SUMMARY
Pyruvate transport across the inner mitochondrial membrane is believed to be a prerequisite step for gluconeogenesis in hepatocytes, which is important for maintenance of normoglycemia during prolonged food deprivation, but also contributes to hyperglycemia in diabetes. To determine the requirement for mitochondrial pyruvate import in gluconeogenesis, mice with liver-specific deletion of mitochondrial pyruvate carrier 2 (LS-Mpc2−/−) were generated. Loss of MPC2 impaired, but did not completely abolish, hepatocyte pyruvate metabolism, labelled pyruvate conversion to TCA cycle intermediates and glucose, and glucose production from pyruvate. Unbiased metabolomic analyses of livers from fasted LS-Mpc2−/− mice suggested that alterations in amino acid metabolism, including pyruvate-alanine cycling, might compensate for loss of MPC2. Indeed, inhibition of pyruvate-alanine transamination further reduced mitochondrial pyruvate metabolism and glucose production by LS-Mpc2−/− hepatocytes. These data demonstrate an important role for MPC2 in controlling hepatic gluconeogenesis and illuminate a compensatory mechanism for circumventing a block in mitochondrial pyruvate import.
Introduction
Hepatic glucose production is a critical physiologic process that is required for maintaining normoglycemia during periods of nutrient deprivation. However, under conditions of insulin deficiency or resistance, hepatic glucose overproduction contributes to the hyperglycemia of diabetes. Metformin, which acts in the liver to inhibit gluconeogenesis (Foretz et al., 2010), has been used for years to lower blood glucose in type 2 diabetic patients. There are three pathways for hepatic glucose production: breakdown of glycogen (glycogenolysis), gluconeogenesis from glycerol, and gluconeogenesis from lactate/pyruvate/amino acids. Of these pathways, gluconeogenesis via pyruvate/lactate/amino acids predominates during prolonged food deprivation (Burgess et al., 2006) and is deranged in diabetic liver (Satapati et al., 2012).
Pyruvate carboxylation to oxaloacetate is required for gluconeogenesis from pyruvate and the enzyme that mediates this reaction, pyruvate carboxylase, is exclusively localized to the mitochondrial matrix. Thus, it is believed that the transport of pyruvate across the inner mitochondrial membrane is a prerequisite step in gluconeogenesis from pyruvate/lactate. The existence of carrier-assisted inner mitochondrial membrane pyruvate transport has been known for many years, but the proteins that facilitate pyruvate import into the mitochondrial matrix have only recently been identified (Bricker et al., 2012; Herzig et al., 2012). Our current understanding suggests that the mammalian mitochondrial pyruvate carrier (MPC) is composed of two proteins, MPC1 and MPC2, which form a hetero-oligomeric complex in the inner mitochondrial membrane and that both proteins are required for MPC complex activity and stability (Bricker et al., 2012; Herzig et al., 2012). Indeed, deletion of either MPC1 or MPC2 destabilizes the MPC complex and leads to loss of both proteins (Bricker et al., 2012; Colca et al., 2013; Herzig et al., 2012; Vigueira et al., 2014).
Despite the predicted importance of mitochondrial pyruvate import in gluconeogenesis, little is known regarding the requirements for, and regulatory effects of, the MPC in this process. Early work suggested that MPC activity was increased by glucagon and inhibited by insulin (Titheradge and Coore, 1976a, b), which would fit with the known effects of these hormones on gluconeogenesis. Other studies using isolated liver preparations showed an acute inhibition of hepatic glucose output by chemical inhibitors of MPC activity, including a potent inhibitor known as UK-5099 (Martin-Requero et al., 1986; Rognstad, 1983; Thomas and Halestrap, 1981). Similarly, several compounds containing thiazolidine rings, including the insulin-sensitizing thiazolidinediones, bind and inhibit the MPC (Colca et al., 2013; Divakaruni et al., 2013; Hildyard et al., 2005). Thiazolidinediones (TZDs), which canonically act as ligands for the nuclear receptor PPARγ (Lehmann et al., 1995), have been shown to suppress glucose production in perfused livers or isolated hepatocytes within a matter of minutes (Adams et al., 1998; Raman et al., 1998; Raman and Judd, 2000), suggesting a non-genomic, acute, and direct hepatic effect of these compounds.
However, whether carrier-mediated pyruvate transport by MPC has significant control strength over gluconeogenesis has been questioned in other studies using chemical inhibitors of MPC (Groen et al., 1986; Groen et al., 1983; Halestrap and Armston, 1984). Our recent work in an MPC2 hypomorphic mouse model showed that modest attenuation of MPC2 activity by genetic means did not impair flux of pyruvate into newly synthesized glucose (Vigueira et al., 2014). Thus, the influence of MPC2 on this process remains unclear and the data in hand suggest that severe impairment of MPC activity may be needed to reduce hepatic gluconeogenesis. Unfortunately, complete deletion of MPC2 in a global manner is lethal at early embryonic stages (Vigueira et al., 2014).
To circumvent the lethality associated with constitutive MPC deletion and to address the role of mitochondrial pyruvate import in liver intermediary metabolism, we generated mice with liver-specific deletion of MPC2 (LS-Mpc2−/− mice) by using Cre-LoxP methodology. The initial characterization of LS-Mpc2−/− mice detected significant impairments in hepatic mitochondrial pyruvate metabolism and gluconeogenesis that were similar to mice with liver-specific deletion of the other protein of the MPC complex, MPC1 (Gray et al., co-submitted). However, we also determined that significant gluconeogenesis and mitochondrial metabolism of pyruvate occurred in hepatocytes with MPC2 deficiency, suggesting an alternative or circumventing pathway was compensating for loss of MPC activity. Metabolomic analyses suggested that residual glucose production in LS-Mpc2−/− livers may result from altered amino acid metabolism. Indeed, mechanistic studies demonstrated that pyruvate-alanine cycling can partially compensate for the defect in mitochondrial pyruvate transport in liver-specific MPC2 knockout mice. Altogether, these studies confirm an important role for mitochondrial pyruvate transport in hepatic gluconeogenesis, but also unveil compensatory pathways that contribute to glucose production when MPC activity is inhibited.
Results
Generation of LS-Mpc2−/− Mice
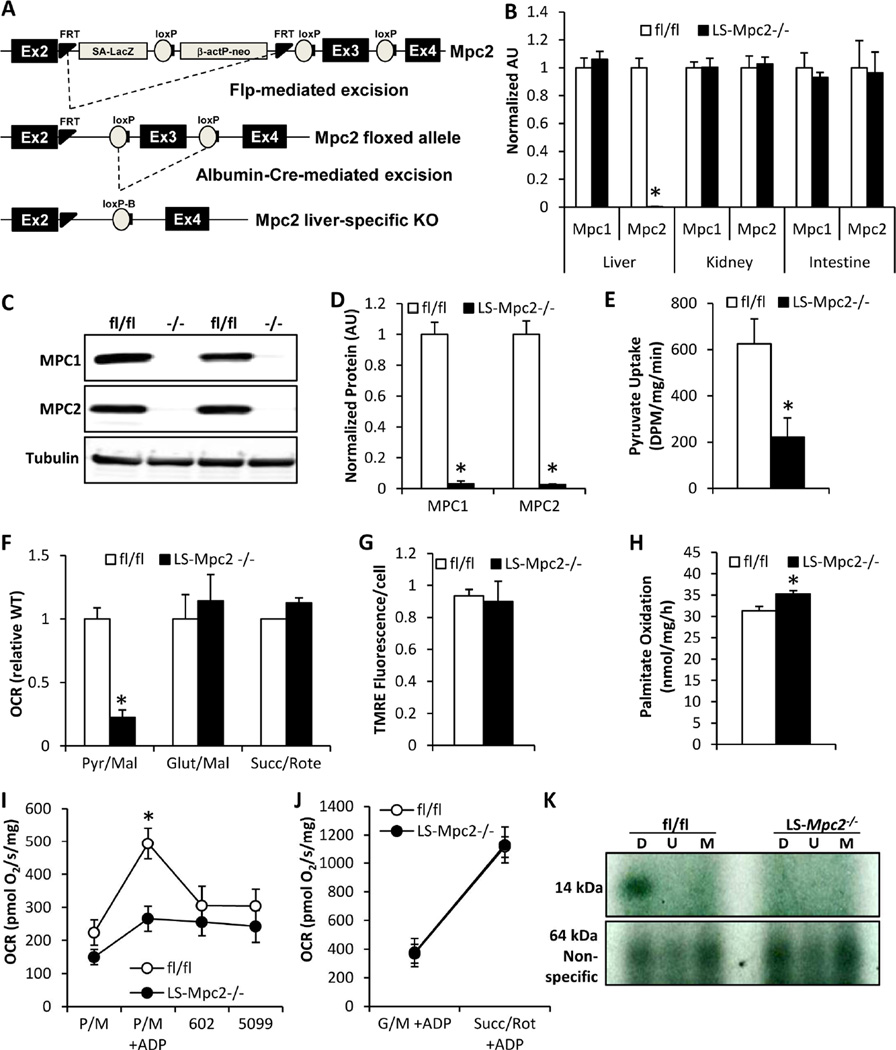
Mice with liver-specific deletion of MPC2 (LS-Mpc2−/− mice) were generated using a conditional allele of Mpc2 (Figure 1A) as described in the Experimental Procedures. LS-Mpc2−/− mice were viable and overtly indistinguishable from their littermate control mice, which were also homozygous for the floxed allele (fl/fl) but did not express Cre. LS-Mpc2−/− mice exhibited no differences in body weight, liver weight, resting energy expenditure, activity, or food or water intake (Figure S1A–C). qRT-PCR analyses confirmed that Mpc2 mRNA was dramatically reduced in LS-Mpc2−/− liver compared to controls, while Mpc1 and Mpc2 mRNA remained unaltered in kidney and intestine, consistent with liver-specific Mpc2 deletion (Figure 1B). Western blotting of isolated hepatocytes confirmed a loss of both MPC2 and MPC1 proteins in LS-Mpc2−/− liver (Figures 1C, D), which is consistent with previous studies suggesting that both proteins are required for MPC complex stability (Bricker et al., 2012; Herzig et al., 2012; Vigueira et al., 2014).
Figure 1. Generation and characterization of LS Mpc2−/− mice.
(A) Gene-targeting strategy to delete exon 3 of Mpc2 in a liver-specific manner by Cre-recombinase expressed under the control of the albumin promoter. (B) Expression of Mpc1 and Mpc2 mRNA in liver, kidney, and intestine in fl/fl and LS-Mpc2−/− mice. (C) Representative western blot images for MPC2 and MPC1 protein in isolated hepatocytes. (D) Quantification of MPC1 and MPC2 protein expression in isolated hepatocytes as in (C). (E) Uptake of 14C-pyruvate by isolated mitochondria. (F) ADP-supported oxygen consumption rates by isolated, permeabilized hepatocytes in the presence of the indicated substrates. (G) Mitochondrial membrane potential in LS-Mpc2−/− and fl/fl hepatocytes as assessed by TMRE staining. (H) Oxidation of 3H-palmitate in LS-Mpc2−/− and fl/fl hepatocytes. (I) Oxygen consumption rates of isolated mitochondria from fl/fl and LS-Mpc2−/− livers stimulated by 5 mM pyruvate and 2 mM malate before and after addition of 15 µM MSDC-0602 or 10 µM UK-5099. (J) Oxygen consumption rates of isolated mitochondria stimulated by 10 mM glutamate/2 mM malate or 5 mM succinate/0.5 µM rotenone. Data presented as mean + S.E.M. *p ≤ 0.05 for fl/fl vs LS-Mpc2−/−. (K) Photoaffinity crosslinking of an iodinated 125I-TZD photoprobe, MSDC-1101, specifically labels a 14 kDa protein in vehicle-treated (labeled D for DMSO) fl/fl hepatocyte mitochondrial membranes. Co-incubation with 30 µM unlabeled UK-5099 (labeled U) or MSDC-0602 (labeled M) prevent the labeling.
Defective Hepatic Pyruvate Metabolism in LS-Mpc2−/− Mice
Accumulation of 14C-pyruvic acid in mitochondria isolated from LS-Mpc2−/− livers was markedly decreased compared to fl/fl liver mitochondria in a pyruvate transport assay (Figure 1E). Permeabilized hepatocytes from LS-Mpc2−/− mice also displayed a strong defect in pyruvate-stimulated, ADP-supported, oxygen consumption rate (OCR) compared to fl/fl controls, while glutamate- and succinate-stimulated respiration remained normal (Figure 1F). Mitochondrial membrane potential was unaffected in LS-Mpc2−/− hepatocytes (Figure 1G); possibly indicating that increased metabolism of other substrates maintains mitochondrial viability and cellular homeostasis. Accordingly, fatty acid oxidation is slightly, yet significantly enhanced in LS-Mpc2−/− hepatocytes compared to controls (Figure 1H). Loss of the MPC also impaired ADP-stimulated OCR in isolated mitochondria when pyruvate was supplied as substrate (Figure 1I), but did not affect maximal respiration using glutamate or succinate as substrates (Figure 1J). Altogether, these results suggest that loss of the MPC in hepatocytes results in the expected decrease in mitochondrial pyruvate transport and metabolism.
MSDC-0602 Alters Pyruvate Metabolism and Decreases Glucose Production in an Mpc2-Dependent Manner
Using a 125I-radiolabeled photoaffinity probe, we recently discovered that the MPC was directly bound by insulin-sensitizing thiazolidinediones (TZDs) (Colca et al., 2013), including MSDC-0602, a novel PPARγ-sparing TZD currently in clinical trials (Colca et al., 2014). Consistent with the specific binding of the TZD-based probe to the MPC, photoaffinity crosslinking of a 14 kDa band, which corresponds to the molecular weight of MPC2, was completely lost in LS-Mpc2−/− hepatocyte mitochondrial membranes (Figure 1K). Unlabeled UK-5099 or MSDC-0602 effectively competed with 125I-labeled TZD crosslinking to the 14 kDa band, but not a nonspecific band at 64 kDa, in fl/fl hepatocytes (Figure 1K). Consistent with a direct effect on the MPC and a competitive interaction with pyruvate, UK-5099 and MSDC-0602 reduced pyruvate-stimulated OCR in fl/fl liver mitochondria, but not LS-Mpc2−/− mitochondria (Figure 1I).
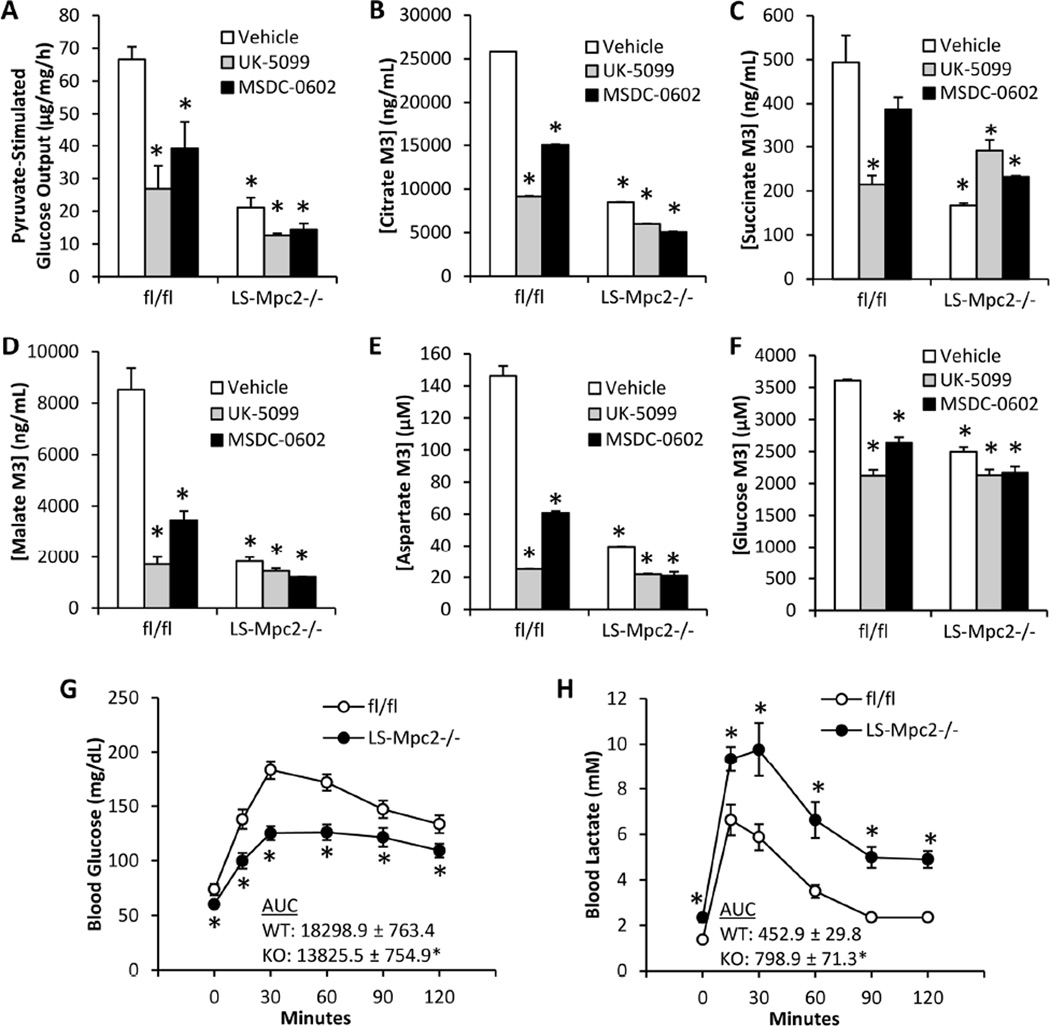
Given the predicted role for mitochondrial pyruvate import in gluconeogenesis from pyruvate, we next evaluated glucose production by hepatocytes with genetic or pharmacologic inhibition of MPC activity. Isolated hepatocytes from LS-Mpc2−/− mice produced significantly less glucose compared to fl/fl hepatocytes when stimulated with pyruvate in vitro (Figure 2A). Chemical inhibition of pyruvate import by UK-5099 or MSDC-0602 also decreased glucose production in fl/fl hepatocytes, but were largely without effect on glucose production by LS-Mpc2−/− hepatocytes (Figure 2A). To further assess pyruvate entry into the mitochondria and carbon flux into TCA cycle intermediates and glucose, isolated hepatocytes were cultured with 2.5 mM U-13C-pyruvate and 2.5 mM pyruvate, and incorporation of 13C-labelled carbon into intracellular citrate, succinate, malate, and aspartate, as well as media glucose was measured by mass spectrometry. As predicted, loss of MPC2 led to a significant impairment in 13C incorporation into each of these metabolites (Figures 2B–F). Chemical inhibition of the MPC with UK-5099 or MSDC-0602 also decreased 13C flux into TCA cycle intermediates and glucose in fl/fl hepatocytes, but had little effect in LS-Mpc2−/− hepatocytes (Figures 2B–F). Table S1 details the 13C enrichment and concentration of a broad panel of intermediates from this study. These results using genetic and pharmacologic approaches strongly suggest that mitochondrial pyruvate transport plays a role in gluconeogenesis from pyruvate.
Figure 2. Decreased mitochondrial pyruvate metabolism and glucose production with chemical or genetic MPC inhibition.
(A) Glucose concentrations in the media of cultured hepatocytes after stimulation by glucagon and 5 mM pyruvate in the presence or absence of UK-5099 (2.5 µM) or MSDC-0602 (15 µM). (B–F) 13C-pyruvate flux into cellular (B) citrate, (C) succinate, (D) malate, (E) aspartate, or (F) medium glucose measured by mass spectrometry in fl/fl and LS-Mpc2−/− hepatocytes. (G) Blood glucose concentrations after overnight fast and throughout a pyruvate tolerance test (PTT). (H) Blood lactate concentrations after overnight fast and throughout a PTT. Data presented as mean + S.E.M. *p ≤ 0.05 vs vehicle-treated fl/fl hepatocytes.
Decreased Gluconeogenesis in LS-Mpc2−/− Mice
To determine the effects of liver MPC2 deletion on gluconeogenesis in vivo, we subjected LS-Mpc2−/− mice to an i.p. pyruvate tolerance test (PTT) after an overnight fast. Fasting blood glucose concentrations at time 0 were significantly lower in LS-Mpc2−/− mice compared to fl/fl controls (Figure 2G). Glucose concentrations were also lower in LS-Mpc2−/− mice compared to fl/fl controls at all times after bolus pyruvate injection, consistent with impaired gluconeogenesis from pyruvate (Figure 2G). LS-Mpc2−/− mice were also observed to have increased blood lactate concentrations compared to fl/fl controls after fasting and throughout the PTT (Figure 2H). This could be due to impaired lactate uptake by liver or increased hepatic lactate production. Importantly, the differences in blood glucose concentration were not a result of altered glucose or insulin tolerance, as blood glucose concentrations were similar during glucose tolerance tests (GTT) (Figure S1D) and insulin tolerance tests (ITT) (Figure S1F). Blood lactate concentrations were found to be elevated in LS-Mpc2−/− mice during GTT and ITT (Figure S1E and G, respectively), again consistent with impaired hepatic lactate metabolism.
Loss of MPC in liver leads to protection from hyperglycemia
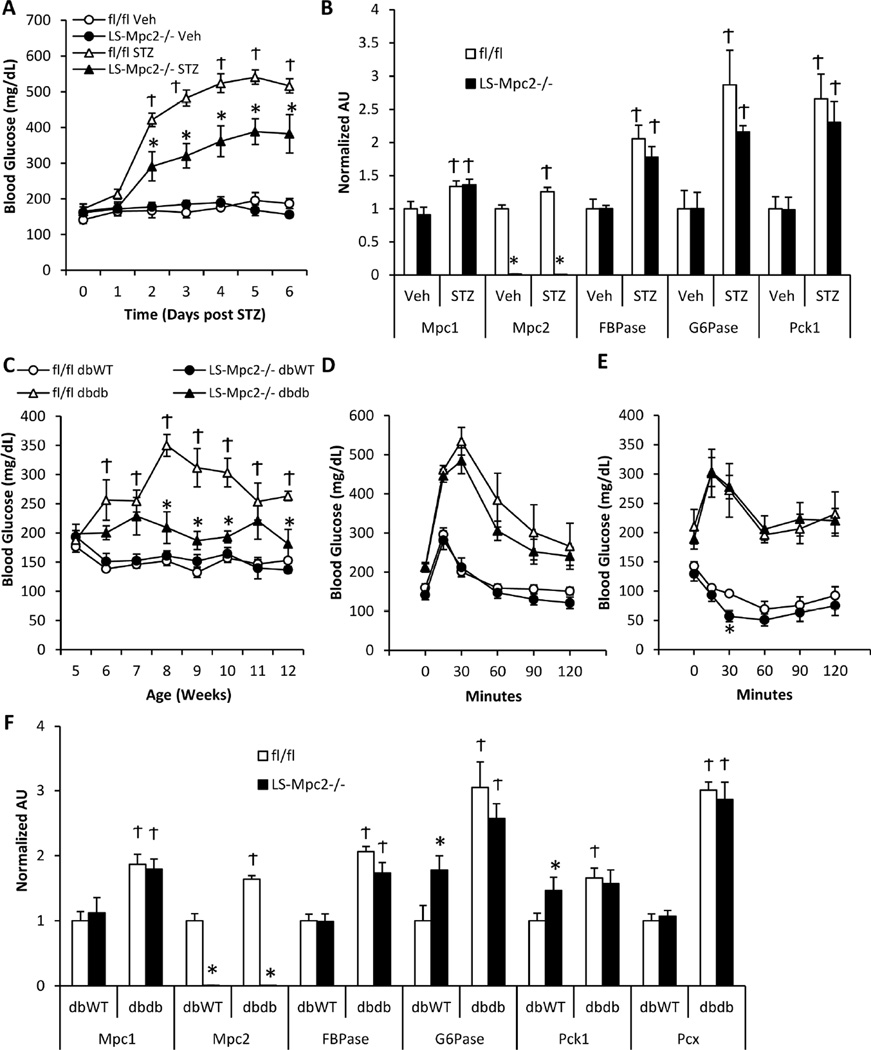
Hepatic gluconeogenesis is markedly increased in insulin deficiency and contributes to the hyperglycemia of diabetes. We rendered mice insulin-deficient with a single dose of 180 µg/g streptozotocin (STZ), which reduced insulin concentrations similarly in LS-Mpc2−/− and fl/fl mice (Table S2). As expected, random fed blood glucose concentrations were significantly elevated in STZ-treated animals compared to saline vehicle control treated mice within a few days of STZ treatment (Figure 3A). However, compared to fl/fl mice, LS-Mpc2−/− mice had significantly lower blood glucose concentrations after STZ injection (Figure 3A). Plasma free fatty acids, total ketone bodies, triglycerides, and cholesterol were all increased by STZ treatment, but no significant differences between fl/fl and LS-Mpc2−/− mice were detected (Table S2). STZ-induced insulin-deficiency enhanced the expression of several gluconeogenic genes, including Mpc1 and Mpc2, in the liver with no differences between fl/fl and LS-Mpc2−/− mice other than the loss of Mpc2 (Figure 3B).
Figure 3. LS Mpc2−/− mice are protected from hyperglycemia.
(A) Blood glucose concentrations after induction of streptozotocin (STZ)-induced insulin deficiency in ad libitum fed mice. (B) mRNA expression from livers of vehicle or STZ-injected mice. Data presented as mean + S.E.M. *p ≤ 0.05 for fl/fl vs LS-Mpc2−/−. Ϯp ≤ 0.05 for STZ and vehicle. (C) Blood glucose concentrations in ad libitum fed fl/fl or LS-Mpc2−/− mice crossed into the db/db background. (D) Blood glucose concentrations during an i.p. glucose tolerance test. (E) Blood glucose concentrations during an i.p. insulin tolerance test. (F) mRNA expression from livers of fl/fl or LS-Mpc2−/− mice crossed into the db/db background. Data presented as mean + S.E.M. *p ≤ 0.05 for fl/fl vs LS-Mpc2−/−. Ϯp ≤ 0.05 for db/WT vs db/db.
We also intercrossed mice harboring the floxed allele into the db/db genetic background. Liver-specific deletion of Mpc2 markedly reduced random fed blood glucose concentrations in this model of genetic obesity and diabetes (Figure 3C), without affecting body weight gain or fat pad mass (Figure S2A–C). Loss of liver MPC activity also did not affect liver weights or development of hepatic steatosis, which was considerable in the db/db mice (Figure S2D–E), but did cause higher blood lactate levels (Figure S2F). Moreover, loss of Mpc2 in liver did not affect glucose tolerance (Figure 3D), insulin tolerance (Figure 3E), or the transcriptional activation of genes encoding gluconeogenic enzymes (Figure 3F) in the livers of the db/db model, suggesting a specific effect on hepatic glucose output rather than general insulin responsiveness. Altogether, these data suggest that hepatic mitochondrial pyruvate import plays an important role in hepatic gluconeogenesis, which contributes to the hyperglycemia of diabetes.
Effect of 24 h Food Deprivation on Liver Metabolism in LS-Mpc2−/− Mice
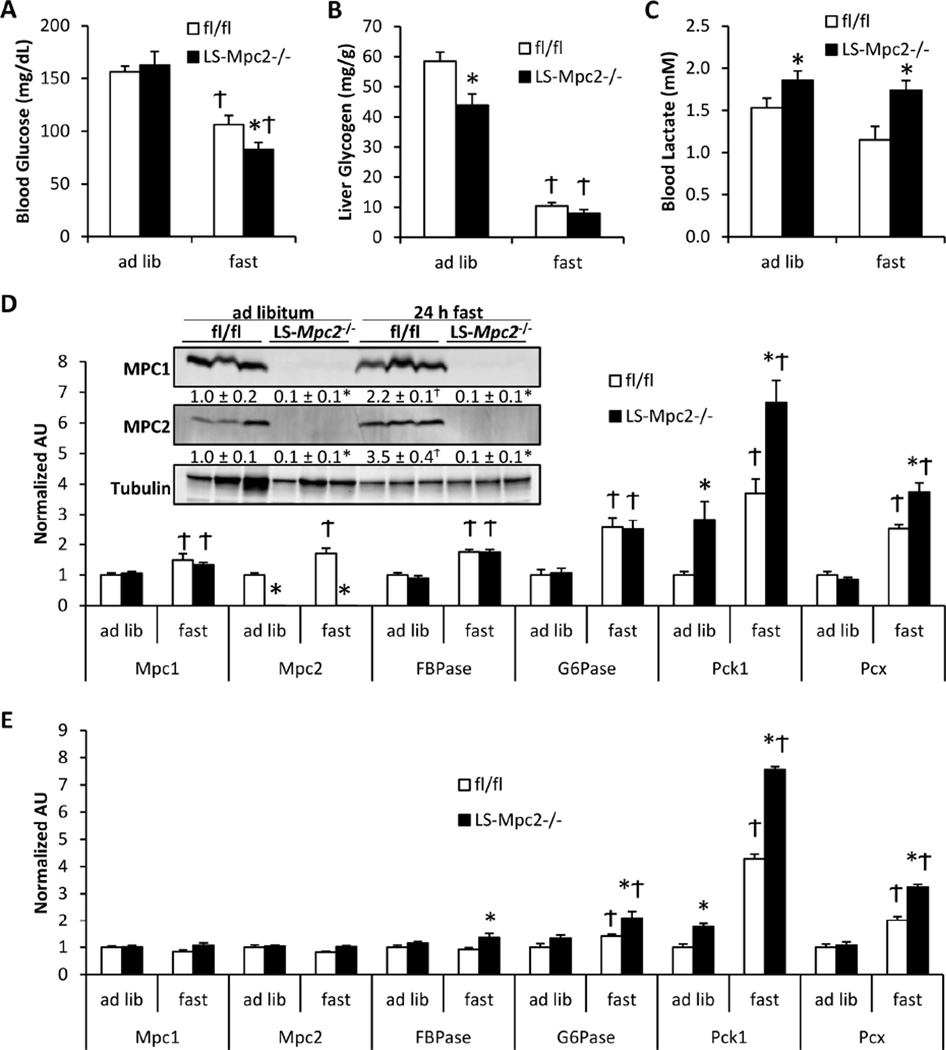
Gluconeogenesis is a critical component of the hepatic response to prolonged food deprivation. LS-Mpc2−/− and fl/fl mice were either given ad libitum access to normal chow diet or subjected to a 24 h fast. As expected, the 24 h fast lowered blood glucose concentrations in both fl/fl and LS-Mpc2−/− mice. Blood glucose concentrations in LS-Mpc2−/− mice were slightly, but significantly, lower compared to fl/fl mice after 24 h of fasting (Figure 4A). Interestingly, LS-Mpc2−/− mice displayed significantly reduced liver glycogen in the fed state (Figure 4B), possibly suggesting alterations in liver glycogen metabolism to maintain normoglycemia or diminished synthesis of glycogen from the indirect pathway, as was detected in Pck1 knockout mice (She et al., 2003). LS-Mpc2−/− mice displayed elevated blood lactate concentrations independent of whether the mice were ad libitum fed or fasted (Figure 4C). The effects of fasting on plasma insulin, free fatty acid, and total ketone body concentrations were not different between genotypes (Table S3). Fasting also resulted in similar liver glycogen depletion (Figure 4B) and TAG accumulation (Table S3) in fl/fl and LS-Mpc2−/− mice. Fasting modestly increased the hepatic expression of Mpc1 and Mpc2 mRNA and protein in fl/fl mice (Figure 4D). Loss of MPC2 did not impair the expression of genes encoding gluconeogenic enzymes (Fbpase, G6pc, Pck1, and Pcx), which was significantly elevated by fasting, and liver of LS-Mpc2−/− mice exhibited higher expression of Pck1 and Pcx compared to fl/fl controls under fasted conditions and Pck1 under fed conditions as well (Figure 4D). LS-Mpc2−/− mice also displayed elevated expression of some gluconeogenic enzymes in the kidney compared to fl/fl mice (Figure 4E), perhaps suggesting that glucose production from the kidneys may partially compensate for the loss of MPC2 in the liver. These data suggest that loss of the MPC in liver leads to modest hypoglycemia under fasting conditions without affecting other aspects of the hepatic fasting response.
Figure 4. Fasting response of LS Mpc2−/− mice.
LS-Mpc2−/− and fl/fl mice were either given ad libitum access to food or fasted 24 h. Graphs depict (A) blood glucose concentration, (B) liver glycogen, and (C) blood lactate concentration. (D) qRT-PCR analyses in livers from fed vs. fasted fl/fl and LS-Mpc2−/− mice. Inset: western blot of liver lysates from fed and fasted mice, with quantification in text below blot. (E) qRT-PCR analyses of kidneys from fed vs. fasted fl/fl and LS-Mpc2−/− mice. Data presented as mean + S.E.M. *p ≤ 0.05 for fl/fl vs. LS-Mpc2−/−. Ϯp ≤ 0.05 for fed vs fasted.
Metabolomic Profiling of the Fasting Response in LS-Mpc2−/− Mice
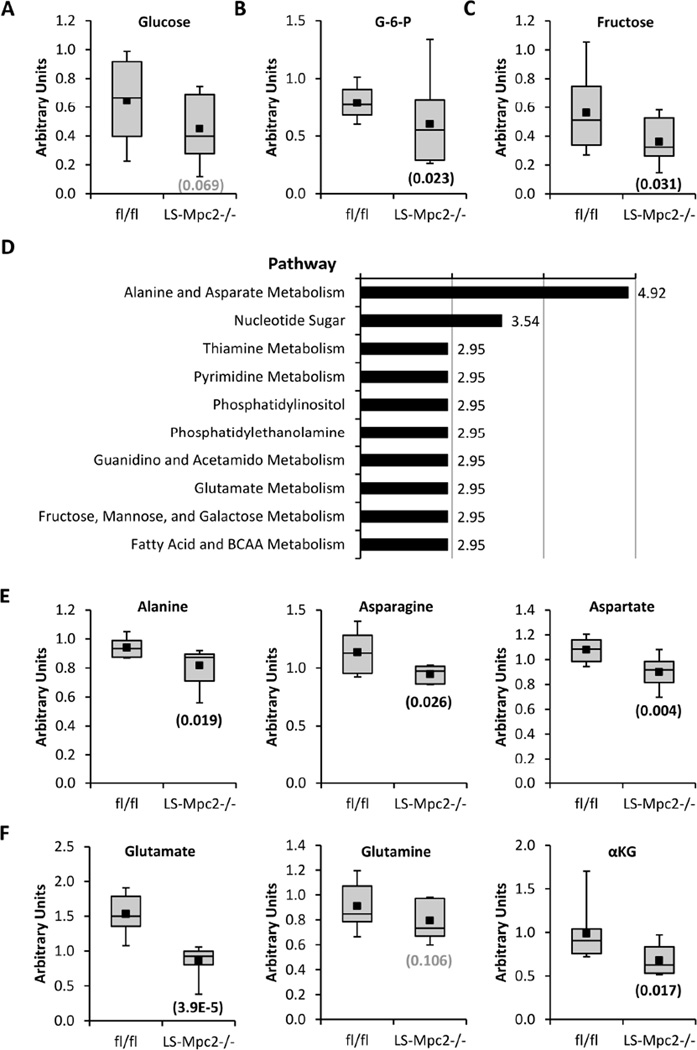
Although fasted LS-Mpc2−/− mice were significantly hypoglycemic compared to fl/fl controls, the magnitude of the effect of MPC deletion on glycemia was less than we expected. To screen for potential hepatic compensatory mechanisms involved in the maintenance of blood glucose levels, liver lysates from fasted mice were analyzed by unbiased multiplatform metabolomics. A total of 549 metabolites were measured and when fl/fl and LS-Mpc2−/− livers were compared in the fasted condition, 33 metabolites were significantly (p < 0.05) increased and 60 metabolites were significantly decreased in LS-Mpc2−/− livers compared to fl/fl comparators (Table S4). Among the metabolites that were altered, we detected an expected decrease in liver glucose (p=0.069; Figure 5A), glucose-6-phosphate (Figure 5B), and fructose (Figure 5C) content in LS-Mpc2−/− livers. Pathway analysis of altered metabolites suggested effects on several pathways in LS-Mpc2−/− livers (Figure 5D), including alterations in amino acid metabolism. Alanine, asparagine, and aspartate (Figure 5E), and glutamate, glutamine, and alpha-ketoglutarate (Figure 5F) were all significantly or tended to be decreased in fasted LS-Mpc2−/− liver compared to fl/fl liver. This observation was interesting in light of the knowledge that alanine and glutamine can be used as gluconeogenic substrates and was the basis for the next series of studies.
Figure 5. Metabolomic analyses of fasted fl/fl and LS-Mpc2−/− livers.
Liver (A) glucose, (B) glucose-6-phosphate (G-6-P), and (C) fructose content in fasted LS-Mpc2−/− compared to fl/fl mice. (D) Metabolic pathways that were most significantly affected by genotype in fasted mouse liver. (E) Alanine, asparagine, and aspartate content and (F) glutamate, glutamine, and alpha-ketoglutarate (αKG), content in fasted LS-Mpc2−/− and fl/fl livers. Data presented as box and whisker plot with minus error bar as the minimum of distribution, bottom of box as lower quartile, line bisecting the box as the median, the black square as the mean, the top of the box as the upper quartile, and the positive error bar as the maximum of distribution. Number in parentheses is p value for fasted fl/fl vs LS-Mpc2−/− liver metabolite.
Alanine Transamination Supports Gluconeogenesis in LS-Mpc2−/− Mice
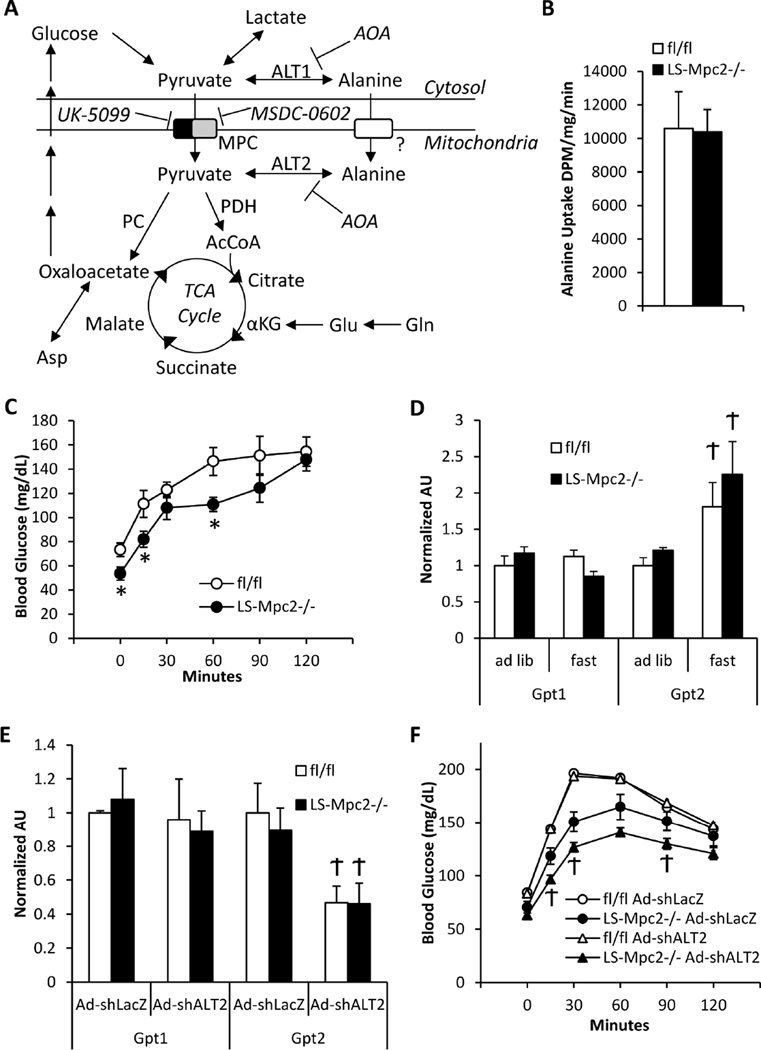
For alanine to be used for gluconeogenesis it must be converted to pyruvate. Alanine-pyruvate interconversion is catalyzed by two alanine transaminase (ALT) enzymes that are robustly expressed in liver (Figure 6A). ALT1 is localized to the cytosol and ALT2 is localized within the mitochondrial matrix (Yang et al., 2009). Previous reports of alanine transport into mitochondria (Cybulski and Fisher, 1977; Dieterle et al., 1978) and alanine transaminase localized to the mitochondrial matrix (ALT2) (Yang et al., 2009) led us to hypothesize an alternative route of pyruvate-carbon entry into mitochondrial pathways in LS-Mpc2−/− mice. Consistent with previous reports (Cybulski and Fisher, 1977; Dieterle et al., 1978), uptake of radiolabelled alanine was substantial in isolated liver mitochondria, and was unaltered by Mpc2 deletion (Figure 6B). LS-Mpc2−/− and fl/fl mice were next subjected to bolus i.p. L-alanine injection in an alanine tolerance test (ATT). Blood glucose concentrations were significantly lower in LS-Mpc2−/− mice after the overnight fast, but blood glucose increased similarly in both fl/fl and LS-Mpc2−/− mice by the 2 h time point after alanine injection (Figure 6C).
Figure 6. Gluconeogenesis from alanine is normal in LS-Mpc2−/− mice.
(A) Schematic depicting mitochondrial alanine metabolism during defective mitochondrial pyruvate transport. (B) Alanine uptake by isolated mitochondria from fl/fl and LS-Mpc2−/− livers. (C) Blood glucose concentrations after i.p. L-alanine injection during an alanine tolerance test (ATT). (D) qRT-PCR analyses for Gpt1 and Gpt2 in fed vs. fasted livers. (E) Liver qRT-PCR analyses after injection of control adenovirus encoding an shRNA against LacZ (Ad-shLacZ) or adenovirus expressing an shRNA against Gpt2 (Ad-shALT2). (F) PTT analysis performed 5 days after in vivo knockdown of ALT2. Data represented as mean ± S.E.M. *p ≤ 0.05 for fl/fl vs. LS-Mpc2−/−. Ϯp ≤ 0.05 for fed vs. fasted or for Ad-shLacZ vs Ad-shALT2.
To begin to investigate the role of pyruvate-alanine cycling in the compensatory phenotype of LS-Mpc2−/− mice, the expression of the genes (Gpt1 and Gpt2) encoding ALT1 and ALT2 enzymes, respectively, were examined in liver. Gpt2, but not Gpt1, was induced by fasting in mice, but was not affected by loss of Mpc2 (Figure 6D). However, in support of increased transamination and resulting urea production in LS-Mpc2−/− mice, liver citrulline and homocitrulline content was significantly elevated compared to fl/fl control liver (Table S4), which could suggest increased urea cycle activity. We next knocked-down liver Gpt2 expression in vivo by injection of adenovirus expressing a shRNA construct targeting Gpt2. Knockdown of Gpt2 mRNA by 55% in the liver had no effect on the blood glucose response during a PTT in fl/fl mice, but further reduced the glucose concentration in LS-Mpc2−/− mice (Figure 6E–F). These results indicate gluconeogenesis from alanine is unimpaired in LS-Mpc2−/− mice and that intramitochondrial transamination of alanine to pyruvate may contribute to gluconeogenesis when mitochondrial pyruvate import is inhibited.
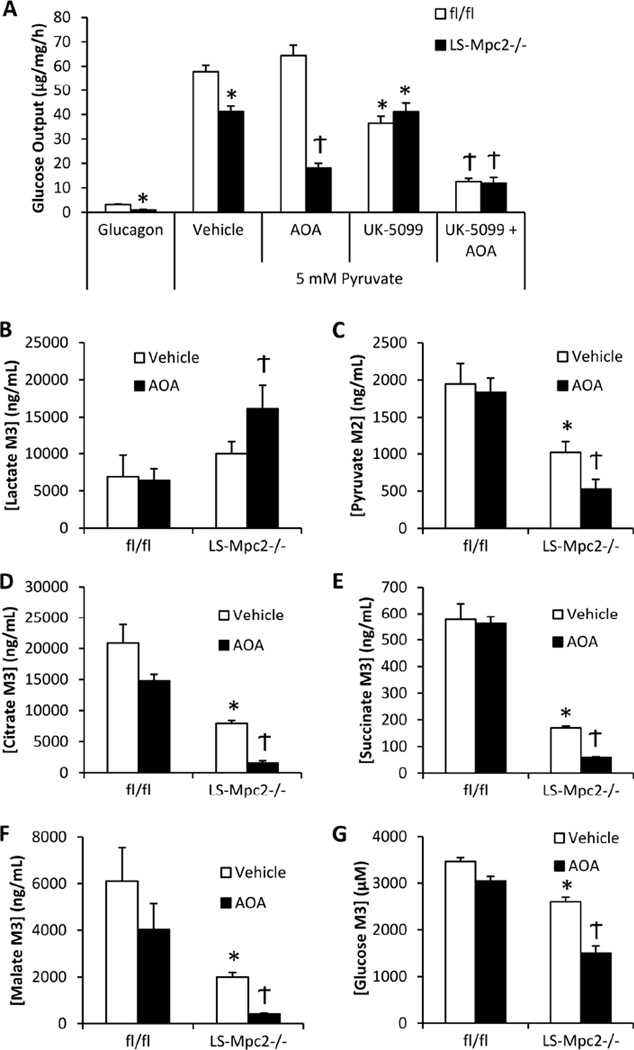
To further assess the possibility that pyruvate-alanine transamination compensates for diminished mitochondrial pyruvate import, isolated hepatocytes were assessed for their ability to produce glucose when stimulated with pyruvate in conjunction with ALT inhibition. When solely treated with glucagon, little glucose was released into the media by hepatocytes of any genotype. However, the LS-Mpc2−/− hepatocytes displayed significantly lower glucose output in this condition (Figure 7A), possibly due to lower glycogen content (Figure 4B). Glucagon supplemented with 5 mM pyruvate evoked an increase in glucose production in both fl/fl and LS-Mpc2−/− hepatocytes, but LS-Mpc2−/− hepatocytes produced significantly less glucose compared to fl/fl hepatocytes, and this effect could be mimicked by the MPC inhibitor UK-5099 in fl/fl hepatocytes (Figure 7A). Incubation with amino-oxyacetate (AOA), an inhibitor of alanine transaminases (Gonzalez et al., 2012), further reduced the amount of glucose produced from pyruvate in LS-Mpc2−/− hepatocytes (Figure 7A). AOA treatment had no effect on glucose produced from pyruvate in fl/fl hepatocytes, unless used in combination with the pyruvate transport inhibitor, UK-5099 (Figure 7A). These results strongly suggest that while mitochondrial pyruvate transport plays an important role in gluconeogenesis, genetic or pharmacologic inhibition of the MPC can be circumvented by an increased dependence on pyruvate-alanine transamination.
Figure 7. Pyruvate transamination to alanine compensates for decreased mitochondrial pyruvate transport in LS-Mpc2−/− hepatocytes.
(A) Glucose production from pyruvate by LS-Mpc2−/− and fl/fl hepatocytes cultured in the presence or absence of the alanine transaminase inhibitor amino-oxyacetate (AOA; 500 µM) and/or UK-5099. (B–G) U13C-pyruvate flux into lysate (B) lactate, (C) M2 pyruvate, (D) citrate, (E) succinate, (F) malate, or (G) medium glucose measured by mass spectrometry using LS-Mpc2−/− and fl/fl hepatocytes cultured in the presence or absence of AOA. Data expressed as mean + S.E.M. *p ≤ 0.05 for fl/fl vs. LS-Mpc2−/−. Ϯp ≤ 0.05 for vehicle vs AOA.
Pyruvate Transport or Alanine Transamination Regulate Flux of Pyruvate Carbon into TCA Cycle and Glucose
To confirm these findings, we again measured incorporation of the carbon contained in pyruvate into downstream intermediates by using isolated hepatocytes cultured with 2.5 mM pyruvate + 2.5 mM U-13C-pyruvate and mass spectrometry. 13C-labelled carbon incorporation into lactate was slightly increased in LS-Mpc2−/− hepatocytes compared to fl/fl controls (Figure 7B). Hepatocyte content of pyruvate containing two labeled carbons [M2] (Figure 7C), which requires mitochondrial TCA cycle metabolism to remove one labeled carbon, and [M3] TCA cycle intermediates (citrate, succinate, and malate; (Figure 7D–F)) were significantly reduced in LS-Mpc2−/− hepatocytes compared to fl/fl controls. Importantly, loss of MPC2 also reduced [M3] glucose concentration in the medium compared to fl/fl controls (Figure 7G). AOA treatment of LS-Mpc2−/− hepatocytes further increased [M3] lactate content and reduced 13C incorporation into TCA cycle intermediates or medium glucose (Figures 7B–G). In contrast, AOA was largely without effect in fl/fl hepatocytes (Figures 7B–G), indicating that transaminase activity is not essential for pyruvate metabolism when the MPC is intact. Table S5 details the 13C enrichment and concentration of a broad panel of intermediates from this experiment. Altogether, these pharmacologic and genetic data provide strong evidence that the MPC is required for full gluconeogenic capacity, but also suggest that pyruvate-alanine cycling is an important alternative pathway when MPC activity is impaired.
Discussion
Hepatic glucose production from pyruvate/lactate is believed to be a critical metabolic function that prevents hypoglycemia in starved conditions and is an important component of the Cori cycle. Dogma dictates that gluconeogenesis from pyruvate and lactate should require mitochondrial pyruvate import because the synthesis of pyruvate from PEP is not a reversible reaction and the carboxylase enzyme required for the metabolism of pyruvate is only localized in the mitochondrial matrix (Figure 6A). In this work, we show that MPC deletion in a liver-specific manner has marked effects on gluconeogenic flux and mitochondrial intermediary metabolism of pyruvate. Taken together with previous work, these data suggest that mitochondrial pyruvate import is required for high rates of gluconeogenesis, but also that the capacity for this process is likely far in excess of that required for robust gluconeogenic flux and that pathways to partially overcome MPC inhibition exist in hepatocytes.
While several studies have shown that chemical inhibition of MPC activity decreases liver glucose production (Lima et al., 2006; Martin-Requero et al., 1986; Rognstad, 1983; Thomas and Halestrap, 1981) other studies using small molecule inhibitors of the MPC (Rognstad, 1983) or hepatocytes expressing a hypomorphic MPC2 allele (Vigueira et al., 2014), failed to demonstrate a strong effect of MPC impairment on gluconeogenesis. Our studies may explain a lack of effect of MPC inhibition on gluconeogenesis by elucidating a compensatory pathway that is operant when mitochondrial pyruvate flux is impaired. Metabolomic profiling of fasted LS-Mpc2−/− liver tissue detected significant reductions in the concentrations of several amino acids related to the metabolism of alanine and glutamine, which can also be used as gluconeogenic substrates. While our paper focused on pyruvate-alanine cycling, a companion paper (Gray et al., co-submitted) clearly showed adaptive use of glutamine to maintain TCA cycle flux in MPC1-deficient liver. We found modest changes in the expression of genes encoding enzymes of the TCA cycle proximal to the entry of glutamine/glutamate at α-ketoglutarate (Idh2 and Sdha) in livers of fast MPC2-deficient mice, but no effect on enzymes directly involved in glutaminolysis (Table S3). It is likely that alterations in the metabolism of glutamine and related amino acids, as well as altered alanine utilization, are all present in MPC2-deficient mice since we detected a depletion of glutamine, glutamate, and α-ketoglutarate. Given that mitochondrial metabolism of pyruvate is such an important process in intermediary metabolism, it is not unexpected that compensatory adaptations would exist. Indeed, recent work in cultured cells has shown that enhanced use of these amino acids as TCA cycle substrates help to allow cells to survive in the context of MPC deficiency (Du et al., 2013; Vacanti et al., 2014; Yang et al., 2014) and the present in vivo studies are consistent with this.
Alanine can be transported across the inner mitochondrial membrane by a carrier-mediated mechanism (Cybulski and Fisher, 1977; Dieterle et al., 1978). The MPC does not mediate this transport activity since we found that mitochondrial alanine import and its stimulatory effects on gluconeogenesis were unaffected by pharmacologic inhibition or genetic loss of MPC2. Previous work also showed that very high (mM) concentrations of cinnamate MPC inhibitors did not markedly affect hepatic glucose production from alanine (Groen et al., 1982; Patel and Olson, 1985). Although alanine transamination to pyruvate can occur in the cytosol (DeRosa and Swick, 1975), several previous studies indicate an important role for the mitochondrial alanine transaminase (ALT2) in gluconeogenesis from alanine by transaminating alanine to pyruvate in the mitochondrial matrix (DeRosa and Swick, 1975). The data obtained with LS-Mpc2−/− hepatocytes or UK-5099 in combination with AOA are also consistent with intramitochondrial alanine deamination.
Hepatic glucose overproduction contributes to hyperglycemia in uncontrolled diabetes. Given the ability of MPC inhibition or deletion to suppress gluconeogenesis from pyruvate/lactate and prevent hyperglycemia, there may be utility in targeting the MPC complex as a glucose-lowering strategy. Several cinnamate analogues (including UK-5099) (Halestrap, 1975) and thiazolidine ring-containing compounds designed as KATP channel agonists (Hildyard et al., 2005) and insulin-sensitizing thiazolidinediones (troglitazone, rosiglitazone, pioglitazone, MSDC-0160 and MSDC-0602) (Divakaruni et al., 2013) inhibit the MPC. The PPARγ-sparing TZDs, MSDC-0602 and MSDC-0160, have been specifically crosslinked to the MPC complex in liver mitochondria (Colca et al., 2013) and suppressed mitochondrial pyruvate oxidation in cultured cells in an MPC-dependent manner (Divakaruni et al., 2013). In this work, we show that MSDC-0602 and UK-5099 suppress hepatocyte glucose production, pyruvate-stimulated mitochondrial OCR, and 13C-pyruvate incorporation into TCA cycle intermediates or glucose via a mechanism that requires the MPC suggesting that, at least in vitro, this effect of the drugs could be mediated by an interaction with the MPC. This finding also provides an explanation and mechanism for our previous observation that suppression of glucose production by the novel TZD, MSDC-0602, was mediated independently of PPARγ, since MSDC-0602 was able to suppress glucose production by hepatocytes from LS-PPARγ−/− mice (Chen et al., 2012). Whether interaction with the MPC is required for the insulin-sensitizing effects of these PPARγ-sparing TZDs in vivo remains to be determined. Unfortunately, this proof of concept study is complicated by effects of TZDs in multiple tissues and the fact that whole body MPC2 knockout in mice leads to embryonic lethality (Vigueira et al., 2014).
In conclusion, we present evidence that liver-specific deficiency in the MPC, which is a target for anti-diabetic drugs, leads to impairments in hepatic gluconeogenesis in mice. Despite a defect in gluconeogenesis from pyruvate, LS-Mpc2−/− mice are outwardly normal, display no evidence of overt liver pathology, and exhibit only modest hypoglycemia during prolonged fasting studies. We also demonstrate that pyruvate-alanine cycling in LS-Mpc2−/− mice likely constitutes an alternative pathway for gluconeogenesis from pyruvate/lactate to proceed by circumventing the MPC complex. Future work will be needed to determine whether selectively targeting the MPC complex is a viable strategy for treating diabetes and other associated metabolic diseases.
Experimental Procedures
Additional Procedures can be found as Supplemental Materials
Animal Studies
All experiments were conducted with 6–12 week old mice of both sexes. All animal experiments were approved by the Animal Studies Committee of the Washington University School of Medicine.
Generation of Mpc2 flox and LS-Mpc2−/− mice
C57/Bl6 mouse embryonic stem cells containing a “knockout first” allele of Mpc2 were obtained from EUCOMM (IKMC Project: 89918; catalog number MAE-1900). This construct contains a targeted allele of Mpc2 containing inserted cassettes for promoter-driven LacZ and Neo cDNAs flanked by frt sites upstream of exon 3 of the Mpc2 gene, which is flanked by LoxP sites. Propagated ES cells were injected into developing embryos and then implanted into pseudopregnant females. Chimeric offspring were mated to establish germline transmission. Resulting heterozygous offspring were mated with C57/Bl6 mice expressing Flp recombinase in a global manner (chicken alpha actin promoter-driven transgenic) to remove LacZ and Neo cassettes and generate mice harboring the conditional floxed allele (Figure 1A). Mpc2 floxed mice were then crossed with hemizygous C57/Bl6 mice expressing Cre under the Albumin promoter to create LS-Mpc2−/− mice. Littermate mice not expressing Cre (fl/fl mice) were used as control mice in all experiments. For db/db intercross experiments, C57/Bl6 Leprdb/+ mice were obtained from the Jackson Laboratory and crossed with LS-Mpc2−/− mice.
Phenomaster Metabolic Profiling
Indirect calorimetry, energy expenditure, spontaneous activity, and food/water consumption were measured in Phenomaster TSE® cages similar to as previously reported (Tang et al., 2015). Data was measured and collected at 10 minute intervals.
13C-Labeled Pyruvate Hepatocyte Studies
Primary adult hepatocytes were isolated and cultured as described previously (Chen et al., 2008). Hepatocytes were isolated and cultured in 60-mm dishes for 12 h as described above. Hepatocytes were washed twice with PBS and incubated in HBSS for 2 h and then washed with fresh HBSS. The hepatocytes were then incubated for 4 h in HBSS containing 2.5 mM U13C-labeled sodium pyruvate (Cambridge Isotope Lab) plus 2.5 mM unlabeled sodium pyruvate or 5 mM unlabeled sodium pyruvate (to correct for background enrichment) in combination with 10 µM UK-5099, 15 µM MSDC-0602, or 500 µM AOA. Cell culture media was removed and frozen, and cells were washed with ice-cold PBS, fixed immediately in 20°C 100% methanol, scraped from the dishes, and frozen. Cell lysates and media were analyzed by gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-mass spectrometry (LC-MS) to determine 13C enrichment and concentration as previously described for glucose (Sunny and Bequette, 2010), organic acids (Des Rosiers et al., 1994), and amino acids (Casetta et al., 2000; Fuzfai et al., 2004). Data are reported as 13C enrichment × concentration.
TZD Photoaffinity Crosslinking
The iodinated (125I) photoprobe, MSDC-1101, was used for photoaffinity crosslinking of crude mitochondrial membranes from isolated fl/fl and LS-Mpc2−/− hepatocytes as previously described (Colca et al., 2013; Colca et al., 2004). The addition of cold UK-5099 or MSDC-0602 (30 µM) were used as competitors for the MPC2 binding site prior to photoactivation.
Non-biased Comprehensive Metabolomic Analysis
Frozen liver chunks (~100 mg) from ad libitum fed or 24 h fasted mice was shipped frozen to Metabolon (Durham, NC), extracted, and analyzed as previously described (Hatori et al., 2012). Three independent platforms (ultrahigh performance liquid chromatography/ tandem mass spectrometry optimized for basic species and acidic species and gas chromatography/ mass spectrometry) were utilized for biochemical detection. Metabolites were identified by automated comparison of ion features in samples to a reference library of chemical standards entries including retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra. Values below limits of detection were ascribed with compound minimum. ANOVA of log transformed data was performed to compare data between groups. Multiple comparisons were accounted for by estimating false discovery rates.
Adenoviral Construction and in vivo Adenoviral Infection
Adenovirus expressing GFP reporter and a validated shRNA targeting murine Gpt2 (glutamic pyruvate transaminase, also known as ALT2) was obtained from Vector Biolabs (shADV-260703). An adenoviral-driven shRNA construct targeting LacZ and GFP reporter was used as a vector control (Finck et al., 2006). Mice were infected by retro-orbital injection of 150–200 µl high-titer adenoviruses.
Statistical Analyses
Statistical comparisons were made using t-test or ANOVA where appropriate. All data unless specified are presented as mean ± SEM, with a statistically significant difference defined as p ≤ 0.05.
Supplementary Material
Acknowledgements
This work was supported by NIH grants R01 DK078187 and R01 DK104735 to BNF and R42 AA021228 to RFK and BNF. A grant from the Barnes Jewish Hospital Foundation and the core services of the Digestive Diseases Research Core Center (P30 DK52574), Diabetes Research Center (P30 DK20579), and the Nutrition Obesity Research Center (P30 DK56341) at the Washington University School of Medicine also supported this work. SCB is supported by NIH grants R01 DK078184 and P01 DK058398, and the Robert A. Welch Foundation (I-1804-01). KSM is a Diabetes Research Postdoctoral Training Program fellow (T32 DK07296 and DK007120). WGM, JRC, and RFK are founders, employees, and significant stockholders of Metabolic Solutions Development Company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
K.S.M. and Z.C. conceived and performed experiments, analyzed data, generated figures, and wrote and edited the manuscript. X.F. performed experiments and edited the manuscript. W.G.M., J.R.C., R.F.K., S.C.B., and B.N.F. conceived experiments, analyzed data, and edited the manuscript.
References
- Adams MD, Raman P, Judd RL. Comparative effects of englitazone and glyburide on gluconeogenesis and glycolysis in the isolated perfused rat liver. Biochemical pharmacology. 1998;55:1915–1920. doi: 10.1016/s0006-2952(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. The Journal of biological chemistry. 2006;281:19000–19008. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casetta B, Tagliacozzi D, Shushan B, Federici G. Development of a method for rapid quantitation of amino acids by liquid chromatography-tandem mass spectrometry (LC-MSMS) in plasma. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2000;38:391–401. doi: 10.1515/CCLM.2000.057. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gropler MC, Norris J, Lawrence JC, Jr, Harris TE, Finck BN. Alterations in hepatic metabolism in fld mice reveal a role for lipin 1 in regulating VLDL-triacylglyceride secretion. Arterioscler Thromb Vasc Biol. 2008;28:1738–1744. doi: 10.1161/ATVBAHA.108.171538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Vigueira PA, Chambers KT, Hall AM, Mitra MS, Qi N, McDonald WG, Colca JR, Kletzien RF, Finck BN. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor gamma-sparing thiazolidinedione. The Journal of biological chemistry. 2012;287:23537–23548. doi: 10.1074/jbc.M112.363960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, McDonald WG, Cavey GS, Cole SL, Holewa DD, Brightwell-Conrad AS, Wolfe CL, Wheeler JS, Coulter KR, Kilkuskie PM, et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PloS one. 2013;8:e61551. doi: 10.1371/journal.pone.0061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. American journal of physiology. Endocrinology and metabolism. 2004;286:E252–E260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- Colca JR, Tanis SP, McDonald WG, Kletzien RF. Insulin sensitizers in 2013: new insights for the development of novel therapeutic agents to treat metabolic diseases. Expert Opin Investig Drugs. 2014;23:1–7. doi: 10.1517/13543784.2013.839659. [DOI] [PubMed] [Google Scholar]
- Cybulski RL, Fisher RR. Mitochondrial neutral amino acid transport: evidence for a carrier mediated mechanism. Biochemistry. 1977;16:5116–5120. doi: 10.1021/bi00642a026. [DOI] [PubMed] [Google Scholar]
- DeRosa G, Swick RW. Metabolic implications of the distribution of the alanine aminotransferase isoenzymes. The Journal of biological chemistry. 1975;250:7961–7967. [PubMed] [Google Scholar]
- Des Rosiers C, Fernandez CA, David F, Brunengraber H. Reversibility of the mitochondrial isocitrate dehydrogenase reaction in the perfused rat liver. Evidence from isotopomer analysis of citric acid cycle intermediates. The Journal of biological chemistry. 1994;269:27179–27182. [PubMed] [Google Scholar]
- Dieterle P, Brawand F, Moser UK, Walter P. Alanine metabolism in rat liver mitochondria. European journal of biochemistry / FEBS. 1978;88:467–473. doi: 10.1111/j.1432-1033.1978.tb12471.x. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Cleghorn WM, Contreras L, Lindsay K, Rountree AM, Chertov AO, Turner SJ, Sahaboglu A, Linton J, Sadilek M, et al. Inhibition of mitochondrial pyruvate transport by zaprinast causes massive accumulation of aspartate at the expense of glutamate in the retina. The Journal of biological chemistry. 2013;288:36129–36140. doi: 10.1074/jbc.M113.507285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC, Jr, Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell metabolism. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. The Journal of clinical investigation. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzfai Z, Katona ZF, Kovacs E, Molnar-Perl I. Simultaneous identification and quantification of the sugar, sugar alcohol, and carboxylic acid contents of sour cherry, apple, and ber fruits, as their trimethylsilyl derivatives, by gas chromatography-mass spectrometry. Journal of agricultural and food chemistry. 2004;52:7444–7452. doi: 10.1021/jf040118p. [DOI] [PubMed] [Google Scholar]
- Gonzalez JD, Caballero A, Viegas I, Meton I, Jones JG, Barra J, Fernandez F, Baanante IV. Effects of alanine aminotransferase inhibition on the intermediary metabolism in Sparus aurata through dietary amino-oxyacetate supplementation. The British journal of nutrition. 2012;107:1747–1756. doi: 10.1017/S000711451100496X. [DOI] [PubMed] [Google Scholar]
- Groen AK, Sips HJ, Vervoorn RC, Tager JM. Intracellular compartmentation and control of alanine metabolism in rat liver parenchymal cells. European journal of biochemistry / FEBS. 1982;122:87–93. doi: 10.1111/j.1432-1033.1982.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Groen AK, van Roermund CW, Vervoorn RC, Tager JM. Control of gluconeogenesis in rat liver cells. Flux control coefficients of the enzymes in the gluconeogenic pathway in the absence and presence of glucagon. The Biochemical journal. 1986;237:379–389. doi: 10.1042/bj2370379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen AK, Vervoorn RC, Van der Meer R, Tager JM. Control of gluconeogenesis in rat liver cells. I. Kinetics of the individual enzymes and the effect of glucagon. The Journal of biological chemistry. 1983;258:14346–14353. [PubMed] [Google Scholar]
- Halestrap AP. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. The Biochemical journal. 1975;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Armston AE. A re-evaluation of the role of mitochondrial pyruvate transport in the hormonal control of rat liver mitochondrial pyruvate metabolism. The Biochemical journal. 1984;223:677–685. doi: 10.1042/bj2230677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell metabolism. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- Hildyard JC, Ammala C, Dukes ID, Thomson SA, Halestrap AP. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochimica et biophysica acta. 2005;1707:221–230. doi: 10.1016/j.bbabio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) The Journal of biological chemistry. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Lima LC, Buss GD, Ishii-Iwamoto EL, Salgueiro-Pagadigorria C, Comar JF, Bracht A, Constantin J. Metabolic effects of p-coumaric acid in the perfused rat liver. Journal of biochemical and molecular toxicology. 2006;20:18–26. doi: 10.1002/jbt.20114. [DOI] [PubMed] [Google Scholar]
- Martin-Requero A, Ayuso MS, Parrilla R. Rate-limiting steps for hepatic gluconeogenesis. Mechanism of oxamate inhibition of mitochondrial pyruvate metabolism. The Journal of biological chemistry. 1986;261:13973–13978. [PubMed] [Google Scholar]
- Patel TB, Olson MS. A reexamination of the role of the cytosolic alanine aminotransferase in hepatic gluconeogenesis. Archives of biochemistry and biophysics. 1985;240:705–711. doi: 10.1016/0003-9861(85)90079-7. [DOI] [PubMed] [Google Scholar]
- Raman P, Foster SE, Stokes MC, Strenge JK, Judd RL. Effect of troglitazone (Rezulin) on fructose 2,6-bisphosphate concentration and glucose metabolism in isolated rat hepatocytes. Life sciences. 1998;62:PL89–PL94. doi: 10.1016/s0024-3205(97)01177-6. [DOI] [PubMed] [Google Scholar]
- Raman P, Judd RL. Role of glucose and insulin in thiazolidinedione-induced alterations in hepatic gluconeogenesis. European journal of pharmacology. 2000;409:19–29. doi: 10.1016/s0014-2999(00)00806-2. [DOI] [PubMed] [Google Scholar]
- Rognstad R. The role of mitochondrial pyruvate transport in the control of lactate gluconeogenesis. The International journal of biochemistry. 1983;15:1417–1421. doi: 10.1016/0020-711x(83)90073-3. [DOI] [PubMed] [Google Scholar]
- Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. Journal of lipid research. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Burgess SC, Shiota M, Flakoll P, Donahue EP, Malloy CR, Sherry AD, Magnuson MA. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52:1649–1654. doi: 10.2337/diabetes.52.7.1649. [DOI] [PubMed] [Google Scholar]
- Sunny NE, Bequette BJ. Gluconeogenesis differs in developing chick embryos derived from small compared with typical size broiler breeder eggs. J Anim Sci. 2010;88:912–921. doi: 10.2527/jas.2009-2479. [DOI] [PubMed] [Google Scholar]
- Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nature medicine. 2015;21:173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- Thomas AP, Halestrap AP. The role of mitochondrial pyruvate transport in the stimulation by glucagon and phenylephrine of gluconeogenesis from L-lactate in isolated rat hepatocytes. The Biochemical journal. 1981;198:551–560. doi: 10.1042/bj1980551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titheradge MA, Coore HG. Hormonal regulation of liver mitochondrial pyruvate carrier in relation to gluconeogenesis and lipogenesis. FEBS letters. 1976a;72:73–78. doi: 10.1016/0014-5793(76)80901-5. [DOI] [PubMed] [Google Scholar]
- Titheradge MA, Coore HG. The mitochondrial pyruvate carrier, its exchange properties and its regulation by glucagon. FEBS letters. 1976b;63:45–50. doi: 10.1016/0014-5793(76)80191-3. [DOI] [PubMed] [Google Scholar]
- Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, Murphy AN, Metallo CM. Regulation of Substrate Utilization by the Mitochondrial Pyruvate Carrier. Molecular cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigueira PA, McCommis KS, Schweitzer GG, Remedi MS, Chambers KT, Fu X, McDonald WG, Cole SL, Colca JR, Kletzien RF, et al. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell reports. 2014;7:2042–2053. doi: 10.1016/j.celrep.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, et al. Glutamine Oxidation Maintains the TCA Cycle and Cell Survival during Impaired Mitochondrial Pyruvate Transport. Molecular cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, Gong DW. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology. 2009;49:598–607. doi: 10.1002/hep.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.