Abstract
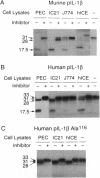
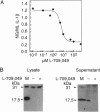
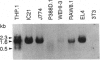
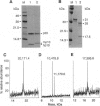
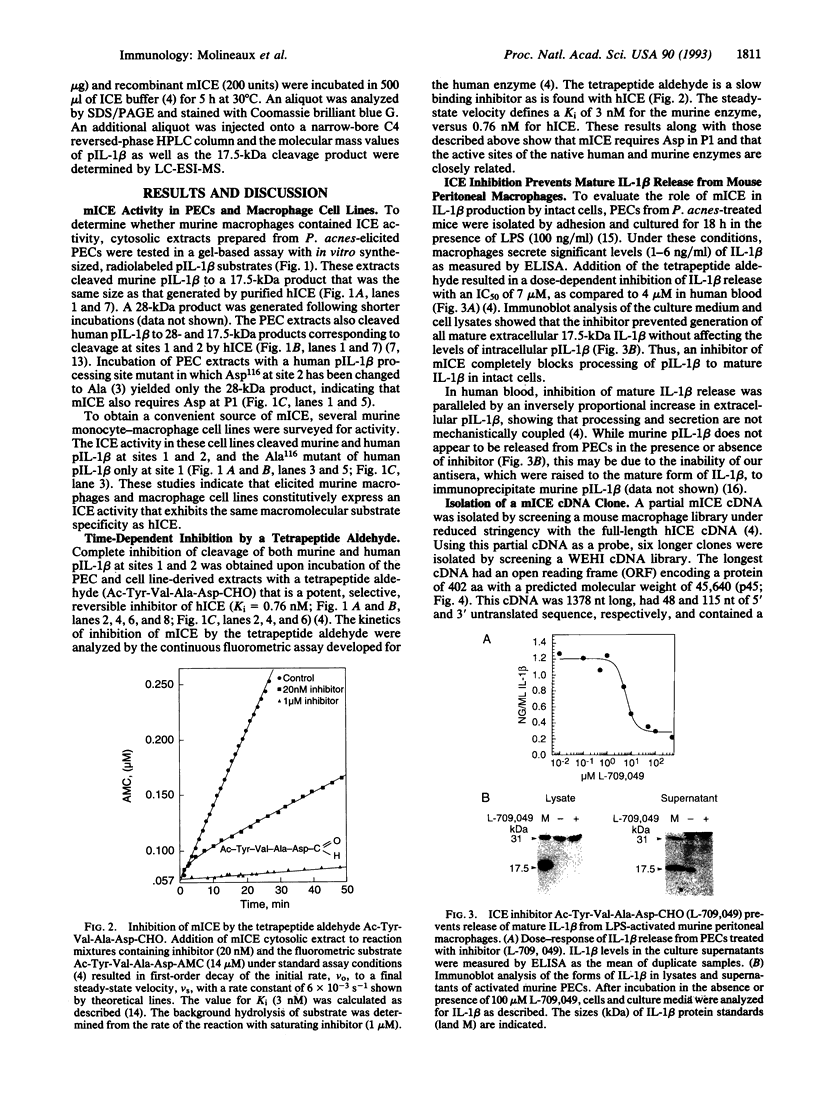
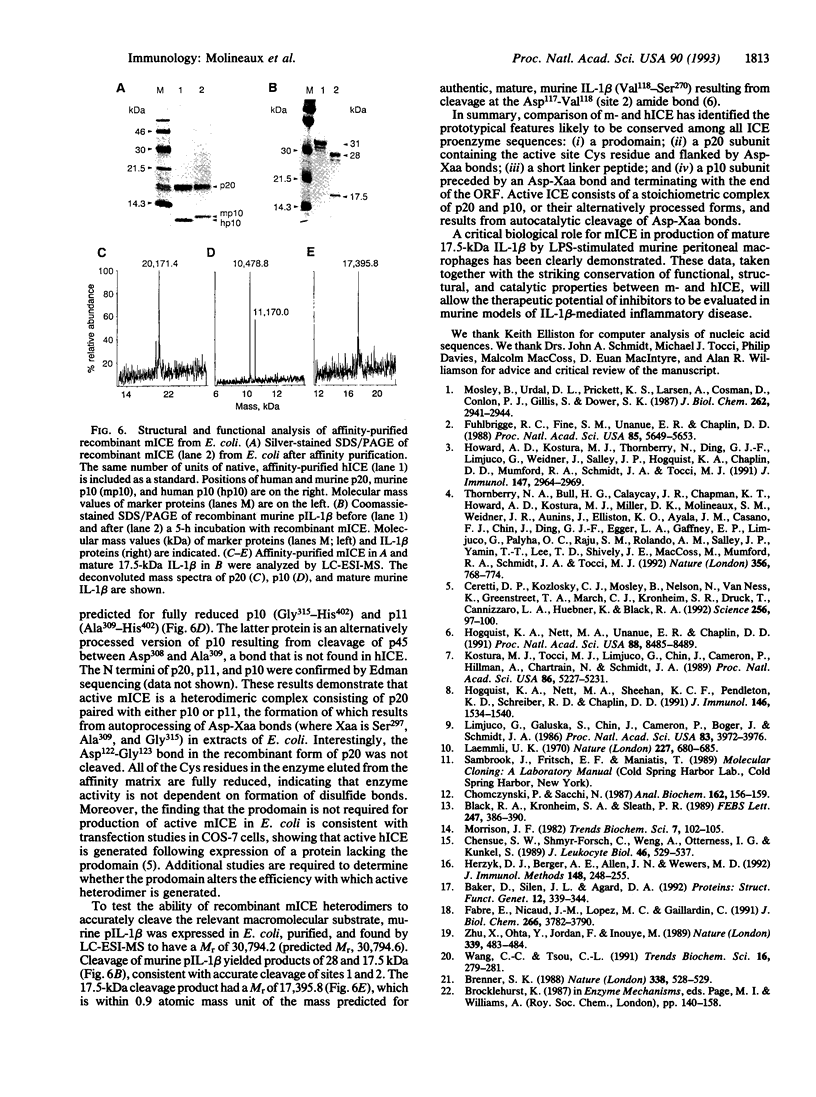
Murine interleukin 1 beta (IL-1 beta) convertase (mICE) was identified in cytosolic extracts of peritoneal exudate cells (PECs) and macrophage cell lines. mICE cleaves both the human and mouse IL-1 beta precursors (pIL-1 beta) at sites 1 and 2 but fails to cleave a human pIL-1 beta (Asp116 to Ala) mutant at site 2, indicating that Asp is required to the left of the scissile bond. Ac-Tyr-Val-Ala-Asp-amino-4-methyl coumarin, patterned after site 2 of human pIL-1 beta, is a fluorogenic substrate for mICE, while the tetrapeptide aldehyde Ac-Tyr-Val-Ala-Asp-CHO is a potent inhibitor (Ki = 3 nM) that prevents generation and release of mature IL-1 beta by PECs (IC50 = 7 microM). Cloning of a full-length 1.4-kb cDNA shows that mICE is encoded as a 402-aa proenzyme (p45) that can be divided into a prodomain (Met1-Asp122), followed by a p20 subunit (Gly123-Asp296), a connecting peptide (Ser297-Asp314), and a p10 subunit (Gly315-His402). At the amino acid level, p45, p20, and p10 are 62%, 60%, and 81% identical with human IL-1 beta convertase (hICE). The active site Cys284 lies within a completely conserved stretch of 18 residues; however, Ser289 in hICE, which aligns with the catalytic region of serine and viral cysteinyl proteases, is absent from mICE. Expression in Escherichia coli of a truncated cDNA encoding Asn119-His402 generated active enzyme, which was autocatalytically processed at three internal Asp-Xaa bonds to generate a p20 subunit (Asn119-Asp296) complexed with either p11 (Ala309-His402) or p10. Recombinant mICE cleaves murine pIL-1 beta accurately at the Asp117-Val118 bond. The striking similarities of the human and murine enzymes will make it possible to assess the therapeutic potential of hICE inhibitors in murine models of disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D., Silen J. L., Agard D. A. Protease pro region required for folding is a potent inhibitor of the mature enzyme. Proteins. 1992 Apr;12(4):339–344. doi: 10.1002/prot.340120406. [DOI] [PubMed] [Google Scholar]
- Black R. A., Kronheim S. R., Sleath P. R. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989 Apr 24;247(2):386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988 Aug 11;334(6182):528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Kozlosky C. J., Mosley B., Nelson N., Van Ness K., Greenstreet T. A., March C. J., Kronheim S. R., Druck T., Cannizzaro L. A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992 Apr 3;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Shmyr-Forsch C., Weng A., Otterness I. G., Kunkel S. L. Biologic and immunohistochemical analysis of macrophage interleukin- 1 alpha, - 1 beta, and tumor necrosis factor production during the peritoneal exudative response. J Leukoc Biol. 1989 Dec;46(6):529–537. doi: 10.1002/jlb.46.6.529. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fabre E., Nicaud J. M., Lopez M. C., Gaillardin C. Role of the proregion in the production and secretion of the Yarrowia lipolytica alkaline extracellular protease. J Biol Chem. 1991 Feb 25;266(6):3782–3790. [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K. A., Nett M. A., Sheehan K. C., Pendleton K. D., Schreiber R. D., Chaplin D. D. Generation of monoclonal antibodies to murine IL-1 beta and demonstration of IL-1 in vivo. J Immunol. 1991 Mar 1;146(5):1534–1540. [PubMed] [Google Scholar]
- Hogquist K. A., Nett M. A., Unanue E. R., Chaplin D. D. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. D., Kostura M. J., Thornberry N., Ding G. J., Limjuco G., Weidner J., Salley J. P., Hogquist K. A., Chaplin D. D., Mumford R. A. IL-1-converting enzyme requires aspartic acid residues for processing of the IL-1 beta precursor at two distinct sites and does not cleave 31-kDa IL-1 alpha. J Immunol. 1991 Nov 1;147(9):2964–2969. [PubMed] [Google Scholar]
- Kostura M. J., Tocci M. J., Limjuco G., Chin J., Cameron P., Hillman A. G., Chartrain N. A., Schmidt J. A. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limjuco G., Galuska S., Chin J., Cameron P., Boger J., Schmidt J. A. Antibodies of predetermined specificity to the major charged species of human interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3972–3976. doi: 10.1073/pnas.83.11.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Urdal D. L., Prickett K. S., Larsen A., Cosman D., Conlon P. J., Gillis S., Dower S. K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987 Mar 5;262(7):2941–2944. [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Tsou C. L. The insulin A and B chains contain sufficient structural information to form the native molecule. Trends Biochem Sci. 1991 Aug;16(8):279–281. doi: 10.1016/0968-0004(91)90114-b. [DOI] [PubMed] [Google Scholar]
- Zhu X. L., Ohta Y., Jordan F., Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989 Jun 8;339(6224):483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]