Abstract
Gene targeting techniques have led to the phenotypic characterization of numerous genes; however, many genes show minimal to no phenotypic consequences when disrupted, despite many having highly conserved sequences. The standard explanation for these findings is functional redundancy. A competing hypothesis is that these genes have important ecological functions in natural environments that are not needed under laboratory settings. Here we discriminate between these hypotheses by competing mice (Mus musculus) whose Hoxb1 gene has been replaced by Hoxa1, its highly conserved paralog, against matched wild-type controls in seminatural enclosures. This Hoxb1A1 swap was reported as a genetic manipulation resulting in no discernible embryonic or physiological phenotype under standard laboratory tests. We observed a transient decline in first litter size for Hoxb1A1 homozygous mice in breeding cages, but their fitness was consistently and more dramatically reduced when competing against controls within seminatural populations. Specifically, males homozygous for the Hoxb1A1 swap acquired 10.6% fewer territories and the frequency of the Hoxb1A1 allele decreased from 0.500 in population founders to 0.419 in their offspring. The decrease in Hoxb1A1 frequency corresponded with a deficiency of both Hoxb1A1 homozygous and heterozygous offspring. These data suggest that Hoxb1 and Hoxa1 are more phenotypically divergent than previously reported and support that sub- and/or neofunctionalization has occurred in these paralogous genes leading to a divergence of gene function and incomplete redundancy. Furthermore, this study highlights the importance of obtaining fitness measures of mutants in ecologically relevant conditions to better understand gene function and evolution.
Keywords: fitness assay, functional redundancy, Hoxa1, Hoxb1, intraspecific competition, subfunctionalization
GENE targeting techniques have led to the phenotypic characterization of thousands of genes across eukaryotes (for reviews see Thorneycroft et al. 2001; Capecchi 2005; Collins et al. 2007) and this characterization continues as this invaluable technology develops (e.g., Meyer et al. 2012; Hsu et al. 2014). However, an estimated 10–15% of mouse genes show minimal to no phenotypic consequences when disrupted (mouse appears normal), despite many having highly conserved sequences (Barbaric et al. 2007). One explanation for these findings is functional redundancy—genes throughout the genome, typically paralogs of disrupted genes, code for the same, or at least overlapping, functions (Nowak et al. 1997; Kafri et al. 2009). A competing explanation for “no phenotype” gene disruptions is that these genes have important ecological functions in natural environments that are not needed, or are of minimal importance, within laboratory settings. Here we discriminate between these hypotheses by using mice that have experienced a manipulation previously reported to have no embryonic or physiological phenotype, wherein the coding sequence of the Hoxb1 gene has been replaced by that of its paralog Hoxa1 (Tvrdik and Capecchi 2006).
The traditional explanation for why redundant genes cannot be maintained over evolutionary time is because accumulation of degenerative mutations, leading to nonfunctionalization, will occur within the genome (Ohno 1970). However, incomplete or partial redundancy could result through several mechanisms: convergent evolution of unrelated genes, recent duplications that have not accumulated enough mutations to be completely nonfunctional, duplicates that have taken on new, but similar, functions through neofunctionalization, and through a process known as subfunctionalization (for reviews see Prince and Pickett 2002; Innan and Kondrashov 2010). Subfunctionalization mediated through the duplication–degeneration–complementation (DDC) model, which predicts that degenerative mutations in regulatory elements increase duplicate gene preservation by partitioning ancestral functions, has been invoked as the most likely explanation for the maintenance of paralogous Hox genes and experiments demonstrating functional redundancy between Hox mutants have been used to support the DDC model (Force et al. 1999). Hox genes encode proteins that act as transcription factors for cellular specification and have undergone two duplication events in tetrapods from the ancestral chordate state (Gehring and Hiromi 1986; Levine and Hoey 1988; Manley and Capecchi 1997, 1998; Chen et al. 1998; Chen and Capecchi 1999; Manzanares et al. 2000). Hox genes are phylogenetically conserved, especially regarding their collinear order and the DNA-binding homeodomains of their proteins (McGinnis and Krumlauf 1992; Lutz et al. 1996; Rijli and Chambon 1997).
Hoxa1 and Hoxb1 are involved in the patterning of the brainstem. At the open neural tube stage, both genes display a similar expression pattern, encompassing the posterior and central hindbrain. At later stages [embryonic day 9.5 (E9.5) in the mouse], the expression of Hoxa1 declines, while Hoxb1 becomes strongly activated in the central segment of the developing hindbrain, the rhombomere 4 (Tvrdik and Capecchi 2006). This activation is dependent on an autoregulatory loop resulting from binding of the HoxB1 protein to its own unique enhancer (Popperl et al. 1995). Hoxb1 expression in r4 persists until E13 and modulates neurogenesis in this segment. In the Hoxa1 mutant mouse, expression of Hoxb1 and other downstream genes are altered, the brainstem respiratory circuits are malformed, and Hoxa1 newborn mutants die of apnea (del Toro et al. 2001; Tvrdik and Capecchi 2006). The Hoxb1 mutant, on the other hand, is viable but displays facial paralysis due to the absence of the seventh cranial nerve originating from rhombomere 4 (Goddard et al. 1996). In humans, homozygous missense mutations in HOXB1 cause bilateral facial palsy, hearing loss, and strabismus, correlating extensively with the mouse Hoxb1 null phenotype (Webb et al. 2012). Homozygous HOXA1 mutations, which have occurred in several human populations, are viable but cause either Bosley-Salih-Alorainy syndrome or Athabascan brainstem dysgenesis syndrome (Bosley et al. 2008; Bertrand et al. 2011). Surprisingly, homozygous Hoxb1A1 swapped mice (i.e., mice expressing HoxA1 protein from both Hoxb1 alleles, with no expression of HoxB1) show no detectable phenotypic change relative to wild type under laboratory conditions despite a 15% amino acid sequence difference at the homeodomains and a mere 49% identity overall (Remacle et al. 2004; Tvrdik and Capecchi 2006). In hemizygous animals, which express only one Hoxb1A1 swapped allele over Hoxb1 null, fewer facial motor neurons are generated, resulting in hypomorphism of the seventh cranial nerve. However, the homozygous Hoxb1A1/A1 swaps never displayed facial paralysis and the allele segregated normally in the laboratory population (Tvrdik and Capecchi 2006). Thus, the laboratory phenotypic assessment suggested that if expressed at sufficient levels, either protein could correctly execute the developmental program carried out by the other paralog.
To determine if mice homozygous for the Hoxb1A1 swap suffer from cryptic negative phenotypes we utilized organismal performance assays (OPAs). Within OPAs, treatment and control mice compete directly for resources, territories, and mates under seminatural conditions. Mouse fitness is largely based upon intraspecific competition and can be measured directly in terms of reproductive success or indirectly through key fitness components such as survival and competitive ability. OPAs have previously been used to detect and quantify fitness costs of both cousin- and sibling-level inbreeding, the cost of bearing a selfish genetic element (t complex), and health consequences of added sugar consumption and pharmaceutical exposure (Meagher et al. 2000; Carroll et al. 2004; Ilmonen et al. 2008; Ruff et al. 2013, 2015; Gaukler et al. 2015). In all cases, OPAs revealed major fitness deficiencies that analyses with conventional, laboratory-based methods failed to detect.
Here we use OPAs to test if mice homozygous for Hoxb1A1 express adverse phenotypes relative to matched, wild-type controls. OPA endpoint measures include survival, male competitive ability, and reproductive success (measured in terms of both allelic frequencies and genotypic counts of offspring born within OPA enclosures). Corresponding laboratory measures of reproductive success are assessed by comparing litter sizes from Hoxb1A1 homozygotes and heterozygotes to genetically matched wild-type controls and by analyzing genotypic frequencies of offspring produced in Hoxb1A1 heterozygous breedering cages for anomalies. If no differences are observed between Hoxb1A1 and control mice then near-complete functional redundancy at both the proximate and the ultimate level will be supported. However, if OPAs reveal differential gene function at the ultimate (i.e., fitness) level, then previous measures of functional redundancy based on proximate measures will have been overestimated, highlighting the importance of a naturalistic environment when quantifying differential performance between mutants and controls.
Materials and Methods
Animals
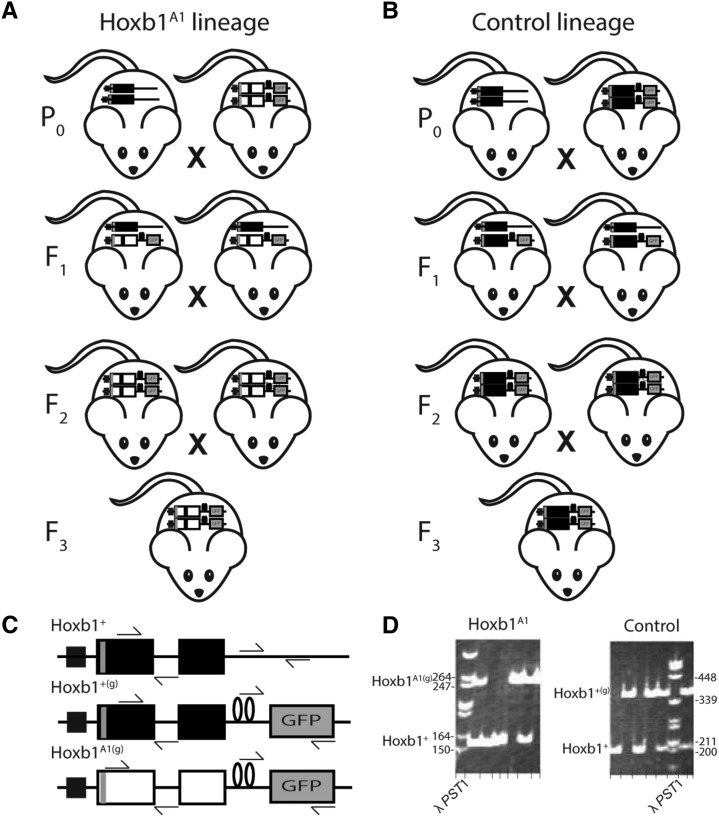
Many laboratory strains of mice do not possess the natural and functional behaviors required for OPA assessment (Manning et al. 1992; Nelson et al. 2013); therefore, suitable mice with the Hoxb1A1 swap and an appropriate control had to be generated (Figure 1). Specifically, a Hoxb1A1 treatment lineage was bred starting with 16 Hoxb1A1(g)/A1(g)-harboring 129 × C57BL/6 hybrid mice, generated by homologous recombination in 129 R1 ES cells (see reference Tvrdik and Capecchi 2006 for a detailed description), were bred to genetically diverse wild-derived mice and the resulting Hoxb1A1(g)/+ heterozygotes (F1) were crossed (n = 93) to establish the next (F2) generation (Figure 1A). Progeny were genetically screened and only Hoxb1A1(g)/A1(g) individuals were selected as OPA founders and are hereon referred to as Hoxb1A1 founders. Three OPA populations were established with these F2 animals and three more were established with F3 animals produced from F2 Hoxb1A1(g)/A1(g) homozygous breeding pairs (n = 16). A control lineage of animals was bred to rule out potential confounding effects due to differential genetics surrounding the swapped region. To achieve this, 12 Hoxb1+(g)/+(g) 129 × C57BL/6 hybrid mice were crossed with the same wild stock used in the Hoxb1A1 treatment lineage. Hoxb1+(g)/+(g) 129 × C57BL/6 hybrid mice were generated in the same manner as the Hoxb1A1 swaps and tagged with the same internal ribosome entry site (IRES)–τ-GFP marker, but expressing the normal HoxB1 protein from the Hoxb1 locus. The resulting F1 Hoxb1+(g)/+ generation (n = 55) were then crossed to produce the F2 generation (Figure 1B). Only Hoxb1+(g)/+(g) mice were selected as control OPA founders and are hereafter referred to as controls. Three OPA populations were established with these F2 animals and three more were established with F3 animals produced from F2 Hoxb1+(g)/+(g) homozygous breeders (n = 12). Therefore, except for the Hox gene region of interest, both Hoxb1A1 and control founders had the same background genetics on average, since other parts of the genome were expected to segregate randomly. Wild-derived animals were from the eighth generation of the colony originally described by Meagher et al. (2000). All P0, F1 animals as well F2 and F3 animals (prior to OPA release) were housed according to standard protocols under a 12:12 h light:dark cycle with food and water available ad libitum. All protocols were approved by the animal care guidelines of the Institutional Animal Care and Use Committee at the University of Utah.
Figure 1.
Breeding design for production of Hoxb1A1 and control founders. (A) To produce animals bearing Hoxb1A1swaps that also possess the functional behaviors needed for OPAs, Hoxb1A1(g)/A1(g) 129 × C57BL/6 mice were bred to outbred, wild-derived mice. The resulting Hoxb1A1(g)/+ heterozygotes were crossed to establish the next (F2) generation. Progeny were genetically screened and only Hoxb1A1(g)/A1(g) individuals were selected as OPA founders. Three OPA populations were established with these F2 animals and three more were established with F3 animals produced from F2 Hoxb1A1(g)/A1(g) homozygous breeding pairs. (B) To control for potential confounding effects due to differential genetics surrounding the swap control animals were bred by crossing Hoxb1+(g)/+(g) 129 × C57BL/6 mice with the same wild stock used in the Hoxb1A1 treatment lineage. The Hoxb1+(g)/+ were then crossed to produce the F2 generation. Only Hoxb1+(g)/+(g) mice were selected as OPA founders. Three OPA populations were established with these F2 animals and three more were established with F3 animals produced from F2 Hoxb1+(g)/+(g) homozygous breeders. (C) Illustrations of wild type (Hoxb1+), wild type with the IRES–τ-GFP tag (Hoxb1+(g)), and Hoxb1A1 swap with the IRES–τ-GFP tag (Hoxb1A1(g)) are provided. Large rectangles represent exons 1 and 2 of Hoxb1 (black) and Hoxa1 (white). The Hoxb1 promoter is conserved across all genotypes and solid squares represent the Hoxb1 autoregulatory enhancer. Loops separating the τ-GFP tag from the second exon depict the IRES. Arrows approximate primer binding sites. Illustrations are not to scale. (D) Image of polyacrylamide gel discrimination between Hoxb1A1(g) and Hoxb1+ (left) and between Hoxb1+(g) and Hoxb1+(right) alleles.
Genotyping
Hoxb1 genotype was determined using a three primer PCR amplification system where a 3′ common primer (5′-AAA TAT CTG CTG ACT TGA ACC C) anneals between exons 1 and 2 within the bridging intron and specific 5′ primers, which anneal within exon 1—for Hoxb1+/+ (5′-GAG TGT GAT CAC GAT CGT GAA AC) and for Hoxb1A1/A1 (5′-AAT AAC TCC TTA TCC CCT CTC C)—yield a 157-bp and 258-bp fragment, respectively. These amplicons were visualized on 5% polyacrylamide gels (Figure 1D). Likewise, to distinguish between τ-GFP-tagged wild-type and true wild-type individuals a similar genotyping system was used. A 3′ common primer (5′-CCA TCA ATC ATC CCT CCA CC) and a 5′-specific primer for Hoxb1+(g)/+(g) (5′-ACA ACC ACT ACC TGA GCA CC) located within the τ-GFP site and for Hoxb1+/+ (5′-TCC ATC ACC TCT TGA ATT GAA C), located 5′ of where the τ-GFP insertion within animals possessing it, yield a 366-bp and 204-bp fragment, respectively, which were visualized on 5% polyacrylamide gels (Figure 1D).
A combination of both genotyping systems was used to genotype all F2 progeny, F2 and F3 founders, and all pups from OPA enclosures. A total of 1145 genotypes were obtained for 1155 F2 offspring for a success rate of 99.1%. Similarly, all F2 and F3 OPA founders’ genotypes were confirmed before release. Regarding OPA pups, 1145 genotypes of the 1194 individuals were determined representing a success rate of 95.9%.
OPA enclosures
OPA enclosures are 30 m2 and are subdivided into six subsections by wire mesh to create environmental complexity and promote territory formation. Subsections have food and water sources provided ad libitum that are associated with a set of nest boxes in either one of the four “optimal” territories (with enclosed nest boxes) or two “suboptimal” territories (with exposed nest boxes). Photographs of OPA enclosures and detailed descriptions may be found elsewhere (Ruff et al. 2013, 2015; Gaukler et al. 2015). OPAs are designed to promote natural mouse mating behavior wherein males compete for territories, a limited resource, which attracts high-quality females; these competitive interactions structure the base unit of house mouse biology—demes (for reviews see Sage 1981; Berdoy and Drickamer 2007).
Six independent OPA enclosures were founded by populations of 28–30 individuals, 8–10 males and 18–20 females for a total of 176 individuals (58 male and 118 female). Populations were created in two sets with three being founded with F2 animals and the remaining three with F3 founders. Equal numbers of Hoxb1A1 and control founders were represented in each sex within all populations. To prevent confounding behaviors associated with relatedness, no male individual was related at the cousin level or above to any other individual within a given population. Relatedness between female founders was also avoided, though sister pairs were included in the second series of populations, which is common in nature. When this was the case, sister pairs were balanced across treatments. Mean age of F2 founders was 37.3 ± 1.2 (M ± SD) weeks for females and 37.5 ± 0.6 weeks for males at the time of release and for F3 founders, females were 33.1 ± 5.8 weeks old and males were 31.4 ± 6.1. To prevent incidental breeding before the establishment of male territories, unmanipulated females were released with the male Hoxb1A1 founders at the onset of each population to allow male territory formation prior to release of female Hoxb1A1 treatment and control founders. After 1 week, the unmanipulated females were removed and the female Hoxb1A1 treatment and control founders released, marking the start (week 1) of the study. OPA populations were maintained for 25 weeks.
OPA measures
Survival:
Survivorship of population founders was determined by periodic checks in each enclosure. Dead founders were identified by passive integrated transponder (PIT) tags and personalized ear markings. Date of death was estimated based on three factors: date of last check, the last date an animal was recorded feeding, and corpse condition. To avoid altering territorial dynamics and influencing infanticide, researchers entered OPAs only to rotate PIT-tag readers between pens, refresh food and water, and conduct pup sweeps (described in paragraph below). Corpses were therefore collected in a variety of conditions that precluded necropsies.
Reproductive success:
To determine founder reproductive success, tissue samples were gathered during “pup sweeps” in which pups born during the previous cycle were removed from the population. Sweeps occurred every 5 weeks to prevent offspring born in enclosures from breeding. In all six populations, five pup sweeps occurred. A total of 1194 individual samples were collected with 199.7 ± 70.6 (M ± SD) per population. Population level reproductive success was determined for Hoxb1A1 and control founders, using the genotyping technique described above.
Male competitive ability:
One week prior to entrance, founders of both sexes were implanted with unique PIT tags (TX1400ST, BioMark, Boise ID). A set of PIT antennae and readers (FS2001F-ISO, BioMark) were rotated through the populations throughout the study and placed at each of the feeders; data were streamed to a computer equipped with data-logging software (Minimon, Culver City, CA). Readers were rotated between populations as only two sets of readers were available, and more than two populations were running concurrently. As dominant males do not tolerate competitors within their territories, dominance was assigned when a male had >80% of all male PIT-tag reads at a single location over the course of a multiday (minimum of 3 day) reader session. Thus, paired measurements of the number of territories controlled by Hoxb1A1 and control males were gathered for each population multiple times throughout the 25-week study. Female behavioral data were also acquired, but are not presented here.
Statistical methods
Breeding cage measures:
To assess for genotype frequency differences in Hoxb1A1 treatment and control lineage heterozygote breeding cages, comparisons were made between the specific homozygotes and between the summed homozygote vs. heterozygote counts. As reproduction data are discrete counts, we modeled offspring counts within the first litter using a generalized linear mixed model (GLMM) with a Poisson distribution and a logarithmic link. Genotype was modeled as a fixed effect while individual breeding cage was modeled as a random effect as two measures were taken from a single breeding cage. Both models for Hoxb1A1 treatment lineage breeding cages were based on 186 observations from 93 breeding cages, while models for control lineage breeding cages were based on 110 observations from 55 cages. This method of analysis was selected as individual pups can be grouped by breeding cage and it is appropriate for the Poisson distribution of litter count data.
As only one measure per breeding cage was used to compare litter sizes between Hoxb1A1 treatment and control lineage heterozygous breeding cages, a Mann–Whitney U-test was conducted. To compare litter sizes between Hoxb1A1 treatment and control lineage homozygous breeders, multiple litters (up to three) from individual breeding cages were used, therefore a GLMM assuming a Poisson distribution and using logarithmic link was based on 75 observations from 28 breeding cages. The model predicts litter size with the main effects of genotype, litter parity, and their interaction on litter size. Breeding cage was modeled as a random effect with a random slope generated for each.
OPA survival:
Survivorship of the 176 OPA founders was analyzed by Cox proportional hazard models. Week one was defined as when Hoxb1A1 and control female founders entered OPA enclosures. A multivariate model was used to assess the effects of genotype, population, and their interaction on survival. Individuals that survived the duration of the trial or that were removed from the study were censored. There were 30 mortality events and 146 censorings.
Male competitive ability:
To assess the effects of genotype, time, and time-by-genotype interaction on male competitive ability, we used a GLMM to predict the probability of territory ownership. As a territory can only be defended or not, we used a binomial distribution with a logit link. Territorial control within populations by each genotype was assessed multiple times throughout the study for a total of 124 observations. The intercept of the model was set at the grand mean (week 13.44). Time, genotype, and their interaction were treated as fixed effects and population was modeled as a random effect with a random intercept calculated for each.
OPA allele frequencies:
A linear mixed-effects model (LMM) was used to assess the frequencies of Hoxb1A1(g) and Hoxb1+(g) in the offspring of OPA founders across the five pup sweeps. The model predicted the main effects of allele [Hoxb1A1(g) vs. Hoxb1+(g)], time, and their interaction on allele frequency across the six populations. Paired gene frequencies were predicted five times at 5-week intervals for a total of 60 observations. Time, allele, and the interaction, were modeled as fixed effects and population was modeled as a random effect, to control for repeated measures with a random intercept and slope calculated for each. The intercept was set at the grand mean (week 15).
OPA genotypic counts of offspring:
As reproduction data are discrete counts we modeled offspring counts over time in a GLMM with a Poisson distribution and a logarithmic link. We predicted population-level fitness across the six populations by modeling the main effects of genotype [Hoxb1A1(g)/A1(g) vs. Hoxb1+(g)/+(g)], time, and their interaction. Offspring genotypes were measured five times at 5-week intervals for a total of 60 observations. Time, genotype, and the interaction were modeled as fixed effects and population was modeled as a random effect with a random intercept. The intercept was set at the grand mean (week 15).
To test for a deficiency (or excess) of heterozygotes within OPAs, a GLMM with the same intercept and distribution as in the specific homozygote comparison was used. However, four distinct genotype groups were assessed: observed heterozygotes, summed homozygotes, and the expected (2×) count of heterozygotes based upon each of the homozygote counts; 30 observations were available for each. Time, genotype, and their interaction were modeled as fixed effects and population was modeled as a random effect with a random intercept and slope.
All mixed-effects models were fit in R using the lmer or glmer function of the “lme4” library (Bates et al. 2014; R Development Core Team 2015). For all mixed-effects models several candidate models for the random effects terms were generated, including models estimating both intercept and/or slope for random effects. In all cases the model that explained at least some of the variance with random effects and had the lowest Akaike’s information criterion score was selected. Degrees of freedom and resulting P values for LMMs were determined with a Satterthwaite approximation using the lmerTest library (Kuznetsova et al. 2014). Estimating degrees of freedom in LMMs remains controversial, but all effects deemed statistically significant on the basis of P values also possess a t value >|2|. This conservative criterion for significance is recommended by the library’s authors. All α-values were 0.05 and tests were two tailed.
Data availability
All pertinent data from breeding cage and OPA studies presented in this manuscript are available in File S1. This Includes litter size and pup genotypes from Hoxb1A1 treatment and control lineage breeding cages, male competitive ability within OPAs, genotypic counts of offspring born within OPAs, and survival data for OPA founders.
Results
No deficiencies of homozygous or heterozygous F2 offspring were observed from F1 heterozygous Hoxb1A1 treatment or control lineage breeding cages; however, litter sizes were larger in Hoxb1A1 treatment breeding cages (Table 1). In F1 Hoxb1A1 treatment lineage heterozygous [Hoxb1A1(g)/+] breeding cages (n = 93), no deficiency of Hoxb1A1(g)/A1(g) homozygotes was observed relative to the count of Hoxb1+/+ offspring (GLMM; Z = 1.12, P = 0.262) and no deficiency of Hoxb1A1(g)/+ heterozygotes was seen relative to the number of summed homozygotes (GLMM; Z = 0.44, P = 0.663). In Hoxb1+(g)/+ control lineage breeding cages (n = 55) no deficiency of Hoxb1+(g)/+(g) homozygotes was observed relative to Hoxb1+/+ (GLMM; Z = −1.64, P = 0.101) and no deficiency of heterozygotes was observed (GLMM; Z = −0.36, P = 0.723). However, litter sizes from the Hoxb1A1 treatment lineage heterozygous breeding cages were larger (8.20 ± 0.20; M ± SEM) than from control lineage breeding cages (7.13 ± 0.35) (Mann–Whitney; U = 1851, P = 0.005). For mixed model results see Supporting Information, Table S1.
Table 1 Summary of genotypic counts and litter sizes of Hoxb1A1 treatment and control lineage breeding cages.
| Lineage | Design (N) | Mutant homozygotes | Wild-type homozygotes | Heterozygotes | Totala |
|---|---|---|---|---|---|
| F1 heterozygous breeding cages | |||||
| Hoxb1A1 treatment | Hoxb1A1(g)/+ × Hoxb1A1(g)/+ (55) | 2.0 ± 0.1b | 2.2 ± 0.2 | 4.0 ± 0 2 | 8.2 ± 0.2A |
| Control | Hoxb1+(g)/+ × Hoxb1+(g)/+ (93) | 1.9 ± 0.2 | 1.5 ± 0.2 | 3.6 ± 0 3 | 7.1 ± 0.4B |
| F2 homozygous breeding cages | |||||
| Hoxb1A1 treatment | Hoxb1A1(g)/A1(g) × Hoxb1A1(g)/A1(g) (16) | 4.7 ± 0.5 | 4.7 ± 0.5A | ||
| Control | Hoxb1+(g)/+(g) × Hoxb1+(g)/+(g) (12) | 7.7 ± 0.1 | 7.7 ± 0.1B | ||
Hoxb1A1 treatment lineage F2 homozygous breeders produced fewer F3 offspring, at least initially, than did homozygous control lineage breeders (Table 1). In first litters (the model intercept) Hoxb1A1(g)/A1(g) breeders produced 4.66 (+0.52, –0.46; M ± SEM) offspring per breeding cage, while Hoxb1+(g)/+(g) homozygous control breeders produced 7.66 (+1.18, –1.02) offspring per breeding cage (GLMM; Z = 3.46, P = 0.001); asymmetric SEMs are reported as they are back transformed from logarithmic values. However, it was observed that control lineage breeders had decreased rates of reproduction in progressing litters (GLMM; Z = −3.01, P = 0.003) and with post hoc Mann–Whitney tests it was confirmed that there was only a significant (P < 0.05) difference between breeding groups during the first litter. For mixed model results see Table S2.
Within OPA enclosures no difference in survival was observed between Hoxb1A1 and control founders [proportional hazards (PH); χ2 = 0.002, P = 0.964; Figure S1). Furthermore, survival did not differ among OPA populations (PH; χ2 = 4.21, P = 0.519), nor did the effect of genotype differ by population (PH; χ2 = 7.76, P = 0.170).
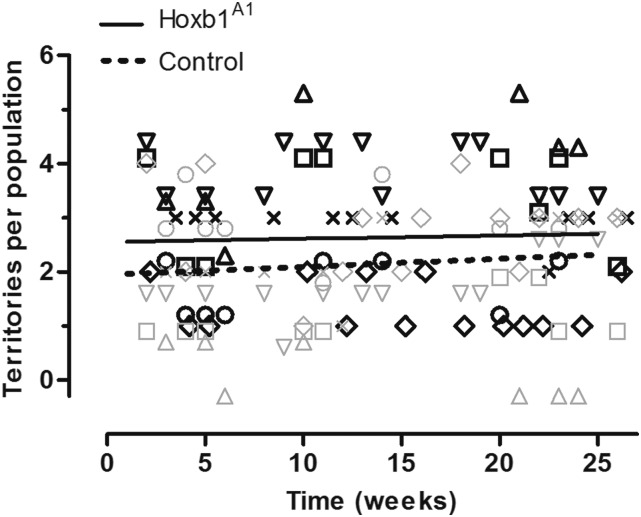
The probability of territorial ownership was lower for male Hoxb1A1 founders than for control founders (Figure 2). At the model intercept (week 13.44), the probability that a territory was dominated by a male Hoxb1A1 founder was 44.7%, while for controls it was 55.3% (GLMM; Z = 2.56, P = 0.010). Neither time (GLMM; Z = 0.318, P = 0.751), nor genotype by time affected territorial acquisition (GLMM; Z = −0.45, P = 0.653), indicating that the competitive disadvantage of male Hoxb1A1 founders persisted over the course of the study. For mixed model results see Table S3.
Figure 2.
Competitive ability of Hoxb1A1 and control founders in OPAs. A 10.6% reduction in the probability of territorial ownership of Hoxb1A1 founders was observed relative to control founders (GLMM; Z = 2.56, P = 0.010). Competitive ability of both groups was assessed at multiple time points across populations (n = 6) for a total of 124 observations. Provided lines are simple linear regressions based upon raw data to help illustrate overall trends. Observations from each population are demarcated by shape and paired at each time point (solid outline for control and shaded outline for Hoxb1A1 founders) and cannot sum to more than six, as this is the maximum number of territories per population. If more than one population was assessed at a given time, then data points from multiple populations appear in a column; vertical scatter was added to prevent overlap and aid in visualization.
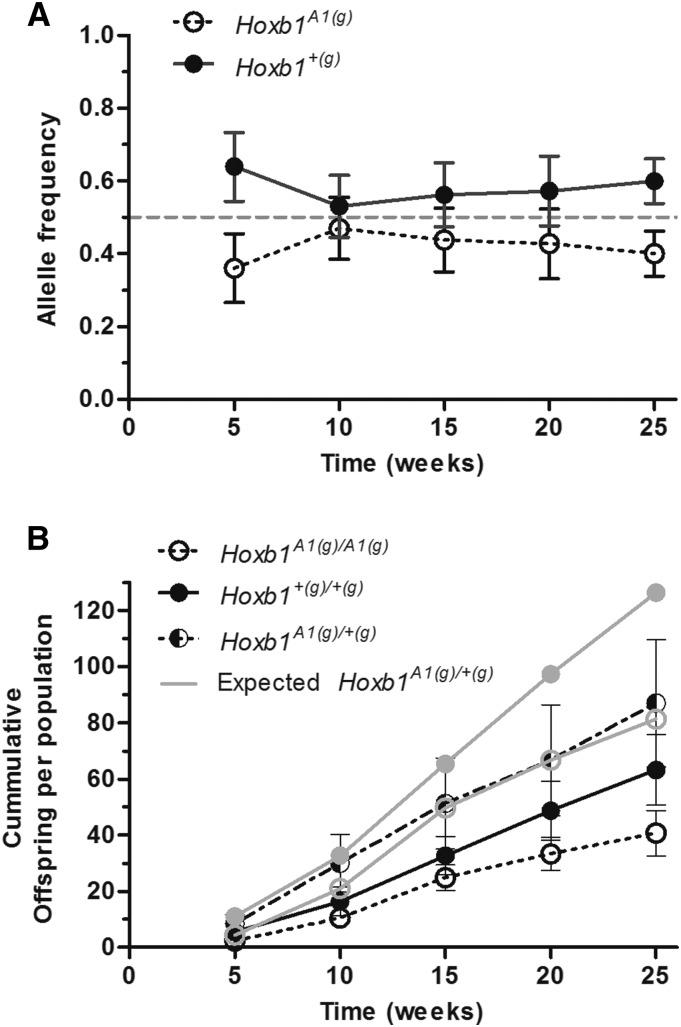
The Hoxb1A1(g) allele was selected against within OPA enclosures. The initial frequency of Hoxb1A1(g) was 0.500 in population founders; however, in offspring born within OPAs, this frequency was reduced to 0.419 ± 0.037 (M ± SEM), resulting in a selection coefficient (s) of 0.162 (Figure 3A). This difference was found to be statistically significant (LMM; t = 3.102, P = 0.003). For mixed model results see Table S4.
Figure 3.
Allele frequencies (A) and genotypes of offspring born within OPAs (B). (A) The Hoxb1A1(g) allele was selected against (s = 0.162) within OPAs as the frequency of the mutant allele decreased in offspring born to population founders (LMM; t = 3.10, P = 0.003). Founders possessed the mutant allele at a frequency of 0.500 (signified by the shaded dashed line). (B) Hoxb1A1 founders had 64.4% of the reproduction achieved by controls as measured by homozygous offspring within OPA enclosures (GLMM; Z = 3.52, P < 0.001). Likewise, a 16.2% deficiency of Hoxb1A1(g)/+(g) offspring was observed as compared to summed homozygotes (GLMM; Z = 2.66, P = 0.008). The observed number of heterozygotes was lower than expected levels based on Hoxb1+(g)/+(g) homozygote counts (GLMM; Z = 6.01, P < 0.001; shaded line with closed circles), but did not differ from those predicted by counts of Hoxb1A1(g)/A1(g) pups (shaded line with open circles). All genotypes were assessed at multiple times across populations (n = 6) for a total of 30 observations. Black lines connect population means and error bars represent standard error.
Hoxb1A1 founders contributed only 64.4% of the reproduction enjoyed by controls as measured by homozygous offspring (Figure 3B). At the model intercept (week 15), 7.82 (+0.90, –0.80) Hoxb1A1(g)/A1(g) offspring per population were produced in OPAs, while 12.18 (+0.81, –1.20) Hoxb1+(g)/+(g) offspring per population were produced (GLMM; Z = 5.03, P < 0.001). Both groups of founders increased reproductive output over time (GLMM; Z = 2.84, P = 0.005), and as there was no interaction between time and genotype (GLMM; Z = 0.951, P = 0.342) the decreased reproduction of Hoxb1A1 founders at the intercept was maintained throughout the study. For mixed model results see Table S4.
A 16.2% deficiency of Hoxb1A1(g)/+(g) heterozygotes was also observed in OPAs compared to the observed number of summed homozygotes (Figure 3B; GLMM; Z = 2.66, P = 0.008). At the model intercept (week 15), 16.74 (+1.70, –1.55) Hoxb1A1(g)/+(g) offspring per population were produced, while 19.65 (+1.22, –1.15) homozygous offspring were produced. As expected from the homozygote comparisons, both the number of heterozygotes and homozygotes produced increased over time (GLMM; Z = 2.40, P = 0.016). There was no time-by-genotype interaction (GLMM; Z = 1.48, P = 0.139), indicating that the decreased production of heterozygotes present at the intercept lasted throughout the study. The observed numbers of heterozygotes produced in OPAs are lower than those expected based on the counts of Hoxb1+(g)/+(g) offspring (GLMM; Z = 6.01, P < 0.001), but do not differ from expected levels predicted by observed Hoxb1A1(g)/A1(g) homozygotes (GLMM; Z = −1.16, P = 0.246). For mixed model results see Table S4.
Discussion
Within breeding cages, no deleterious effects of possessing a single Hoxb1A1(g) allele (i.e., being heterozygous) were observable, but being homozygous did decrease the size of first litters in the Hoxb1A1 treatment lineage. In Hoxb1A1 treatment and control lineage heterozygous breeding cages no deficiency of either Hoxb1A1(g)/A1(g) or Hoxb1+(g)/+(g) homozygotes was observed relative to the wild type (Hoxb1+/+), and no deficiency of heterozygotes was detected in relation to the homozygous offspring. This indicates that neither the IRES–τ-GFP-tagged control nor the IRES–τ-GFP-tagged Hoxb1A1 swap contributed to embryonic mortality. However, litters from Hoxb1A1 treatment lineage breeding cages with Hoxb1A1(g)/+ heterozygous pairs were larger than those from control lineage breeding cages (Hoxb1+(g)/+), suggesting that animals heterozygous for the Hoxb1A1(g) swap were more fit, at least in the breeding cage environment, than those bearing the control allele. Conversely, Hoxb1A1 treatment lineage homozygous Hoxb1A1(g)/A1(g) pairings produced fewer offspring than did mice in Hoxb1+(g)/+(g) control lineage breeding cages; this effect was only observed in first litters and disappeared in subsequent litters. The findings from heterozygous breeders and from all but the first litter of homozygous breeders are in accordance with previous investigations of this transgenic line that support a near-complete degree of functional redundancy between these paralogous genes (Tvrdik and Capecchi 2006); however, the decreased size of first litters experienced by Hoxb1A1(g)/A1(g) breeders argues for subtle reproductive impairment in these mice.
Within OPAs, Hoxb1A1 founders were outcompeted by control founders as measured by competitive ability and fitness. Specifically, male Hoxb1A1 founders were less likely to acquire a territory than control founders and the frequency of the Hoxb1A1(g) allele decreased from 0.500 in population founders to 0.419 in their offspring. The declining allelic frequency of Hoxb1A1(g) is driven by decreased reproduction of Hoxb1A1 founders who only produced 64% as many offspring as control founders, as measured by homozygous offspring. It is likely that the decreased competitive ability of Hoxb1A1 male founders contributed to the marked decrease in reproduction. It has been shown that dominant males sire the majority (∼80%) of pups within OPA enclosures (Carroll et al. 2004); however, as the discrepancy in territorial acquisition was small (∼10%), decreased competitive ability is insufficient to explain the observed differences in reproduction. Furthermore, the measures of decreased performance of Hoxb1A1 founders should be considered robust, as the high level of genetic diversity in wild-derived mice should make the influence of the Hoxb1A1 swap more difficult to detect.
A possible mechanism for the decreased reproductive success of Hoxb1A1 founders within OPAs is embryonic lethality, especially as Hox genes are of critical developmental importance. However, lethality is not supported by breeding cage data, which indicate equal frequencies of Hoxb1A1(g)/A1(g) and wild-type homozygotes. Litter sizes were reduced from Hoxb1A1 treatment lineage homozygous [Hoxb1A1(g)/A1(g)] breeding cages relative to homozygous [Hoxb1+(g)/+(g)] control lineage breeding cages similar to the degree observed in OPAs. However, as this effect was only present in first litters, it cannot explain the decreased reproductive success of Hoxb1A1 founders across the 25-week study. It is possible that the natural stressors present within OPAs could exacerbate embryonic or early neonatal death or reproductive impairment, as has been seen with caloric restriction, territorial instability, and increased exposure to pathogens, all of which are elevated within OPAs relative to standard breeding cages (Bruce 1959; Rivers and Crawford 1974; Ilmonen et al. 2008).
Nonrandom mating has been observed in OPAs previously and could also explain the reproductive deficit of Hoxb1A1 founders (Potts et al. 1991). Specifically, the deficiency of Hoxb1A1(g)/A1(g) homozygotes and Hoxb1A1(g)/+(g) heterozygous offspring could be explained by control founders mating preferentially with each other, leaving Hoxb1A1 founders to mate randomly with remaining partners. This assertion is supported by the finding that the number of heterozygous pups observed within OPAs match expected values based on the number of Hoxb1A1(g)/A1(g) homozygotes, but not the levels expected based on Hoxb1+(g)/+(g) homozygotes. Additional mechanisms leading to reproductive decline are likely at work in Hoxb1A1 founders, and though they have yet to be determined, the characterization of the organismal phenotype should hasten their discovery.
Though it has been argued that many Hox paralog swaps, including Hoxb1A1, are functionally redundant, data presented here indicate that the degree of functional redundancy has been overestimated by proximate assessments. Though many proximate defects associated with Hoxb1−/− complete knockouts may be masked by the Hoxb1A1(g) swap, the animals fail to achieve equal levels of Darwinian fitness under seminatural conditions and exhibit hints of fitness declines in laboratory cages. This fitness inequality could explain the extreme conservation seen in Hox genes across taxa, including Hoxb1 and Hoxa1, as purifying selection will remove allelic variants from populations when their fitness is lower; variants do not need to possess gross alteration in morphological or behavioral traits to be selected against, but only need to be less fit than alternatives.
Paralogous Hox genes have provided a case study to further understand how duplicated genes can avoid nonfunctionalization and be maintained across evolutionary time, with the leading explanation being subfunctionalization via the DDC model (Prince and Pickett 2002). Examples of functional redundancy between paralogous genes have been cited as evidence for this model, though if subfunctionalization has occurred, one would expect incomplete redundancy. In this case, the level of redundancy is inversely related to the level of subfunctionalization that has occurred. Therefore, our findings, that the degree of redundancy between Hoxb1 and Hoxa1 is lower than previously acknowledged, does not argue against the DDC model, but suggests that there is less overlap in gene function between the paralogs tested than previously thought. Likewise, illustrating decreased redundancy does not necessarily help distinguish between candidate explanations for the maintenance of duplicated genes, such as neofunctionalization or subfunctionalization (either via the DDC model or the escape from adaptive conflict model) (Des Marais and Rausher 2008), but it does argue that for these paralogs, the degree to which these processes have altered gene function is higher than initially conceived.
With OPAs we are able to characterize inequalities between mice homozygous for a Hoxb1A1 swap and control mice that are missed by more traditional proximate investigations. This is likely due to the competitive nature of house mice, which vigorously compete with one another over resources. Differences in physiological performance that are too cryptic, diffuse, or subtle to cause gross defects may nonetheless lower fitness in a competitive environment and this concept has driven the use of fitness assays in Drosophila, RNA virus, and yeast communities (e.g., Shabalina et al. 1997; Thatcher et al. 1998; Lauring et al. 2012); unfortunately, similar approaches have not been adopted by those working with vertebrate model systems (with the notable exception of genes involved in sperm function or competition, e.g., Sutton et al. 2008). In addition to the Hoxb1A1 phenotypes herein, OPAs have revealed adversities associated with three other genetic treatments, including cousin- and sibling-level inbreeding and bearing the selfish genetic element known as the t complex, which had escaped detection for decades (Meagher et al. 2000; Carroll et al. 2004; Ilmonen et al. 2008). Examples such as these give weight to the argument that fitness assays are necessary for functional genomics, especially when confronted with no-phenotype knockouts, swaps, enhancers, deletions, and other mutants (Carroll and Potts 2006).
Supplementary Material
Acknowledgments
We thank M. Capecchi for graciously providing transgenic mice; D. Cornwall for help in figure design; and C. Davis, J. Timm, R. Ussing, and S. Wright for data collection. We also acknowledge two anonymous reviewers whose comments greatly improved this manuscript. This work was supported by National Institutes of Health (NIH) grant R01-GM039578 and was partially conducted while W.K.P was supported by National Science Foundation (NSF) grant DEB 09-18969 and NIH grant R01-GM109500. L.L.R. was supported by the NSF-funded Western Alliance to Expand Student Opportunities.
Footnotes
Communicating editor: B. A. Payseur
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.178079/-/DC1.
Literature Cited
- Bates, D., M. Maechler, B. M., Bolker and S. Walker, 2014 Lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7. Available at: http://CRAN.R-project.org/package=lme4.
- Barbaric I., Miller G., Dear T. N., 2007. Appearances can be deceiving: phenotypes of knockout mice. Brief. Funct. Genomic Proteomic. 6: 91–103. [DOI] [PubMed] [Google Scholar]
- Bertrand N., Roux M., Ryckebusch L., Niederreither K., Dolle P., et al. , 2011. Hox genes define distinct progenitor sub-domains within the second heart field. Dev. Biol. 353: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoy M., Drickamer L. C., 2007. Comparative social organization and life history of Rattus and Mus, pp. 380–392 in Rodent Societies: An Ecological and Evolutionary Perspective, edited by Wolff J. O., Sherman P. W. University of Chicago Press, Chicago. [Google Scholar]
- Bosley T. M., Alorainy I. A., Salih M. A., Aldhalaan H. M., Abu-Amero K. K., et al. , 2008. The clinical spectrum of homozygous hoxa1 mutations. Am. J. Med. Genet. A. 146A: 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce H. M., 1959. An exteroceptive block to pregnancy in the mouse. Nature 164: 105. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., 2005. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat. Rev. Genet. 6: 507–512. [DOI] [PubMed] [Google Scholar]
- Carroll L. S., Potts W. K., 2006. Functional genomics requires ecology. Adv. Stud. Behav. 36: 173–215. [Google Scholar]
- Carroll L. S., Meagher S., Morrison L., Penn D. J., Potts W. K., 2004. Fitness effects of a selfish gene are revealed in an ecological context. Evolution 58: 1318–1328. [DOI] [PubMed] [Google Scholar]
- Chen F., Capecchi M. R., 1999. Paralogous mouse hox genes, hoxa9, hoxb9, and hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc. Natl. Acad. Sci. USA 96: 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Greer J., Capecchi M. R., 1998. Analysis of hoxa7/hoxb7 mutants suggests periodicity in the generation of the different sets of vertebrae. Mech. Dev. 77: 49–57. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Rossant J., Wurst W., 2007. A mouse for all reasons. Cell 128: 9–13. [DOI] [PubMed] [Google Scholar]
- del Toro E. D., Borday V., Davenne M., Neun R., Rijli F. M., et al. , 2001. Generation of a novel functional neuronal circuit in hoxa1 mutant mice. J. Neurosci. 21: 5637–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais D. L., Rausher M. D., 2008. Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature 454: 762–765. [DOI] [PubMed] [Google Scholar]
- Force A., Lynch M., Pickett F. B., Amores A., Yan Y. L., et al. , 1999. Preservation of duplicate genes by complementary, degenerative mutations. Genet. 151: 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaukler S. M., Ruff J. S., Galland T., Kandaris K. A., Underwood T. K., et al. , 2015. Low-dose paroxetine exposure causes lifetime declines in male mouse body weight, reproduction and competitive ability as measured by the novel organismal performance assay. Neurotoxicol. Teratol. 47: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. J., Hiromi Y., 1986. Homeotic genes and the homeobox. Annu. Rev. Genet. 20: 147–173. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Rossel M., Manley N. R., Capecchi M. R., 1996. Mice with targeted disruption of Hoxb-1 fail to form the motor nucleus of the VIIth nerve. Development 122: 3217–3228. [DOI] [PubMed] [Google Scholar]
- Hsu P. D., Lander E. S., Zhang F., 2014. Development and applications of crispr-cas9 for genome engineering. Cell 157: 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmonen P., Penn D. J., Damjanovich K., Clarke J., Lamborn D., et al. , 2008. Experimental infection magnifies inbreeding depression in house mice. J. Evol. Biol. 21: 834–841. [DOI] [PubMed] [Google Scholar]
- Innan H., Kondrashov F., 2010. The evolution of gene duplications: classifying and distinguishing between models. Nat. Rev. Genet. 11: 97–108. [DOI] [PubMed] [Google Scholar]
- Kafri R., Springer M., Pilpel Y., 2009. Genetic redundancy: new tricks for old genes. Cell 136: 389–392. [DOI] [PubMed] [Google Scholar]
- Kuznetsova, A., P. B. Brockhoff and R. H. B. Christensen, 2014 lmerTest: tests in linear mixed effects models. R package version 2.0–20. Available at: http://CRAN.R-project.org/package=lmerTest.
- Lauring A. S., Acevedo A., Cooper S. B., Andino R., 2012. Codon usage determines the mutational robustness, evolutionary capacity, and virulence of an rna virus. Cell Host Microbe 12: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Hoey T., 1988. Homeobox proteins as sequence-specific transcription factors. Cell 55: 537–540. [DOI] [PubMed] [Google Scholar]
- Lutz B., Lu H. C., Eichele G., Miller D., Kaufman T. C., 1996. Rescue of Drosophila labial null mutant by the chicken ortholog hoxb-1 demonstrates that the function of hox genes is phylogenetically conserved. Genes Dev. 10: 176–184. [DOI] [PubMed] [Google Scholar]
- Manley N. R., Capecchi M. R., 1997. Hox group 3 paralogous genes act synergistically in the formation of somitic and neural crest-derived structures. Dev. Biol. 192: 274–288. [DOI] [PubMed] [Google Scholar]
- Manley N. R., Capecchi M. R., 1998. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev. Biol. 195: 1–15. [DOI] [PubMed] [Google Scholar]
- Manning C. J., Potts W. K., Wakeland E. K., Dewsbury D. A., 1992. What’s wrong with MHC mate choice experiments? pp. 229–235 in Chemical Signals in Vertebrates, edited by Doty R. L., Müller-Schwarze D. Plenum, New York. [Google Scholar]
- Manzanares M., Wada H., Itasaki N., Trainor P. A., Krumlauf R., et al. , 2000. Conservation and elaboration of hox gene regulation during evolution of the vertebrate head. Nature 408: 854–857. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R., 1992. Homeobox genes and axial patterning. Cell 68: 283–302. [DOI] [PubMed] [Google Scholar]
- Meagher S., Penn D. J., Potts W. K., 2000. Male-male competition magnifies inbreeding depression in wild house mice. Proc. Natl. Acad. Sci. USA 97: 3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Ortiz O., Hrabe De Angelis M., Wurst W., Kuhn R., 2012. Modeling disease mutations by gene targeting in one-cell mouse embryos. Proc. Natl. Acad. Sci. USA 109: 9354–9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. C., Cauceglia J. W., Merkley S. D., Youngson N. A., Oler A. J., et al. , 2013. Reintroducing domesticated wild mice to sociality induces adaptive transgenerational effects on mup expression. Proc. Natl. Acad. Sci. USA 110: 19848–19853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. A., Boerlijst M. C., Cooke J., Smith J. M., 1997. Evolution of genetic redundancy. Nature 388: 167–171. [DOI] [PubMed] [Google Scholar]
- Ohno S., 1970. Evolution by Gene Duplication, Springer-Verlag, New York. [Google Scholar]
- Potts W. K., Manning C. J., Wakeland E. K., 1991. Mating patterns in seminatural populations of mice influenced by mhc genotype. Nature 352: 619–621. [DOI] [PubMed] [Google Scholar]
- Prince V. E., Pickett F. B., 2002. Splitting pairs: the diverging fates of duplicated genes. Nat. Rev. Genet. 3: 827–837. [DOI] [PubMed] [Google Scholar]
- Popperl H., Bienz M., Studer M., Chan S. K., Aparicio S., et al. , 1995. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell 81: 1031–1042. [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2015 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
- Remacle S., Abbas L., De Backer O., Pacico N., Gavalas A., et al. , 2004. Loss of function but no gain of function caused by amino acid substitutions in the hexapeptide of hoxa1 in vivo. Mol. Cell. Biol. 24: 8567–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijli F. M., Chambon P., 1997. Genetic interactions of hox genes in limb development: learning from compound mutants. Curr. Opin. Genet. Dev. 7: 481–487. [DOI] [PubMed] [Google Scholar]
- Rivers J. P., Crawford M. A., 1974. Maternal nutrition and the sex ratio at birth. Nature 252: 297–298. [DOI] [PubMed] [Google Scholar]
- Ruff J. S., Suchy A. K., Hugentobler S. A., Sosa M. M., Schwartz B. L., et al. , 2013. Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat. Commun. 4: 2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff J. S., Hugentobler S. A., Suchy A. K., Sosa M. M., Tanner R. E., et al. , 2015. Compared to sucrose, previous consumption of fructose and glucose monosaccharides reduces survival and fitness of female mice. J. Nutr. 145: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage, R. D., 1981 Wild mice, pp. 40–90 in The Mouse in Biomedical Research, edited by H. L. Foster, J. D. Small, and J. G. Fox. Academic Press, New York. [Google Scholar]
- Shabalina S. A., Yampolsky L., Kondrashov A. S., 1997. Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proc. Natl. Acad. Sci. USA 94: 13034–13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton K. A., Jungnickel M. K., Florman H. M., 2008. A polycystin-1 controls postcopulatory reproductive selection in mice. Proc. Natl. Acad. Sci. USA 105: 8661–8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher J. W., Shaw J. M., Dickinson W. J., 1998. Marginal fitness contributions of nonessential genes in yeast. Proc. Natl. Acad. Sci. USA 95: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneycroft D., Sherson S. M., Smith S. M., 2001. Using gene knockouts to investigate plant metabolism. J. Exp. Bot. 52: 1593–1601. [PubMed] [Google Scholar]
- Tvrdik P., Capecchi M. R., 2006. Reversal of hox1 gene subfunctionalization in the mouse. Dev. Cell 11: 239–250. [DOI] [PubMed] [Google Scholar]
- Webb B. D., Shaaban S., Gaspar H., Cunha L. F., Schubert C. R., et al. , 2012. Hoxb1 founder mutation in humans recapitulates the phenotype of hoxb1−/− mice. Am. J. Hum. Genet. 91: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All pertinent data from breeding cage and OPA studies presented in this manuscript are available in File S1. This Includes litter size and pup genotypes from Hoxb1A1 treatment and control lineage breeding cages, male competitive ability within OPAs, genotypic counts of offspring born within OPAs, and survival data for OPA founders.