16 genomic locus encodes three groups of transcripts (a, b, and d/f/h) that are transcribed from distinct promoters (Lin et al. 1997; Ogg et al. 1997; Kwon et al. 2010). The encoded proteins possess phosphorylation sites conserved in humans and other species (Supporting Information, Figure S1, A and B). daf-16d, f, and h are transcribed from the same promoter but have distinct 5′ ends and translational start sites (Figure S1C; Figure S2; WormBase, www.wormbase.org) (Kwon et al. 2010; Murphy and Hu 2013). For clarity, we refer to the d/f/h group of transcripts and polypeptides collectively as daf-16f and DAF-16F, respectively. In animals with diminished DAF-2/IGFR signaling, mutations that reduce daf-16a and f but not daf-16b activity shorten life span to the same extent as daf-16 null mutations (Lee et al. 2001). Furthermore, overexpression of DAF-16B under the control of its endogenous promoter does not extend the life span of daf-16/FoxO null mutants (Lee et al. 2001; Kwon et al. 2010). These data implicate DAF-16A and DAF-16F as the critical targets of DAF-2/IGFR in life span control. Analysis of functional isoform-specific DAF-16::GFP reporters demonstrates that DAF-16A and DAF-16F have overlapping spatiotemporal expression patterns and are both expressed in multiple tissues (Kwon et al. 2010). Notably, although the set of genes collectively regulated by DAF-16/FoxO is well defined, the roles of specific DAF-16/FoxO isoforms in gene regulation are obscure.
In light of the differential influence of DAF-16/FoxO isoforms on longevity (Kwon et al. 2010), we hypothesized that detailed characterization of isoform-specific daf-16/FoxO mutants would illuminate the molecular basis for DAF-16/FoxO action in life span control by enabling the prioritization of a subset of DAF-16/FoxO target genes for further investigation.
Materials and Methods
C. elegans strains and maintenance
Strains used in this study are listed in Table S17; Table S20. Animals were maintained at 15° on nematode growth media (NGM) plates seeded with Escherichia coli OP50. Double mutants were constructed using standard genetic techniques. Genotypes were confirmed by PCR amplification to detect restriction fragment length or PCR polymorphisms. Percival I-36NL incubators (Percival Scientific, Perry, IA) were used for maintenance, dauer arrest assays, and life span assays.
RNA isolation
Animals were washed twice in M9 buffer. Total RNA was isolated using TRIzol reagent (Invitrogen) and purified using an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions.
Quantitative real-time reverse-transcriptase PCR
cDNA was synthesized using a SuperScript III Reverse Transcriptase kit and random hexamers (Invitrogen, Carlsbad, CA). Real-time PCR was then performed in triplicate using Power SYBR Green PCR master mix (Applied Biosystems, Warrington, UK) and a Mastercycler ep realplex thermal cycler (Eppendorf North America, Westbury, NY). A total of 10 ng of cDNA was used as a template in 15 µl reaction volume. Primers were selected initially using GETPrime (Gubelmann et al. 2011) and Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) and subsequently validated by melt curve analysis and agarose gel electrophoresis. Primer sequences are listed in Table S21. Relative expression levels and technical error were determined by the ΔΔ2Ct method (Nolan et al. 2006). Gene expression levels were normalized to actin (act-1), and the ratio of expression relative to act-1 was then compared to the same ratio in N2 Bristol wild type. Statistical analysis was performed in GraphPad Prism (GraphPad Software, La Jolla, CA) using the paired ratio t-test.
Rapid amplification of complementary DNA (cDNA) ends
Total RNA was isolated from young adult animals. daf-16a and daf-16f cDNA was prepared using a 5′ rapid amplificiation of cDNA ends (RACE) system version 2.0 (Invitrogen). First-strand cDNA was synthesized using a daf-16a/f gene-specific primer and SuperScript II. The original mRNA template was degraded by RNase H and RNase T1. After purification, a homopolymeric tail was added using terminal deoxynucleotidyl transferase to the 3′ end of cDNA. Standard PCR was performed using Taq DNA polymerase, a nested daf-16a or daf-16f-specific primer, and the abridged anchor primer complementary to the homopolymeric tail. After visualization of products on a standard agarose gel, the reaction mix was cloned into pCR4-TOPO vector using a TOPO TA Cloning kit (Invitrogen) and transformed into chemically competent E. coli DH5α. Clones were selected on LB plates containing 50 µg/ml ampicillin, and plasmids were analyzed by Sanger sequencing. Numbers of clones analyzed for each strain are indicated in Table S22.
Dauer arrest assays
Dauer assays were performed at 25° as previously described (Hu et al. 2006). Briefly, animals were synchronized in a 4-hr egglay at 15° and grown at 25° on NGM plates. Animals were scored when wild-type animals were gravid adults and daf-2 animals had arrested as dauers (∼60–72 h after egglay). Statistical significance was assessed using a two-tailed, unpaired t-test with Welch’s correction.
Life span assays
Life span assays were performed as previously described (Chen et al. 2013a), with minor modifications. Animals derived from a synchronized 4-hr egglay were grown at 15° until the L4 larval stage and then shifted to 20°. Plates harboring any males were discarded. Animals were grown for an additional 20–24 hr to day 1 of adulthood and then placed on life span plates containing 25 μg/ml 5-fluoro-2′-deoxyuridine (FUDR) (Sigma-Aldrich, St. Louis) to prevent progeny growth. glp-1 mutant animals were raised for 48 hr at the restrictive temperature (25°) to ablate the germline and then shifted to 20°. Statistical significance was assessed using the standard chi-square-based log-rank test in GraphPad Prism.
RNAi
RNAi clones were identical to previously published isoform-specific and pan-daf-16 RNAi clones (Kwon et al. 2010). Feeding RNAi was performed using standard procedures (Kamath et al. 2001). All RNAi NGM plates contained 5 mM IPTG and 25 μg/ml carbenicillin. NGM plates were seeded with an overnight culture of E. coli HT115 with either control L4440 vector or RNAi plasmid. For RNAi life span assays, HT115 was concentrated 5×. Plasmids from E. coli clones were sequenced for every experimental replicate to confirm their identity.
MosI-mediated single copy insertion
Using the full build service platform at Knudra Transgenics, the plasmids pNU164 and pNU191 were constructed by inserting daf-16 cargo into MosI-mediated single copy insertion (MosSCI) vector backbones. For pNU164, the daf-16a cDNA was PCR amplified from yk13f11/yk1006c10 plasmid, and the full-length daf-16 3′ UTR was PCR amplified from wild-type genomic DNA. These two were ligated by PCR-mediated overlap extension (Heckman and Pease 2007) and cloned into a pCFJ151 plasmid. A hybrid segment composed of the 3′ portion of the cDNA and the 3′ UTR was amplified from this plasmid, while daf-16a promoter segments and partial daf-16a genomic sequence was PCR amplified from wild-type genomic DNA. All parts were then inserted into the multiple cloning site (MCS) of pCFJ151 via Gibson ligation. For pNU191, the promoter and unique daf-16f exons were PCR amplified from wild-type genomic DNA. The common daf-16a/f coding segment and the end of the coding region through the 3′ UTR were amplified from pNU164. All parts were then inserted into the MCS of pCFJ151 via Gibson ligation. All coding regions of plasmids were verified by Sanger sequencing. For production of transgenic animals, plasmids were used in a MosSCI injection mix and used to create single copy insertions at the ttTi5605 locus as per published methods (Frokjaer-Jensen et al. 2008).
Stress-resistance assays
For all stress assays, animals derived from a synchronized 4-hr egglay were grown at 15° until the late L3 larval stage and then shifted to 20° and grown for an additional 12 hr until the L4 larval stage. Plates harboring any males were discarded.
For oxidative stress, animals were transferred to plates containing 7.5 mM tert-butyl hydroperoxide (t-BOOH) (Sigma-Aldrich) and scored approximately three times per day for survival. For ultraviolet (UV) stress, animals were transferred to plates lacking bacteria and irradiated with 1200 J/m2 UV-C using a Stratalinker 2400 UV crosslinker (Stratagene). UV-treated animals were then transferred to seeded plates containing 25 μg/ml FUDR and scored daily for survival. For thermotolerance, animals were transferred to seeded plates containing 25 μg/ml FUDR, grown for an additional 12 hr at 20°, shifted to 33°, and scored approximately four times per day for survival. For all assays, animals that dessicated on the side of plates were censored. Statistical significance was assessed in GraphPad Prism using the standard chi-square-based log-rank test.
Whole transcriptome profiling (RNA-Sequencing)
Animals were grown as described for life span assays. After picking a subset of the population for life span assays, the remaining animals were harvested for isolation of total RNA. The Agilent TapeStation was used to assess RNA quality. Samples with RNA integrity numbers (RINs) of eight or greater were prepped using the Illumina TruSeq mRNA Sample Prep v2 kit (catalog nos. RS-122-2001 and RS-122-2002). Messenger RNA (mRNA) was isolated from 0.1 to 3 μg of total RNA by polyA+ purification, fragmented, and copied into first-strand cDNA using reverse transcriptase and random primers. The 3′ cDNA ends were then adenylated and adapters were ligated. One of the adapters contained a six-nucleotide barcode to enable multiplexing of samples. Products were purified and enriched by PCR to create the final cDNA library. Libraries were checked for quality and quantity by Agilent TapeStation and quantitative PCR (qPCR) using a library quantification kit for Illumina sequencing platforms (catalog no. KK4835, Kapa Biosystems, Wilmington, MA). Clonal clusters were generated using cBot (Illumina, San Diego). Quadriplexed samples were sequenced using the HiSequation 2000 system (Illumina) with a 100-cycle paired-end run in high output mode using version 3 reagents according to manufacturer’s protocols.
RNA-seq analysis
Individual read files for each sample were concatenated into a single .fastq file. Raw reads data for each sample were checked using FastQC (version 0.10.0, Babraham Bioinformatics, Cambridge, United Kingdom; http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/) to identify features potentially indicative of quality issues (e.g., low-quality scores, overrepresented sequences, and inappropriate GC content). We used the Tuxedo Suite (Langmead et al. 2009; Trapnell et al. 2009, 2013) for alignment, differential expression analysis, and postanalysis diagnostics. Briefly, reads were aligned to the reference genome (University of California Santa Cruz, UCSC ce10; http://genome.ucsc.edu/) using TopHat (version 2.0.9) (Trapnell et al. 2009) and Bowtie (version 2.1.0.0) (Langmead 2010). Alignments were performed using default parameter settings, with the exception of “–b2-very-sensitive” and “–no-coverage-search.” Additionally, the “max_intron_length” parameter was set to 25 kb to minimize false positive splice junction discovery. A second round of quality control was then performed using FastQC to ensure that only high-quality data were analyzed further. Cufflinks/CuffDiff (version 2.1.1) (Trapnell et al. 2013) was used for quantification of expression and differential expression analysis, using UCSC ce10.fa as the reference genome and UCSC ce10.gtf as the reference transcriptome (http://genome.ucsc.edu/). For this analysis, we used parameter settings “–multi-read-correct” to adjust expression calculations for reads that map to more than one locus, as well as “–compatible-hits-norm” and “–upper-quartile –norm” for normalization of expression values. We generated diagnostic plots using the CummeRbund package (Trapnell et al. 2012) as a quality check on normalized data.
RNA-seq reads were examined using the Integrative Genomics Viewer version 2.3.39 (Broad Institute) (Robinson et al. 2011; Thorvaldsdottir et al. 2013). The annotated gene expression data output from CuffDiff was read into R (http://www.r-project.org/) for six comparisons: daf-2(e1370) compared to wild type, daf-16(mu86);daf-2, daf-16a/f(mg54);daf-2, daf-16a(tm5030);daf-2, daf-16a(tm5032);daf-2, and daf-16f(tm6659);daf-2. DAF-16A/F targets were defined by the following criteria: (1) fold change (FC) ≥ ±1.5 for wild type vs. daf-2, and (2) FC ≥ 2 in the opposite direction as wild type vs. daf-2 for both daf-2 vs. daf-16(mu86);daf-2 and daf-2 vs. daf-16a/f(mg54);daf-2. We required FDR < 0.05 for only one of the three comparisons for
three reasons: (1) nearly all genes showed concordance in FC for all three comparisons even if they did not satisfy FDR < 0.05 for all three; (2) some known DAF-16/FoxO targets only satisfied FDR < 0.05 in one or two comparisons; and (3) the choice of requiring FDR < 0.05 for one, two, or three comparisons did not significantly affect the categorization of DAF-16A/F target genes (Table S23; see below). FC ≥ 2 instead of 1.5 was chosen for the daf-2 vs. daf-16;daf-2 comparisons to improve quantification of the effects of daf-16a and daf-16f mutations on gene expression.
For functional enrichment testing, we identified enriched Gene Ontology (GO) biological process terms (Ashburner et al. 2000) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (Kanehisa and Goto 2000) using LRpath, a logistic regression method that overcomes limitations associated with the use of arbitrary significance cutoff values by taking into account the distribution of significance levels of all profiled genes (Sartor et al. 2009). Enriched GO terms and KEGG pathways were defined as those having a false discovery rate of <0.01. We used a directional LRpath test to distinguish between up-regulated and down-regulated functions. Overlapping and redundant GO terms were clustered semantically with REVIGO (Supek et al. 2011). We employed a semantic similarity (SimRel) cutoff of 0.7 to define representative terms. LRpath and REVIGO outputs are shown in Table S15.
To define classes of DAF-16A/F targets, A and F indices (IA and IF) were calculated for 399 DAF-16A/F targets. Given high correlation in gene expression between daf-16a(tm5030);daf-2 and daf-16a(tm5032);daf-2 (Figure S17), a combined daf-16a;daf-2 gene expression profile was generated by calculating the mean fragments per kilobase per million reads (FPKM) for each gene. IA for each gene was defined as the absolute FPKM difference between daf-2(e1370) and daf-16a;daf-2, divided by the absolute FPKM difference between daf-2(e1370) and daf-16a/f(mg54);daf-2. Likewise, IF for each gene was defined as the FPKM difference between daf-2(e1370) and daf-16f(tm6659);daf-2, divided by the FPKM difference between daf-2(e1370) and daf-16a/f(mg54);daf-2.
Classes of DAF-16A/F targets were defined by the following criteria: DAF-16A-specific, IA > 0.8 and IF < 0.2; DAF-16F-specific, IF > 0.8 and IA < 0.2; and redundantly regulated, IA < 0.2 and IF < 0.2. Genes not binned into these three categories were partitioned into one of the following three groups: shared A-dominant (shared A > F), IA/IF > 2; shared F-dominant (shared F > A), IF/IA > 2; or equally shared (shared A = F), 0.5 ≤ IA/IF ≤ 2.
RNAi-defective assays
Assays were performed essentially as described (Billi et al. 2012). L1 larvae were picked onto RNAi plates (5 mM IPTG, 25 μg/ml carbenicillin) seeded with an overnight culture of E. coli HT115 containing a pos-1 RNAi plasmid, which causes embryonic lethality (Tabara et al. 1999). rde-4(ne301) was used as an RNAi-defective (rde) control (Tabara et al. 2002). Animals were grown for 84 hr at 20° to allow for development and egglaying and then removed from the plate. Eggs were counted immediately, live progeny were counted 48 hr later, and percentage of nonviable eggs was calculated.
Data availability
Strains are available upon request. Whole transcriptome profiling data are available at GEO with the accession number: GSE72426.
Results
Molecular characterization of isoform-specific daf-16/FoxO mutants
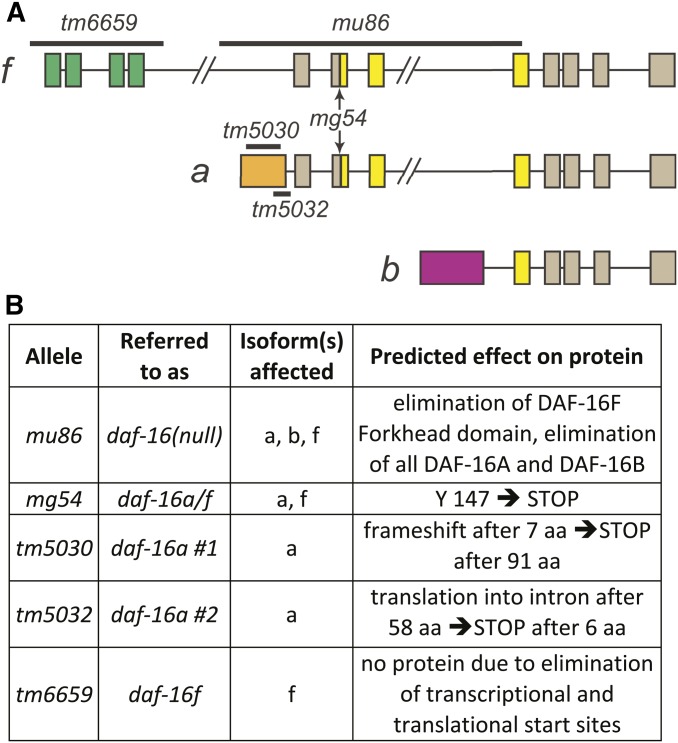
To isolate daf-16a- and daf-16f-specific mutant alleles, we screened for mutants with deletions in the unique N-terminal exons of each isoform (Gengyo-Ando and Mitani 2000). Two independent alleles, tm5030 and tm5032, are deletions in the daf-16a-specific exon (Figure 1). The 5′ RACE analysis shows that these mutations result in frameshifts and predicted early translation termination (Figure 1B, Figure S3, Figure S4, and Figure S5). As expected, both tm5030 and tm5032 significantly reduced daf-16a transcript levels as measured by quantitative real-time reverse transcriptase PCR (qPCR; Figure S6 A and B; Table S1), consistent with nonsense-mediated decay secondary to premature translation termination. Neither mutation influenced the integrity or quantity of daf-16b or daf-16f transcripts (Figure S6, C and D; Table S1). tm6659 is a deletion that spans all four daf-16f-specific exons, including all three putative transcriptional and translational start sites (Figure 1A; Figure S1C) (Kwon et al. 2010; Murphy and Hu 2013). daf-16f transcripts were undetectable in animals harboring tm6659 by 5′ RACE and qPCR (Figure S3; Figure S6D; Table S1), and neither daf-16a nor daf-16b transcripts were affected (Figure S6 A–C; Table S1). Total daf-16 levels are sharply reduced in tm6659 mutants (Figure S6 E and F; Table S1). Importantly, our data show no compensatory increase in daf-16f transcripts in either daf-16a(tm5030) or daf-16a(tm5032) (Figure S6D) and no increase in daf-16a transcripts in daf-16f(tm6659) (Figure S6, A and B).
Figure 1.
daf-16/FoxO isoforms and isoform-specific mutations. (A) daf-16/FoxO genomic structure with isoform-specific mutations. Colors indicate unique N-terminal exons (green and orange) and Forkhead domains (yellow) that correspond to DAF-16/FoxO protein domains in Figure S1A. (B) Summary of isoform-specific mutant alleles. See text for details.
Taken together, these data strongly suggest that tm5030, tm5032, and tm6659 are bona fide isoform-specific loss-of-function alleles. These isoform-specific alleles are henceforth referred to as daf-16a no. 1 (tm5030), daf-16a no. 2 (tm5032), and daf-16f (tm6659).
DAF-16A promotes dauer arrest in animals with reduced DAF-2/IGFR signaling
In response to adverse environmental conditions, C. elegans larvae undergo developmental arrest in an alternative larval stage known as dauer (Riddle 1988). daf-2/IGFR and daf-16/FoxO mutants were first isolated in genetic screens for animals with dauer-constitutive (Daf-c) and dauer-defective (Daf-d) phenotypes, respectively (Riddle et al. 1981). daf-2/IGFR mutants constitutively arrest as dauers at 25° in a daf-16/FoxO-dependent manner (Riddle et al. 1981; Vowels and Thomas 1992; Gottlieb and Ruvkun 1994).
To examine the roles of distinct DAF-16/FoxO isoforms in dauer regulation, we determined the influence of isoform-specific daf-16/FoxO mutations on dauer-constitutive phenotypes in two daf-2/IGFR mutant backgrounds: e1368, a missense mutation in the DAF-2/IGFR extracellular ligand-binding domain, and e1370, a missense mutation in the cytoplasmic tyrosine kinase domain (Kimura et al. 1997). e1370 may be a stronger loss-of-function allele than e1368, as it causes a more penetrant dauer-constitutive phenotype and extends life span to a greater extent than e1368 (Figure 2 and Figure 3) (Gems et al. 1998).
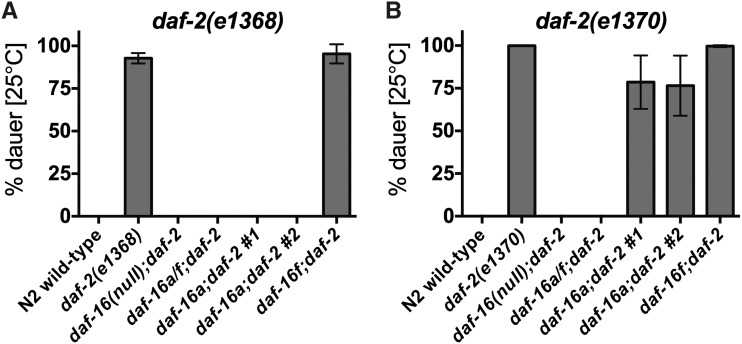
Figure 2.
daf-16a-specific mutations suppress dauer arrest in daf-2/IGFR mutants. (A) The dauer-constitutive phenotype of daf-2(e1368) animals is fully suppressed by daf-16a/f mutation and both daf-16a mutations but is unaffected by daf-16f mutation. (B) The dauer-constitutive phenotype of daf-2(e1370) animals is fully suppressed by daf-16a/f mutation, partially suppressed by both daf-16a mutations, and unaffected by daf-16f mutation. Mean and standard deviation for at least three biological replicates are presented. Statistics and raw data are presented in Table S2; Table S3; Table S4.
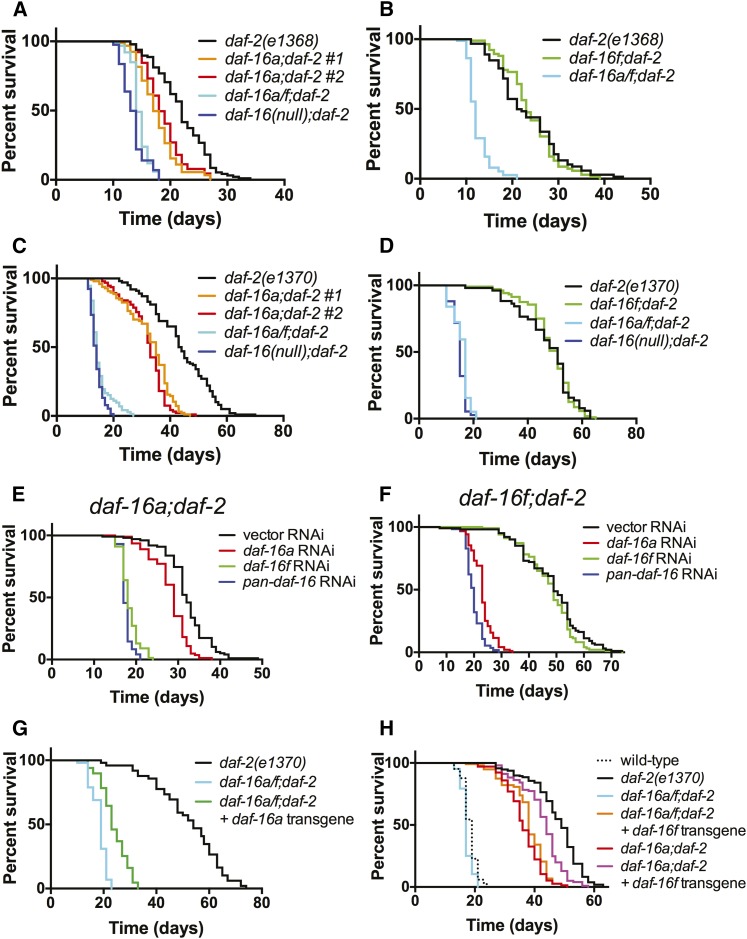
Figure 3.
Effects of daf-16a- and daf-16f-specific mutations, RNAi, and single-copy transgenic rescues on daf-2/IGFR life span. (A–D) Effects of daf-16a (A and C) and daf-16f (B and D) mutations on life spans of daf-2(e1368) (A and B) and daf-2(e1370) (C and D). (E and F) daf-16f is required for daf-16a;daf-2 longevity and vice versa. Survival curves are presented for (E) daf-16a;daf-2(e1370) and (F) daf-16f;daf-2(e1370) mutant animals upon exposure to isoform-specific daf-16 RNAi. Figure S7A shows control daf-2(e1370) survival when treated with isoform-specific daf-16 RNAi. (G) Effect of a single-copy daf-16a transgene on daf-16a/f;daf-2(e1370) life span. (H) Effect of a single-copy daf-16f transgene on daf-16a/f;daf-2(e1370) and daf-16a;daf-2(e1370) life span. See text for details. Statistics and raw data are presented in Table S5; Table S6; Table S7; Table S8.
As previously shown, the dauer-constitutive phenotype caused by both daf-2/IGFR mutations is fully suppressed by the null daf-16/FoxO allele mu86 (Lin et al. 1997) as well as by mg54 (Figure 2; Table S2; Table S3; Table S4), a nonsense mutation that affects daf-16a and daf-16f but not daf-16b (Figure 1, A and B) (Ogg et al. 1997; Lee et al. 2001). daf-16(mu86) and daf-16(mg54) are henceforth referred to as “daf-16 null” and “daf-16a/f mutation” for purposes of clarity. These results indicate that DAF-16B does not suffice to promote dauer arrest in animals with reduced DAF-2/IGFR signaling, implicating DAF-16A and/or DAF-16F in dauer regulation.
Both daf-16a mutations completely suppress the dauer-constitutive phenotype of daf-2(e1368) mutants, whereas daf-16f mutation does not influence daf-2(e1368) dauer arrest (Figure 2A; Table S2; Table S3). Thus, in this context, DAF-16A is the critical isoform that regulates dauer arrest. This result is consistent with the observation that in daf-2(e1368) animals, DAF-16A translocates to nuclei, whereas DAF-16F remains cytoplasmic (Bansal et al. 2014). In daf-2(e1370) mutants, daf-16a no. 1 and no. 2 mutations suppress dauer arrest by 22% (P = 0.0204) and 24% (P = 0.0408), respectively, whereas daf-16f mutation has no effect on dauer arrest (Figure 2B; Table S2; Table S4). Since daf-16a/f mutation fully suppresses daf-2(e1370) dauer arrest (Figure 2B; Table S2; Table S4), DAF-16A and DAF-16F act redundantly to promote dauer arrest in daf-2(e1370) mutant animals.
DAF-16A promotes longevity in animals with reduced DAF-2/IGFR signaling
DAF-16/FoxO is required for life span extension both in animals with reduced DAF-2/IGFR activity (Kenyon et al. 1993) and in animals lacking a germline (Hsin and Kenyon 1999). Experiments involving RNAi-based knockdown and transgenic overexpression of DAF-16/FoxO isoforms suggest that both DAF-16A and DAF-16F promote longevity in daf-2/IGFR mutants (Kwon et al. 2010). We determined the effect of daf-16a and daf-16f mutations on life span in daf-2(e1368) and daf-2(e1370) mutants. As previously shown, life span extension induced by daf-2/IGFR mutation was fully suppressed by daf-16 null mutation as well as by daf-16a/f mutation (Figure 3, A–D; Table S5; Table S6) (Lee et al. 2001; Lin et al. 2001).
daf-16a no. 1 and no. 2 mutations partially reduced mean life spans of both daf-2(e1368) and daf-2(e1370) mutants, whereas daf-16a/f mutation decreased mean life spans to the same extent as daf-16 null mutation (Figure 3, A and C; Table S5; Table S6). These results indicate that DAF-16A is partially required for the longevity of daf-2/IGFR mutants. In contrast, daf-16f mutation did not reproducibly influence life span in either daf-2/IGFR mutant background (Figure 3, B and D; Table S5; Table S6). This finding was unexpected in light of previous studies implicating DAF-16F in life span control by the DAF-2/IGFR pathway (Kwon et al. 2010; Bansal et al. 2014). Taken with our finding that the tm6659 mutation likely fully eliminates daf-16f activity (Figure 1, A and B; Figure S1C; Figure S6D), our data are consistent with a hierarchical model of DAF-16/FoxO isoform function in promoting longevity in the context of reduced DAF-2/IGFR signaling. DAF-16A is necessary for full life span extension. However, DAF-16F is dispensable for longevity, as DAF-16A is sufficient to fully extend life span even when daf-16f is mutated.
Given that daf-16a/f mutation shortens life span in animals with reduced DAF-2/IGFR activity to a much greater extent than daf-16a mutation alone (Figure 3, A and C; Table S5; Table S6), we tested the possibility that DAF-16F is required for life span extension in the absence of DAF-16A by performing isoform-specific daf-16/FoxO RNAi (Kwon et al. 2010). As previously shown, inactivation of either daf-16a or daf-16f alone by RNAi had a modest effect on the longevity of daf-2/IGFR mutant animals compared to RNAi of all daf-16/FoxO isoforms (“pan-daf-16 RNAi”; Figure S7A; Table S7) (Kwon et al. 2010). However, daf-16f RNAi shortened the mean life span of daf-16a;daf-2 double mutant animals to nearly the same extent as pan-daf-16 RNAi (Figure 3E; Table S7). Therefore, although DAF-16F is dispensable for longevity in the presence of DAF-16A, it is required for life span extension in animals lacking DAF-16A. The life span shortening effect of daf-16a RNAi in daf-16a;daf-2 double mutants may be a consequence of off-target RNAi effects (Ma et al. 2006).
We also wished to determine the extent to which longevity in daf-16f;daf-2 double mutants requires DAF-16A. To address this question, we performed isoform-specific daf-16/FoxO RNAi on daf-16f;daf-2 double mutants. daf-16a RNAi shortened the mean life span of daf-16f;daf-2 double mutant animals by nearly the same amount as pan-daf-16 RNAi (Figure 3F; Table S7). In contrast, daf-16f RNAi had a negligible effect on daf-16f;daf-2 mean life span. Therefore, when DAF-16F is absent, DAF-16A is likely the sole FoxO isoform that promotes longevity. In aggregate, our results indicate that DAF-16A is the primary isoform that controls C. elegans aging in the context of reduced DAF-2/IGFR signaling. DAF-16F is not required for longevity when DAF-16A is present, but it promotes long life in the absence of DAF-16A.
Rescue of mutant phenotypes with single-copy isoform-specific DAF-16/FoxO transgenes
Our data on DAF-16/FoxO isoform function are in agreement with a previous study implicating both DAF-16A and DAF-16F in dauer regulation in the daf-2(e1370) mutant background (Kwon et al. 2010). However, our results contradict the contention that DAF-16F plays a more prominent role in life span control than DAF-16A (Kwon et al. 2010; Bansal et al. 2014). This discrepancy may be a consequence of distinct experimental strategies used to assess the function of DAF-16/FoxO isoforms in life span control. We used isoform-specific deletion mutants in which other daf-16/FoxO isoforms remained under the control of endogenous regulatory elements and continued to be expressed at physiological levels (Figure S6). In contrast, Kwon et al. (2010) based their analysis on strains harboring both a daf-16/FoxO null mutation and transgenes overexpressing cDNAs encoding individual daf-16/FoxO isoforms.
In an effort to reconcile our results with those of Kwon et al. (2010), we generated integrated transgenic strains expressing either daf-16a or daf-16f under the control of their native promoters (Figure S8). These transgenes were modeled after those reported by Kwon et al. (2010). To minimize potentially confounding influences pertaining to overexpression and/or differences in genomic integration sites, we constructed these strains using MosSCI (Frokjaer-Jensen et al. 2008). We crossed these single-copy transgenes into daf-16a/f and daf-16a mutant backgrounds to assess their relative activity in promoting longevity and dauer arrest.
To test the daf-16a transgene in life span control, we crossed it into daf-16a/f;daf-2 double mutant animals that lack both DAF-16A and DAF-16F. daf-16a/f;daf-2 double mutant animals containing the single-copy daf-16a transgene lived longer than nontransgenic siblings but significantly shorter than daf-2 single mutants (Figure 3G; Figure S9A; Table S8) (and by inference, daf-16f;daf-2 double mutants) (Figure 3D). Furthermore, this transgene did not fully rescue life span extension in daf-16a;daf-2 double mutants (Figure S9, B and C; Table S8). Therefore, the daf-16a transgene promotes life span extension modestly in comparison to endogenous daf-16a, which extends life span fully in daf-16f;daf-2 double mutants (Figure 3F). Similarly, although this daf-16a transgene rescued the dauer-constitutive phenotype of daf-16a;daf-2(e1370) double mutants (Figure S10B; Table S11), it did not rescue dauer arrest in daf-16a;daf-2(e1368) double mutants (Figure S10D; Table S11), nor did it fully rescue dauer arrest in daf-16a/f;daf-2 double mutants (Figure S10, A and C; Table S11). In concert, these data indicate that this single-copy daf-16a transgene is less active than the endogenous daf-16a locus.
Promoter-swap experiments show that daf-16a or daf-16f transgenes driven by the daf-16f promoter rescue longevity to a greater extent than the same transgenes driven by the daf-16a promoter (Kwon et al. 2010). Intriguingly, WormBase (www.wormbase.org) annotations document the existence of daf-16a transcripts that originate from the daf-16f promoter. We verified this in two ways. First, we used PCR amplification and sequencing to identify splice junctions unique to these transcripts (Figure S11, A and B). Furthermore, we identified sequence reads in whole transcriptome profiling data that correspond to such transcripts (Figure S11B). Therefore, daf-16a is transcribed from two promoters, one of which is the same promoter that drives expression of daf-16f. As primary transcripts originating from the daf-16f promoter contain all daf-16a exons (Figure 1A), alternative splicing of such pre-mRNAs may generate mature daf-16a transcripts. Taken together with the relative importance of the daf-16f promoter in extending life span (Kwon et al. 2010), this finding could explain why our single-copy daf-16a transgene (Figure S8) has less activity than the endogenous daf-16a locus (Figure 3G; Figure S9, A–C; Figure S10, A–D).
To test the activity of our single-copy daf-16f transgene, we constructed daf-16a/f;daf-2(e1368) and daf-16a/f;daf-2(e1370) animals harboring the daf-16f transgene. The life spans of these animals were comparable to the life spans of daf-16a;daf-2 double mutant animals (Figure 3H; Figure S9D; Table S8). Since daf-16f activity is the major contributor to longevity in daf-16a;daf-2 double mutants (Figure 3E), the activity of daf-16f encoded by this single-copy transgene is comparable to the activity of the endogenous daf-16f locus. Dauer arrest phenotypes of these animals also mirrored those of daf-16a;daf-2 double mutants (Figure S10, E and F). As previously observed in strains harboring multicopy daf-16 transgenes (Kwon et al. 2010), animals with the daf-16f transgene lived much longer than those with the daf-16a transgene (Figure 3, G and H; Table S8). Notably, the daf-16f transgene significantly increased both the life span and the penetrance of the dauer-constitutive phenotype of daf-16a;daf-2(e1370) double mutant animals that contain intact endogenous daf-16f (Figure 3H; Figure S10E). Therefore, the longevity and dauer-constitutive phenotypes of daf-2 mutant animals are highly sensitive to daf-16f gene dosage. Collectively, our results with single-copy isoform-specific daf-16/FoxO transgenes, while consistent with published results using multicopy transgenes (Kwon et al. 2010; Bansal et al. 2014), strongly suggest that the previously reported difference in longevity-promoting activity of multicopy daf-16a- and daf-16f-specific transgenes (Kwon et al. 2010; Bansal et al. 2014) is due to both overexpression of daf-16f as well as the failure of the daf-16a-specific transgene to recapitulate endogenous daf-16a activity.
DAF-16A promotes longevity in animals lacking a germline
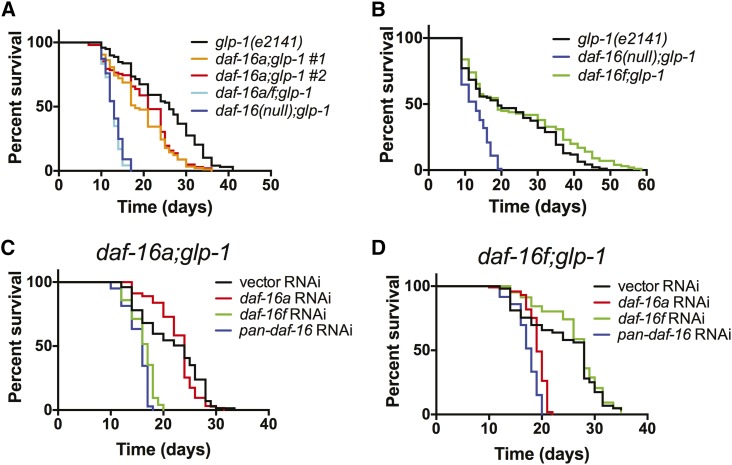
Although DAF-16/FoxO is also required for longevity in animals lacking a germline (Hsin and Kenyon 1999), life span extension caused by germline ablation requires molecules such as KRI-1 and TCER-1 that are not necessary for longevity in daf-2/IGFR mutants (Berman and Kenyon 2006; Ghazi et al. 2009). These observations suggest that DAF-2/IGFR and the germline could control life span by coupling to distinct DAF-16/FoxO isoforms. To test this hypothesis, we determined the effect of isoform-specific daf-16/FoxO mutations on life span in animals harboring a temperature-sensitive glp-1 mutation that develops without a germline when grown at 25° (Arantes-Oliveira et al. 2002). As we observed in animals with reduced DAF-2/IGFR activity, the life spans of germline-ablated animals were modestly reduced by daf-16a mutation (Figure 4A; Table S9). daf-16f mutation did not influence the life span of germline-ablated animals in a consistent manner (Figure 4B; Table S9).
Figure 4.
Effects of daf-16a- and daf-16f-specific mutations and RNAi on life span in animals lacking a germline. (A and B) Effects of daf-16a (A) and daf-16f (B) mutations on life spans of germline-ablated glp-1(e2141) animals. (C and D) daf-16f is required for daf-16a;glp-1 longevity and vice versa. Survival curves are presented for (C) daf-16a;glp-1 and (D) daf-16f;glp-1 mutant animals upon exposure to isoform-specific daf-16 RNAi. Figure S7B shows control glp-1(e2141) survival when treated with isoform-specific daf-16 RNAi. See text for details. Statistics and raw data are presented in Table S9; Table S10.
RNAi-based inactivation of either daf-16a or daf-16f in animals lacking a germline revealed phenotypes similar to those observed in daf-2/IGFR mutants. Compared to pan-daf-16 RNAi, daf-16a, or daf-16f RNAi partially shortened median life spans of germline-ablated animals (Figure S7B; Table S10). In contrast, daf-16f RNAi shortened the life span of daf-16a;glp-1 double mutant animals to the same extent as pan-daf-16 RNAi (Figure 4C; Table S10). Likewise, daf-16a RNAi suppressed longevity to nearly the same degree as pan-daf-16 RNAi in daf-16f;glp-1 double mutants (Figure 4D; Table S10).
Collectively, these data show that DAF-16A is also the main FoxO isoform that promotes longevity in animals lacking a germline, while DAF-16F is not required for life span extension in this context. Furthermore, they demonstrate that the distinct molecular requirements for life span extension in daf-2/IGFR mutants and germline-ablated animals (Berman and Kenyon 2006; Ghazi et al. 2009) cannot be explained by the coupling of these upstream pathways to disparate DAF-16/FoxO isoform outputs.
DAF-16A promotes stress resistance in animals with reduced DAF-2/IGFR signaling
As DAF-16/FoxO is required for the resistance of daf-2/IGFR mutants to various environmental insults (Murakami and Johnson 1996; Honda and Honda 1999; McColl et al. 2010), we determined the impact of daf-16a- and daf-16f-specific mutations on the sensitivity of daf-2(e1370) mutants to heat, oxidative stress, and UV radiation. The effect of these mutations on stress tolerance was similar in each condition. As expected, the daf-2/IGFR mutant was significantly more resistant to heat, oxidative stress, and UV radiation (Figure S12; Table S12) than wild-type animals. daf-16a/f mutation completely abolished the resistance of the daf-2/IGFR mutant to heat and oxidative stress and strongly suppressed its resistance to UV radiation. In all three contexts, daf-16a mutation partially suppressed stress resistance of the daf-2/IGFR mutant, whereas daf-16f mutation did not influence its ability to withstand insult. These results mirror the impact of isoform-specific mutations on life span (Figure 3; Figure 4).
DAF-16/FoxO target gene regulation by DAF-16A and DAF-16F
To illuminate the mechanistic basis for DAF-16/FoxO isoform-specific functions in life span control, we performed whole transcriptome profiling of young adult daf-2(e1370) mutant animals in the context of wild-type daf-16/FoxO and isoform-specific daf-16/FoxO mutant alleles. A subset of animals from each experimental replicate was subjected to life span assays to confirm that all mutants from which RNA was isolated had the expected life span phenotypes. Identification of genes that were differentially expressed in wild type and daf-2(e1370) and differentially expressed in the opposite direction in both daf-16(null);daf-2 and daf-16a/f;daf-2 double mutants compared to daf-2 mutants defined a set of 399 genes that are targets of DAF-16A and/or DAF-16F (Figure 5; Table S13; henceforth referred to as “DAF-16A/F target genes”). We validated our profiling results by measuring transcript levels corresponding to 15 genes that emerged from this analysis using qPCR. DAF-16/FoxO-dependent regulation was confirmed for all 15 of these genes (Figure 6; Figure S13; Table S14).
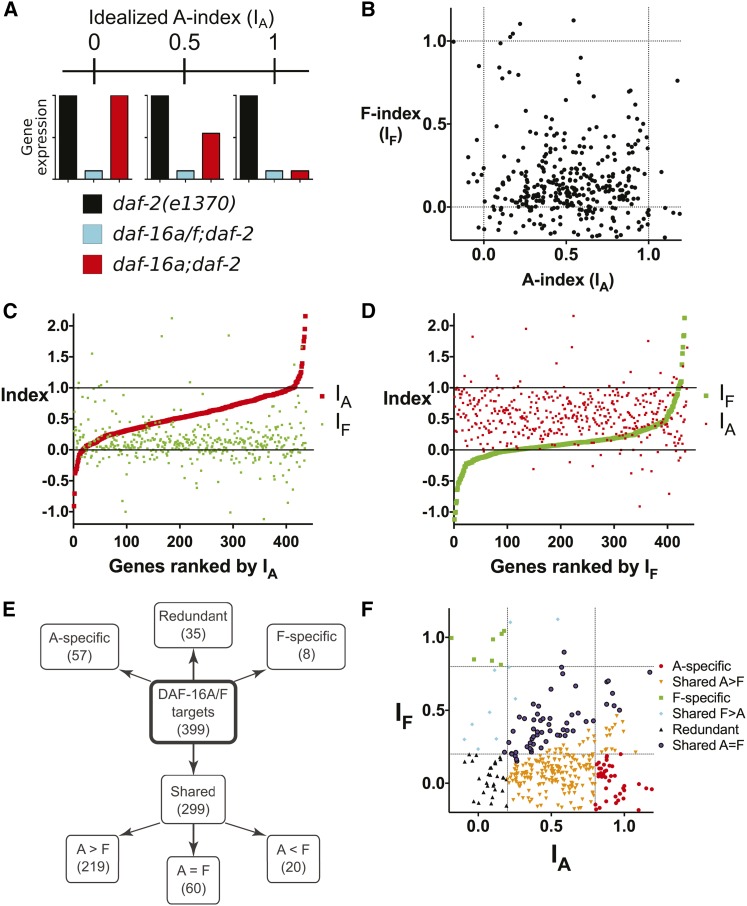
Figure 5.
DAF-16A and DAF-16F target genes identified by whole transcriptome profiling. (A) Depiction of the A-index (IA) for three hypothetical target genes with IA = 0, 0.5, and 1.0. Idealized expression profiles in daf-2(e1370), daf-16a/f;daf-2, and daf-16a;daf-2 are shown for all three genes. (B) Scatterplot comparing IA and IF for DAF-16A/F target genes. Dashed lines indicate IA and IF = 0 or 1. Only genes with indices from −0.2 to 1.2 are shown; a scatterplot with a wider range of indices is shown in Figure S15. IA and IF for all genes are listed in Table S13. (C and D) Plots of IA and IF for all DAF-16A/F target genes from lowest to highest IA (C) or IF (D). Solid lines correspond to indices of 0 and 1. Three genes with indices >2.2 or < −1.2 were omitted for presentation purposes. Gene rankings are listed in Table S13. (E) Tree diagram summarizing the categorization system for DAF-16A and DAF-16F target genes. See text and Materials and Methods for details. (F) Scatterplot from B, with individual genes color coded according to categories depicted in E. Dashed lines indicate IA or IF values of 0.2 or 0.8, corresponding to the cutoffs used to define redundantly regulated, A-specific, and F-specific targets.
Figure 6.
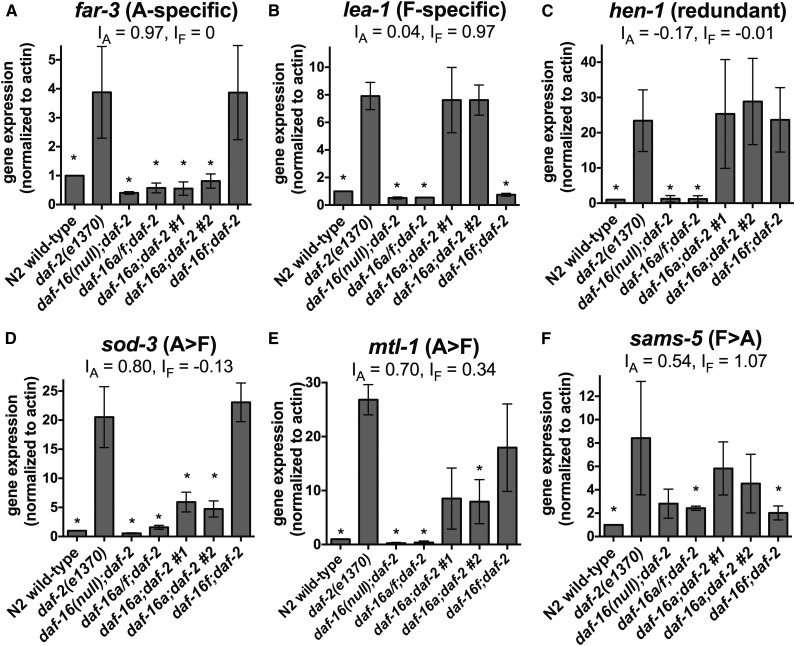
qPCR validation of target gene regulation by DAF-16A and DAF-16F. (A–F) Expression of six DAF-16A/F target genes quantified by qPCR using RNA isolated from day 1 young adult animals. Values represent the mean from three biological replicates. Error bars represent standard deviation. Asterisks indicate statistically significant changes (P < 0.05 by paired ratio t-test). IA and IF were calculated using mean expression values measured by qPCR, and correlate with indices calculated using RNA-seq measurements (Table S16). Statistics and data are summarized in Table S14.
To estimate the influence of specific DAF-16/FoxO isoforms on aspects of whole organism physiology, we performed gene set enrichment analysis of DAF-16A/F target genes using GO biological process terms (Ashburner et al. 2000) and KEGG pathways (Kanehisa and Goto 2000) (Figure S14; Table S15). GO analysis revealed specific up-regulation of ribosome biogenesis genes and specific down-regulation of replication and cell death genes in the daf-16f mutant. Immune response genes were up-regulated in daf-16a mutants and down-regulated in the daf-16f mutant (Figure S14A; Table S15). KEGG analysis also unveiled enrichment of genes involved in ribosome biogenesis in the daf-16f mutant while showing reduction of genes involved in glycolysis and gluconeogenesis in daf-16a mutants. Genes involved in cysteine and methionine metabolism were depleted in the daf-16f mutant but enriched in daf-16a mutants (Figure S14B; Table S15).
To gain insight into the relative magnitude of DAF-16A- and DAF-16F-specific contributions to the regulation of individual DAF-16A/F target genes, we compared the effect of either daf-16a or daf-16f mutation on DAF-2/IGFR-dependent gene regulation to the effect of daf-16a/f mutation on DAF-2/IGFR-dependent gene regulation, thus calculating an “A-index” (IA) and “F-index” (IF) for each DAF-16A/F target gene (see Materials and Methods). Hypothetically, an idealized DAF-16A-specific target gene would have IA = 1.0 and IF = 0, whereas a DAF-16F-specific target gene would have IA = 0 and IF = 1.0 (Figure 5A). IA and IF calculated using FPKM values from whole transcriptome profiling correlated well with IA and IF derived from qPCR data for 15 genes tested (Figure 6; Figure S13; Table S13; Table S16).
We first generated a scatterplot of IA and IF for the entire set of 399 DAF-16A/F target genes (Figure 5B; Figure S15). This depiction indicates that IA > IF for most genes, suggesting that DAF-16A plays a larger role in gene regulation than DAF-16F. To further explore this question, we plotted IA and IF of the entire set of DAF-16A/F target genes from lowest to highest IA (Figure 5C) and IF (Figure 5D). This allowed us to visualize the magnitude of isoform-specific regulation for both DAF-16A and DAF-16F across the entire set of target genes while potentially revealing relationships between the isoforms in target gene regulation. These illustrations confirm that for most target genes, DAF-16A has a greater impact on expression than DAF-16F. Furthermore, they reveal no obvious global relationship between the degree of regulation of any single gene by one isoform and the impact of the other isoform on its expression.
Categorization of DAF-16A/F target genes
The life span phenotypes of daf-16a/f, daf-16a, and daf-16f mutants (Figure 3) suggested that sorting of DAF-16A/F target genes into categories based on their regulation by DAF-16A and/or DAF-16F might help to prioritize subsets of DAF-16/FoxO genes that are likely to contribute significantly to longevity. Therefore, we placed each DAF-16A/F target gene into one of four categories based on the impact of each isoform on expression: DAF-16A-specific (IA > 0.8 and IF < 0.2), DAF-16F-specific (IF > 0.8 and IA < 0.2), redundant (IA and IF both < 0.2), and shared (all others). We further subdivided genes in the “shared” category into three subgroups: genes for which DAF-16A plays a greater role than DAF-16F in regulation (shared A > F; IA/IF > 2.0), genes for which DAF-16F has a greater impact on regulation than DAF-16A (shared F > A; IF/IA > 2.0), and genes that are regulated by both isoforms (shared A = F; 0.5 ≤ IA/IF ≤ 2.0) (Figure 5, E and F).
Fifty-seven genes are DAF-16A-specific targets (Figure 5, E and F; Table S13). This group includes far-3 (Figure 6A; Table S14), which encodes a fatty acid/retinol binding protein (Garofalo et al. 2003), as well as the lipl-1 gene encoding a lysosomal acid lipase, which is transcriptionally up-regulated and promotes the mobilization of lipid stores in response to starvation (O’Rourke and Ruvkun 2013). Overexpression of lipl-1 extends life span (O’Rourke and Ruvkun 2013). Eight genes are DAF-16F-specific targets (Figure 5, E and F; Table S13), including lea-1 (Figure 6B; Table S14), which encodes a homolog of human perilipin-4 that promotes resistance to dehydration stress (Gal et al. 2004).
Most DAF-16A/F target genes are regulated by both DAF-16A and DAF-16F (Figure 5, E and F). Thirty-five genes are redundantly regulated by DAF-16A and DAF-16F (Figure 5, E and F; Table S13), including hen-1 (Figure 6C; Table S14), a secreted protein required for sensory integration and learning (Ishihara et al. 2002), and the established DAF-16/FoxO target genes lys-7 and dod-17 (Figure S13, D–E; Table S14) (Murphy et al. 2003).
The remaining 299 DAF-16A/F target genes are categorized as those with shared regulation by DAF-16A and DAF-16F (Figure 5, E and F; Table S13). A total of 73% of these (219/299) are primarily regulated by DAF-16A (Figure 5, E and F; Table S13). This subgroup comprises the largest subset of target genes and includes the established DAF-16/FoxO targets sod-3 (Figure 6D; Table S14), mtl-1 (Figure 6E; Table S14), fat-7 (Figure S13F; Table S14), hsp-12.6, dod-3, dod-23, and dod-24 (Murphy et al. 2003) and the lipase-like gene lipl-2 (Figure S13G; Table S14). It also includes aakg-4, which encodes an atypical AMP kinase gamma subunit that participates in a positive feedback loop to promote DAF-16/FoxO activity (Chen et al. 2013b; Tullet et al. 2014), akt-2, which acts together with akt-1 to inhibit DAF-16/FoxO (Paradis and Ruvkun 1998), and ins-7, which encodes an insulin-like peptide that is repressed by DAF-16/FoxO as part of a feedback loop that coordinates organism-wide DAF-16/FoxO activation (Murphy et al. 2003, 2007). Among the 20 shared target genes that are primarily regulated by DAF-16F are the S-adenosyl methionine synthase gene sams-5 (Figure 6F; Table S14) and five collagen genes (Figure 5, E and F; Table S13). Finally, 60 target genes are regulated to a comparable extent by DAF-16A and DAF-16F (Figure 5, E and F; Table S13). Within this subgroup are lipl-3 and lipl-4, which encode lipases that, along with the DAF-16A-specific target lipl-1, are transcriptionally induced in response to fasting (O’Rourke and Ruvkun 2013), and mdl-1, a Mad transcription factor family member that promotes DAF-16/FoxO-dependent longevity (Riesen et al. 2014).
Collectively, these classifications indicate that DAF-16A plays an important role in regulating 93% of DAF-16A/F target genes (“DAF-16A specific,” “redundant,” “shared A > F,” and “shared A = F” categories; 371/399 genes), whereas DAF-16F strongly influences the expression of just over 30% of target genes (“DAF-16F specific,” redundant, “shared F > A,” and shared A = F categories; 123/399 genes).
Two DAF-16A-specific target genes play important roles in life span control
Our main goal at the outset of this line of inquiry was to prioritize a tractable subset of DAF-16/FoxO target genes for functional analysis. Based on the life span phenotypes of isoform-specific daf-16/FoxO mutants (Figure 3; Figure 4), we surmised that the subgroups of 57 DAF-16A-specific and 35 redundant target genes had a high probability of containing genes that would impact longevity significantly. DAF-16A-specific genes likely contribute to the life span differential between daf-16a;daf-2 and daf-2 mutants, whereas redundantly regulated genes might be expected to account for the difference in the life spans of daf-16a/f;daf-2 and daf-16a;daf-2 double mutants (Figure 3, A and C).
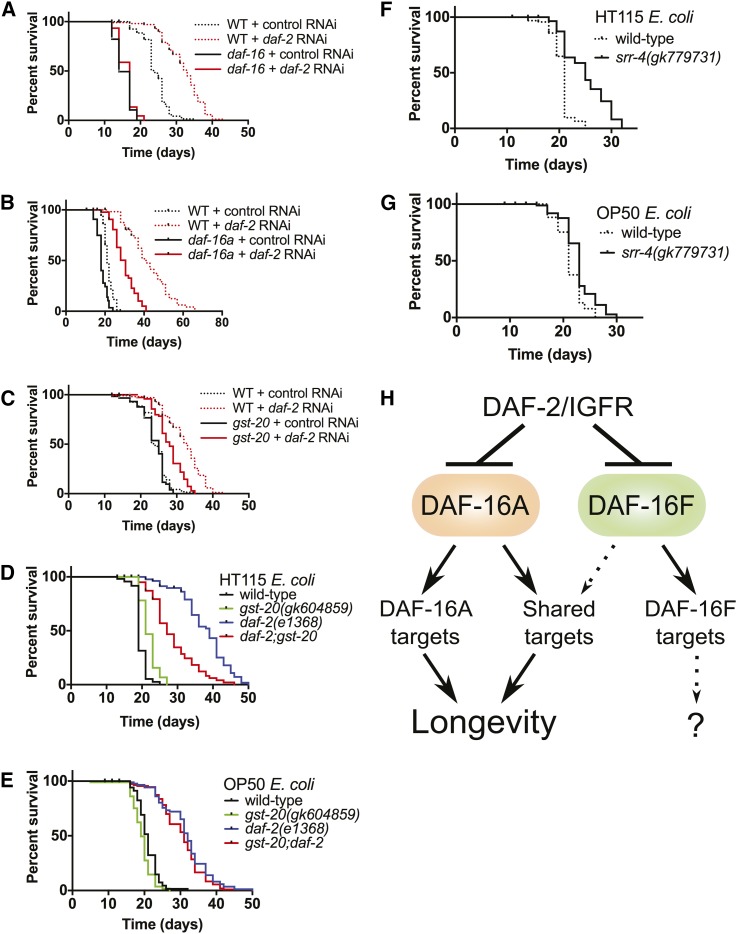
To test this hypothesis, we obtained strains readily available to the community that harbored probable strong loss-of-function mutations in twenty DAF-16A-specific, two DAF-16F-specific, and nine redundant target genes (Table S17). Life spans were assayed after exposure to either control bacteria or bacteria engineered to synthesize double-stranded RNA corresponding to the daf-2 gene (daf-2 RNAi). In control experiments, life span extension induced by daf-2 RNAi was completely abrogated in daf-16 null mutants and partially reduced in daf-16a-specific mutants (Figure 7, A and B).
Figure 7.
DAF-16A-specific targets influence life span. daf-16 (A) and daf-16a (B) are required for longevity induced by daf-2/IGFR RNAi. (C) The DAF-16A up-regulated target gene gst-20 is required for full life span extension induced by daf-2/IGFR RNAi. gst-20 promotes longevity in daf-2(e1368) mutants grown on E. coli HT115 (D) but not in animals grown on E. coli OP50 (E). The DAF-16A down-regulated target gene srr-4 limits longevity in animals grown on HT115 (F) but does not influence life span in animals grown on OP50 (G). (H) Model of life span control and gene regulation by DAF-16A and DAF-16F. See text for details. Life span statistics and data are presented in Table S18.
Among 19 strains harboring mutations in genes induced by DAF-16/FoxO, daf-2-RNAi-induced life span extension was significantly less than that observed in wild-type controls in a strain harboring a mutation in gst-20 (Figure 7C; Table S18). This strain was not RNAi defective (Rde; Figure S16), indicating that the reduced life span extension upon exposure to daf-2 RNAi is unlikely to be a consequence of a background mutation that reduces the efficiency of daf-2 inactivation by RNAi. Furthermore, examination of the mutational load in this strain, which was generated as part of the Million Mutation Project (Thompson et al. 2013), revealed no obvious strong loss-of-function mutations in genes known to influence aging or RNAi (Table S19).
To verify this result, we constructed a gst-20;daf-2(e1368) double mutant and compared its life span to that of gst-20 and daf-2 single mutant siblings. gst-20 mutation did not significantly shorten life span in animals with wild-type daf-2 (Figure 7, D and E; Table S18), indicating that this mutation does not cause general frailty or sickness. Intriguingly, gst-20 mutation significantly reduced life span extension caused by daf-2 mutation when animals were fed E. coli HT115 (Figure 7D; Table S18) but did not influence the life span of daf-2(e1368) mutants when they were fed E. coli OP50 (Figure 7E; Table S18).
gst-20 encodes a glutathione-S-transferase family member homologous to human hematopoietic prostaglandin D synthase, an enzyme that catalyzes the glutathione-dependent isomerization of prostaglandin H2 to prostaglandin D2 (Kanaoka and Urade 2003). We hypothesize that the induction of gst-20 by DAF-16A (Figure S13A; Table S14) contributes to DAF-16/FoxO-dependent life span extension in a manner dependent upon bacterial food source.
Among 12 strains with mutations in genes repressed by DAF-16/FoxO, one strain containing a mutation in srr-4 lived significantly longer than wild-type animals exposed to control RNAi. This strain also did not contain strong loss-of-function mutations in genes that affect aging (Table S19). Life span assays with outcrossed srr-4 mutant animals and wild-type siblings verified that srr-4 mutation extends life span (Figure 7F; Table S18). As observed for gst-20 mutation, we found that the impact of srr-4 mutation on life span was also dependent upon bacterial food source; srr-4 mutation extended life span significantly when animals were fed E. coli HT115 (Figure 7F; Table S18) but had less of an effect on longevity when animals were fed E. coli OP50 (Figure 7G; Table S18). srr-4 encodes a putative seven-transmembrane G-protein-coupled receptor that is repressed by DAF-16A (Figure S13B; Table S14). The SRR-4 protein is conserved in other Caenorhabditis species and is predicted to contain a DUF267 (domain of unknown function) domain. We postulate that DAF-16A may promote longevity in part by reducing srr-4 expression.
Discussion
Over 20 yr after the initial demonstration that DAF-16/FoxO promotes longevity (Kenyon et al. 1993), the question of which DAF-16/FoxO target genes are important for life span extension remains unanswered. Efforts to address this important issue have been hindered by the prodigious number of genes that are regulated by DAF-16/FoxO. Most of the hundreds of DAF-16/FoxO target genes that are regulated by DAF-2/IGFR signaling (Lee et al. 2003; McElwee et al. 2003; Murphy et al. 2003) have not been interrogated for roles in life span control.
Approaches to prioritize subsets of DAF-16/FoxO target genes for detailed investigation have succeeded in identifying DAF-16/FoxO target genes with previously unappreciated functions in promoting longevity (Schuster et al. 2010; Tullet et al. 2014). We have combined phenotypic analysis and whole transcriptome profiling of isoform-specific daf-16/FoxO mutants to define a subset of 92 DAF-16/FoxO target genes (composed of 57 DAF-16A-specific and 35 redundant target genes; Figure 5, E and F; Table S13) that we have prioritized for further study. We hypothesize that this subset may be enriched in genes likely to play important roles in life span control; regulation of DAF-16A-specific genes may account for the difference in life span between daf-2 mutants and daf-16a;daf-2 double mutants (Figure 3, A and C), and regulation of redundant genes may contribute to life span differences between daf-16a/f;daf-2 double mutants and both daf-16a;daf-2 (Figure 3, A and C) and daf-16f;daf-2 double mutants (Figure 3, B and D).
Our initial phenotypic analysis of 29 strains harboring strong loss-of-function mutations in genes from this subset has revealed potentially important functions for two DAF-16/FoxO target genes, gst-20 and srr-4, in controlling life span. Neither of these genes had previously been implicated in DAF-16/FoxO-dependent longevity. Our discovery of these new longevity genes underscores the efficacy of our integrative strategy in identifying DAF-16/FoxO target genes that are likely to influence life span. We anticipate that functional analysis of the remaining 63 genes within this prioritized subset of DAF-16/FoxO targets will reveal further insights into the mechanistic basis for life span extension by DAF-16/FoxO. As most transcription factors regulate the expression of hundreds of genes, our strategy may be generally applicable to the study of target genes and their relative importance in mediating transcription factor function.
Notably, our life span assays revealed diet-dependent effects of gst-20 and srr-4 on longevity. Mutations in both genes influenced life span when animals were fed E. coli HT115 but did not significantly affect longevity when animals were fed E. coli OP50 (Figure 7; Table S18). Given that the main RNAi library used in the C. elegans field was constructed using E. coli HT115 (Kamath et al. 2003), which is distinct from the standard OP50 strain used in C. elegans studies, our findings underscore the importance of verifying RNAi-based life span assays in the context of E. coli OP50.
Although we have focused our efforts on genes within the DAF-16A-specific and redundant categories of DAF-16/FoxO targets (Figure 5E; Table S13), genes in other categories may also influence life span significantly. Indeed, aakg-4, which encodes an atypical AMP-activated protein kinase gamma isoform that promotes longevity in the context of reduced DAF-2/IGFR activity (Chen et al. 2013b; Tullet et al. 2014), emerged from our analysis in the shared A > F category, which was the largest subset of DAF-16/FoxO targets (Figure 5E; Table S13). We are exploring complementary integrative approaches to define other subsets of DAF-16/FoxO target genes that may direct our attention to specific genes within the larger subsets generated in this study.
Our analysis of isoform-specific daf-16/FoxO mutants indicates that DAF-16A is the major FoxO isoform that controls dauer arrest (Figure 2), longevity (Figure 3; Figure 4), and stress resistance (Figure S12). In the presence of physiologic levels of DAF-16A, DAF-16F is dispensable for dauer regulation, life span control, and stress resistance. In the absence of DAF-16A, DAF-16F promotes dauer arrest and longevity, as demonstrated by the incomplete suppression of dauer-constitutive and life span extension phenotypes by daf-16a mutation (Figure 2B; Figure 3, A and C; Figure 4A) and the influence of daf-16f RNAi on life span in daf-2/IGFR mutant animals and germline-ablated animals that lack daf-16a (Figure 3E; Figure 4C).
These conclusions are at odds with previous suggestions that DAF-16F is the major isoform that promotes longevity in the context of reduced DAF-2/IGFR signaling (Kwon et al. 2010; Bansal et al. 2014). As this interpretation of the primacy of DAF-16F in life span control was based in large part on life span phenotypes of transgenes overexpressing either daf-16a or daf-16f (Kwon et al. 2010; Bansal et al. 2014), we conducted our own investigations of single-copy daf-16a and daf-16f transgenes driven by the same promoters used by Kwon et al. (2010) (Figure S8). Our results (Figure 3, G and H; Figure S9; Figure S10) strongly suggest that the difference in the longevity-promoting activities of daf-16a and daf-16f transgenes that they observed is a consequence of both the sensitivity of the daf-16f transgene to dosage (Figure 3H; Figure S10E) as well as the failure of the daf-16a transgene to fully recapitulate the activity of the endogenous daf-16a locus (Figure 3G; Figure S9A; Figure S10, A–D), rather than greater longevity-promoting activity per se of daf-16f compared to daf-16a.
The expression profiling experiments presented here are the first to define the relative contributions of endogenous DAF-16/FoxO isoforms to the regulation of DAF-16/FoxO target genes. They reveal a dominant role for DAF-16A relative to DAF-16F in regulating gene expression in young adult animals (Figure 5; Table S13) that is commensurate with the relative influence of DAF-16A and DAF-16F on adult life span (Figure 3; Figure 4). These results further support the conclusion that DAF-16A is the major FoxO isoform that promotes longevity in C. elegans.
Based on our results, we propose a hierarchical model of DAF-16/FoxO isoform function in life span control (Figure 7H). DAF-2/IGFR inhibits both DAF-16A and DAF-16F (Kwon et al. 2010). In the context of reduced DAF-2/IGFR signaling, both DAF-16A and DAF-16F contribute to longevity and the regulation of DAF-16/FoxO target genes. In the absence of DAF-16A, the altered expression of genes regulated mainly by DAF-16A shortens life span. When DAF-16F is inactive, genes regulated primarily by DAF-16F are misregulated, but this does not influence life span. When neither DAF-16A nor DAF-16F is present, DAF-2/IGFR mutation does not promote longevity due to the misregulation of both DAF-16A-specific target genes as well as genes that are regulated by both DAF-16A and DAF-16F.
The conserved role of IGFR signaling and FoxO transcription factors in life span control was first discovered in C. elegans (Friedman and Johnson 1988a,b; Johnson 1990; Kenyon et al. 1993). Decades later, how FoxO transcription factors extend life span remains poorly understood. Here we report a novel approach to delineate the contributions of specific FoxO target genes to FoxO-dependent longevity. IGFR signaling is now known to influence aging in mammals (Bluher et al. 2003; Holzenberger et al. 2003) and possibly humans (Suh et al. 2008; Tazearslan et al. 2011), and the prolongevity function of FoxO that is conserved in invertebrates (Kenyon et al. 1993; Giannakou et al. 2004; Hwangbo et al. 2004; Slack et al. 2011; Yamamoto and Tatar 2011) and mice (Shimokawa et al. 2015) is likely relevant to human aging and aging-related diseases. By directing focus to a subset of target genes, our strategy will facilitate the elucidation of the molecular basis for life span extension by FoxO transcription factors. Further investigation of DAF-16/FoxO target gene function in C. elegans promises to illuminate phyletically general functions of FoxO transcription factors in controlling aging.
Supplementary Material
Acknowledgments
We thank Robert Lyons and Jeanne Geskes at the University of Michigan (UM) DNA Sequencing Core for their assistance with whole transcriptome profiling, the UM Bioinformatics Core and Mallory Freeberg for help with analysis of transcriptome profiling data, and John Kim and Colin Delaney for comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). This work was supported by the Biology of Aging training grant T32AG000114, awarded to the UM Geriatrics Center by the National Institute on Aging (A.T.-Y.C.), the Functional Assessment Core of the UM Nathan Shock Center of Excellence in the Basic Biology of Aging, research scholar grant DDC-119640 from the American Cancer Society (P.J.H.), and R01 grant AG041177 from the NIH (P.J.H.).
Footnotes
Communicating editor: D. Greenstein
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.177998/-/DC1.
Literature Cited
- Accili D., Arden K. C., 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117: 421–426. [DOI] [PubMed] [Google Scholar]
- Ambrogini E., Almeida M., Martin-Millan M., Paik J. H., Depinho R. A., et al. , 2010. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 11: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi C. V., Malovini A., Roncarati R., Novelli V., Villa F., et al. , 2009. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 12: 95–104. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N., Apfeld J., Dillin A., Kenyon C., 2002. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295: 502–505. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., et al. , 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A., Kwon E. S., Conte D., Jr, Liu H., Gilchrist M. J., et al. , 2014. Transcriptional regulation of Caenorhabditis elegans FOXO/DAF-16 modulates lifespan. Longev. Healthspan 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel A., Schmoll D., Unterman T. G., 2005. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 16: 183–189. [DOI] [PubMed] [Google Scholar]
- Berman J. R., Kenyon C., 2006. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124: 1055–1068. [DOI] [PubMed] [Google Scholar]
- Billi A. C., Alessi A. F., Khivansara V., Han T., Freeberg M., et al. , 2012. The Caenorhabditis elegans HEN1 ortholog, HENN-1, methylates and stabilizes select subclasses of germline small RNAs. PLoS Genet. 8: e1002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M., Kahn B. B., Kahn C. R., 2003. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572–574. [DOI] [PubMed] [Google Scholar]
- Chen A. T., Guo C., Dumas K. J., Ashrafi K., Hu P. J., 2013a Effects of Caenorhabditis elegans sgk-1 mutations on lifespan, stress resistance, and DAF-16/FoxO regulation. Aging Cell 12: 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Li P. W., Goldstein B. A., Cai W., Thomas E. L., et al. , 2013b Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Reports 5: 1600–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Guo S., Copps K., Dong X., Kollipara R., et al. , 2009. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat. Med. 15: 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. C., Copps K. D., Guo S., Li Y., Kollipara R., et al. , 2008. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 8: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F., Caliebe A., Kleindorp R., Blanche H., von Eller-Eberstein H., et al. , 2009. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl. Acad. Sci. USA 106: 2700–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. B., Johnson T. E., 1988a A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. B., Johnson T. E., 1988b Three mutants that extend both mean and maximum life span of the nematode, Caenorhabditis elegans, define the age-1 gene. J. Gerontol. 43: B102–B109. [DOI] [PubMed] [Google Scholar]
- Frokjaer-Jensen C., Davis M. W., Hopkins C. E., Newman B. J., Thummel J. M., et al. , 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40: 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal T. Z., Glazer I., Koltai H., 2004. An LEA group 3 family member is involved in survival of C. elegans during exposure to stress. FEBS Lett. 577: 21–26. [DOI] [PubMed] [Google Scholar]
- Gan B., Lim C., Chu G., Hua S., Ding Z., et al. , 2010. FoxOs enforce a progression checkpoint to constrain mTORC1-activated renal tumorigenesis. Cancer Cell 18: 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo A., Rowlinson M. C., Amambua N. A., Hughes J. M., Kelly S. M., et al. , 2003. The FAR protein family of the nematode Caenorhabditis elegans. Differential lipid binding properties, structural characteristics, and developmental regulation. J. Biol. Chem. 278: 8065–8074. [DOI] [PubMed] [Google Scholar]
- Gems D., Sutton A. J., Sundermeyer M. L., Albert P. S., King K. V., et al. , 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengyo-Ando K., Mitani S., 2000. Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 269: 64–69. [DOI] [PubMed] [Google Scholar]
- Ghazi A., Henis-Korenblit S., Kenyon C., 2009. A transcription elongation factor that links signals from the reproductive system to lifespan extension in Caenorhabditis elegans. PLoS Genet. 5: e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou M. E., Goss M., Junger M. A., Hafen E., Leevers S. J., et al. , 2004. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 305: 361. [DOI] [PubMed] [Google Scholar]
- Gottlieb S., Ruvkun G., 1994. daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics 137: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubelmann C., Gattiker A., Massouras A., Hens K., David F., et al. , 2011. GETPrime: a gene- or transcript-specific primer database for quantitative real-time PCR. Database (Oxford) 2011: bar040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman K. L., Pease L. R., 2007. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2: 924–932. [DOI] [PubMed] [Google Scholar]
- Henderson S. T., Johnson T. E., 2001. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11: 1975–1980. [DOI] [PubMed] [Google Scholar]
- Holzenberger M., Dupont J., Ducos B., Leneuve P., Geloen A., et al. , 2003. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421: 182–187. [DOI] [PubMed] [Google Scholar]
- Honda Y., Honda S., 1999. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 13: 1385–1393. [PubMed] [Google Scholar]
- Hsin H., Kenyon C., 1999. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399: 362–366. [DOI] [PubMed] [Google Scholar]
- Hu P. J., Xu J., Ruvkun G., 2006. Two membrane-associated tyrosine phosphatase homologs potentiate C. elegans AKT-1/PKB signaling. PLoS Genet. 2: e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo D. S., Gershman B., Tu M. P., Palmer M., Tatar M., 2004. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429: 562–566. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Iino Y., Mohri A., Mori I., Gengyo-Ando K., et al. , 2002. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 109: 639–649. [DOI] [PubMed] [Google Scholar]
- Johnson T. E., 1990. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science 249: 908–912. [DOI] [PubMed] [Google Scholar]
- Junger M. A., Rintelen F., Stocker H., Wasserman J. D., Vegh M., et al. , 2003. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser, and J. Ahringer, 2001 Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2: RESEARCH0002. [DOI] [PMC free article] [PubMed]
- Kanaoka Y., Urade Y., 2003. Hematopoietic prostaglandin D synthase. Prostaglandins Leukot. Essent. Fatty Acids 69: 163–167. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Goto S., 2000. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., 2010. The genetics of ageing. Nature 464: 504–512. [DOI] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R., 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464. [DOI] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G., 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Nakae J., Kitamura Y., Kido Y., Biggs W. H., 3rd, et al. , 2002. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J. Clin. Invest. 110: 1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon E. S., Narasimhan S. D., Yen K., Tissenbaum H. A., 2010. A new DAF-16 isoform regulates longevity. Nature 466: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead, B., 2010 Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinformatics Chapter 11: Unit 11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S. L., 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. Y., Hench J., Ruvkun G., 2001. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 11: 1950–1957. [DOI] [PubMed] [Google Scholar]
- Lee S. S., Kennedy S., Tolonen A. C., Ruvkun G., 2003. DAF-16 target genes that control C. elegans life-span and metabolism. Science 300: 644–647. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang W. J., Cao H., Lu J., Wu C., et al. , 2009. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum. Mol. Genet. 18: 4897–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Dorman J. B., Rodan A., Kenyon C., 1997. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278: 1319–1322. [DOI] [PubMed] [Google Scholar]
- Lin K., Hsin H., Libina N., Kenyon C., 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28: 139–145. [DOI] [PubMed] [Google Scholar]
- Lunetta K. L., D’Agostino R. B., Sr, Karasik D., Benjamin E. J., Guo C. Y., et al. , 2007. Genetic correlates of longevity and selected age-related phenotypes: a genome-wide association study in the Framingham Study. BMC Med. Genet. 8(Suppl 1): S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Creanga A., Lum L., Beachy P. A., 2006. Prevalence of off-target effects in Drosophila RNA interference screens. Nature 443: 359–363. [DOI] [PubMed] [Google Scholar]
- McColl G., Rogers A. N., Alavez S., Hubbard A. E., Melov S., et al. , 2010. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab. 12: 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee J., Bubb K., Thomas J. H., 2003. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2: 111–121. [DOI] [PubMed] [Google Scholar]
- Murakami S., Johnson T. E., 1996. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 143: 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. T., Hu P. J., 2013. Insulin/insulin-like growth factor signaling in C. elegans. WormBook 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. T., McCarroll S. A., Bargmann C. I., Fraser A., Kamath R. S., et al. , 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283. [DOI] [PubMed] [Google Scholar]
- Murphy C. T., Lee S. J., Kenyon C., 2007. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104: 19046–19050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J., Biggs W. H., 3rd, Kitamura T., Cavenee W. K., Wright C. V., et al. , 2002. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 32: 245–253. [DOI] [PubMed] [Google Scholar]
- Nolan T., Hands R. E., Bustin S. A., 2006. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 1: 1559–1582. [DOI] [PubMed] [Google Scholar]
- O’Rourke E. J., Ruvkun G., 2013. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat. Cell Biol. 15: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., et al. , 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. [DOI] [PubMed] [Google Scholar]
- Paik J. H., Kollipara R., Chu G., Ji H., Xiao Y., et al. , 2007. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S., Ruvkun G., 1998. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12: 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowska L., Hu D., Huntsman S., Sung A., Chu C., et al. , 2009. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8: 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rached M. T., Kode A., Xu L., Yoshikawa Y., Paik J. H., et al. , 2010. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 11: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle, D. L., 1988 The dauer larva, pp. 393–412 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Plainview, NJ. [Google Scholar]
- Riddle D. L., Swanson M. M., Albert P. S., 1981. Interacting genes in nematode dauer larva formation. Nature 290: 668–671. [DOI] [PubMed] [Google Scholar]
- Riesen M., Feyst I., Rattanavirotkul N., Ezcurra M., Tullet J. M., et al. , 2014. MDL-1, a growth- and tumor-suppressor, slows aging and prevents germline hyperplasia and hypertrophy in C. elegans. Aging (Albany, N.Y.) 6: 98–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. T., Thorvaldsdottir H., Winckler W., Guttman M., Lander E. S., et al. , 2011. Integrative genomics viewer. Nat. Biotechnol. 29: 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor M. A., Leikauf G. D., Medvedovic M., 2009. LRpath: a logistic regression approach for identifying enriched biological groups in gene expression data. Bioinformatics 25: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster E., McElwee J. J., Tullet J. M., Doonan R., Matthijssens F., et al. , 2010. DamID in C. elegans reveals longevity-associated targets of DAF-16/FoxO. Mol. Syst. Biol. 6: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I., Komatsu T., Hayashi N., Kim S. E., Kawata T., et al. , 2015. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell 14: 707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Giannakou M. E., Foley A., Goss M., Partridge L., 2011. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell 10: 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y., Atzmon G., Cho M. O., Hwang D., Liu B., et al. , 2008. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl. Acad. Sci. USA 105: 3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F., Bosnjak M., Skunca N., Smuc T., 2011. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6: e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes S. M., Lane S. W., Bullinger L., Kalaitzidis D., Yusuf R., et al. , 2011. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias. Cell 146: 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H., Sarkissian M., Kelly W. G., Fleenor J., Grishok A., et al. , 1999. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99: 123–132. [DOI] [PubMed] [Google Scholar]
- Tabara H., Yigit E., Siomi H., Mello C. C., 2002. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell 109: 861–871. [DOI] [PubMed] [Google Scholar]
- Tazearslan C., Huang J., Barzilai N., Suh Y., 2011. Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles. Aging Cell 10: 551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O., Edgley M., Strasbourger P., Flibotte S., Ewing B., et al. , 2013. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 23: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdottir H., Robinson J. T., Mesirov J. P., 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14: 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., et al. , 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Hendrickson D. G., Sauvageau M., Goff L., Rinn J. L., et al. , 2013. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Tanaka J., Shuiqing Y., Welch C. L., DePinho R. A., et al. , 2012. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 15: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Westerterp M., Murphy A. J., Subramanian V., Ferrante A. W., Jr, et al. , 2013. Expanded granulocyte/monocyte compartment in myeloid-specific triple FoxO knockout increases oxidative stress and accelerates atherosclerosis in mice. Circ. Res. 112: 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet J. M., Araiz C., Sanders M. J., Au C., Benedetto A., et al. , 2014. DAF-16/FoxO directly regulates an atypical AMP-activated protein kinase gamma isoform to mediate the effects of insulin/IGF-1 signaling on aging in Caenorhabditis elegans. PLoS Genet. 10: e1004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A., Burgering B. M., 2007. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 8: 440–450. [DOI] [PubMed] [Google Scholar]
- Vowels J. J., Thomas J. H., 1992. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 130: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox B. J., Donlon T. A., He Q., Chen R., Grove J. S., et al. , 2008. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 105: 13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R., Tatar M., 2011. Insulin receptor substrate chico acts with the transcription factor FOXO to extend Drosophila lifespan. Aging Cell 10: 729–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. Whole transcriptome profiling data are available at GEO with the accession number: GSE72426.