Abstract
Objectives
Our primary aim of this pilot study was to test feasibility of the planned design, the interventions (education plus telephone coaching), and the outcome measures, and to facilitate a power calculation for a future randomized controlled trial to improve adherence to recovery goals following hip fracture.
Design
This is a parallel 1:1 randomized controlled feasibility study.
Setting
The study was conducted in a teaching hospital in Vancouver, BC, Canada.
Participants
Participants were community-dwelling adults over 60 years of age with a recent hip fracture. They were recruited and assessed in hospital, and then randomized after hospital discharge to the intervention or control group by a web-based randomization service. Treatment allocation was concealed to the investigators, measurement team, and data entry assistants and analysts. Participants and the research physiotherapist were aware of treatment allocation.
Intervention
Intervention included usual care for hip fracture plus a 1-hour in-hospital educational session using a patient-centered educational manual and four videos, and up to five postdischarge telephone calls from a physiotherapist to provide recovery coaching. The control group received usual care plus a 1-hour in-hospital educational session using the educational manual and videos.
Measurement
Our primary outcome was feasibility, specifically recruitment and retention of participants. We also collected selected health outcomes, including health-related quality of life (EQ5D-5L), gait speed, and psychosocial factors (ICEpop CAPability measure for Older people and the Hospital Anxiety and Depression Scale).
Results
Our pilot study results indicate that it is feasible to recruit, retain, and provide follow-up telephone coaching to older adults after hip fracture. We enrolled 30 older adults (mean age 81.5 years; range 61–97 years), representing a 42% recruitment rate. Participants excluded were those who were not community dwelling on admission, were discharged to a residential care facility, had physician-diagnosed dementia, and/or had medical contraindications to participation. There were 27 participants who completed the study: eleven in the intervention group, 15 in the control group, and one participant completed a qualitative interview only. There were no differences between groups for health measures.
Conclusion
We highlight the feasibility of telephone coaching for older adults after hip fracture to improve adherence to mobility recovery goals.
Keywords: feasibility, recruitment, hip fracture, telephone follow-up, patient education, coaching
Video abstract
Introduction
Hospitalization and recovery for a fall-related hip fracture often pose significant challenges for older adults. A third of older adults with hip fracture will be readmitted to hospital with avoidable complications within 30 days of their return home.1,2 The vulnerability that precipitates a fall and fracture is compounded by the myriad of physiological stressors that hospitalization itself imposes, creating a transient acquired period of susceptibility to further complications, and hospital readmission, known as the “posthospital syndrome.”3 The devastating consequences of hip fracture on mobility and independence can also create challenges for older adults to self-manage their recovery after hospitalization, and this is compounded if discharge teaching is inadequate, leading to increased risk of harm and hospital readmission.4 Although several hospital transitional programs to reduce readmissions have shown some success,5–7 30-day hospital readmission rate in Canada is ~8.5%, and costs an estimated CAN $1.8 billion per year.1
Many factors contribute to the risk of hospital readmission including health system factors (eg, access to ambulatory care), hospital factors (postdischarge follow-up), patient factors (health literacy, comorbidities, severity of illness, health behavior engagement), social factors (family and caregiver engagement), and home environment.8,9 For example, some older adults after hip fracture risk hospital readmission because they do not have access to or are unaware of community resources, have significant barriers to the success of the transition home such as a decline in mobility status but the same home environment (eg, no longer able to independently climb stairs but live in a townhouse), and/or little or no social support.6,10,11 Thus, despite well-designed transition plans, sometimes, older adults “fall through the cracks.” In the context of financial constraints and resource allocation, some transition programs7,12 have implemented follow-up phone calls after discharge to problem-solve barriers to self-management, as is the case with Project Re-Engineered Discharge (Project RED).12 Although telephone calls to support transitions are feasible, identify clinical problems early, and encourage health-promoting behaviors,13 it is not known whether alone they are successful in reducing readmissions to acute care settings.14,15 Contributing to the challenges encountered during the hospital-to-home transition may be that older adults are not certain of what to expect after hip fracture, for example, how to preempt and problem-solve common medical complications, what milestones and targets to aim for in the recovery process, how to stay on track with health goals (if present), and how to translate these goals into actual behaviors.
To respond to this, we developed and tested a patient-centered toolkit16 for older adults after hip fracture, based on the “words of wisdom” shared by older adults and family members who previously experienced a hip fracture.17 Using these lived experiences, as well as consulting with a broad range of clinical experts, allowed us to tailor our postoperative hip fracture toolkit to target gaps in patient knowledge postfracture, as well as behavioral strategies, including setting goals for recovery. Since pilot studies are “an almost essential requirement” prior to larger scale trials,18 in advance of a larger study to look at the effect of this model on quality of life, functional outcomes, and hospital readmissions, we completed pilot work to discern key trial feasibility information such as the following: Can we recruit participants into the study as planned during the acute hospital stay? How many older adults are eligible for the study (eg, who would benefit from the intervention in its current form)? Was the intervention acceptable to recently discharged older adults with hip fracture?
Methods
Trial design
This was a single-site parallel 1:1 randomized controlled trial (RCT) to test feasibility of an enhanced discharge support model for older adults with a recent hip fracture that included an educational toolkit and telephone follow-up. The design, conduct, analysis, and reporting of this clinical trial followed the published guidelines in the Consolidated Standards of Reporting Trials (CONSORT 2010) Statement.19 We obtained approval from the University of British Columbia and Vancouver Coastal Health Research Institute hospital ethics boards to conduct this study, and all participants gave written consent prior to enrollment. The ClinicalTrials.gov identifier is NCT01930409.
Participants
We recruited community-dwelling older adults aged 60+ years who sustained a surgically repaired fall-related hip fracture and invited them to enroll in this feasibility study. We excluded older adults with hip fracture who sustained a fracture related to metastatic disease or bisphosphonate use, did not return home in the community after hospital discharge, had medical contraindications restricting activity and exercise (eg, significant cardiovascular disease or neurological degenerative disease), did not understand or speak English well enough to benefit from the telephone intervention, had hearing or speech impairments precluding telephonic communication, or had a physician-diagnosed dementia.
Setting
Participants were recruited from the acute orthopedic unit at a Metro Vancouver teaching hospital following surgical repair of the hip fracture.
Recruitment and data collection
After assessing for study eligibility, the staff occupational therapist approached the participant as soon as he/she was clinically stable postoperatively, and provided details of the study. When the participant expressed interest in enrolling, the research physiotherapist (RPT) scheduled an in-hospital appointment to fully explain the study details. We assessed participants at two time points: baseline (on the orthopedic unit within 7 days following hip fracture) and final (at 4 months after hospital discharge). After written informed consent was obtained, the RPT completed the baseline assessment. A different RPT completed the final assessment in the participant’s home. Training in the study protocols was completed together by both RPTs prior to collecting outcome data.
Intervention
Participants who were randomized to the intervention group received usual care for their hip fracture, and they received a 1-hour in-hospital educational session with a trained health professional (RPT), using the hip fracture recovery manual and four educational videos (Fracture Recovery for Seniors at Home [FReSH Start]). The content of this education program followed a standard format as guided by the manual but was individualized for each participant, including a description of the type of hip fracture sustained, how it was surgically fixated, red flags to watch out for during recovery, an exercise program (home-based exercises reduce both the rate and risk of future falls),20 practical information about future falls prevention, review of home safety and environmental hazards, and mobility and recovery goal setting. The videos were viewed at the bedside using a tablet and headphones. The RPT used a “teach-back” approach,21 clarified and checked participants’ understanding of materials, and ensured that participants were able to provide a verbal summary of the education provided to them. On discharge, usual care included follow-up physician and surgeon visits, and usual rehabilitation and home care, if appropriate, as determined by the health care team. Following discharge, we also adopted elements of Project RED12 for our study protocol. The RPT telephoned participants up to five times in the first 4 months following hip fracture to provide further encouragement, falls prevention information, coaching to remain active, problem-solving skills, mobility goal setting, and advice to help participants maintain and increase their prescribed home exercises. Total telephone intervention time over the five calls averaged 151 minutes (42–286 minutes) at an average cost of $125 per participant. Table 1 describes the content of the sessions classified according to the CALO-RE (Coventry, Aberdeen and London – Refined) taxonomy of behavior change techniques.10 At the first call, within 1 week of discharge from acute care, the RPT used techniques in the spirit of motivational interviewing,22 to facilitate participants to set recovery goals. This included developing plans (action and coping) to adhere to an exercise program, to remain as active as possible, and to help resolve any mobility problems, such as restrictions resulting from pain or fatigue. At the third, fourth, or fifth telephone calls, as deemed appropriate, the participant and RPT discussed recovery, and jointly set longer term individualized mobility goals, including plans for community reintegration and prevention of future falls and fractures. We also completed an in-depth semi-structured interview with participants in the intervention group and key informant health professionals to determine acceptability of the intervention, barriers to participation, as well as their experience of the transition home; however, this report is beyond the scope of the current paper.
Table 1.
Content of intervention by session based on the CALO-RE taxonomy of behavior change techniques10
| Behavior change technique | Session 1
|
Session 2
|
Session 3
|
Session 4
|
Session 5
|
Session 6
|
|---|---|---|---|---|---|---|
| In person | Telephone | Telephone | Telephone | Telephone | Telephone | |
| Shaping knowledge (ie, hip fracture, information about health consequences) | x | |||||
| Goal setting (behavior) | x | x | x | x | x | x |
| Goal setting (outcome) | x | x | x | x | x | x |
| Action planning | x | x | x | x | x | |
| Prompt practice (ie, exercise) | x | |||||
| Barrier identification/problem solving | x | x | x | x | x | x |
| Shaping knowledge (instruction on how to perform exercise behavior) | x | |||||
| Social support (practical) | x | x | x | x | x | x |
| Social support (emotional) | x | x | x | x | x | x |
| Use of follow-up prompts | x | x | x | x | x | |
| Review of goals | x | x | x | x | ||
| Relapse prevention/coping planning | x | x | x | x |
Notes: Session 1 was a 1-hour individual session during hospitalization with a trained health professional including four videos. Session 2 included first phone call within 1 week of discharge. Sessions 3–6 were follow-up phone calls in the first 4 months following hip fracture. Session 1 was provided to all participants. Phone calls were provided to the intervention group only (bold).
Abbreviation: CALO-RE, Coventry, Aberdeen and London – Refined.
Control
Participants who were randomized to the control group received usual care for their hip fracture as part of the established hospital hip fracture care pathway and were given a 1-hour individualized in-hospital teaching session using the FReSH Start manual and videos with the RPT as described in the Intervention section.
Primary outcome
Our primary outcome of this trial was feasibility measured by recruitment rate and participant retention. We considered success as 30% recruitment rate, and 90% retention of study participants at 4 months (final assessment).
Secondary outcomes
Participant demographic measures including sex, age, living situation, and pre-fracture mobility were taken at baseline. Our secondary outcomes were outcomes of interest for a proposed future larger RCT. Thus, we determined statistical trends on quality of life at 4 months post fall-related hip fracture in community-dwelling older adults, as measured by EQ5D-5L.23 This five-question measure is easy to administer and can be used in cost-effectiveness studies to evaluate clinical interventions.23 Using this measure, respondents are asked to rate their health states for five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has five levels with one indicating no problems and five indicating extreme problems. In addition, respondents are asked to rate their health on a visual analog scale, a 20 cm scale with the bottom endpoint labeled “the worst health you can imagine” and the top labeled “the best health you can imagine” (EQ-VAS [Euro-Qol Visual Analogue Scale for health related quality of life]).
We requested participants to complete the following self-report questionnaires: Hospital Anxiety and Depression Scale, Short-form Falls Efficacy Scale – International,24 and ICEpop Capability measure for Older people.25,26 We also measured participants’ grip strength using the standardized method as described by the American Society of Hand Surgery27 and the 4 m gait speed test28 and report the mean of three trials. We assigned a clinical frailty score (ranging from 1, very fit to 9, terminally ill) to each participant based on his/her function in the week prior to admission,29 as well as at 4 months after surgery. We monitored falls (defined as “any event when the participant unexpectedly came to rest on the ground, floor, or another lower level”20) via a prospective self-reported daily falls diary, provided to each participant at discharge, and monitored monthly by telephone calls by a research assistant blinded to group allocation.
Sample size
We aimed to recruit sufficient participants for our feasibility measures and to calculate estimates of variability for the outcome measures, and to generate a preliminary estimate of effect for the intervention.
Randomization
All participants were randomly assigned 1:1 to the intervention or control group by remote allocation following baseline assessment, and hospital discharge. Treatment allocation was concealed, as an independent statistician from an off-site consulting firm generated the allocation sequence using randomized blocks of varying size. We stratified randomization by sex, to allow for equal numbers of men in each group, and by age group, that is, 60–75 years and 76+ years.
Blinding
The study coordinator maintained the randomization outcome for all participants, and treatment allocation was concealed to the investigators, measurement team, and data entry assistants/analysts. Participants, the study coordinator, and the RPT who was delivering the intervention were not blinded to treatment allocation.
Statistical analysis
We summarized the sociodemographic characteristics of the study participants using counts and proportions for categorical data and means and standard deviations for continuous data or medians and 10th and 90th percentiles if appropriate. To evaluate feasibility, we calculated recruitment and retention rates and report percentage. For the health outcome variables, we estimated average change by fitting separate linear regression models for each of the health outcome variables using group allocation as the only independent variable including baseline values as covariates. Second, we estimated the average change in outcome measures across control and intervention group. We report the regression coefficients and P-values for the group allocation variable, and R2 values from the regression analyses to provide an estimate of model fit. Further, we estimated confidence intervals and standard errors of intervention effects and changes over time irrespective of intervention group for these variables through nonparametric bootstrapping using 1,000 resamples with random seed set to a value of 2014. We used Stata version 12 (StataCorp, College Station, TX, USA).
Results
Primary outcome
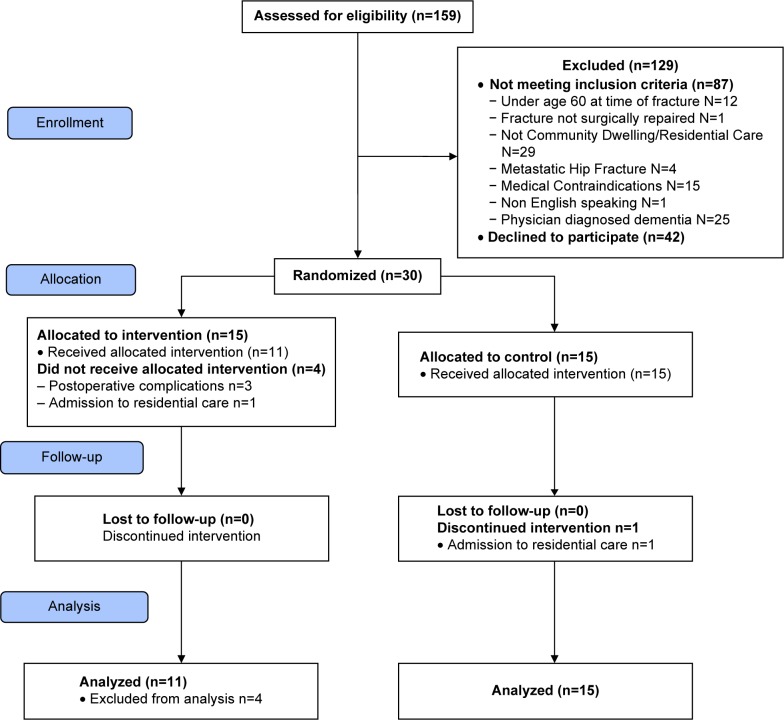
Our primary outcome for this trial was feasibility measured by recruitment rate and participant retention. We screened 159 older adults with hip fracture for eligibility from November 2013 to May 2014. There were 87 people who did not meet our inclusion criteria, and the main reasons for exclusion were the following: participant was not community dwelling (n=29), had physician-diagnosed dementia (n=25), or had medical contraindications precluding participation (n=15). Of the eligible participants, 42 declined to enroll in the study, primarily due to feeling overwhelmed by the sudden hospitalization. There were 30 participants who consented to the study, representing a 42% recruitment rate. The retention rate at 4 months was 90% (27/30 participants) including N=15 control and N=11 intervention participants (Figure 1). (One intervention participant remained in the study but completed only a qualitative interview, declining final assessment at 4 months due to a medical concern).
Figure 1.
Back to the future flow diagram.
Secondary outcomes
Demographic and descriptive characteristics of participants allocated to each group are summarized in Table 2. At baseline, participants in the intervention group did not differ significantly from participants in the control group with regard to their demographic data.
Table 2.
Baseline demographic descriptives and objective and self-reported secondary outcomes at two time points: baseline and final assessment at 4 months
| Baseline
|
Final
|
|||
|---|---|---|---|---|
| Intervention (N=15) | Control (N=15) | Intervention (N=11) | Control (N=15) | |
| Age, mean (SD) (years) | 83 (8) | 82 (10) | 82 (9) | 81 (10) |
| Sex | ||||
| Female | 11 | 8 | 7 | 8 |
| Male | 4 | 7 | 4 | 7 |
| Living situation | ||||
| Alone | 9 | 4 | 5 | 5 |
| With others | 6 | 11 | 6 | 10 |
| Pre-fracture mobility aid | ||||
| Y | 3 | 4 | 0 | 4 |
| N | 12 | 11 | 11 | 11 |
| Grip strength, mean (SD) | 20.49 (8.65) | 20.25 (13.15) | 20.39 (10.48) | 15.92 (9.23) |
| Gait speed (in m/s), mean (SD) | 0.22 (0.12) | 0.26 (0.13) | 0.83 (0.24) | 0.83 (0.29) |
| EQ-VAS (out of 100), mean (SD) | 60.00 (18.98) | 56.87 (20.07) | 75.72 (16.14) | 77.00 (17.09) |
| ICECAP-O, mean (SD) | 0.79 (0.14) | 0.73 (0.16) | 0.85 (0.12) | 0.88 (0.12) |
Abbreviations: SD, standard deviation; VAS, visual analog scale; ICECAP-O, ICEpop CAPability measure for Older people; EQ-VAS, EuroQol visual analogue scale.
As a pilot study, we were not powered to detect significant differences in study outcomes, and there were no statistically significant differences between groups at follow-up for the objective and self-reported secondary outcome measures (Tables 3 and 4). There was no difference in the number of falls between groups: one participant in each of the control and intervention groups fell twice during the study data collection.
Table 3.
Average difference between groups for follow-up measures adjusted for baseline
| Adjusted for baseline | |
|---|---|
| Grip strength | |
| P-value | 0.10 |
| Model R2 | 0.49 |
| β coefficient [95% CI] | −5.12 [−11.12, 0.90] |
| Gait speed | |
| P-value | 0.70 |
| Model R2 | 0.20 |
| β coefficient [95% CI] | −0.04 [−0.24, 0.17] |
| EQ-VAS | |
| P-value | 0.85 |
| Model R2 | 0.01 |
| β coefficient [95% CI] | 1.28 [−12.95, 13.54] |
| ICECAP-O | |
| P-value | 0.71 |
| Model R2 | 0.01 |
| β coefficient [95% CI] | 0.02 [−0.09, 0.16] |
Abbreviations: CI, confidence interval; VAS, visual analog scale; ICECAP-O, ICEpop CAPability measure for Older people; EQ-VAS, EuroQol visual analogue scale.
Table 4.
Average change over time (all participants)
| Mean change over time (baseline minus follow-up)
|
|
|---|---|
| Mean [95% CI] | |
| Grip strength | −3.09 [−6.93, 0.76] |
| Gait speed | −0.59 [−0.68, −0.49]* |
| EQ-VAS | 18.19 [7.62, 28.77]* |
| ICECAP-O | 0.12 [0.04, 0.20]* |
Note:
Significant difference between baseline and 4-month follow-up.
Abbreviations: CI, confidence interval; VAS, visual analog scale; ICECAP-O, ICEpop CAPability measure for Older people; EQ-VAS, EuroQol visual analogue scale.
There was a statistically significant difference between baseline and 4 months for gait speed and measures of quality of life for all participants (Table 4). There were no 30-day readmissions of study participants in either group.
Discussion
Our findings support the feasibility of in-hospital recruitment and retention of older adults in a research study designed to evaluate the delivery of a face-to-face hip fracture self-management intervention and follow-up telephone calls. Our recruitment rate, at 42%, can be attributed to working closely with the clinical staff, surveillance, ensuring that participants were medically and psychologically ready before approaching them with study details, as well as to almost daily communication between the research and clinical teams. Further, although our study was not powered to detect differences between groups, valuable information was gathered to generate future hypotheses, and plan for a larger trial. In particular, we are interested in the effect of telephone coaching on functional outcomes, specifically gait speed. Our pilot study had 15.3 (or a B=0.153) power to detect a clinically important difference (at P<0.05) of 0.1 m/s in gait speed between the study groups. To detect a statistically significant between-group difference of 1 m/s (or d=0.37) at P<0.05, with 80% power (or B=0.80), results of the power analysis that use pooled standard deviations obtained from the pilot indicate that at least 116 participants per group would be required to show a minimal clinically important difference between groups for gait speed in a definitive RCT. However, as pilot studies may be imprecise in the calculation of sample size, it may be preferable to inflate these estimates to adjust for this lack of precision when planning for larger scale trials.30
Our study suggests that telephone coaching is feasible to promote health behaviors for older adults after a recent hip fracture. However, the skill of the therapist delivering the intervention must be considered in behavioral change protocols, since a strong therapeutic alliance is likely to show more favorable treatment outcomes.31 The physiotherapist providing the intervention in our study was an experienced orthopedic clinician, and we ensured capability of delivering the intervention as planned by conducting training in advance of, and during the study. This included coaching sessions in health-related behaviors, discussions on the theoretical underpinnings of transitions in care, and behavioral change theory and relapse prevention training.10 Detailed training was also provided on the content of each of the five telephone follow-up calls (Table 1), and a comprehensive protocol manual and skills checklist were provided to the research therapist to ensure protocol fidelity.32
Also, we note that telephone-delivered health behavior change interventions can be integrated within health care and population health delivery systems to close health care gaps and enhance the transition from hospital to home.
It takes considerable physical and mental effort to be able to manage recovery after hip fracture: coordinating medications, exercises, awareness of warning signs that may signal health deterioration, and accessing and using health care services create considerable burden, and this effort is increased in people with multi-morbidity.33,34 Thus, our study was motivated by the knowledge that older adults and their caregivers require abundant support to be able to self-manage after hospital discharge. However, despite best intentions, the implementation of discharge plans may be hampered by both person factors (such as distraction, being overwhelmed, or poor health literacy), as well as staff and system factors (such as lack of time for individualized and personalized teaching).35,36 Therefore, our study was designed with both staff and person factors in mind, to assess feasibility and acceptability of the delivery of enhanced discharge teaching materials, plus additional telephone support to lessen the burden of self-care after discharge. Evidence supporting the efficacy of interventions to support capacity to enact self-care after hospital discharge is particularly emphasized in a recent meta-analysis and systematic review analyzing transition support to reduce the risk of rehospitalization.33
Adherence and fidelity to the prescribed program is an important feature of behavioral modification studies.32 All participants in our study were individually prescribed and taught exercises for mobility and strength recovery in the acute hospital setting, and encouraged to gradually increase their walking tolerance at home. Adherence to the in-hospital prescribed exercises, and participant goal attainment, was monitored by the RPT at each follow-up telephone call via a daily exercise log sheet and falls diary, and assessment and feedback were provided to problem-solve barriers to participation (such as pain, fatigue, and lack of motivation).
We also included a number of safeguards to our protocol to ensure that the intervention was delivered as designed. Adherence and fidelity to the program delivery was monitored by the number of telephone sessions, as well as length (in minutes) of telephone contact, a comprehensive written report and checklist of the content delivered and discussed, including action and coping plans. During each phone call, the previous goal attainment and future goal setting plans by each participant was recorded. We also consider that adherence to the protocol may have been enhanced by the personalized in-hospital visit and assessment of participants by the research therapist, when mutual trust could be developed prior to the follow-up phone calls in the community setting. This strategy has been used successfully in other transition programs.6
Clinical questions requiring further clarity include investigating whether there may be a dose–response relationship of postdischarge telephone coaching, the effects of individual participants pre-fracture mobility, resilience, disposition, and preferences: although a recent systematic review emphasizes the effectiveness of telephone interventions for promotion of physical activity, including in older adults,37 we cannot extrapolate these successes to older adults who have been recently hospitalized with hip fracture, and who are often struggling to assume independent care despite pain, mobility challenges, cognitive impairment, and fatigue.3 Moreover, there is limited evidence as to optimal timing, delivery, and duration of telephone follow-up, with some studies showing clinically equivalent results for telephone follow-up and control groups.14
We note several limitations. Our feasibility study was underpowered to detect statistically significant differences between groups. Both groups received the 1-hour education intervention, videos, and teach-back components; thus, we believe that usual care was altered. In addition, most of our participants in both the control and intervention groups also received community-based physiotherapy either in the home or in outpatient setting, and these factors may have diluted our results. This ready access to professional assistance may not be so readily available in rural settings; thus, our findings are not generalizable. In addition, older adults may not benefit from telephone interventions after hospitalization if they are at extreme ends of the spectrum, that is, if they are already functioning very well, or conversely, if they have unrecognized cognitive impairment and multi-morbidity.38
A further limitation to our pilot study was that adherence to the prescribed amount of physical activity was subjectively reported by participants.
Last, the participants in our study did not receive a standardized “dose” of telephone interventions, as the telephone call length varied from person to person and time to time, based on clinical reasoning and participant preferences. Our future trial should investigate whether there is a dose–response relationship (complex behavioral changes may require longer telephone interventions), and further examine the fidelity of adherence and implementation.37
Conclusion
The specific aims of this pilot study were to evaluate the feasibility of recruitment and retention to a complex intervention including education and telephone coaching after hip fracture to determine if the study components could be delivered as expected. Patient education is a cornerstone of effective care, yet it may be ineffectively delivered in the shuffle of a busy clinical unit with competing demands. This intervention was delivered in a “real-world” setting, illustrating feasibility of recruiting and retaining older adults in the acute setting after hip fracture to deliver individualized bedside education, and follow-up phone calls after hospitalization to support recovery and mobility goals. Our pilot study supports a larger investigation to determine whether personalized bedside education and postdischarge telephone follow-up influence quality of life, functional outcomes, and short-term readmission rates, and future work will also include optimizing delivery of material, investigating the older adults retention of, and satisfaction with the delivery of, educational material for self-management and recovery after hip fracture.
Acknowledgments
The authors extend sincere thanks to study participants for their generosity with their time, and the staff of the hospital unit where the study took place. The authors also thank Dr Pierre Guy for his support and advice, Mr Joseph Puyat for his assistance with study design and data analyses, research assistants Samantha Gray and Megan McAllister, and Chantalle Jack, librarian at Vancouver Coastal Health for assistance retrieving papers for the literature review. The authors gratefully acknowledge financial support from the Vancouver Coastal Health Research Institute/North Shore Health Research Foundation, North Vancouver (F13-02469), the Vancouver General Hospital Foundation, and the University of British Columbia Foundation, and career award support to Dr Ashe from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. The sponsors had no role in the study design, analysis, or preparation of this manuscript.
Footnotes
Author contributions
Authors DPL and MCA contributed to the study concept and design. DPL, KCB, MF, ML, JL, and KP contributed to the acquisition of subjects and/or data. All authors contributed to the analysis and interpretation of data, and took part in either drafting or revising the manuscript.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Canadian Institute for Health Information (CIHI) All-Cause Readmission to Acute Care and Return to the Emergency Department. 2012. 1823. [Accessed December 21, 2014]. Available from: https://secure.cihi.ca/estore/productFamily.htm?locale=en&pf=PFC.
- 2.Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305–1311. doi: 10.1001/archinte.167.12.1305. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM. Post-hospital syndrome – an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makaryus AN, Friedman EA. Patients’ understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994. doi: 10.4065/80.8.991. [DOI] [PubMed] [Google Scholar]
- 5.Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52(11):1817–1825. doi: 10.1111/j.1532-5415.2004.52504.x. [DOI] [PubMed] [Google Scholar]
- 6.Coleman EA. The Care Transitions Program. 2013. [Accessed December 21, 2014]. Available from: http://www.caretransitions.org/definitions.asp.
- 7.Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–427. doi: 10.1002/jhm.2054. [DOI] [PubMed] [Google Scholar]
- 8.Dhalla IA, O’Brien T, Morra D, et al. Effect of a postdischarge virtual ward on readmission or death for high-risk patients: a randomized clinical trial. JAMA. 2014;312(13):1305–1312. doi: 10.1001/jama.2014.11492. [DOI] [PubMed] [Google Scholar]
- 9.Rennke S, Ranji SR. Transitional care strategies from hospital to home: a review for the neurohospitalist. Neurohospitalist. 2015;5(1):35–42. doi: 10.1177/1941874414540683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- 11.Hesselink G, Zegers M, Vernooij-Dassen M, et al. European HANDOVER Research Collaborative Improving patient discharge and reducing hospital readmissions by using Intervention Mapping. BMC Health Serv Res. 2014;14:389. doi: 10.1186/1472-6963-14-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goode AD, Reeves MM, Eakin EG. Telephone-delivered interventions for physical activity and dietary behavior change: an updated systematic review. Am J Prev Med. 2012;42(1):81–88. doi: 10.1016/j.amepre.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006;4:CD004510. doi: 10.1002/14651858.CD004510.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N, Fujimoto J, Karliner L. Evaluation of a primary care-based post-discharge phone call program: keeping the primary care practice at the center of post-hospitalization care transition. J Gen Intern Med. 2014;29(11):1513–1518. doi: 10.1007/s11606-014-2942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Center for Hip Health and Mobility FReSH START Toolkit: A Fracture Recovery Guide for Patients and Families. 2014. [Accessed March, 2015]. Available from: http://www.hiphealth.ca/blog/FReSHStart.
- 17.Schiller C, Franke T, Belle J, Sims-Gould J, Sale J, Ashe MC. Words of wisdom – patient perspectives to guide recovery for older adults after hip fracture: a qualitative study. Patient Prefer Adherence. 2015;9:57–64. doi: 10.2147/PPA.S75657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- 22.Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 24.Kempen GI, Yardley L, van Haastregt JC, et al. The Short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing. 2008;37(1):45–50. doi: 10.1093/ageing/afm157. [DOI] [PubMed] [Google Scholar]
- 25.Coast J, Flynn TN, Natarajan L, et al. Valuing the ICECAP capability index for older people. Soc Sci Med. 2008;67(5):874–882. doi: 10.1016/j.socscimed.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Coast J, Peters TJ, Natarajan L, Sproston K, Flynn T. An assessment of the construct validity of the descriptive system for the ICECAP capability measure for older people. Qual Life Res. 2008;17(7):967–976. doi: 10.1007/s11136-008-9372-z. [DOI] [PubMed] [Google Scholar]
- 27.American Society for Surgery of the Hand . The Hand: Examination and Diagnosis. New York: Churchill Livingstone; 1990. [Google Scholar]
- 28.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15:264. doi: 10.1186/1745-6215-15-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J Consult Clin Psychol. 2000;68(3):438–450. [PubMed] [Google Scholar]
- 32.Borrelli B, Sepinwall D, Ernst D, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol. 2005;73(5):852–860. doi: 10.1037/0022-006X.73.5.852. [DOI] [PubMed] [Google Scholar]
- 33.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sav A, Kendall E, McMillan SS, et al. ‘You say treatment, I say hard work’: treatment burden among people with chronic illness and their carers in Australia. Health Soc Care Community. 2013;21(6):665–674. doi: 10.1111/hsc.12052. [DOI] [PubMed] [Google Scholar]
- 35.Flacker J, Park W, Sims A. Hospital discharge information and older patients: do they get what they need? J Hosp Med. 2007;2(5):291–296. doi: 10.1002/jhm.166. [DOI] [PubMed] [Google Scholar]
- 36.Naylor MD. Transitional care of older adults. Annu Rev Nurs Res. 2002;20:127–147. [PubMed] [Google Scholar]
- 37.Goode AD, Winkler EA, Lawler SP, Reeves MM, Owen N, Eakin EG. A telephone-delivered physical activity and dietary intervention for type 2 diabetes and hypertension: does intervention dose influence outcomes? Am J Health Promot. 2011;25(4):257–263. doi: 10.4278/ajhp.090223-QUAN-75. [DOI] [PubMed] [Google Scholar]
- 38.Goldman LE, Sarkar U, Kessell E, et al. Support from hospital to home for elders: a randomized trial. Ann Intern Med. 2014;161(7):472–481. doi: 10.7326/M14-0094. [DOI] [PubMed] [Google Scholar]