Abstract
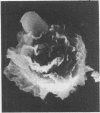
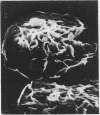
Pulmonary alveolar macrophages were obtained by saline lavage from 23 healthy male volunteers--10 non-smokers and 13 cigarette smokers. Lavage produced three times as many alveolar macrophages in smokers than in non-smokers. When macrophages from smokers and from non-smokers were incubated in vitro, more cells from smokers adhered to glass, spread out, and showed enhanced nitroblue tetrazolium (NBT) reduction. The surface morphology of alveolar macrophages from smokers showed more with a plate like appearance and ridge like membrane surface, while the macrophages from non-smokers were predominantly spherical with ruffles. The proportions of cells which stained highly for beta galactosidase were 55% in smokers and 11% in non-smokers. Thus, in a resting state in vitro, alveolar macrophages from smokers were more active than those from non-smokers. When, however, macrophages from smokers and non-smokers were incubated with immunobeads and with opsonised or non-opsonised BCG, the phagocytic activity and stimulated NBT reduction of alveolar macrophages from smokers were similar to or somewhat less than those of non-smokers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M. Macrophage activation in tuberculin reactions of rabbits with primary BCG infection and reinfection. J Reticuloendothel Soc. 1973 Aug;14(2):132–145. [PubMed] [Google Scholar]
- Ando M., Suga M., Shima K., Sugimoto M., Higuchi S. Different effects of phytohemagglutinin-activated lymphocytes and their culture supernatants on macrophage function. Infect Immun. 1976 May;13(5):1442–1448. doi: 10.1128/iai.13.5.1442-1448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Suga M., Shima K., Sugimoto M., Tokuomi H. Activation of alveolar macrophages exposed to lavage-procured immunoglobulin G obtained from normal rabbit lungs. Infect Immun. 1978 May;20(2):476–484. doi: 10.1128/iai.20.2.476-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Suga M., Sugimoto M., Tokuomi H. Superoxide production in pulmonary alveolar macrophages and killing of BCG by the superoxide-generating system with or without catalase. Infect Immun. 1979 May;24(2):404–410. doi: 10.1128/iai.24.2.404-410.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Murrmann S. K., Davis J., Johnston R. B., Jr The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975 Sep;56(3):571–576. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest G. B., Hudson L. D., Altman L. C. Impaired alveolar macrophage chemotaxis in patients with acute smoke inhalation. Am Rev Respir Dis. 1979 Feb;119(2):279–286. doi: 10.1164/arrd.1979.119.2.279. [DOI] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L., Huber G. L. The effects of experimental exposure to tobacco smoke on the oxidative metabolism of alveolar macrophages. J Reticuloendothel Soc. 1979 Jun;25(6):597–604. [PubMed] [Google Scholar]
- Finch G. L., Fisher G. L., Hayes T. L., Golde D. W. Morphological studies of cultured human pulmonary macrophages. Scan Electron Microsc. 1980;(3):315–326. [PubMed] [Google Scholar]
- Finch G. L., Fisher G. L., Hayes T. L., Golde D. W. Surface morphology and functional studies of human alveolar macrophages from cigarette smokers and nonsmokers. J Reticuloendothel Soc. 1982 Jul;32(1):1–23. [PubMed] [Google Scholar]
- Fisher G. L., McNeill K. L., Finch G. L., Wilson F. D., Golde D. W. Functional evaluation of lung macrophages from cigarette smokers and nonsmokers. J Reticuloendothel Soc. 1982 Oct;32(4):311–321. [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. O., Swenson E. W., Johnson J. E., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Niewoehner D. E. Lung phagocyte recruitment and metabolic alterations induced by cigarette smoke in humans and in hamsters. Am Rev Respir Dis. 1982 Sep;126(3):548–552. doi: 10.1164/arrd.1982.126.3.548. [DOI] [PubMed] [Google Scholar]
- Horio S., Ando M., Ukeshima A., Sugimoto M., Tokuomi H. Surface morphology and function of alveolar macrophages exposed to lung lavage fluids and serum from normal rabbits. J Reticuloendothel Soc. 1981 Feb;29(2):137–152. [PubMed] [Google Scholar]
- Imanishi K., Ando M., Ideta T., Tokuomi H. Histochemical studies of lysosomal enzyme and nitroblue tetrazolium reduction of phagocytes in the cerebrospinal fluid of patients with infectious diseases of the central nervous system. Acta Neurol Scand. 1981 Jul;64(1):54–65. doi: 10.1111/j.1600-0404.1981.tb04385.x. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann P. E., Cohen A. B., Finley T. N., Ladman A. J. Alveolar macrophages. Structural and functional differences between nonsmokers and smokers of marijuana and tobacco. Lab Invest. 1971 Aug;25(2):111–120. [PubMed] [Google Scholar]
- Martin R. R. Altered morphology and increased acid hydrolase content of pulmonary macrophages from cigarette smokers. Am Rev Respir Dis. 1973 Apr;107(4):596–601. doi: 10.1164/arrd.1973.107.4.596. [DOI] [PubMed] [Google Scholar]
- Park B. H., Fikrig S. M., Smithwick E. M. Infection and nitroblue-tetrazolium reduction by neutrophils. A diagnostic acid. Lancet. 1968 Sep 7;2(7567):532–534. doi: 10.1016/s0140-6736(68)92406-9. [DOI] [PubMed] [Google Scholar]
- Rasp F. L., Clawson C. C., Hoidal J. R., Repine J. E. Reversible impairment of the adherence of alveolar macrophages from cigarette smokers. Am Rev Respir Dis. 1978 Dec;118(6):979–986. doi: 10.1164/arrd.1978.118.6.979. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Kazmierowski J. A., Newball H. H. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975 Aug;56(2):376–385. doi: 10.1172/JCI108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., White R. R., Senior R. M., Levine E. A. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. doi: 10.1126/science.910131. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Higuchi S., Ando M., Horio S., Tokuomi H. The effect of cytochalasin B on the superoxide production by alveolar macrophages obtained from normal rabbit lungs. J Reticuloendothel Soc. 1982 Feb;31(2):117–130. [PubMed] [Google Scholar]
- Territo M. C., Golde D. W. The function of human alveolar macrophages. J Reticuloendothel Soc. 1979 Jan;25(1):111–120. [PubMed] [Google Scholar]
- Warr G. A., Martin R. R. Chemotactic responsiveness of human alveolar macrophages: effects of cigarette smoking. Infect Immun. 1974 Apr;9(4):769–771. doi: 10.1128/iai.9.4.769-771.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G. A., Martin R. R. Histochemical staining and in vitro spreading of human pulmonary alveolar macrophages: variability with cigarette smoking status. J Reticuloendothel Soc. 1978 Jan;23(1):53–62. [PubMed] [Google Scholar]