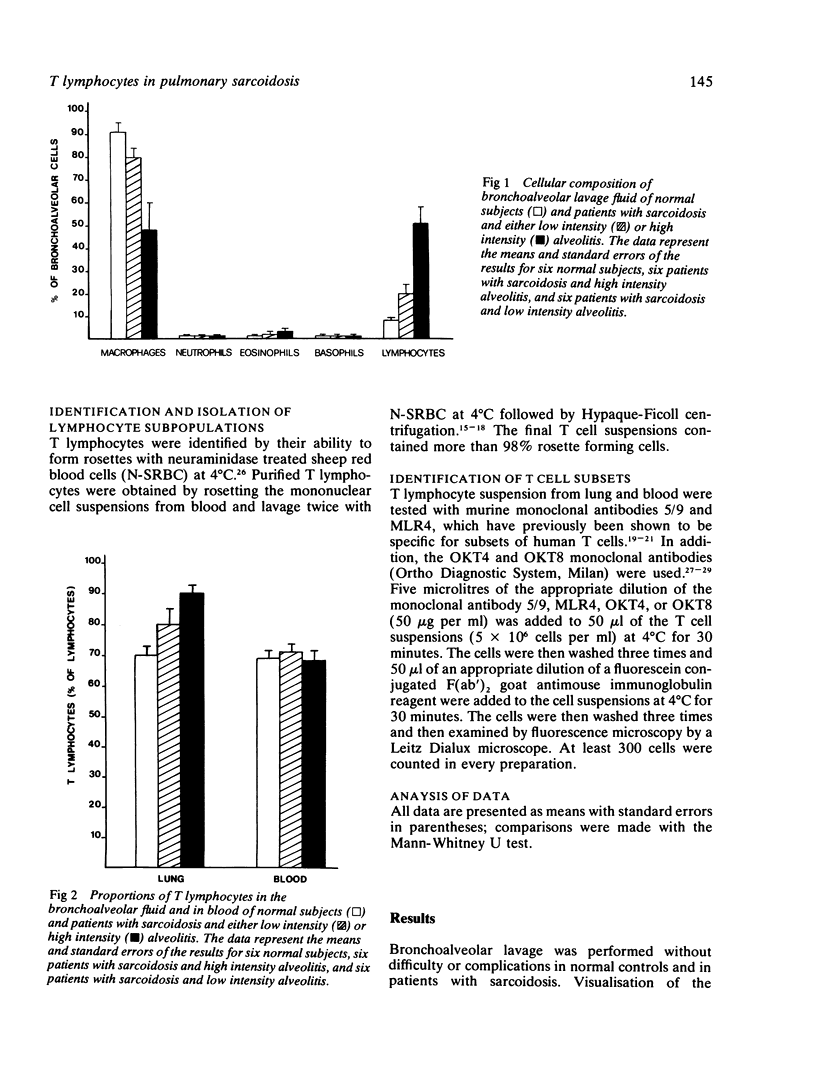
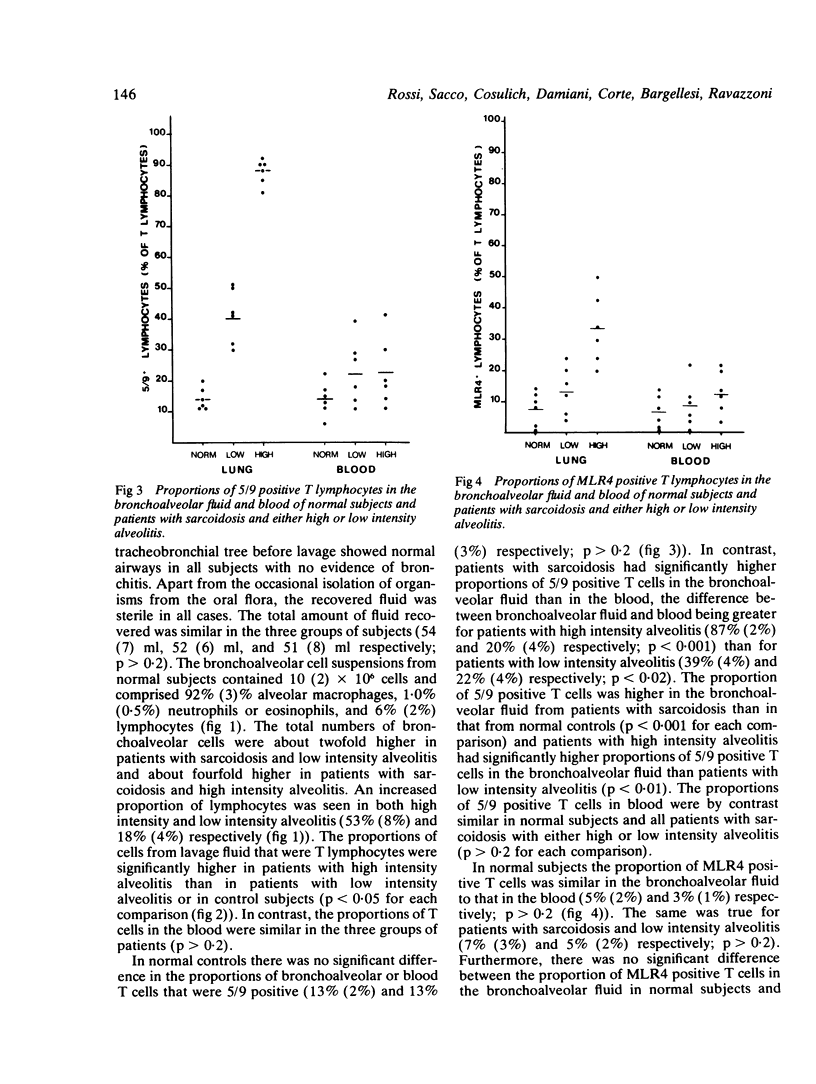
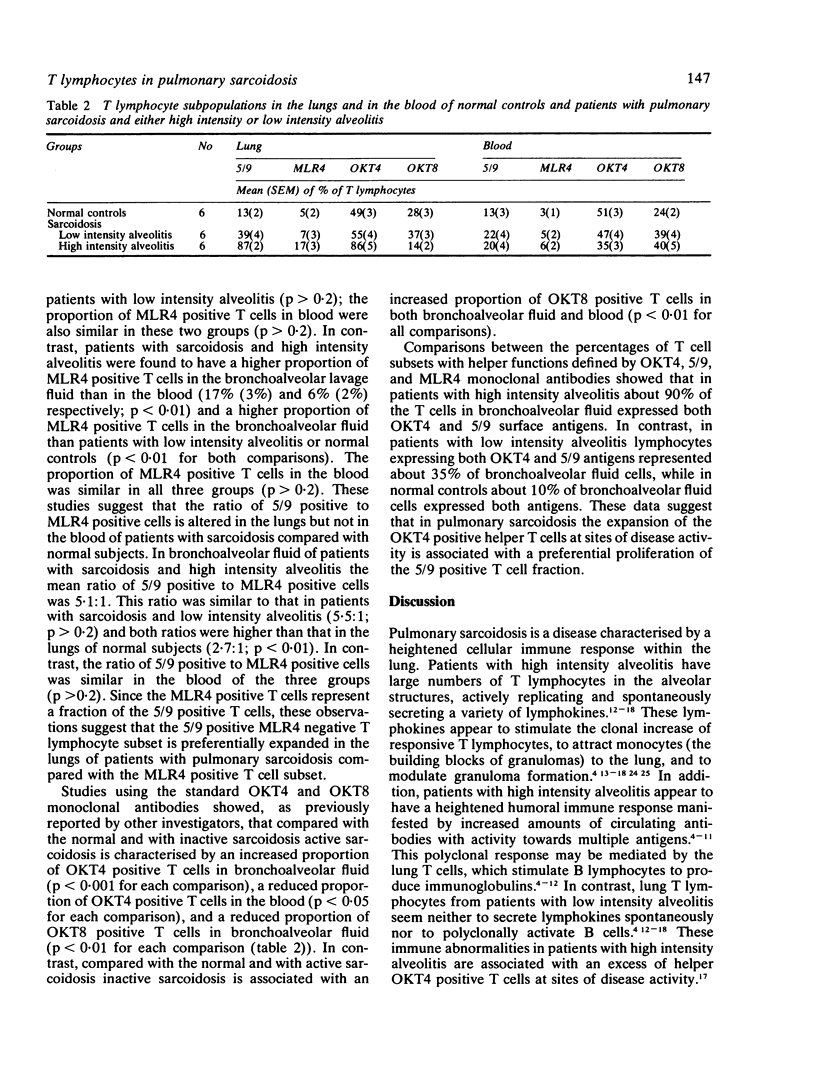
Abstract
Different lymphocyte subpopulations have been evaluated in bronchoalveolar fluid and blood obtained from six patients with active and six with inactive pulmonary sarcoidosis and from six normal subjects by means of two recently described monoclonal antibodies, 5/9 and MLR4. The percentages of OKT4 positive (helper) and OKT8 positive (suppressor) T cells were also determined. Patients with active sarcoidosis had significantly higher proportions of 5/9 positive T cells in the bronchoalveolar fluid than patients with inactive disease (p less than 0.01) or normal subjects (p less than 0.001). In contrast, the proportions of 5/9 positive blood T cells were similar in the three groups studied. Patients with active sarcoidosis had also a greater proportion proportion of MLR4 positive T lymphocytes in bronchoalveolar fluid than patients with inactive disease or normal subjects (p less than 0.01 for each comparison), but similar proportions of MLR4 positive blood T cells were found in each group. The ratio of 5/9 positive to MLR4 positive T cells was higher in the bronchoalveolar fluid (but not in the blood) in patients with either active or inactive sarcoidosis than in normal subjects. These observations suggest that the MLR4 negative fraction rather than the MLR4 positive fraction of the 5/9 positive T cells is preferentially expanded in the lungs of patients with pulmonary sarcoidosis and may indicate a secondary role for the MLR4 positive T cells in producing lung injury in this disorder. Comparisons of the OKT4 positive and 5/9 positive T cells showed that in patients with active disease most of the lung T lymphocytes expressed both the OKT4 and the 5/9 surface antigens, so the 5/9 monoclonal antibody may be considered a good marker of activity in this disorder. Pulmonary sarcoidosis may be characterised by the preferential expansion of helper T cell subsets at sites of disease activity.
Full text
PDF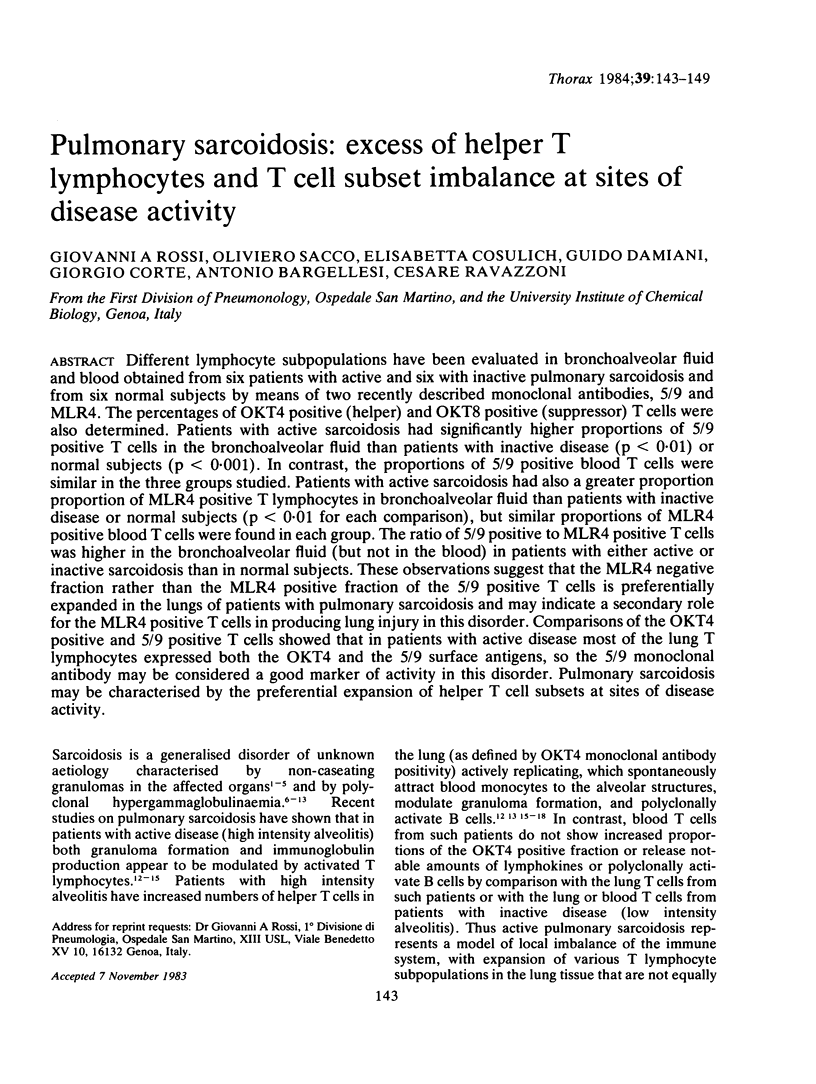



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Canonica G. W., Bagnasco M., Corte G., Ferrini S., Ferrini O., Giordano G. Circulating T lymphocytes in Hashimoto's disease: imbalance of subsets and presence of activated cells. Clin Immunol Immunopathol. 1982 Jun;23(3):616–625. doi: 10.1016/0090-1229(82)90324-5. [DOI] [PubMed] [Google Scholar]
- Carrington C. B. Structure and function in sarcoidosis. Ann N Y Acad Sci. 1976;278:265–283. doi: 10.1111/j.1749-6632.1976.tb47038.x. [DOI] [PubMed] [Google Scholar]
- Corte G., Mingari M. C., Moretta A., Damiani G., Moretta L., Bargellesi A. Human T cell subpopulations defined by a monoclonal antibody. I. A small subset is responsible for proliferation to allogeneic cells or to soluble antigens and for helper activity for B cell differentiation. J Immunol. 1982 Jan;128(1):16–19. [PubMed] [Google Scholar]
- Corte G., Moretta L., Damiani G., Mingari M. C., Bargellesi A. Surface antigens specifically expressed by activated T cells in humans. Eur J Immunol. 1981 Feb;11(2):162–164. doi: 10.1002/eji.1830110220. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Rossman M. D. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980 Mar;92(3):406–416. doi: 10.7326/0003-4819-92-3-406. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., McMillan L. J., Dauber J. H., Rossman M. D. Immune complexes in sarcoidosis: a correlation with activity and duration of disease. Chest. 1978 Sep;74(3):261–264. doi: 10.1378/chest.74.3.261. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Kueppers F., DeRemee R. A., Huston K. A., McDuffie F. C. Pulmonary and extrapulmonary sarcoidosis in relation to circulating immune complexes: a quantification of immune complexes by two radioimmunoassays. Am Rev Respir Dis. 1977 Aug;116(2):261–266. doi: 10.1164/arrd.1977.116.2.261. [DOI] [PubMed] [Google Scholar]
- Hedfors E., Norberg R. Evidence for circulating immune complexes in sarcoidosis. Clin Exp Immunol. 1974 Mar;16(3):493–496. [PMC free article] [PubMed] [Google Scholar]
- Hirshaut Y., Glade P., Vieira B. D., Ainbender E., Dvorak B., Siltzbach L. E. Sarcoidosis, another disease associated with serologic evidence for herpes-like virus infection. N Engl J Med. 1970 Sep 3;283(10):502–506. doi: 10.1056/NEJM197009032831002. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Mechanisms of hypergammaglobulinemia in pulmonary sarcoidosis. Site of increased antibody production and role of T lymphocytes. J Clin Invest. 1981 Jan;67(1):86–92. doi: 10.1172/JCI110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fulmer J. D., Young R. C., Jr, Gadek J. E., Crystal R. G. Localization of the immune response in sarcoidosis. Am Rev Respir Dis. 1979 Jul;120(1):49–57. doi: 10.1164/arrd.1979.120.1.49. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Keogh B. A., Hunninghake G. W., Line B. R., Crystal R. G. The alveolitis of pulmonary sarcoidosis. Evaluation of natural history and alveolitis-dependent changes in lung function. Am Rev Respir Dis. 1983 Aug;128(2):256–265. doi: 10.1164/arrd.1983.128.2.256. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Line B. R., Hunninghake G. W., Keogh B. A., Jones A. E., Johnston G. S., Crystal R. G. Gallium-67 scanning to stage the alveolitis of sarcoidosis: correlation with clinical studies, pulmonary function studies, and bronchoalveolar lavage. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):440–446. doi: 10.1164/arrd.1981.123.4.440. [DOI] [PubMed] [Google Scholar]
- Mitchell D. N., Scadding J. G. Sarcoidosis. Am Rev Respir Dis. 1974 Dec;110(6):774–802. doi: 10.1164/arrd.1974.110.6P1.774. [DOI] [PubMed] [Google Scholar]
- Moretta A., Corte G., Mingari M. C., Moretta L. Human T cell subpopulations defined by a monoclonal antibody. II. Evidence for cell cooperation in the response to alloantigens and generation of cytolytic cells. J Immunol. 1982 Jan;128(1):20–23. [PubMed] [Google Scholar]
- NIH conference. Pulmonary sarcoidosis: a disease characterized and perpetuated by activated lung T-lymphocytes. Ann Intern Med. 1981 Jan;94(1):73–94. doi: 10.7326/0003-4819-94-1-73. [DOI] [PubMed] [Google Scholar]
- Oreskes I., Siltzbach L. E. Changes in rheumatoid factor activity during the course of sarcoidosis. Am J Med. 1968 Jan;44(1):60–67. doi: 10.1016/0002-9343(68)90237-4. [DOI] [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Rankin J. A., Naegel G. P., Schrader C. E., Matthay R. A., Reynolds H. Y. Air-space immunoglobulin production and levels in bronchoalveolar lavage fluid of normal subjects and patients with sarcoidosis. Am Rev Respir Dis. 1983 Apr;127(4):442–448. doi: 10.1164/arrd.1983.127.4.442. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Rosen Y., Athanassiades T. J., Moon S., Lyons H. A. Nongranulomatous interstitial pneumonitis in sarcoidosis. Relationship to development of epithelioid granulomas. Chest. 1978 Aug;74(2):122–125. doi: 10.1378/chest.74.2.122. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]


