Abstract
Major scholars in the field, based on a 3-day consensus, created an in-depth review of current knowledge on the role of diet in CVD, the changing global food system and global dietary patterns, and potential policy solutions. Evidence from different countries, age/race/ethnicity/socioeconomic groups suggest the health effects studies of foods, macronutrients, and dietary patterns on CVD appear to be far more consistent though regional knowledge gaps are highlighted. There are large gaps in knowledge about the association of macronutrients to CVD in low- and middle-income countries (LMIC), particularly linked with dietary patterns are reviewed. Our understanding of foods and macronutrients in relationship to CVD is broadly clear; however major gaps exist both in dietary pattern research and ways to change diets and food systems. Based on the current evidence, the traditional Mediterranean-type diet, including plant foods/emphasizing plant protein sources, provides a well-tested healthy dietary pattern to reduce CVD.
Keywords: diet, food consumption, low and middle income countries, food system, cardiovascular disease, climate change
I. INTRODUCTION
There is much controversy surrounding the optimal diet for cardiovascular (CV) health. Data relating diet to cardiovascular diseases (CVDs) has predominantly been generated from high-income countries (HIC), but over 80% of CVD deaths occur in low- and middle-income countries (LMIC). Relatively sparse data on diet and CVD exist from these countries though new data sources are rapidly emerging (1,2). Non-communicable diseases (NCDs) are forecasted to increase substantially in LMIC because of lifestyle transitions associated with increasing urbanization, economic development and globalization. The Global Burden of Disease study cites diet as a major factor behind the rise in hypertension, diabetes, obesity, and other CVD components (3). There are an estimated over 500 million obese (4,5) and close to 2 billion overweight or obese individuals worldwide (6). Furthermore, unhealthy dietary patterns have negative environmental impacts, notably on climate change.
Poor quality diets are high in refined grains and added sugars, salt, unhealthy fats and animal-source foods; and low in whole grains, fruits, vegetables, legumes, fish and nuts. They are often high in processed food products – typically packaged and often ready to consume – and light on whole foods and freshly-prepared dishes. These unhealthy diets are facilitated by modern food environments, a problem that is likely to become more widespread as food environments in LMIC shift to resemble those of HIC (5,7,8).
In this paper, we summarize the evidence relating food to CVD, and the powerful forces that underpin the creation of modern food environments -- what we call the global food system -- to emphasize the importance of identifying systemic solutions to diet-related health outcomes. We do this in the context of increasing global attention to the importance of improving food systems by the international development and nutrition community (9–11). While the “food system” may feel remote to a clinician sitting in an office seeing a patient, its impacts on the individuals they are trying to treat are very real. The paper is based on a World Heart Federation international workshop to review the state of knowledge on this topic. This review of diet, dietary patterns and CVD is not based on new systematic reviews or meta-analyses but represents a careful review of many published meta-analyses, seminal primary studies, and recent research by the scholars who participated in the Consensus conference.
The paper presents: 1) an overview of the development of the modern, globalized food system and its implications for the food supply; 2) a consensus on the evidence relating various macronutrients and foods to CVD and its related comorbidities, and 3) an outline of how changes to the global food system can address current diet-related public health problems, and simultaneously have beneficial impacts on climate change.
II. THE CHANGING FOOD SYSTEM AND FOOD SUPPLY AND IMPLICATIONS FOR DIETS AND THE ENVIRONMENT
The development of the modern, globalized food system
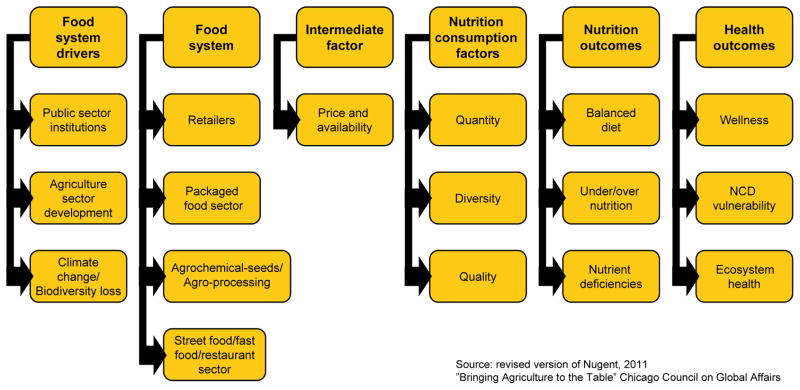
Food systems were once dominated by local production for local markets, with relatively little processing before foods reached the household (see Supplementary Box 1) (12). In contrast, the modern food system is characterized by a global web of interactions between multiple actors from farm to fork, geared towards maximizing efficiency to reduce costs and increase production (Figure 1). The major actors who control this system have changed dramatically in HIC and LMIC, as described below (13).
Figure 1.
Food System Impact on Nutrition-Related Non-Communicable Diseases
The shift to a global food system started in the US and other high income industrialized countries, and was driven initially by government investment and intervention in markets, infrastructure and research intended to raise farm-sector productivity. Building on actions taken in the late 19th century (14), policies on agricultural research and supporting on-farm production introduced in the 1930–60 period in the US (14) and Europe focused on few major crops, particularly grains (e.g., wheat, corn, rice), oilseeds (e.g., soybeans), livestock (e.g., pigs, poultry, and cattle) and critical cash crops, especially sugar cane and other sources of sugar (15–18). State intervention in most LMICs, took a different form, such as policies to subsidize food, taxes on agricultural producers; and systems to control the supply and marketing of key commodities (19–22). The 1960s also saw the start of significant agricultural transformation in LMIC, with the “Green Revolution” in which focused on increasing productivity of corn, rice and wheat.
These investments and changes in production systems were designed to make calories from staples (e.g., wheat, corn, rice) cheaply available, in order to simultaneously address hunger in LMIC and national food insecurity in HICs (23). In addition to vastly increasing the calorie supply, the ensuing productivity boom also provided the basis of cheap feed for livestock and cheap inputs for processed foods, in turn creating incentives for the growth of manufacturers of processed foods (24). This coincided with huge technological innovations in food processing, (24–28), the rise of to mass marketing to persuade consumers to eat more, supermarket retailing and fast food (29) (30), As a result of these changes, the transformation of raw commodities into food and the distribution of consumable food items beyond the farm gate has become far more important (31). Today integration and control of our farm to fork food supply by major agribusinesses, food manufacturers, retailers, food service companies is more the rule that the exception (13). Meanwhile, production of less processed foods such as coarse grains (e.g., millet, sorghum), roots, tubers, and legumes has declined (32,33) while animal source food production grown dramatically (34).
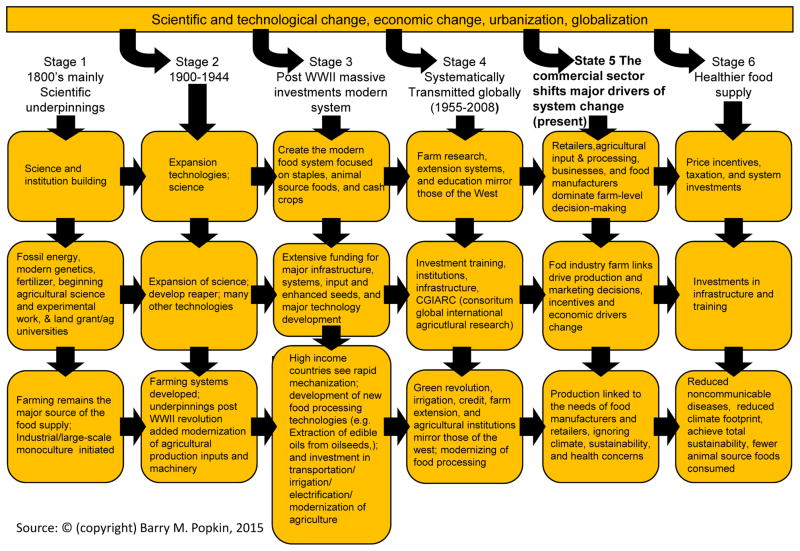
Figure 2 sets out the stages of change involved in leading to this modern food system. This model has spread unevenly to most LMIC (35–37). Many countries retain various forms of state intervention in agriculture and food systems (18,38–41), but policies to liberalize trade and private sector investment have revolutionized the entire sector in many regions (13,42). Retailing has been transformed in LMIC through the growth of supermarkets (18,38–41). While this process originated with companies in industrialized countries looking for growth in foreign markets, companies based in LMICs are now also investing back into HICs.
Figure 2.
Stages of Global Agricultural System’s Development
Dietary impacts
The way people eat has changed greatly across the globe; moreover the pace of change in LMIC’s is quickening. Snacking and snack foods have grown in frequency and number (43–48); eating frequency has increased; away-from-home-eating in restaurants, in fast food outlets, and from take-out meals is increasing dramatically in LMIC; both at home and away-from-home-eating increasingly involve fried and processed food (47,49); and the overall proportion of highly processed food in diets has grown (50,51).
These changes in the global food system coupled with these food behavior shifts have enabled some critical changes to the global food supply, all with dietary implications. First is the shift to refined carbohydrates – refined grains and added sugars. Rapidly increasing production of starchy staples combined with processing technologies mean that refined flour is increasingly dominant in diets. White bread, for example, once rarely consumed in Latin America, became widespread after the introduction of high-yield wheat varieties. In Asia, white rice became dominant as a staple over legumes and coarse grains, with a more recent trend being rapidly rising consumption of instant noodles as a staple (52,53). Since 1964, average total carbohydrate intake in the US has increased from about 375 g/d to 500 g/d (from 2 to 6 kg/year of ready-to-eat cereals), but the percent of carbohydrate that is fiber has not substantially changed over this time, reflecting increased refined carbohydrates and sugar sweetened beverages (SSBs) is high in HIC (54). In the 1985 to 2005 period extensive added sugar intake occurred across HIC (55) but more recently large increases have occurred in LMIC, particularly in consumption of sugar-sweetened beverages and processed foods (56–59). Today in the US packaged and processed food supply over 75% of foods have some form of added sugar (60). With urbanization there is some evidence to show that refined carbohydrate consumption is increasing, whereas consumption of traditional grains (i.e. millet, maize) is decreasing in LMIC (61,62).
A second key change has been the increasing intake of vegetable oils, including processed vegetable oils, and a decline in consumption of animal fats (2,63). This was initially driven by rising production of soybeans in the US, later in Argentina and Brazil, and then palm oil in East Asia. Oilseeds are now among the most widely traded crops, and are also processed to create margarines and vegetable shortenings and into partially hydrogenated fats and bleached deodorized oils for use in processed foods, (see also Supplementary Box 2). Between 1958 and 1996, a major global shift occurred in the amount and types of available fats, with soybean, palm, and rapeseed/canola oils replacing butter, tallow, and lard. In 1958–1962, soybean, palm, and rapeseed/canola oils represented 20% of the 29 million metric tons of fat produced globally/year, while butter, lard, and tallow represented 37%. By 1996–2001, these oils accounted for 52% of the 103 million metric tons of fat produced globally/year, while butter, lard, and tallow contributed 20% (64). This has implications for consumption of fatty acids. Palm oil (deodorized) has become an increasing source of saturated fatty acids; and partially hydrogenated fats are the main source of trans-fatty acids Between 1990 and 2010, global saturated fat, dietary cholesterol, and trans-fat intakes remained stable (trans-fats going down in HIC and up in LMIC), while n-6, seafood n-3, and plant n-3 fat intakes each increased (65). Vegetable oil consumption remains two times higher in HIC than in LMIC. However, trans-fat consumption is very high in many LMIC while decreasing markedly in HIC. In India, for example, vanaspati, a vegetable ghee used in bakery products, fried snacks and foods sold by street vendors is a leading source of trans-fats, as is bakery shortening. In 75 countries representing 61.8% of the world’s adult population, global saturated fat consumption was ≈9% of energy; though considerable variation existed across countries (range: 2 to 28%). Country-specific omega 6 consumption ranged from ≈1 to 13% (global mean: 6%) of energy; trans-fat consumption ranged from ≈0.2 to 6.5% (global mean: 1%) of energy; dietary cholesterol consumption ranged from ≈97 to 440 mg (global mean: 228 mg) per day; seafood n-3 ranged from ≈35 to 3,886 mg (global mean: 163 mg) per day; and plant n-3 ranged from <≈100 to 5,542 mg (global mean: 1,371 mg) per day (65).
A third key change has been the increasing global consumption of meat, which has been made economically feasible by subsidized production of crops for animal feed – most importantly corn and soybeans (soybean oil is a byproduct of soymeal production for animals) (66–69). At very low levels of intake animal food consumption may not induce harm, providing high quality protein and iron, whereas excess animal food intake in HIC may be linked to adverse health outcomes, particularly from processed meats (70). Meat consumption has increased considerably worldwide, and there is substantially greater production of meat in HIC than in LMIC (71). North and South America, Europe, and Australia/New Zealand have the highest meat intake, whereas Asia and Africa have the lowest (72,73). Processed meats (which refers to post-butchering modifications of foods such as curing, smoking, or addition of sodium nitrate), account for a modest proportion of all meat consumed in HIC (unpublished data from the PURE study) (71).
Dietary consumption patterns of other protein sources have been mixed. Between 1973 and1997, dairy consumption per capita (kg) increased in LMIC by ~48% and is projected to almost double (93% increase) by 2020. On average, fish consumption is 2 to 3 times higher in HIC than LMIC (74), although with marked heterogeneity within income categories. China has the highest per capita consumption of fish in the world, followed by Oceania, North America, and Europe (74). Globally, although there has been little or no increase in sea fish consumption per capita since the 1960s, catches per year have risen exponentially (75) and freshwater fish intake has intake has increased during this time (71). Eggs are similarly consumed in higher quantities (2–6 times) in HIC relative to LMIC, with a 14% decline in consumption in HIC observed between 1980–2000, and no change was observed in LMIC (76). The consumption of legumes declined in the US from 1960 and into the 1980’s, with reduced consumption patterns observed globally (8). Relatively, HIC such as Canada, US, and Western Europe, tend to consume the lowest quantities of legumes per capita in the world, whereas LMIC within Africa and India consume the greatest quantities of legumes, along with certain South American countries where beef is uncommon, such as Colombia and Peru (77–79). Globally, pulse consumption has decreased since 1961, from ≈9.5 kg/person/year in 1961 to 6.5 kg/person/year in 2006. In LMIC countries pulses contributed ≈4% of energy to the diets, and just 1% of energy to diets of HIC (80). Total production of tree nuts in 2012 was 3.5 million metric tons, a 5.5% increase from 2011. World consumption of tree nuts in 2011 exceeded 3 million metric tons (81).
A fourth key change is the marked growth of purchases of all packaged foods and beverages (all categories of processing). This process is accelerating across all LMIC markets (13,82,83). For example, 58% of calories consumed by Mexicans come from packaged foods and beverages, which is similar throughout the Americas (83) and even with the US (66%) (65,84). The proportion for China is 28.5% and rising rapidly (36,82,83). The component that is “ultra-processed” – ready to eat, of snack, foods – varies depending on the method of measurement but is increasing wherever it is studied at all income levels (50,85,86). The shift to ultra-processed foods has not just affected the food available for consumption but also the way food is consumed (87). The way people eat has changed greatly across the globe and the pace of change is quickening. Snacking and snack foods have grown in frequency and number (43–48); eating frequency has increased; away-from-home-eating in restaurants, in fast food outlets, and from take-out meals is increasing dramatically in LMIC; both at home and away-from-home-eating increasingly involve fried and processed food (47); and the overall proportion of highly processed food in diets has grown (50,51).
A fifth trend noted above in relation to the added sugar change is the shift in the way LMIC are experiencing a marked increase in added sugar in beverages. In the 1985 to 2005 period extensive added sugar intake occurred across HIC (55) but more recently large increases have occurred in LMIC’s, particularly in consumption of sugar-sweetened beverages and ultra-processed foods (56–59). Today in the US packaged and processed food supply over 75% of foods have some form of added sugar (60).
In addition, fruit and vegetable intake has remained inadequate. Fruit and vegetable consumption is substantially higher in HIC compared to LMIC (88). Analysis of 52 LMIC countries taking part in the World Health Survey (2002–2003) (89) found that low fruit and vegetable consumption (i.e., less than 5 fruits and vegetables per day) prevalence ranged from 36.6% (Ghana) to 99.2% (Pakistan) for men and from 38.0% (Ghana) to 99.3% (Pakistan) for women. Overall, 77.6% of men and 78.4% of women consumed less than the minimum recommended five daily servings of fruits and vegetables. In the US, 32.6% of adults consumed fruit two or more times per day and 27.2% ate vegetables three or more times per day (90). In 2012, 40.6% of Canadians aged 12 and older, reported consuming fruit and vegetables five or more times per day (91).
While all of these changes across LMIC display great heterogeneity (92), the global food system has clearly reached all corners of the LMIC urban and rural sector and major shifts in diets appear to be accelerating.
Implications for environmental impacts
The modern food system is a major force in a range of serious environmental problems, including climate change [as a leading source of greenhouse gas (GHG) emissions, including carbon dioxide, methane, and nitrous oxide], the loss of biodiversity, the strain on freshwater resources, and the release of persistent toxins, excess nitrates and phosphates (from fertilizer and concentrated livestock operations, causing widespread problems of eutrophication), and animal pharmaceutical residues into waterways (12,93–95).
The major causes are beef and other large animals for GHG and the metabolic losses associated with shifting the product of nearly one third of the world’s arable land to concentrated animals, which effectively magnifies the resource budgets and pollution loads of industrial monocultures (34). The expansion of low-input agriculture and extensive ranching are also major factors in deforestation, which bear heavily on both climate change (as carbon is released from vegetation and soils and sequestration capacity diminishes) and biodiversity loss.
In addition to being a major force in many environmental problems, world agriculture is also extremely vulnerable to climate change, biodiversity loss, declining freshwater availability, and the inevitable limits of non-renewable resources (e.g., fossil energy, high grade phosphorous) although vulnerability is highly uneven on a world scale (96). Many of the world’s poorest regions are poised to be most adversely affected by rising average temperatures, aridity, and water stress, as well as through increasingly severe extreme weather events like drought or flooding; in fact some feel this is already occurring (97–99).
It is also noteworthy that the FAO estimates that one-third of all food produced for human consumption globally is wasted before it is consumed, which has both social and environmental costs that have been precisely measured in the US (100–102). Waste affects the entire food system from production to post harvest (including inside the home) (102). Hall’s estimate to home food waste of about 1400 kcal/capita for the US adds up to 25–45% waste of total food for some HIC (101).
III. MACRONUTRIENTS, FOODS, AND CVD RISK FACTORS
Carbohydrates
Refined Carbohydrates
a) CVD Risk Factors
Ecological evidence from the US demonstrates an association of refined carbohydrate (as corn syrup) with type 2 diabetes (T2DM) and obesity (54). Robust data from systematic reviews and high-quality RCTs support a harmful effect of highly-refined, high-glycemic-load carbohydrates. A meta-analysis of observational studies indicated that high–glycemic index (GI) foods are associated with T2DM (103). Proof-of-concept studies have used alpha-glucosidase inhibitors, such as acarbose, to lower the glycemic index of the foods consumed; for example, in the STOP-NIDDM trial, acarbose reduced progression to T2DM by 25% compared with placebo (104). T2DM risk in individuals with the highest glycemic load (GL) and lowest cereal fiber is 2.5-fold that of those with the lowest GL and highest cereal fiber diet (105). A large Danish prospective cohort study of the impact of replacing saturated fats with high-GI carbohydrates found that when high-GI carbohydrates replace saturated fat, myocardial infarction (MI) risk increases 33% (106). A meta-analysis of 10 prospective cohort studies (n=296,849) (107) found increased GL associated with a 27% increased coronary heart disease (CHD) and MI risk (108). In controlled-feeding studies, replacing saturated fat with carbohydrates lowers low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol and increases triglycerides (109). Dietary interventions which raise HDL-C may not necessarily translate into CVD risk reduction, as serum HDL-c has recently been called into question regarding its role in the causal pathway of CVD (110). However, in a 5-week controlled-feeding trial of 163 generally healthy but overweight adults, low compared with high-glycemic index diets did not improve insulin sensitivity, lipid levels, or systolic blood pressure in the context of a healthy, DASH-like dietary pattern, and a low compared with high-glycemic index diet increased LDL-C when the carbohydrate content of the diet was high (109). This study suggests that the adverse effect on CHD risk may not be mediated by short term effects on classical risk factors, but that postprandial hyperglycemia and hyperinsulinemia or hyperlipidemia may play a mediating role; further work is needed to clarify the effect of the glycemic index on LDL-C and related lipid risk factors.
b) LMIC
Highly refined carbohydrates include polished white rice, cornstarch, and white wheat flour with reduced fiber content. Carbohydrate refinement is common in HICs and increasing in LMIC (111,112). Traditional diets in LMIC, once rich in whole grains and dietary fiber, now include highly refined carbohydrates, such as polished white rice and refined flours. In East Asian countries, white rice consumption is associated with a 55% higher T2DM risk (111,112). In the cross-sectional CURES 57 study, higher refined grain intake was associated with higher waist circumference, systolic and diastolic blood pressure (SBP and DBP), fasting glucose, triglycerides, and insulin resistance and lower HDL-C levels (61). Replacing white with brown rice (50 grams/day) reduced T2DM risk by 16% (113), and substituting beans for white rice reduced the odds of metabolic syndrome by 35% (108). However, a 16-week randomized trial of replacing white with brown rice in middle-aged Chinese men and women with or at high risk for T2DM only improved HDL-C and reduced DBP in the brown rice group. Data from the international Prospective Urban and Rural Epidemiological Study (PURE; 138,926 individuals in 628 communities 17 countries) suggest a need to consider the context and availability of specific foods before making food choice recommendations (114). Increasing whole grain and cereal fiber consumption, while decreasing total and high-GI carbohydrate, are helpful strategies to prevent T2DM and CVD in the general population (115). Furthermore, low-glycemic index diets improve glycemic control and serum lipids in RCTs of participants with T2DM with major implications for CHD risk reduction in this vulnerable segment of the population whose numbers are increasing rapidly globally (116–119).
Sugar-Sweetened Beverages
a) SSB and CV Risk Factors
Sugar-sweetened beverage (SSB) consumption accounts for up to 50% of added sugar in the American diet (120,121). The epidemiological relationships between SSB consumption, overweight, obesity, hypertension, and T2DM are strong (122). In a meta-analysis of prospective studies of SSB and hypertension, CHD, and stroke, the RR for a 1-serving increase in SSB/d was 1.17 (95 % CI 1.10, 1.24) for CHD and 1.08 (95% CI: 1.04 to 1.12) for incident hypertension, but no clear effect was seen for total stroke (RR 1.06, 95 % CI 0.97, 1.15) (123). A meta-analysis of seven cohort studies and five randomized controlled trials (RCTs) found SSB’s increased weight by 0.12 kg/serving/year (95% confidence interval [CI]: 0.10–0.14) in adults (124). A World Health Organization (WHO) systematic review reported similar positive associations (125). In a pooled analysis of Nurses’ Health (NHS) and Health Professionals Follow-up Study (HPFS) data, a one SSB serving/day increment is associated with a 1-kg weight gain over a four-year period (126), possibly due to incomplete compensation for this energy at other meals. After adjusting for important potential confounders, including body mass index (BMI), ≥ 1/d versus < 1 SSB/mo increased T2DM by 39% (127). A meta-analysis of 310,819 participants and 15,043 cases of T2DM reported a 26% increased T2DM risk among those consuming 1–2 SSB servings/day compared to non-consumers (128). A meta-analysis of 4 cohort studies reported a linear association between SSB and hypertension risk [RR: 1.08 (95 % CI: 1.04, 1.12) per serving/d] (129). The Framingham Offspring Study reported a 22% higher incidence of hypertension among those consuming ≥ 1 SSB serving/day compared with non-consumers (130). A potential explanation of the SSB-T2DM association is the high content of rapidly-absorbed sugar from corn syrup, which increases blood glucose and insulin and de novo lipogenesis –which may contribute to pancreatic beta cell dysfunction and eventually T2DM (131). In the National Health and Nutrition Examination Survey III (NHANES III), with 831 CVD deaths during 163,039 person-years of follow-up, consumption of ≥ 7 SSB servings/week was associated with a 29% increased CVD mortality risk compared with < 1 serving/week (120), with no increased risk up to 6 drinks/week. Few studies have investigated the association between SSBs and CVD events. Those that have report increased CHD and stroke risk with SSB consumption. A 2015 meta-analysis reported a relative risk for incident CHD of 1.17 (95 % CI: 1.10, 1.24) per serving/d increase in SSB consumption (129). Both the HPFS and the NHS find a ≈20% increased CHD risk in the highest category of SSB consumption compared with the lowest category (132,133); and, after adjusting for dietary and non-dietary cardiovascular risk factors, a 16% increased stroke risk (134). Similarly, two Swedish prospective cohort studies in women and men reported a relative risk (RR) of 1.19 (95% CI: 1.04 and 1.36) for stroke among those consuming ≥ 2 SSB servings/day (135).
b) LMIC
Facing health concerns, the beverage industry is shifting from full-calorie carbonated soft drinks (CSDs) to lower-calorie CSDs, coffees, and teas. According to the NHANES, between 1999 and 2006 the average US full-calorie CSD intake decreased, while intake of diet CSDs, low-calorie fruit drinks, and other sweetened beverages increased (122). These products will gradually enter the markets in China, Brazil, and other LMICs. However, there are practically no data on the effects of SSBs on health outcomes from LMIC (136,137).
Fats and Oils
a) CVD Risk Factors
Vegetable oils that are primarily comprised of mono- (e.g., olive oil) and polyunsaturated fatty acids appear to reduce CHD risk, and sources of the n-3 polyunsaturated fat alpha-linolenic acid (ALA), such as rapeseed or canola oil, are cardioprotective. Replacing saturated fat with monounsaturated or polyunsaturated fat reduces low-density lipoprotein cholesterol (LDL-C) and preserves HDL-C (109). Further, canola oil, as part of a low-glycemic index diet, improves glycemic control and blood lipids in type 2 diabetes (138). Trans-fatty acids increase CHD risk compared with other macronutrients, with strong evidence of adverse effects of small amounts of trans-fats on lipids (109,139) and CVD risk (140,141).
Though total fat (142), and specifically saturated fats, have generally been considered to be deleterious to insulin sensitivity (143), in large cohort studies, saturated fat is not associated with development of T2DM, after adjustment for BMI, total dietary fiber, or magnesium intake (144–149). Macronutrient exchange generally does not influence markers of glucose homeostasis, though in two relatively large trials, replacing SFA with either MUFA or carbohydrate improved indices of glucose homeostasis (150,151). Associations have been seen between major food sources of saturated fat, such as red and processed meat, and development of T2DM (152,153), though dairy products, notably fermented dairy, may be protective (154,155).
b) CVD
Saturated fats have not been consistently associated with CVD in meta-analyses of cohort studies (odds ratio [OR]: 1.07; 95% CI: 0.96–1.19) of higher compared with lower intakes (156). However, in most of these studies the association of high saturated fat intake largely represents replacing highly refined carbohydrates. Replacing saturated fat with highly refined carbohydrate is not associated with lower CHD risk, whereas replacing saturated fat with polyunsaturated fat reduces CHD risk (157,158). This benefit of polyunsaturated fat includes the primary n-6 polyunsaturated fatty acid, linoleic acid (158,159). Replacing saturated fat with high-GI carbohydrate increases MI risk by 33%, whereas replacing with low- and medium-GI carbohydrates appears neutral (106). Emerging evidence suggests that the effect of a saturated fat on CHD may depend on the type of fatty acid and the specific food source (i.e., dairy vs. meat) (160,161). Consumption of non-hydrogenated vegetable oils appears to be superior to consumption of animal fats (162). Consumption of plant oils in a Mediterranean diet reduced CVD in two RCTs. The Lyon Diet Heart Study found regular consumption of ALA (canola oil) significantly reduced cardiac deaths and nonfatal CHD (163), while the PREDIMED RCT found that a Mediterranean diet (50 grams/d extra virgin olive oil) reduced CVD events by 30% in over a five-year period (164).
Palm oil is the dominant fat globally and is relatively high in saturated fat. Based on controlled-feeding studies examining changes in blood lipids, replacing palm oil with unsaturated fatty acids would be expected to lower CHD risk (109), but palm oil would be preferred to partially hydrogenated oils high in trans-fatty acids. Few studies have directly compared palm oil with other oils for CHD risk. One large case-control study in Costa Rica found that soybean oil consumption was associated with lower acute MI risk compared to palm oil consumption (165).
Summary of Fats and Oils
compared to saturated fat, vegetable oils rich in polyunsaturated fats reduce the TC:HDL-C ratio and CHD incidence; inclusion of n-3 fatty acids (ALA) with the vegetable oils is important for CHD prevention. The effect of replacing saturated fat with carbohydrate on CHD risk appears to depend on the quality of the carbohydrate. Prospective studies consistently indicate adverse effects of trans-fats on CHD. Effects of monounsaturated fat from plant sources require further study; extra virgin olive oil appears to reduce CVD.
Protein sources
Meats
a) Nutrients and CVD Risk Factors
Meat is rich in protein, iron, zinc, and B-vitamins, but can also contain significant amounts of cholesterol and saturated fatty acids, which raise LDL-C and lower triglyceride (157). A high red meat intake (rich in heme iron), increases endogenous formation of N-nitroso compounds in the gastrointestinal tract that are associated with increased epithelial proliferation, oxidative stress, and iron-induced hypoxia signaling (166–168).
In a meta-analysis of 17 cohort studies (16 from Western countries), consumption of red and processed meat increased T2DM and CHD risk; but few included studies examined unprocessed red meat (169). Intake of red or processed meat was not associated with stroke, but only three studies evaluated these relationships (169). In more recent analyses, red meats, particularly processed red meats, were associated with increased CVD, CHD, stroke, and cancer mortality, while poultry was not (170–172). Potential mechanisms linking unprocessed red meat with CVD include saturated fat, cholesterol, iron, phosphatidylcholine, and carnitine; and cooking methods (e.g., barbecuing), that increase heterocyclic amine content and N-nitroso compounds, also implicated in colorectal cancer (167). The evidence suggests that processed meat consumption increases CHD risk, while unprocessed meat consumption has a small or no association with CHD, mainly when compared to refined starch and sugar. Both unprocessed and processed red meats are associated with greater CVD risk compared to poultry, fish, or vegetable protein sources. Both types of meat are associated with higher T2DM risk, although gram-for-gram the effect size is notably larger for processed meats.
b) LMIC
Data relating meat consumption to CVD risk in LMICs is limited. A recent pooled analysis of data from 296,721 individuals from Asian countries (i.e., Bangladesh, mainland China, Japan, Korea, and Taiwan) found no association between red meat and poultry consumption and CVD, cancer mortality, or all-cause mortality (173). Red meat intake is generally much lower in these areas than in HICs however, and current consumption does not likely reflect long-term patterns.
Dairy
a) CVD Risk Factors
The consumption of dairy products has been associated with weight loss in small studies (174), but the overall literature does not confirm an important effect on body weight. However, increased low-fat dairy consumption is associated with lower LDL-C, triglycerides, plasma insulin, insulin resistance, waist circumference, BMI, possibly blood pressure (BP); and reduced diabetes risk (174–182). In a large meta-analysis of cohort studies (13,000 incident cases), and the EPIC InterAct case-cohort study (12,000 incident cases), fermented dairy (i.e., yogurt, cheese, and thick fermented milk), but not total dairy, was inversely associated with T2DM (155,183). In a meta-analysis of prospective cohort studies, milk consumption was inversely associated with total CVD in a small subset of studies with few cases, but using a larger body of data with more specific endpoints, milk was not associated with CHD or stroke (173). In a prospective cohort study of 53,387 Japanese men and women, higher dairy calcium (173 mg/d vs. none) reduced risk for hemorrhagic stroke, ischemic stroke, and stroke mortality by ≈50% over a 10-year follow-up (184). Collectively, these studies do not suggest a strong or consistent relationship between consumption of dairy products and T2DM and CVD risk.
b) LMIC
Data on dairy consumption and CVD in LMICs are limited. In a prospective cohort of 2,091 middle-aged Chinese men and women monitored for six years, individuals who reported consuming > 1 dairy serving/day were 35% less likely to develop T2DM (RR: 0.65; 95% CI: 0.49 and 0.85) than nonconsumers (185).
Egg
a) CVD Risk Factors
Eggs are a relatively inexpensive and low-calorie source of protein, folate, and B vitamins (186). Eggs are also a source of dietary cholesterol (a medium egg contains ~225 milligrams of cholesterol) (187). A meta-analysis showed that eggs increase TC, HDL-C, and TC:HDL-C,(188) but five RCTs subsequently reported that egg consumption did not significantly alter these parameters (189–191) or endothelial function (192,193). No RCT has tested the effect of egg consumption on CVD events. In a meta-analysis (194) of 16 prospective cohort studies (90,735 participants) (191), egg consumption was not associated with overall CVD or CHD, stroke, or CHD or stroke mortality; but was associated with T2DM. Overall, consumption of eggs in moderation (one egg/day) is likely neutral for CVD. However, relative to other protein-rich foods that lower LDL cholesterol, such as whole grains and nuts, eggs would likely increase CVD risk.
b) LMIC
Unpublished data from three large international studies, PURE, ONTARGET, and INTERHEART, with collectively 200,000 individuals and 22,000 CVD events from regions including China, India, and Africa, show that moderate egg consumption appears to be neutral or protective against CVD. However, significant variations exist across regions, with a benefit of daily egg consumption in China but possible harm in South Asia.
Fish
a) CVD Risk Factors
Fish are a source of protein, vitamin D, multiple B vitamins, essential amino acids, and trace elements; and the long-chain omega-3 (n-3) fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (195,196), though amounts vary over tenfold across seafood species. Fatty fish, such as salmon, sardines, trout, white tuna, anchovies, and herring, have the highest concentrations. In clinical trials and meta-analyses of these trials the long-chain omega-3 polyunsaturated fatty acids in fish reduced multiple CVD risk factors, including vascular resistance, BP, inflammation, serum lipids, and endothelial function (197).
Some prospective cohort studies find an inverse association between fish intake and CVD mortality, whereas others do not (198). Available evidence suggests cardiovascular benefits with fish consumption in secondary prevention, but the evidence is inconsistent regarding primary prevention. Possible explanations include differences in the amounts and types of fish consumed, cooking methods, and background fish consumption. Fifteen of the 16 cohort studies [exception (199)] were conducted in North America and European countries, where deep-frying fish is common. The Diet and Reinfarction Trials (DART-1), a secondary prevention trial and DART-2, in men with stable angina, are the only randomized trial of fish intake and CVD outcomes. They arrive at opposite conclusions. In DART-1, fish lowered all-cause mortality and trended towards reducing CVD events after 2 y (194). In DART-2 oily fish did not affect all-cause mortality or CVD events after 3–9y, and increased sudden cardiac death, largely confined to the subgroup given fish oil capsules (200). Differential behavioral change or CVD stage may explain the discrepancy (201). Follow-up of DART-1 at 5y also showed increased rates of CVD in the fish/fish oil group that did not persist through the 10y assessment (202). We know of no primary prevention trial on fish intake and CVD outcomes, but a meta-analysis of fish oil supplement RCTs is neutral (203).
b) Low-income Countries and HICs
Most of the data indicating that fish is protective comes from studies in HICs (204–208). (204,205). Unpublished data from three large international studies (PURE, ONTARGET, and INTERHEART) reflect considerable heterogeneity in the association between fish intake and CVD outcomes. In the PURE study fish intake was inversely associated with CVD outcomes in South America, China, North America, and Europe (RR: 0.76–0.84) but positively associated in South Asia (RR: 1.97). However, no associations between fish consumption and CVD outcomes were observed in a high-risk secondary population in ONTARGET. INTERHEART found fish intake beneficial in North America and Europe (RR: 0.73; 95% CI: 0.62–0.87) but harmful in the Middle East (RR: 1.59; 95% CI: 1.32–1.93). More work is needed in China and India to understand the effects of regional types and preparation methods of fish a.
Nuts
a) CVD Risk Factors
In clinical trials nuts improve serum lipids (209) (210,211). In observational studies, nuts lower CHD (196,212,213) and hypertension risk (213); but not stroke (207, (213). In one meta-analysis of prospective cohorts, nuts were not associated with T2DM (214), but in another, they were protective (207). Despite their high energy density, nuts do not contribute to weight gain, changes in waist circumference, or obesity perhaps owing to their satiating effects and increased fecal energy losses. (215,216). In observational studies (133,217–220) and RCTs (164,221–223) a Mediterranean diet including nuts lowers CVD risk. However, no RCTs have assessed the effects of nut consumption alone on CVD events. Taken together, the published data from observational studies and clinical trials support nuts for lowering CVD risk.
b) LMIC
Unpublished data from the PURE cohort indicate that nut consumption is very low in LMIC (60% of individuals consume ≤ 1 nut serving/week). No associations between nut consumption and CVD outcomes is seen at this low level.
Legumes
a) CVD Risk Factors
In observational studies and RCTs consumption of legumes improves CVD risk factors, such as waist circumference, cholesterol, BP, C-reactive protein, glucose; and is protective against T2DM (224–231). A meta-analysis of 26 RCTs (1,037 participants) finds that 130 grams legumes/d (~1 serving) reduced LDL-C by 0.17 mmol/L (95% CI: −0.25, −0.09) (228). Legumes also reduce SBP (by 2 mmHg) and lowered mean arterial pressure(231) in a meta-analysis of eight RCTs. A legume-rich diet reduced HbA1c (−0.3%; 95% CI: −1.4, −0.1%) in an RCT in participants with diabetes (226).
b) CVD
In a meta-analysis of five observational studies, 100 grams of legumes four times/week is inversely associated with CHD (RR: 0.86; 95% CI: 0.78–0.94) (232). However, that study and another meta-analysis of eight prospective cohort studies found no association between legumes and diabetes (207) or stroke (232,233). A large prospective study of two cohorts of US health professionals found a 45% increased risk of ischemic stroke per daily serving of legumes (RR: 1.45; 95% CI: 1.06–2.00) (234). This suggests that while legumes are valuable to reduce CHD risk, more research is required to understand their impact on total stroke risk.
c) LMIC
In a cohort of Chinese men and women, soy (the primary legume consumed in China) was negatively correlated with TC and LDL-C (235). A population-based cross-sectional study in India found that women consuming legumes once/day were less likely to develop T2DM (OR: 0.55; 95% CI: 0.34–0.88; n=99,574). A similar but non-significant trend was observed in men (OR: 0.70; 95% CI: 0.39–1.26; n=56,742) (236). In a prospective cohort study of middle-aged Shanghai women, a ~50 gram/day increase in legume consumption over 4.6 years reduced T2DM risk by 38% (RR: 0.62; 95% CI: 0.51–0.74) (237). A prospective cohort study of 64,915 Chinese women aged 40 to 70 (n=62 cases; follow-up 2 y) found that individuals consuming ≥ 11.2 grams/day of soy protein were less likely to develop CHD (RR: 0.25; 95% CI: 0.10 to 0.63) than those consuming ≤ 4.5 grams/day (238).
Summary of major protein sources
Reducing red meats, especially processed meats, and increasing fish, nuts, legumes, and possibly fermented dairy products are likely beneficial. Sustainability issues discussed in this paper must also be addressed specifically in relation to meat, fish, and dairy foods (12,34,239–241).
Fruits and Vegetables
a) CVD Risk Factors
In the Dietary Approaches to Stop Hypertension (DASH) RCT, higher intake of fruits and vegetables, either as part of a typical Western diet or the DASH eating plan, reduced BP, TC, LDL-C, and HDL-C without affecting triglycerides (242). A meta-analysis of three prospective cohorts (3,415 cases) found high adherence to the DASH eating reduced T2DM risk by 27% (243). A strong evidence base from observational studies indicates that high consumption of vegetables and fruits reduces CHD and stroke (196).
b) CVD in HIC and LMIC
Large global studies and systematic reviews of prospective cohorts generally support a protective role of fruits and vegetables against CVD (244–246). In the global INTERSTROKE study (3,000 stroke cases and 3,000 controls), compared with fewer than 1 serving per day, 1 serving of fruit was protective against stroke (OR: 0.61, 95% CI: 0.50 to 0.73), but the benefits were not clear with higher consumption of up to 3 servings/d of vegetables was not t (OR: 0·91; 95% CI: 0.75 to 1.10) (244). A meta-analysis of 20 prospective cohort studies (16,981 stroke events) found that fruit and vegetable consumption was associated with decreased stroke risk (RR: 0.79; 95% CI: 0.75–0.84 for highest vs. lowest categories), as were fruits (RR: 0.77; 95% CI: 0.71–0.84) and vegetables (RR: 0.86; 95% CI: 0.79–0.93) separately (245). In unpublished INTERHEART data a one-serving/day increase in fruit decreased MI risk by 12% (RR: 0.88; 95% CI: 0.84–0.92); a one-serving/day increase in vegetables decreased MI risk by 5% (RR: 0.95; 95% CI: 0.92–0.97). Large meta-analyses of observational studies support a ≈5–10% reduction in CVD mortality per serving per day (246), and a protective association of green leafy vegetables with T2DM (RR per 0.2 servings/day: 0.87; 95% CI: 0.81–0.93) (247). The beneficial effects of fruits and vegetables appear consistent across regions of the world. A major issue has been the lack of success in encouraging increased fruit and vegetable consumption by the citizens of western nations (248).
Overall Summary
Increased refined grains, starches, and added sugar (i.e., carbohydrates of lower quality) has paralleled the rise in obesity and T2DM. Collectively, the data support emphasizing low-GI whole grains, legumes, fruits, and vegetables and minimizing high-GI refined grains and foods with added sugars, including SSBs.
Dietary Sodium
Sodium is an essential nutrient required for normal physiological function (249) (250,251). Increasing sodium intake well beyond physiological requirements increases BP (241–243) and CVD mortality (95–97). In LMIC sodium sources include table salt and salt additives and spices; while processed foods are the primary source in HICs. Sodium consumption has declined slightly in LMIC as refrigeration has replaced salted-preserved foods. RCTs have reported reductions in BP with reduced sodium intake to < 1.5 grams/day. Population- recommendations for low sodium intake (252–254) (< 2.0 grams/day) have been achieved in short-term feeding clinical trials (254), but not sustained in longer-term clinical trials (> 6 months) (255) (256,257). No RCTs have determined whether low sodium intake reduces CVD events or deaths compared with moderate intake (249). Meta-analyses of CVD event trials did not report a significant reduction in CVD with lower sodium intake, but the included trials were underpowered to detect moderate risk reductions (258). Prospective cohort studies suggest a J-shaped association between sodium intake and CVD events, consistent across methods of sodium estimation (259–265). In a recent Cochrane Review (266) of 23 epidemiological studies (n=274,683), the lowest risk of CVD events and deaths occurs at an intake between 2.7 and 5.0 grams/day. PURE study findings (263) are consistent with this evidence, with both high (> 6 grams/day) and low (< 3 grams/day) sodium excretion associated with higher mortality and CVD events compared to average (4.00–5.99 grams/day) sodium excretion despite a positive association between sodium excretion and BP (251,263). The increased risk of CVD events with higher sodium intake (> 5 grams/day) was most prominent in those with hypertension (263). However, methodological issues make studies of sodium intake particularly challenging (267). Large RCTs are needed for definitive evidence on optimal sodium intake for preventing CVD events (249,268–270).
Alcohol
a) Consumption patterns
Greater amounts of alcohol are consumed per capita by adults in HIC (9.6 litres/y) than in LMIC (4.1 litres/y), or LIC (3.1 litres/y), although this may be an underestimate in LMIC and LIC due to the use of homemade or unsafe alcohol products (e.g., industrial or medical) (271). In the PURE study, 31% of participants identified as current drinkers, with considerable variation across income regions (13–80%). In all regions, men were more likely to be current drinkers than women (272).
b) CVD Risk Factors
In a meta-analysis of randomized controlled intervention studies of 2–8 weeks’ duration (273), moderate alcohol consumption (up to one drink or 15 g/d in women; or up to 2 drinks or 30 g/d in men) compared with no alcohol: raised HDL-C, Apo-A1, and adiponectin and reduced LDL-C and fibrinogen. Total cholesterol, triglycerides, and lp (a) were not significantly affected. Results stratified by beverage type (wine/beer/spirits) were similar to the pooled analyses. The changes in lipid and hemostatic risk factors align with lower cardiovascular risk.
c) CVD
The European Prospective Investigation on Cancer and nutrition (EPIC) study monitored 380,395 participants for 13 years (4,187 CVD deaths) (274). Female never-drinkers were at increased risk of CVD death (RR: 1.31; 95% CI: 1.13 to 1.53) compared with moderate drinkers (0.1 to 4.9 g/d); but the association was not significant for male never-drinkers (RR: 1.20; 95% CI: 0.89 to 1.62). In a dose-response meta-analysis of 24 prospective cohort studies (275), the association of drinking with CHD was J-shaped: non-drinkers were at slightly increased risk; in men the greatest protection was at 31 g/d with a trend for increased risk beginning at 63 g/d; for women, the greatest protection was at 11 g/d with a trend for increased risk beginning at 14 g/d. In a meta-analysis of 27 prospective studies of alcohol and stroke, no association was found between light or moderate alcohol use and stroke, but heavy alcohol use (>45 g/d) was shown to increase total stroke risk (RR: 1.20; 95% CI: 1.01 to 1.43), particularly hemorrhagic stroke (RR: 1.29; 95% CI: 0.98 to 1.71) (275). Patterns of alcohol consumption may be a particularly important determinant of the magnitude of the association—in a meta-analysis of 10 prospective cohort and 4 case-control studies, irregular “binge” drinking (>60 g of pure alcohol or >=5 drinks per occasion at least monthly) increased CHD risk by 45% (RR: 1.45; 95% CI: 1.24 to 1.70) (276). In summary, small and inconsistent cardiovascular benefit is seen in moderate drinkers, but a strong increase in CVD and stroke risk is seen with regular heavy alcohol consumption and binge drinking.
d) LMIC
In HIC/UMIC in the PURE study (272), current drinking was protective against MI, but not in LIC/LMIC (p=0·02 for interaction); a similar trend was seen for stroke, but the between-region difference was not significant (P=0.15 for interaction). In LIC and LMIC, heavy episodic drinking pattern and higher intakes of alcohol were associated with a composite outcome of mortality, MI, stroke, cancer, injury, and hospitalization. Current drinking was associated with an increased risk of alcohol-related cancers.
Summary of alcohol
The CVD risk benefits of moderate alcohol consumption (up to 5 g/d) compared with abstinence are small but consistent within individual study populations. Higher levels of consumption increase CVD risk, beginning at 30 g/d in women and 45 g/d in men. Therefore, recommendations for moderate alcohol consumption for CVD prevention in people who already drink must consider individual preferences, population-specific responses, and risk of comorbid conditions, such as addiction and cancers. It not recommended that non-drinkers take up drinking.
Dietary Patterns
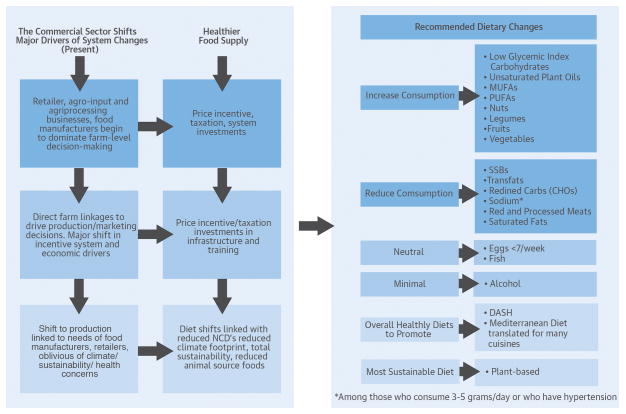
Dietary pattern analysis assesses overall diet quality and is easier to translate into dietary recommendations than recommendations for single foods or nutrients (277). The U.S. Dietary Guidelines Advisory Committee modeled 3 dietary patterns--the Healthy-US type Pattern, the Healthy Mediterranean style Pattern and the Healthy Vegetarian Pattern--for healthfulness and nutritional adequacy. All 3 include components of a dietary pattern associated with health benefits (277). The DASH diet emphasizes fruits, vegetables, whole grains, low-fat dairy products and discourages sugar-sweetened foods and beverages, red meat, and added fats (254). The DASH diet was associated with lower SBP, DBP, and cholesterol. However, the DASH trials were not designed to evaluate the impact of this diet on CVD clinical events. Further improvements in cardiovascular risk factors were seen when part of the carbohydrate in DASH was replaced with either monounsaturated fat or protein (278). In prospective cohort studies, a higher adherence to a DASH dietary pattern is associated with reduced stroke, as well as all-cause, CVD, and cancer mortality (279,280). The Portfolio Diet (281), which includes 30 grams/day of nuts, 20 grams/day of viscous fiber (i.e., oats, barley, legumes), 80 grams/day of vegetable protein (soy, beans, chickpeas, lentils), and 2 grams/day of plant sterols (plant sterol margarine) from plant foods lowers LDL-C by up to 30% within four weeks, similar to statins (281). Reductions of 13–23% are maintained when this diet was extended to 12 months (282,283). However, no RCT has evaluated the impact of this diet on CVD clinical events. Strong evidence from prospective cohort studies and randomized trials shows reduced CHD with the consumption of a higher-fat Mediterranean diet (196). In the PREDIMED randomized trial a plant-based diet high in nuts or extra virgin olive oil, vegetables, fruits, legumes, fish, and poultry but low in red meats, sweets, and whole-fat dairy was superior to the control group assigned to a low-fat Western diet in preventing CVD (164). It bears mention that low-fat diets have not been particularly effective for long-term CVD risk reduction, partly due to the difficulty sustaining such a diet long-term (284,285). Table S1 translates the essence of the Mediterranean diet to other regions and cultures, suggesting replacement foods that maintain the major food groups in particular regions. Randomized trials of CVD outcomes are needed in different regions of the world. Supplementary table 1 provides a Mediterranean Style Diet adapted to various regions of the world. Figure 3 provides an overview of all the components of the food system and the summary of the dietary changes that need to come from food system changes.
Figure 3.
Global food system changes needed to meet create a sustainable healthy diet
IV. HEALTHIER FOOD SYSTEMS
If more people made healthier choices, the food systems would have greater incentives to produce healthier them. At the same time, a healthier food supply enables individuals to make healthier choices. Developing food systems that underpin healthier dietary patterns based on our consensus noted above involves improving the food supply by producing more heart-healthy foods and fewer foods associated with CVD. It extends beyond merely growing and raising these foods to ensuring they reach people everywhere in a still healthy form in a way that is accessible and acceptable, including groups of lower-socioeconomic status. Changing food systems thus extends beyond agricultural production to what happens between farm and fork (31). This is challenging in the modern global food system given the often extensive distance between production and consumption; multiple and complex transformations in ingredients and foods between farm and fork; and a multitude of other forces discouraging diversity and freshness. Producing more poultry, for example, may be seen as a way to enhance the quality of the food supply since it is healthier relative to red meat, but much chicken is transformed into fast food and other calorie-rich, ultra-processed, heavily-advertised presentations (286). Additional public health impacts of industrial poultry production are also well-reported, such as food borne disease (e.g., e coli, salmonella, listeria), antimicrobial residues, and avian flu (287). Clearly families and individuals also play a major role in selecting the quality of their diet, particularly in HIC and among middle- and higher-income LMIC, but our focus herein is on the broader environmental factors affecting this system.
There are also huge political, economic and environmental challenges to changing the food system, not least the capacity of governments to implement change (288). Yet change is possible. It can start with a wider understanding of how the modern food system shapes dietary patterns and eating behaviors, as supported by education that makes clear the interrelationship between food systems, food environments, diets and adverse public health and environmental outcomes, and the potential for better diets to lead to major improvements in public health and environmental sustainability. The pace and effectiveness of improvements will largely depend on political will, encouraged and reinforced by the work of civil society, with health-related professionals having important contributions to make and Dietary Guidelines sensitive to these issues playing a major role to play.
Identifying food system solutions
The task may be daunting, but there are many ways to move towards healthier food systems. There is no one solution but a multitude of both top-down and bottom-up starting points. Many changes need not be drastic and costly, and small modifications can improve nutritional intakes and overall diet quality (see Box 2 for the trans-fat story).
The first “top down” step is to undertake rigorous analytical work at the global level in order to identify the main levers for change for each of the food groups and provide a framework more conducive to the success of bottom-up approaches (289). The bottom up approach starts with engaging communities in finding their own solutions to food system related problems with an emphasis on overall dietary patterns (277,290–292).
Experience indicates actions by the following key actors have potential to improve food systems, many of which can simultaneously have health and environmental benefits:
Governments can review policies in various sectors which influence food systems for coherence with dietary objectives and realign policies so they “do no harm” (293–296); explore fiscal incentives from farm to fork to realign food and agricultural systems; and set a coherent framework of standards and regulations for large transnational food companies to disincentivize the production, sale and marketing of foods high in refined carbohydrates and low in heart-healthy elements, including clear standards for promotional marketing (297,298). Continued efforts are needed to develop a systems-oriented, mutually reinforcing approach to reduce excessive consumption of SSBs (299).
Communities can identify community-based solutions to poor food access, such as through engaging local/small/family farmers and/or innovative community-oriented production and marketing of wholegrains, fruits, vegetables legumes and nuts in low income communities, and developing local food policy councils (300).
Farmers and food producers can leverage the potential of traditional/indigenous crops as part of biodiverse food systems, and support urban food production. Low-input, biodiverse farming systems are more sustainable than high-input monocultures and tend to be more productive per land area, making greater use of ecological space.
Agricultural and rural development agencies and programs can, where wholegrains, fruits, vegetables, legumes and nuts are in short supply in LMIC, invest in transportation, distribution networks, procurement logistics and price information systems to remove supply bottlenecks.
Investors in agriculture and food research and development can shift investments and research toward the production, distribution and consumption of healthier, more diverse sources of nutrients and agricultural programs; make national and international investments in food research aligned with a healthy food supply; and invest in pre- and post-harvest systems to reduce food waste and focusing on recycling in home food waste (101,301).
Food processors and manufacturers of all scales can ensure food processing does not strip fiber and positive nutrients from foods; direct research and marketing to enhance the desirability of nutritious foods to consumers.
Educational institutions can improve curricula and build capacity among nutrition and public health students and professionals to identify and implement multi-sectorial, food systems solutions to unhealthy diets.
In addition, middle- and high-income countries have introduced measures to improve food environments, summarized at www.wcrf.org/NOURISHING. Actions in key countries, mainly introduced in the context of obesity, include (302):
Taxation of sugar-sweetened beverages (SSB’s) and unessential foods (junk foods in general) in Mexico. A number of other LMIC’s are considering these taxes, and several European countries have introduced taxes on different food groups.
Front-of-pack labels (FOP) on packaged food products considered healthy (303) or unhealthy (304). Several Latin American countries have regulated FOP labels, including Ecuador’s traffic light label (implemented in 2014), and Chile’s warning label (being implemented over the next 4 years). Several Asian countries are developing a healthier choices option.
Mandatory School food standards are the most widely implemented action, in around 11 middle-income countries and 15 high-income countries. However the direction of the mandate must be appropriate.
Effective marketing controls of unhealthy foods and beverages have not been widely implemented. Chile’s new program is the most extensive (implemented over the 2015–19 period). Mexico, South Korea, the UK and Ireland present rare exceptions, but the regulations have loopholes and do not comprehensively restrict marketing. Efforts to restrict marketing in other countries through regulation have been met with strong opposition from the food and advertising industries. A critical issue is to go beyond marketing focused at children’s TV to all TV viewed during key hours or full time and also to consider all other media. A second issue is linking marketing controls with the FOP labeling efforts so either only healthy foods are marketed or all unhealthy foods are not allowed to be marketed.
Encouraging food processers to produce vegetable-protein rich foods as meat and dairy alternatives with subsidies/tax incentives, etc.
While the evidence base for these policy option is growing, more, and more rigorous, evaluation of these actions are needed, Econometric evaluations of the Mexican 10% SSB tax and 8% junk food taxes are underway using longitudinal nationally representative scanned food purchase data at the household level. A critical need is much more evaluation of all these options, including to assessments of how they feedback to effect the food system (302,305). Nutrition labels, for example, have been shown to have a clear impact on reformulation (303,306).
V. CONCLUSIONS
This paper has provided a state-of-the-art review of the link between specific macronutrients and foods and CVD and summarized how the global food system contributes to dietary patterns that greatly increase the risks for the population to experience ill health. While many perceive food consumption as an individual choice driven by the desires of consumers for tasty food, linked with rising incomes and urbanization, we have shown that the food system and all the stakeholders within it should play a major role (307).
Short term controlled-feeding studies with CVD risk factors as outcomes, long term prospective cohort studies with CHD, stroke and T2DM as outcomes, and a limited number of RCTs with CVD as the outcome collectively show that multiple aspects of diet substantially influence CVD risk. However, similar data are needed from LMIC as dietary patterns differ in various regions of the world and the context in which foods are accessed differs markedly.
Based on the current evidence, the optimal dietary pattern to reduce CVD is one that emphasizes whole grains, fruits and vegetables, legumes, nuts, fish, poultry, and moderate dairy and heart-healthy vegetable oil intake; this pattern will likely reduce the CVD risk by about a third. This healthy dietary pattern needs also to be low in refined grains, added sugars, trans-fats, SSBs, and red and processed meats. The traditional Mediterranean-type diet provides a well-tested prototype for this healthy dietary pattern. Given that we now understand the components of this diet sufficiently, it may be possible to translate this pattern to other regions, with appropriate similar food replacements based on food availability and preferences (see attached Table S1). Despite significant advances in our understanding of optimal dietary patterns to prevent CVD, additional research including large cohort studies and RCTs of dietary patterns are needed in different regions of the world to address existing knowledge gaps. This includes evaluation of the impact of specific fruits and vegetables, types of dairy foods, types and amount of carbohydrate, optimal cooking oils, regional specific dietary patterns, and cooking methods. Finally, we acknowledge that while this paper is focused on CVD, dietary choices and recommendations should also be made with consideration of the environmental implications of food choice (i.e., the environmental impact of poultry, livestock/cattle production, and diminishing wild fish stocks), and the role of diet in other disease processes. Human health must be linked to environmental health—the basis of the new Sustainable Development Goals (241). Additionally, modifications to our recommendations may provide improved protection against cancer or neurodegenerative or auto-immune diseases.
Policy actions and interventions that improve food supplies and dietary patterns have social, cultural and environmental benefits. There are many opportunities to increase access to healthy food that are also likely to have significant environmental benefits. Health providers throughout the world can lead by advocating for action in the food system, as well as in food environments and behavior change communication. So too can other professionals, civil society and public interest organizations, influential writers and journalists, and organizations of chefs and gastronomes. A synergistic systems approach is essential. The challenge to create and sustain what is healthy and change what is unhealthy is compelling because improving the nourishment that goes into our bodies can have wide-ranging benefits in improving the health of societies and environments. A second major challenge is to identify and implement effective food systems and solutions and evaluate the national and local level policy actions underway to improve our diets.
Supplementary Material
Table 1.
Summary of evidence of associations between major foods and cardiovascular disease.
| Food | HIC | L/MIC | Sustainability | ||||
|---|---|---|---|---|---|---|---|
| Definitely Known | Suggested | Strength of Evidence | Definitely | Suggested | Strength of Evidence | ||
| Refined carbohydrates (solid foods) | Refined, high-glycemic carbohydrates are associated with T2DM and CHD | Refined, high-glycemic carbohydrates adversely affect the lipid profile | High (Prospective cohort, RCT, SRMA of both RCT and Prospective cohorts) | None | Refined, high-glycemic index carbohydrates are associated with T2DM, markers of central adiposity, blood pressure, insulin, and lipids. | Moderate (cross sectional, RCT) | Moderate |
| Sugar-sweetened beverages | Excessive sugar-sweetened beverage consumption is associated with CVD mortality, stroke, obesity, hypertension, and T2DM. | None | High (Prospective cohort, RCT, SRMA of both RCT and prospective cohorts) | None | None | Absent | Low |
| Saturated fats | None | Replacement of saturated fats with unsaturated plant fats or low-glycemic carbohydrates reduces the risk of cardiovascular disease, and reduces LDL-cholesterol, without adversely affecting HDL-C. | High (Prospective cohort, RCT, SRMA of both RCT and prospective cohorts) | None | None | None | Low; higher saturated fat from palm oil is unsustainable |
| Trans fatty acids | Replacement of trans fatty acids with unsaturated plant fats or low-glycemic carbohydrates reduces the risk of CVD, and reduces LDL-cholesterol, without adversely affecting HDL-C. | None | High (Prospective cohort, RCT, SRMA of both RCT and prospective cohorts) | None | None | None | low |
| Unsaturated fatty acids (MUFA, PUFA) | Diets higher in unsaturated plant fats (mono-unsaturated and polyunsaturated fats from plants and fish) reduce risk of CVD, and improve the lipid profile. | None | High (Prospective cohort, RCT, SRMA of both RCT and prospective cohorts) | None | None | None | Low |
| Red and processed meat | Red and processed meats are associated with CHD and T2DM | Red and processed meats are associated with stroke. | Moderate (SRMA of prospective cohorts, prospective cohorts) | None | No association between red meat consumption and CVD in LMIC. | Low (one pooled analysis) | Very HighMeat production is harmful to the environment |
| Dairy | None | Higher low-fat and fermented dairy products are inversely associated with CVD, blood pressure, and improved serum lipids. | Moderate (SRMA of prospective cohorts, prospective cohorts, RCTs, SRMA of RCTs) | None | Consuming dairy is associated with reduced risk of T2DM | Low (single prospective cohort) | Very High Cow’s milk production is harmful to the environment; resource -intensive |
| Eggs | None | Consuming <7 eggs per week does not increase risk of CVD, nor do they affect the lipid profile | Low (SRMA of prospective cohorts, SRMA of RCTS on lipids) | None | Moderate egg consumption appears protective against CVD | Low (unpublished case-control data from 3 large international studies) | Moderate |
| Fish | None | Long chain omega-3 fats from fish reduce multiple CVD risk factors | Low (SRMA of prospective cohorts, large RCTs that conflict) | None. | Considerable heterogeneity in the association between fish and CVD outcomes | Low (unpublished case-control data from 3 large international studies) | Sustainability of fish is challenging |
| Nuts | Many varieties of nuts show beneficial effects on LDL-C, the LDL-C:HDL-C ratio, TC, and triglycerides; and nuts reduce CHD risk and hypertension, but not stroke. | Nuts may reduce risk of T2DM | High (Prospective cohort, RCT, SRMA of both RCT and prospective cohorts) | None | Nuts are not associated with CVD outcomes | Weak (single observational study) | Sustainable alternative protein source to meat |
| Legumes | Legumes show beneficial effects on LDL-C, the LDL-C:HDL-C ratio, TC, and triglycerides; and nuts reduce CHD risk and hypertension, but not stroke. | Legumes reduce CHD risk, but not stroke risk | High (Prospective cohort, RCT, SRMA of both RCT and prospective cohorts) | None | Legume consumption reduces risk of CHD, type 2 diabetes. | Weak (small number of prospective cohort studies, cross sectional association) | Sustainable alternative protein source to meat |
| Fruits and vegetables | Diets high in fruits and vegetables reduce BP, TC, LDL-C, and HDL-C and protect against CVD. | None | High (Prospective cohort, RCT, SRMA of both RCT and prospective cohorts) | None | Protective role of fruits and vegetables against CVD | Weak (small number of prospective studies) | Sustainable source of carbohydrate energy and micronutrients |
| Sodium | None | Lower sodium diets reduce blood pressure, but the association between lower sodium and CVD is unclear. | Low (conflicting observational and RCT data) | None | Excessive or low-sodium diets are associated with CVD | Low (single multi-country study) | Low |
| Alcohol | Compared with non-alcoholic beverages, alcoholic beverages raise HDL-C. | Compared with not drinking, moderate drinking (up to 5 g ethanol/d) may reduce risk of CVD; but higher intakes(>30 g/d and above) increase risk of hemorrhagic stroke and cancers | Low (Prospective cohort, SRMA of prospective cohorts, SRMA of RCT) | None | Alcohol does not reduce risk of MI in LMIC | Low (single multi-country study) | Low |
| DASH Dietary Pattern | Following a DASH dietary pattern lowers blood pressure and serum lipid risk factors for CVD. | Higher DASH diet scores are associated with reduced all-cause mortality, CVD, and cancer mortality. | Moderate (RCT data, prospective cohorts, but no long-term event trials) | None | None | None | Low if only plant-based; Moderate when uses low-fat dairy as key aspect |
| Dietary Portfolio | Following the Dietary Portfolio approach lowers blood pressure and serum lipid risk factors for CVD. | none | Moderate (strong RCT data, but no event trials) | None | None | None | Focus on plant foods enhances sustainability |
| Mediterranean Diet | Following a Mediterranean Diet is associated with reduced CVD, blood pressure and serum lipid risk factors for CVD. | none | High (SRMA of both RCT and prospective cohorts, prospective cohorts, RCTs) | None | None | None | Low |
Acknowledgments
This review comes out of a World Heart Federation consensus conference health held May 14–16 at the Population Health Research Institute, Hamilton Health Sciences and McMaster University in Hamilton, Ontario, Canada. We thank all participants and presenters for their thoughtful discussion.
Abbreviations
- CVD
cardiovascular disease
- LMIC
low and middle income countries
- HIC
high income country
- GI
glycemic index
- GL
glycemic load
- T2DM
type 2 diabetes
- MI
myocardial infarction
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- TC
total cholesterol
- CURES 57
Chennai Urban Rural Epidemiology Study 57
- BP
blood pressure
- SBP
diastolic blood pressure
- DBP
diastolic blood pressure
- PURE
Prospective Urban and Rural Epidemiological Study
- SSB
sugar-sweetened beverage
- NHANES
National Health and Nutrition Examination Survey
- RCT
randomized controlled trial
- CI
confidence interval
- WHO
World Health Organization
- BMI
body mass index
- mm Hg
millimeters of mercury
- CHD
coronary heart disease
- NHS
Nurses’ Health Study
- HPFS
Health Professionals Follow-up Study
- RR
risk ratio
- CSD
carbonated soft drink
- OR
odds ratio
- HR
hazard ratio
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- ALA
alpha-linolenic acid
- DART
Diet and Reinfarction Trial
- DART-2
Diet and Angina Randomized Trial
- mmol/L
milimol/liter
- HbA1c
glycated hemoglobin
Footnotes
The 2014 Consensus Conference on Nutrition was held May 14–16, 2014 at The Population Health Research Institute - 20 Copeland Avenue, Hamilton, Ontario, Canada.
No funding and conflicts of interest exist among the authors related to this paper.
DUALITY OF INTEREST STATEMENT
Dr. Ronald Krauss reports holding grants from the US National Dairy Council and the Almond Board of California.
Dr. Miguel Martinez reports a research contract with Danone to support research on yogurt in the SUN cohort, and a departmental grant from the International Nut Council
Dr. Russell de Souza reports serving as an external resource person on trans and saturated fats to the World Health Organization’s Nutrition Guidelines Advisory Group.
Dr. David Jenkins reports serving on the Scientific Advisory Board of Unilever, Sanitarium Company, California Strawberry Commission, Loblaw Supermarket, Herbal Life International, Nutritional Fundamental for Health, Pacific Health Laboratories, Metagenics, Bayer Consumer Care, Orafti, Dean Foods, Kellogg’s, Quaker Oats, Procter & Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Pulse Canada, Saskatchewan Pulse Growers, and Canola Council of Canada; receiving honoraria for scientific advice from the Almond Board of California, International Tree Nut Council Nutrition Research and Education Foundation, Barilla, Unilever Canada, Solae, Oldways, Kellogg’s, Quaker Oats, Procter & Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Canola Council of Canada, Dean Foods, California Strawberry Commission, Haine Celestial, and Alpro Foundation; being on the speakers panel for the Almond Board of California; receiving research grants from Loblaw Brands Ltd, Unilever, Barilla, Almond Board of California, Solae, Haine Celestial, Sanitarium Company, Orafti, International Tree Nut Council, and Peanut Institute; and receiving travel support to attend meetings from the Almond Board of California, Unilever, Alpro Foundation, and International Tree Nut Council, Canadian Institutes for Health Reseach, Canada Foundation for Innovation, and the Ontario Research Fund. Dr. Jenkins receives salary support as a Canada Research Chair from the federal government of Canada. Dr Jenkins’ wife is a director of Glycemic Index Laboratories, Toronto, Ontario, Canada.
Dr. Dariush Mozzafarrian reports being on the scientific advisory board of Unilever North America; and ad hoc honoraria or consulting from Bunge, Nutrition Impact, Amarin, Astra Zeneca, and Life Sciences Research Organization.
Dr. Barry Popkin has received funding to speak on SSB behaviors globally from Danome water research center at two international conferences in the past 5 years and was a co-investigator to a water vs SSB RCT funded by this same organization to the Mexican National Institute of Public Health in Cuernevaca..
Drs. Sonia Anand, Adam Bernstein, Vasanti Malik, Walter Willett, Salim Yusuf, Andrew Mente, Corrina Hawkes, Rachel Nugent, Tony Weis, Michael Zulyniak, Daan Kromhout, and Mahshid Deghan declare no duality of interest in relation to the contents of this paper..
References
- 1.Imamura F, Micha R, Khatibzadeh S, et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Global Health. 2015;3:e132–e142. doi: 10.1016/S2214-109X(14)70381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micha R, Khatibzadeh S, Shi P, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. 2014 doi: 10.1136/bmj.g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Fact Sheet No. 311. 2014. Obesity and overweight. [Google Scholar]
- 5.Fund WCR, editor. WCRF-AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. London: World Cancer Research Fund; 2007. p. 517. [Google Scholar]
- 6.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 8.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Agricultural Organization of the United Nations. Second International Conference on Nutrition (ICN2) Rome: FAOUN; 2014. [Google Scholar]
- 10.Fan S, Brzeska J. Building a resilient global food system by lowering food price spikes and volatility. Washington DC: International Food Policy Reseasrch Institute; 2014. p. 4. [Google Scholar]
- 11.Babu SC, Blom S. Resilience Conference Brief. Washington DC: International Food Policy Research Institute; 2014. Strengthening capacity for resilient food systems. [Google Scholar]
- 12.Weis T. The Global Food Economy: The Battle for the Future of Farming. London: Zed Books; 2007. [Google Scholar]
- 13.Reardon T, Chen KZ, Minten B, et al. The quiet revolution in Asia’s rice value chains. Ann NY Acad Sci. 2014;1331:106–118. doi: 10.1111/nyas.12391. [DOI] [PubMed] [Google Scholar]
- 14.Gardner BL. American agriculture in the twentieth century: how it flourished and what it cost. Cambridge, MA: Harvard University Press; 2002. [Google Scholar]
- 15.Yudkin J. Sweet and Dangerous: The New Facts About the Sugar You Eat As a Cause of Heart Disease, Diabetes, and Other Killers. New York City: Bantam Books; 1972. [Google Scholar]
- 16.Mintz S. Sweetness and Power: The Place of Sugar in Modern History. New York City: Penguin; 1986. [Google Scholar]
- 17.Galloway J. Sugar. In: Kiple K, Ornelas K, editors. The Cambridge World History of Food 2000. New York, NY: Cambridge University Press; 2000. pp. 437–49. [Google Scholar]
- 18.Popkin B. The World Is Fat: The Fads, Trends, Policies, and Products That Are Fattening the Human Race. New York: Avery-Penguin Group; 2008. [Google Scholar]
- 19.Rashid S, Gulati A, Cummings R. From Parastatals to Private Trade: Lessons from Asian Agriculture. Baltimore: Johns Hopkins University Press; 2008. [Google Scholar]
- 20.Anderson K. The Political Economy of Agricultural Price Distortions. Cambridge University Press; 2010. [Google Scholar]
- 21.World Bank. World development report 2008. Agriculture for development; World Bank; 2007. [Google Scholar]
- 22.Pinstrup-Andersen P. Food Subsidies in Developing Countries: Costs, Benefits, and Policy Options. Johns Hopkins University Press; 1988. [Google Scholar]
- 23.Lang T, Heasman M. Food Wars: The Global Battle for Mouths Minds and Market. London: Routledge; 2003. [Google Scholar]
- 24.Connor J, Schiek W. Food Processing: An Industrial Powerhouse in Transition. NYC: John Wiley and Sons; 1997. [Google Scholar]
- 25.Wilkinson J. The food processing industry, globalization and developing countries. Electron J Agr Dev Econ. 2004;1:184–201. [Google Scholar]
- 26.Welch R, Mitchell P. Food processing: a century of change. Br Med Bull. 2000;56:1–17. doi: 10.1258/0007142001902923. [DOI] [PubMed] [Google Scholar]
- 27.Fellows P. Food Processing Technology: Principles and Practic. 3. Cambridge, UK: Woodhead Publishing; 2009. [Google Scholar]
- 28.Moss M. Salt Sugar Fat: How the Food Giants Hooked Us. New York City: Random House; 2013. [Google Scholar]
- 29.Packard V. The Hidden Persuaders. New York: David McKay Company; 1957. [Google Scholar]
- 30.Burch D, Lawrence G. Supermarkets and Agri-Food Supply Chains: Transformations in the Production and Consumption of Foods. Cheltenham UK: Edward Elgar Publishing Ltd; 2007. [Google Scholar]
- 31.Hawkes C, Friel S, Lobstein T, Lang T. Linking agricultural policies with obesity and noncommunicable diseases: A new perspective for a globalising world. Food Policy. 2012;37:343–353. [Google Scholar]
- 32.Starmer E, Witteman A, Wise TA. GDAE Working Paper. Medford, MA: Tufts University; 2006. Feeding the Factory Farm: Implicit Subsidies to the Broiler Chicken Industry. [Google Scholar]
- 33.Schaffer HD, Hunt DB, Ray DE. US Agricultural Commodity Policy and its Relationship to Obesity. Agricultural Policy Analysis Center University of Tennessee; Knoxville: 2007. p. 31. [Google Scholar]
- 34.Weis T. The Ecological Hoofprint: The Global Burden of Industrial Livestock. London: Zed Books; 2013. [Google Scholar]
- 35.Neven D, Odera M, Reardon T, Wang H. Kenyan supermarkets, emerging middle-class horticultural farmers, and employment impacts on the rural poor. World Dev. 2009;37:1802–1811. [Google Scholar]
- 36.Reardon T, Chen KZ, Minten B, et al. The quiet revolution in Asia’s rice value chains. Ann NY Acad Sci. 2014:106–118. doi: 10.1111/nyas.12391. [DOI] [PubMed] [Google Scholar]
- 37.Reardon T, Timmer CP. The economics of the food system revolution. Ann Rev Res Econ. 2012;4:225–264. [Google Scholar]
- 38.Khandelwal S, Reddy K. Eliciting a policy response for the rising epidemic of overweight-obesity in India. Obes Rev. 2013;14:114–125. doi: 10.1111/obr.12097. [DOI] [PubMed] [Google Scholar]
- 39.Wilde PE. Food Policy in the United States: An Introduction. New York: Routledge/Earthscan; 2013. [Google Scholar]
- 40.Institute for Agriculture and Trade Policy. Food without Thought: How US Farm Policy Contributes to Obesity. Minneapolis: The Institute for Agriculture and Trade Policy; 2006. p. 14. [Google Scholar]
- 41.Hawkes C, Murphy S. An overview of global food trade. In: Hawkes C, Blouin C, Henson S, Drager N, Dubé L, editors. Trade, Food, Diet and Health: Perspectives and Policy Options. Wiley-Blackwell; 2010. pp. 16–32. [Google Scholar]
- 42.Bruinsma J. World agriculture: towards 2015/2030 Summary Report. Rome: Food and Agriculture Organization; 2003. p. 97. [Google Scholar]
- 43.Adair LS, Popkin BM. Are child eating patterns being transformed globally? Obes Res. 2005;13:1281–99. doi: 10.1038/oby.2005.153. [DOI] [PubMed] [Google Scholar]
- 44.Duffey K, Pereira R, Popkin B. Prevalence and energy intake from snacking in Brazil: analysis of the first nationwide individual survey. Eur J Clin Nutr. 2013;67:868–74. doi: 10.1038/ejcn.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng S, Zaghloul S, Ali H, et al. Nutrition transition in the United Arab Emirates. Eur J Clin Nutr. 2011 doi: 10.1038/ejcn.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popkin B, Duffey K. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. Am J Clin Nutr. 2010;91:1342–7. doi: 10.3945/ajcn.2009.28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Zhai F, Du S, Popkin B. Dynamic shifts in Chinese eating behaviors. Asia Pac J Clin Nutr. 2008;17:123–30. [PubMed] [Google Scholar]
- 48.Duffey KJ, Rivera J, Popkin B. Snacking is prevalent in Mexico. J Nutr. 2014:144. doi: 10.3945/jn.114.198192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monteiro C, Gomes F, Cannon G. The snack attack. Am J Public Health. 2010;100:975–981. doi: 10.2105/AJPH.2009.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monteiro C, Moubarac J, Cannon G, Ng S, Popkin BM. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14:21–28. doi: 10.1111/obr.12107. [DOI] [PubMed] [Google Scholar]
- 51.Poti JM, Mendez MA, Ng SW, Popkin BM. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr. 2015:101. doi: 10.3945/ajcn.114.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231–7. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 53.Reddy S, Katan M. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004;7:167–86. doi: 10.1079/phn2003587. [DOI] [PubMed] [Google Scholar]
- 54.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–9. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 55.Duffey KJ, Popkin BM. High-fructose corn syrup: is this what’s for dinner? Am J Clin Nutr. 2008;88:1722S–1732S. doi: 10.3945/ajcn.2008.25825C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu S, Yoffe P, Hills N, Lustig R. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. Plos One. 2013;8:e57873. doi: 10.1371/journal.pone.0057873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleiman S, Ng SW, Popkin B. Drinking to our health: can beverage companies cut calories while maintaining profits? Obes Rev. 2012;13:258–74. doi: 10.1111/j.1467-789X.2011.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14:21–28. doi: 10.1111/obr.12107. [DOI] [PubMed] [Google Scholar]
- 59.Monteiro CA, Levy RB, Claro RM, de Castro IR, Cannon G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. 2011;14:5–13. doi: 10.1017/S1368980010003241. [DOI] [PubMed] [Google Scholar]
- 60.Ng SW, Slining MM, Popkin BM. Use of caloric and noncaloric sweeteners in US consumer packaged foods, 2005–2009. J Acad Nutr Diet. 2012;112:1828–1834. doi: 10.1016/j.jand.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57) Metabolism. 2009;58:675–681. doi: 10.1016/j.metabol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 62.Wang L. Report of China nationwide nutrition and health survey 2002 (1): summary report. Beijing: People’s Medical Publishing House; 2005. pp. 18–45. [Google Scholar]
- 63.Drewnowski A, Popkin BM. The nutrition transition: new trends in the global diet. Nutr Rev. 1997;55:31–43. doi: 10.1111/j.1753-4887.1997.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 64.Aldersey C. Consumer Trends & Usage of Fats & Oils. 14th Biennial Sunflower Conference June 2003; Australia. 2003. [Google Scholar]
- 65.Micha R, Khatibzadeh S, Shi P, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. Br Med J. 2014:348. doi: 10.1136/bmj.g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delgado C, Rosegrant M, Steinfield H, Ehui S, Courbois C. Livestock to 2020: The Next Food Revolution. Food, Agriculture, and the Environment; Washington, D.C: International Food Policy Research Institute; 1999. [Google Scholar]
- 67.Delgado CL. Rising consumption of meat and milk in developing countries has created a new food revolution. J Nutr. 2003;133:3907S–3910S. doi: 10.1093/jn/133.11.3907S. [DOI] [PubMed] [Google Scholar]
- 68.Popkin BM, Du S. Dynamics of the nutrition transition toward the animal foods sector in China and its implications: a worried perspective. J Nutr. 2003;133:3898S–3906S. doi: 10.1093/jn/133.11.3898S. [DOI] [PubMed] [Google Scholar]
- 69.Food and Agricultural Organization of the United Nations. Livestock’s long shadow: environmental issues and options. Rome: Food and Agricultural Organization United Nations; 2007. [Google Scholar]
- 70.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: A prospective study of over half a million people. Arch Intern Med. 2009;169:562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci. 2010;365:2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anand SS, Dehghan M, de Souza R, et al. Dietary Patterns and Macronutrients and CVD – A Summary Statement. The World Heart Federation Nutrition Conference; Ontario, Canada. 2014. [Google Scholar]
- 73.Food and Agriculture Organization of the United Nations. The State of Food and Agriculture, 2009. Rome: 2009. [Google Scholar]
- 74.Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. Rome: 2012. [Google Scholar]
- 75.Watson R, Pauly D. Systematic distortions in world fisheries catch trends. Nature. 2001;414:534–536. doi: 10.1038/35107050. [DOI] [PubMed] [Google Scholar]
- 76.Economic Research Service/USDA. International Agriculture and Trade Outlook. 2003. p. 47. [Google Scholar]
- 77.Schneider AVC. Overview of the market and consumption of puises in Europe. Br J Nutr. 2002;88:243–250. doi: 10.1079/BJN2002713. [DOI] [PubMed] [Google Scholar]
- 78.Oniang O, Mutuku J, Malaba SJ. Contemporary African food habits and their nutritional and health implications. Asia Pac J Clin Nutr. 2003;12:331–336. [PubMed] [Google Scholar]
- 79.Aykroyd WR, Doughty J, Walker A. Legumes in Human Nutrition. Food & Agriculture Organization; 1982. [PubMed] [Google Scholar]
- 80.Maredia M. Global pulse production and consumption trends: the potential of pulses to achieve ‘feed the future’ food and nutritional security goals. 2012 [Google Scholar]
- 81.International Nutrition & Dried Fruit Council. Global Statistical Review 2007–2012. 2012. [Google Scholar]
- 82.Zhou Y, Du S, Su C, Zhang B, Wang H, Popkin B. The food retail revolution in China and its association with diet and health. Food Policy. doi: 10.1016/j.foodpol.2015.07.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Popkin BM. Nutrition, agriculture and the global food system in low and middle income countries. Food Policy. 2014;47:91–96. doi: 10.1016/j.foodpol.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Slining MM, Ng SW, Popkin BM. Food companies’ calorie-reduction pledges to improve U.S. diet. Am J Prev Med. 2013;44:174–84. doi: 10.1016/j.amepre.2012.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monteiro CA, Cannon G. The impact of transnational “Big Food” cmpanies on the south: A view from Brazil. PLoS Med. 2012;9:e1001252. doi: 10.1371/journal.pmed.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monteiro C, Levy R, Claro R, de Castro I, Cannon G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. 2011;14:5–13. doi: 10.1017/S1368980010003241. [DOI] [PubMed] [Google Scholar]
- 87.Ng SW, Popkin BM. The Healthy Weight Commitment Foundation Marketplace Commitment and Consumer Packaged Goods purchased by US households with children. Am J Prev Med. 2014;47:508–519. doi: 10.1016/j.amepre.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blisard WN, Stewart H, Jolliffe D. Agriculture UDo, editor. Low-income households’ expenditures on fruits and vegetables. Washington, DC: Economic Research Service; 2004. [Google Scholar]
- 89.Hall K, Guo J, Dore M, Chow C. The progressive increase of food waste in America and its environmental impact. Plos One. 2009;4:e7940. doi: 10.1371/journal.pone.0007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention. Fruit and vegetable consumption among adults--United States, 2005. Morb Mortal Wkly Rep. 2007;56:213–7. [PubMed] [Google Scholar]
- 91.Wang Y, Ge K, Popkin BM. Why do some overweight children remain overweight, whereas others do not? Public Health Nutr. 2003;6:549–58. doi: 10.1079/phn2003470. [DOI] [PubMed] [Google Scholar]
- 92.Imamura F, Micha R, Khatibzadeh S, et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. The Lancet Global Health. 2015;3:e132–e142. doi: 10.1016/S2214-109X(14)70381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McMichael AJ, Powles JW, Butler CD, Uauy R. Food, livestock production, energy, climate change, and health. Lancet. 2007;370:1253–1263. doi: 10.1016/S0140-6736(07)61256-2. [DOI] [PubMed] [Google Scholar]
- 94.Hoekstra A, Chapagain A. Globalization of Water: Sharing the Planet’s Freshwater Resources. New York City: Wiley-Blackwell; 2008. [Google Scholar]
- 95.Hoekstra AY, Chapagain AK. Water footprints of nations: Water use by people as a function of their consumption pattern. Water Resour Manage. 2007;21:35–48. [Google Scholar]
- 96.McIntyre B, Herren H, Wakhungu J, Watson R. International Assessment of Agricultural Knowledge, Science and Technology for Development: Agriculture at a Crossroads. Washington DC: Island Press; 2009. [Google Scholar]
- 97.Hertel TW, Burke MB, Lobell DB. The poverty implications of climate-induced crop yield changes by 2030. Global Environ Change. 2010;20:577–585. [Google Scholar]
- 98.Cline WR. Global Warming and Agriculture: Impact Estimates by Country. Washington DC: Peter G. Peterson Institute for International Economics; 2007. [Google Scholar]
- 99.Pachauri R, Meyer L, editors. Intergovernmental Panel on Climate Change, Working Groups I IaIttFARotIPoCC. Climate Change 2014: Synthesis Report. Geneva, Switzerland: 2014. p. 151. [Google Scholar]
- 100.Food and Agricultural Organization. Food wastage footprint: Impacts on natural resources: Summary Report. Rome: Food and Agricultural Organizaiton of the United Nations; 2013. p. 61. [Google Scholar]
- 101.Hall KD, Guo J, Dore M, Chow CC. The progressive increase of food waste in America and its environmental impact. Plos One. 2009;4:e7940. doi: 10.1371/journal.pone.0007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parfitt J, Barthel M, Macnaughton S. Food waste within food supply chains: quantification and potential for change to 2050. Phil Trans R Soc B: Bio Sci. 2010;365:3065–3081. doi: 10.1098/rstb.2010.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100:218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 105.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–7. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 106.Jakobsen MU, Dethlefsen C, Joensen AM, et al. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010;91:1764–8. doi: 10.3945/ajcn.2009.29099. [DOI] [PubMed] [Google Scholar]
- 107.Mirrahimi A, de Souza RJ, Chiavaroli L, et al. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc. 2012;1:e000752. doi: 10.1161/JAHA.112.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mattei J, Hu FB, Campos H. A higher ratio of beans to white rice is associated with lower cardiometabolic risk factors in Costa Rican adults. Am J Clin Nutr. 2011;94:869–76. doi: 10.3945/ajcn.111.013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 110.Voight B, Peloso G, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28:845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 113.Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170:961–9. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–27. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 115.Mattei J, Malik V, Wedick NM, et al. A symposium and workshop report from the Global Nutrition and Epidemiologic Transition Initiative: nutrition transition and the global burden of type 2 diabetes. Br J Nutr. 2012;108:1325–1335. doi: 10.1017/S0007114512003200. [DOI] [PubMed] [Google Scholar]
- 116.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low–Glycemic Index Diets in the Management of Diabetes: A meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 117.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87:258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 118.Jenkins DA, Kendall CC, McKeown-Eyssen G, et al. Effect of a low–glycemic index or a high–cereal fiber diet on type 2 diabetes: A randomized trial. JAMA. 2008;300:2742–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- 119.Goff LM, Cowland DE, Hooper L, Frost GS. Low glycaemic index diets and blood lipids: A systematic review and meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis. 2013;23:1–10. doi: 10.1016/j.numecd.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 120.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174:516–24. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.National Cancer Institute. Sources of calories from added sugars among the US population, 2005–06. 2014. [Google Scholar]
- 122.Kleiman S, Ng SW, Popkin B. Drinking to our health: can beverage companies cut calories while maintaining profits? Obes Rev. 2012;13:258–274. doi: 10.1111/j.1467-789X.2011.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xi B, Huang Y, Reilly KH, et al. Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr. 2015;113:709–17. doi: 10.1017/S0007114514004383. [DOI] [PubMed] [Google Scholar]
- 124.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. Br Med J. 2013:346. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 126.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 128.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–83. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xi B, Huang Y, Reilly KH, et al. Sugar-sweetened beverages and risk of hypertension and CVD: a dose–response meta-analysis. Br J Nutr. 2015;113:709–717. doi: 10.1017/S0007114514004383. [DOI] [PubMed] [Google Scholar]
- 130.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 131.Janssens JP, Shapira N, Debeuf P, et al. Effects of soft drink and table beer consumption on insulin response in normal teenagers and carbohydrate drink in youngsters. Eur J Cancer Prev. 1999;8:289–296. doi: 10.1097/00008469-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 132.De Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125:1735–1741. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95:1190–9. doi: 10.3945/ajcn.111.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Larsson SC, Åkesson A, Wolk A. Sweetened beverage consumption is associated with increased risk of stroke in women and men. J Nutr. 2014;144:856–860. doi: 10.3945/jn.114.190546. [DOI] [PubMed] [Google Scholar]
- 136.Odegaard AO, Koh W-P, Arakawa K, Yu MC, Pereira MA. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: The Singapore Chinese Health Study. Am J Epidemiol. 2010;171:701–708. doi: 10.1093/aje/kwp452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hernández-Cordero S, Barquera S, Rodríguez-Ramírez S, et al. Substituting water for sugar-sweetened beverages reduces circulating triglycerides and the prevalence of metabolic syndrome in obese but not in overweight Mexican women in a randomized controlled trial. J Nutr. 2014;144:1742–1752. doi: 10.3945/jn.114.193490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jenkins DJA, Kendall CWC, Vuksan V, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: A randomized controlled trial. Diabetes Care. 2014;37:1806–14. doi: 10.2337/dc13-2990. [DOI] [PubMed] [Google Scholar]
- 139.Brouwer IA, Wanders AJ, Katan MB. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans--a quantitative review. Plos One. 2010;5:e9434. doi: 10.1371/journal.pone.0009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bendsen NT, Christensen R, Bartels EM, Astrup A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: a systematic review and meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65:773–783. doi: 10.1038/ejcn.2011.34. [DOI] [PubMed] [Google Scholar]
- 141.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 142.Marshall JA, Hamman RF, Baxter J. High-fat, low-carbohydrate diet and the etiology of non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol. 1991;134:590–603. doi: 10.1093/oxfordjournals.aje.a116132. [DOI] [PubMed] [Google Scholar]
- 143.Riserus U. Fatty acids and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2008;11:100–5. doi: 10.1097/MCO.0b013e3282f52708. [DOI] [PubMed] [Google Scholar]
- 144.Lindstrom J, Peltonen M, Eriksson JG, et al. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia. 2006;49:912–20. doi: 10.1007/s00125-006-0198-3. [DOI] [PubMed] [Google Scholar]
- 145.Meyer K, Kushi L, Jacobs D, Folsom A. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care. 2001;24:1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- 146.Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73:1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 147.Simila ME, Kontto JP, Valsta LM, Mannisto S, Albanes D, Virtamo J. Carbohydrate substitution for fat or protein and risk of type 2 diabetes in male smokers. Eur J Clin Nutr. 2012;66:716–21. doi: 10.1038/ejcn.2012.24. [DOI] [PubMed] [Google Scholar]
- 148.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The Women’s Health study. Diabetes Care. 2004;27:2108–2115. doi: 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- 149.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25:417–424. doi: 10.2337/diacare.25.3.417. [DOI] [PubMed] [Google Scholar]
- 150.Vessby B, Uusitupa M, Hermansen K, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44:312–9. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 151.Perez-Jimenez F, Lopez-Miranda J, Pinillos MD, et al. A Mediterranean and a high-carbohydrate diet improve glucose metabolism in healthy young persons. Diabetologia. 2001;44:2038–43. doi: 10.1007/s001250100009. [DOI] [PubMed] [Google Scholar]
- 152.Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA Intern Med. 2013;173:1328–35. doi: 10.1001/jamainternmed.2013.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes--an updated review of the evidence. Curr Atheroscler Rep. 2012;14:515–24. doi: 10.1007/s11883-012-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao D, Ning N, Wang C, et al. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. Plos One. 2013;8:e73965. doi: 10.1371/journal.pone.0073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sluijs I, Forouhi NG, Beulens JW, et al. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr. 2012;96:382–390. doi: 10.3945/ajcn.111.021907. [DOI] [PubMed] [Google Scholar]
- 156.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–46. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–32. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farvid MS, Ding M, Pan A, et al. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.de Oliveira Otto MC, Mozaffarian D, Kromhout D, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012;96:397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu FB, Stampfer MJ, Manson JE, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–8. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- 162.Halton TL, Willett WC, Liu S, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 163.de Lorgeril M, Renaud S, Salen P, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 164.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 165.Kabagambe EK, Baylin A, Ascherio A, Campos H. The type of oil used for cooking is associated with the risk of nonfatal acute myocardial infarction in Costa Rica. J Nutr. 2005;135:2674–2679. doi: 10.1093/jn/135.11.2674. [DOI] [PubMed] [Google Scholar]
- 166.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002;132:3522S–3525S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 167.Bastide NM, Pierre FHF, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res. 2011;4:177–184. doi: 10.1158/1940-6207.CAPR-10-0113. [DOI] [PubMed] [Google Scholar]
- 168.Joosen AMCP, Kuhnle GGC, Aspinall SM, et al. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009;30:1402–1407. doi: 10.1093/carcin/bgp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rohrmann S, Overvad K, Bueno-de-Mesquita HB, et al. Meat consumption and mortality--results from the European prospective investigation into cancer and nutrition. BMC Med. 2013;11:63. doi: 10.1186/1741-7015-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–63. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–83. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee JE, McLerran DF, Rolland B, et al. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr. 2013;98:1032–1041. doi: 10.3945/ajcn.113.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:735–747. doi: 10.3945/ajcn.112.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kai SHY, Bongard V, Simon C, et al. Low-fat and high-fat dairy products are differently related to blood lipids and cardiovascular risk score. Eur J Prev Cardiol. 2013;21:1557–67. doi: 10.1177/2047487313503283. [DOI] [PubMed] [Google Scholar]
- 176.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 177.Fumeron F, Lamri A, Khalil CA, et al. Dairy consumption and the incidence of hyperglycemia and the metabolic syndrome results from a French prospective study, Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2011;34:813–817. doi: 10.2337/dc10-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rideout TC, Marinangeli C, Martin H, Browne RW, Rempel CB. Consumption of low-fat dairy foods for 6 months improves insulin resistance without adversely affecting lipids or bodyweight in healthy adults: a randomized free-living cross-over study. Nutr J. 2013;12:1–9. doi: 10.1186/1475-2891-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: A prospective study. Arch Internal Med. 2005;165:997–1003. doi: 10.1001/archinte.165.9.997. [DOI] [PubMed] [Google Scholar]
- 180.Liu S, Choi HK, Ford E, et al. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29:1579–1584. doi: 10.2337/dc06-0256. [DOI] [PubMed] [Google Scholar]
- 181.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. 2010;45:925–939. doi: 10.1007/s11745-010-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65:1027–1031. doi: 10.1038/ejcn.2011.62. [DOI] [PubMed] [Google Scholar]
- 183.Chen M, Sun Q, Giovannucci E, et al. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12:215. doi: 10.1186/s12916-014-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Umesawa M, Iso H, Date C, et al. Dietary intake of calcium in relation to mortality from cardiovascular disease the JACC Study. Stroke. 2006;37:20–26. doi: 10.1161/01.STR.0000195155.21143.38. [DOI] [PubMed] [Google Scholar]
- 185.Zong G, Sun Q, Yu D, et al. Dairy consumption, type 2 diabetes and changes in cardiometabolic traits-a prospective cohort study of middle-aged and older Chinese in Beijing and Shanghai. Diabetes Care. 2013;37:56–63. doi: 10.2337/dc13-0975. [DOI] [PubMed] [Google Scholar]
- 186.Qureshi AI, Suri MFK, Ahmed S, Nasar A, Divani AA, Kirmani JF. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med Sci Monit. 2006;13:CR1–CR8. [PubMed] [Google Scholar]
- 187.McCance RA, Widdowson EM. Medical Research Council, editor. The composition of foods. 1960. [PubMed] [Google Scholar]
- 188.Weggemans RM, Zock PL, Katan MB. Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: a meta-analysis. Am J Clin Nutr. 2001;73:885–891. doi: 10.1093/ajcn/73.5.885. [DOI] [PubMed] [Google Scholar]
- 189.Reaven GM, Abbasi F, Bernhart S, et al. Insulin resistance, dietary cholesterol, and cholesterol concentration in postmenopausal women. Metabolism. 2001;50:594–597. doi: 10.1053/meta.2001.22559. [DOI] [PubMed] [Google Scholar]
- 190.Goodrow EF, Wilson TA, Houde SC, et al. Consumption of one egg per day increases serum lutein and zeaxanthin concentrations in older adults without altering serum lipid and lipoprotein cholesterol concentrations. J Nutr. 2006;136:2519–2524. doi: 10.1093/jn/136.10.2519. [DOI] [PubMed] [Google Scholar]
- 191.Wenzel AJ, Gerweck C, Barbato D, Nicolosi RJ, Handelman GJ, Curran-Celentano J. A 12-wk egg intervention increases serum zeaxanthin and macular pigment optical density in women. J Nutr. 2006;136:2568–2573. doi: 10.1093/jn/136.10.2568. [DOI] [PubMed] [Google Scholar]
- 192.Katz DL, Evans MA, Nawaz H, et al. Egg consumption and endothelial function: a randomized controlled crossover trial. Int J Cardiol. 2005;99:65–70. doi: 10.1016/j.ijcard.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 193.Njike V, Faridi Z, Dutta S, Gonzalez-Simon AL, Katz DL. Daily egg consumption in hyperlipidemic adults-Effects on endothelial function and cardiovascular risk. Nutr J. 2010:9. doi: 10.1186/1475-2891-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:146–59. doi: 10.3945/ajcn.112.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012;15:725–737. doi: 10.1017/S1368980011002254. [DOI] [PubMed] [Google Scholar]
- 196.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–69. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 197.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 198.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 199.Yuan J-M, Ross RK, Gao Y-T, Mimi CY. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 200.Ness AR, Gallacher JEJ, Bennett PD, et al. Advice to eat fish and mood: a randomised controlled trial in men with angina. Nutr Neurosci. 2003;6:63–65. doi: 10.1080/1028415021000056069. [DOI] [PubMed] [Google Scholar]
- 201.Burr ML. Secondary prevention of CHD in UK men: the Diet and Reinfarction Trial and its sequel. Proceedings of the Nutrition Society. 2007;66:9–15. doi: 10.1017/S0029665107005241. [DOI] [PubMed] [Google Scholar]
- 202.Ness ARHJ, Elwood PC, Whitley E, Smith GD, Burr ML. The long-term effect of dietary advice in men with coronary disease: follow-up of the Diet and Reinfarction trial (DART) Eur J Clin Nutr. 2002;56:512–8. doi: 10.1038/sj.ejcn.1601342. [DOI] [PubMed] [Google Scholar]
- 203.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 204.Wallin A, Di Giuseppe D, Orsini N, Patel PS, Forouhi NG, Wolk A. Fish Consumption, Dietary Long-Chain n-3 Fatty Acids, and Risk of Type 2 Diabetes Systematic review and meta-analysis of prospective studies. Diabetes Care. 2012;35:918–929. doi: 10.2337/dc11-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu JHY, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107:S214–S227. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yamagishi K, Iso H, Date C, et al. Fish, ω-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women: The JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 207.Iso H, Kobayashi M, Ishihara J, et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 208.Nakamura Y, Ueshima H, Okamura T, et al. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med. 2005;118:239–245. doi: 10.1016/j.amjmed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 209.Kris-Etherton PM, Hu FB, Ros E, Sabaté J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138:1746S–1751S. doi: 10.1093/jn/138.9.1746S. [DOI] [PubMed] [Google Scholar]
- 210.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 211.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev. 2001;59:103–11. doi: 10.1111/j.1753-4887.2001.tb06996.x. [DOI] [PubMed] [Google Scholar]
- 213.Zhou D, Yu H, He F, et al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2014;100:270–7. doi: 10.3945/ajcn.113.079152. [DOI] [PubMed] [Google Scholar]
- 214.Guo K, Zhou Z, Jiang Y, Li W, Li Y. Meta-analysis of prospective studies on the effects of nut consumption on hypertension and type 2 diabetes mellitus. J Diabetes. 2015;7:202–12. doi: 10.1111/1753-0407.12173. [DOI] [PubMed] [Google Scholar]
- 215.Flores-Mateo G, Rojas-Rueda D, Basora J, Ros E, Salas-Salvado J. Nut intake and adiposity: meta-analysis of clinical trials. Am J Clin Nutr. 2013;97:1346–55. doi: 10.3945/ajcn.111.031484. [DOI] [PubMed] [Google Scholar]
- 216.Smith JD, Hou T, Ludwig DS, et al. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr. 2015;101:1216–24. doi: 10.3945/ajcn.114.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. New Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 218.Dilis V, Katsoulis M, Lagiou P, Trichopoulos D, Naska A, Trichopoulou A. Mediterranean diet and CHD: the Greek European prospective investigation into cancer and nutrition cohort. Br J Nutr. 2012;108:699–709. doi: 10.1017/S0007114512001821. [DOI] [PubMed] [Google Scholar]
- 219.Mitrou PN, Kipnis V, Thiébaut ACM, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. 2007;167:2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 220.Buckland G, González CA, Agudo A, et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am J Epidemiol. 2009;170:1518–29. doi: 10.1093/aje/kwp282. [DOI] [PubMed] [Google Scholar]
- 221.de Lorgeril M, Salen P, Martin J-L, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction. Heart Failure. 1999;11:6. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 222.Barzi F, Woodward M, Marfisi RM, Tavazzi L, Valagussa F, Marchioli R. Mediterranean diet and all-causes mortality after myocardial infarction: results from the GISSI-Prevenzione trial. Eur J Clin Nutr. 2003;57:604–611. doi: 10.1038/sj.ejcn.1601575. [DOI] [PubMed] [Google Scholar]
- 223.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455–1461. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 224.Venn BJ, Perry T, Green TJ, et al. The effect of increasing consumption of pulses and wholegrains in obese people: a randomized controlled trial. J Am Coll Nutr. 2010;29:365–372. doi: 10.1080/07315724.2010.10719853. [DOI] [PubMed] [Google Scholar]
- 225.Hermsdorff HHM, Zulet MÁ, Abete I, Martínez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50:61–69. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- 226.Jenkins DJA, Kendall CWC, Augustin LSA, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Internal Med. 2012;172:1653–1660. doi: 10.1001/2013.jamainternmed.70. [DOI] [PubMed] [Google Scholar]
- 227.Mollard RC, Luhovyy BL, Panahi S, Nunez M, Hanley A, Anderson GH. Regular consumption of pulses for 8 weeks reduces metabolic syndrome risk factors in overweight and obese adults. Br J Nutr. 2012;108:S111–S122. doi: 10.1017/S0007114512000712. [DOI] [PubMed] [Google Scholar]
- 228.Liu J, Mazzone PJ, Cata JP, et al. Serum free fatty acid biomarkers of lung cancer. Chest. 2014;146:670–679. doi: 10.1378/chest.13-2568. [DOI] [PubMed] [Google Scholar]
- 229.Anderson JW, Major AW. Pulses and lipaemia, short-and long-term effect: potential in the prevention of cardiovascular disease. Br J Nutr. 2002;88:263–271. doi: 10.1079/BJN2002716. [DOI] [PubMed] [Google Scholar]
- 230.Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:94–103. doi: 10.1016/j.numecd.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jayalath VH, de Souza RJ, Sievenpiper JL, et al. Effect of dietary pulses on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Am J Hypertens. 2013;27:56–64. doi: 10.1093/ajh/hpt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014:101. doi: 10.3945/ajcn.113.076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shi ZQ, Tang JJ, Wu H, Xie CY, He ZZ. Consumption of nuts and legumes and risk of stroke: A meta-analysis of prospective cohort studies. Nutr Metab Cardiovas Dis. 2014;24:1262–71. doi: 10.1016/j.numecd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 234.Bernstein AM, Pan A, Rexrode KM, et al. Dietary protein sources and the risk of stroke in men and women. Stroke. 2012;43:637–44. doi: 10.1161/STROKEAHA.111.633404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ho SC, Woo JLF, Leung SSF, Sham ALK, Lam TH, Janus ED. Intake of soy products is associated with better plasma lipid profiles in the Hong Kong Chinese population. J Nutr. 2000;130:2590–2593. doi: 10.1093/jn/130.10.2590. [DOI] [PubMed] [Google Scholar]
- 236.Agrawal S, Ebrahim S. Association between legume intake and self-reported diabetes among adult men and women in India. BMC Public Health. 2013;13:706–19. doi: 10.1186/1471-2458-13-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Villegas R, Gao Y-T, Yang G, et al. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am J Clin Nutr. 2008;87:162–167. doi: 10.1093/ajcn/87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhang X, Shu XO, Gao Y-T, et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–2878. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 239.Worm B, Barbier EB, Beaumont N, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 240.Jenkins DJA, Sievenpiper JL, Pauly D, Sumaila UR, Kendall CWC, Mowat FM. Are dietary recommendations for the use of fish oils sustainable? Canadian Med Assoc J. 2009;180:633–637. doi: 10.1503/cmaj.081274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hawkes C, Popkin BM. Can the sustainable development goals reduce the burden of nutrition-related non-communicable diseases without truly addressing major food system reforms? BMC Med. 2015;13:1–3. doi: 10.1186/s12916-015-0383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 243.Esposito K, Chiodini P, Maiorino MI, Bellastella G, Panagiotakos D, Giugliano D. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine. 2014:1–10. doi: 10.1007/s12020-014-0264-4. [DOI] [PubMed] [Google Scholar]
- 244.O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 245.Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke a meta-analysis of prospective cohort studies. Stroke. 2014;45:1613–1619. doi: 10.1161/STROKEAHA.114.004836. [DOI] [PubMed] [Google Scholar]
- 246.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. Br Med J. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nerbass FB, Pecoits-Filho R, McIntyre NJ, McIntyre CW, Willingham FC, Taal MW. Demographic associations of high estimated sodium intake and frequency of consumption of high-sodium foods in people with chronic kidney disease stage 3 in England. J Renal Nutr. 2014;24:236–242. doi: 10.1053/j.jrn.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 248.Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832–1838. doi: 10.3945/jn.110.124826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:2531–2. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 250.Powles J, Fahimi S, Micha R, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mente A, O’Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–11. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 252.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011:CD004022. doi: 10.1002/14651858.CD004022.pub3. [DOI] [PubMed] [Google Scholar]
- 253.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;4:CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 255.TOHP Investigators. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–67. [PubMed] [Google Scholar]
- 256.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–9. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Coxson PG, Cook NR, Joffres M, et al. Mortality benefits from US population-wide reduction in sodium consumption: projections from 3 modeling approaches. Hypertension. 2013;61:564–70. doi: 10.1161/HYPERTENSIONAHA.111.201293. [DOI] [PubMed] [Google Scholar]
- 258.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34:703–9. doi: 10.2337/dc10-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–38. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 261.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–85. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 262.Thomas MC, Moran J, Forsblom C, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34:861–6. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 264.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail. 2014;16:394–402. doi: 10.1002/ejhf.56. [DOI] [PubMed] [Google Scholar]
- 265.Saulnier PJ, Gand E, Hadjadj S, Group SS. Sodium and cardiovascular disease. N Engl J Med. 2014;371:2135–6. doi: 10.1056/NEJMc1412113. [DOI] [PubMed] [Google Scholar]
- 266.Graudal N, Jurgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014;27:1129–37. doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]
- 267.Cobb LK, Anderson CA, Elliott P, et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–86. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 268.Mitka M. IOM report: Evidence fails to support guidelines for dietary salt reduction. JAMA. 2013;309:2535–6. doi: 10.1001/jama.2013.7110. [DOI] [PubMed] [Google Scholar]
- 269.O’Donnell M, Mente A, Yusuf S. Sodium intake and cardiovascular health. Circ Res. 2015;116:1046–57. doi: 10.1161/CIRCRESAHA.116.303771. [DOI] [PubMed] [Google Scholar]
- 270.Oparil S. Low sodium intake--cardiovascular health benefit or risk? N Engl J Med. 2014;371:677–9. doi: 10.1056/NEJMe1407695. [DOI] [PubMed] [Google Scholar]
- 271.World Health Organization; World Health Organization, editor. Global Status Report on Alcohol and Health 2014. 2014. [Google Scholar]
- 272.Smyth A, Teo K, Rangarajan S, et al. Alcohol consumption and cardiovascular disease, cancer, injury, hospitalization, and mortality. Lancet. 2015 doi: 10.1016/S0140-6736(15)00235-4. In Press. [DOI] [PubMed] [Google Scholar]
- 273.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ferrari P, Licaj I, Muller DC, et al. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open. 2014;4:e005245. doi: 10.1136/bmjopen-2014-005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhang C, Qin YY, Chen Q, et al. Alcohol intake and risk of stroke: a dose-response meta-analysis of prospective studies. Int J Cardiol. 2014;174:669–77. doi: 10.1016/j.ijcard.2014.04.225. [DOI] [PubMed] [Google Scholar]
- 276.Roerecke M, Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am J Epidemiol. 2010;171:633–44. doi: 10.1093/aje/kwp451. [DOI] [PubMed] [Google Scholar]
- 277.U.S. Department of Health and Human Services and the US Department of Agriculture; Promotion OoDPaH, editor. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington DC: Office of Disease Preventio and Health Promotion USDHHS; 2015. p. 571. [Google Scholar]
- 278.Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 279.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144:881–9. doi: 10.3945/jn.113.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Struijk EA, May AM, Wezenbeek NL, et al. Adherence to dietary guidelines and cardiovascular disease risk in the EPIC-NL cohort. Int J Cardiol. 2014;176:354–9. doi: 10.1016/j.ijcard.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 281.Jenkins DJ, Kendall CW, Marchie A, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–10. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- 282.Jenkins DJ, Jones PJ, Lamarche B, et al. Effect of a dietary portfolio of cholesterol-lowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia: a randomized controlled trial. JAMA. 2011;306:831–9. doi: 10.1001/jama.2011.1202. [DOI] [PubMed] [Google Scholar]
- 283.Jenkins DJ, Kendall CW, Faulkner DA, et al. Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am J Clin Nutr. 2006;83:582–91. doi: 10.1093/ajcn.83.3.582. [DOI] [PubMed] [Google Scholar]
- 284.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–66. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 285.Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Hawkes C. The influence of trade liberalization on global dietary change: the case of vegetable oils, meat and highly processed foods. In: Hawkes C, Blouin C, Henson S, Drager N, Dubé L, editors. Trade, Food, Diet and Health: Perspectives and Policy Options. New York, NY: Wiley-Blackwell; 2010. pp. 36–59. [Google Scholar]
- 287.Davis M. The Monster at Our Door: The Global Threat of Avian Flu. New York, NY: Holt Paperbacks; 2006. [Google Scholar]
- 288.Babu SC, Blom S. 2020 Conference Brief 6. Washington DC: International Food Policy Research Institute; 2014. Building resilience for food and nutrition security; pp. 1–4. [Google Scholar]
- 289.Hawkes C, Thow A, Downs S, et al. Identifying effective food systems solutions for nutrition and noncommunicable diseases: creating policy coherence in the fats supply chain. SCN News. 2013;40:39–47. [Google Scholar]
- 290.Winne M. Closing the Food Gap: Resetting the Table in the Land of Plenty. New York City: Beacon Press; 2009. [Google Scholar]
- 291.Chavasit V, Kasemsup V, Tontisirin K. Thailand conquered under-nutrition very successfully but has not slowed obesity. Obes Rev. 2013;14:96–105. doi: 10.1111/obr.12091. [DOI] [PubMed] [Google Scholar]
- 292.Coitinho D, Monteiro C, Popkin B. What Brazil is doing to promote healthy diets and active lifestyles. Public Health Nutr. 2002;5:263–7. doi: 10.1079/phn2001302. [DOI] [PubMed] [Google Scholar]
- 293.Unit for Policy Coherence for Development; Secretary-General OOot, editor. Working Paper No 1. Paris: Organization for Economic Cooperation and Development; 2012. Policy Framework For Policy Coherence For Development; p. 46. [Google Scholar]
- 294.Blouin C. Trade policy and health: from conflicting interests to policy coherence. Bull World Health Org. 2007;85:169–173. doi: 10.2471/BLT.06.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Blouin C, Chopra M, Hoeven R. Trade and health 3: trade and the social determinants of health. Lancet. 2009;373:502–507. doi: 10.1016/S0140-6736(08)61777-8. [DOI] [PubMed] [Google Scholar]
- 296.Nilsson M, Zamparutti T, Petersen JE, Nykvist B, Rudberg P, McGuinn J. Understanding policy coherence: Analytical framework and examples of sector–environmental policy interactions in the EU. Envir Policy Governance. 2012;22:395–423. [Google Scholar]
- 297.Igumbor EU, Sanders D, Puoane TR, et al. “Big Food,” the consumer food environment, health, and the policy response in South Africa. PLoS Med. 2012;9:e1001253. doi: 10.1371/journal.pmed.1001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 299.Thow AM, Hawkes C. Global sugar guidelines: an opportunity to strengthen nutrition policy. Public Health Nutr. 2014;17:2151–2155. doi: 10.1017/S1368980014001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Burgan M, Winne M. Doing food policy councils eight: A guide to development and action. Santa Fe: Mark Winne Associaties; 2012. [Google Scholar]
- 301.Parfitt J, Barthel M, Macnaughton S. Food waste within food supply chains: quantification and potential for change to 2050. Phil Trans R Soc B: Bio Sci. 2010;365:3065–3081. doi: 10.1098/rstb.2010.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Popkin B, Monteiro C, Swinburn B. Overview: Bellagio conference on program and policy options for preventing obesity in the low- and middle-income countries. Obes Rev. 2013;14:1–8. doi: 10.1111/obr.12108. [DOI] [PubMed] [Google Scholar]
- 303.Roodenburg A, Popkin B, Seidell J. Development of international criteria for a front of package nutrient profiling system: international Choices Programme. Eur J Clin Nutr. 2011;65:1190–1200. doi: 10.1038/ejcn.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Corvalán C, Reyes M, Garmendia ML, Uauy R. Structural responses to the obesity and non-communicable diseases epidemic: The Chilean law of food labeling and advertising. Obes Rev. 2013;14:79–87. doi: 10.1111/obr.12099. [DOI] [PubMed] [Google Scholar]
- 305.Hawkes C, Smith TG, Jewell J, et al. Smart food policies for obesity prevention. Lancet. 2015 doi: 10.1016/S0140-6736(14)61745-1. [DOI] [PubMed] [Google Scholar]
- 306.Vyth EL, Steenhuis IH, Roodenburg AJ, Brug J, Seidell JC. Front-of-pack nutrition label stimulates healthier product development: a quantitative analysis. Int J Behav Nutr Phys Act. 2010;7:65. doi: 10.1186/1479-5868-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Godfray H, Crute IR, Haddad L, et al. The future of the global food system. Phil Trans R Soc B: Bio Sci. 2010;365:2769–2777. doi: 10.1098/rstb.2010.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.