Summary
Dietary lipids may influence the abundance of circulating inflammatory microbial factors. Hence, inflammation in white adipose tissue (WAT) induced by dietary lipids may be partly dependent on their interaction with the gut microbiota. Here, we show that mice fed lard for 11 weeks have increased Toll-like receptor (TLR) activation and WAT inflammation and reduced insulin sensitivity compared with mice fed fish oil and that phenotypic differences between the dietary groups can be partly attributed to differences in microbiota composition. Trif−/− and Myd88−/− mice are protected against lard-induced WAT inflammation and impaired insulin sensitivity. Experiments in germ-free mice show that an interaction between gut microbiota and saturated lipids promotes WAT inflammation independent of adiposity. Finally, we demonstrate that the chemokine CCL2 contributes to microbiota-induced WAT inflammation in lard-fed mice. These results indicate that gut microbiota exacerbates metabolic inflammation through TLR signaling upon challenge with a diet rich in saturated lipids.
Graphical Abstract
Highlights
-
•
The gut microbiota contributes to phenotypic differences in mice fed lard or fish oil
-
•
Mice lacking MyD88 or TRIF are protected against WAT inflammation
-
•
Microbial-derived factors induce CCL2 in adipocytes through TLR4, MyD88, and TRIF
-
•
Microbial-induced CCL2 enhances macrophage accumulation in WAT
Caesar et al. reveal how saturated lipids in lard affect gut microbial composition to promote obesity and WAT inflammation via TLR signaling and CCL2; in contrast, mice fed a fish-oil diet enriched in polyunsaturated fatty acids are protected. Transfer of microbiota from fish-oil-fed mice dampens lard-induced inflammation.
Introduction
Diets rich in saturated dietary lipids are associated with increased white adipose tissue (WAT) inflammation and metabolic disease (Kennedy et al., 2009), whereas diets rich in polyunsaturated fatty acids have been shown to counteract inflammation and promote a lean and metabolically healthy phenotype (Buckley and Howe, 2009; Calder, 2006; Oh et al., 2010). Host diet also has a major impact on gut microbial composition (Scott et al., 2013), and changes in gut ecology can affect the inflammatory and metabolic properties of the gut microbiota and thereby host physiology (Tremaroli and Bäckhed, 2012). Studies showing that germ-free (GF) mice are protected against diet-induced obesity and exhibit reduced WAT inflammation and insulin resistance (Bäckhed et al., 2007; Caesar et al., 2012; Ding et al., 2010; Rabot et al., 2010) have led to the suggestion that microbial factors may directly contribute to WAT inflammation and adverse metabolic consequences. Circulating microbial factors have, indeed, been identified in healthy humans and mice (Caesar et al., 2010). Furthermore, an increased influx of microbial factors has been linked to inflammation and impaired glucose metabolism through activation of Toll-like receptor (TLR)-dependent signaling (Cani et al., 2007; Henao-Mejia et al., 2012). Several genetic mouse models have shown that deletion of components of the TLR signaling pathway is associated with protection against WAT inflammation and/or rescue of metabolically perturbed phenotypes (Jin and Flavell, 2013). However, although TLR ligands may be of bacterial origin, they may also come from the diet or the host (Yu et al., 2010), and, thus, gnotobiotic models are required to determine how the gut microbiota contributes to WAT inflammation upon diet change.
In the present paper, we aim to determine whether WAT inflammation induced by dietary lipids is mediated through the gut microbiota and to identify molecular mechanisms through which the gut microbiota induces macrophage accumulation in WAT.
Results
Impact of Lard versus Fish-Oil Diet on Gut Microbiota
To assess how the dietary fat sources affects the microbiota, we fed mice isocaloric diets that differed only in fat composition (either lard or fish oil, which are rich in saturated and polyunsaturated lipids, respectively) (Table S1) for 11 weeks. First, we showed that lard-fed mice gained more weight (Figure 1A), consumed more food, and had increased feed efficiency (Figures S1A and S1B) compared with mice fed fish oil. Lard-fed mice had reduced respiratory quotient (RQ) (Figure S1C), indicative of increased fat utilization, both after 2 days and 5 weeks of high-fat diet. The fat source did not change locomotory activity (Figure S1C), but mice fed fish oil utilized more energy for locomotory activity (Figure S1D). As expected, the lard-fed mice had higher fasting insulin and glucose levels, as well as impaired insulin sensitivity, compared to mice fed fish oil (Figures S1E–S1G).
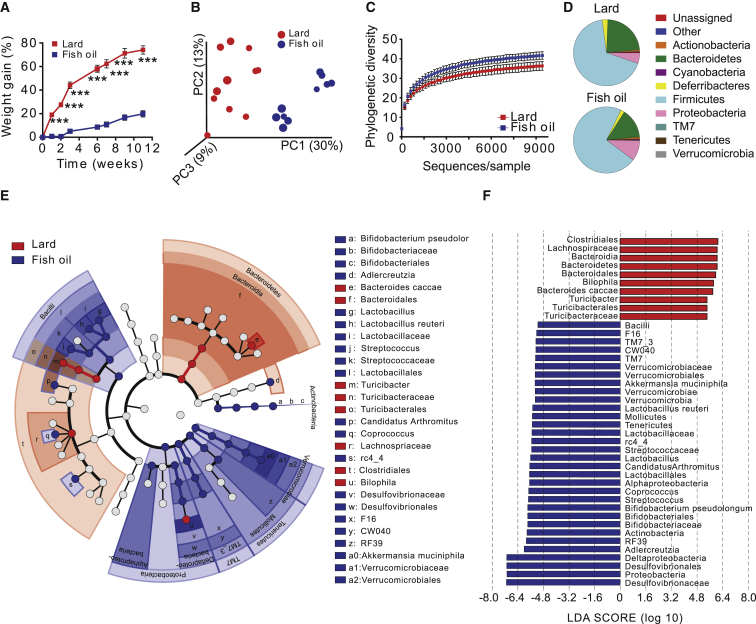
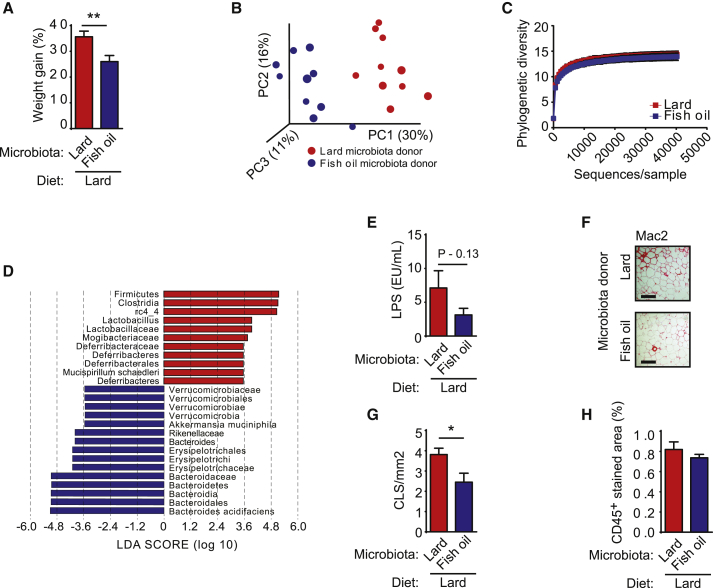
Figure 1.
Mice Fed a Lard Diet Have Increased Adiposity and Distinct Gut Microbiota Composition Compared to Mice Fed an Isocaloric Fish-Oil Diet
Mice were fed high-fat diets for 11 weeks.
(A) Body weight gain of mice fed lard or fish oil (n = 15). Data indicate means ± SEM. ∗∗∗p < 0.001.
(B) Principal coordinate analysis of gut microbiota composition based on unweighted UniFrac in mice fed lard or fish oil (n = 10–11 mice per group).
(C) Rarefaction curves for phylogenetic diversity in microbiota from mice fed lard or fish oil (10–9,410 sequences per sample). Data indicate means ± SD.
(D) Pie charts of gut microbial phyla composition in mice fed lard or fish oil for 11 weeks (n = 9–10 mice per group) (see Table S2 for the list of differentially abundant taxa grouped at the phylum and genus level).
(E) Cladogram generated from LEfSe analysis, showing the most differentially abundant taxa enriched in microbiota from mice fed lard (red) or fish oil (blue).
(F) LDA scores of the differentially abundant taxa shown in (E). Taxa enriched in microbiota from mice fed lard (red) or fish oil (blue) are indicated with a positive or negative LDA score, respectively (taxa with LDA score >2 and significance of α < 0.05 determined by Wilcoxon signed-rank test).
See also Table S2.
We analyzed gut microbiota composition by 454 pyrosequencing of the 16S rRNA gene in cecal contents of these mice and observed dramatic changes in the microbial ecology according to the type of dietary fat (Figures 1B–1F; Table S2). Principal coordinate analysis of the unweighted UniFrac showed significant clustering of samples according to diet (Figure 1B), and multivariate non-parametric ANOVA (adonis, 999 permutations) showed that fat source explained about 24% of the variability in microbiota composition (R2 = 0.24, p = 0.001). Diversity within samples was also affected by the type of fat, as shown by a significant decrease in phylogenetic diversity in samples from mice fed lard versus fish oil (Figure 1C; p = 0.001 non-parametric p value calculated using 999 Monte Carlo permutations at the maximum sampled depth). Linear discriminant analysis (LDA) effect size indicated that the genera Bacteroides, Turicibacter, and Bilophila were increased in lard-fed mice, while Actinobacteria (Bifidobacterium and Adlercreutzia), lactic acid bacteria (Lactobacillus and Streptococcus), Verrucomicrobia (Akkermansia muciniphila), Alphaproteobacteria, and Deltaproteobacteria were increased in fish-oil-fed mice (Figures 1E and 1F).
Using qPCR, we confirmed the increase in Akkermansia and Lactobacillus in the cecal contents of mice fed fish oil compared to lard for 11 weeks; we also observed a significant increase in Lactobacillus, but not in Akkermansia, in fish-oil-fed mice after 3 weeks (Figures S1H and S1I).
Impact of Lard versus Fish-Oil Diet on TLR Activation in Systemic Circulation
Despite the marked differences in the gut microbiota, short-chain fatty acid (SCFA) levels were similar in cecal samples from mice fed lard or fish oil for 11 weeks, with a minor increase only observed for acetate relative to the total SCFAs (C2 C4) in mice fed lard (Figures S2A–S2I). Because it is known that obesity and high-fat diets rich in saturated lipids are associated with increased intestinal absorption of microbial products (Caesar et al., 2010; Cani et al., 2007), we measured the potential of serum from vena cava to activate innate immunity receptors. We found that TLR2 and TLR4—but not TLR5, TLR9, or NOD2—were activated by serum from mice fed lard (Figures 2A–2E), suggesting that a lard diet promotes an increased influx of microbial factors into the systemic circulation. In agreement with these results, we found a trend toward increased levels of the TLR4 ligand LPS (lipopolysaccharide) in mice fed lard for 11 weeks (Figure 2F); however, there was no difference in LPS concentration between mice fed lard and fish oil for 3 weeks (Figure S2J).
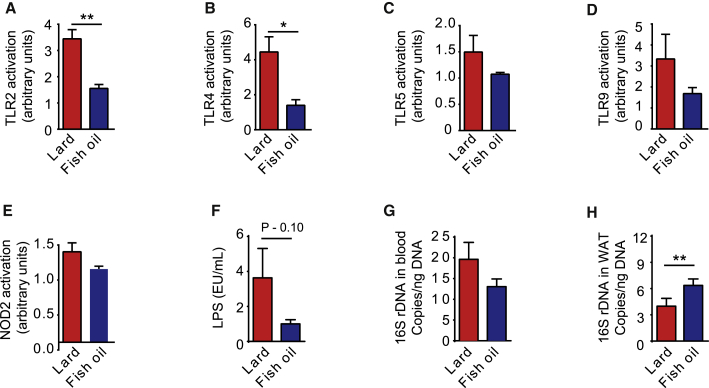
Figure 2.
Presence of TLR Ligands, LPS, and Bacterial DNA in Blood and Bacterial DNA in WAT of Mice Fed Lard and Fish Oil
(A–E) Activation of innate immunity receptors induced by stimulation with serum isolated from CONV-R mice fed lard or fish oil for 11 weeks (n = 3).
(F) Concentrations of LPS in serum isolated from CONV-R mice fed lard or fish oil for 11 weeks (n = 11–13 mice per group).
(G) Levels of 16S rDNA in blood from CONV-R mice fed lard or fish oil for 11 weeks (n = 15 mice per group).
(H) Levels of 16S rDNA in WAT from CONV-R mice fed lard or fish oil for 11 weeks (n = 15 mice per group).
Mean values ± SEM are plotted. ∗p < 0.05; ∗∗p < 0.01.
Impaired metabolism has been associated with bacterial translocation from the intestine (Amar et al., 2011). Therefore, we determined the levels of bacterial DNA in blood and epididymal WAT using qPCR. We found that the bacterial DNA load was similar in blood samples from mice fed lard or fish oil for 11 weeks (Figure 2G). We observed a small increase in bacterial load in WAT from mice fed fish oil compared to lard, but the levels in both groups were very low (Figure 2H). We profiled the 16S rRNA genes by Illumina sequencing and observed no significant differences in diversity and composition in either blood or WAT between mice fed lard or fish oil for 11 weeks (Figures S3A–S3F). Thus, our data suggest that the gut microbiota impairs glucose metabolism by stimulating inflammation through their pro-inflammatory molecules rather than by translocation from the intestine.
Lard Diet Promotes WAT Inflammation through TLR Signaling
The increased activation of TLR in the systemic circulation of lard-fed mice prompted us to investigate the potential role of TLR signaling in the development of diet-induced WAT inflammation. To test this, we fed lard or fish oil for 11 weeks to mice lacking the TLR adaptor molecules MyD88 or TRIF. As expected, mice fed fish oil gained less body weight and had smaller adipocytes than mice fed lard, regardless of the genotype (Figures 3A–3C). Lard-fed Myd88−/− mice were protected against diet-induced obesity and had decreased adipocyte size compared with lard-fed wild-type mice (Figures 3A–3C). By contrast, TRIF deficiency did not affect body weight or adipocyte size upon exposure to lard (Figures 3A–3C).
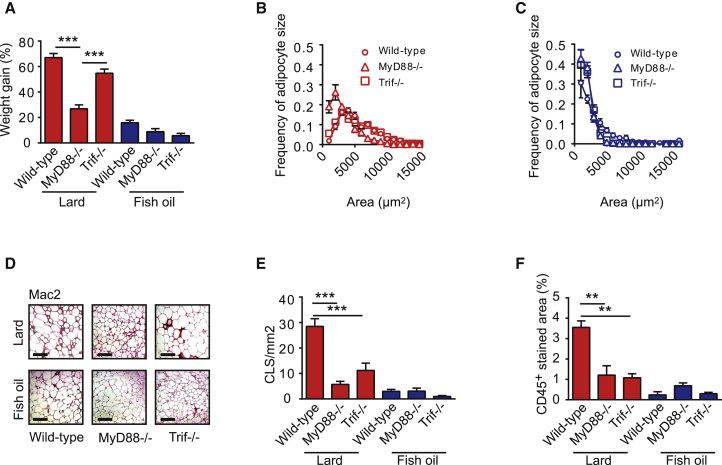
Figure 3.
Mice Lacking MyD88 or TRIF Are Protected against Lard-Diet-Induced WAT Inflammation
(A) Body weight gain of wild-type, Myd88−/−, and Trif−/− mice fed lard or fish oil for 11 weeks. ns = 23 (wild-type lard), 7 (Myd88−/− lard), 8 (Trif−/− lard), 22 (wild-type fish oil), 6 (Myd88−/− fish oil), and 9 (Trif−/− fish oil).
(B) Distribution of adipocyte sizes in wild-type, Myd88−/− and Trif−/− mice fed lard (n = 4–6 mice per group).
(C) Distribution of adipocyte sizes in wild-type, Myd88−/−, and Trif−/− mice fed fish oil. n = 8 for wild-type, and n = 4 for Myd88−/− and Trif−/−.
(D) Representative Mac-2 immunostaining of WAT from wild-type, Myd88−/−, and Trif−/− mice fed lard or fish oil. Scale bars, 100 μm.
(E) Quantification of CLS. ns = 7 (wild-type lard), 7 (Myd88−/− lard), 5 (Trif−/− lard), 7 (wild-type fish oil), 5 (Myd88−/− fish oil), and 4 (Trif−/− fish oil).
(F) Percentage of area occupied by CD45+ cells in WAT of wild-type, Myd88−/−, and Trif−/− mice. ns = 7 (wild-type lard), 7 (Myd88−/− lard), 5 (Trif−/− lard), 3 (wild-type fish-oil), 4 (Myd88−/− fish oil), and 4 (Trif−/− fish oil).
Mean values ± SEM are plotted. ∗∗p < 0.01; ∗∗∗p < 0.001.
Fat type had a dramatic effect on WAT inflammation: the number of crown-like structures (CLS; indicative of macrophage abundance in adipose tissue and WAT inflammation) (Cinti et al., 2005) and accumulation of CD45+ cells (leukocytes) in WAT increased dramatically between 3 and 11 weeks on a lard diet, whereas fish oil did not induce inflammation (Figures S4A and S4B). The numbers of CLS and CD45+ leukocytes were significantly lower in Myd88−/− and Trif−/− mice than in wild-type mice after 11 weeks on a lard diet (Figures 3D–3F). We also showed that fasting insulin levels were lower and that insulin sensitivity was improved in lard-fed Myd88−/− and Trif−/− mice compared to lard-fed wild-type mice (Figures S4C–S4F).
These data suggest that dietary saturated fatty acids might mediate WAT inflammation and impaired metabolic phenotypes through TRIF and MyD88 and that the TRIF-mediated effects are independent of the degree of adiposity.
Interaction between Dietary Lipids and the Gut Microbiota Affects WAT Inflammation
Our observation that knockout models for TLR signaling are protected against lard-induced WAT inflammation is consistent with previous reports showing that mice lacking TLR4 have reduced WAT inflammation (Kim and Sears, 2010) and implicates microbial components as mediators of the inflammatory phenotype. To investigate the role of the microbiota in diet-induced WAT inflammation, we fed CONV-R and GF mice lard or fish oil for 11 weeks. Diet had the largest impact on weight gain and lard-fed GF mice gained significantly more weight than fish-oil–fed GF mice (Figure 4A). However, GF mice gained less body weight than their CONV-R counterparts when fed either lard or fish oil (Figure 4A). Increased adiposity, per se, may promote WAT inflammation (Weisberg et al., 2003). Thus, to study the influence of gut microbiota on WAT inflammation independent of differences in adiposity, in subsequent analyses, we used GF and CONV-R mice with matching body weights and equal adipocyte size distribution at the end of the feeding period for each diet (Figures 4B and 4C).
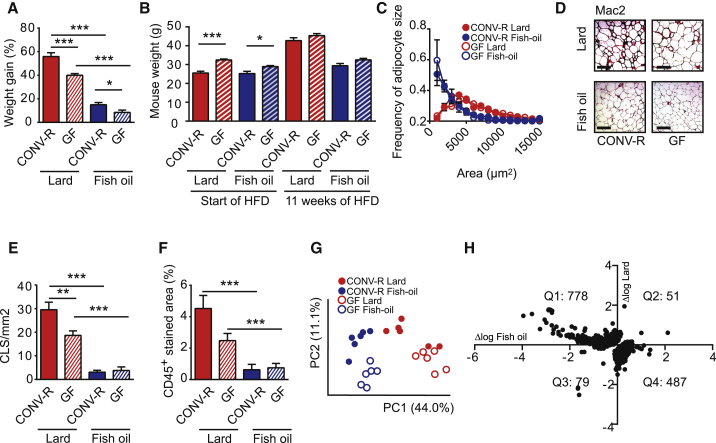
Figure 4.
Gut Microbiota and Dietary Lipid Interact to Regulate WAT Inflammation Independent of Body Weight and Adipocyte Size
(A) Body weight gain of CONV-R and GF mice fed lard or fish oil for 11 weeks. ns = 34 (CONV-R lard), 19 (GF lard), 34 (CONV-R fish oil), and 18 (GF fish oil).
(B) Initial and final body weight of mice used for analysis of WAT inflammation (n = 6).
(C) Distribution of adipocyte sizes in mice used for analysis of inflammation and metabolic perturbations. ns = 4 (CONV-R lard), 5 (GF lard), 6 (CONV-R fish oil), and 6 (GF fish oil).
(D) Representative Mac-2 immunostaining of WAT from CONV-R and GF mice fed lard or fish oil. Scale bars, 100 μm.
(E) Quantification of CLS (n = 6 mice per group).
(F) Percentage of area occupied by CD45+ cells in WAT from CONV-R and GF mice fed lard or fish oil (n = 5–6 mice per group).
(G) Principal-component analysis of global gene expression in WAT from CONV-R and GF mice fed lard or fish oil (n = 6 mice per group).
(H) Genes that are regulated by the interaction between diet and gut microbiota. WAT genes induced by the gut microbiota in mice fed lard are plotted on the y axis, and WAT genes induced by the gut microbiota in mice fed fish oil are plotted on the x axis (n = 6 mice per group). Interaction was determined by two-way ANOVA.
Means ± SEM are plotted. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
To investigate how dietary lipids and gut microbiota affect WAT inflammation, we performed immunohistochemistry to analyze WAT from CONV-R and GF mice fed a lard or fish-oil diet. As expected, we noted increased numbers of CLS and levels of CD45+ cells in lard-fed versus fish-oil-fed mice, both for CONV-R and GF mice (Figures 4D–4F). Importantly, we also found that the number of CLS was higher in lard-fed CONV-R versus GF mice and a trend (p = 0.08) toward increased levels of CD45+ cells in CONV-R versus GF mice (Figures 4D–4F).
To further investigate how dietary lipids and gut microbiota affect WAT inflammation metabolism, we performed a microarray analysis of WAT from CONV-R and GF mice fed a lard or fish-oil diet. Principal-component analysis of gene expression data revealed that mice separated on diet in the first dimension and on microbial status in the second dimension (Figure 4G). We observed increased expression of genes involved in immune processes and decreased expression of genes involved in energy generation and metabolism in lard-fed versus fish-oil-fed CONV-R mice (Table S3). To compare how the gut microbiota affects WAT gene expression in mice fed lard or fish oil, differences in expression level between CONV-R and GF mice were plotted for significantly regulated genes, with values for mice fed fish oil on the x axis and values for mice fed lard on the y axis (Figure S5). The majority of genes demonstrated a positive linear relationship, indicating that many genes are regulated by the microbiota independently of dietary lipids. Gene ontology analysis showed that the microbiota induced expression of genes involved in, e.g., RNA processing and mitochondrial organization in both dietary groups (Table S4). However, expression of genes involved in immune processes was decreased by the gut microbiota in fish-oil-fed mice and increased by the gut microbiota in lard-fed mice (Table S4). We applied a two-way ANOVA to identify genes with expression levels controlled by the interaction between dietary lipids and gut microbiota (Figure 4H). The subset of these genes located in Q1 (i.e., upregulated by the gut microbiota in lard-fed mice and downregulated in fish-oil-fed mice) was enriched in functional categories associated with immune responses (Table S4). Together, these data suggest that the gut microbiota interacts with dietary lipids to modulate WAT inflammation.
Microbiota from Mice Fed Fish Oil Counteracts Adiposity and Inflammation in Mice Subsequently Fed Lard
To test whether the gut microbiota of mice fed fish oil could attenuate inflammation and protect against diet-induced obesity during lard feeding, we transplanted the cecal microbiota of mice fed fish oil or lard for 11 weeks into recipient mice pretreated with antibiotics and then fed both groups of recipient mice a lard diet for 3 weeks. Strikingly, mice that received fish-oil microbiota gained less weight than mice that received lard microbiota (Figure 5A).
Figure 5.
Gut Microbiota Transplanted from Donor Mice Fed a Fish-Oil Diet Counteract Lard-Diet-Induced Adiposity and WAT Inflammation
Mice colonized with gut microbiota from donor mice were fed either a lard or a fish-oil diet for 11 weeks and fed a lard diet for 3 weeks.
(A) Body weight gain (n = 10 mice per group).
(B and C) Indicated here are the (B) principal coordinate analysis of gut microbiota composition based on unweighted UniFrac and (C) rarefaction curves (10–40,610 sequences per sample) for phylogenetic diversity in microbiota (n = 10).
(D) LDA scores of the differentially abundant taxa in blood. Taxa enriched in microbiota from mice fed lard (red) or fish oil (blue) are indicated with a positive or negative LDA score, respectively (taxa with LDA score >2 and a significance of α < 0.05 determined by Wilcoxon signed-rank test are shown).
(E) Concentrations of LPS in serum (n = 8–10 mice per group).
(F) Representative Mac-2 immunostaining of WAT. Scale bars, 100 μm.
(G) Quantification of CLS (n = 10 mice per group).
(H) Percentage of area occupied by CD45+ cells (n = 10 mice per group).
Mean values ± SEM are plotted. ∗p < 0.05; ∗∗p < 0.01.
Profiling of the 16S rRNA gene by Illumina sequencing of the cecal contents from the recipient mice demonstrated that the composition of the microbiota differed between mice that received fish-oil or lard microbiota, even though all mice were subsequently fed the same lard diet for 3 weeks. Principal coordinate analysis showed significant clustering of samples according to donor diet (Figure 5B), and multivariate non-parametric ANOVA showed that fat source of the donor explained about 27% of the variability in microbiota composition (R2 = 0.27, p = 0.001) Within-sample diversity, as measured by phylogenetic diversity, did not differ between the two recipient groups (Figure 5C). Finally, LDA effect size analysis indicated that taxa belonging to Akkermansia increased in the cecum of mice that received fish-oil microbiota, while Lactobacillus increased in mice that received a lard microbiota (Figure 5D); these results were confirmed by qPCR analysis (Figures S6A and S6B).
We found a trend toward decreased levels of LPS in serum from mice transplanted with microbiota from fish-oil donors (Figure 5E). Analysis of WAT showed that the number of CLS was slightly decreased in mice transplanted with microbiota from fish-oil donors but that the CD45+ cell level was similar in both groups (Figures 5F–5H).
CCL2 in WAT Is Induced by the Gut Microbiota and Mediates WAT Inflammation
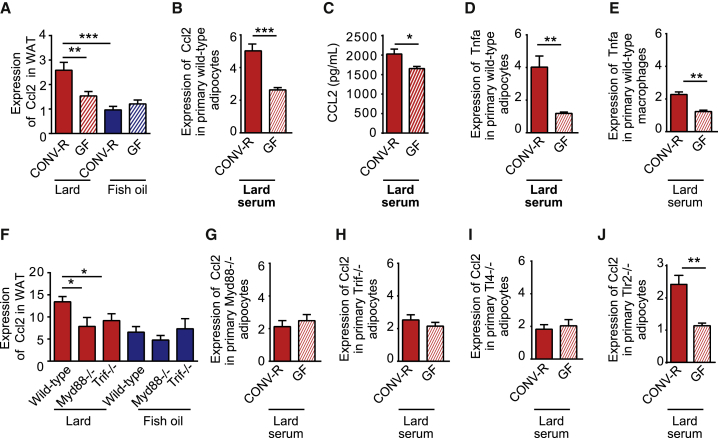
To investigate the mechanisms underlying the gut-microbiota-induced WAT inflammation in lard-fed mice, we searched our WAT microarray dataset for differentially regulated genes that are known to be associated with macrophage recruitment. Many previous reports have suggested that the chemokine CCL2 is a mediator of macrophage accumulation in WAT during obesity (Kamei et al., 2006; Kanda et al., 2006; Weisberg et al., 2006). Indeed, we found that Ccl2 expression in WAT was higher in lard-fed CONV-R mice than in lard-fed GF mice and that both CONV-R and GF mice fed fish oil had low expression of Ccl2 (Figure 6A). Because the WAT microarray dataset is from GF and CONV-R mice with matching body weights (as shown in Figures 4B and 4C), the difference in Ccl2 expression between lard-fed CONV-R and GF mice was independent of body weight and adipocyte size.
Figure 6.
CCL2 Production in WAT Is Induced by the Gut Microbiota through Activation of MyD88, TRIF, and TLR4
(A) Expression of Ccl2 in WAT from CONV-R and GF mice fed lard or fish oil for 11 weeks (n = 6).
(B) Expression of Ccl2 in primary adipocytes stimulated for 4 hr with 2% serum from the vena cava of CONV-R or GF mice fed lard (n = 5–6 mice per group).
(C) Secretion of CCL2 from primary wild-type primary adipocytes stimulated for 4 hr with 2% serum isolated from the vena cava of CONV-R and GF mice fed lard (n = 5–6).
(D and E) Expression of Tnfα in primary (D) adipocytes and (E) macrophages stimulated for 4 hr with 2% serum isolated from the vena cava of CONV-R and GF mice fed lard (n = 5–6).
(F) Expression of Ccl2 in WAT from CONV-R wild-type, Myd88−/−, and Trif−/− mice fed lard or fish oil for 11 weeks. ns = 6 (wild-type lard), 6 (Myd88−/− lard), and 4 (Trif−/− lard).
(G–J) Expression of Ccl2 in (G) Myd88−/−, (H) Trif−/−, (I) Tlr4−/−, and (J) Tlr2−/− primary adipocytes stimulated for 4 hr with 2% serum isolated from the vena cava of CONV-R or GF mice fed lard (n = 5–6).
Mean values ± SEM are plotted. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
To test the hypothesis that factors in the serum of lard-fed CONV-R mice promote inflammation in WAT, primary adipocytes and macrophages were exposed to serum from CONV-R or GF mice fed lard. Gene expression and secretion of CCL2 were increased in adipocytes, but not macrophages, treated with serum from lard-fed CONV-R mice compared to cells treated with serum from lard-fed GF mice (Figures 6B and 6C; Figures S7A and S7B). We showed that treatment with serum from lard-fed CONV-R mice increased expression of Tnfα in both adipocytes and macrophages (Figures 6D and 6E), indicating that circulating microbial factors may trigger a general inflammatory response in both these cell types.
To study the role of innate immunity signaling in the regulation of Ccl2, we analyzed Ccl2 expression in WAT from Myd88−/− and Trif−/− mice fed lard or fish oil. Ccl2 expression was lower in WAT from lard-fed Myd88−/− and Trif−/− mice than that from lard-fed wild-type mice, while Ccl2 expression in mice fed fish oil was low regardless of genotype (Figure 6F). We also showed that Ccl2 expression was not increased when primary Myd88−/−, Trif−/−, or Tlr4−/− adipocytes were exposed to serum from lard-fed CONV-R mice compared to adipocytes treated with serum from GF mice (compare Figures 6G–6I with 6B). In contrast, Ccl2 expression in primary Tlr2−/− adipocytes was increased by exposure to serum from lard-fed CONV-R mice compared to exposure to serum from GF mice (Figure 6J). These data indicate that there are factors present in the blood of CONV-R mice that induce Ccl2 expression through pathways involving TLR4, MyD88, and TRIF but not TLR2.
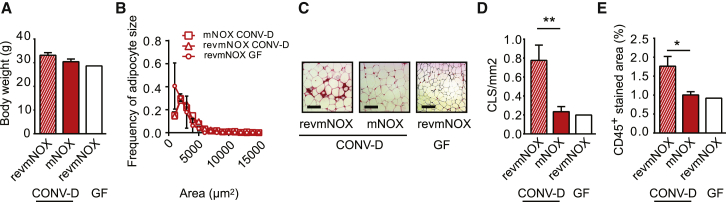
To investigate whether CCL2 is involved in the development of WAT inflammation induced by the gut microbiota and lard diet, GF mice were conventionalized and fed a lard diet for 4 weeks in the presence of either the CCL2 inhibitor mNOX-E36 or the nonfunctional control substance revmNOX-E36. Mice treated with mNOX-E36 and revmNOX-E36 did not differ in body weight or adipocyte size (Figures 7A and 7B). Strikingly, however, conventionalized mice treated with mNOX-E36 had decreased abundance of CLS and CD45+ cells in WAT compared to mice treated with revmNOX-E36 (Figures 7C–7E), indicating a role of CCL2 in macrophage recruitment to WAT.
Figure 7.
CCL2 Mediates WAT Inflammation
(A and B) Indicated here are the (A) body weight and (B) distribution of adipocyte size in WAT from CONV-D mice fed lard for 28 days and treated with either revmNOX or mNOX and from GF mice fed lard for 28 days and treated with revmNOX. n = 8 (CONV-D revmNOX and CONV-D mNOX), and n = 2 (GF revmNOX).
(C) Representative Mac-2 immunostaining of WAT from CONV-D mice fed lard and treated with either revmNOX or mNOX and from GF mice fed lard and treated with revmNOX. Scale bars, 100 μm.
(D and E) Indicated here are the (D) quantification of CLS and (E) percentage of area occupied by CD45+ cells in WAT from CONV-D mice fed lard and treated with either revmNOX or mNOX and from GF mice fed lard and treated with revmNOX. n = 8 (CONV-D revmNOX and CONV-D mNOX), and n = 2 (GF revmNOX).
Mean values ± SEM are plotted; ∗p < 0.05; ∗∗p < 0.01.
Taken together, these data suggest that CCL2 is induced by factors in the blood of CONV-R mice fed a lard diet through mechanisms involving MyD88, TRIF, and TLR4 and that CCL2 facilitates accumulation of macrophages in WAT.
Discussion
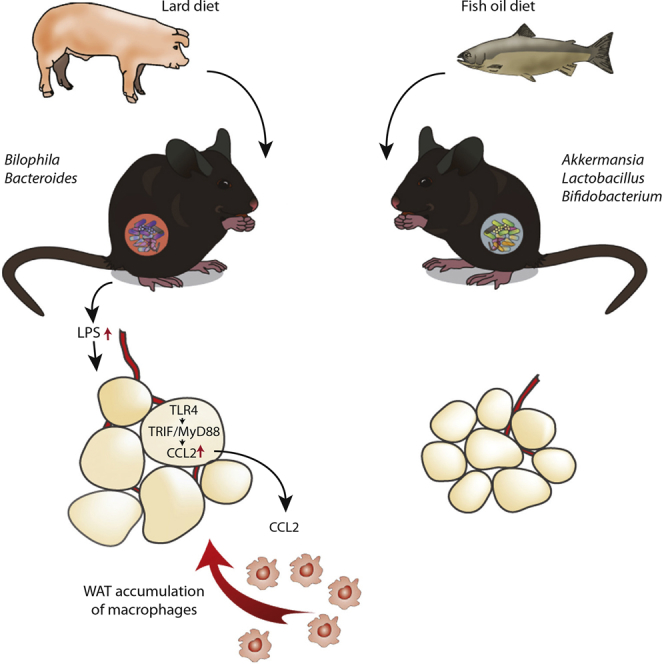
In the present study, we demonstrate that the type of dietary lipids affects the gut microbiota and that the gut microbiota contributes to the phenotypic differences between mice fed lard and mice fed fish oil. Mice fed a lard diet have increased TLR activation in the systemic circulation, increased WAT inflammation, and impaired insulin sensitivity compared to mice fed fish oil. We found that mice lacking MyD88 or TRIF are protected against lard-induced WAT inflammation and metabolic perturbations and that saturated dietary lipids and the gut microbiota interact to induce WAT inflammation. In addition, we show that CCL2 is required for microbiota-induced macrophage recruitment to WAT in mice on a lard diet and that expression of CCL2 in adipocytes is induced by factors in the blood of lard-fed CONV-R mice through a mechanism involving MyD88, TRIF, and TLR4.
We showed that the type of dietary fat is a major driver of community structure, affecting both the composition and diversity of the gut microbiota. Results from 454 pyrosequencing and qPCR showed that mice fed fish oil had increased levels of taxa from the genera Lactobacillus, a known probiotic that has been linked to reduced inflammation and mucosal lesion scores in several models of inflammatory bowel diseases (Guarner et al., 2005), and Akkermansia muciniphila, which has been shown to reduce fat mass gain and WAT macrophage infiltration and improve gut barrier function and glucose metabolism when administered to mice with diet-induced obesity (Everard et al., 2013). By contrast, mice fed lard had increased levels of taxa related to Bilophila. Previous studies have shown that Bilophila increases in mice and humans after consumption of diets rich in saturated fats of animal origin (David et al., 2014; Devkota et al., 2012), and Bilophila wadsworthia has been shown to exacerbate colitis in genetically susceptible models (Devkota et al., 2012).
To determine whether the microbiota of fish-oil-fed mice could confer protection against lard-diet-induced adiposity and inflammation, we transplanted microbiota from lard- or fish-oil-fed mice into antibiotic-treated mice that were then fed a lard diet for 3 weeks. Here, we used antibiotic-treated mice rather than GF mice because it is known that GF mice have an underdeveloped immune system (Hooper et al., 2012), which could potentially confound this analysis. Mice that received microbiota from a lard-fed donor showed increased adiposity and inflammation, together with a significant increase in Lactobacillus, compared to mice that received microbiota from a fish-oil-fed donor. Therefore, these data do not provide evidence for a role of Lactobacillus in reducing inflammation. However, we found that the enrichment of Akkermansia co-occurred with partial protection against adiposity and inflammation in mice transplanted with fish-oil microbiota and fed a lard diet, highlighting Akkermansia as a potential mediator of the improved inflammatory and metabolic phenotype of mice fed fish oil. This finding is in agreement with previous findings linking Akkermansia muciniphilia with protection to diet-induced obesity (Everard et al., 2013; Shin et al., 2014).
Serum from mice fed lard had increased capacity to activate TLR4, which has been linked to WAT inflammation and metabolic perturbations (Caesar et al., 2012; Cani et al., 2007; Creely et al., 2007). Furthermore, we found that mice deficient in either of the two TLR adaptor molecules MyD88 and TRIF were protected from lard-induced WAT inflammation and insulin sensitivity. These findings are consistent with earlier studies showing reduced inflammation in mouse models lacking functional MyD88 (Björkbacka et al., 2004; Everard et al., 2014; Michelsen et al., 2004) or TRIF (Richards et al., 2013). One report has shown that MyD88 protects against glucose homeostasis perturbations and liver disease during a high-fat diet (Hosoi et al., 2010). Inconsistencies in reports on the role of MyD88 may be due to environmental factors at different animal facilities. For example, the presence of segmented filamentous bacteria, which are enriched in Myd88−/− mice (Larsson et al., 2012) and have a major impact on host immunity (Ivanov et al., 2009), differs between animal facilities (Kriegel et al., 2011). Importantly, the TRIF-deficient mice in our study had the same body weight and adipocyte size as the wild-type mice, showing that protection against WAT inflammation was not dependent on reduced adiposity.
TLR signaling can be activated by both microbial and endogenous ligands, and some investigators have suggested that saturated fatty acids promote inflammation and insulin resistance in obesity through TLR4 (Shi et al., 2006). The gut microbiota modulates host lipid metabolism (Velagapudi et al., 2010); therefore, protection against WAT inflammation in Myd88−/− and Trif−/− mice fed lard might be due to decreased TLR signaling induced by ligands originating from the host or from the diet. Here, we showed that serum levels of LPS were higher in mice fed lard compared to mice fed fish oil, indicating that microbial factors are present in the periphery that may directly affect WAT inflammation. However, we cannot exclude the possibility that other factors, such as saturated lipids, also directly contribute to the inflammatory response by activating TLR signaling (Huang et al., 2012). Furthermore, to determine the specific impact of the gut microbiota on lard-induced WAT inflammation, we compared the effects of lard and fish oil in CONV-R versus GF mice. As expected, GF mice were partly protected against lard-induced WAT inflammation, although the protection against obesity in GF mice was less than that observed in previous studies (Bäckhed et al., 2007; Caesar et al., 2012; Ding et al., 2010; Rabot et al., 2010). This is likely due to the reduced sucrose levels in the high-fat diets used in the present study. Sucrose levels have previously been shown to have a major impact on microbiota-induced obesity (Fleissner et al., 2010). We used this fact to our benefit, as we could use weight-matched mice to try and untangle whether the microbiota modulated WAT inflammation by weight-dependent or -independent mechanisms. Importantly, we observed an adiposity-independent link between the gut microbiota and WAT inflammation, which may implicate microbially derived products as mediators of inflammation through TLRs. However, we also showed that GF mice fed lard had increased WAT inflammation compared to GF mice fed fish oil, indicating that microbiota-independent mechanisms also contribute to accumulation of immune cells in WAT.
Previous studies have demonstrated that gut-microbiota-derived factors can induce inflammation and Tnfα expression in WAT (Caesar et al., 2012; Cani et al., 2007), and we showed that serum from lard-fed CONV-R mice compared to GF mice had an increased capacity to induce expression of Tnfα in both adipocytes and macrophages. CCL2 is the only chemokine that has been shown to mediate inflammation in a WAT-specific knockout model (Lee and Lee, 2014), and TLR ligands have been shown to induce secretion of CCL2 from 3T3-L1 adipocytes (Kopp et al., 2009). Here, we found that Ccl2 expression in primary adipocytes and WAT was induced by microbial factors in serum and required the presence of MyD88, TRIF, and TLR4. Overexpression of Ccl2 in adipocytes has been shown to result in WAT inflammation and insulin resistance without obesity (Kamei et al., 2006; Kanda et al., 2006), and mice deficient in CCL2, or its receptor chemokine (C-C motif) receptor 2 (CCR2), have reduced WAT inflammation and insulin resistance during a high-fat diet (Kanda et al., 2006; Weisberg et al., 2006). A recent study also reported that CCL2 promotes local proliferation of macrophages in WAT (Amano et al., 2014). By using the specific pharmacological CCL2 inhibitor mNOX-E36 (Kulkarni et al., 2007, 2009; Wlotzka et al., 2002), we demonstrated that CCL2 is essential for WAT macrophage accumulation in our model and, therefore, constitutes a putative mediator of gut-microbiota-induced WAT inflammation. In addition, we found that GF mice fed lard or fish oil had similar expression levels of Ccl2 in WAT, suggesting that microbiota-independent WAT inflammation is not mediated through CCL2.
Taken together, our data show that interaction between the gut microbiota and dietary lipids induces WAT inflammation. We also identify putative mechanisms, including the role of cell signaling components and regulation of chemokine expression. The study establishes the gut microbiota as an independent factor aggravating inflammation during diet-induced obesity and, therefore, a suitable target for therapies against associated metabolic perturbations.
Experimental Procedures
Wild-type C57Bl/6, Myd88−/−, and Trif−/−, Tlr4−/−, and Tlr2−/− mice were maintained under standard specific-pathogen-free (SPF) or GF conditions as described previously (Caesar et al., 2012). All mice were males and 11–14 weeks of age at the start of the experiments. Mice were weight matched at the start of the experiments, except when the aim was to compare weight-matched mice at the end of the experiment as indicated in the text. Mice were fed irradiated isocaloric diets (45% kcal fat) of identical composition except for the source of fat, which was either menhaden fish oil (Research Diets, D05122102) or lard (Research Diets, D10011202) (Table S1), for 11 weeks unless otherwise indicated. The mice were fasted for 4 hr before they were killed. Blood samples and epididymal WAT samples were harvested at the end of the experiment. Weekly food consumption was measured cage-wise.
To study the role of CCL2 in WAT inflammation during conventionalization, three groups of GF mice were injected subcutaneously with 20 mg/kg of the CCL2 inhibitor mNOX-E36 (Baeck et al., 2012) or the nonfunctional control substance revmNOX-E36 (both NOXXON Pharma) three times per week for 30 days. From day 3, the mice were fed a lard diet, and two groups of mice (one group receiving mNOX-E36 and the other receiving revmNOX-E36) were transferred to a conventional environment and gavaged with cecal content (isolated from a SPF 12-week-old male C57Bl/6 mouse) suspended in 200 μl PBS. The third group of mice (receiving revmNOX-E36) remained in a GF environment.
Gut microbiota transplantation with cecal content from donor mice fed lard or fish oil for 11 weeks was performed on male 12-week-old mice. Before the microbial transplantation, recipient mice were treated with a 200 μl antibiotic cocktail (ampicillin, 1 g/l; metronidazole, 1 g/l; vancomycin, 0.5 g/l; neomycin, 0.5 g/l) administrated by oral gavage once a day for 3 days. During the last 8 hr of antibiotic treatment, mice were fed either lard or fish oil to facilitate subsequent colonization. Half a frozen cecum was suspended in 5 ml of PBS containing 0.2 g/l Na2S and 0.5 g/l cysteine as reducing agents in Hungate tubes. Mice were colonized by oral gavage with 200 μl of cecal suspension after a 4-hr fast once a week. After the first microbiota gavage, all mice were fed a lard diet for 3 weeks. All experiments were performed with protocols approved by the University of Gothenburg Animal Studies Committee.
Statistical Analysis
Data are shown as means ± SEM. Statistical comparison of two groups was performed using a Student’s t test; comparisons of three or more groups were analyzed by one-way ANOVA with ad hoc Tukey post tests; analysis of datasets containing multiple measurements from each mouse (weight gain, ITT, diet consumption, and feeding efficiency) was performed with a two-way ANOVA for repeated measurements; analysis of interaction was performed with a two-way ANOVA; and analysis of covariance was performed on linear regression. Statistical analysis was performed in GraphPad Prism 6 unless otherwise stated.
Additional experimental procedures are described in the Supplemental Experimental Procedures.
Author Contributions
R.C.: designed, performed, and analyzed experiments. V.T., P.K.D., and P.D.C.: performed and analyzed experiments. F.B.: conceived the project and designed and analyzed experiments. R.C. and F.B. wrote the paper. All authors commented and approved the paper.
Acknowledgments
We thank Rosie Perkins (Wallenberg Laboratory, University of Gothenburg) for editing the manuscript; Anna Hallén, Gunnel Östergren Lunden, Carina Arvidsson, and Alexandra Ferraro Werling for superb technical assistance; Rozita Akrami for bioinformatics assistance; and NOXXON Pharma for providing mNOX-E36 and revmNOX-E36. We thank Professor Willem de Vos (Wageningen University) for donating Akkermansia muciniphila, Professor Jan Borén (Wallenberg Laboratory, University of Gothenburg) for donating Tlr4−/− mice, and Professor Carina Mallard (Institute of Neuroscience and Physiology, University of Gothenburg) for donating Tlr2−/− mice. This work was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Swedish Diabetes Foundation, the Swedish Heart Lung Foundation, the Torsten Söderbergs and Ragnar Söderbergs Foundations, the Novo Nordisk Foundation, the Knut and Alice Wallenberg Foundation, TORNADO (FP7-KBBE-222720; http://www.fp7tornado.eu/), the EU-funded ETHERPATHS project (FP7-KBBE-222639; http://www.etherpaths.org), and a LUA-ALF grant from Västra Götalandsregionen. The computations were performed on resources provided by Swedish National Infrastructure for Computing (SNIC) through the Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX). F.B. is a recipient of a European Research Council (ERC) Consolidator grant (615362 - METABASE). P.D.C. is a research associate at FRS-FNRS (Fonds de la Recherche Scientifique) and recipient of a FRFS-WELBIO under grant (WELBIO-CR-2012S-02R) and an ERC Starting Grant 2013 (ERC, Starting grant 336452-ENIGMO).
Published: August 27, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.cmet.2015.07.026.
Contributor Information
Robert Caesar, Email: robert.caesar@wlab.gu.se.
Fredrik Bäckhed, Email: fredrik.backhed@wlab.gu.se.
Accession Numbers
Microarray data have been uploaded to the Gene Expression Omnibus (GEO) database with accession number GSE70922, and bacterial DNA sequencing data have been uploaded to NCBI as PRJNA289917.
Supplemental Information
References
- Amano S.U., Cohen J.L., Vangala P., Tencerova M., Nicoloro S.M., Yawe J.C., Shen Y., Czech M.P., Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermúdez-Humarán L.G., Smirnova N., Bergé M., Sulpice T., Lahtinen S. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeck C., Wehr A., Karlmark K.R., Heymann F., Vucur M., Gassler N., Huss S., Klussmann S., Eulberg D., Luedde T. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- Björkbacka H., Kunjathoor V.V., Moore K.J., Koehn S., Ordija C.M., Lee M.A., Means T., Halmen K., Luster A.D., Golenbock D.T., Freeman M.W. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- Buckley J.D., Howe P.R. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes. Rev. 2009;10:648–659. doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- Caesar R., Fåk F., Bäckhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J. Intern. Med. 2010;268:320–328. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- Caesar R., Reigstad C.S., Bäckhed H.K., Reinhardt C., Ketonen M., Lundén G.Ö., Cani P.D., Bäckhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61:1701–1707. doi: 10.1136/gutjnl-2011-301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A.S., Obin M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Creely S.J., McTernan P.G., Kusminski C.M., Fisher M., Da Silva N.F., Khanolkar M., Evans M., Harte A.L., Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Chi M.M., Scull B.P., Rigby R., Schwerbrock N.M.J., Magness S., Jobin C., Lund P.K. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Geurts L., Caesar R., Van Hul M., Matamoros S., Duparc T., Denis R.G.P., Cochez P., Pierard F., Castel J. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat. Commun. 2014;5:5648. doi: 10.1038/ncomms6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner C.K., Huebel N., Abd El-Bary M.M., Loh G., Klaus S., Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 2010;104:919–929. doi: 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- Guarner F., Perdigon G., Corthier G., Salminen S., Koletzko B., Morelli L. Should yoghurt cultures be considered probiotic? Br. J. Nutr. 2005;93:783–786. doi: 10.1079/bjn20051428. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T., Thaiss C.A., Kau A.L., Eisenbarth S.C., Jurczak M.J. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T., Yokoyama S., Matsuo S., Akira S., Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS ONE. 2010;5:e12537. doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Rutkowsky J.M., Snodgrass R.G., Ono-Moore K.D., Schneider D.A., Newman J.W., Adams S.H., Hwang D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Flavell R.A. Innate sensors of pathogen and stress: linking inflammation to obesity. J. Allergy Clin. Immunol. 2013;132:287–294. doi: 10.1016/j.jaci.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Kamei N., Tobe K., Suzuki R., Ohsugi M., Watanabe T., Kubota N., Ohtsuka-Kowatari N., Kumagai K., Sakamoto K., Kobayashi M. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A., Martinez K., Chuang C.-C., LaPoint K., McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J. Nutr. 2009;139:1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Sears D.D. TLR4 and insulin resistance. Gastroenterol. Res. Pract. 2010;2010:11. doi: 10.1155/2010/212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., Buechler C., Neumeier M., Weigert J., Aslanidis C., Schölmerich J., Schäffler A. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 2009;17:648–656. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- Kriegel M.A., Sefik E., Hill J.A., Wu H.-J., Benoist C., Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni O., Pawar R.D., Purschke W., Eulberg D., Selve N., Buchner K., Ninichuk V., Segerer S., Vielhauer V., Klussmann S., Anders H.J. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 2007;18:2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- Kulkarni O., Eulberg D., Selve N., Zöllner S., Allam R., Pawar R.D., Pfeiffer S., Segerer S., Klussmann S., Anders H.J. Anti-Ccl2 Spiegelmer permits 75% dose reduction of cyclophosphamide to control diffuse proliferative lupus nephritis and pneumonitis in MRL-Fas(lpr) mice. J. Pharmacol. Exp. Ther. 2009;328:371–377. doi: 10.1124/jpet.108.142711. [DOI] [PubMed] [Google Scholar]
- Larsson E., Tremaroli V., Lee Y.S., Koren O., Nookaew I., Fricker A., Nielsen J., Ley R.E., Bäckhed F. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61:1124–1131. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.-C., Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen K.S., Wong M.H., Shah P.K., Zhang W., Yano J., Doherty T.M., Akira S., Rajavashisth T.B., Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot S., Membrez M., Bruneau A., Gérard P., Harach T., Moser M., Raymond F., Mansourian R., Chou C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- Richards M.R., Black A.S., Bonnet D.J., Barish G.D., Woo C.W., Tabas I., Curtiss L.K., Tobias P.S. The LPS2 mutation in TRIF is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate Immun. 2013;19:20–29. doi: 10.1177/1753425912447130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.-R., Lee J.-C., Lee H.-Y., Kim M.-S., Whon T.W., Lee M.-S., Bae J.-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- Tremaroli V., Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Velagapudi V.R., Hezaveh R., Reigstad C.S., Gopalacharyulu P., Yetukuri L., Islam S., Felin J., Perkins R., Borén J., Orešič M., Bäckhed F. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 2010;51:1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R.L., Ferrante A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlotzka B., Leva S., Eschgfäller B., Burmeister J., Kleinjung F., Kaduk C., Muhn P., Hess-Stumpp H., Klussmann S. In vivo properties of an anti-GnRH Spiegelmer: an example of an oligonucleotide-based therapeutic substance class. Proc. Natl. Acad. Sci. USA. 2002;99:8898–8902. doi: 10.1073/pnas.132067399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wang L., Chen S. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010;14:2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.