Abstract
The cell envelopes of many Gram-positive bacteria contain wall teichoic acids (WTAs). Staphylococcus aureus WTAs are composed of ribitol phosphate (RboP) or glycerol phosphate (GroP) backbones substituted with d-alanine and N-acetyl-d-glucosamine (GlcNAc) or N-acetyl-d-galactosamine (GalNAc). Two WTA glycosyltransferases, TarM and TarS, are responsible for modifying the RboP WTA with α-GlcNAc and β-GlcNAc, respectively. We recently reported that purified human serum anti-WTA IgG specifically recognizes β-GlcNAc of the staphylococcal RboP WTA and then facilitates complement C3 deposition and opsonophagocytosis of S. aureus laboratory strains. This prompted us to examine whether anti-WTA IgG can induce C3 deposition on a diverse set of clinical S. aureus isolates. To this end, we compared anti-WTA IgG-mediated C3 deposition and opsonophagocytosis abilities using 13 different staphylococcal strains. Of note, the majority of S. aureus strains tested was recognized by anti-WTA IgG, resulting in C3 deposition and opsonophagocytosis. A minority of strains was not recognized by anti-WTA IgG, which correlated with either extensive capsule production or an alteration in the WTA glycosylation pattern. Our results demonstrate that the presence of WTAs with TarS-mediated glycosylation with β-GlcNAc in clinically isolated S. aureus strains is an important factor for induction of anti-WTA IgG-mediated C3 deposition and opsonophagocytosis.
INTRODUCTION
Staphylococcus aureus can cause serious infections of the skin, soft tissues, and bloodstream in the community and in hospitalized patients (1). To establish successful infection, S. aureus deploys a variety of survival and immune evasion strategies, such as the acquisition of essential nutrients and expression of adhesins, which promote colonization and survival, and the production of virulence factors, such as capsules and toxins, which aid host immune evasion (2, 3). The recent spread of methicillin-resistant S. aureus (MRSA) increases the necessity of treating infections better. Unfortunately, many efforts to develop an efficacious vaccine against S. aureus have failed (4, 5). The putative reasons for this failure in vaccine clinical trials were assumed to be due to a focus on vaccines with single target antigens stimulating humoral defense rather than vaccines with a combination of target antigens stimulating both humoral and cellular immunity.
S. aureus is a Gram-positive bacterial pathogen that is surrounded by glycopolymers, including wall teichoic acid (WTA), peptidoglycan, lipoteichoic acid, and capsular polysaccharide (CP). These bacterial surface glycopolymers are recognized by serum antibodies and a variety of pattern recognition molecules, including mannose-binding lectin (MBL) (6, 7). Bacterial WTAs are involved in bacterial cell wall maintenance, susceptibility to antimicrobial molecules, biofilm formation, and host interaction (8, 9). Most MRSA strains, such as USA300, COL, and MW2, express poly(ribitol phosphate) (RboP) WTA, which is composed of 10 to 40 RboP repeating units (10). The hydroxyls on the RboP repeats are modified with d-alanine and N-acetylglucosamines (GlcNAc) via an α- or β-GlcNAc anomer (11). However, some strains, such as S. aureus PS187 (ST395 lineage), have recently been found to produce a unique poly(glycerol phosphate) (GroP) WTA modified with N-acetyl-d-galactosamine (GalNAc) (12). Furthermore, the molecular elucidation of WTA biosynthesis pathways in S. aureus paved the way for the identification of two WTA glycosyltransferases, TarM and TarS, responsible for modifying RboP with either α-GlcNAc or β-GlcNAc, respectively (13, 14). In addition, analysis of the WTA biosynthesis pathway in the S. aureus sequence type 395 (ST395) lineage revealed a novel WTA glycosyltransferase, TagN, which is involved in modification of GroP WTA with α-GalNAc (12, 15). These studies help provide an understanding of how S. aureus cells produce variable WTA types and elucidate the functional importance of WTA structure variation during infections.
The human complement system is the first line of host defense responses to invading pathogens (16). Pathogen-specific serum antibodies activate the classical complement pathway (17). Human serum MBL binds to a mannose or GlcNAc residue of bacterial surface sugar chains (18) and functions as an opsonin activating the lectin complement pathway (6). The activation of the classical and lectin pathways mediates opsonization by complement fragments, such as C4b and C3b. The opsonized pathogens are engulfed by phagocytes, which are recruited by C3a and C5a anaphylatoxins (17). Therefore, functional determination of the bacterial ligand moiety recognized by serum antibody during opsonophagocytosis is important for understanding the host-microbe interaction and for prevention of S. aureus infections.
Recently, we reported that S. aureus WTA functions as a ligand of MBL (19). Intriguingly, serum MBL from infants who had not yet fully developed adaptive immunity could bind to S. aureus WTA and induce complement C3 deposition. Additionally, the purified anti-WTA IgG from adults' sera strongly induced activation of the classical complement pathway, leading to the opsonophagocytosis of S. aureus cells (20). We further determined that anti-WTA IgG and MBL require the GlcNAc residues of S. aureus WTAs for complement activation (21). Namely, although anti-WTA IgG-mediated classical and MBL-mediated lectin complement activation and opsonophagocytosis are required for the β-GlcNAc residue of WTA, α-GlcNAc residues of WTA have hardly any and only a low capacity to activate both the classical and lectin complement pathways (21). Also, we have demonstrated in vivo the protective efficacy of anti-WTA antibodies against two clinical MRSA strains, COL and MW2 (22).These studies reveal that the sugar moiety of WTA is an important molecular determinant in host immune responses to S. aureus infection in vitro and in vivo.
However, we wondered whether our purified human anti-WTA IgG can recognize other clinically isolated S. aureus strains, which may harbor different WTA backbone structures, different glycosylation patterns, or different amounts of CPs on the bacterial surfaces (15, 23). We assumed that the determination of a spectrum of staphylococci recognized by serum anti-WTA IgG may be valuable for designing efficacious passive immunization against infections caused by different staphylococci. Also, to design an efficient WTA vaccine target antigen, it is vital to determine the exact WTA glycosylation pattern and WTA backbone structures of diverse staphylococcal strains.
The staphylococcal CPs play important roles in pathogenesis during S. aureus infection by impeding phagocytosis, resulting in bacterial persistence in the bloodstream of infected host organisms (24). Two major staphylococcal CPs, serotype 5 CP (CP5) and serotype 8 CP (CP8), predominate among clinically isolated strains from humans (24). These CPs have been reported to decrease in vitro complement-mediated opsonophagocytosis and to increase lethality in a mouse infection model (25). Previously, a careful study was carried out to estimate the ability of the complement C3 component to bind to different S. aureus strains by injection of six different encapsulated S. aureus strains into intact and C3-depleted mice (26). However, in that study, the molecular reasons why there was no straight relationship between the CP amounts of S. aureus strains and the deposited C3 amounts were not clearly answered. Therefore, we supposed that elucidation of the reason why serum antibody-mediated C3 deposition was not induced on some CP-producing strains is important for understanding the molecular interaction between host and microbes.
To examine how the amounts of CP produced, different WTA backbone structures, and the WTA glycosylation pattern affect anti-WTA IgG recognition of 13 different S. aureus strains, we analyzed anti-WTA IgG-mediated C3 deposition and opsonophagocytosis, the phage susceptibility, and the genotypes of 13 different staphylococcal strains. Those 13 strains consisted of 6 CP-producing strains, 1 CP-deficient mutated strain, 1 S. aureus ST395 clone (PS187), 1 S. epidermidis strain, 3 MRSA strains, and 1 laboratory strain (Table 1). Of note, complement C3 deposition on three strains failed, including S. aureus Lowenstein and PS187 and S. epidermidis, while anti-WTA IgG-mediated opsonophagocytosis of five strains was not induced. Genotyping and phage susceptibility patterns revealed that rare alterations in the WTA backbone structure, glycosylation pattern, or overt CP production can interfere with the binding of anti-WTA IgG to a certain extent. Our combined analytical techniques revealed possible reasons why C3 deposition and opsonophagocytosis were not induced for some of the strains, providing valuable information offering an understanding of the molecular interaction between host and microbes.
TABLE 1.
S. aureus and S. epidermidis strains used in this study
| Strain | Relevant characteristicsa | Reference |
|---|---|---|
| S. aureus RN4220 | Restriction mutant | 39 |
| S. aureus M0107 | RN4220 Δspa::Phl | 40 |
| S. aureus T790 | M0107 ΔtarM::Erm | 21 |
| S. aureus T803 | M0107 ΔtarS::Km | 21 |
| S. aureus T807 | M0107 ΔtarM::Erm ΔtarS::Km | 21 |
| S. aureus USA300 | CA-MRSA | 14 |
| S. aureus USA300 ΔtarM | USA300 ΔtarM | 41 |
| S. aureus USA300 ΔtarS | USA300 ΔtarS | 14 |
| S. aureus USA300 ΔtarMS | USA300 ΔtarM ΔtarS | 41 |
| S. aureus COL | HA-MRSA | 42 |
| S. aureus MW2 | CA-MRSA | 43 |
| S. aureus Becker | CP8 | 44 |
| S. aureus Reynolds | CP5 | 44 |
| S. aureus JL022 | CP deficient | 31 |
| S. aureus M (NCTC 10649) | CP1 | 45 |
| S. aureus Wright | CP8 | 46 |
| S. aureus Smith diffuse | CP2 | 47 |
| S. aureus Lowenstein | CP5 | 48 |
| S. aureus PS187 | ST395 isolate | 12 |
| S. epidermidis ATCC 14990 (Fussel) | Coagulase negative | 49 |
CA-MRSA, community-associated MRSA; HA-MRSA, health care-associated MRSA.
MATERIALS AND METHODS
Ethics statement.
We obtained approval for this study specifically from the Institutional Review Board of Pusan National University. For the collection of human polymorphonuclear leukocytes (PMNs) from adults, we also obtained written informed content from all healthy participants.
Proteins, sera, bacteria, and reagents.
Native human MBL/MBL-associated serine protease (MASP) complex was purified from human serum as described previously (27). S. aureus-treated serum was prepared as described previously (20) using the S. aureus M0107 strain (Δspa), which is deficient in immunoglobulin-binding protein A. S. aureus strains were grown at the appropriate temperature in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 1% NaCl) containing, where appropriate, 100 μg/ml ampicillin, 10 μg/ml erythromycin, 50 μg/ml kanamycin, 12.5 μg/ml chloramphenicol, or 20 μg/ml phleomycin. Encapsulated strains were cultivated in Columbia medium supplemented with 2% NaCl to enhance capsule production. The bacteria were fixed with ethanol in order to (i) inhibit bacterial cell growth, which has been shown in previous studies to be optimal for the in vitro interaction of S. aureus cells with serum antibodies and human MBL (19–21), and to (ii) exclude the effects of bacterial secreted protein and cell surface molecules, which might be factors differentially released from various S. aureus strains and affect complement activation and opsonophagocytosis.
Purification of WTA from S. aureus.
S. aureus WTA was prepared as described previously (28) with some modifications. In brief, WTA-bound insoluble peptidoglycan was prepared and treated with 5% (wt/vol) trichloroacetic acid for 18 h at room temperature to release the WTA from peptidoglycan. The obtained WTA was further purified by anion-exchange column chromatography as described previously (21).
Purification of anti-WTA IgG.
Anti-WTA IgG was purified from commercially available human intravenous IgG (IVIG; Green Cross, South Korea) using a WTA-coated nitrocellulose membrane as described previously (19) with the following modifications. Briefly, 100 μg of peptidoglycan-linked WTA in 200 μl of phosphate-buffered saline (PBS) was prepared from S. aureus strain T384 (RN4220 Δlgt::Phl ΔoatA::Erm) as described previously (19), spotted onto a nitrocellulose membrane (10 by 90 mm; pore size, 0.45 μm; Whatman), and baked at 100°C for 1 h. The membranes were washed with buffer A (20 mM Tris-HCl, pH 7.4, 250 mM NaCl) and blocked with buffer B (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% bovine serum albumin [BSA]) for 2 h at 4°C. Three sheets of the membrane were incubated with 50 mg of IVIG in 40 ml of buffer C (10 mM Tris-HCl, pH 7.5, 140 mM NaCl, 1% BSA) for 2 h at 4°C. After washing with buffer A, bound IgGs were eluted with 1 ml of 0.1 M glycine (pH 2.8) and immediately neutralized with 1 M KOH to pH 7.5. The glycine in the eluted IgG fraction was removed by passing the fraction through a Vivaspin 20 centrifugal concentrator (Sartorius) three times in the presence of buffer A. To remove the anti-peptidoglycan IgGs, the IgG fraction obtained was incubated at 4°C with S. aureus Δspa ΔtagO double mutant cells that had been prefixed with formaldehyde. Then, the S. aureus double mutant cells were pelleted by centrifugation, and the supernatant was collected, concentrated with the Vivaspin 20 concentrator, and used as a purified anti-WTA IgG fraction.
Flow cytometry analysis of S. aureus cells.
The amounts of bound IgG on S. aureus cells were measured as described previously (21). Briefly, ethanol-fixed S. aureus cells (4 μl of a suspension with an A600 of 3) were incubated with human anti-WTA IgG (50 ng) in 20 μl of incubation buffer (10 mM Tris-HCl, pH 7.4, 140 mM NaCl, 10 mM CaCl2, 1% BSA) on ice. The cells were washed with washing buffer (10 mM Tris-HCl, pH 7.4, 140 mM NaCl, 10 mM CaCl2) and incubated with mouse anti-human IgG monoclonal antibody (MAb; diluted 1:200; Sigma) as the primary antibody, followed by goat F(ab′)2 anti-mouse IgG antibodies conjugated with fluorescein 5-isothiocyanate (FITC; diluted 1:200; Beckman Coulter). The washed S. aureus cells were sonicated for 15 s to disperse clumped cells before flow cytometry analyses (Accuri C6; Beckman Coulter). To detect C3 deposition, ethanol-fixed cells were incubated with S. aureus-treated serum (10%) with or without MBL/MASP (10 ng) or anti-WTA IgG (50 ng) in 20 μl of incubation buffer for 60 min at 37°C. Then, bound C3b was detected using mouse anti-human C3 MAb conjugated with FITC (diluted 1:200; Beckman Coulter).
Isolation of human PMNs and opsonophagocytosis assay.
PMNs were isolated from healthy donors using Polymorphprep solution (Nycomed Pharm As, Torshov, Norway) as described previously (20). The PMNs were 99% viable, as shown in a trypan blue dye exclusion test. An opsonophagocytosis assay was performed with minor modifications as described previously (20). S. aureus cells grown to a postexponential growth phase in LB or Columbia medium were washed, killed with 70% ethanol, labeled with 0.1 mM FITC (Sigma) in 0.1 M Na2CO3 buffer (pH 8.5) for 30 min at room temperature, and resuspended in Hanks' balanced salt solution. FITC-labeled bacteria (in an amount equivalent to 1.5 × 107 CFU) were opsonized with 10% S. aureus-treated serum with purified anti-WTA IgG in 20 μl of Hanks' balanced salt solution containing 2 mM CaCl2, 1 mM MgCl2, 150 mM NaCl, and 0.4% BSA for 30 min at 37°C with shaking. A PMN suspension (1.5 × 105 cells, 35 μl) was added to 5 μl of opsonized bacteria (corresponding to 3.7 × 106 CFU; multiplicity of infection, ∼25) and incubated at 37°C for 60 min with shaking. The phagocytosed FITC-labeled S. aureus cells in the PMNs were counted by fluorescent phase-contrast microscopy. More than 100 PMNs were counted. Extracellular FITC-labeled S. aureus cells were quenched by 0.2% trypan blue.
Experiments with phages.
Phage susceptibility was analyzed using a soft-agar spot assay as described previously (13). Briefly a phage panel including the broad-host-range phages ΦK (13) and Φ812 (29), serogroup B phage Φ11 (13), and serogroup L phage Φ187 (29) was used, and lysates were spotted onto bacterial lawns and analyzed after overnight incubation for macroplaque formation. Phage adsorption was determined as described previously (12), except that the multiplicity of infection was set equal to 0.1 for both phages Φ11 and Φ187. Phages ΦK, Φ812, and Φ11 were propagated on S. aureus RN4220 cells. Phage Φ187 was propagated on S. aureus PS187 cells as described previously (12).
Molecular genotyping of S. aureus WTA glycosyltransferases.
The tarM, tarS, and tagN genes were amplified from genomic DNA using the primers (Eurofins Genomics, Germany) listed in Table S1 in the supplemental material. Publicly available reference genomes were analyzed for the presence of tarM, tarS, or tagN using the BLAST program (30).
Sequence analysis of the tarS deletion in S. aureus Lowenstein.
Whole-genome sequencing (WGS) was performed using an Illumina MiSeq system (Illumina, CA). Standard Illumina libraries for two 250-bp runs were made using a Nextera XT kit according to the manufacturer's instructions (Illumina, CA). CLC Bio's Genomic Workbench (version 8.0.1; CLC Bio, Denmark) was used for the de novo assembly and alignment of contigs against the S. aureus NCTC 8325 reference genome (GenBank accession no. NC_007795).
Processing and statistical analysis.
The results from the quantitative analyses are expressed as the mean ± standard deviation (SD) of the data from at least three independent experiments, unless otherwise stated. Other data were representative of those from at least three independent experiments that yielded similar results. The statistical analyses were performed using Student's t test. P values of less than 0.05 were considered statistically significant and are indicated in the figures. The statistical analyses were performed using SPSS statistical analysis software. Differences between groups were analyzed by an unpaired Student's t test. P values of less than 0.05 were considered significant and are indicated in the figures.
Accession number.
Raw reads of whole-genome sequence data for S. aureus Lowenstein were deposited in the NCBI Short Read Archive under the study accession number SRP061258.
RESULTS
WTA β-GlcNAc governs human anti-WTA IgG-mediated C3 deposition against USA300.
First, because we used ethanol-fixed bacteria to examine anti-WTA IgG-mediated C3 deposition, it was necessary to address the effects of ethanol treatment on the S. aureus strains. For this experiment, we used two different S. aureus strains under two different conditions: live and alcohol-fixed S. aureus strains RN4220 and USA300. After incubation of these four bacterial samples with 10% Δspa mutant-treated human serum and anti-WTA IgG, C3 deposition capabilities were examined via fluorescence-activated cell sorter analyses. As shown in Fig. S1 in the supplemental material, C3 deposition was satisfactory under all four conditions, while the amount of bound C3 was lower in the live bacteria (groups b and d) than alcohol-fixed bacteria (groups a and c), confirming that ethanol treatment of S. aureus cells leads to a clearer C3 deposition capacity and does not inhibit serum-mediated complement activation.
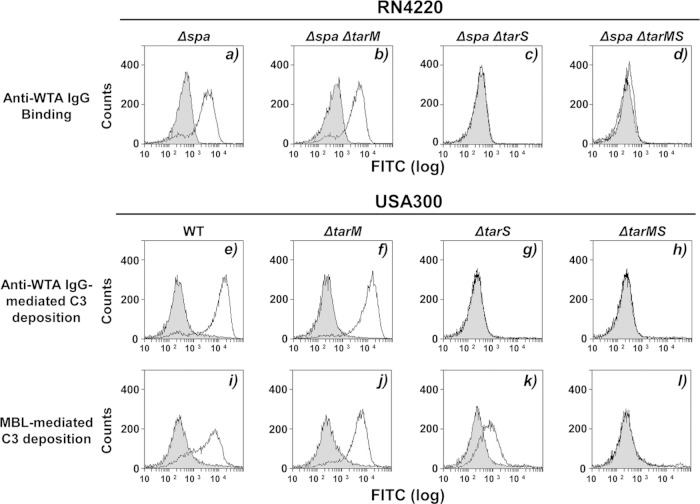
In our previous study, we used cells of the S. aureus RN4220 parental strain, a α-GlcNAc-deficient ΔtarM mutant, a β-GlcNAc-deficient ΔtarS mutant, and a ΔtarMS double mutant to examine the relationship between WTA glycosylation and human MBL- or anti-WTA IgG-dependent complement activation (19–21). Since RN4220 is a laboratory strain previously mutagenized with a chemical mutagen and we did not examine purified anti-WTA IgG-dependent C3 deposition on clinical strains in our previous study (21), we assumed that C3 deposition and opsonophagocytosis should be retested with clinically important strains, such as the USA300 strain. For this purpose, the binding specificity of purified human serum anti-WTA IgG was addressed (Fig. 1a to d). As described previously, purified anti-WTA IgG bound to RN4220 lacking the IgG-binding protein A (Δspa mutant) and to the Δspa ΔtarM double mutant but not to the Δspa ΔtarS mutant or the Δspa ΔtarMS mutant (Fig. 1a to d), suggesting that the purified anti-WTA IgG has a strong binding specificity for staphylococcal WTA β-GlcNAc residues.
FIG 1.
WTA glycosylation-dependent C3 deposition via purified anti-WTA IgG or MBL on S. aureus MRSA strain USA300. (a to d) Ethanol-killed S. aureus RN4220 mutant cells were incubated without (gray area) or with (area outlined by a black line) anti-WTA IgG (50 ng) in 20 μl of buffer, and bound IgG was detected by flow cytometric analysis. (e to l) Measurement of C3 deposition on USA300 mutant strains incubated in 10% Δspa mutant-treated human serum without (gray area) or with (area outlined by a black line) anti-WTA IgG (50 ng) in 20 μl of buffer. C3 was detected by flow cytometric analysis with specific antibodies. The method used for the preparation of Δspa mutant-treated human serum is described in Materials and Methods. The results are representative of those from three independent experiments.
Next, we examined anti-WTA IgG- or human MBL-mediated C3 deposition on the USA300 wild type and corresponding mutants (Fig. 1e to l). Anti-WTA IgG induced C3 deposition on both the USA300 parental and ΔtarM strains but not on the ΔtarS and ΔtarMS mutants (Fig. 1e to h). Human MBL induced C3 deposition on the parental USA300 strain and the ΔtarM or ΔtarS mutants but not on the ΔtarMS double mutant. Of note, β-GlcNAc WTA has a stronger capacity to stimulate MBL-dependent complement activation than α-GlcNAc, as described in our previous study (21). These results suggest that human anti-WTA IgG is specific for β-GlcNAc-modified RboP WTA of the USA300 strain.
C3 deposition and opsonophagocytosis are induced for the majority of S. aureus isolates recognized by anti-WTA IgG.
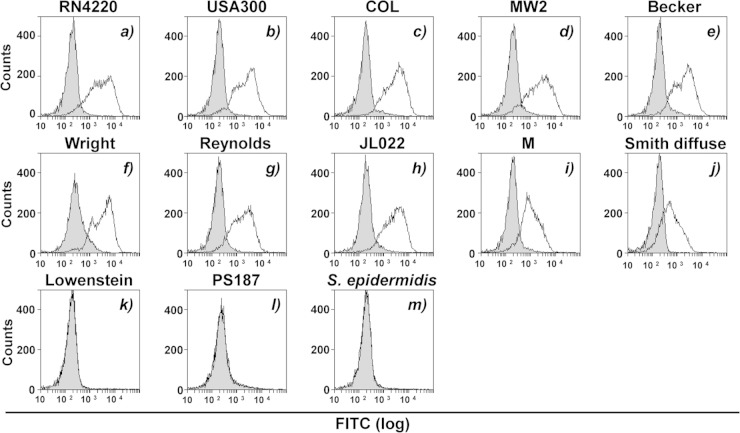
We wondered whether purified anti-WTA IgG can also induce complement-mediated C3 deposition on diverse S. aureus strains. Four mildly CP-producing strains, S. aureus Becker (CP8), Wright (ATCC 49525, CP8), Lowenstein (ATCC 49521, CP5), and Reynolds (CP5), were selected. Also, the two heavily CP-producing strains M (ATCC 49951, CP1) and Smith diffuse (ATCC 13709, CP2) were selected. As a control, a CP-deficient strain (JL022) constructed from the Reynolds strain by allelic replacement mutagenesis of a CP biosynthesis gene was included (31). Also, RN4220 and three clinically isolated MRSA strains (USA300, COL, and MW2), which are known to produce β-GlcNAc- and α-GlcNAc-substituted RboP backbones of WTA (13–15), were included. Finally, two special staphylococcal strains, S. aureus ST395 isolate PS187 and S. epidermidis strain ATCC 14990, both of which produce distinct WTA backbone structures and glycosylation patterns (12, 32), were included in the analyses.
As shown in Fig. 2a to j, anti-WTA IgG-mediated C3 deposition was induced on the eight strains, supporting the possibility of the presence of exposed WTAs on their bacterial surfaces. However, C3 deposition failed on the three strains S. aureus Lowenstein and PS187 and S. epidermidis ATCC 14990 (Fig. 2k to m). The failure of C3 deposition on the PS187 and S. epidermidis strains was expected due to the previously reported production of GroP WTA types modified with either GalNAc or α-Glc/α-GlcNAc (12, 32), respectively, highlighting the specificity of our anti-β-GlcNAc antibody for RboP-GlcNAc WTA types. The reason why C3 deposition was not induced on the mildly CP-producing Lowenstein strain is provided below.
FIG 2.
Anti-WTA IgG-mediated C3 deposition on various staphylococcal strains. Cultured bacterial cells were collected by centrifugation, washed with PBS three times, and treated with ethanol for further experiments. C3 deposition was measured in 10% Δspa mutant-treated human serum without (gray area) or with (area outlined by a black line) anti-WTA IgG (50 ng). Bound C3 was detected with specific antibodies by flow cytometric analysis. The results are representative of those from three independent experiments.
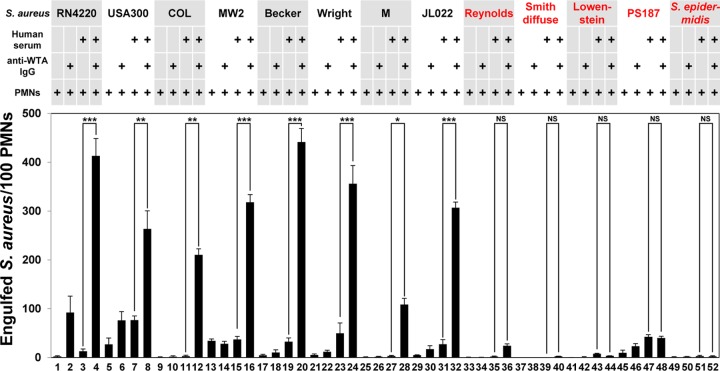
To further address the inability of C3 to be deposited on these three strains, we next examined anti-WTA IgG-mediated opsonophagocytosis (Fig. 3). To estimate anti-WTA IgG-mediated opsonophagocytosis, we counted the FITC-labeled bacterial cells engulfed by 100 PMNs under a fluorescence microscope. Anti-WTA IgG-mediated opsonophagocytosis of RN4220 (Fig. 3, column 4) and three MRSA strains (Fig. 3, columns 8, 12, and 16) was induced. Opsonophagocytosis of mildly CP-producing strains, such as Becker and Wright, and the heavily CP-producing M strain also occurred (Fig. 3, columns 20, 24, and 28), reflecting C3 deposition. However, although C3 deposition was induced on the mildly CP-producing Reynolds and heavily CP-producing Smith diffuse strains (Fig. 2g and j), anti-WTA IgG-mediated opsonophagocytosis of these two strains was not induced in a statistically significant fashion (Fig. 3, columns 36 and 40), indicating that CP production by these two strains may protect these bacteria from opsonophagocytosis. Under the same conditions, the JL022 strain, a CP-deficient mutant constructed from strain Reynolds, was opsonophagocytosed by PMNs (Fig. 3, column 32). As expected, anti-WTA IgG-mediated opsonophagocytosis of three strains, strains Lowenstein and PS187 and the S. epidermidis strain, was not induced, as found as described above for C3 deposition (Fig. 3, columns 44, 48, and 52). Taken together, our results suggest that PMN-mediated opsonophagocytosis cannot be directly correlated with CP production on some S. aureus strains.
FIG 3.
Anti-WTA IgG does not induce opsonophagocytosis of some strains. Ethanol-killed bacterial cells were labeled with FITC (0.1 mM) and opsonized without or with Δspa mutant-treated human serum (10%). Purified anti-WTA IgG (50 ng) was simultaneously added as indicated. Opsonized FITC-labeled bacterial cells were incubated with human PMNs (1 × 105) at a multiplicity of infection of 25 in RPMI 1640 medium at 37°C for 1 h. The number of phagocytosed S. aureus cells per 100 PMNs was counted by fluorescent phase-contrast microscopy. Data are presented as the means ± SDs (error bars) of the results from three independent experiments. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
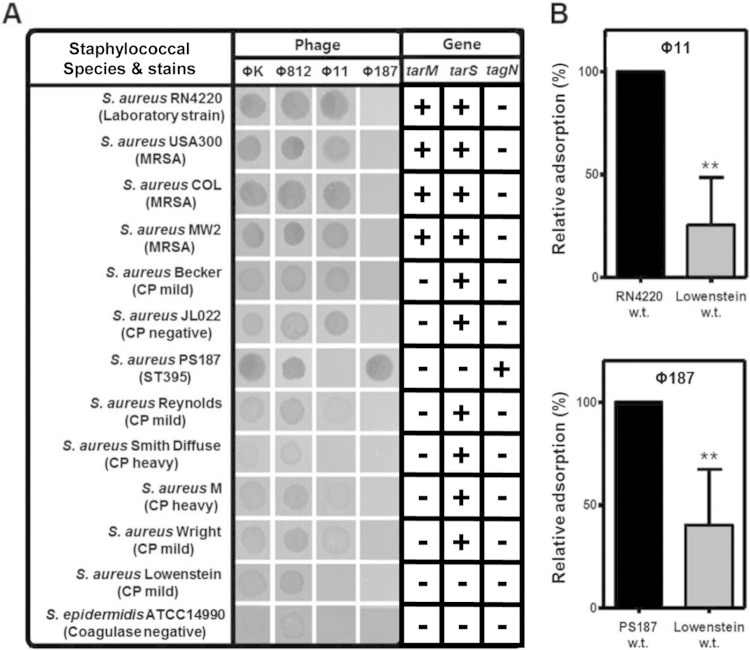
Complement C3 deposition strongly correlates with phage Φ11 susceptibility and glycosylated β-GlcNAc (β-GlcNAcylated) RboP WTA.
To further elucidate the relationship between C3 deposition and WTA backbone structures or WTA glycosylation patterns, we used a set of 4 different staphylococcal phages, including ΦK, Φ812, Φ11, and Φ187. Lytic S. aureus phages, such as ΦK and Φ812, are known to recognize diverse WTA backbones of either the RboP or GroP type (12, 13, 33, 34), while Φ11 recognizes GlcNAc-modified RboP WTA for efficient absorption and infection (12, 33). In contrast, phage Φ187 is specific for GalNAc-modified GroP WTA (34). As expected, all 13 tested strains were susceptible to ΦK and Φ812, suggesting that WTAs are produced on their surfaces (Fig. 4A). Among them, complement-activating strains, such as RN4220, the three MRSA strains tested, Becker, and CP-negative strain JL022 were susceptible to Φ11, indicating that these strains express GlcNAc-modified RboP WTA on their cell surfaces. However, other CP-producing strains, the Reynolds, M, and Wright strains, retained only weak susceptibility to phage Φ11, supporting the notion that these strains produce GlcNAc-modified RboP WTA but that production of mild CPs may interfere with the absorption of Φ11. Interestingly, Smith diffuse, which was sensitive to C3 deposition, was susceptible to ΦK and Φ812 but resistant to Φ11 and Φ187. Since Smith diffuse bears tarS within the WTA tar gene cluster, facilitating β-GlcNAc-modified RboP WTA biosynthesis, β-GlcNAc-harboring RboP WTA is likely produced (see below; Fig. 4A). The nonsusceptibility of the Smith diffuse strain to phage Φ11 might be caused by heavy CP production. On the other hand, the Lowenstein strain was resistant to C3 deposition, Φ11, and Φ187, suggesting that either (i) CP production may mask WTA glycosylation or (ii) a distinct WTA glycosylation type might be present on this strain (Fig. 4A). When the relative absorption rate of phage Φ11 or Φ187 for S. aureus Lowenstein was determined, the relative adsorption rate was decreased about 75% and 60% compared to that for the RN4220 and PS187 strains, respectively (Fig. 4B), supporting the results presented in Fig. 4A. The only Φ187-susceptible strain was PS187, which was in agreement with previous observations (12). Finally, S. epidermidis strain ATCC 14990 weakly retained susceptibility to phages ΦK and Φ812 but was resistant to phages Φ11 and Φ187, which is consistent with its WTA structure having a GroP WTA backbone with a α-Glc/α-GlcNAc modification (32).
FIG 4.
Correlation between phage susceptibility and the presence of WTA glycosyltransferases. The phage susceptibilities of various S. aureus wild-type strains and S. epidermidis strain ATCC 14990 were determined. Lysates were spotted onto bacterial lawns, and macroplaque formation was analyzed after overnight incubation. The presence (+) or absence (−) of the WTA glycosyltransferase-encoding genes tarM, tarS, and tagN in the corresponding genome is indicated. The tarM, tarS, and tarN genes were amplified from genomic DNA by using the corresponding primers listed in Table S1 in the supplemental material. (B) Rates of phage Φ11 or Φ187 absorption by the S. aureus Lowenstein wild-type (w.t.) strain. The results are representative of those from three independent experiments. The rate of phage adsorption relative to the rate of adsorption of phage Φ11 or Φ187 by strain RN4220 or PS187, which was set equal to 100%, is shown, and results are given as means ± SDs (n = 3). Statistically significant differences compared with the results for the strains that absorbed the phages were determined by the unpaired two-tailed Student's t test. **, P < 0.001 to 0.01.
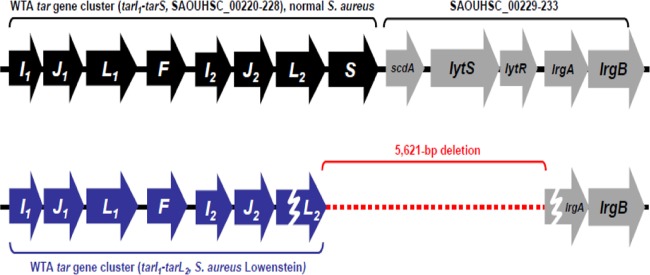
To explain the different phage susceptibilities of the S. aureus strains and their correlation with complement-mediated C3 deposition and opsonophagocytosis, we further checked for the presence or absence of WTA glycosyltransferases encoded by the tarM, tarS, and tagN genes via PCR (Fig. 4A). Notably, all strains on which C3 was deposited, such as RN4220, the three MRSA strains, Becker, M, Wright, and JL022, carried tarS, and some also carried tarM. However, despite the presence of tarS, the heavily CP-producing Smith diffuse strain was resistant to Φ11 and anti-WTA IgG-mediated opsonophagocytosis, suggesting that β-GlcNAc-modified RboP WTA might be masked by surface CPs. Other complement-evading strains were either positive for tagN (PS187) or negative for all WTA glycosyltransferases described so far, TagN, TarS, and/or TarM. Moreover, whole-genome sequencing of S. aureus strain Lowenstein, on which complement was not deposited and whose genome lacked tarM and tagN, revealed a unique 5,621-bp deletion encompassing the tarS-encoding region (Fig. 4A and 5). The deletion also comprised the 3′ end of tarL2 as well as scdA, lytS, lytR, and the 5′ end of lrgA, suggesting that this strain synthesizes nonglycosylated WTA. Accordingly, altered WTA structures strongly correlate with complement evasion (12, 32). Taken together, these results demonstrate that anti-WTA IgG-mediated C3 deposition and opsonophagocytosis strongly correlate with phage Φ11 susceptibility and RboP WTA glycosylated with β-GlcNAc.
FIG 5.
The complement-evading S. aureus strain Lowenstein carries a large deletion in the tarS-encoding region. Whole-genome sequencing revealed a 5,621-bp deletion in the tarS-encoding region which includes the 3′ end of tarL2, scdA, lytS, lytR, and the 5′ end of lrgA. The genetic organization of the WTA tar gene cluster of S. aureus reference strain NCTC 8325 (top) was compared to that of the WTA tar gene cluster of strain Lowenstein (bottom). tarL2 and lrgA most likely represent pseudogenes in strain Lowenstein (broken arrows). Gene locus numbers are indicated (note that, for some reason, SAOUHSC_002224 is missing in NCTC 8325).
DISCUSSION
Our combined analyses of anti-WTA IgG-mediated C3 deposition and phage susceptibility and genotyping of 13 different staphylococcal strains provided us with invaluable information about WTA glycosylation and the WTA backbone structures of these strains (Table 2). Previous studies have shown that injection of S. aureus teichoic acids into humans or rabbits results in the induction of circulating antibodies against β-GlcNAc or α-GlcNAc WTA (35, 36). Until recently, the lack of availability of purified homogeneous GlcNAc WTAs from S. aureus mutant cells hampered efforts to determine the exact epitope of anti-WTA antibodies. In our previous study (21), the availability of S. aureus ΔtarM, ΔtarS, and ΔtarMS mutant cells and WTAs purified from these mutant cells enabled us to determine the exact antigenic determinant of anti-WTA antibodies and MBL. The current study further confirms that human serum anti-WTA IgG recognizes the β-GlcNAc of WTA of clinically isolated staphylococcal strains. Also, we demonstrate that the S. aureus β-GlcNAc WTA recognized by serum anti-WTA IgG specifically induces the opsonophagocytosis of diverse S. aureus strains harboring the β-GlcNAc residue of WTA. Interestingly, tarM was absent from five CP-producing S. aureus strains. The absence of tarM has also been reported in several health care-associated MRSA strains, such as N315, Mu50, Mu3, and JH1 (15), indicating that the absence of tarM is a common feature of certain sequence types. Evolutionarily, it remains unclear if the loss or gain of tarM could be an advantage during colonization and infection.
TABLE 2.
Predicted WTA structures of various staphylococcal strains
| Species/strain | WTA type (glycosylation) | Anti-WTA IgG-mediated C3 depositionb | Reference or source |
|---|---|---|---|
| S. aureus RN4220 | RboP (α- and β-GlcNAc) | ○ | 13 |
| S. aureus USA300 | RboP (α- and β-GlcNAc) | ○ | This study |
| S. aureus COL | RboP (α- and β-GlcNAc) | ○ | This study |
| S. aureus MW2 | RboP (α- and β-GlcNAc) | ○ | 14 |
| S. aureus Becker | RboP (β-GlcNAc) | ○ | This study |
| S. aureus Reynolds | RboP (β-GlcNAc) | ○ | This study |
| S. aureus JL022 | RboP (β-GlcNAc) | ○ | This study |
| S. aureus M | RboP (β-GlcNAc) | ○ | This study |
| S. aureus Wright | RboP (β-GlcNAc) | ○ | This study |
| S. aureus Smith diffuse | RboP (β-GlcNAc) | ○ | This study |
| S. aureus Lowenstein | RboP (unknowna) | X | Unknown |
| S. aureus PS187 | GroP (α-GalNAc) | X | 12 |
| S. epidermidis ATCC 14990 | GroP (α-Glc, α-GlcNAc) | X | 32 |
Most likely produces a nonglycosylated RboP WTA.
○, deposition; X, no deposition.
Recent studies suggested that an effective vaccine to prevent S. aureus infections must contain multiple antigens that are carefully selected to interrupt S. aureus pathogenesis (37). Our study shows that human anti-WTA IgG recognizes the β-GlcNAc of WTA of most of the clinically isolated staphylococcal strains tested. If staphylococcal WTA is proven to be a valuable vaccine target antigen, a mixture of WTA derivatives, such as RboP WTA modified with β-GlcNAc residues and GroP WTA modified with GalNAc residues, should be considered active vaccine candidates that should permit active immunization against infections caused by diverse S. aureus strains. Also, because S. aureus can persistently colonize the human body, a constant interaction and adaptation between the bacteria and the host immune system will occur. This is supported by the observation that all adults have preexisting serum antibodies capable of binding to S. aureus cell surface antigens (38). However, the exact ligand molecule(s) recognized by antibodies preexisting in serum in vivo has not yet been determined. Since we demonstrated that the β-GlcNAc WTA recognized by serum IgG specifically induces the opsonophagocytosis of diverse S. aureus strains in vitro, purified anti-WTA IgG will be a valuable tool for determination of the exact ligand motif of staphylococcal surface molecules which are recognized by serum antibodies in vivo. Also, since S. aureus is a major pathogen that can be difficult to treat due to drug resistance or its presence at sites of infection that are difficult to reach, the development of a vaccine may help to prevent S. aureus infections. Our current work provides some important findings that may help with the identification of a possible vaccine target, and it presents a panel of methods that may be useful for the typing of strains found in the clinic.
Finally, combined technologies, such as analysis of anti-WTA IgG-mediated C3 deposition and opsonophagocytosis, phage susceptibility testing, and genotyping of diverse staphylococcal strains, will be useful tools for determination of the staphylococcal WTA backbone structure and WTA glycosylation patterns in newly emerging drug-resistant S. aureus strains.
Supplementary Material
ACKNOWLEDGMENTS
We deeply thank Jean C. Lee of Harvard Medical School and K. M. Cunnion of Eastern Virginia Medical School for providing capsule-producing S. aureus strains, Misao Matsushita for providing purified human MBLs, and Petra Kühner for excellent technical support.
This work was supported by a grant (NRF-2015R1A2A01005247) from the Korean National Research Foundation to B.L.L. and by grants from the German Research Council (TRR34, SFB766) and from the German Center for Infection Research to A.P.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00767-15.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.DeLeo FR, Diep BA, Otto M. 2009. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am 23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker D, Prince A. 2012. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin Immunopathol 34:281–297. doi: 10.1007/s00281-011-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler VG Jr, Proctor RA. 2014. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 20(Suppl 5):S66–S75. doi: 10.1111/1469-0691.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 6.Fujita T. 2002. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol 2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 7.Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. 2009. Mannose-binding lectin and innate immunity. Immunol Rev 230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 8.Weidenmaier C, Peschel A. 2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev 6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 9.Brown S, Santa Maria JP Jr, Walker S. 2013. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swoboda JG, Campbell J, Meredith TC, Walker S. 2010. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 11:35–45. doi: 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia G, Kohler T, Peschel A. 2010. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol 300:148–154. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Winstel V, Liang C, Sanchez-Carballo P, Steglich M, Munar M, Broker BM, Penades JR, Nubel U, Holst O, Dandekar T, Peschel A, Xia G. 2013. Wall teichoic acid structure governs horizontal gene transfer between major bacterial pathogens. Nat Commun 4:2345. doi: 10.1038/ncomms3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia G, Maier L, Sanchez-Carballo P, Li M, Otto M, Holst O, Peschel A. 2010. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J Biol Chem 285:13405–13415. doi: 10.1074/jbc.M109.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown S, Xia G, Luhachack LG, Campbell J, Meredith TC, Chen C, Winstel V, Gekeler C, Irazoqui JE, Peschel A, Walker S. 2012. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc Natl Acad Sci U S A 109:18909–18914. doi: 10.1073/pnas.1209126109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winstel V, Xia G, Peschel A. 2014. Pathways and roles of wall teichoic acid glycosylation in Staphylococcus aureus. Int J Med Microbiol 304:215–221. doi: 10.1016/j.ijmm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Ricklin D, Hajishengallis G, Yang K, Lambris JD. 2010. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daha NA, Banda NK, Roos A, Beurskens FJ, Bakker JM, Daha MR, Trouw LA. 2011. Complement activation by (auto-) antibodies. Mol Immunol 48:1656–1665. doi: 10.1016/j.molimm.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Weis WI, Drickamer K. 1996. Structural basis of lectin-carbohydrate recognition. Annu Rev Biochem 65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 19.Park KH, Kurokawa K, Zheng L, Jung DJ, Tateishi K, Jin JO, Ha NC, Kang HJ, Matsushita M, Kwak JY, Takahashi K, Lee BL. 2010. Human serum mannose-binding lectin senses wall teichoic acid glycopolymer of Staphylococcus aureus, which is restricted in infancy. J Biol Chem 285:27167–27175. doi: 10.1074/jbc.M110.141309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung DJ, An JH, Kurokawa K, Jung YC, Kim MJ, Aoyagi Y, Matsushita M, Takahashi S, Lee HS, Takahashi K, Lee BL. 2012. Specific serum Ig recognizing staphylococcal wall teichoic acid induces complement-mediated opsonophagocytosis against Staphylococcus aureus. J Immunol 189:4951–4959. doi: 10.4049/jimmunol.1201294. [DOI] [PubMed] [Google Scholar]
- 21.Kurokawa K, Jung DJ, An JH, Fuchs K, Jeon YJ, Kim NH, Li X, Tateishi K, Park JA, Xia G, Matsushita M, Takahashi K, Park HJ, Peschel A, Lee BL. 2013. Glycoepitopes of staphylococcal wall teichoic acid govern complement-mediated opsonophagocytosis via human serum antibody and mannose-binding lectin. J Biol Chem 288:30956–30968. doi: 10.1074/jbc.M113.509893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K, Kurokawa K, Moyo P, Jung DJ, An JH, Chigweshe L, Paul E, Lee BL. 2013. Intradermal immunization with wall teichoic acid (WTA) elicits and augments an anti-WTA IgG response that protects mice from methicillin-resistant Staphylococcus aureus infection independent of mannose-binding lectin status. PLoS One 8:e69739. doi: 10.1371/journal.pone.0069739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuchscherr L, Loffler B, Buzzola FR, Sordelli DO. 2010. Staphylococcus aureus adaptation to the host and persistence: role of loss of capsular polysaccharide expression. Future Microbiol 5:1823–1832. doi: 10.2217/fmb.10.147. [DOI] [PubMed] [Google Scholar]
- 24.O'Riordan K, Lee JC. 2004. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakker M, Park JS, Carey V, Lee JC. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 66:5183–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunnion KM, Lee JC, Frank MM. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immun 69:6796–6803. doi: 10.1128/IAI.69.11.6796-6803.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita M, Endo Y, Fujita T. 2000. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol 164:2281–2284. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 28.Shiratsuchi A, Shimizu K, Watanabe I, Hashimoto Y, Kurokawa K, Razanajatovo IM, Park KH, Park HK, Lee BL, Sekimizu K, Nakanishi Y. 2010. Auxiliary role for d-alanylated wall teichoic acid in Toll-like receptor 2-mediated survival of Staphylococcus aureus in macrophages. Immunology 129:268–277. doi: 10.1111/j.1365-2567.2009.03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantucek R, Doskar J, Ruzickova V, Kasparek P, Oracova E, Kvardova V, Rosypal S. 2004. Identification of bacteriophage types and their carriage in Staphylococcus aureus. Arch Virol 149:1689–1703. doi: 10.1007/s00705-004-0335-6. [DOI] [PubMed] [Google Scholar]
- 30.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 31.Portoles M, Kiser KB, Bhasin N, Chan KH, Lee JC. 2001. Staphylococcus aureus Cap5O has UDP-ManNAc dehydrogenase activity and is essential for capsule expression. Infect Immun 69:917–923. doi: 10.1128/IAI.69.2.917-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endl J, Seidl PH, Fiedler F, Schleifer KH. 1984. Determination of cell wall teichoic acid structure of staphylococci by rapid chemical and serological screening methods. Arch Microbiol 137:272–280. doi: 10.1007/BF00414557. [DOI] [PubMed] [Google Scholar]
- 33.Xia G, Corrigan RM, Winstel V, Goerke C, Grundling A, Peschel A. 2011. Wall teichoic acid-dependent adsorption of staphylococcal siphovirus and myovirus. J Bacteriol 193:4006–4009. doi: 10.1128/JB.01412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winstel V, Sanchez-Carballo P, Holst O, Xia G, Peschel A. 2014. Biosynthesis of the unique wall teichoic acid of Staphylococcus aureus lineage ST395. mBio 5(2):e00869–14. doi: 10.1128/mBio.00869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juergens WG, Sanderson AR, Strominger JL. 1963. Chemical basis for an immunological specificity of a strain of Staphylococcus aureus. J Exp Med 117:925–935. doi: 10.1084/jem.117.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colque-Navarro P, Jacobsson G, Andersson R, Flock JI, Mollby R. 2010. Levels of antibody against 11 Staphylococcus aureus antigens in a healthy population. Clin Vaccine Immunol 17:1117–1123. doi: 10.1128/CVI.00506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, Jansen KU. 2012. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother 8:1585–1594. doi: 10.4161/hv.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holtfreter S, Kolata J, Broker BM. 2010. Towards the immune proteome of Staphylococcus aureus—the anti-S. aureus antibody response. Int J Med Microbiol 300:176–192. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oku Y, Kurokawa K, Matsuo M, Yamada S, Lee BL, Sekimizu K. 2009. Pleiotropic roles of poly-glycerolphosphate synthase of lipoteichoic acid in the growth of Staphylococcus aureus cells. J Bacteriol 191:141–151. doi: 10.1128/JB.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winstel V, Kuhner P, Salomon F, Larsen J, Skov R, Hoffmann W, Peschel A, Weidenmaier C. 2015. Wall teichoic acid glycosylation governs Staphylococcus aureus nasal colonization. mBio 6(4):e00632–15. doi: 10.1128/mBio.00632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami K, Tomasz A. 1989. Involvement of multiple genetic determinants in high-level methicillin resistance in Staphylococcus aureus. J Bacteriol 171:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819–1827. doi: 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 44.Karakawa WW, Vann WF. 1982. Capsular polysaccharides of Staphylococcus aureus, p 285–293. In Robbins JB, Hill JC, Sadoff JC (ed), Seminars in infectious diseases, vol IV Bacterial vaccines. Thieme-Stratton Inc., New York, NY. [Google Scholar]
- 45.Scott AC. 1969. A capsulate Staphylococcus aureus. J Med Microbiol 2:253–260. doi: 10.1099/00222615-2-3-253. [DOI] [PubMed] [Google Scholar]
- 46.Fournier JM, Vann WF, Karakawa WW. 1984. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun 45:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt GA, Moses AJ. 1958. Acute infection of mice with Smith strain of Staphylococcus aureus. Science 128:1574–1575. doi: 10.1126/science.128.3338.1574. [DOI] [PubMed] [Google Scholar]
- 48.Karakawa WW, Sutton A, Schneerson R, Karpas A, Vann WF. 1988. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun 56:1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones D, Deibel RH, Niven CF Jr. 1963. Identity of Staphylococcus epidermidis. J Bacteriol 85:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.